Abstract
Drug resistant epilepsy affects ~30% of people with epilepsy and is associated with epilepsy syndromes with frequent and multiple types of seizures, lesions or cytoarchitectural abnormalities, increased risk of mortality and comorbidities such as cognitive impairment and sleep disorders. A limitation of current preclinical models is that spontaneous seizures with comorbidities take time to induce and test, thus making them low-throughput. Kcna1-null mice exhibit all the characteristics of drug resistant epilepsy with spontaneous seizures and comorbidities occurring naturally; thus, we aimed to determine whether they also demonstrate pharmacoresistanct seizures and the impact of medications on their sleep disorder comorbidity. In this exploratory study, Kcna1-null mice were treated with one of four conventional antiseizure medications, carbamazepine, levetiracetam, phenytoin, and phenobarbital using a moderate throughput protocol (vehicle for 2 days followed by 2 days of treatment with high therapeutic doses selected based on published data in the 6 Hz model of pharmacoresistant seizures). Spontaneous recurrent seizures and vigilance states were recorded with video-EEG/EMG. Carbamazepine, levetiracetam and phenytoin had partial efficacy (67%, 75% and 33% were seizure free, respectively), whereas phenobarbital was fully efficacious and conferred seizure freedom to all mice. Thus, seizures of Kcna1-null mice appear to be resistant to three of the drugs tested. Levetiracetam failed to affect sleep architecture, carbamazepine and phenytoin had moderate effects, and phenobarbital, as predicted, restored sleep architecture. Data suggest Kcna1-null mice may be a moderate throughput model of drug resistant epilepsy useful in determining mechanisms of pharmacoresistance and testing novel therapeutic strategies.
Keywords: phenobarbital, phenytoin, carbamazepine, levetiracetam, diurnal, Kv1.1
Graphical Abstract
1. Introduction
Most people with epilepsy attain seizure freedom with antiseizure medications, however, one-third of patients have seizures that are refractory to current antiseizure medications (Kwan and Brodie, 2000; Chen et al., 2018; Kalilani et al., 2018; Blond et al., 2020). Epilepsy is considered drug-resistant when sustained seizure freedom is not accomplished with the trial of two adequately dosed medications (Kwan et al., 2010). This failure rate is independent of initial drug treatment or subsequent drugs but may be associated with seizure types. In humans, risk analyses have identified that focal and generalized seizures associated with lesions or cytoarchitectural abnormalities, high seizure frequency, high interictal spike frequency and early onset of epilepsy are among the most predictive factors of drug-resistant epilepsy (Kalilani et al., 2018). Drug resistant epilepsy is associated with epilepsy syndromes exhibiting multiple seizure types, increased risk of mortality, sudden unexpected death in epilepsy (SUDEP) and co-morbidities such as cognitive impairment and sleep disorders (Löscher, 2011; Jehi, 2016; Strzelczyk et al., 2017; Gavrilovic et al., 2019; Bergmann et al., 2020).
There are several animal models of drug resistant epilepsy (Löscher, 2011), however, the lack of development of effective therapies for the pharmacoresistant population may be hampered by the reliance on models that do not fully recapitulate the drug resistant epilepsy phenotype. Each of the current models has limitations including having a singular seizure type, limited pharmacoresistance, increased susceptibility to toxicity, or lacking drug resistant epilepsy co-morbidities, among others (Löscher, 2011). The development of additional models that more closely resemble the phenotypic context of drug resistant epilepsy and thus complement the current models may improve the ability to discover and develop effective treatments.
Kcna1 global knockout mice exhibit multiple characteristics associated with drug resistant epilepsy. The KCNA1 gene encodes the alpha subunit of the Kv1.1 voltage-dependent potassium channel. Several point mutations in the KCNA1 gene identified in humans are associated with epilepsy and result in near complete reduction of channel function in a dominant negative manner (Paulhus et al., 2020). Other KCNA1 genetic alterations, such as a de novo gain in copy number variant, are associated with drug resistant epilepsy and sudden unexpected death in epilepsy (SUDEP) (Klassen et al., 2014). Kcna1-null mice experience spontaneous myoclonic, focal and generalized seizures multiple times per day, and die suddenly between the fifth and tenth postnatal week (in our colony 50% of mice die by postnatal day 46), thus these mice are also considered a model of SUDEP (Smart et al., 1998; Wenzel et al., 2007; Moore et al., 2014; Simeone et al., 2016, 2018; Iyer et al., 2020). Seizures range from mild to severe. The focal to bilateral tonic-clonic seizures in these mice have been shown to arise in the hippocampus and propagate to cortical and subcortical regions (Wenzel et al., 2007; Roundtree et al., 2016). Kcna1-null mice develop sleep disorder comorbidities including increased latency to sleep onset, increased time spent awake, and decreased rapid eye movement sleep (REMS) and non-REMS (NREMS) (Roundtree et al., 2016). The co-morbid sleep disorder may worsen seizures and/or vice versa. Kcna1-null mice also have cognitive impairments and co-morbid cardiorespiratory abnormalities that may contribute to SUDEP (Kim et al., 2015; Dhaibar et al., 2019; Simeone et al., 2018; Iyer et al., 2020). These attributes resemble the risk factors associated with human drug resistant epilepsy. Here, in this initial exploratory study to determine whether Kcna1-null mice are resistant to conventional antiseizure medications, we tested phenytoin, carbamazepine, levetiracetam, and phenobarbital. Using a within subject, moderate throughput experimental paradigm, we performed continuous video-EEG/EMG monitoring during multiple days of vehicle and drug administration to determine rates of seizure freedom and effects of the antiseizure medications on the sleep comorbidity.
2. Methods and Methods
2.1. Animals:
Breeding pairs of heterozygous Kcna1-null mice on a C3HeB/FeJ congenic background were purchased from Jackson Laboratories (Bar Harbor, Maine) and the colony has been maintained since 2009 in the Animal Resource Facility at Creighton University School of Medicine. Mice were given food and water ad libitum and kept on a 12-hour light/dark cycle with lights on at zeitgeber time 00:00 hr and lights off at ZT12:00 hr. Tail clips were collected on postnatal day 12–15 and genotypes were determined by Transnetyx, Inc (Cordova, TN, USA). Experimental groups contained equal numbers of male and female mice. All experiments conformed to NIH guidelines in accordance with the United States Public Health Service’s Policy on Humane Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Creighton University School of Medicine (Protocol No. 0875).
2.2. Electroencephalography (EEG) and electromyography (EMG) neurosurgeries:
Mice (postnatal day 30–33) were allowed to habituate for 5 days in a round (10” diameter × 10” height) transparent plexiglass cage used during tethered EEG recordings and. On postnatal day 35–38, mice were implanted with EEG/EMG electrodes as we have previously described (Fenoglio-Simeone et al., 2009a,b; Roundtree et al., 2016; Simeone et al., 2014, 2016, 2017). Mice were anesthetized with isoflurane (5% initiation and 3% maintenance) and two subdural, ipsilateral cortical electrodes were implanted at 1.2 mm anterior to Bregma and 1 mm lateral to midline. A reference electrode was implanted 1.5 mm posterior to bregma and 1 mm lateral to midline, contralateral from recording electrodes. EMG electrode wires were inserted into the nuchal muscles in all mice. Electrodes were soldered to the head mount (Pinnacle Technologies, Inc., Lawrence, KS, USA), and secured to the skull with dental cement. During 5–7-day recovery and throughout the experiment, mice were housed individually and were given access to food and water ad libitum.
2.3. Drug administration:
Following recovery, mice (postnatal day 42–43) were injected intraperitoneally with the appropriate vehicle matching the subsequent antiseizure medication (sterile saline or 0.5% carboxy methylcellulose (CMC)) once daily between zeitgeber time 01:30 – 02:00 hrs for two consecutive days, followed by two days of once daily phenobarbital (30 mg kg−1 in saline), phenytoin (30 mg kg−1 in 0.5% CMC), carbamazepine (30 mg kg−1 in 0.5% CMC) or levetiracetam (200 mg kg−1 in saline). These doses represent high therapeutic doses in other models of drug resistance in mice and rats (Koneval et al., 2018; Metcalf et al., 2019; Thomson et al., 2020). Video-EEG-EMG recordings were started after injection using a time-synched infrared video surveillance system (Pinnacle Technology, Inc.) as we have described (Fenoglio-Simeone et al., 2009a,b; Roundtree et al., 2016; Simeone et al., 2014, 2016, 2017).
2.4. Seizure analysis:
EEG recordings were imported into Spike2 v7 software (Cambridge Electronic Design, Cambridge, England. U.K.) for initial seizure identification using short-time fast Fourier transform time-frequency analysis. Subsequently, behavioral aspects of EEG seizures were confirmed using video recordings and Sirenia software (Pinnacle Technology, Inc.). Behavioral manifestations were manually scored and verified by 2 blinded investigators. Seizures were identified based on ictal cortical EEG activity, high EMG activity and previously defined seizure behaviors for this mouse strain. Seizure behavior was scored using a modified Racine scale for generalized seizures: Type 1 – myoclonic jerk; Type 2 – head stereotypy; Type 3 – bilateral clonus manifested as hunched, forelimb clonus with or without rearing; Type 4 – hindlimb clonus with a head tilt, tail extension with 1 or 2 rearing and falling events; Type 5 – bilateral clonus and continuous rearing and falling of 3 or more times; Type 6 – tonic-clonic seizures involving running, energetic myoclonic jumping, falling, limb tonus and clonus. Seizure burden (Σseizure Type2–6 × seizure duration) was calculated as we have previously described (Roundtree et al., 2016; Simeone et al., 2014, 2016, 2017).
2.5. Sleep analyses:
Using Sirenia Sleep Pro (v1.4.2, Pinnacle Technologies, Inc.), recordings were divided into 10s epochs for sleep state scoring and analyzed using a semi-automated method as we have described previously (Roundtree et al., 2016). Recordings were initially scored into different sleep states based on EEG power in the delta band of the anterior cortical electrode, located over the motor cortices and the corpus callosum, and EMG power (10–50 Hz). Sleep states were defined as follows: Wake = low delta power, high EMG; NREMS = high delta power, low to medium EMG power; REMS = low delta power, low EMG. Scoring based on EEG and EMG was then manually verified with video recording. Importantly, the epochs during which an animal was seizing were excluded from the analyses.
2.6. Statistics:
Seizure and sleep data were analyzed using a one-way ANOVA with Sidak’s or Dunnett’s post hoc test or paired t-test where appropriate. For correlation analyses, statistical dependence was determined using Spearman’s non-parametric rank correlation coefficient. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Dependence of seizure phenotypes on state of vigilance.
The epilepsy phenotype of Kcna1-null mice consists of interictal spikes, focal seizures and multiple generalized seizure types. Here, we focused on five different types of generalized seizures (Type 2–6). The EEG signature of all seizures began with an initial spike, followed by a brief quiescence and then high frequency activity with a concentrated band of beta frequency with (Types 2–4) or without (Types 5–6) harmonics in gamma or scattered increases in all frequencies in between (Types 3–6; Fig. 1A–E). Type 2 seizures were associated with behavioral automatisms and stereotypy consisting of head-bobbing and swaying (Fig. 1A). Type 3 seizures displayed bilateral clonus manifesting as hunched, forelimb clonus (Fig. 1B). Type 3 seizures could progress into Type 4 seizures with hindlimb clonus, a head tilt, tail extension and 1 or 2 rearing and falling events (Fig. 1C). Type 5 seizures involved bilateral clonus and continuous rearing and falling of 3 or more times (Fig. 1D). The most severe Type 6 seizures were tonic-clonic seizures that involved running, energetic myoclonic jumping, falling, limb tonus and clonus, and ended with the mouse going limp, then quickly recovering (Fig. 1E). On the EEG, high frequency activity ended abruptly for all seizure types. Type 3–6 seizures with clonus had post-ictal periodic spikes with delta representation which in some cases would lead into EEG depression.
Figure 1.
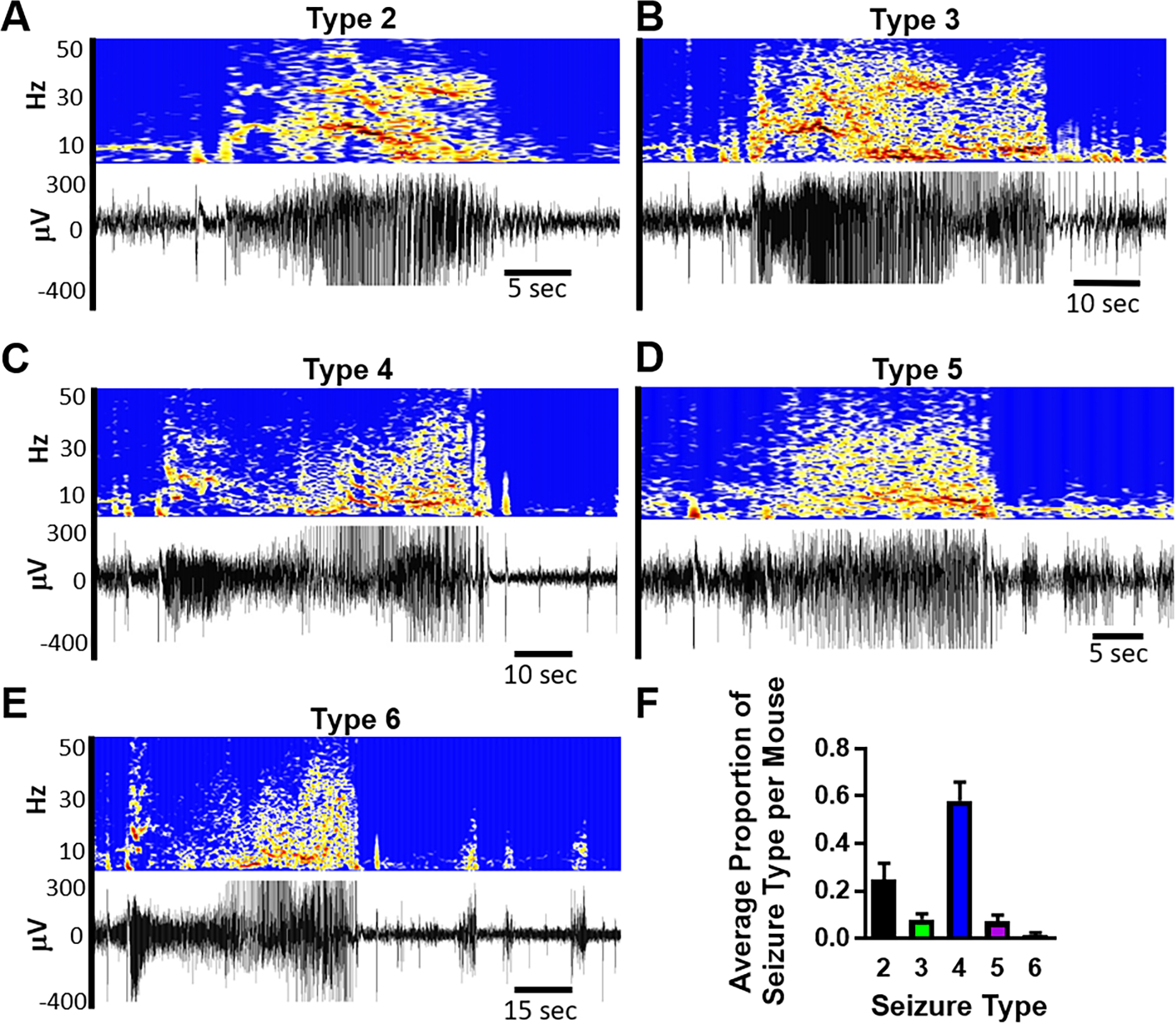
Kcna1-null mice exhibit multiple types of generalized seizures. (A-E) Examples of EEG recordings and spectrograms of Types 2–6 generalized seizures. (F) The proportion of seizure types expressed in individual mice (n = 22).
The most prevalent seizures on average for individual mice were Type 4 followed by Type 2 which together constitute about 80% of seizures (Fig. 1F). The daily frequency of generalized seizures varied between mice (5.2 ± 1.5 day−1; range 1 – 33; coefficient of variation = 1.36; n = 22), but the within mouse daily seizure frequency was more consistent with a smaller coefficient of variation of 0.53 ± 0.11. Daily seizure severity (averaged Racine Scale) was 3.48 ± 0.15 and daily seizure burden, a metric for accounting for the severity and duration of individual seizures (ΣType2–6 × duration), was 1052 ± 195.
We examined the generalized seizures during the rest and active phases. Generalized seizures occurred in a diurnal rhythm with a periodicity of 23.83 ± 0.35 hours, the zenith was at zeitgeber time 8:00 and nadir at zeitgeber time 20:00 (Fig. 2A), similar to previous reports for Kcna1-null mice (Fenoglio-Simeone et al., 2009b; Wright et al., 2016). Approximately two-thirds of generalized seizures occurred during the rest (light) phase (Fig. 2B).
Figure 2.
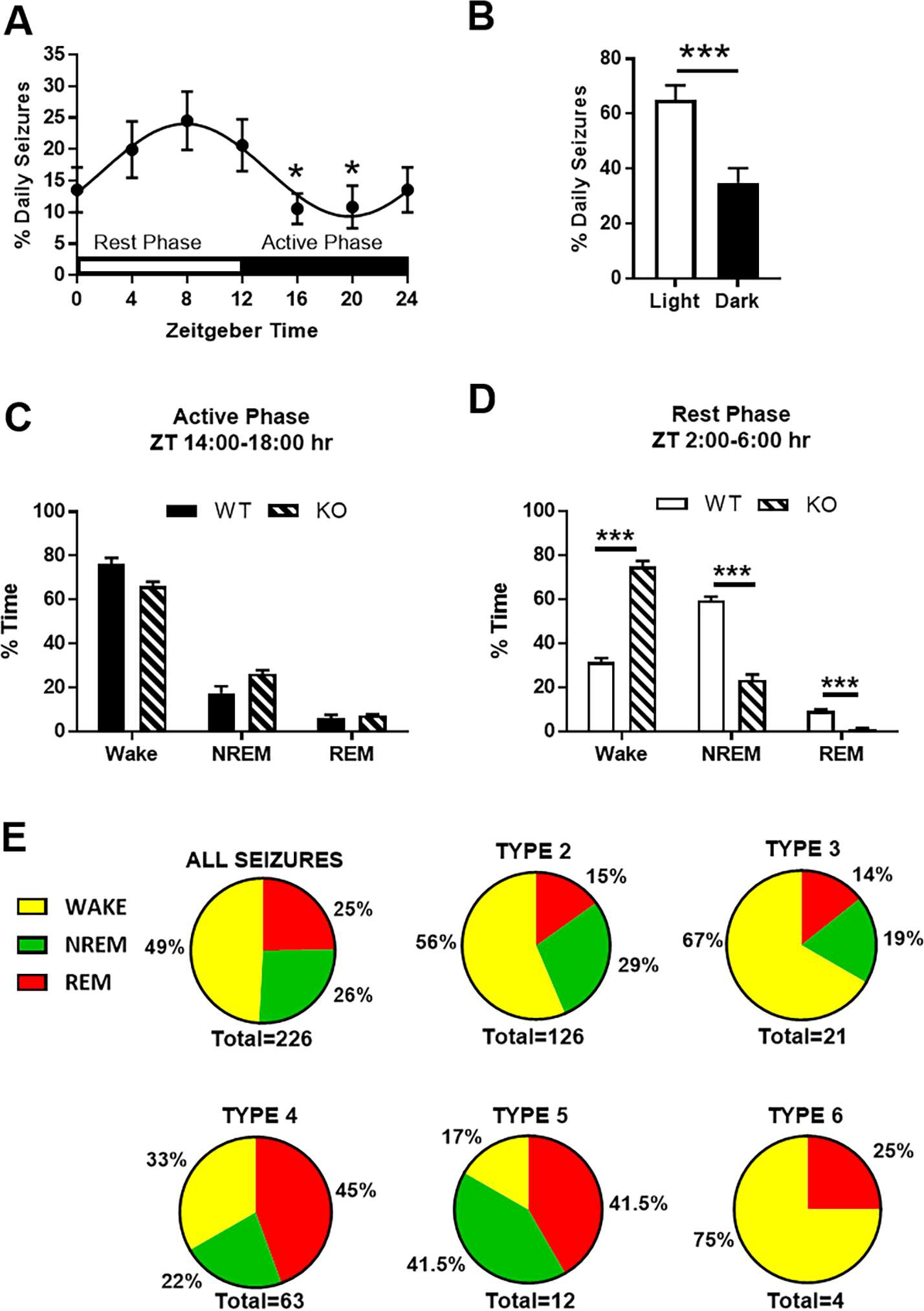
The diurnal relationship of seizures and sleep in Kcna1-null mice. (A) Seizures occurred with a diurnal rhythm with periodicity of 23.83 ± 0.35 hrs (non-linear regression: sine wave with non-zero baseline, wavelength constraint > 23 and phase shift constraint = −3.65). The zenith was at ZT8:00 and nadir at ZT20:00 (one-way repeated measures ANOVA, F = 2.285, p = 0.05; followed by Dunnett’s multiple comparisons test, *p < 0.05 vs ZT8:00; n =22 mice). (B) Overall, more seizures occurred during the light (rest) phase (paired t-test, ***p < 0.001; n = 22 mice). (C) Comparison of vigilance states between genotypes during the active (dark) phase (unpaired t-test, p > 0.05; n = 22 mice). (D) Comparison of vigilance states between genotypes during the rest (light) phase (unpaired t-test, ***p < 0.001; n = 22 mice). (E) Relationship of seizure types and vigilance states for all 226 seizures detected in 22 mice.
Sleep architecture was determined during the active (dark) and rest phases. Importantly, any epoch that contained a seizure was removed from sleep architecture analyses and data were normalized to the total number of epochs. During the active phase, Kcna1-null and WT littermates spent similar amounts of time awake and in NREMS and REMS (Fig. 2C). In contrast, during the rest phase Kcna1-null mice spent more time awake and less time in NREMS and REMS when compared with WT (Fig. 2D) supporting our previous findings [9,33]. These data highlight that Kcna1-null mice do not have a typical 24-hr sleep-wake cycle. Therefore, irrespective of the diurnal phase, we determined whether specific seizure types arose during specific states of vigilance. When considering all seizure types, half of seizures occurred during wake and half occurred during sleep evenly split between NREMS and REMS (Fig. 2E). Type 2 and 3 occurred more during wake, whereas Type 4 and 5 seizures were more prominent during sleep, specifically during REMS. Interestingly, of the 4 severe Type 6 seizures that were recorded, 3 occurred during wake while the remaining seizure occurred during REMS.
3.2. Antiseizure medication Effects on Kcna1-null seizures.
Antiseizure medications were administered between zeitgeber time 01:30 and 02:00 hrs. First, we pooled all the vehicle data and compared responses of all subjects for each drug. To account for differential pharmacokinetics, we examined seizure frequency in 4-hr bins from the time of injection, constructed the average daily cumulative frequency (Fig 3A) and determined the area under the curve (Fig 3B). These data suggest that only phenobarbital prevented generalized seizures in every mouse for the entire 24-hr period post-injection, whereas carbamazepine, phenytoin, and levetiracetam resulted in non-significant reductions of seizures in the first few time bins. However, when the data is pooled in this manner, which is common practice, it was unclear whether carbamazepine, phenytoin or levetiracetam resulted in seizure freedom in any of the mice. Therefore, we depicted seizure frequency as heatmaps which visually indicated that individual mice responded to carbamazepine, phenytoin or levetiracetam with either seizure freedom, decreased seizures, increased seizures or no effect (Fig. 4A; Table 1). To quantify these differences, seizure frequency was normalized during drug treatment within subject to their respective vehicle in each 4-hr bin.
Figure 3.
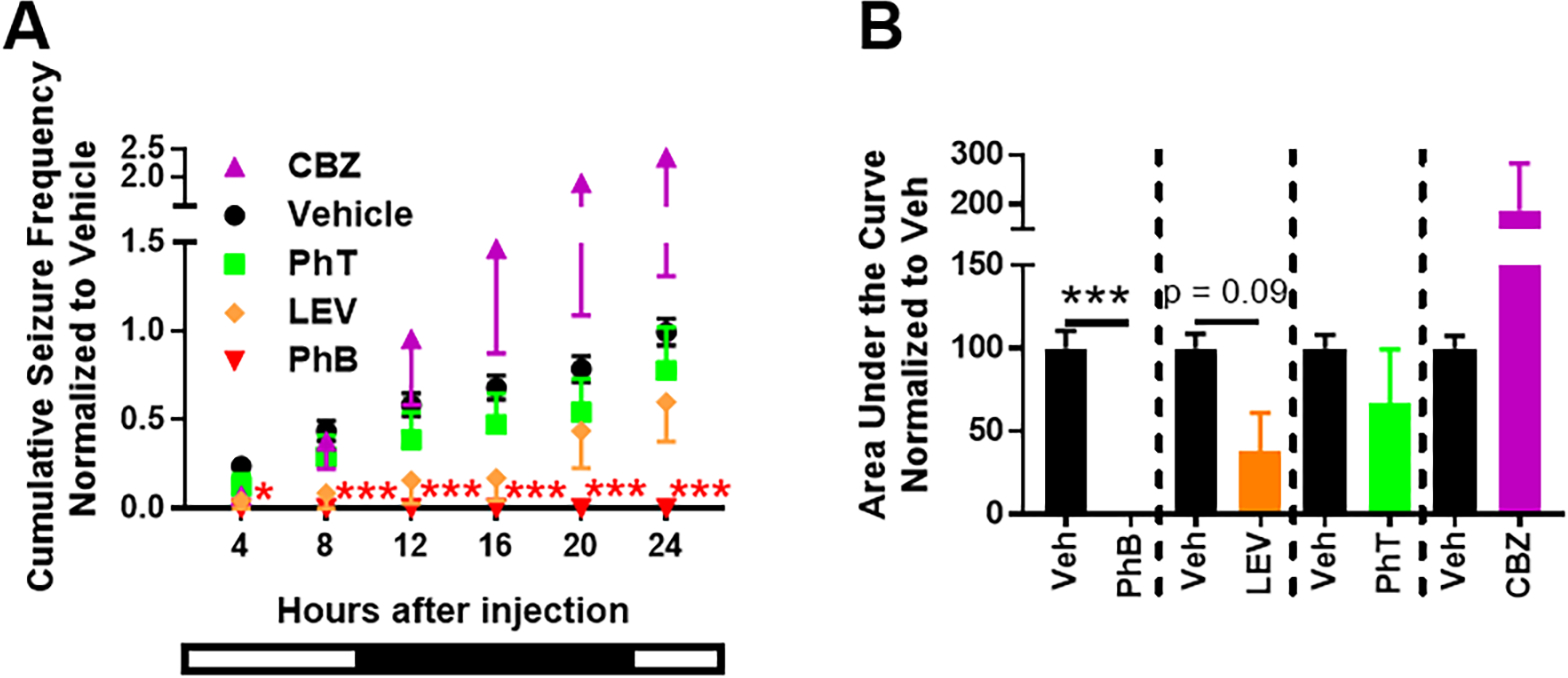
Pharmacoresponsivess of Kcna1-null seizure frequency. (A) Cumulative seizure frequency over 24 hrs post-injection of vehicle or antiseizure medication presented in 4 hr bins. Two-way repeated measures ANOVA for each treatment vs. vehicle over time; carbamazepine (CBZ): F(5, 110) = 2.151, p = 0.065; time: F(5, 110) = 6.8, p < 0.0001, n = 6; phenytoin (PhT): F(5, 110) = 0.19, p = 0.97; time: F(5, 110) = 20.9, p < 0.0001, n = 6; levetiracetam (LEV): F(5, 70) = 0.91, p = 0.48; time: F(5, 70) = 12.8, p < 0.0001, n = 4; phenobarbital (PhB): F(5, 110) = 11.8, p < 0.0001; time: F(5, 110) = 11.9, p < 0.0001, n = 6; followed by Sidak’s multiple comparisons test, *p < 0.05, ***p < 0.001 vs. vehicle. Bottom bar represents light (empty)-dark (filled) phases associated with the hours post-injection. (B) Calculated area under the curve for graph in A (paired t-test, ***p< 0.001).
Figure 4.
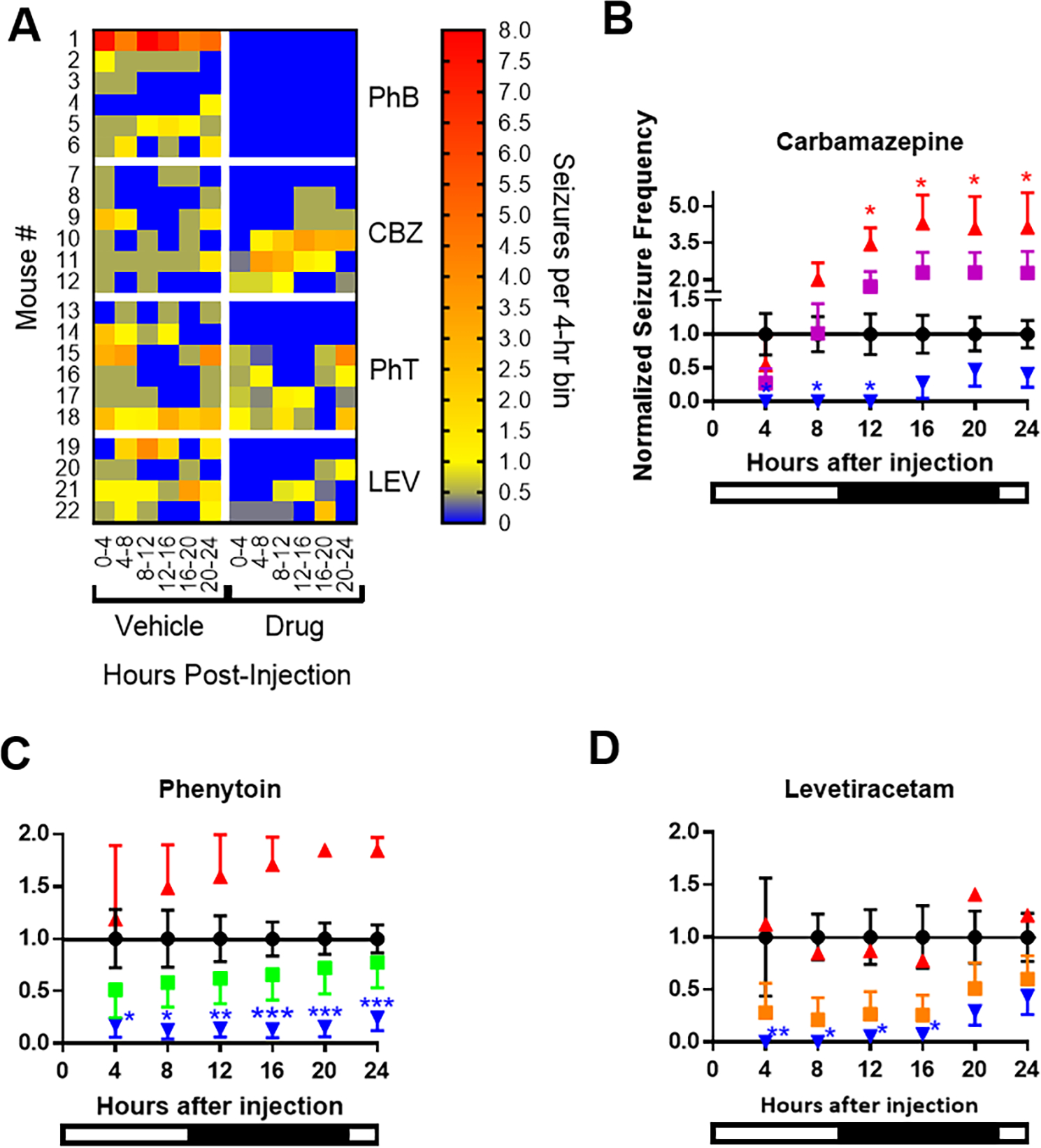
Individual variation of drug effects. (A) Heat maps of seizure frequency of individual mice after injection of vehicle or drug. (B-D) The mean effect for all mice for each drug is depicted in by the squares (CBZ is purple, n = 6; PhT is green, n = 6; LEV is orange, n = 4). Stratification of antiseizure medication effects on seizure frequency normalized to vehicle for each 4 hr bin. Red triangles indicate seizures increased or did not change (CBZ, n = 3; PhT, n = 2; LEV, n = 1). Blue triangles indicate seizures were reduced (CBZ, n = 3; PhT, n = 4; LEV, n = 3). Two-way repeated measures ANOVA for each treatment vs. vehicle over time followed by Sidak’s multiple comparisons test, *p < 0.05, **, p < 0.01, ***p < 0.001. Bottom bar represents light-dark phases associated with the hours post-injection.
Table 1.
Antiseizure Medication Effect on Seizure Frequency
| seizure free | ≥75% reduction | ≥50% reduction | seizure increase | |
|---|---|---|---|---|
|
| ||||
| 4hr post-injection | ||||
|
| ||||
| Vehicle | 3/22 | |||
| Carbamazepine | 4/6* | 4/6 | 5/6 | 1/6 |
| Levetiracetam | 3/4* | 3/4 | 3/4 | 0/4 |
| Phenytoin | 2/6 | 2/6 | 4/6 | 1/6 |
| Phenobarbital | 6/6** | 6/6 | 6/6 | 0/6 |
| All Drugs | 15/22*** | 15/22 | 18/22 | 1/22 |
|
| ||||
| 8hr post-injection | ||||
|
| ||||
| Vehicle | 1/22 | |||
| Carbamazepine | 3/6* | 3/6 | 3/6 | 3/6 |
| Levetiracetam | 3/4** | 3/4 | 3/4 | 0/4 |
| Phenytoin | 2/6 | 3/6 | 4/6 | 1/6 |
| Phenobarbital | 6/6*** | 6/6 | 6/6 | 0/6 |
| All Drugs | 14/22*** | 15/22 | 16/22 | 4/22 |
|
| ||||
| 24hr post-injection | ||||
|
| ||||
| Vehicle | 0/22 | |||
| Carbamazepine | 1/6 | 2/6 | 2/6 | 3/6 |
| Levetiracetam | 1/4 | 2/4 | 2/4 | 0/4 |
| Phenytoin | 2/6* | 2/6 | 4/6 | 2/6 |
| Phenobarbital | 6/6*** | 6/6 | 6/6 | 0/6 |
| All Drugs | 10/22*** | 12/22 | 14/22 | 5/22 |
Drug vs. vehicle
p < 0.5
p < 0.01
p < 0.001, Fisher’s exact test.
As depicted in Figures 4B–D seizure frequency is plotted as a group average and separated into groups based on whether drug treatment increased or decreased seizures. Carbamazepine significantly reduced seizures for 12 hrs post-injection in 3/6 mice, whereas seizures increased in 3/6 mice (Fig. 4B). Phenytoin significantly reduced seizures for 24 hrs post-injection in 4/6 mice, whereas seizures increased in 2/6 mice (Fig. 4C). Levetiracetam achieved significantly reduced seizures for 16 hrs post-injection in 3/4 mice, whereas no effect was seen in 1/4 mice (Fig. 4D).
Previous studies determining the pharmacological characteristics of current and potential models of drug resistant epilepsy have defined full efficacy as achieving seizure freedom in all mice, whereas anything less, even if seizures are reduced, is partial efficacy (Barton et al., 2001; Duveau et al., 2016; Metcalf et al., 2017, 2019; Koneval et al., 2018; Thomson et al., 2020; West et al., 2020; Wilcox et al., 2020; Pernici et al., 2021). To determine changes to the cumulative rate of seizure freedom, we compared the proportion of mice that remain seizure free by a given 4hr-bin post-injection of vehicle or drug. This can be seen in the heat map of Figure 4A and in Table 1. After vehicle injection 14% mice were seizure free for the first 4 hrs which fell to 5% by 8 hrs and all mice had seizures by 24 hrs. The rates of seizure freedom were significant for 12 hrs for carbamazepine (50% of mice) and 16 hrs for levetiracetam (50–75% of mice) (p ≤ 0.05, Fisher’s exact test). Phenytoin failed to induce significant seizure freedom. Thus, carbamazepine, levetiracetam and phenytoin exerted partial efficacy, whereas phenobarbital had full efficacy.
Additional measures of antiseizure medication impact on seizures are changes in severity and seizure. Regardless of the effects on seizure frequency, phenytoin and levetiracetam shifted seizure severity from Type ≥ 4 seizures to Type 2 and 3 seizures (Fig. 5A). Within subject changes are plotted in Figure 5B to highlight individual changes in severity and burden. Carbamazepine did not affect the overall proportion of seizure Types, average severity within subject or overall seizure burden (Fig. 5A, B).
Figure 5.
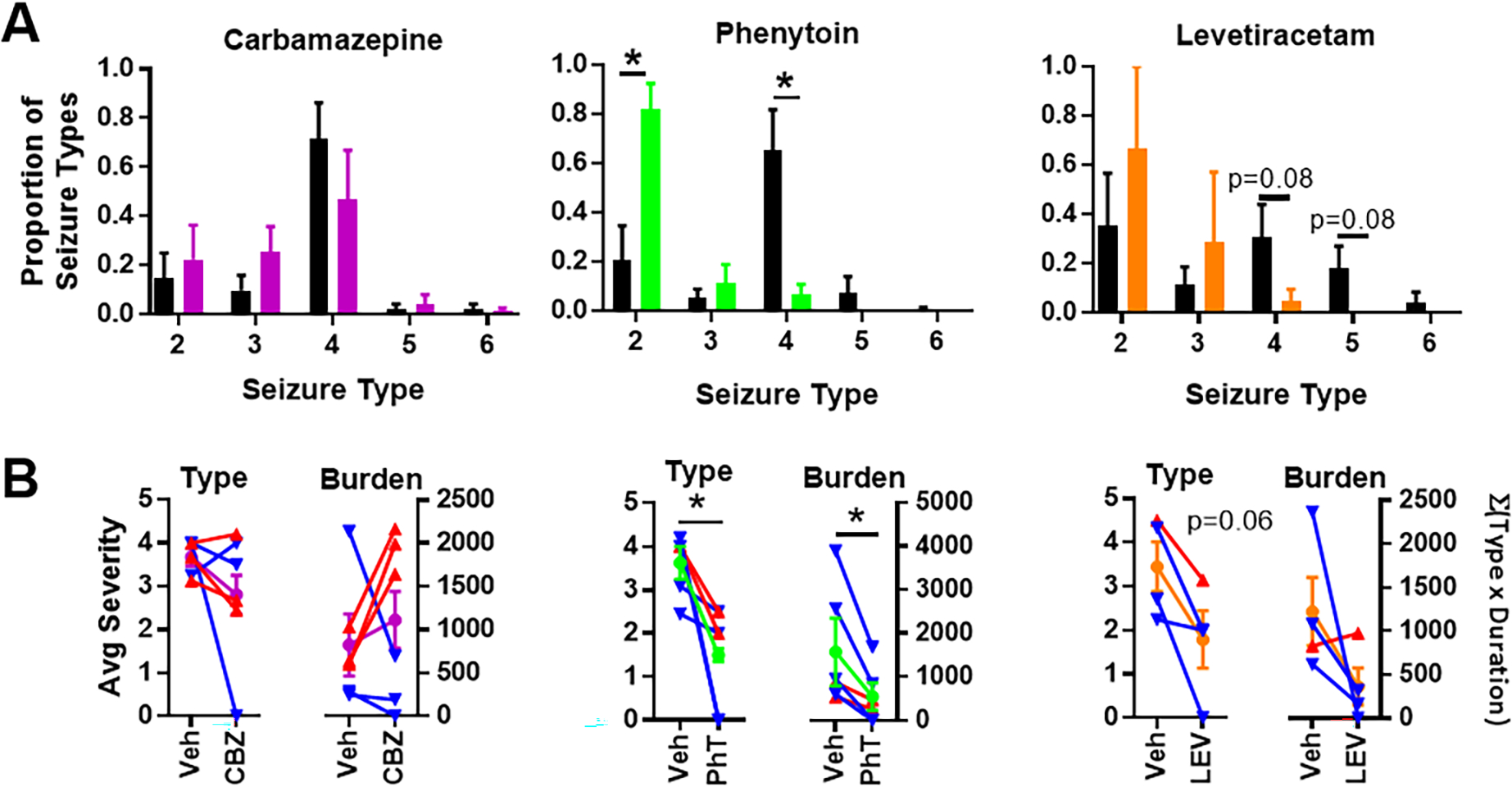
Carbamazepine, phenytoin, levetiracetam effects on seizure type and burden. (A) Average proportion of seizure types for individual mice during vehicle and antiseizure medication treatment (paired t-tests, *p< 0.05). (B) Average seizure types and burdens during vehicle and antiseizure medication (circle symbols; CBZ is purple, PhT is green and LEV is orange, paired t-test, *p< 0.05). In addition, the responses of individual mice in which seizure frequency increased or did not change (red triangles: CBZ, n = 3; PhT, n = 2; LEV, n = 1) or was reduced (blue triangles: CBZ, n = 3; PhT, n = 4; LEV, n = 3) are also indicated.
3.3. Antiseizure medication Effects on Kcna1-null comorbid sleep disorder
The comorbid sleep disorder of Kcna1-null mice involves increased wake and decreased non-REM and REM sleep during the light (rest) phase (Roundtree et al., 2016). The hypnotic effect of each antiseizure medication was determined during the rest phase (the first 4 hrs post injection, zeitgeber time 02:00–06:00 hrs) and during the active phase (12–16 hrs post injection, zeitgeber time 14:00–18:00 hrs). During the rest (light) phase, phenobarbital was the only antiseizure medication to restore sleep architecture to levels that did not differ from WT littermates (Fig. 6A). Carbamazepine was effective in increasing NREMS and reducing wake (Fig. 6B). Within subject analyses indicated that phenobarbital and carbamazepine treatment increased the number of REMS bouts (p < 0.01 and p < 0.05, respectively) and the amount of time mice stayed in NREMS and REMS (p < 0.01 and p < 0.05, respectively; Fig. 6Ai, Bi). Phenytoin reduced wake (Fig. 6C), but along with levetiracetam was unsuccessful in restoring sleep architecture (Fig. 6D).
Figure 6.
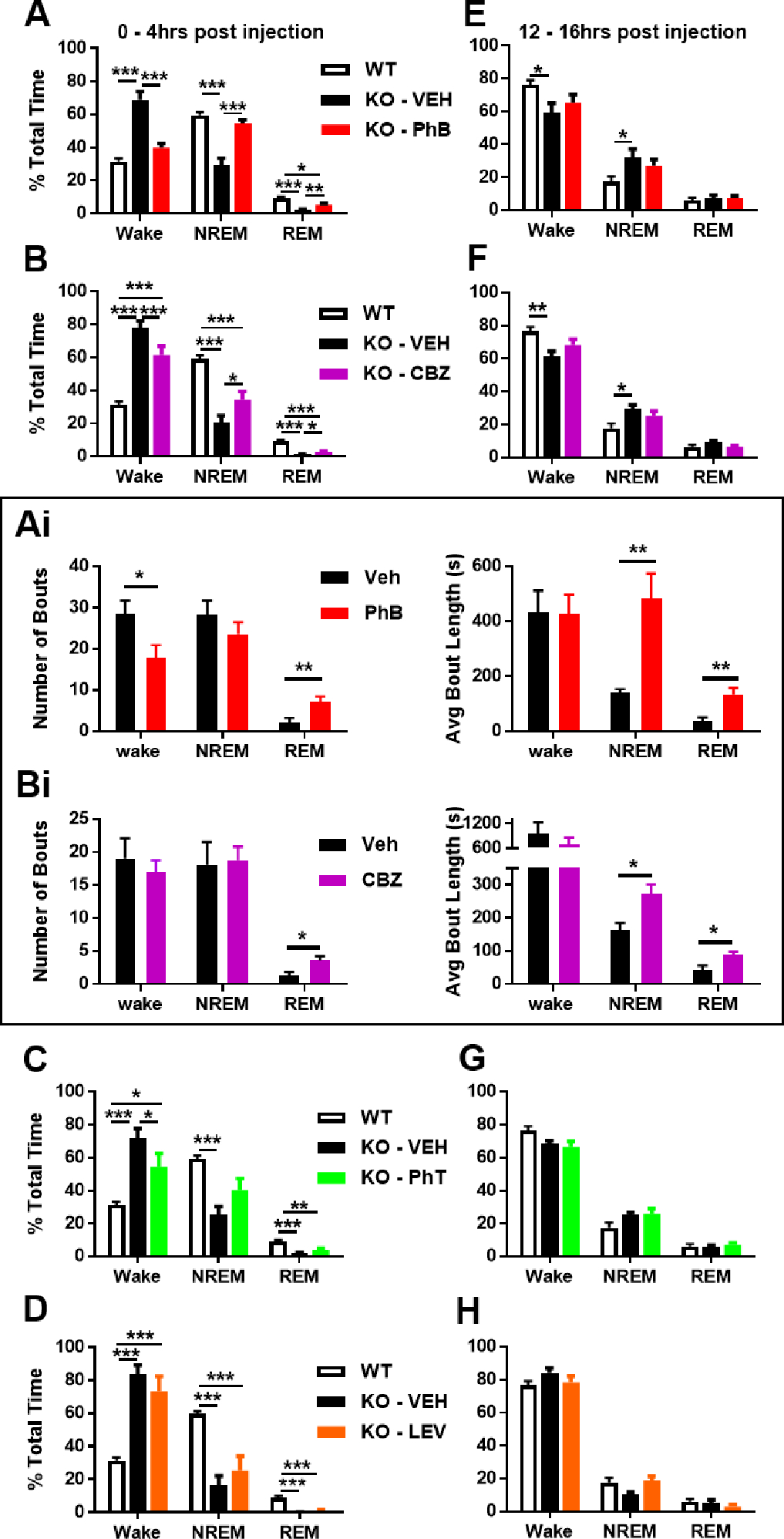
Pharmacoresponsivess of Kcna1-null sleep architecture. (A-D) In the first four hours post-antiseizure medication injection and in the normal rest (light) phase, phenobarbital, CBZ and PhT decreased wake and phenobarbital and CBZ significantly increased NREMS and REMS. (Ai) Phenobarbital achieved a strong effect on sleep architecture (left) by increasing the number of REMS bouts by 250% and reducing wake bouts by 40% and (right) by increasing the average time spent in a bout of NREMS and REMS by 250% and 270%, respectively. (Bi) Moderate effects on sleep by CBZ were achieved (left) by increasing the number of REMS bouts by 188%, and (right) by increasing the average length of NREMS and REMS bouts by 67% and 112%. (E-H) Twelve hrs later, in the active (dark) phase, no antiseizure medication affected wake, NREMS or REMS. One-way ANOVA for each vigilance state followed by Tukey’s multiple comparisons test, *p < 0.05, **, p < 0.01, ***p < 0.001 (PhB, n = 6; CBZ, n = 6; PhT, n = 6; LEV, n = 4).
During the active (dark) phase, Kcna1-null mice and WT have similar vigilant state proportions (Fig. 2C) and the antiseizure medications did not influence these proportions (Fig. 6E–H). For the most part, antiseizure medications also did not affect the vigilant state out of which seizures occurred (Fig. 7A–D). The exception was phenytoin which increased the proportion of seizures that occurred during NREMS (Fig. 7C).
Figure 7.
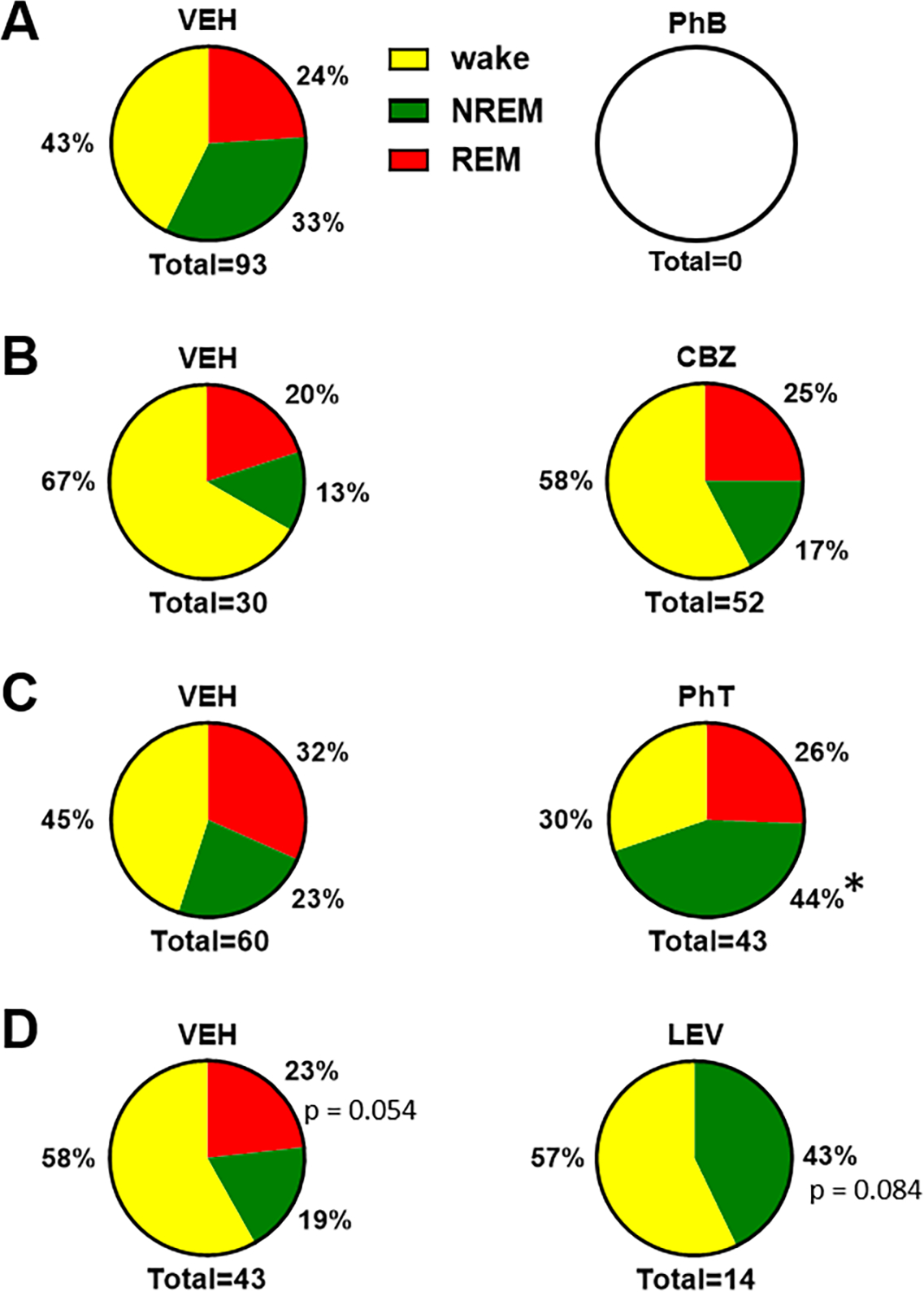
Antiseizure medication effects on the emergence of seizures from vigilance states. (A) phenobarbital prevented seizures in all vigilance states. (B) CBZ did not affect the proportion of seizures occurring in wake, NRMS or REMS. (C) PhT significantly increased the proportion of seizures occurring in NREMS. (D) There was a trend for LEV to prevent seizures from occurring in REMS in favor of NREMS. (A-D) Fisher’s exact test, *p < 0.05.
Lastly, whether the Kcna1-null sleep disorder correlated with generalized seizure frequency during the rest phase was determined. When examined alone each treatment failed to reveal a correlation (Veh: r = 0, p = 0.95, n = 44 days, 22 mice; carbamazepine: r = 0.26, p = 0.48, n = 12 days, 6 mice; phenytoin: r = 0.48, p = 0.14, n = 12 days, 6 mice; phenobarbital: vertical line, n = 12 days, 6 mice; levetiracetam: r = 0.65, p = 0.33, n = 8 days, 4 mice) indicating the antiseizure medication effects on seizures were independent of their effects on sleep. However, when the treatments were pooled to provide more power, a slight negative correlation was found (r = 0.51, p < 0.001, n = 44 treatments, 22 mice) indicating decreased time spent in NREMS is associated with higher seizure frequency in general.
4. Discussion
4.1. Seizures
In nearly a quarter century of research utilizing Kcna1-null mice, it has been determined that these mice exhibit a multitude of attributes that are risk factors for drug resistant epilepsy; however, there has been no consistent effort to determine whether these mice are resistant to current pharmacotherapies or meet the definition of drug resistant epilepsy. According to the International League Against Epilepsy clinical description of pharmacoresistance or drug resistant epilepsy is the “failure of adequate trials of two tolerated, appropriately chosen and used antiepileptic drug schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom” (Kwan and Brodie, 2000; Kwan et al., 2010; Chen et al., 2018; Blond et al., 2020) [4,5,21,22]. In the context of animal models this has been adapted to mean that at least two conventional antiseizure medications fail to provide seizure freedom to all animals tested, and that multiple drugs do not need to be tested in the same animal (Barton et al., 2001; Duveau et al., 2016; Metcalf et al., 2017; Koneval et al., 2018; Thomson et al., 2020; West et al., 2020). As no model is perfect, the collective results from multiple models can provide insight for anti-seizure efficacy of a drug. There are five models of drug resistant epilepsy that are currently employed by the NINDS preclinical Epilepsy Therapy Screening Program: the 6 Hz mouse and rat models, the lamotrigine-resistant kindled rat model, the intrahippocampal kainate mouse model and the kainic acid-induced status epilepticus rat model of spontaneous recurrent seizures (Wilcox et al., 2020). Many, if not all, clinically available antiseizure medications have been tested in these models.
The drug resistant epilepsy models have advantages and disadvantages. In three models, 6 Hz mouse, 6 Hz rat and lamotrigine-resistant kindled rat, subjects are moderate to high throughput for in vivo drug screening, however, they do not experience spontaneous recurrent seizures or comorbidities: The 6 Hz mouse and rat models measure seizure threshold of acute, focal seizures, and the lamotrigine-resistant kindled rat model of temporal lobe epilepsy uses the acquisition of a lowered seizure threshold through repeated electrical stimulation. The intrahippocampal kainate mouse model and the kainic acid-induced status epilepticus rat model induce focal and generalized spontaneous recurrent seizures, respectively, and have been reported to have cognitive comorbidities, however, the lag time for spontaneous recurrent seizures onset and potentially infrequent seizures, make them comparatively lower throughput to test anti-seizure efficacy of a drug. Here, we propose that Kcna1-null mice may be a moderate throughput model that complements current drug resistant epilepsy models. Kcna1-null mice have multiple daily spontaneous recurrent seizures (i.e. no lag time) with focal and generalized seizures, which allows for relatively quick within subject comparisons of vehicle and drug treatments. In addition to spontaneous recurrent seizures, Kcna1-null mice experience cognitive and sleep comorbidities that are associated with human drug resistant epilepsy providing additional opportunities for drug testing (Löscher et al., 2011; Kim et al., 2015; Jehi, 2016; Roundtree et al., 2016; Strzelczyk et al., 2017; Kalilani et al., 2018; Gavrilovic et al., 2019; Bergmann et al., 2020)
Each drug resistant epilepsy model has a specific pharmacoresponsive profile (Barton et al., 2001; Duveau et al., 2016; Metcalf et al., 2017, 2019; Thomson et al., 2020; Wilcox et al., 2020). It is important to note that in all of the current drug resistant epilepsy models, many antiseizure medications provide seizure freedom to some mice and/or reduce seizure burden in other mice (i.e., partial efficacy) and a few antiseizure medications provide complete seizure freedom in every subject (i.e., full efficacy); they are still considered models of pharmacoresistance because at least two antiseizure medications failed to exert full efficacy. In the present study, we systematically evaluated four conventional antiseizure medications at high therapeutic doses (Koneval et al., 2018; Metcalf et al., 2019; Thomson et al., 2020) for effects on spontaneous recurrent seizures (seizure frequency, severity, and burden) and on the sleep comorbidity. We found that Kcna1-null mice are resistant to carbamazepine, phenytoin and levetiracetam, supporting previous reports in drug resistant epilepsy models. Carbamazepine, phenytoin and levetiracetam have partial efficacy in all five current drug resistant epilepsy models, the 6 Hz mouse and rat models, the lamotrigine-resistant kindled rat model, intrahippocampal kainate mouse model and the kainic acid-induced status epilepticus model. In addition, similar results have been reported for other drug resistant epilepsy models under development such as the lamotrigine-resistant corneal kindled mouse, and against spontaneous recurrent seizures of intra-amygdala kainic acid injected mouse and hyperthermia-induced seizures in Scn1aA1783V/WT mice (Koneval et al., 2018; West et al., 2020; Pernici et al., 2021).
Of the four antiseizure medications tested, we found that phenobarbital provided seizure freedom to all Kcna1-null mice. Similarly, phenobarbital fully protected against seizures in the 6 Hz mouse, lamotrigine-resistant kindled rat and intrahippocampal kainate mouse models; sub-chronic treatment with phenobarbital had partial efficacy with seizure freedom for 10 of 12 kainic acid-induced status epilepticus rats (83%); and phenobarbital failed to provide full protection in the 6 Hz rat model at nontoxic doses. The efficacy of phenobarbital strengthens the translational relevance of Kcna1-null as human drug resistant convulsive and non-convulsive status epilepticus often respond well to phenobarbital (Brodie and Kwan, 2012). In addition, the intrinsic severity hypothesis of drug refractoriness posits that drug resistance is related to epilepsy severity and suggests that in models of epilepsy with frequent spontaneous recurrent seizures (or high seizure susceptibility) no drug may be fully efficacious (Rogawski and Johnson, 2013; Löscher et al., 2020); thus, demonstration that at least one current antiseizure medication has full efficacy against seizures in drug resistant epilepsy models strengthens the notion that other as yet unknown compounds will also provide pharmacotherapy.
Potential mechanisms of drug resistant epilepsy have recently been elegantly and comprehensively reviewed by Löscher et al. (2020). Evidence of varying strength have led to the proposal of multiple hypotheses involving drug targets, drug uptake, pharmacokinetics, neural networks, intrinsic epilepsy severity, gene variants, epigenetics, neuoroinflammation and blood-brain barrier dysfunction. However, drug resistant epilepsy is most likely multifactorial and could involve several of these mechanisms. Although not previously investigated in the context of drug resistant epilepsy, studies have thus far demonstrated that Kcna1-null mice display intrinsic epilepsy severity, neural network hyperexcitability, neuroinflammation, blood-brain barrier dysfunction and epigenetic alterations (Smart et al., 1998; Wenzel et al., 2007; Kim et al., 2015; Roundtree et al., 2016; Wright et al., 2016; Simeone et al., 2013, 2014, 2016, 2017). It is unknown whether drug targets, drug uptake or pharmacokinetics are altered in Kcna1-null mice. It will be important to determine whether any of these play a role in the pharmacoresponsiveness of Kcna1-null mice. The multitude of disease-related physiological alterations and comorbidities in Kcna1-null mice may lend this model to the dissection of individual mechanistic contributions to overall drug resistant epilepsy and/or development of new hypotheses of drug resistant epilepsy.
Three limitations of this study are that (i) each drug was given at one high-therapeutic dose, (ii) once daily and (iii) only for two days. However, this relatively simple within subject vehicle-drug administration design provides key advantages. First, it allowed the model to be used at a moderate throughput rate and second, it was able to stratify mice into responders and non-responders. As genetic mice are inbred clones and testing a single drug on individual mice can equate to testing multiple drugs on each mouse, the overall failure rate of Kcna1-null mice after testing the four drugs is 36% in the first 8 hours (Table 1), similar to the calculated ~30% of people with epilepsy that continue to have seizures after trying 4 drugs (Kwan et al., 2000; Chen et al., 2018; Blond et al., 2020). Third, this simple administration design was also capable of identifying the duration of drug efficacy. Considering only mice that experienced seizure reduction or freedom, the rank order of the duration of efficacy is phenobarbital (24 hrs) > phenytoin (20 hrs) > levetiracetam (16 hrs) > carbamazepine (12 hrs) (Figs 4B–D and Table 1), which is similar to the rank order of published mouse and rat t1/2s for each drug, 9–20 hrs, 5–16 hrs, 2–3 hrs and 1–3.5 hrs, respectively (Markowitz et al., 2010; Fortuna et al., 2013; Thomson et al., 2020). This suggests a reasonable chronic dosing schedule would be phenobarbital qd, phenytoin and levetiracetam bid and carbamazepine tid, which is in precise agreement with previous reports of dosing schedules (Metcalf et al., 2019; Thomson et al., 2020).
Although previous studies in mice and rats indicate that the antiseizure medication doses used in this study represent high-therapeutic doses (Koneval et al., 2018; Metcalf et al., 2019; Thomson et al., 2020), it is acknowledged that this must be established in Kcna1-null mice and represents another limitation. Future studies will perform dose responses to determine adequate dosing, use more frequent dosing to establish steady state concentrations, and expand the antiseizure medications tested to determine the pharmacoresponsiveness of the Kcna1-null model more fully. Interestingly, as the primary purpose of drug resistant epilepsy models is to identify novel and non-traditional anti-seizure therapeutics, thus far, Kcna1-null mice have identified antiseizure efficacy with unconventional and novel pharmacotherapies including almorexant (dual orexin recptor antagonist), ramelteon (melatonin receptor agonist), pioglitazone (PPARgamma agonist), vorinostat (broad HDAC inhibitor), RG2833 (HDAC1 and HDAC3 inhibitor), b-hydroxybutyrate (ketone body), NIM811 (cyclophilin D inhibitor) (Fenoglio-Simeone et al., 2009a; Kim et al., 2015; Roundtree et al., 2016; Simeone et al., 2017; Ibhazehiebo et al., 2018); and metabolic therapies including the ketogenic diet (Kim et al., 2015; Simeone et al., 2016, 2017), and supplementation with pyruvate, alpha-tocopherol and ascorbic acid (Simeone et al., 2014).
4.2. Sleep
Apart from the relative consistent development of and frequency of seizures, an additional advantage of using Kcna1-null mice is the presence of multiple comorbidities. The Kcna1-null sleep comorbidity manifests as a deficiency in NREM sleep and near loss of REM sleep in the rest phase compared to non-epileptic wildtype littermates. The dynamic interdependency of seizures and sleep remains unclear and is not readily distinguishable, however, we did observe a diurnal rhythm to seizures as previously described (Fenoglio-Simeone et al., 2009b; Wright et al., 2016). In humans, seizures may arise during wake or sleep, but with increased occurrence in NREMS and few occurring during REMS (Ng et al., 2013). Preclinical studies using epileptic mice suggest that most spontaneous recurrent seizures occur during sleep, similar to humans, however, they occur in either equal proportions between NREMS and REMS or predominantly in REMS (Bastlund et al., 2005; Strohl et al., 2007; Sedigh-Sarvestani et al., 2014). In our study, Kcna1-null mice experienced approximately ¼ of seizures out of NREMS and ¼ out of REMS, and the rest during wake. We did observe a trend towards type 4 and 5 seizures arising from sleep, and type 2,3, and 6 arising primarily out of wake, raising the possibility that different seizure types are promoted in different vigilance states.
Antiseizure medications have varying effects on sleep in patients with epilepsy which is not fully understood. It is thought the differential effects likely depend on the type of seizures, mono- vs poly-therapy, acute vs chronic treatment and dose (Bazil, 2003; Jain and Glauser, 2014). The sodium channel blockers, carbamazepine and phenytoin, either increase or decrease NREMS depending on dose or length of treatment (Wolf et al, 1984; Gigli et al., 1988; Manni et al., 1990; Bazil, 2003; Legros and Bazil, 2003). Phenobarbital increases NREMS and reduces arousals during sleep in healthy adults and in people with epilepsy (Karacan et al., 1981; Prinz et al., 1981; Wolf et al, 1984). This is not surprising given its positive modulatory action on inhibitory GABAA receptors and use as a sedative hypnotic. In contrast, levetiracetam, which binds to the synaptic vesicle protein SV2A, does not significantly affect sleep in epilepsy (Cho et al., 2011). Here, in Kcna1-null mice, carbamazepine and phenytoin moderately reduced wake and improved NREMS, phenobarbital fully restored sleep architecture and levetiracetam did not have any significant effects on sleep patterns. Only phenytoin significantly altered the occurrence of seizures during vigilance states to a greater proportion during NREMS. This may have been due to the ability of phenytoin to shift the major seizure type from Type 4 which occur during REMS to Type 2 which occur during NREMS.
Individually, antiseizure medication effects on sleep did not correlate with seizures; however, pooling all treatment data suggest that low seizure frequency correlated with increased NREMS. This suggests that reducing seizures may improve sleep and vice versa. Thus, intentionally treating sleep may be a therapeutic strategy to indirectly (or directly) improve seizures in epilepsy patients. Our previous studies support this notion as we found that modern sleep aids including the melatonin receptor agonist ramelteon and the dual orexin receptor antagonist almorexant effectively improved sleep of Kcna1-null mice and improved seizure profiles (Fenoglio-Simeone et al., 2009a; Roundtree et al., 2016). The mechanism of action of these drugs is not directly related to seizure control, thus providing probationary evidence that improved sleep could result in reducing some seizure phenotypes. Additionally, metabolic antiseizure therapeutics, including the ketogenic diet, have been shown to reduce seizures and improve sleep in preclinical models and clinical studies (Hallböök et al., 2015; Simeone et al., 2014, 2016, 2017; Iyer et al., 2018). Thus, Kcna1-mice may aid in understanding the interconnection of sleep and epilepsy.
4.3. Conclusions
The current pharmacological characterization of Kcna1-null mice suggests a degree of drug resistance comparable to the human epilepsy experience. In addition, previous studies have found that Kcna1-null mice exhibit multiple factors associated with drug resistant epilepsy. Kcna1-null mice have multiple spontaneous recurrent seizures types from focal seizures and myoclonic jerks (not studied here) to severe generalized seizures which occur numerous times per day (Smart et al., 1998). They exhibit neuropathology risk factors for drug resistant epilepsy like mossy fiber sprouting, hippocampal sclerosis and cell loss, which are markers of TLE, as well as hippocampal hyperexcitability, mitochondrial dysfunction, and increased oxidative stress (Wenzel et al., 2007; Simeone et al., 2013, 2014, 2021; Kim et al., 2015). Kcna1-null mice have CNS comorbidities such as cognitive memory deficits (Kim et al., 2015; Scantlebury et al., 2017), and a sleep disorder that progressively worsens with age (Iyer et al., 2018). Additionally, Kcna1-null mice exhibit cardiac and respiratory abnormalities and experience SUDEP, of which drug resistant epilepsy is a risk factor (Moore et al., 2014; Iyer et al., 2018, 2020; Simeone et al., 2018; Dhaibar et al., 2019). Although the short life span prevents reuse of mice and limits the number of drugs tested on any individual mouse, it has proven beneficial when studying therapeutic effects on longevity and disease progression. The findings of this exploratory study suggest Kcna1-null mice are pharmacoresistant to certain antiseizure medications and support the need for future studies to fully characterize the pharmacoresponsivenss of Kcna1-null mice. Currently, the multitude of overt, clinically relevant comorbidities present in Kcna1-null mice are not exhibited by other proposed drug resistant epilepsy models suggesting that Kcna1-null mice may have added value in differentiating promising compounds.
Acknowledgements
The authors would like to thank Segewkal Heruye and Joseph Kostansek IV for contributions to the graphical abstract.
Funding Sources
This work was supported by Citizens United for Research in Epilepsy Foundation (TAS), NIH NS085389(TAS), NIH NS072179 (KAS) and NIH NS111389 (KAS). The project described was also supported by the National Center for Research Resources grant G20RR024001. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Abbreviations:
- NREMS
non-rapid eye movement sleep
- REMS
rapid eye movement sleep
- SUDEP
sudden unexpected death in epilepsy
Footnotes
Declaration of Interest
None of the authors has any conflict of interest to disclose.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Chemical Compounds: Chemical compounds studied in this article are phenobarbital (PubChem CID: 4763); phenytoin (PubChem CID: 1775); carbamazepine (PubChem CID: 2554); levetiracetam (PubChem CID: 5284583).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001. Dec;47(3):217–27. doi: 10.1016/s0920-1211(01)00302-3. PMID: 11738929. [DOI] [PubMed] [Google Scholar]
- [2].Bastlund JF, Jennum P, Mohapel P, Penschuck S, Watson WP Spontaneous epileptic rats show changes in sleep architecture and hypothalamic pathology, Epilepsia. 46 (2005) 934–938. 10.1111/j.1528-1167.2005.63204.x [DOI] [PubMed] [Google Scholar]
- [3].Bazil CW Epilepsy and sleep disturbance, Epilepsy Behav. Suppl 2 (2003) S39–45. 10.1016/j.yebeh.2003.07.005 [DOI] [PubMed] [Google Scholar]
- [4].Bergmann M, Prieschl M, Stefani A, Heidbreder A, Walser G, Frauscher B, Unterberger I, Högl B A prospective controlled study about sleep disorders in drug resistant epilepsy, Sleep Med. 75 (2020) 434–440. 10.1016/j.sleep.2020.09.001 [DOI] [PubMed] [Google Scholar]
- [5].Blond BN, Hirsch LJ,Mattson RH Misperceptions on the chance of seizure freedom with antiseizure medications after two failed trials, Epilepsia. 61 (2020) 1789–1790. 10.1111/epi.16594 [DOI] [PubMed] [Google Scholar]
- [6].Brodie MJ, Kwan P Current position of phenobarbital in epilepsy and its future, Epilepsia. 53 (2012) 40–46. 10.1111/epi.12027 [DOI] [PubMed] [Google Scholar]
- [7].Chen Z, Brodie MJ, Liew D, Kwan P Treatment Outcomes in Patients With Newly Diagnosed Epilepsy Treated With Established and New Antiepileptic Drugs: A 30-Year Longitudinal Cohort Study, JAMA Neurol. 75 (2018) 279–286. 10.1001/jamaneurol.2017.3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cho YW, Kim DH, Motamedi GK The effect of levetiracetam monotherapy on subjective sleep quality and objective sleep parameters in patients with epilepsy: compared with the effect of carbamazepine-CR monotherapy, Seizure. 20 (2011) 336–339. 10.1016/j.seizure.2011.01.006 [DOI] [PubMed] [Google Scholar]
- [9].Dhaibar H, Gautier NM, Chernyshev OY, Dominic P, Glasscock E Cardiorespiratory profiling reveals primary breathing dysfunction in Kcna1-null mice: Implications for sudden unexpected death in epilepsy, Neurobiol Dis. 127 (2019) 502–511. 10.1016/j.nbd.2019.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Duveau V, Pouyatos B, Bressand K, Bouyssières C, Chabrol T, Roche Y, Depaulis A, Roucard C. Differential Effects of Antiepileptic Drugs on Focal Seizures in the Intrahippocampal Kainate Mouse Model of Mesial Temporal Lobe Epilepsy. CNS Neurosci Ther. 2016. Jun;22(6):497–506. doi: 10.1111/cns.12523. Epub 2016 Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fenoglio-Simeone K, Mazarati A, Sefidvash-Hockley S, Shin D, Wilke J, Milligan H, Sankar R, Rho JM, Maganti R Anticonvulsant effects of the selective melatonin receptor agonist ramelteon, Epilepsy Behav. 16 (2009a) 52–57. 10.1016/j.yebeh.2009.07.022 [DOI] [PubMed] [Google Scholar]
- [12].Fenoglio-Simeone KA, Wilke JC, Milligan HL, Allen CN, Rho JM, Maganti RK Ketogenic diet treatment abolishes seizure periodicity and improves diurnal rhythmicity in epileptic Kcna1-null mice, Epilepsia. 50 (2009b) 2027–2034. 10.1111/j.1528-1167.2009.02163.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fortuna A, Alves G, Soares-da-Silva P, Falcão A. Pharmacokinetics, brain distribution and plasma protein binding of carbamazepine and nine derivatives: new set of data for predictive in silico ADME models. Epilepsy Res. 2013. Nov;107(1–2):37–50. doi: 10.1016/j.eplepsyres.2013.08.013. Epub 2013 Sep 3. PMID: 24050973. [DOI] [PubMed] [Google Scholar]
- [14].Gavrilovic A, Toncev G, Boskovic Matic T, Vesic K, Ilic Zivojinovic J, Gavrilovic J Impact of epilepsy duration, seizure control and EEG abnormalities on cognitive impairment in drug-resistant epilepsy patients, Acta Neurol Belg. 119 (2019) 403–410. 10.1007/s13760-019-01090-x [DOI] [PubMed] [Google Scholar]
- [15].Gigli GL, Gotman J, Thomas ST Sleep alterations after acute administration of carbamazepine in cats, Epilepsia. 29 (1988) 748–752. 10.1111/j.1528-1157.1988.tb04230.x [DOI] [PubMed] [Google Scholar]
- [16].Hallböök T, Sjölander A, Åmark P, Miranda M, Bjurulf B, Dahlin M Effectiveness of the ketogenic diet used to treat resistant childhood epilepsy in Scandinavia. Eur J Paediatr Neurol. 19 (2015) 29–36. 10.1016/j.ejpn.2014.09.005 [DOI] [PubMed] [Google Scholar]
- [17].Ibhazehiebo K, Gavrilovici C, de la Hoz CL, Ma SC, Rehak R, Kaushik G, Meza Santoscoy PL, Scott L L, Nath N, Kim DY, Rho JM, Kurrasch DM A novel metabolism-based phenotypic drug discovery platform in zebrafish uncovers HDACs 1 and 3 as a potential combined anti-seizure drug target, Brain. 141 (2018) 744–761. 10.1093/brain/awx364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Iyer SH, Aggarwal A, Warren TJ, Hallgren J, Abel PW, Simeone TA, Simeone KA Progressive cardiorespiratory dysfunction in Kv1.1 knockout mice may provide temporal biomarkers of pending sudden unexpected death in epilepsy (SUDEP): The contribution of orexin, Epilepsia. 61 (2020) 572–588. 10.1111/epi.16434 [DOI] [PubMed] [Google Scholar]
- [19].Iyer SH, Matthews SA, Simeone TA, Maganti R, Simeone KA Accumulation of rest deficiency precedes sudden death of epileptic Kv1.1 knockout mice, a model of sudden unexpected death in epilepsy, Epilepsia. 59 (2018) 92–105. 10.1111/epi.13953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jain SV, Glauser TA Effects of epilepsy treatments on sleep architecture and daytime sleepiness: an evidence-based review of objective sleep metrics, Epilepsia. 55 (2014) 26–37. 10.1111/epi.12478 [DOI] [PubMed] [Google Scholar]
- [21].Jehi L Neurology’s Silent Killer: Drug-Resistant Epilepsy, Epilepsy Curr 16 (2016) 232–233. 10.5698/1535-7511-16.4.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Klassen TL, Bomben VC, Patel A, Drabek J, Chen TT, Gu W, Zhang F, Chapman K, Lupski JR, Noebels JL, Goldman AM High-resolution molecular genomic autopsy reveals complex sudden unexpected death in epilepsy risk profile, Epilepsia. 55 (2014) e6–12. 10.1111/epi.12489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kalilani L, Sun X, Pelgrims B, Noack-Rink M, Villanueva V The epidemiology of drug-resistant epilepsy: A systematic review and meta-analysis, Epilepsia. 59 (2018) 2179–2193. 10.1111/epi.14596 [DOI] [PubMed] [Google Scholar]
- [24].Karacan I, Orr W, Roth T, Kramer M, Thornby J, Bingham S, Kay D Dose-related effects of phenobarbitone on human sleep-waking patterns, Br J Clin Pharmacol. 12 (1981) 303–313. 10.1111/j.1365-2125.1981.tb01218.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kim DY, Simeone KA, Simeone TA, Pandya JD, Wilke JC, Ahn Y, Geddes JW, Sullivan PG, Rho JM Ketone bodies mediate antiseizure effects through mitochondrial permeability transition, Ann Neurol. 78 (2015) 77–87. 10.1002/ana.24424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Koneval Z, Knox KM, White HS, Barker-Haliski M. Lamotrigine-resistant corneal-kindled mice: A model of pharmacoresistant partial epilepsy for moderate-throughput drug discovery. Epilepsia. 2018. Jun;59(6):1245–1256. doi: 10.1111/epi.14190. Epub 2018 May 11. PMID: 29750337. [DOI] [PubMed] [Google Scholar]
- [27].Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshé SL, Perucca E, Wiebe S, French J Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies, Epilepsia. 51 (2010) 1069–1077. 10.1111/j.1528-1167.2009.02397.x [DOI] [PubMed] [Google Scholar]
- [28].Kwan P, Brodie MJ Early identification of refractory epilepsy, N Engl J Med. 342 (2000) 314–319. 10.1056/NEJM200002033420503 [DOI] [PubMed] [Google Scholar]
- [29].Legros B, Bazil CW Effects of antiepileptic drugs on sleep architecture: a pilot study, Sleep Med. 4 (2003) 51–55. 10.1016/s1389-9457(02)00217-4 [DOI] [PubMed] [Google Scholar]
- [30].Löscher W Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs, Seizure. 20 (2011) 359–368. 10.1016/j.seizure.2011.01.003 [DOI] [PubMed] [Google Scholar]
- [31].Löscher W, Potschka H, Sisodiya SM, Vezzani A Drug Resistance in Epilepsy: Clinical Impact, Potential Mechanisms, and New Innovative Treatment Options, Pharmacol Rev. 72 (2020) 606–638. 10.1124/pr.120.019539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Manni R, Galimberti CA, Zucca C, Parietti L, Tartara A Sleep patterns in patients with late onset partial epilepsy receiving chronic carbamazepine (CBZ) therapy, Epilepsy Res. 7 (1990) 72–76. 10.1016/0920-1211(90)90056-2 [DOI] [PubMed] [Google Scholar]
- [33].Markowitz GJ, Kadam SD, Boothe DM, Irving ND, Comi AM. The pharmacokinetics of commonly used antiepileptic drugs in immature CD1 mice. Neuroreport. 2010. Apr 21;21(6):452–6. doi: 10.1097/wnr.0b013e328338ba18. PMID: 20848732; PMCID: PMC3220279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Metcalf CS, Huff J, Thomson KE, Johnson K, Edwards SF, Wilcox KS Evaluation of antiseizure drug efficacy and tolerability in the rat lamotrigine- resistant amygdala kindling model, Epilepsia Open. 4 (2019) 452–463. 10.1002/epi4.12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Metcalf CS, West PJ, Thomson KE, Edwards SF, Smith MD, White HS, Wilcox KS. Development and pharmacologic characterization of the rat 6 Hz model of partial seizures. Epilepsia. 2017. Jun;58(6):1073–1084. doi: 10.1111/epi.13764. Epub 2017 Apr 27. PMID: 28449218; PMCID: PMC5469205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Moore BM, Jerry Jou C, Tatalovic M, Kaufman ES, Kline DD, Kunze DL The Kv1.1 null mouse, a model of sudden unexpected death in epilepsy (SUDEP), Epilepsia. 55 (2014) 1808–1816. 10.1111/epi.12793 [DOI] [PubMed] [Google Scholar]
- [37].Ng M, Pavlova M Why are seizures rare in rapid eye movement sleep? Review of the frequency of seizures in different sleep stages, Epilepsy Res Treat. (2013) 932790. 10.1155/2013/932790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Paulhus K, Ammerman L, Glasscock E Clinical Spectrum of KCNA1 Mutations: New Insights into Episodic Ataxia and Epilepsy Comorbidity, Int J Mol Sci 21 (2020) 2802. 10.3390/ijms21082802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pernici CD, Mensah JA, Dahle EJ, Johnson KJ, Handy L, Buxton L, Smith MD, West PJ, Metcalf CS, Wilcox KS. Development of an antiseizure drug screening platform for Dravet syndrome at the NINDS contract site for the Epilepsy Therapy Screening Program. Epilepsia. 2021. Jul;62(7):1665–1676. doi: 10.1111/epi.16925. Epub 2021 May 17. PMID: 34002394; PMCID: PMC8360068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Prinz PN, Vitiello MV, Roehrs TA, Linnoila M, Weitzman ED Effect of phenobarbital on sleep and nighttime plasma growth hormone and cortisol levels, Can J Physiol Pharmacol. 59 (1981) 1139–45. 10.1139/y81-176 [DOI] [PubMed] [Google Scholar]
- [41].Rogawski MA, Johnson MR Intrinsic severity as a determinant of antiepileptic drug refractoriness, Epilepsy Curr. 8 (2013) 127–130. 10.1111/j.1535-7511.2008.00272.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Roundtree HM, Simeone TA, Johnson C, Matthews SA, Samson KK, Simeone KA Orexin Receptor Antagonism Improves Sleep and Reduces Seizures in Kcna1-null Mice, Sleep. 39 (2016) 357–368. 10.5665/sleep.5444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Scantlebury MH, Chun KC, Ma SC, Rho JM, Kim DY Adrenocorticotropic hormone protects learning and memory function in epileptic Kcna1-null mice, Neurosci Lett. 645 (2017) 14–18. 10.1016/j.neulet.2017.02.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sedigh-Sarvestani M, Thuku GI, Sunderam S, Parkar A, Weinstein SL, Schiff SJ, Gluckman BJ Rapid eye movement sleep and hippocampal theta oscillations precede seizure onset in the tetanus toxin model of temporal lobe epilepsy. J Neurosci. 34 (2014) 1105–1114. 10.1523/JNEUROSCI.3103-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Simeone KA, Hallgren J, Bockman CS, Aggarwal A, Kansal V, Netzel L, Iyer SH, Matthews SA, Deodhar M, Oldenburg PJ, Abel PW, Simeone TA Respiratory dysfunction progresses with age in Kcna1-null mice, a model of sudden unexpected death in epilepsy, Epilepsia. 59 (2018) 345–357. 10.1111/epi.13971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Simeone KA, Matthews SA, Rho JM, Simeone TA Ketogenic diet treatment increases longevity in Kcna1-null mice, a model of sudden unexpected death in epilepsy, Epilepsia. 57 (2016) e178–82. 10.1111/epi.13444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Simeone KA, Matthews SA, Samson KK, Simeone TA Targeting deficiencies in mitochondrial respiratory complex I and functional uncoupling exerts anti-seizure effects in a genetic model of temporal lobe epilepsy and in a model of acute temporal lobe seizures, Exp Neurol. 251 (2014) 84–90. 10.1016/j.expneurol.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Simeone KA, Wilke JC, Matthews SA, Simeone TA, Rho JM. Ketogenic diet-mediated seizure reduction preserves CA1 cell numbers in epileptic Kcna1-null mice: An unbiased stereological assessment. Epilepsia. 2021. Aug;62(8):e123–e128. doi: 10.1111/epi.16983. Epub 2021 Jul 7. PMID: 34231878. [DOI] [PubMed] [Google Scholar]
- [49].Simeone TA, Matthews SA, Samson KK, Simeone KA Regulation of brain PPARgamma2 contributes to ketogenic diet anti-seizure efficacy, Exp Neurol. 287 (2017) 54–64. 10.1016/j.expneurol.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Simeone TA, Simeone KA, Samson KK, Kim DY, Rho JM Loss of the Kv1.1 potassium channel promotes pathologic sharp waves and high frequency oscillations in in vitro hippocampal slices, Neurobiol Dis. 54 (2013) 68–81. 10.1016/j.nbd.2013.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Smart SL, Lopantsev V, Zhang CL, Robbins CA, Wang H, Chiu SY, Schwartzkroin PA, Messing A, Tempel BL Deletion of the K(V)1.1 potassium channel causes epilepsy in mice, Neuron. 20 (1998) 809–819. 10.1016/s0896-6273(00)81018-1 [DOI] [PubMed] [Google Scholar]
- [52].Strohl KP, Gallaugher L, Lynn A, Friedman L, Hill A, Singer JB, Lander ES, Nadeau J Sleep-related epilepsy in the A/J mouse, Sleep. 30 (2007) 169–176. 10.1093/sleep/30.2.169 [DOI] [PubMed] [Google Scholar]
- [53].Strzelczyk A, Griebel C, Lux W, Rosenow F, Reese JP The Burden of Severely Drug-Refractory Epilepsy: A Comparative Longitudinal Evaluation of Mortality, Morbidity, Resource Use, and Cost Using German Health Insurance Data, Front Neurol. 8 (2017) 712. 10.3389/fneur.2017.00712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Thomson KE, Metcalf CS, Newell TG, Huff J, Edwards SF, West PJ, Wilcox KS Evaluation of subchronic administration of antiseizure drugs in spontaneously seizing rats, Epilepsia. 61 (2020) 1301–1311. 10.1111/epi.16531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wenzel HJ, Vacher H, Clark E, Trimmer JS, Lee AL, Sapolsky RM, Tempel BL, Schwartzkroin PA Structural consequences of Kcna1 gene deletion and transfer in the mouse hippocampus, Epilepsia. 48 (2007) 2023–2046. 10.1111/j.1528-1167.2007.01189.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].West Peter J., Thomson Kyle, Billingsley Peggy, Pruess Timothy, Rueda Carlos, Saunders Gerald W., Smith Misty D., Metcalf Cameron S., Wilcox Karen S. Spontaneous recurrent seizures in an intra-amygdala kainate microinjection model of temporal lobe epilepsy are differentially sensitive to antiseizure drugs. bioRxiv 2020.12.03.410266; doi: 10.1101/2020.12.03.410266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wilcox KS, West PJ, Metcalf CS The current approach of the Epilepsy Therapy Screening Program contract site for identifying improved therapies for the treatment of pharmacoresistant seizures in epilepsy, Neuropharmacol. 166 (2020) 107811. 10.1016/j.neuropharm.2019.107811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wright S, Wallace E, Hwang Y, Maganti R Seizure phenotypes, periodicity, and sleep-wake pattern of seizures in Kcna-1 null mice, Epilepsy Behav. 55 (2016) 24–29. 10.1016/j.yebeh.2015.11.028 [DOI] [PubMed] [Google Scholar]
- [59].Wolf P, Röder-Wanner UU, Brede M Influence of therapeutic phenobarbital and phenytoin medication on the polygraphic sleep of patients with epilepsy, Epilepsia. 25 (1984) 467–475. 10.1111/j.1528-1157.1984.tb03445.x [DOI] [PubMed] [Google Scholar]


