Abstract
Context
Chronic exposure to glucocorticoids (GCs) or stress increases the risk of medical disorders, including cardiovascular and neuropsychiatric disorders. GCs contribute to accelerated aging; however, while the link between chronic GC exposure and disease onset is well established, the underpinning mechanisms are not clear.
Objective
We explored the potential nexus between GCs or stress exposure and telomere length.
Methods
In addition to rats exposed to 3 weeks of chronic stress, an iatrogenic mouse model of Cushing syndrome (CS), and a mouse neuronal cell line, we studied 32 patients with CS and age-matched controls and another cohort of 75 healthy humans.
Results
(1) Exposure to stress in rats was associated with a 54.5% (P = 0.036) reduction in telomere length in T cells. Genomic DNA (gDNA) extracted from the dentate gyrus of stressed and unstressed rats showed 43.2% reduction in telomere length (P = 0.006). (2) Mice exposed to corticosterone had a 61.4% reduction in telomere length in blood gDNA (P = 5.75 × 10-5) and 58.8% reduction in telomere length in the dentate gyrus (P = 0.002). (3) We observed a 40.8% reduction in the telomere length in patients with active CS compared to healthy controls (P = 0.006). There was a 17.8% reduction in telomere length in cured CS patients, which was not different from that of healthy controls (P = 0.08). For both cured and active CS, telomere length correlated significantly with duration of hypercortisolism (R2 = 0.22, P = 0.007). (4) There was a 27.6% reduction in telomere length between low and high tertiles in bedtime cortisol levels of healthy participants (P = 0.019).
Conclusion
Our findings demonstrate that exposure to stress and/or GCs is associated with shortened telomeres, which may be partially reversible.
Keywords: telomere length, stress, cortisol, glucocorticoids, allostatic load, cellular aging, Cushing syndrome
Allostatic load refers to the lifetime accumulation of disease burden in an individual’s effort to adapt to environmental demands of daily living. Glucocorticoid (GC) exposure has become a major focus as a vital contributor to allostatic load because prolonged exposure to GCs from stress, iatrogenic GC administration, or hypercortisolism from endogenous Cushing syndrome increases the risk for type 2 diabetes mellitus, cardiovascular events, metabolic disorders, and neuropsychiatric disorders (1-9). Other molecular substrates have also gained traction over the years as determinants of accelerated aging and accumulated disease burden. One of the most widely used and recognized molecular tools for biological age estimation is telomere length (10).
Telomeres are DNA-protein amalgams that overlay the ends of chromosomes (11), and the structures thus formed serve to protect the integrity of chromosome termini (12). The shielding of the chromosome terminus promotes cell stability and prevents premature senescence by mitigating the deterioration of chromosomal structures (13). When telomeres are prematurely shortened to a threshold length, the risk for premature cell senescence resulting in degenerative and aging-related diseases increases (14). Interestingly, there is a significant body of literature linking stress and telomere length (15). Telomere length attrition (TLA) has been measured in individuals with various stress-related conditions including post-traumatic stress disorder (16), major depression (17, 18), dementia (19), bipolar disorder (20), and schizophrenia (21). In fact, while TLA is inversely associated with chronological age, it has even a stronger relationship to health status (22).
It is clear that telomere shortening is associated with different forms of stress, although the exact role of GCs in this process and the mechanism by which GCs shorten telomeres have not been fully elucidated. Several studies have linked telomere length with cortisol reactivity (23-25) or psychosocial stress exposure (26, 27), although negative findings have been reported (28, 29). Other studies have observed reduced telomerase activity following cortisol exposure (30), whereas some have observed increased activity especially in the acutely stressed individuals (31). Despite these promising lines of investigation, no study to date has examined telomere length in human or animal cohorts where chronic exposure to GCs has been determined. Moreover, no study has examined whether telomeres can increase in length if excess GC exposure is terminated. Therefore, we investigated the conditions of GC excess and normalization of excessive GC exposure in a cross-species study. We selected mouse, rat, and human and studied 2 types of tissues (blood and brain). For the brain, we were interested in the hippocampal dentate, as it serves a pivotal role in learning and memory, undergoes neurogenesis despite the postmitotic state of neuronal cells elsewhere, and is particularly susceptible to the impact of GC exposure (32). We also used several different models to study GC exposure: (1) patients with both active and cured Cushing syndrome; (2) an iatrogenic mouse model of Cushing syndrome; (3) a mouse neuronal cell line; (4) rats exposed to 3 weeks of chronic variable stress; and (5) a healthy human cohort.
We hypothesized that, in the rodent models, serum corticosterone levels would be negatively correlated with TLA and that this would be associated with decreased expression of 2 genes that are vital for telomere integrity, namely Tert and Dkc1.
For the human studies, we hypothesized that patients with active Cushing syndrome would have shorter telomeres compared with cured patients as well as compared with a healthy control group. Finally, we measured TLA in a healthy human sample where 30 consecutive days of cortisol metrics were obtained. We hypothesized that given the narrow range of cortisol levels in a healthy cohort, there would not be a significant correlation between 30-day cortisol exposure and TLA.
Methods
Mice
Four-week-old male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were housed in conventional polycarbonate mouse cages in a temperature- and humidity-controlled room on a 12-hour light, 12-hour dark cycle with light onset at 6:00 am. All animals received ad libitum access to water and standard laboratory chow (Harlan Teklad 2018, Indianapolis, IN) for 1 week after arrival in the laboratory. At 5 weeks of age, animals were given ad libitum access to solutions containing corticosterone (Sigma-Aldrich, St. Louis, MO; 100 μg/mL with 1% ethanol; corticosterone [CORT] group) or vehicle (1% ethanol; control group) in place of their normal drinking water, and this treatment continued for 4 weeks. The CORT and vehicle solutions were made fresh daily, and the animals were given ad libitum access to 0.9% saline solution to prevent symptoms of adrenal insufficiency (33).
Rats
Young adult male Sprague-Dawley rats at 6 weeks of age (~170 g) were purchased from Charles River Laboratories (Wilmington, MA). Rats (N = 10; 2 per cage) underwent 3 weeks of chronic variable stress. Age-matched control group (N = 10; 2 per cage) were also used for comparison. Brain tissues were harvested from these animals at the conclusion of the 3-week stress regimen, following blood collection for DNA extraction and corticosterone measurements. All procedures for the animals were approved by the Institutional Animal Care and Use Committee at Johns Hopkins University School of Medicine and were performed in accordance with guidelines established in the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Chronic Variable Stress
Chronic variable stress (CVS) regimens including restraint, cold temperature exposure, and cage shaking were administered once in the morning (8-11 am) and once in the afternoon (1-4 pm) at irregular times to keep the routine unpredictable. The CVS regimen included (1) a 3-hour period in a restraint cylinder; (2) 10 minutes forced swimming; (3) 3 hours tilted cage; (4) 1 hour on shaking platform; and (5) 1 hour in the 4 oC cold room. Overnight stressors included social crowding, food restriction, wet bedding, and light being turned on. Animals were individually housed over the weekend to induce social isolation stress.
Human Participants
Healthy control participants
We utilized DNA from a previously described cohort (34) consisting of 75 participants of self-reported European ancestry (mean age [SD] 34.5 [12.9] years for 44 female participants and 30.0 [9.1] years for 31 males). In-person assessments were conducted by a Master’s-level interviewer and included contact and demographic information (ie, date of birth, race/ethnicity, education level, employment status), recent alcohol and drug use assessed using the 90-day Timeline Followback (35) and breathalyzer and urine toxicology screens, and past year mental health symptoms obtained using the MINI International Neuropsychiatric Interview for DSM-IV (36). People were excluded from study participation if they reported: a serious medical condition; a medication in the past 3 months that could affect cortisol assay measurements (including antidepressants and anti-anxiety medications); met criteria for a current DSM-5 diagnosis; tested positive for or self-reported recent drug use; exceeded National Institute on Alcohol Abuse and Alcoholism guidelines for social drinking; or were currently or had been pregnant or breast feeding within the past 3 months. The study protocol consisted of an in-person study visit 1, followed by approximately 30-plus days of at-home saliva collection at bedtime and awakening prompted by text messaging. Blood samples were collected for genomic DNA (gDNA) extraction at Visit 2. DNA and salivary cortisol were assayed as previously described (34). All study procedures were reviewed and approved by the Johns Hopkins School of Medicine Institutional Review Board.
Cushing syndrome and matched controls
We utilized DNA from a previously described cohort of 27 females and 5 males with Cushing syndrome (37). Nine patients had active Cushing syndrome and 23 patients were cured of Cushing syndrome. The average age for the patients was 45 ± 12 years. The disease duration was mean (SD) 7.1 (2.2) years. DNA was also obtained from 32 healthy controls matched for age, sex, and years of education. All patients and controls signed an informed consent after study approval by the Sant Pau Hospital Ethics Committee. None of the participants had a past medical history of head injury, cerebrovascular disease, mental illness, or psychiatric disorders and none were taking tranquilizers or GCs.
Mouse Hippocampal Cell Line
The HT-22 cell line derived from the mouse hippocampus was cultured using DMEM (ThermoFisher, Waltham, MA) supplemented with 10% fetal bovine serum (Sigma-Aldrich) under standard conditions (5% CO2, 37 oC). Cells were trypsinized and plated in triplicate in 25 cm2 vented flasks before treatment with 1 μM dexamethasone (DEX; Sigma-Aldrich). Control samples were treated with an equal volume of ethanol as those treated with DEX. Cells were split on the third day of treatment to maintain them in the log phase of growth. After 7 days of treatment, cells were harvested for genomic DNA. A fraction of the cells was cultured for an additional 4 weeks in the absence of DEX, during which additional DNA was harvested.
Rodent Blood Collection
At the conclusion of the GC or stress regimen, blood samples were collected at the onset of the light cycle (9:00 am) for genomic DNA extraction. Samples were centrifuged at 4 oC, and plasma was collected and frozen at −80 oC for CORT determination. A second blood sample (~70 μL whole blood) was collected immediately after the first for genomic DNA extraction.
Corticosterone Assays
For CVS, small volumes of blood (~100 μL) were sampled at 8 time points during the 3-week experiment. The first time point was prior to the CVS regimen and served as the baseline for the subsequent time point values. Blood was sampled on Wednesday morning and on Monday morning following each week of CVS, prior to the stress regimen of the following week. The last time point was on the morning prior to euthanasia. Blood samples were centrifuged (600g, 4 oC, 10 minutes) to separate the plasma from the blood cells. Plasma samples were used for the determination of corticosterone by radioimmunoassay, according to the manufacturer’s instructions (MP Biomedicals, Santa Ana, CA).
Antibodies used in this study (listed on antibodyregistry.org) were Human Cortisol, MP Biomedical (RRID AB 2801525) and Mouse Corticosterone: MP Biomedical (RRID AB 2783720).
DNA Extraction From Blood and Brain Tissues
After blood collection, red blood cells were lysed using 3.5 times the volume of ACK Lysing Buffer (Quality Biological, Gaithersburg, MD). Remaining white blood cells were pelleted by centrifugation at 300g for 5 minutes at 4 oC. T cells of stressed rats were further isolated from the white blood cells using the Miltenyi Biotec Pan T Cell MicroBeads according to the manufacturer’s protocol (Cambridge, MA). T cells were selected because we previously demonstrated that they were most affected by GC-induced change in DNA methylation (38). We also tested DNA from the dentate gyrus of the hippocampus, where glucocorticoids are thought to not lead to changes in cell type composition and can undergo appreciable neurogenesis (38, 39).
Mouse and rat whole brains were mounted on a cryostat, and the hippocampal dentate was dissected using the Mouse and Rat Brain Atlas. The coordinates were AP-2.3; ML, ±1.5; DV-1.7 for the mouse (40) and AP-3.8; ML, ±1.8; DV-3.0 for the rat (41). Genomic DNA was extracted using the MasterPure Complete DNA Extraction kit (Epicentre, Madison, WI) according to the manufacturer’s protocol. DNA samples were resuspended or eluted in TE buffer, and their concentrations were determined by Qubit 2.0 Fluorometer (ThermoFisher).
Telomere Assays for Human, Mouse, and Rat Genomic DNA
The assay for determining telomere length has been adapted for quantitative polymerase chain reaction (qPCR) to measure telomere quantity and demonstrated to show a high correlation (R2=0.844) to telomere length determination by the telomere restriction fragment (TRF) assay (42). The albumin locus (ALB) was used as the single copy gene assay for normalization of telomere quantity. For each assay, 50 ng of gDNA was plated in triplicate and subjected to qPCR using the SYBR Green Master Mix and QuantStudio 5 Real-time PCR System (MMqPCR) (Applied Biosystems, Foster City, CA). To calculate telomere quantity, the -ΔΔCt method was used (43), where triplicate Ct values for each sample were averaged and subtracted from those derived from the single copy locus ALB (albumin). The Ct difference for a calibrator sample was subtracted from those of the test samples and the resulting -ΔΔCt values were raised to a power of 2 to determine relative telomere length. Primers used for the telomere and ALB assays are listed in Table 1. For the telomere assay, same primer sets were employed for all 3 species due to sequence conservation.
Table 1.
Primers used for the assessment of telomere quantity
| Species | Albumin | Telomere |
|---|---|---|
| Human | F - AAATGCTGCACAGAATCCTTG | F - ACACTAAGGTTTGGGTTT |
| R - GAAAAGCATGGTCGCCTGTT | GGGTTTGGGTTTGGGTTAGTGT | |
| Mouse | F - GCTGACTGCTGTACAAAACAAGAG | R - TGTTAGGTATCCCTATCCC |
| R - TGACTATCAGCATAAGTGTTACTA | TATCCCTATCCCTATCCCTAACA | |
| Rat | F - AAAGCTGGTAGGGTTCTC | |
| R - GATCTGACCCTCTCTGTC |
Expression of Genes Associated With the Telomerase Component
Mouse genes encoding TERT and DKC1 were chosen based on experimental evidence and literature. Both genes harbored binding sites for the glucocorticoid receptor (NR3C1) identified by ChIP-Seq experiments that were performed by HudsonAlpha on 2 different cell lines: the A549 lung carcinoma epithelial cell line and the ECC-1 endometrial adenocarcinoma epithelial cell line. The data are available on the UCSC Genome Browser (human GRCh37/hg19 assembly) and from a ChIP-Seq study on the glucocorticoid receptor (44). In addition, the selection of these genes was made from corroborating studies implicating their activities from exposure to glucocorticoids or oxidative stress. Ct values from TERT and DKC1 were normalized against those obtained from the housekeeping gene Actb. Relative expression levels of each sample were determined using the QuantStudio 5 Real-time PCR System and the -ΔΔCt method.
Data Analysis
For rodent and cell line data, differences in telomere lengths and their association with plasma corticosterone levels were analyzed by Student’s t tests and linear regression, respectively. The human data was analyzed using linear regression with both unadjusted and adjusted models controlling for age and sex. A threshold of P < 0.05 was used to define statistical significance, and all analyses were carried out in either Stata or R.
Results
Telomere Length Is Associated With Exposure to Stress in Rats
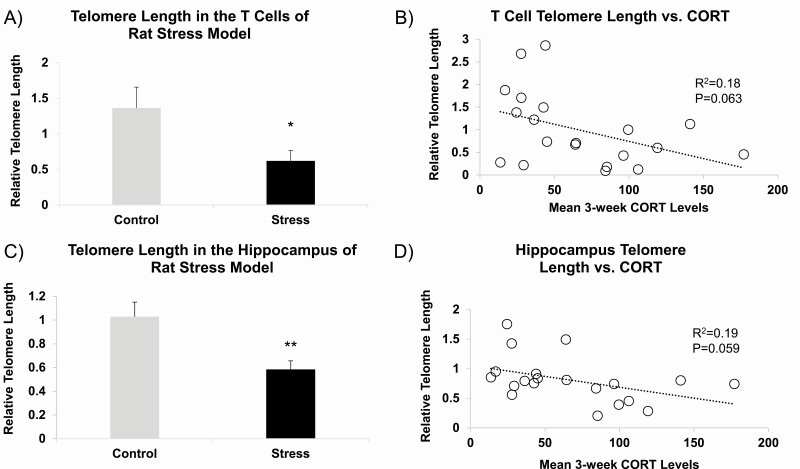
In the first study, we tested the effects of our rat chronic variable stress (CVS) model on telomere length attrition (TLA). To preclude the possible shift in the white cell composition of blood during CVS, rat blood was further processed to isolate T cells followed by genomic DNA extraction. In the T cells, we observed a 54.5% (P = 0.036) reduction in relative telomere length in the stressed animals (Fig. 1A). Theorizing that GCs may be one of the active substrates inducing TLA in the CVS model, the mean 3-week corticosterone levels were determined for each animal and between the CVS and control groups (101.6 ± 12.0 ng/mL for CVS and 32.9 ± 4.7 ng/mL for control groups, mean ± SEM, N = 10 per group, P = 4.7 × 10-5). The relative telomere length in the stressed rats correlated with the mean 3-week corticosterone levels with borderline significance (R2 = 0.18, P = 0.063, Fig. 1B).
Figure 1.
Telomere length in the rat T cells and hippocampus and its relationship with mean 3-week plasma corticosterone levels. (A) Telomere length was assessed in T-cell DNA obtained from rats exposed to 3-week chronic variable stress or no stress (N = 10 per group). (B) T-cell telomere lengths were correlated with mean 3-week plasma corticosterone levels calculated from 8 samplings during the CVS regimen. (C) Telomere length assessed in hippocampal DNA showed similar stress-induced reduction. (D) Similar association between hippocampal telomere length and corticosterone levels was observed. Bar graphs are presented as mean ± SEM. *P < 0.05 and **P < 0.01.
Genomic DNA extracted from the dentate gyrus of stressed and unstressed rats showed 43.2% reduction in relative telomere length (P = 0.006, Fig. 1C), suggesting that stress-induced telomere reduction is still observed in a tissue that does not undergo appreciable changes in cellular heterogeneity. Telomere length values from the hippocampus were correlated against the 3-week mean corticosterone levels for each animal, and a borderline significant correlation was observed (R2 = 0.185, P = 0.059, Fig. 1D).
Glucocorticoids Reduce Telomere Length in Mouse and Mouse Cell Line Models
The correlation between relative telomere length and mean CORT levels in the rat CVS model suggested that despite the many endocrine and immune factors that mediate the stress response, GCs may be an important substrate mediating TLA. To this end, we selected to work with our mouse model of iatrogenic Cushing syndrome (33, 45). Mice were exposed to corticosterone (CORT) or vehicle for 4 weeks producing supraphysiological mean serum levels of CORT (454.7 ± 78.3 ng/mL for CORT and 22.9 ± 6.0 ng/mL for VEHICLE groups, mean ± SEM, N = 12 per group, P = 8.9 × 10-5). This treatment produced a 61.4% reduction in telomere length in the blood gDNA of mice exposed to CORT (P = 5.75 × 10-5, Fig. 2A). T cells were not isolated for analysis due to the volume of blood necessary for T-cell isolation and genomic DNA extraction. Since mammalian blood can undergo glucocorticoid-induced changes in cell type composition (38, 46), there was a possibility that the observed reduction in telomere length may be due to glucocorticoid-induced increase in the proportion of a cell type with shorter telomeres. Therefore, as in the rat CVS model, the dentate gyrus of the hippocampus was isolated for analysis. There was a 58.8% reduction in telomere length following CORT treatment compared with vehicle-treated animals (P = 0.002, Fig. 2A). To identify potential mechanisms that can mediate the impact of glucocorticoids on telomere length, expression levels of Tert and Dkc1, 2 genes involved in telomere lengthening and stability, respectively, were assayed by qPCR. CORT treatment led to a 77.9% reduction in the expression level of Tert (P = 1.41 × 10-4) and a 54.1% reduction in the expression level of Dkc1 (P = 1.35 × 10-4, Fig. 2B) in the hippocampus. To complement the in vivo model, a neuronal cell line was utilized to study TLA in a purely homogenous population of cells. Treatment of the HT-22 mouse hippocampal cell line with different doses of dexamethasone (DEX) or vehicle for 7 days showed a dose-dependent reduction in telomere length in the DEX-treated samples with the highest dose of 1 μM DEX leading to 71.1% reduction in telomere length compared with vehicle-treated samples (P = 0.007, Fig. 2C). We also tested whether cells undergo any reversal of DEX-induced TLA following the withdrawal of GCs. We observed a recovery up to 68.2% of the telomere length in control samples after 1 week of recovery and more than 80% of the control telomere length during weeks 2 to 4 (Fig. 2D).
Figure 2.
Telomere length measurements in glucocorticoid-treated mice and cell line. (A) Telomere length was assessed in whole blood and hippocampal DNA of mice treated with corticosterone (CORT) or vehicle solution for 30 days (N = 12 per group). (B) Normalized gene expression levels of telomere-associated genes Tert and Dkc1 in the hippocampus of CORT-treated mice. (C) Dose-dependent reduction in telomere length was observed in the hippocampal cell line HT-22 treated with dexamethasone (DEX). (D) DEX-induced TLA was normalized following withdrawal of DEX from the media. Bar and line graphs are represented as mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001.
Telomere Length Is Associated With Cushing Syndrome Status
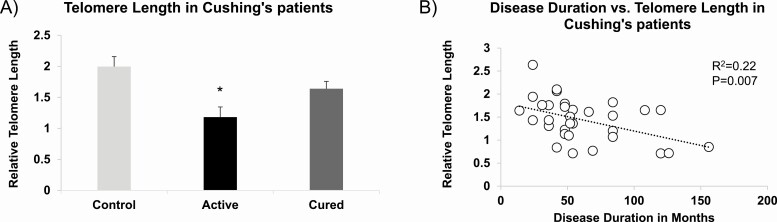
With evidence that both stress and GC exposure induce TLA in mouse and rat, we analyzed blood genomic DNA obtained from a cohort of patients with Cushing syndrome (37) to determine whether relative telomere length was shorter in individuals with endogenous hypercortisolism. We observed a 40.8% reduction in the telomere length in patients with active Cushing compared with healthy controls (Fig. 3A), which was significant even after adjusting for age and sex (P = 0.006). By contrast, there was only a 17.8% reduction in telomere length in Cushing patients with cured hypercortisolism that was no longer significantly different from healthy controls after adjusting for age and sex (P = 0.08). Interestingly, for both cured and active Cushing patients, telomere length correlated significantly with the duration of hypercortisolism (R2=0.22, P = 0.007, Fig. 3B).
Figure 3.
Telomere length attrition (TLA) in patients with Cushing syndrome. (A) Telomere length was assessed in leukocyte DNA obtained from patients with active Cushing syndrome (N = 9), those who have been cured (N = 23), and healthy controls (N = 32). (B) Telomere length correlated significantly with the duration of hypercortisolism in patients with active Cushing syndrome and patients cured of the disorder. Bar graph is represented as mean ± SEM. *P < 0.05.
Telomere Length Is Associated With Mean 30-Day Bedtime Cortisol Levels
With the finding that high levels of GC are associated with shortened telomeres in a patient population, we investigated whether telomere length correlated with cortisol levels in a healthy, human cohort. Previous characterization of this cohort of healthy individuals showed a normal range of awakening and bedtime cortisol levels over 30+ days (34). Relative telomere lengths were calculated from blood gDNA of the participants and compared against mean 30+ day awakening and bedtime cortisol levels. There was a 27.6% reduction in relative telomere length observed between low and high tertiles in bedtime cortisol levels (Fig. 4A) that was significant even after controlling for age and sex (P = 0.019). Analysis of the full samples also showed a significant relationship between telomere length and continuous bedtime cortisol levels (R2=0.1, P = 0.006, Fig. 4B). Methylation levels of several CpGs that were used as surrogates for assessing the cellular composition of blood (47) in this cohort showed no differences between the 2 cortisol tertiles (34).
Figure 4.
Telomere length in healthy individuals. (A) Telomere length in leukocyte DNA was analyzed from 75 healthy participants whose DNA samples were divided into tertiles based on their mean 30-day bedtime salivary cortisol levels. Shorter overall telomere lengths were observed in the high cortisol tertile compared with the low cortisol tertile. (B) Telomere lengths correlated significantly with bedtime cortisol levels for all 75 participants. Bar graph is represented as mean ± SEM. *P < 0.05.
Discussion
In this study, we used animal models of stress and corticosterone (CORT) exposure as well as human clinical samples to establish a link between telomere length and GC exposure. To test whether we can detect telomere attrition during exposure to stress, a rat model of chronic variable stress was used (48). We isolated T cells for this analysis of TLA following the stress procedure so that a homogeneous population of cells could be investigated. This precludes the possibility the results were due to shifting white blood cell populations. We found that an unpredictable but mild stress regimen administered over a 3-week period elicited elevated CORT levels and TLA in both T-cell gDNA and hippocampus of stress-exposed rats. Further, the telomere lengths in both tissues correlated significantly with mean 3-week CORT levels. The correlation between relative telomere length and mean CORT levels in the rat CVS model suggested that despite the sundry endocrine and immune factors that mediate the stress response, GCs may be an important substrate mediating TLA. Therefore, we used an established mouse model of Cushing syndrome (45) to determine if telomere length was impacted by high serum CORT levels. Mice were exposed to CORT or vehicle for 4 weeks. We measured telomere lengths in both blood and brain, a tissue that does not undergo appreciable changes in cellular composition following glucocorticoid treatment (38). T cells were not isolated from blood for analysis due to reduced blood volume of mice. Once again, we observed telomere shortening of gDNA from blood and from hippocampal dentate gyrus, suggesting that the observation was due to a decrease in telomere lengths rather than changes in the cellular composition. Interestingly, reduction of telomere length was greater in the hippocampus than blood, suggesting that the telomeres in this tissue may be more vulnerable to the impact of glucocorticoids.
To be more certain that glucocorticoid-induced changes in telomere length did not reflect shifting cell populations, we treated a hippocampal cell line with dexamethasone (DEX). After 7 days of treatment with DEX, a robust decrease in overall telomere length was observed. This finding shows that similar to our T-cell experiment, glucocorticoids can directly lead to TLA without causing a shift in cellular composition. Interestingly the DEX-treated cells that underwent withdrawal from DEX exposure for a month reversed much of the TLA following the 7-day DEX treatment, suggesting that telomere shortening was a reversible process, although incomplete. The cause of TLA in our studies is unclear. However, we did observe that glucocorticoid treatment led to the downregulation of Tert and Dkc1 genes that are involved in telomere elongation and stability, respectively. Glucocorticoid-regulation of genes such as Tert and Dkc1 may be crucial in our understanding of telomere length restoration. Studies have shown that the TERT locus is under the control of GCs as well as sex hormones such as estrogen (30, 49).
In a recent study, a transcriptomic comparison of GC-resistant vs GC-sensitive groups implicated multiple transcripts involved in telomere maintenance (50). Our study shows that additional genes such as Dkc1 may be co-regulated. It is plausible that glucocorticoids lead to accelerated cellular aging in part by disrupting multiple components involved in telomere maintenance and elongation. It is unclear whether suppression of gene expression persists with prolonged exposure to stress or glucocorticoids. Previous work has demonstrated that glucocorticoids can regulate gene function through epigenetic mechanisms that can persist beyond the exposure period (45, 51, 52). Additional work is needed to examine the persistent effects of GC exposure and the altered presence of these factors at the telomere termini.
With evidence that both stress and GC exposure induce TLA in mouse and rat, we measured TLA in gDNA from human cohorts we previously characterized (34, 37). First, we investigated TLA from patients with active Cushing syndrome, age- and sex-matched with patients cured of the disorder, as well as age- and sex-matched with healthy control participants. Previous work employing the TRF assay on a larger set of Cushing syndrome samples that included our samples reported no significant differences in telomere length between Cushing syndrome and healthy controls and no correlation between telomere length and duration of hypercortisolism (46). In our study, we observed a more than 40% reduction in telomere length between Cushing and healthy controls. Furthermore, disease duration was negatively correlated with telomere length, and those who had been cured of hypercortisolism showed telomere lengths between those of healthy controls and active Cushing syndrome patients. Importantly, cured patients showed no significant differences in neutrophil or lymphocyte count compared with the healthy controls (46). The discrepancies in the results between the 2 studies are likely due to the differences in both the method of telomere length measurement and sensitivities afforded by the two methods. Our finding that samples from cured Cushing patients exhibit longer telomere lengths compared with those of the active Cushing patients can recapitulate what we observed during recovery from DEX exposure in the hippocampal cell line. Assuming that individuals cured of hypercortisolism had telomere lengths similar to those with active Cushing disease prior to treatment, this finding raises questions about the potential mechanisms underlying the reversibility of TLA. This scenario would imply that telomere maintenance is a steady-state process that strikes a balance between telomere lengthening and shortening. The steady-state level would shift in one direction based on certain conditions such as during glucocorticoid exposure and shift back when glucocorticoid levels return to baseline. Importantly, TLA reversal in human lymphocytes is facilitated by telomerases that may be expressed under certain conditions such as mitogenic stimulation (53).
With the finding that high levels of GC are associated with shortened telomeres in a patient population, we investigated whether telomere length correlated with endogenous cortisol levels in a healthy, human cohort. Specifically, we asked whether telomere lengths correlated with cortisol exposure in a human cohort from which 30+ consecutive days of awakening and bedtime cortisol levels had been recorded. In contrast to our hypothesis, we found a significant association between mean 30+ day bedtime cortisol levels and telomere length even when the data was age- and sex-adjusted. Once again, we made certain that the finding was not the result of differences in white blood cell populations in the low and high cortisol tertile groups. Previously, we tested the absence of such a change by confirming similar methylation levels of epigenetic markers of different cell types in the blood between individuals exposed to low vs high cortisol levels (34). Other studies have shown TLA as an indicator of disease burden in chronic disease states such as diabetes, cancer, and HIV infection, with notable exceptions (54-57). Our study is the first to demonstrate such a relationship in healthy individuals and highlights the dynamic role of cortisol on telomere length determination in the absence of additional disease burden from chronic conditions.
There could be different hypotheses to explain the association between excessive GC concentrations and telomere attrition, such as the direct effect of increased oxidative stress caused by GC or the reduction of resources available for telomere maintenance through changes in the metabolic machinery (58).
The relationship between stress hormones and oxidative stress has been clearly demonstrated at the cellular level. GCs increase oxidative stress damage to neurons, in part by increasing glutamate and calcium and decreasing antioxidant enzymes (59, 60). The GC effect on oxidative stress depends on the duration of treatment and is larger in long-term experiments. Importantly, there was a significant effect on tissue, with brain and heart being the most and the least susceptible to GC-induced oxidative stress, respectively (60).
Data on the effects of oxidative stress and in Cushing syndrome are scarce. A study showed an enhanced oxidative injury and platelet aggregation in patients affected by Cushing syndrome (61). Moreover, despite long-term cure, patients who have suffered CS exhibit persistent accumulation of central fat, as during active hypercortisolemia, with consequent unfavorable adipokine profile, leading to a state of low-grade inflammation (62). Dyslipidemia and chronic inflammation markers are also correlated with telomere length shortening in Cushing syndrome. (63). Thus, the shortening of telomere length during stressful periods and/or exposure to excess of GCs can be mediated by metabolic dysregulations. All of these factors can determine a persistent and increased cardiovascular risk and can reduce telomere length in these patients.
Our current work has several notable features. One strength is the translational nature of the finding where three species had a similar response to glucocorticoid exposure. Second, a clinically relevant cohort of patients with Cushing syndrome was used to demonstrate the role of endogenous hypercortisolism on telomere length. We then expanded the inquiry to include a cohort of healthy participants. To our knowledge, our study is the first to show a correlation between glucocorticoid exposure and TLA in a healthy human population, by leveraging cortisol measurements collected over 30+ consecutive days. Third, findings in the brain were further supported by a hippocampal cell line that demonstrated the direct impact of glucocorticoids on telomere length as well as the reversible nature of the phenomenon. Fourth, all of the samples were examined using the same primer set for telomere length determination. This was made possible by the identical telomere repeat sequence among human, mouse, and rat and helped to increase the translatability of the animal findings.
Our study has several limitations. First, although we addressed the issue of whether the shortening of telomeres in this study was happening in all cell types in a given sample or was an artifact of shifting cell populations, more experiments can still be performed to clarify this point. In addition, although the cured Cushing samples and the cell line model provided some evidence of reversibility, it is still unclear how long TLA persist in vivo and which mechanistic factors may be involved in its reversal.
Second, we also acknowledge that telomere dynamics are different among the 3 species and between tissues and immortalized cell lines. For instance, telomere lengths in laboratory mice range from 50 to 150 kilobases long, whereas those in humans range from 5 to 12 kilobases (64). It is unclear how these observations can be reconciled with the significant differences in longevity between the two species and whether different mechanisms exist to buffer the corrosion of telomeres by chronic GC exposure. In addition, immortalized cells such as the HT-22 hippocampal cell line exhibit robust telomerase activity (65). Although the HT-22 cell line has been used here to demonstrate GC-induced TLA in a homogeneous population of cells, reversal of TLA is likely due to its abundance of telomerases, and therefore of limited relevance to the hippocampus of mice and humans, except perhaps in areas of active neurogenesis (66, 67).
Third, we did not assess oxidative damage caused by reactive oxygen species (ROS) metabolites and changes in mitochondrial metabolism; glucocorticoid-induced telomere attrition might be associated with both. This is especially important given that genes such as Dkc1 may be involved in GC-induced oxidative stress in addition to direct regulation by GC exposure alone (68).
Fourth, although the Tert and Dkc1 genes were chosen based on experimental evidence of regulation by GCs and studies implicating them in GC action or oxidative stress (31, 68-71), there could be multiple genes that can potentially play a role in GC-induced telomere attrition. As GC receptor binding is highly cell type–specific (72), the ability of GCs to regulate telomere-associated proteins in different cell types or tissues are currently unknown. A comprehensive study examining expression levels of the components of the telomerase complex and telomere protection genes in the context of brain GC signaling are needed.
Last, there are inherent limitations associated with the MMqPCR method (42) employed in the current study. While the qPCR platform enables a highly sensitive and quantitative assessment of telomere “length,” it does not allow for direct length measurement as in the TRF method (73, 74). Instead, the MMqPCR measures the “amount” of telomere repeats present. Furthermore, it is difficult to compare across multiple studies without the rigorous use of standards and adjustment for assay-specific batch effects. Nevertheless, the MMqPCR method is commonly employed for its ease of use and the need for only several nanograms of DNA. Since its publication, this method has garnered much traction and has been employed in a number of studies that have examined telomere lengths in numerous areas, including obesity (75), cancer (76), cardiovascular disease (77, 78), diabetes (79), and psychiatric disorders (80).
In summary, the results from the current cross-species study demonstrate that telomere length is strongly impacted by GCs across species. Telomere length attrition may be reversible under certain conditions. Taken together, the findings suggest telomere length may be used as a proxy for long-term glucocorticoid exposure. The observation of reversibility of GC-induced TLA also raises the prospect of using treatments that lower serum cortisol levels to increase telomere length and possibly reverse cellular senescence.
Acknowledgments
This study was funded by the Baker Foundation (R.S.L.), Rales Family Foundation (R.S.L. and G.S.W.), and NIAAA U01 AA020890 (G.S.W.)
Glossary
Abbreviations
- CORT
corticosterone
- CS
Cushing syndrome
- CVS
chronic variable stress
- DEX
dexamethasone
- GC
glucocorticoid
- qPCR
quantitative polymerase chain reaction
- TLA
telomere length attrition
- TRF
telomere restriction fragment
Contributor Information
Richard S Lee, Department of Psychiatry and Behavioral Sciences, Johns Hopkins School of Medicine, Baltimore, MD 21205, USA.
Peter P Zandi, Department of Psychiatry and Behavioral Sciences, Johns Hopkins School of Medicine, Baltimore, MD 21205, USA; Department of Mental Health, Johns Hopkins School of Public Health, Baltimore, MD 21205, USA.
Alicia Santos, Endocrinology/Medicine Department, Hospital Sant Pau, Centro de Investigación Biomédica en Red de Enfermedades Raras (CIBER-ER, Unit747), IIB-Sant Pau, ISCIII and Universitat Autònoma de Barcelona (UAB), Barcelona, Spain.
Anna Aulinas, Endocrinology/Medicine Department, Hospital Sant Pau, Centro de Investigación Biomédica en Red de Enfermedades Raras (CIBER-ER, Unit747), IIB-Sant Pau, ISCIII and Universitat Autònoma de Barcelona (UAB), Barcelona, Spain.
Jenny L Carey, Department of Psychiatry and Behavioral Sciences, Johns Hopkins School of Medicine, Baltimore, MD 21205, USA.
Susan M Webb, Endocrinology/Medicine Department, Hospital Sant Pau, Centro de Investigación Biomédica en Red de Enfermedades Raras (CIBER-ER, Unit747), IIB-Sant Pau, ISCIII and Universitat Autònoma de Barcelona (UAB), Barcelona, Spain.
Mary E McCaul, Department of Psychiatry and Behavioral Sciences, Johns Hopkins School of Medicine, Baltimore, MD 21205, USA.
Eugenia Resmini, Endocrinology/Medicine Department, Hospital Sant Pau, Centro de Investigación Biomédica en Red de Enfermedades Raras (CIBER-ER, Unit747), IIB-Sant Pau, ISCIII and Universitat Autònoma de Barcelona (UAB), Barcelona, Spain.
Gary S Wand, Department of Psychiatry and Behavioral Sciences, Johns Hopkins School of Medicine, Baltimore, MD 21205, USA; Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD 21205, USA.
Additional Information
Disclosures: The authors declare no conflicts of interest
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci U S A. 2001;98(8):4770-4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arnsten AF. Stress weakens prefrontal networks: molecular insults to higher cognition. Nat Neurosci. 2015;18(10):1376-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bose M, Oliván B, Laferrère B. Stress and obesity: the role of the hypothalamic-pituitary-adrenal axis in metabolic disease. Curr Opin Endocrinol Diabetes Obes. 2009;16(5):340-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brydon L, Magid K, Steptoe A. Platelets, coronary heart disease, and stress. Brain Behav Immun. 2006;20(2):113-119. [DOI] [PubMed] [Google Scholar]
- 5. Fardet L, Petersen I, Nazareth I. Suicidal behavior and severe neuropsychiatric disorders following glucocorticoid therapy in primary care. Am J Psychiatry. 2012;169(5):491-497. [DOI] [PubMed] [Google Scholar]
- 6. Hackett RA, Steptoe A. Psychosocial factors in diabetes and cardiovascular risk. Curr Cardiol Rep. 2016;18(10):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kelly SJ, Ismail M. Stress and type 2 diabetes: a review of how stress contributes to the development of type 2 diabetes. Annu Rev Public Health. 2015;36:441-462. [DOI] [PubMed] [Google Scholar]
- 8. McKlveen JM, Morano RL, Fitzgerald M, et al. Chronic stress increases prefrontal inhibition: a mechanism for stress-induced prefrontal dysfunction. Biol Psychiatry. 2016;80(10):754-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rahman MM, Callaghan CK, Kerskens CM, Chattarji S, O’Mara SM. Early hippocampal volume loss as a marker of eventual memory deficits caused by repeated stress. Sci Rep. 2016;6:29127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6(8):611-622. [DOI] [PubMed] [Google Scholar]
- 11. Coimbra BM, Carvalho CM, Moretti PN, Mello MF, Belangero SI. Stress-related telomere length in children: a systematic review. J Psychiatr Res. 2017;92:47-54. [DOI] [PubMed] [Google Scholar]
- 12. Koliada AK, Krasnenkov DS, Vaiserman AM. Telomeric aging: mitotic clock or stress indicator? Front Genet. 2015;6:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Houben JM, Moonen HJ, van Schooten FJ, Hageman GJ. Telomere length assessment: biomarker of chronic oxidative stress? Free Radic Biol Med. 2008;44(3):235-246. [DOI] [PubMed] [Google Scholar]
- 14. Belsky DW, Caspi A, Houts R, et al. Quantification of biological aging in young adults. Proc Natl Acad Sci U S A. 2015;112(30):E4104-E4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: an overview. Biol Psychiatry. 2013;73(1):15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lohr JB, Palmer BW, Eidt CA, et al. Is post-traumatic stress disorder associated with premature senescence? A review of the literature. Am J Geriatr Psychiatry. 2015;23(7):709-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cai N, Chang S, Li Y, et al. Molecular signatures of major depression. Curr Biol. 2015;25(9):1146-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gotlib IH, LeMoult J, Colich NL, et al. Telomere length and cortisol reactivity in children of depressed mothers. Mol Psychiatry. 2015;20(5):615-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Honig LS, Kang MS, Schupf N, Lee JH, Mayeux R. Association of shorter leukocyte telomere repeat length with dementia and mortality. Arch Neurol. 2012;69(10):1332-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lima IM, Barros A, Rosa DV, et al. Analysis of telomere attrition in bipolar disorder. J Affect Disord. 2015;172:43-47. [DOI] [PubMed] [Google Scholar]
- 21. Kao HT, Cawthon RM, Delisi LE, et al. Rapid telomere erosion in schizophrenia. Mol Psychiatry. 2008;13(2):118-119. [DOI] [PubMed] [Google Scholar]
- 22. Muzumdar R, Atzmon G. Telomere length and aging. In: Li B, ed. Reviews on Selected Topics of Telomere Biology. Rijeka: InTech; 2012:3-30. [Google Scholar]
- 23. Provenzi L, Giorda R, Fumagalli M, et al. Telomere length and salivary cortisol stress reactivity in very preterm infants. Early Hum Dev. 2019;129:1-4. [DOI] [PubMed] [Google Scholar]
- 24. Nelson BW, Allen NB, Laurent H. Infant HPA axis as a potential mechanism linking maternal mental health and infant telomere length. Psychoneuroendocrinology. 2018;88:38-46. [DOI] [PubMed] [Google Scholar]
- 25. Kroenke CH, Epel E, Adler N, et al. Autonomic and adrenocortical reactivity and buccal cell telomere length in kindergarten children. Psychosom Med. 2011;73(7):533-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101(49):17312-17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Puterman E, Lin J, Blackburn E, O’Donovan A, Adler N, Epel E. The power of exercise: buffering the effect of chronic stress on telomere length. Plos One. 2010;5(5):e10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Woody A, Hamilton K, Livitz IE, Figueroa WS, Zoccola PM. Buccal telomere length and its associations with cortisol, heart rate variability, heart rate, and blood pressure responses to an acute social evaluative stressor in college students. Stress. 2017;20(3):249-257. [DOI] [PubMed] [Google Scholar]
- 29. Jodczyk S, Fergusson DM, Horwood LJ, Pearson JF, Kennedy MA. No association between mean telomere length and life stress observed in a 30 year birth cohort. Plos One. 2014;9(5):e97102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Choi J, Fauce SR, Effros RB. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav Immun. 2008;22(4):600-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Epel ES, Lin J, Dhabhar FS, et al. Dynamics of telomerase activity in response to acute psychological stress. Brain Behav Immun. 2010;24(4):531-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sapolsky RM. Stress and the brain: individual variability and the inverted-U. Nat Neurosci. 2015;18(10):1344-1346. [DOI] [PubMed] [Google Scholar]
- 33. Lee RS, Tamashiro KL, Yang X, et al. A measure of glucocorticoid load provided by DNA methylation of Fkbp5 in mice. Psychopharmacology (Berl). 2011;218(1):303-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee RS, Mahon PB, Zandi PP, et al. DNA methylation and sex-specific expression of FKBP5 as correlates of one-month bedtime cortisol levels in healthy individuals. Psychoneuroendocrinology. 2018;97:164-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sobell L, Sobell M. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, eds. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowas, NJ: Humana Press; 1992:41-72. [Google Scholar]
- 36. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22-33;quiz 34. [PubMed] [Google Scholar]
- 37. Resmini E, Santos A, Aulinas A, et al. Reduced DNA methylation of FKBP5 in Cushing’s syndrome. Endocrine. 2016;54(3):768-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seifuddin F, Wand G, Cox O, et al. Genome-wide Methyl-Seq analysis of blood-brain targets of glucocorticoid exposure. Epigenetics. 2017;12(8):637-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433-1438. [DOI] [PubMed] [Google Scholar]
- 40. Franklin KBJ, Paxinos G. Paxinos and Franklin’s The Mouse Brain in Stereotaxic Coordinates. Amsterdam: Academic Press, an imprint of Elsevier; 2013. [Google Scholar]
- 41. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Amsterdam/Boston: Academic Press/Elsevier; 2007. [Google Scholar]
- 42. Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37(3):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101-1108. [DOI] [PubMed] [Google Scholar]
- 44. Reddy TE, Pauli F, Sprouse RO, et al. Genomic determination of the glucocorticoid response reveals unexpected mechanisms of gene regulation. Genome Res. 2009;19(12):2163-2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee RS, Tamashiro KL, Yang X, et al. Chronic corticosterone exposure increases expression and decreases deoxyribonucleic acid methylation of Fkbp5 in mice. Endocrinology. 2010;151(9):4332-4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aulinas A, Ramírez MJ, Barahona MJ, et al. Telomere length analysis in Cushing’s syndrome. Eur J Endocrinol. 2014;171(1):21-29. [DOI] [PubMed] [Google Scholar]
- 47. Jaffe AE, Irizarry RA. Accounting for cellular heterogeneity is critical in epigenome-wide association studies. Genome Biol. 2014;15(2):R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carey JL, Cox OH, Seifuddin F, et al. A rat methyl-seq platform to identify epigenetic changes associated with stress exposure. J Vis Exp. 2018;24(140):58617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Calado RT, Yewdell WT, Wilkerson KL, et al. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood. 2009;114(11):2236-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nicolaides NC, Polyzos A, Koniari E, et al. Transcriptomics in tissue glucocorticoid sensitivity. Eur J Clin Invest. 2019;49(8):e13129. [DOI] [PubMed] [Google Scholar]
- 51. Niwa M, Jaaro-Peled H, Tankou S, et al. Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science. 2013;339(6117):335-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat Neurosci. 2010;13(11):1351-1353. [DOI] [PubMed] [Google Scholar]
- 53. Hiyama K, Hirai Y, Kyoizumi S, et al. Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. J Immunol. 1995;155(8):3711-3715. [PubMed] [Google Scholar]
- 54. Bao Y, Prescott J, Yuan C, et al. Leucocyte telomere length, genetic variants at the TERT gene region and risk of pancreatic cancer. Gut. 2017;66(6):1116-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pathai S, Lawn SD, Gilbert CE, et al. Accelerated biological ageing in HIV-infected individuals in South Africa: a case-control study. AIDS. 2013;27(15):2375-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shen Q, Zhao X, Yu L, et al. Association of leukocyte telomere length with type 2 diabetes in mainland Chinese populations. J Clin Endocrinol Metab. 2012;97(4):1371-1374. [DOI] [PubMed] [Google Scholar]
- 57. You NC, Chen BH, Song Y, et al. A prospective study of leukocyte telomere length and risk of type 2 diabetes in postmenopausal women. Diabetes. 2012; 61(11):2998-3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McIntosh LJ, Sapolsky RM. Glucocorticoids may enhance oxygen radical-mediated neurotoxicity. Neurotoxicology. 1996; 17(3-4):873-882. [PubMed] [Google Scholar]
- 59. Patel R, McIntosh L, McLaughlin J, et al. Disruptive effects of glucocorticoids on glutathione peroxidase biochemistry in hippocampal cultures. J Neurochem. 2002; 82: 118-125. [DOI] [PubMed] [Google Scholar]
- 60. Costantini D, Marasco V, Møller AP. A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J Comp Physiol B. 2011;181(4):447-456. [DOI] [PubMed] [Google Scholar]
- 61. Karamouzis I, Pervanidou P, Berardelli R, et al. Enhanced oxidative stress and platelet activation combined with reduced antioxidant capacity in obese prepubertal and adolescent girls with full or partial metabolic syndrome. Horm Metab Res. 2011;43(9):607-613. [DOI] [PubMed] [Google Scholar]
- 62. Barahona MJ, Sucunza N, Resmini E, et al. Persistent body fat mass and inflammatory marker increases after long-term cure of Cushing’s syndrome. J Clin Endocrinol Metab. 2009;94(9):3365-3371. [DOI] [PubMed] [Google Scholar]
- 63. Aulinas A, Ramírez MJ, Barahona MJ, et al. Dyslipidemia and chronic inflammation markers are correlated with telomere length shortening in Cushing’s syndrome. Plos One. 2015;10(3):e0120185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Calado RT, Dumitriu B. Telomere dynamics in mice and humans. Semin Hematol. 2013;50(2):165-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Holt SE, Wright WE, Shay JW. Regulation of telomerase activity in immortal cell lines. Mol Cell Biol. 1996;16(6):2932-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Caporaso GL, Lim DA, Alvarez-Buylla A, Chao MV. Telomerase activity in the subventricular zone of adult mice. Mol Cell Neurosci. 2003;23(4):693-702. [DOI] [PubMed] [Google Scholar]
- 67. Ostenfeld T, Caldwell MA, Prowse KR, Linskens MH, Jauniaux E, Svendsen CN. Human neural precursor cells express low levels of telomerase in vitro and show diminishing cell proliferation with extensive axonal outgrowth following transplantation. Exp Neurol. 2000;164(1):215-226. [DOI] [PubMed] [Google Scholar]
- 68. Ibáñez-Cabellos JS, Pérez-Machado G, Seco-Cervera M, Berenguer-Pascual E, García-Giménez JL, Pallardó FV. Acute telomerase components depletion triggers oxidative stress as an early event previous to telomeric shortening. Redox Biol. 2018;14:398-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Beery AK, Lin J, Biddle JS, Francis DD, Blackburn EH, Epel ES. Chronic stress elevates telomerase activity in rats. Biol Lett. 2012;8(6):1063-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ichiyoshi H, Kiyozuka Y, Kishimoto Y, Fukuhara S, Tsubura A. Massive telomere loss and telomerase RNA expression in dexamethasone-induced apoptosis in mouse thymocytes. Exp Mol Pathol. 2003;75(2):178-186. [DOI] [PubMed] [Google Scholar]
- 71. Ibáñez-Cabellos JS, Seco-Cervera M, Picher-Latorre C, Pérez-Machado G, García-Giménez JL, Pallardó FV. Acute depletion of telomerase components DKC1 and NOP10 induces oxidative stress and disrupts ribosomal biogenesis via NPM1 and activation of the P53 pathway. Biochim Biophys Acta Mol Cell Res. 2020;1867(12):118845. [DOI] [PubMed] [Google Scholar]
- 72. John S, Sabo PJ, Thurman RE, et al. Chromatin accessibility pre-determines glucocorticoid receptor binding patterns. Nat Genet. 2011;43(3):264-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345(6274):458-460. [DOI] [PubMed] [Google Scholar]
- 74. Ouellette MM, Liao M, Herbert BS, et al. Subsenescent telomere lengths in fibroblasts immortalized by limiting amounts of telomerase. J Biol Chem. 2000;275(14):10072-10076. [DOI] [PubMed] [Google Scholar]
- 75. Martens DS, Plusquin M, Gyselaers W, De Vivo I, Nawrot TS. Maternal pre-pregnancy body mass index and newborn telomere length. BMC Med. 2016;14(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ropio J, Chebly A, Ferrer J, et al. Reliable blood cancer cells’ telomere length evaluation by qPCR. Cancer Med. 2020;9(9):3153-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Peng H, Mete M, Desale S, et al. Leukocyte telomere length and ideal cardiovascular health in American Indians: the strong heart family study. Eur J Epidemiol. 2017;32(1):67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. García-Calzón S, Martínez-González MA, Razquin C, et al. Mediterranean diet and telomere length in high cardiovascular risk subjects from the PREDIMED-NAVARRA study. Clin Nutr. 2016;35(6):1399-1405. [DOI] [PubMed] [Google Scholar]
- 79. Grunnet LG, Pilgaard K, Alibegovic A, et al. Leukocyte telomere length is associated with elevated plasma glucose and HbA1c in young healthy men independent of birth weight. Sci Rep. 2019;9(1):7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Schutte NS, Malouff JM. The association between depression and leukocyte telomere length: a meta-analysis. Depress Anxiety. 2015;32(4):229-238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.