Abstract
Background:
Subjective cognitive decline (SCD) may be an early indicator of cognitive impairment, but depressive symptoms can confound this relationship. Associations may be influenced by differences between individuals (i.e., between-persons) or how each individual changes in their experiences over time (i.e., within-persons).
Objective:
We examined depressive symptoms as a mediator of the between- and within-person associations of SCD and objective memory in older adults.
Methods:
Coordinated analyses were conducted across four datasets drawn from large longitudinal studies. Samples (range: n=1,889 to n=15,841) included participants 65 years of age or older with no dementia at baseline. We used multilevel structural equation modeling to examine the mediation of SCD and objective memory through depressive symptoms, as well as direct relationships among SCD, objective memory, and depressive symptoms.
Results:
Older adults who were more likely to report SCD had lower objective memory on average (between-person associations), and depressive symptoms partially mediated this relationship in three of four datasets. However, changes in depressive symptoms did not mediate the relationship between reports of SCD and declines in objective memory in three of four datasets (within-person associations).
Conclusion:
Individual differences in depressive symptoms, and not changes in an individual’s depressive symptoms over time, partially explain the link between SCD and objective memory. Older adults with SCD and depressive symptoms may be at greater risk for poor cognitive outcomes. Future research should explore how perceived changes in memory affect other aspects of psychological well-being, and how these relationships influence cognitive decline and Alzheimer’s disease risk.
Keywords: memory, cognition, depression, aging
INTRODUCTION
Subjective cognitive decline (SCD), or an individual’s perceived decline in cognitive function, may be a useful early indicator of cognitive impairment and Alzheimer’s disease (AD) risk [1]. Recent initiatives, such as the Subjective Cognitive Decline Initiative (SCD-I) working group, have posited that this stage of self-reported cognitive decline in the absence of observable impairment on cognitive tests may represent one of the earliest transitory stages towards AD [1]. Indeed, community-dwelling older adults with SCD have a two- to four-fold higher risk of developing mild cognitive impairment (MCI) or AD compared to those without SCD [2,3]. Even higher rates have been reported among those who seek help at memory clinics [4,5]. However, many factors contribute to reporting of SCD and can confound investigations of its relationship with objective cognition, including the cognitive symptoms of depression. Depressive symptoms are known to co-occur with SCD [6], and older adults with higher depressive symptoms are more likely to experience current and future cognitive impairment, including AD [7–9]. Therefore, depressive symptoms may play a role in how changes in subjective cognition lead to detectable declines in cognitive performance as individuals age.
Depression and its related symptomatology are particularly difficult to discriminate from SCD as there is considerable overlap between symptoms and their outcomes. In fact, some common measures of depression in later life include perceived memory problems (e.g., Geriatric Depression Scale; [10]) as a possible indicator. SCD and depressive symptoms are consistently related cross-sectionally [11], and a recent longitudinal study found that SCD predicted older adults’ future depressive symptoms [12]. Some researchers theorize that the comorbidity of SCD and depressive symptoms is so widespread that both are the expressed symptoms of an underlying neurodegenerative disease [13–16]. In one estimate, approximately 50% of individuals with AD exhibited signs of clinical depression, with an even higher prevalence of subclinical or other neuropsychological symptoms [17,18]. Other studies suggest that since depression often expresses itself differently in older compared to younger adults, late-onset depression may in fact be a unique risk factor for cognitive decline or AD [19,20]. Therefore, longitudinal investigations of SCD and objective cognition require consideration of depressive symptoms’ potential role.
Several longitudinal studies have attempted to disentangle these relationships, with mixed results. One recent study found that over a 25-year observation period, participants’ decline in objective cognition was not associated with subjective cognitive complaints cross-sectionally or longitudinally; however, greater depressive symptoms were associated with cognitive complaints over 25 years [21]. In comparison, over approximately eight years, Jorm and colleagues [22] found that memory complaints were negatively associated with future objective memory performance. In line with the SCD-I conceptualization, this suggests that personal perceptions of impaired memory functioning may represent early signs of objective cognitive decline [1]. Further, memory complaints were associated with negative affect at the time of assessment, but did not predict later negative affect, and objective memory was not directly associated with negative affect. Finally, another recent study reported that SCD and depression independently predicted MCI and dementia status [23], but the co-occurrence of both depression and SCD led to the highest rates of conversion over a seven-year period. Across all studies, SCD, negative mood states, and depression were associated, yet how they relate to objective cognition was less clear.
The interrelationships among depressive symptoms, SCD, and objective memory are complex, potentially reflecting differences in between-person associations (e.g., cross-sectionally for whom is memory lower) and within-person processes (i.e., when someone’s memory is changing). Between-person variation differentiates people from one another; that is, who is more likely to report SCD, have higher depressive symptoms, and have poorer objective cognitive performance, on average. Between-person variation in longitudinal studies examines aggregated scores across many time points and is interpreted as how individuals differ from each other on average. In contrast, within-person variation describes how individuals change over time and how changes in variables covary. In other words, what is the association between changes in an individual’s report of SCD, changes in their depressive symptoms, and changes in their objective memory performance over time. Investigating these different sources of variability can clarify longitudinal patterns. For example, previous studies have found that depressive symptoms are higher overall in older adults with SCD compared to those without (between-person variation), yet there is a consistent association across older adults such that when they report SCD they also tend to report more depressive symptoms (within-person variation; [12,24]).
Given known relationships between SCD and depressive symptoms as well as depressive symptoms and objective memory, we examined the role of depressive symptoms as a mediator of the between- and within-person effects of SCD on objective memory among older adults without dementia. We hypothesized that:
Older adults who were more likely to report SCD would, on average, have poorer objective memory, and greater depressive symptoms would partially mediate this relationship (between-person association).
When older adults reported SCD, depressive symptoms would increase, and in turn, objective memory would decline (within-person association).
MATERIALS AND METHODS
We conducted a coordinated analysis across four longitudinal datasets to test our hypothesized relationships (See [25] for full study protocol). Coordinated analysis allows examination of the same research questions, using the same analytic approach, across multiple independent datasets, thereby supporting direct comparisons and establishing the generalizability of our findings [26]. When results are consistent across datasets, this suggests a broader phenomenon among individuals with different characteristics (e.g., demographics). Divergent results, however, indicate the need to examine whether the findings are specific to certain subgroups of individuals. The datasets selected for the current study include samples representative of U.S. older adults as well as community-based samples with high proportions of underrepresented groups.
Samples
Samples were drawn from multiple large longitudinal studies of aging: Einstein Aging Study (EAS; [27]), Memory and Aging Project (MAP; [28]), Minority Aging Research Study (MARS; [29]), Health and Retirement Study (HRS; [30,31]), and National Health and Aging Trends Study (NHATS; [32]). Due to the use of similar recruitment techniques, study methods, and the same measures for our concepts of interest across MAP and MARS, these datasets were combined in the current study. Therefore, analyses were coordinated across four independent datasets: EAS, MAP/MARS, HRS, and NHATS. All studies obtained ethics approval from their respective institutional review boards and all participants across studies provided written consent. The current study was approved by the institutional review board at the Pennsylvania State University.
Participants were included in the current study if they were 65 years of age or older, completed all measures of interest in English, did not report having AD or dementia, and had no diagnosis of AD or other dementia at baseline per the parent study protocol (EAS, MAP/MARS). HRS and NHATS do not identify individuals with dementia as part of their protocols; therefore, for these datasets cognitive status was determined based on the Telephone Interview for Cognitive Status (TICSm) measure in HRS and on scores for three cognitive domains (memory, orientation, and executive functioning) in NHATS. In HRS, participants who scored below 7 on the TICSm were classified as having dementia [33]. In NHATS, participants who scored less than 1.5 SD below the mean on at least two of the three cognitive domains were classified as having dementia ([34]; see Measures for more details). For participants who developed dementia during the study period, data from the first wave at which dementia was reported/identified as well as all subsequent waves were excluded from the final analytical datasets. Additionally, the number of participants who did not identify as Black or White (i.e., Hispanic, Other, or did not identify with any race/ethnicity) was small across all datasets (<1% - 8.9%). Therefore, they were excluded from the final analytical datasets to avoid unbalanced racial/ethnic representation that could lead to incorrect inferences about race/ethnicity effects on our variables of interest. A flowchart describing the sample inclusion/exclusion criteria is provided in Figure 1. Below, we provide descriptions of the studies from which samples were drawn for analyses.
Figure 1.
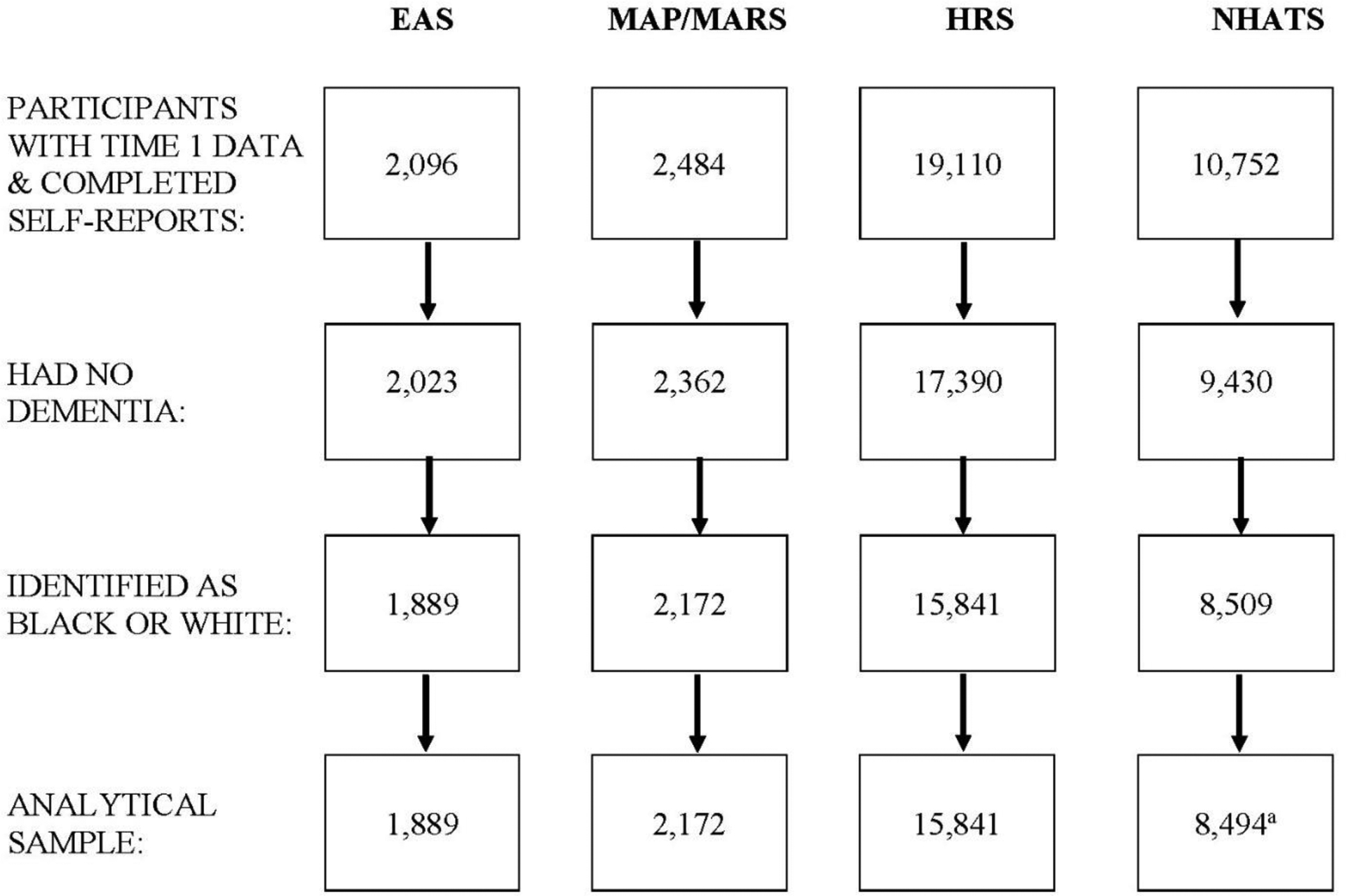
Sample Size from EAS, MAP/MARS, HRS, & NHATS Based on the Inclusion Criteria
aFinal NHATS sample includes participants who had fair to good understanding of questions and completed interviews in English.
EAS
EAS is a longitudinal cohort study of community-dwelling older adults residing in a multi-ethnic urban area of New York City (see [35] for details). To be eligible for participation, individuals must be 70 years or older, English speaking, and live independently. Data collection began in 1993 and occurs annually. Participants complete in-person assessments and comprehensive medical and neuropsychological examinations. The current study included 1,889 EAS participants (62% female; 71% White; 29% Black; Mage = 78.09, SD = 5.34) with up to 11 waves of data (1993 – 2003) per participant.
MAP/MARS
MAP is a longitudinal cohort study that examines chronic conditions, cognitive decline, and Alzheimer’s disease risk in older adults. MAP recruits older adults primarily from retirement communities as well as low-income housing throughout Northwest Illinois (see [28] for details). MARS is a longitudinal cohort study that examines risk factors for cognitive decline in African American older adults (see [29] for details). MARS recruits older adults who self-identify as African American from various settings including retirement communities, churches, clubs, and social service centers in Chicago and surrounding areas. To be eligible for participation in MAP and MARS, participants must be 65 years or older and have no diagnosis of dementia at the time of enrollment. Data collection began in 1997 for MAP and 2004 for MARS and occurs annually via in-person assessments and comprehensive medical and neuropsychological examinations. The current study included 2,172 MAP/MARS participants (75% female; 68% White; 28% Black; Mage = 78.65, SD = 7.26) with up to 21 waves (1997 – 2017) and 15 waves (2004 – 2018) of data per participant from MAP and MARS, respectively.
HRS
HRS is a longitudinal cohort study that focuses on understanding economic, health, and psychological factors associated with aging in individuals ages 50 or older in the United States [31]. It is a nationally representative study and oversamples Black and Hispanic participants. Data collection began in 1992 and occurs biennially via in-person or telephone interviews. The current study included 15,841 HRS participants (58% female; 87% White; 13% Black; Mage = 70.27, SE = 6.48) from cohorts 1 to 4, with up to nine biennial waves of data (1998 – 2014) per participant.
NHATS
NHATS is a longitudinal cohort study of aging that focuses on understanding trends in late-life disability and its social and economic consequences among individuals aged 65 and older who are medical beneficiaries in the United States [34]. NHATS is a nationally representative study and oversamples Black individuals as well as those at older ages. Data collection began in 2011 and occurs annually via in-person interviews. The current study included 8,494 NHATS participants (58% female; 78% White; 22% Black; Mage = 75 – 79) with up to eight waves of data (2011 – 2017) per participant.
Measures
All primary measures of interest (SCD, depressive symptoms, and objective memory) were assessed at each available timepoint (i.e., annually in EAS, MAP/MARS, and NHATS; biennially in HRS).
Subjective cognitive decline (SCD)
SCD was measured as self-reported decline in memory over one year (EAS, NHATS), two years (HRS), or 10 years (MAP/MARS). In EAS, participants were asked, “Compared with one year ago, do you have trouble remembering things more often, less often, or about the same?” Item wording in NHATS, HRS, and MAP/MARS was similar other than the timeframe of recollection: “Compared to one year ago, would you say your memory is much better now, better now, about the same, worse now, or much worse now than it was then?”, “Compared with two years ago, would you say your memory is better now, about the same, or worse than it was then?”, and “Compared to 10 years ago, would you say that your memory is much worse, a little worse, the same, a little better, or much better?”, respectively. Due to the low frequency of participants (~2–4%) reporting an improvement in their memory over time, response options were recoded to dichotomous variables across all datasets: 0 = no decline (i.e., memory is “better,” “much better,” or “about the same”); 1 = decline (i.e., memory is “worse” or “much worse”).
Depressive symptoms
The 15-item Geriatric Depression Scale (GDS; [10]) was used to assess depressive symptoms in EAS. One item in the GDS tapped into subjective memory (“Do you feel you have more problems with memory than most?”); it was therefore not included in the final GDS score. In the other datasets, depressive symptoms were assessed using the 10-item version of Center for Epidemiologic Studies Depression Scale (CES-D; [36]) in MAP/MARS, the 8-item version of the CES-D [36] in HRS, and the Patient Health Questionnaire-2 (PHQ-2; [37]) in NHATS. Response options for the GDS and CES-D were dichotomous (0 = no; 1 = yes) for each item whereas the PHQ-2 used a four-point response scale (1 = not at all – 4 = nearly every day). Across datasets, composite scores were created and ranged from 0–14 for the modified GDS (EAS), 0–10 or 0–8 for the CES-D (MAP/MARS and HRS, respectively), and 2–8 for the PHQ-2 (NHATS). Higher scores indicated more depressive symptoms.
Objective memory
Objective memory was assessed with a composite total recall score, as available across datasets. In EAS, the total score on the free and cued selective reminding tests was used [38]. In MAP/MARS, word list memory and word list recall scores were added to create the total recall score [39]. In HRS and NHATS, the 10-word immediate and delayed recall tests of memory were added to create the total recall score. Higher scores indicated better objective memory performance.
Covariates
Participants’ age, gender (0 = female; 1 = male), race (0 = Black; 1 = White), income, education, and cognitive status were included as covariates. In HRS, cognitive status was determined based on the TICSm measure using three cognitive tests: immediate and delayed recall of 10 words, serial 7s subtraction, and backward counting. The total score could range from 0 – 27. Using validated cutoff criteria [33,40], participants were assigned to groups as follows: scores of 12 or above were considered normal cognition; scores between 7 and 11 were considered mild cognitive impairment (MCI). In NHATS, cognitive status was determined based on participants’ scores in three cognitive domains: memory (immediate and delayed word recall), orientation (date, president, and vice president’s name), and executive functioning (clock drawing) [34]. Participants who scored less than or equal to 1.5 SD below the mean in one of three cognitive domains were coded as MCI; those with scores higher than this in all domains were coded as normal cognition. The final cognitive status variable was assigned a value of 0 if participants had normal cognition at all waves and a value of 1 if they met MCI criteria at any wave. Additionally, cohort was included as a covariate (1 = born before 1924; 2 = children of the depression: born between 1924 – 1930; 3 = born between 1931 – 1941; 4 = war babies: born between 1942 – 1947) for the HRS dataset. To facilitate coordinated analysis, education and income were re-coded into categorical variables with the same response options across datasets (education: 1 = less than high school; 2 = high school; 3 = post-secondary education/associate degree; 4= bachelor’s or higher; income: 1 = < $15K, 2 = $15K - $30K, 3 = > $30K). Age was available as a categorical variable in NHATS (1 = 65–69; 2 = 70–74; 3 = 75–79; 4 = 80–84; 5 = 85–89; 6 = 90+). However, the large number of categories allowed us to use it as a continuous variable in our analyses.
Statistical analyses
Analyses were conducted in a series of steps. First, descriptive statistics and intercorrelations among key study variables were examined. Intraclass correlation coefficients (ICCs) were calculated to determine the percentage of total variability in objective memory that could be attributed to between-person differences and within-person change. Next, we fit multilevel structural equation models (MSEM; [41]) in Mplus v.8 [42] to examine our research questions. The MSEM approach combines multilevel modeling (MLM) and structural equation modeling (SEM) to differentiate between level 1 (within-person) and level 2 (between-person) components. This allows examination of mediation effects in which a single variable can be included as a predictor and an outcome [43–45] while data are nested (e.g., observations nested in persons). Mediation is examined using a series of simultaneous regression analyses (identified as paths) to quantify the indirect and total effects of predictor and mediator variables on an outcome variable (see Figure 2 for visualization of pathways). Given that our variables of interest (SCD, depressive symptoms, and objective memory) were measured within-persons repeatedly across time (level 1), we performed 1-1-1 MSEM [44].
Figure 2.
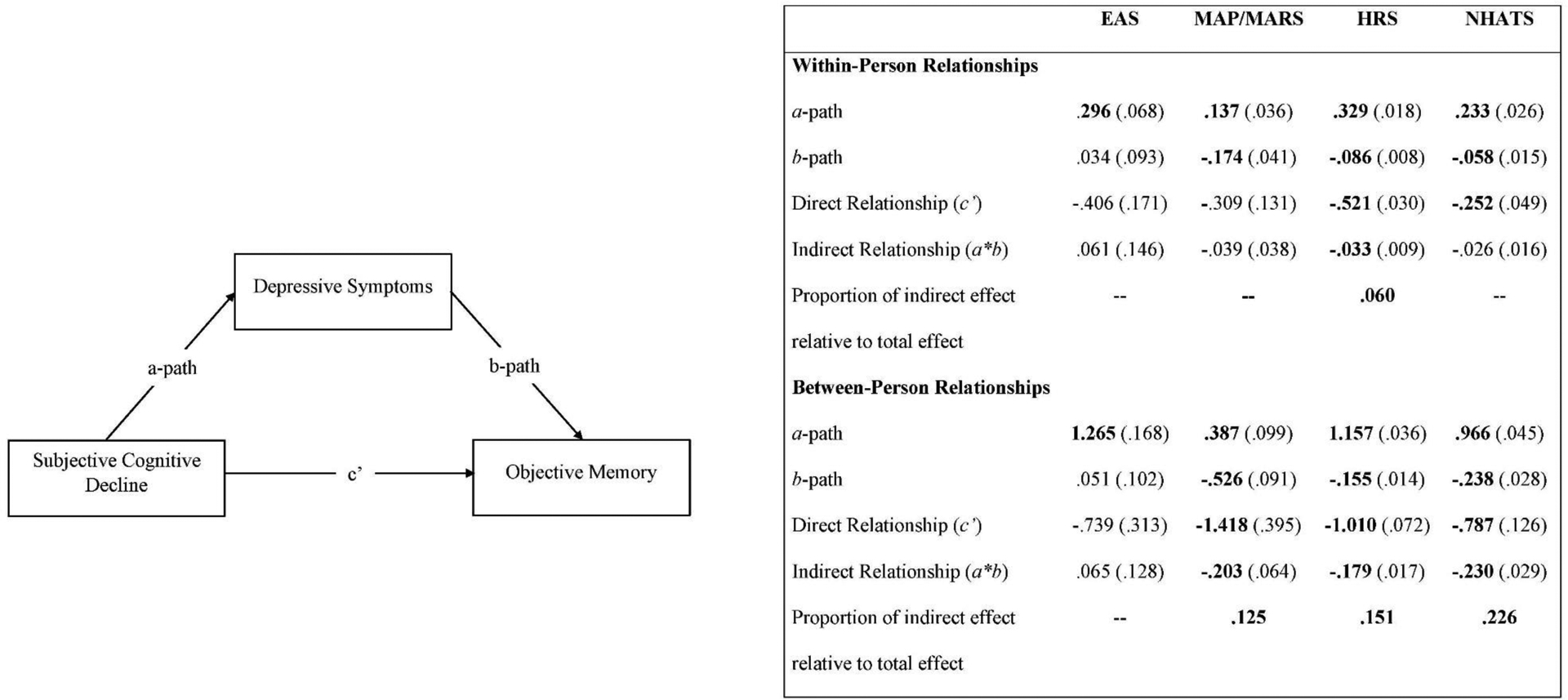
Model of Depressive Symptoms Mediating Subjective Cognitive Decline and Objective Memory with Associated Estimates by Dataset.
Note. Values in parentheses are standard errors. Estimates in bold are significant, p < .01. Proportion of indirect effect relative to total effect calculated for significant indirect effects only.
In 1-1-1 MSEM, between-person and within-person mediational relationships can be tested simultaneously; however, the target of analysis is the within-person associations among variables of interest over time. For direct relationships, we examined how changes in reports of SCD related to changes in depressive symptoms (within-person a-path), how changes in depressive symptoms related to changes in objective memory (within-person b-path), and how changes in reports of SCD related to changes in objective memory (c’). The indirect effect is reflected in the amount of the total relationship between changes in SCD and changes in objective memory accounted for by changes in depressive symptoms (a*b path). All paths were simultaneously tested at the between-person level to examine whether individual differences in depressive symptoms accounted for individual differences in the associations among SCD and objective memory.
Models were fit separately across datasets, in line with our coordinated analysis approach. All models included random intercepts and slopes per the requirements of 1-1-1 mediation. Several covariates were included at the between-person level. Age was grand-mean centered. Gender, race, and cognitive status were included as dichotomous variables. Education and income were effect coded. Grand-mean centered SCD and depressive symptoms variables were included at the between-person level whereas baseline-centered SCD and depressive symptoms variables were included at the within-person level. The MODEL CONSTRAINT command was used to estimate the between- and within-person indirect effects.
Given the large samples and the number of statistical tests conducted, we used a p-value of .01 as our criterion level of significance. As an estimate of effect size, we calculated the proportion of the total relationship between SCD and objective memory (ab + c’) that is uniquely predicted by depressive symptoms (ab) [46].
RESULTS
Descriptive statistics for sample demographics are presented in Table 1. At baseline, SCD was positively correlated with depressive symptoms and negatively correlated with objective memory in all datasets. In MAP/MARS, HRS, and NHATS, depressive symptoms were negatively correlated with objective memory. Inter-correlations among the key study variables are presented in Supplementary Tables 1 and 2.
Table 1.
Baseline Participant Characteristics by Study
| Characteristics | EAS (n= 1,889) | MAP/MARS (n= 2,172) | HRS (n= 15,841) | NHATS (n=8,494) |
|---|---|---|---|---|
| Agea | ||||
| 65–69 | Mage = 78.09 (5.34) | Mage = 78.65 (7.26) | Mage = 70.27 (6.48) | 1986 (23.38) |
| 70–74 | 1957 (23.04) | |||
| 75–79 | 1736 (20.44) | |||
| 80–84 | 1525 (17.95) | |||
| 85–89 | 827 (9.74) | |||
| 90+ | 463 (5.45) | |||
| Education | ||||
| Less than high school, n (%) | 403 (21.33) | 135 (6.22) | 4043 (25.52) | 1556 (18.32) |
| High school, n (%) | 559 (29.59) | 477 (21.96) | 5409 (34.15) | 2438 (28.70) |
| Some college, n (%) | 373 (19.75) | 588 (27.07) | 3316 (20.93) | 2371 (27.91) |
| College and beyond, n (%) | 553 (29.27) | 972 (44.75) | 3070 (19.38) | 2124 (25.01) |
| Missing, n (%) | 1 (00.05) | - | 3 (00.02) | 5 (00.06) |
| Gender | ||||
| Female, n (%) | 1172 (62.04) | 1625 (74.82) | 9115 (57.54) | 4897 (57.65) |
| Male, n (%) | 716 (37.90) | 547 (25.18) | 6726 (42.46) | 3597 (42.35) |
| Missing, n (%) | 1 (0.05) | - | - | - |
| Race | ||||
| White, n (%) | 1339 (70.88) | 1472 (67.77) | 13785 (87.02) | 6640 (78.17) |
| Black, n (%) | 550 (29.12) | 700 (32.23) | 2056 (12.98) | 1854 (21.83) |
| Income | ||||
| Less than $15,000, n (%) | 409 (21.65) | 275 (12.66) | 2853 (18.01) | 4545 (53.51) |
| $15,001 - $30,000, n (%) | 699 (37.00) | 486 (22.38) | 4305 (27.18) | 1153 (13.57) |
| Greater than $30,000, n (%) | 606 (32.08) | 1225 (56.50) | 8683 (54.81) | 2796 (32.92) |
| Missing, n (%) | 175 (9.26) | 186 (8.56) | - | - |
| Cognitive Status | ||||
| Normal | 1209 (64.00) | 988 (45.49) | 8840 (55.80) | 5129 (60.38) |
| MCI | 680 (36.00) | 1184 (54.51) | 7001 (44.20) | 3365 (39.62) |
| Follow-up years, M (SD) | 2.46 (2.55) | 4.07 (3.68) | 2.49 (2.19) | 2.39 (2.10) |
| Subjective Cognitive Decline, n (% yes) | 369 (19.92) | 1675 (77.12) | 3173 (20.03) | 985 (11.60) |
Note.
Age was included as a continuous variable in EAS, MAP/MARS, and HRS.
ICCs derived from unconditional means model showed that, across the four datasets, 44.4% - 70.9% of the total variance in objective memory could be attributed to between-person differences. In other words, 29.1% - 55.6% of the total variance in objective memory was related to change within-persons across time. Next, MSEM models were estimated to examine between- and within-person associations among SCD, depressive symptoms, and objective memory (See Figure 2 for 1-1-1 MSEM model estimates).
Between-person effects in our statistical models describe whether and to what extent individuals differ from each other, on average, across all timepoints. Within-person effects in our statistical models describe whether and to what extent changes among variables covary over time. In other words, when an individual reports SCD (i.e., a change in SCD), what are the associated changes in depressive symptoms and objective memory expected for that individual. These results are discussed in more detail below, specific to the paths tested at each level (between- and within-persons). Due to previous associations with SCD, depressive symptoms, and objective memory [47–49], gender, age, income, education, and cognitive status were included as covariates to limit potential confounding effects. Including covariates in the analyses did not substantively impact the relationship among the main variables of interest. All findings reported below account for associations with all covariates (see Table 2 for the associations of covariates with depressive symptoms and objective memory in the MSEM models). To ensure we were not biasing our results by including individuals with MCI at baseline, a sensitivity analysis was conducted removing those participants; all substantive results remained the same.
Table 2.
Associations of Covariates with Depressive Symptoms (Mediator) and Objective Memory (Outcome)
| Depressive Symptoms (Mediator) | EAS | MAP/MARS | HRS | NHATS |
|---|---|---|---|---|
| b(SE) | b(SE) | b(SE) | b(SE) | |
| Gender (ref = Female) | −0.118 (0.129) | −0.249*** (0.088) | −0.249*** (0.029) | −0.118*** (0.028) |
| Race (ref = Black) | 0.485** (0.150) | −0.204 (0.082) | −0.074 (0.040) | −0.104** (0.032) |
| Age | 0.013 (0.012) | 0.006 (0.006) | 0.009*** (0.002) | −0.029** (0.009) |
| Education | −0.239*** (0.063) | −0.152*** (0.037) | −0.194*** (0.015) | −0.166*** (0.014) |
| Income | −0.512*** (0.094) | −0.304*** (0.049) | −0.378*** (0.019) | −0.059*** (0.017) |
| Cognitive Status (ref = CN) | 0.278* (0.128) | 0.105 (0.073) | 0.167*** (0.029) | 0.112*** (0.028) |
| Objective Memory (Outcome) | ||||
| Gender (ref = Female) | −0.040 (0.142) | −2.751*** (0.265) | −1.151*** (0.030) | −0.984*** (0.050) |
| Race (ref = Black) | 0.081 (0.135) | 0.519 (0.271) | 0.315*** (0.047) | 1.000*** (0.062) |
| Age | −0.019 (0.012) | −0.218*** (0.018) | −0.109*** (0.003) | −0.649*** (0.017) |
| Education | −0.046 (0.069) | 1.192*** (0.126) | 0.346*** (0.015) | 0.584*** (0.024) |
| Income | 0.060 (0.100) | 0.629*** (0.166) | 0.120*** (0.021) | 0.202*** (0.027) |
| Cognitive Status (ref = CN) | −0.701*** (0.189) | −4.671*** (0.228) | −2.460*** (0.032) | −1.294*** (0.049) |
Note. EAS, MAP/MARS, HRS, and NHATS data were analyzed in separate multilevel structural equation models. Results from these models are presented together for ease of comparison. CN = Cognitively Normal. Gender: 0 = Female; 1 = Male; Race: 0 = Black; 1 = White; Cognitive Status: 0 = cognitively normal; 1 = mild cognitive impairment.
p ≤ .001.
p ≤ .01.
SCD and objective memory
Between-person associations of SCD with objective memory were significant in three of the four datasets (MAP/MARS: b = −1.418, SE = 0.395, p < .01; HRS: b = −1.010, SE = 0.072, p < .001; NHATS: b = −0.787, SE = 0.126, p < .001). These associations indicate that, on average, individuals who were more likely to report SCD had lower objective memory compared to others; this association was not significant in EAS. Within-person associations among SCD and objective memory were significant in two of the four datasets (HRS: b = −0.521, SE = 0.030, p < .001; NHATS: b = −0.252, SE = 0.049, p < .001). These associations indicate that when a change in SCD was reported (i.e,, SCD report changed from not present at one assessment to present at the next assessment), objective memory tended to decrease; associations were not significant in EAS or MAP/MARS.
SCD and depressive symptoms
Between-person associations of SCD with depressive symptoms were significant across all datasets (EAS: b = 1.265, SE = 0.168, p < .001; MAP/MARS: b = 0.387, SE = 0.099, p < .001; HRS: b = 1.157, SE = 0.036, p < .001; NHATS: b = 0.966, SE = 0.045, p < .001). These associations indicate that, on average, individuals who were more likely to report SCD had higher depressive symptoms compared to others. Within-person associations of SCD and depressive symptoms were also significant across all datasets (EAS: b = 0.296, SE = 0.068, p < .001; MAP/MARS: b =0.137, SE = 0.036, p < .001; HRS: b = 0.329, SE = 0.018, p < .001; NHATS: b = 0.233, SE = 0.026, p < .001). These associations indicate that when a change in SCD was reported (i.e., SCD report changed from not present at one assessment to present at the next assessment), individuals’ depressive symptoms tended to increase.
Depressive symptoms and objective memory
Between-person associations of depressive symptoms with objective memory were significant in three of four datasets (MAP/MARS: b = −0.526, SE = 0.091, p < .001; HRS: b = −0.155, SE = 0.014, p < .001; NHATS: b = −0.238, SE = 0.028, p < .001). These associations indicate that, on average, individuals with higher depressive symptoms had lower objective memory compared to others. Within-person associations of depressive symptoms with objective memory were significant in three of the four datasets (MAP/MARS: b = −0.174, SE = 0.041, p < .001; HRS: b = −0.086, SE = 0.008, p < .001; NHATS: b = −0.058, SE = 0.015, p < .001). These associations indicate that when depressive symptoms increased, objective memory tended to decrease. Between- and within-person associations of depressive symptoms with objective memory were not significant in EAS.
Indirect effects
Between-person indirect effects convey the extent to which individual differences in the relationship between SCD and objective memory were mediated by depressive symptoms. The between-person indirect effect of SCD on objective memory via depressive symptoms was significant in three of the four datasets (MAP/MARS: b = −0.203, SE = 0.064, p < .01; HRS: b = −0.179, SE = 0.017, p < .001; NHATS: b = −0.230, SE = 0.029, p < .001). These associations indicate that, on average, individuals who were more likely to report SCD had higher depressive symptoms, and in turn, lower objective memory. Across datasets, the proportion of the between-person association of SCD and objective memory uniquely predicted by depressive symptoms ranged from 12% in MAP/MARS, 15% in HRS, to 23% in NHATS. In EAS, the between-person indirect effect of SCD on objective memory through depressive symptoms was not significant.
Within-person indirect effects convey the extent to which depressive symptoms mediate the relationship among changes in reports of SCD and changes in objective memory within individuals over time. The within-person indirect effect of SCD on objective memory via depressive symptoms was significant in HRS only (b = −0.033, SE = 0.009, p < .001). Specifically, when SCD was reported, depressive symptoms tended to increase, and in turn, objective memory tended to decrease. In HRS, the proportion of the within-person association of SCD and objective memory uniquely predicted by depressive symptoms was 6%. These associations were not significant in EAS, MAP/MARS, or NHATS.
DISCUSSION
SCD is often linked with objective cognitive performance in older adults, but across studies, findings are inconsistent and depressive symptoms are a known confounding factor. We aimed to extend understanding of these links by examining how SCD and objective memory are related between- and within-individuals over time, and specifically whether depressive symptoms mediate either of these relationships. By using coordinated analysis across four longitudinal datasets, we immediately replicated our results to enhance generalizability and evaluate consistencies or discrepancies in findings. We found strong evidence to support our first hypothesis: in three of the four datasets, older adults who were more likely to report SCD tended to have lower objective memory on average, and higher depressive symptoms partially mediated this relationship. Our second hypothesis had limited support: depressive symptoms partially mediated the within-person association between SCD and objective memory only in our largest dataset (HRS). Furthermore, depressive symptoms only explained approximately 6% of the within-person relationship in HRS, compared to 12–23% of the between-person relationship in MAP/MARS, HRS, and NHATS. These findings suggest that differences in levels of depressive symptoms help explain individual differences in the link between SCD and objective memory, but changes in depressive symptoms over time play less of a role in how SCD relates to changes in objective memory within individuals’ cognitive trajectories.
Our findings demonstrate that between-person differences help explain why relationships between SCD and objective memory differ across older adults, specific to the role of depressive symptoms. We found that individuals who are more likely to report SCD also report more depressive symptoms, consistent with previous evidence [11,12]. And furthermore, that those depressive symptoms explain a part of why SCD is related to objective memory performance. Although we cannot determine causality based on these results, they do suggest that for some older adults, feelings of depressed mood (e.g., sadness, hopelessness) that accompany perceived memory problems may influence cognition, as previously identified in depressive symptoms generally [19,20]. In a cross-sectional investigation, Seo et al [50] found that memory complaints and objective cognition were only significantly related in older adults with higher depressive symptoms. Although our between-person findings are also specific to individual differences, we examined average associations over multiple years, extending understanding of the stability of individual differences in these relationships. In addition, we replicated our findings in three large datasets, substantially enhancing generalizability and providing strong evidence for a mediation effect. These results help to identify a subgroup of older adults who are more likely to have poorer objective memory performance: those who report SCD with co-occurring depressive symptoms (and not necessarily depression). This group may be important to target with early interventions to improve psychological and cognitive health, thereby reducing risk for cognitive decline and AD.
It is important to note that we did not find evidence of mediation in the dataset with the smallest sample size, EAS. However, factors other than sample size may have influenced our findings, and inconsistent results in a coordinated analysis are a valuable opportunity to explore reasons for discrepancies. EAS participants are recruited from a defined geographic area (Bronx, NY) compared to the nationally representative HRS and NHATS datasets, and EAS uses systematic sampling from registered voter lists, compared to the community-based recruitment methods used in MAP and MARS. These factors may have contributed to the EAS sample being meaningfully different from the others in terms of reporting of depressive symptoms or SCD. Relatedly, measures of both SCD and depressive symptoms differed in EAS compared to the other datasets. Although all SCD measures assessed memory change over time, the question used in EAS asked specifically about “trouble remembering things.” All other questions asked whether “your memory” was better, the same, or worse than a previous time period, a more general question that may have influenced SCD reporting. Previous work has described the importance of question wording in reducing response bias when assessing subjective cognition [51]. Furthermore, measures of depressive symptoms differed across datasets. Depression rating scales, including those used in the current study, vary in their coverage of the different symptoms possible in depressive symptomatology [52].
We also examined within-person relationships (i.e., how individuals change over time), but found little support for within-person mediation of the association between SCD and objective memory by depressive symptoms. In our largest sample, HRS, reports of SCD were associated with increases in individuals’ depressive symptoms and, in turn, declines in their objective memory. Given the size of the dataset (n > 15,000), the lack of replication across the remaining datasets, and the small estimated size of the mediation effect, we cannot conclude that depressive symptoms mediate the relationship between SCD and objective cognition at the within-person level. However, compared to the other datasets, HRS participants were younger on average. It is possible that the within-person mediating effect of depressive symptoms on the relationship between SCD and objective memory is influenced by age. Indeed, in another study, within-person associations between subjective memory and depressive symptoms differed based on age, with older participants having weaker associations [53].
It could be that other factors influencing the relationship between SCD and objective memory, such as age [54,55] or personality [55,56], may play more of a role at the within-person level. That is, while we found that SCD and objective memory were directly related within-persons, depressive symptoms may not be a prime mechanism of this association. One alternative mechanism is activity (dis-) engagement, whereby individuals who perceive declines in their memory functioning reduce engagement in cognitively demanding activities [57]. Reduced activity engagement, in turn, leads to more rapid objective cognitive decline [58]. Further, there was significant variability in relationships among our within-person predictors. This suggests that although there was not strong evidence for mediation at the within-person level across all older adults in our samples, subgroups for whom this relationship holds may still exist (i.e., moderated mediation; [59]).
In addition to our findings regarding mediation, we also found strong evidence for associations among SCD and depressive symptoms, depressive symptoms and objective memory, as well as SCD and objective memory. Although not universal across all datasets, between- and within-person associations among SCD and objective memory were supported overall, particularly in the larger datasets. These findings build on previous research identifying inconsistent relationships, particularly cross-sectionally [60]. Our findings suggest a durable link between SCD and objective memory performance, regardless of depressive symptoms. A single measurement point may be insufficient for capturing cognitive decline risk based on SCD reporting, likely due to the variety of factors that influence subjective cognition (e.g., personality; [61,62]). However, our results suggest that overall, individual differences in SCD do relate to objective cognition when multiple assessment points are considered. Reports of SCD are often clinically meaningful and should be addressed with thorough assessment and tailored intervention, as appropriate, and not immediately discounted when objective cognitive testing is normal. Furthermore, the within-person SCD-objective memory associations support previous longitudinal work demonstrating a higher risk of cognitive impairment and incident dementia in older adults with SCD [63].
Overall, older adults with SCD had more depressive symptoms; this was supported almost universally at both between- and within-person levels. We also found that depressive symptoms were negatively associated with cognition such that individuals with higher depressive symptoms had lower objective memory on average, and when depressive symptoms increased over time, objective memory tended to decrease. These findings are consistent with previous literature [64]. Indeed, previous research found that a majority of older adults with depression also reported memory problems [65]. Further, depressive symptoms were an effect measure modifier such that reports of memory problems were linked to poorer objective cognition among participants with depression. In addition, several studies have also shown a direct link between depressive symptoms, subjective memory, and objective memory. Serra-Blasco et al. [66] reported a correlation between depressive symptoms and objective as well as subjective memory, and Brown et al. [67] found that depression and SCD were related in older adults, irrespective of age group or gender.
The current study enhances our understanding of the interrelationships between SCD, objective memory, and depressive symptoms; however, there are several limitations to consider. First, while the structure of mediation analysis permits inferences into the relationship between SCD and depressive symptoms, and depressive symptoms to objective memory decline, the current study is correlational overall; it is possible that other variables may partially explain these results. Second, due to the nature of secondary data analysis, we were limited in our selection of SCD measures, leading to the usage of single-item questions that assessed perceived changes in memory only. Currently there is no standardized measure of SCD, leading to inconsistencies in characterization [68]. Using one item likely does not capture the full variation in presentation. An important direction for future research is to examine how different facets of SCD (e.g., executive function impairment vs. memory recall) are associated with depressive symptoms and objective cognitive decline. Third, there is an inherent limitation in asking older adults with cognitive impairment to recall their experience of memory decline. Anosognosia, or poor awareness of one’s cognitive deficits, occurs in some individuals with MCI, although there is considerable variation in experiences [69]. Although our analyses accounted for MCI status, it is possible that participants whose objective memory performance declined over the study period were not as accurate or consistent in their reports of SCD, and/or depressive symptoms. Finally, although we replicated analyses across multiple datasets, these included U.S. older adults only. Future cross-national comparisons are important to better understand these relationships among older adults residing in other countries.
This study also has many important strengths, most notably the coordinated data analyses and the disentangling of within- and between-person effects. Coordinated analysis allows for rapid replication and extension of results across multiple datasets with large, targeted samples of interest. This study replicated key findings in samples drawn from demographically diverse and nationally representative studies, which increases the generalizability of and confidence in our results. Additionally, the focus on quantifying between-person and within-person effects allowed us to identify differences in these relationships. Past work has primarily focused on group differences, and indeed evidence for between-person effects is stronger in the current study. However, clarifying the overall lack of within-person effects (i.e., increases in individuals’ depressive symptoms did not explain the association between their reports of SCD and declines in objective memory) also extends current knowledge. Specifically, it appears that one size does not fit all when individuals judge their perceived memory decline; this affects the strength of the relationship with objective memory and may help explain past work detailing inconsistent longitudinal findings of objective performance predicted by subjective memory (e.g., [70–72]).
Our results suggest that for people with SCD, co-occurring depressive symptoms may influence individual differences in objective memory performance. This is particularly relevant as interventions exist that could help older adults manage their depressive symptoms (regardless of cause), which in turn would promote cognitive health [73]. Although our results were only consistent at the between-person level, this work highlights the need to better understand the ways in which perceived memory problems impact older adults. Future research should examine other potential within-person mediators to identify temporally proximal changes that occur for older adults at times when they perceive changes in their memory. Changes in emotional well-being or other psychological states that immediately follow a perceived change in memory would serve as critical targets for interventions that support cognitive functioning and prevent changes in behavior (e.g., withdrawing from activities [57]) that can increase the risk for cognitive decline [74].
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the National Institute on Aging (NIA) of the National Institutes of Health (grant number R01AG055398). The funder had no role in the study design, data analysis, interpretation of data, or writing the manuscript. This study uses secondary data from five NIA-funded studies: Einstein Aging Study (EAS; grant number P01AG003949), Health and Retirement Study (HRS; grant number U01AG009740), National Health and Aging Trends Study (NHATS; grant number U01AG032947), Minority Aging Research Study (MARS; grant number RF1AG22018), and Rush Memory and Aging Project (MAP; grant number R01AG17917). The authors thank the participants and research teams of the EAS, HRS, NHATS, MARS, and MAP studies.
Footnotes
DISCLOSURE STATEMENT
The authors have no conflict of interest to report.
REFERENCES
- [1].Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, Dubois B, Dufouil C, Ellis KA, van der Flier WM Glodzik L, van Harten AC, de Leon MJ, McHugh P, Mielke MM, Molinuevo JL, Mosconi L, Osorio RS, Perrotin A, Petersen RC, Rabin LA, Lorena R, Reisberg B, Rentz DM, Sachdev PS, de la Sayette V, Saykin AJ, Scheltens P, Shulman MB, Slavin MJ, Sperling RA, Stewart R, Uspenskaya O, Vellas B, Visser PJ, Wagner M (2014) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement 10, 844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B (2014) Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand 130, 439–451. [DOI] [PubMed] [Google Scholar]
- [3].Reid LM, Maclullich AMJ (2006) Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord 22, 471–485. [DOI] [PubMed] [Google Scholar]
- [4].Slot RER, Sikkes SAM, Berkhof J, Brodaty H, Buckley R, Cavedo E, Dardiotis E, Guillo-Benarous F, Hampel H, Kochan NA, Lista S, Luck T, Maruff P, Molinuevo JL, Kornhuber J, Reisberg B, Riedel-Heller SG, Risacher SL, Roehr S, Sachdev PS, Scarmeas N, Scheltens P, Shulman MB, Saykin AJ, Vergaillie SCJ, Visser PJ, Vos SJB, Wagner M, Wolfsgruber S, Jessen F, van der Flier WM(2019) Subjective cognitive decline and rates of incident Alzheimer’s disease and non-Alzheimer’s disease dementia. Alzheimers Dement J Alzheimers Assoc 15, 465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Snitz BE, Wang T, Cloonan YK, Jacobsen E, Chang C-CH, Hughes TF, Kamboh MI, Ganguli M (2018) Risk of progression from subjective cognitive decline to mild cognitive impairment: The role of study setting. Alzheimers Dement J Alzheimers Assoc 14, 734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stogmann E, Moser D, Klug S, Gleiss A, Auff E, Dal-Bianco P, Pusswald G, Lehrner J (2015) Activities of daily living and depressive symptoms in patients with subjective cognitive decline, mild cognitive impairment, and Alzheimer’s disease. J Alzheimers Dis 49, 1043–1050. [DOI] [PubMed] [Google Scholar]
- [7].Potter GG, Steffens DC (2007) Contribution of depression to cognitive impairment and dementia in older adults. The Neurologist 13, 105–117. [DOI] [PubMed] [Google Scholar]
- [8].Jorm AF (2001) History of depression as a risk factor for dementia: An updated review. Aust N Z J Psychiatry 35, 776–781. [DOI] [PubMed] [Google Scholar]
- [9].Lockwood KA, Alexopoulos GS, Kakuma T, Van Gorp WG (2000) Subtypes of cognitive impairment in depressed older adults. Am J Geriatr Psychiatry 8, 201–208. [PubMed] [Google Scholar]
- [10].Sheikh JI, Yesavage JA (1986) Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin Gerontol J Aging Ment Health 5, 165–173. [Google Scholar]
- [11].Hill NL, Mogle J, Wion R, Munoz E, DePasquale N, Yevchak AM, Parisi JM (2016) Subjective cognitive impairment and affective symptoms: A systematic review. The Gerontologist 56, e109–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mogle J, Hill NL, Bhargava S, Bell TR, Bhang I (2020) Memory complaints and depressive symptoms over time: a construct-level replication analysis. BMC Geriatr 20, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Amariglio RE, Mormino EC, Pietras AC, Marshall GA, Vannini P, Johnson KA, Sperling RA, Rentz DM (2015) Subjective cognitive concerns, amyloid-β, and neurodegeneration in clinically normal elderly. Neurology 85, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Baquero M, Martín N (2015) Depressive symptoms in neurodegenerative diseases. World J Clin Cases WJCC 3, 682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, Kubera M, Bob P, Lerer B, Maj M (2009) The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis 24, 27–53. [DOI] [PubMed] [Google Scholar]
- [16].Panza F, Frisardi V, Capurso C, D’Introno A, Colacicco AM, Imbimbo BP, Santamato A, Vendemiale G, Seripa D, Pilotto A, Capurso A, Solfrizzi V (2010) Late-Life Depression, Mild Cognitive Impairment, and Dementia: Possible Continuum? Am J Geriatr Psychiatry 18, 98–116. [DOI] [PubMed] [Google Scholar]
- [17].Lyketsos CG, Lee HB (2004) Diagnosis and treatment of depression in alzheimer’s disease. Dement Geriatr Cogn Disord 17, 55–64. [DOI] [PubMed] [Google Scholar]
- [18].Lyketsos CG, Olin J (2002) Depression in Alzheimer’s disease: overview and treatment. Biol Psychiatry 52, 243–252. [DOI] [PubMed] [Google Scholar]
- [19].Heser K, Tebarth F, Wiese B, Eisele M, Bickel H, Köhler M, Mösch E, Weyerer S, Werle J, König H-H, Leicht H, Pentzek M, Fuchs A, Riedel-Heller SG, Luppa M, Prokein J, Scherer M, Maier W, Wagner M (2013) Age of major depression onset, depressive symptoms, and risk for subsequent dementia: Results of the German study on Ageing, Cognition, and Dementia in Primary Care Patients (AgeCoDe). Psychol Med 43, 1597–1610. [DOI] [PubMed] [Google Scholar]
- [20].Rickards H (2006). Depression in neurological disorders: an update. Curr Opin Psychiatry 19, 294–298. [DOI] [PubMed] [Google Scholar]
- [21].Topiwala A, Suri S, Allan C, Zsoldos E, Filippini N, Sexton CE, Mahmood A, Singh-Manoux A, Mackay CE, Kivimäki M, Ebmeier KP (2021) Subjective cognitive Ccmplaints given in questionnaire: Relationship with brain structure, cognitive performance and self-reported depressive symptoms in a 25-year retrospective cohort study. Am J Geriatr Psychiatry 29, 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jorm AF, Christensen H, Korten AE, Jacomb PA, Henderson AS (2001) Memory complaints as a precursor of memory impairment in older people: a longitudinal analysis over 7–8 years. Psychol Med 31, 441–449. [PubMed] [Google Scholar]
- [23].Liew TM (2019) Depression, subjective cognitive decline, and the risk of neurocognitive disorders. Alzheimers Res Ther 11, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bhang I, Mogle J, Hill N, Whitaker EB, Bhargava S (2020) Examining the temporal associations between self-reported memory problems and depressive symptoms in older adults. Aging Ment Health 24, 1864–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hill NL, Mogle J (2018) Alzheimer’s disease risk factors as mediators of subjective memory impairment and objective memory decline: protocol for a construct-level replication analysis. BMC Geriatr. 18, 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hofer SM, Piccinin AM (2009) Integrative data analysis through coordination of measurement and analysis protocol across independent longitudinal studies. Psychol Methods 14, 150–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lipton RB, Katz MJ, Kuslansky G, Sliwinski MJ, Stewart WF, Verghese J, Crystal HA, Buschke H (2003) Screening for dementia by telephone using the memory impairment screen. J Am Geriatr Soc 51, 1382–1390. [DOI] [PubMed] [Google Scholar]
- [28].Bennett D, Schneider J, Buchman A, Barnes L, Boyle P, Wilson R (2012) Overview and findings from the rush memory and aging project. Curr Alzheimer Res 9, 646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA (2012) The Minority Aging Research Study: Ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res 9, 734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Health and Retirement Study, (public survey data: 1998–2014) public use dataset. Produced and distributed by the University of Michigan with funding from the National Institute on Aging (grant number NIA U01AG009740). Ann Arbor, MI. [Google Scholar]
- [31].Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR (2014) Cohort profile: the health and retirement study (HRS). Int J Epidemiol 43, 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].NHATS Public Use Data. Rounds 1–8, sponsored by the National Institute on Aging (grant number NIA U01AG032947) through a cooperative agreement with the Johns Hopkins Bloomberg School of Public Health. Available at www.nhats.org.
- [33].Langa KM, Larson EB, Crimmins EM, Faul JD, Levine DA, Kabeto MU, Weir DR (2017) A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med 177, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kasper JD, Freedman VA (2018) National Health and Aging Trends Study (NHATS) user guide: Rounds 1 – 7 beta release [Internet]. Baltimore: Johns Hopkins University School of Public Health. Available from: www.nhats.org [Google Scholar]
- [35].Katz MJ, Lipton RB, Hall CB, Zimmerman ME, Sanders AE, Verghese J, Dickson DW, Derby CA (2012) Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and alzheimer dementia in Blacks and Whites: a report from the Einstein Aging Study. Alzheimer Dis Assoc Disord 26, 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Radloff LS (1977) The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1, 385–401. [Google Scholar]
- [37].Kroenke K, Spitzer RL, Williams JBW (2003) The Patient Health Questionnaire-2: Validity of a two-item depression screener. Med Care, 41, 1284–1292. [DOI] [PubMed] [Google Scholar]
- [38].Buschke H (1984). Cued recall in amnesia. J Clin Neuropsychol 6, 433–40. [DOI] [PubMed] [Google Scholar]
- [39].Wilson RS, Boyle PA, Yu L, Barnes LL, Sytsma J, Buchman AS, Bennett DA, Schneider JA (2015) Temporal course and pathologic basis of unawareness of memory loss in dementia. Neurology 85, 984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Crimmins EM, Kim JK, Langa KM, Weir DR (2011) Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci 66, i162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Muthén BO, Asparouhov T (2008) Growth mixture modeling: Analysis with non-Gaussian random effects. In Advances in Longitudinal Data Analysis, Fitzmaurice G, Davidian M, Verbeke G, Molenberghs G, eds. Chapman & Hall/CRC Press, Boca Raton, FL: pp. 143–165. [Google Scholar]
- [42].Muthén LK, Muthén BO (2018) Mplus User’s Guide. Eighth Edition. Muthén & Muthén, Los Angeles, CA. [Google Scholar]
- [43].Mehta PD, Neale MC (2005) People are variables too: Multilevel structural equations modeling. Psychol Methods 10, 259–284. [DOI] [PubMed] [Google Scholar]
- [44].Preacher KJ, Zyphur MJ, Zhang Z (2010) A general multilevel SEM framework for assessing multilevel mediation. Psychol Methods 15, 209–233. [DOI] [PubMed] [Google Scholar]
- [45].Robitaille A, Muniz G, Lindwall M, Piccinin AM, Hoffman L, Johansson B, Hofer S (2014) Physical activity and cognitive functioning in the oldest old: within- and between-person cognitive activity and psychosocial mediators. Eur J Ageing 11, 333–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wen Z, Fan X (2015) Monotonicity of effect sizes: Questioning kappa-squared as mediation effect size measure. Psychol Methods 20, 193–203. [DOI] [PubMed] [Google Scholar]
- [47].Jonker C, Launer LJ, Hooijer C, Lindeboom J (1996) Memory complaints and memory impairment in older individuals. J Am Geriatr Soc 44, 44–49. [DOI] [PubMed] [Google Scholar]
- [48].Mojtabai R, Olfson M (2004) Major depression in community-dwelling middle-aged and older adults: prevalence and 2-and 4-year follow-up symptoms. Psychol Med 34, 623–634. [DOI] [PubMed] [Google Scholar]
- [49].McDougall GJ, Pituch KA, Stanton MP, Chang W (2014) Memory performance and affect: Are there gender differences in community-residing older adults? Issues Ment Health Nurs 35, 620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Seo EH, Kim H, Choi KY, Lee KH, Choo IH (2017) Association of subjective memory complaint and depressive symptoms with objective cognitive functions in prodromal Alzheimer’s disease including pre-mild cognitive impairment. J Affect Disord 217, 24–28. [DOI] [PubMed] [Google Scholar]
- [51].Hill NL, Mogle J, Whitaker EB, Gilmore-Bykovskyi A, Bhargava S, Bhang IY, Sweeder L, Tiwari PA, Van Haitsma K (2019) Sources of response bias in cognitive self-report items: “which memory are you talking about?” The Gerontologist 59, 912–924. [DOI] [PubMed] [Google Scholar]
- [52].Balsamo M, Cataldi F, Carlucci L, Padulo C, Fairfield B (2018) Assessment of late-life depression via self-report measures: a review. Clin Interv Aging 13, 2021–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hülür G, Hertzog C, Pearman A, Ram N, Gerstorf D (2014) Longitudinal associations of subjective memory with memory performance and depressive symptoms: Between-person and within-person perspectives. Psychol Aging 29, 814–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Rabbitt P, Maylor E, McInnes L, Bent N, Moore B (1995) What goods can self-assessment questionnaires deliver for cognitive gerontology? Appl Cogn Psychol 9, S127–152. [Google Scholar]
- [55].Hülür G, Hertzog C, Pearman AM, Gerstorf D (2015) Correlates and moderators of change in subjective memory and memory performance: findings from the Health and Retirement Study. Gerontology 61, 232–240. [DOI] [PubMed] [Google Scholar]
- [56].Steinberg SI, Negash S, Sammel MD, Bogner H, Harel BT, Livney MG, McCoubrey H, Wolk DA, Kling MA, Steven AE (2013) Subjective memory complaints, cognitive performance, and psychological factors in healthy older adults. Am J Alzheimers Dis Other Demen 28, 776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wion RK, Hill NL, DePasquale N, Mogle J, Bratlee-Whitaker E (2020) The relationship between subjective cognitive impairment and activity participation: A systematic review. Act Adapt Aging 44, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wilson RS, Bennett DA, Bienias JL, Aggarwal NT, Mendes De Leon CF, Morris MC, Schneider JA, Evans DA (2002) Cognitive activity and incident AD in a population-based sample of older persons. Am Acad Neurol 59, 1910–1914. [DOI] [PubMed] [Google Scholar]
- [59].Kim S, Hong S (2020) Comparing methods for multilevel moderated mediation: A decomposed-first strategy. Struct Equ Model Multidiscip J 27, 661–677. [Google Scholar]
- [60].Burmester B, Leathem J, Merrick P (2016) Subjective cognitive complaints and objective cognitive function in aging: A systematic review and meta-analysis of recent cross-sectional findings. Neuropsychol Rev 26, 376–393. [DOI] [PubMed] [Google Scholar]
- [61].Ausén B, Edman G, Almkvist O, Bogdanovic N (2009) Personality features in subjective cognitive impairment and mild cognitive impairment - early indicators of dementia? Dement Geriatr Cogn Disord 28, 528–535. [DOI] [PubMed] [Google Scholar]
- [62].Hill NL, Mogle J, Bhargava S, Bell TR, Wion RK (2019) The influence of personality on memory self-report among black and white older adults. PloS One 14, e0219712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wang X-T, Wang Z-T, Hu H-Y, Qu Y, Wang M, Shen X-N, Xu W, Dong Q, Tan L, Yu J-T (2021) Association of subjective cognitive decline with risk of cognitive impairment and dementia: A systematic review and meta-analysis of prospective longitudinal studies. J Prev Alzheimers Dis 8, 277–285. [DOI] [PubMed] [Google Scholar]
- [64].Brigola AG, Manzini CSS, Oliveira GBS, Ottaviani AC, Sako MP, Vale FAC (2015) Subjective memory complaints associated with depression and cognitive impairment in the elderly: A systematic review. Dement Neuropsychol 9, 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Chu CS, Sun IW, Begum A, Shen IL, Chang CJ, Chiu WC, Chen CH, Tang H-S, Yang CL, Lin YC, Chiu CC, Stewart R (2017) The association between subjective memory complaint and objective cognitive function in older people with previous major depression. PloS One 12, e0173027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Serra-Blasco M, Torres IJ, Vicent-Gil M, Goldberg X, Navarra-Ventura G, Aguilar E, Via E, Portella MJ, Figuereo I, Palao D, Lam RW, Cardoner N (2019) Discrepancy between objective and subjective cognition in major depressive disorder. Eur Neuropsychopharmacol 29, 46–56. [DOI] [PubMed] [Google Scholar]
- [67].Brown MJ, Hill NL, Haider MR (2020) Age and gender disparities in depression and subjective cognitive decline-related outcomes. Aging Ment Health 16, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Rabin LA, Smart CM, Crane PK, Amariglio RE, Berman LM, Boada M, Buckley RF, Chetelat G, Dubois B, Ellis KA, Gifford KA, Jefferson AL, Jesen F, Katz MJ, Lipton RB, Luck T, Maruff P, Mielke MM, Molinuevo JL, Naeem F, Perrotin A, Peterson RC, Rami L, Reisberg B, Rentz DM, Riedel-Heller SG, Risacher SL, Rodriguez O, Sachdev PS, Saykin AJ, Slavin MJ, Snitz BE, Sperling RA, Tandetnik C, van der Flier WM, Wagner M, Wolfsgruber S, Sikkes SA (2015) Subjective cognitive decline in older adults: an overview of self-report measures used across 19 international research studies. J Alzheimers Dis 48, S63–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Roberts JL, Clare L, Woods B (2009) Subjective memory complaints and awareness of memory functioning in mild cognitive impairment: a systematic review. Dement Geriatr Cogn Disord 28, 95–109. [DOI] [PubMed] [Google Scholar]
- [70].Pearman A, Hertzog C, Gerstorf D (2014) Little evidence for links between memory complaints and memory performance in very old age: longitudinal analyses from the Berlin Aging Study. Psychol Aging 29, 828–842. [DOI] [PubMed] [Google Scholar]
- [71].Parisi JM, Gross AL, Rebok GW, Saczynski JS, Crowe M, Cook SE, Langbaum JBS, Unverzagt FW (2011) Modeling change in memory performance and memory perceptions: Findings from the ACTIVE study. Psychol Aging 26, 518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Brailean A, Steptoe A, Batty GD, Zaninotto P, Llewellyn DJ (2019) Are subjective memory complaints indicative of objective cognitive decline or depressive symptoms? Findings from the English Longitudinal Study of Ageing. J Psychiatr Res 110, 143–151. [DOI] [PubMed] [Google Scholar]
- [73].Kok RM, Reynolds CF (2017) Management of depression in older adults: A review. JAMA 317, 2114–2122. [DOI] [PubMed] [Google Scholar]
- [74].Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S,Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Ogunniyi A, Orgeta V, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N (2020) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 396, 413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


