Abstract
Aims
The angiotensin receptor–neprilysin inhibitor (ARNI), sacubitril/valsartan, confers additional protective effects compared with angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers (ACEIs/ARBs) in terms of reversed left ventricular (LV) remodelling and improves the prognosis of patients with heart failure (HF). However, few studies have examined the effects of ARNI on the left atrium. Accordingly, this study compared the effects of ARNI and ACEI/ARB on left atrial (LA) remodelling in heart failure with reduced ejection fraction (HFrEF).
Methods and results
This was a single‐centre retrospective study of patients with HFrEF hospitalized at the First Affiliated Hospital of Dalian Medical University between 26 February 2016 and 8 July 2020. Patients were classified into ARNI and ACEI/ARB groups and further subgroups based on the left atrial volume index (LAVI): mildly abnormal (29 mL/m2 ≤ LAVI < 34 mL/m2), moderately abnormal (34 mL/m2 ≤ LAVI < 40 mL/m2), and severely abnormal (LAVI ≥ 40 mL/m2). The primary endpoint was changes in LA parameters by echocardiography. The secondary endpoint was all‐cause mortality. A total of 336 patients (mean age: 64.11 ± 12.86, 30.06% female) were included. Except those lost to follow‐up, 274 HFrEF patients remained, with 144 cases in the ARNI group and 130 cases in the ACEI/ARB group. Greater reductions from baseline were seen with ARNI in LA diameter (LAD) (P = 0.013, t‐test), superior and LA superior–inferior diameter (LASID) (P < 0.0001), LA transverse diameter (LATD) (P < 0.0001), LA volume (LAV) (P < 0.0001), LAVI (P < 0.0001), and LA sphericity index (LASI) (P < 0.0001). Over a mean follow‐up of 19.40 months, 97 patients (67.3%) in the ARNI group and 29 patients (22.3%) in the ACEI/ARB group showed LA reverse remodelling (LARR). Kaplan–Meier analysis showed significantly lower overall mortality in the ARNI group compared with the ACEI/ARB group (P = 0.048, log‐rank test). The mildly abnormal LAVI group of ARNI patients showed a reduction in mortality compared with ACEI/ARB patients (P = 0.044). However, no significant difference was observed for the moderately abnormal (P = 0.571) or severely abnormal LAVI groups (P = 0.609), suggesting that early initiation of ARNI was associated with a better prognosis.
Conclusions
In this proof‐of‐concept study, ARNI use showed greater effects on LARR and was associated with a better prognosis compared with ACEI/ARB use in HFrEF. Early initiation of ARNI in the HF disease process may produce greater benefit, but this needs to be confirmed in future studies.
Keywords: Sacubitril/valsartan, Left atrium remodelling, Heart failure, Mortality
Introduction
Adverse cardiac remodelling is an important mechanism leading to the occurrence and progression of chronic heart failure (HF). 1 However, this remodelling can occur not only in the left ventricle (LV) but also in the left atrium (LA), which may have additional impact on disease progression. 2 Echocardiographic indices of the LA have been increasingly recognized as an important part of HF assessment, particularly in patients with HF with preserved ejection fraction (HFpEF) and atrial fibrillation (AF). 3 , 4 Indeed, a holistic approach with multimodality imaging is important for risk stratification and optimizing patient management. 5 , 6 , 7
Sustained activation of renin angiotensin aldosterone system (RAAS) is involved in the pathophysiology of HF and has led to the introduction of drugs inhibiting key components of the RAAS. 8 , 9 , 10 Both angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) have been proven to effectively reduce vascular and LV remodelling and decrease LV hypertrophy. 11 , 12 Recently, a new class termed angiotensin receptor–neprilysin inhibitor (ARNI) has been developed to simultaneously block the RAAS system and augment the natriuretic peptide system. 13 , 14 To date, sacubitril/valsartan is the first ARNI indicated for patients with HF and has shown promising efficacy in reversing LV dysfunction, reducing HF‐related hospitalizations and cardiac mortality without significant side effects. 15 , 16 , 17 Although sacubitril/valsartan has shown good superiority in reversing LV remodelling, there are relatively fewer studies looking at its effects on LA. This study therefore compared the effects of ARNI and ACEI/ARB on LA remodelling.
Methods
Study cohort
All procedures were conducted in accordance with the Declaration of Helsinki, and the study was approved by the Institutional Review Board of Dalian Medical University. The need for informed consent was waived by the committee owing to the retrospective and observational nature. This was a single‐centre retrospective observational study of consecutive patients with heart failure with reduced ejection fraction (HFrEF) who were hospitalized for acute decompensated HF at the First Affiliated Hospital of Dalian Medical University between 26 February 2016 and 8 July 2020. Decompensated HF was defined the presentation with signs and symptoms of HF and worsening HF that required intravenous treatment and hospital admission. Additional inclusion criteria were left ventricular ejection fraction (LVEF) < 40%, patients who are 18 years or older, and exposure to either ARNI or ACEI/ARB was at least 3 months. More patients received ACEI/ARB than ARNI over the same period of recruitment. To reduce the impact of confounding, 1:1 matching between ARNI and ACEI/ARB users was performed based on age, sex, AF, and LVEF. For age and LVEF, a difference less than 3% was considered acceptable. The cohort was subdivided further by left atrial volume index (LAVI): mildly abnormal (29 mL/m2 ≤ LAVI < 34 mL/m2), moderately abnormal (34 mL/m2 ≤ LAVI < 40 mL/m2), and severely abnormal (LAVI ≥ 40 mL/m2) groups.
Clinical data
Patients' demographics, comorbidities, biomarker assessment, arrhythmias, and drug therapies were collected and recorded. The laboratory indicators were measured on admission, which required to fast more than 8 h before venous blood collection. All subjects underwent dynamic electrocardiography to record the occurrence of various arrhythmias before discharge.
Echocardiographic data
Three novel indices of LA remodelling were examined:
LAVI: the European Association of Echocardiography recommended the use of the LAVI as a marker of LA size, with LAVI correlating with other echocardiographic indices of LV diastolic function. 18
Left atrial sphericity index (LASI), defined as the ratio between the transverse and longitudinal diameters of the LA, is another novel index for evaluating left atrial remodelling and may be altered earlier in the disease process than LAVI. 19
Left atrial reverse remodelling (LARR), defined as a reduction >15% in the left atrium end‐systolic volume (LAESV). 20
Other echocardiographic parameters were also recorded, such as LA diameter (LAD), LA superior–inferior diameter (LASID), LA transverse diameter (LATD), mitral Doppler early velocity/mitral annular early velocity (E/e′), LVEF, and left ventricular end‐diastolic dimension (LVEDD).
Follow‐up and study endpoints
As per local practice, all patients hospitalized for HF were offered to attend an outpatient clinic for follow‐up after discharge from the hospital for HF. Those who did not attend in person were interviewed over the telephone annually. The primary endpoint was the follow‐up echocardiographic data (outpatient or inpatient). Notably, the vast majority of echocardiographic findings on follow‐up were obtained during episodes of HF decompensation. Follow‐up data were obtained in the outpatient setting only for 13 patients. The secondary endpoint was all‐cause mortality, with follow‐up of 31 December 2020 or the endpoint, whichever was earlier.
Statistical analysis
Statistical analysis was performed using Statistical Package for Social Sciences, Version 24.0 (SPSS Inc., Chicago, IL). Categorical data were expressed as percentages (%), and chi‐squared test was used for comparison between the groups. Continuous data with non‐normal distributions were expressed as median (interquartile range), and the Kruskal–Wallis test was used. Continuous variables with normal distributions were expressed as mean ± standard deviation, and Student's t‐test was used for between‐group comparison. Kaplan–Meier analysis was performed to calculate the cumulative incidence of all‐cause mortality, with comparisons between groups made using the long‐rank test. All values were two‐tailed, and a P‐value < 0.05 was considered statistically significant.
Results
A flow diagram of inclusion and exclusion of subjects is shown in Figure 1 . Over the recruitment period, more patients received ACEIs/ARBs (n = 564) than ARNI (n = 168). To reduce the imbalances between the groups and reduce confounding, ACEIs/ARBs users were matched to users of ARNI in a 1:1 ratio for age, sex, AF, and LVEF, yielding 168 HFrEF cases in each group. The cohort had a mean age of 64.1 ± 12.9 years old and 30.1% were female. At baseline, most of the clinical, laboratory, and echocardiographic findings were not significantly different between both groups (Table 1 ). Patients who were lost to follow‐up were then further excluded. Subsequently, 274 HFrEF patients remained (ARNI group: n = 144; ACEI/ARB group: n = 130).
Figure 1.
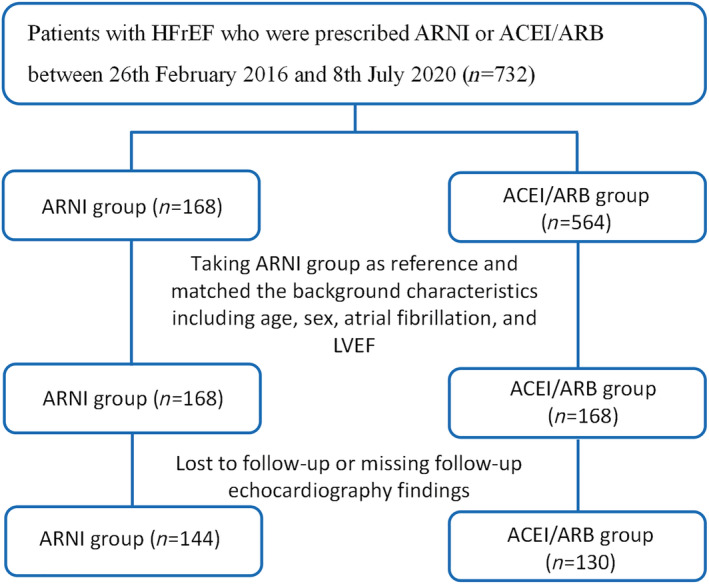
Flowchart of the study protocol.
Table 1.
Baseline characteristics of the study cohort of patients with heart failure with reduced ejection fraction, stratified by angiotensin receptor–neprilysin inhibitor (ARNI) and angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker (ACEI/ARB) groups
| Variables | ARNI group (n = 168) | ACEI/ARB group (n = 168) | P‐value |
|---|---|---|---|
| Age (years) | 62.19 ± 13.85 | 64.11 ± 12.88 | 0.190 |
| Female (n, %) | 52 (31.0) | 49 (29.2) | 0.721 |
| Systolic blood pressure (mmHg) | 128.27 ± 32.67 | 127.36 ± 20.25 | 0.757 |
| Diastolic blood pressure (mmHg) | 79.76 ± 14.40 | 80.13 ± 16.41 | 0.824 |
| Heart rate (b.p.m.) | 83.25 ± 21.64 | 82.08 ± 19.66 | 0.631 |
| QRS duration (ms) | 117.67 ± 38.77 | 116.15 ± 31.09 | 0.735 |
| QTc interval (ms) | 470.02 ± 72.88 | 479.18 ± 42.45 | 0.226 |
| BSA (kg/m2) | 1.85 ± 0.18 | 1.84 ± 0.20 | 0.815 |
| NYHA class | |||
| III (n, %) | 101 (60.1) | 99 (58.9) | 0.824 |
| IV (n, %) | 52 (31.0) | 62 (36.9) | 0.249 |
| Hypertension (n, %) | 99 (58.9) | 80 (47.6) | 0.038 |
| Diabetes mellitus (n, %) | 61 (36.3) | 63 (37.5) | 0.821 |
| Coronary heart disease (n, %) | 79 (47.0) | 80 (47.6) | 0.913 |
| Atrial fibrillation (n, %) | 54 (32.1) | 50 (29.8) | 0.637 |
| Dilated cardiomyopathy | 55 (32.7) | 58 (34.5) | 0.729 |
| Laboratory values | |||
| BNP (pg/mL) | 1019 (470, 2052) | 928 (399, 2096) | 0.551 |
| hs‐TnI (μg/L) | 0.06 (0.02, 0.15) | 0.04 (0.02, 0.09) | 0.021 |
| Glucose (mmol/L) | 5.61 (4.78, 7.91) | 5.44 (4.80, 7.27) | 0.579 |
| Cholesterol (mmol/L) | 4.33 ± 1.25 | 4.28 ± 1.23 | 0.702 |
| Triglyceride (mmol/L) | 1.27 (0.92, 1.72) | 1.16 (0.86, 1.51) | 0.262 |
| LDL‐C (mmol/L) | 2.31 (1.75, 3.00) | 2.37 (1.83, 2.95) | 0.823 |
| HDL‐C (mmol/L) | 0.98 (0.76, 1.16) | 1.02 (0.81, 1.16) | 0.574 |
| Urea (mmol/L) | 8.55 (6.51, 11.66) | 8.33 (6.21, 11.21) | 0.743 |
| Creatinine (mmol/L) | 94 (79, 116) | 93 (72, 108) | 0.701 |
| Uric acid (mmol/L) | 533.69 ± 167.15 | 515.30 ± 173.97 | 0.325 |
| Sodium (mmol/L) | 139.61 ± 10.56 | 141.43 ± 4.20 | 0.400 |
| Potassium (mmol/L) | 4.00 ± 0.59 | 4.03 ± 0.54 | 0.641 |
| Echocardiography findings | |||
| LVEF (%) | 30.49 ± 7.88 | 30.02 ± 8.37 | 0.470 |
| LVEDD (mm) | 61.39 ± 9.13 | 62.49 ± 10.33 | 0.311 |
| E/e′ | 14.73 ± 5.90 | 15.48 ± 5.77 | 0.263 |
| Interventricular septal thickness (mm) | 10.34 ± 2.09 | 10.12 ± 1.96 | 0.330 |
| Left ventricular wall thickness (mm) | 9.72 ± 1.36 | 9.83 ± 1.46 | 0.510 |
| EDT | 165.29 ± 49.47 | 153.24 ± 47.34 | 0.026 |
| LAD (mm) | 44.73 ± 5.82 | 45.84 ± 5.71 | 0.078 |
| LASID (mm) | 48.29 ± 6.14 | 48.18 ± 6.03 | 0.869 |
| LATD (mm) | 62.16 ± 8.09 | 62.59 ± 8.46 | 0.645 |
| LAV (mL) | 72.76 ± 27.16 | 77.05 ± 33.34 | 0.216 |
| LAVI (mL/m2) | 39.55 ± 15.15 | 40.99 ± 14.53 | 0.390 |
| LASI | 1.29 ± 0.08 | 1.30 ± 0.11 | 0.254 |
| Treatments | |||
| Beta‐blockers (n, %) | 154 (91.6) | 133 (79.1) | 0.001 |
| Calcium channel blocker (n, %) | 13 (7.7) | 37 (22.0) | <0.0001 |
| Spironolactone (n, %) | 147 (87.5) | 149 (88.6) | 0.736 |
| Digoxin (n, %) | 31 (18.4) | 61 (36.3) | <0.0001 |
| Loop diuretics (n, %) | 147 (87.5) | 140 (83.3) | 0.279 |
| Subgroup analysis | |||
| Mildly abnormal LAVI (n, %) | 26 (15.5) | 25 (14.9) | 0.879 |
| Moderately abnormal LAVI (n, %) | 32 (19.0) | 28 (16.7) | 0.569 |
| Severely abnormal LAVI (n, %) | 68 (40.5) | 68 (40.5) | 1.000 |
Abbreviations: BNP, B‐type natriuretic peptide; BSA, body surface area; E/e′, mitral Doppler early velocity/mitral annular early velocity; EDT, E peak deceleration time; HDL‐C, high‐density lipoprotein cholesterol; hs‐TNI, high‐sensitivity troponin I; LAD, left atrium diameter; LASI, left atrial sphericity index; LASID, superior and inferior diameter of left atrium; LATD, transverse diameter of left atrium; LAV, left atrial volume; LAVI, left atrial volume index; LDL‐C, low‐density lipoprotein cholesterol; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Echocardiographic data and mortality on follow‐up
At follow‐up, patients in the ARNI group had higher B‐type natriuretic peptide (BNP) levels than those in the ACEI/ARB group, whereas other tests such as high‐sensitivity troponin, lipids, and glycaemic tests were similar between both groups (Table 2 ). Differences in the following echocardiographic parameters between baseline and follow‐up were calculated and compared between the ARNI (n = 144) and ACEI/ARB groups (n = 130): LAD (ARNI group: from 44.97 ± 5.75 to 44.18 ± 6.30 mm vs. ACEI/ARB group: from 46.04 ± 5.89 to 46.37 ± 7.90 mm, P = 0.013), LASID (ARNI group: from 48.47 ± 6.14 to 44.93 ± 6.28 mm vs. ACEI/ARB group: from 48.23 ± 6.17 to 48.03 ± 7.11 mm, P < 0.0001), LATD (ARNI group: from 62.45 ± 8.17 to 53.10 ± 8.78 mm vs. ACEI/ARB group: from 62.71 ± 8.49 to 62.82 ± 9.09 mm, P < 0.0001), LA volume (LAV) (ARNI group: from 73.85 ± 27.41 to 57.15 ± 22.33 mm vs. ACEI/ARB group: from 75.57 ± 26.76 to 77.41 ± 34.25 mm, P < 0.0001), LAVI (ARNI group: from 40.19 ± 15.18 to 31.32 ± 13.18 mm vs. ACEI/ARB group: from 41.27 ± 14.80 to 42.27 ± 18.83 mm, P < 0.0001), LASI (ARNI group: from 1.29 ± 0.083 to 1.19 ± 0.15 vs. ACEI/ARB group: from 1.30 ± 0.115 to 1.31 ± 0.09, P < 0.0001), E/e′ (ARNI group: from 14.67 ± 6.14 to 14.67 ± 7.20 vs. ACEI/ARB group: from 15.69 ± 6.05 to 13.95 ± 5.67, P = 0.426), LVEF (ARNI group: from 30.02 ± 8.09 to 36.26 ± 11.72 vs. ACEI/ARB group: from 30.18 ± 8.92 to 32.48 ± 10.83, P = 0.006), and LVEDD (ARNI group: from 61.64 ± 9.16 to 60.42 ± 9.57 mm vs. ACEI/ARB group: from 61.78 ± 10.46 to 60.55 ± 9.69 mm, P = 0.911) (Figure 2 ). Moreover, 97 patients (67.3%) in the ARNI group and 29 patients (22.3%) in the ACEI/ARB group showed LARR (Figure 3 ).
Table 2.
Follow‐up results for clinical, laboratory, and echocardiographic details
| Variables | ARNI group (n = 144) | ACEI/ARB group (n = 130) | P‐value |
|---|---|---|---|
| Systolic blood pressure (mmHg) | 118.6 ± 27.5 | 121.7 ± 23.4 | 0.435 |
| Diastolic blood pressure (mmHg) | 73.0 ± 14.7 | 75.3 ± 12.8 | 0.305 |
| Heart rate (b.p.m.) | 82.6 ± 23.1 | 79.6 ± 23.2 | 0.425 |
| QRS duration (ms) | 116.6 ± 35.1 | 121.2 ± 37.5 | 0.450 |
| QTc interval (ms) | 436.6 ± 141.6 | 472.6 ± 72.5 | 0.029 |
| BSA (kg/m2) | 1.84 ± 0.18 | 1.84 ± 0.19 | 0.745 |
| Laboratory values | |||
| BNP (pg/mL) | 1396 (507, 3723) | 995 (406, 1820) | 0.029 |
| hs‐TnI (μg/L) | 0.06 (0.03, 0.14) | 0.04 (0.02, 0.14) | 0.183 |
| Creatinine (mmol/L) | 102 (87, 137) | 98 (75, 125) | 0.075 |
| Uric acid (mmol/L) | 537.01 ± 165.81 | 543.79 ± 201.06 | 0.790 |
| Serum potassium (mmol/L) | 4.21 ± 0.71 | 4.13 ± 0.70 | 0.360 |
| Echocardiography findings | |||
| LVEF (%) | 36.26 ± 11.72 | 32.48 ± 10.83 | 0.006 |
| LVEDD (mm) | 60.42 ± 9.57 | 60.55 ± 9.69 | 0.911 |
| E/e′ | 14.67 ± 7.20 | 13.95 ± 5.67 | 0.426 |
| Interventricular septal thickness (mm) | 10.00 ± 1.87 | 10.05 ± 1.93 | 0.867 |
| Left ventricular wall thickness (mm) | 9.65 ± 1.39 | 9.48 ± 1.49 | 0.331 |
| LAD (mm) | 44.18 ± 6.30 | 46.37 ± 7.90 | 0.013 |
| LASID (mm) | 44.93 ± 6.28 | 48.03 ± 7.11 | <0.0001 |
| LATD (mm) | 53.10 ± 8.78 | 62.82 ± 9.09 | <0.0001 |
| LAV (mL) | 57.15 ± 22.33 | 77.41 ± 34.25 | <0.0001 |
| LAVI (mL/m2) | 31.32 ± 13.18 | 42.27 ± 18.83 | <0.0001 |
| LASI | 1.19 ± 0.15 | 1.31 ± 0.09 | <0.0001 |
Abbreviations: BNP, B‐type natriuretic peptide; BSA, body surface area; E/e′, mitral Doppler early velocity/mitral annular early velocity; hs‐TnI, high‐sensitivity troponin I; LAD, left atrium diameter; LASI, left atrial sphericity index; LASID, superior and inferior diameter of left atrium; LATD, transverse diameter of left atrium; LAV, left atrial volume; LAVI, left atrial volume index; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction.
Figure 2.
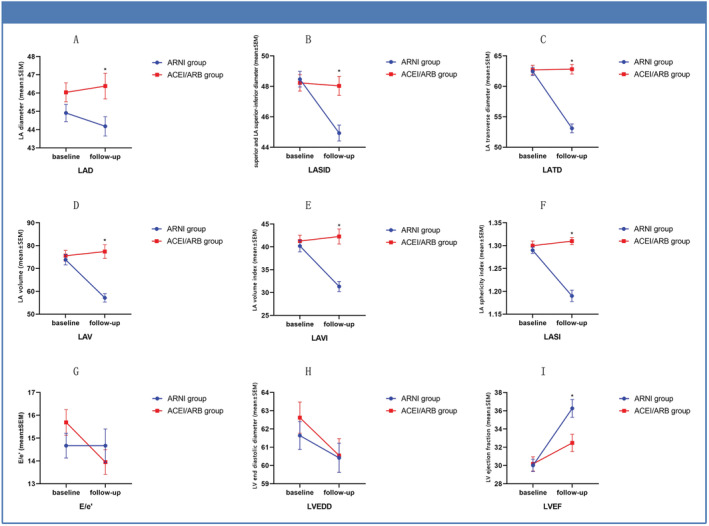
Changes in echocardiographic parameters between baseline and follow‐up for the ARNI and ACEI/ARB groups.
Figure 3.
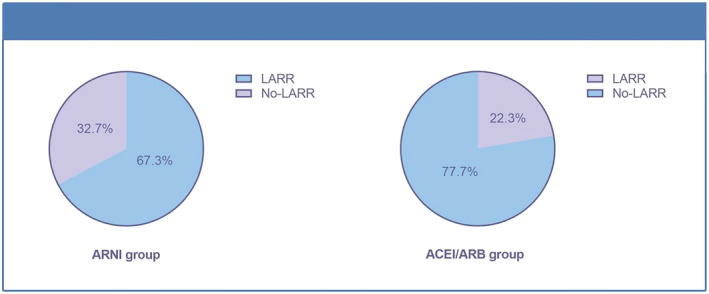
Left atrial reverse remodelling in the ARNI and ACEI/ARB groups.
The number of patients with mortality were 17 (11.8%) and 34 (26.2%) for the ARNI and ACEI/ARB groups, respectively. Kaplan–Meier analysis showed that mortality in the ARNI group was significantly lower than that of the ACEI/ARB group (Figure 4 ).
Figure 4.
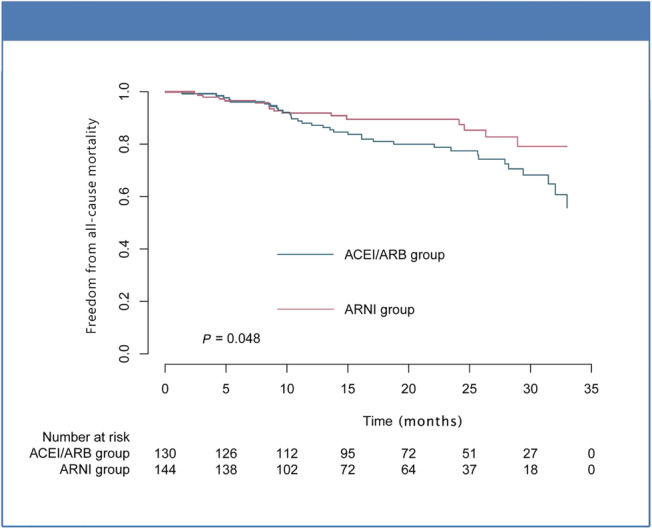
Mortality outcome stratified by ARNI or ACEI/ARB use.
Subgroup analysis
In a subgroup analysis, the number of patients (percentage) in the normal, mildly abnormal, moderately abnormal, and severely abnormal groups of LAVI were 31 (21.5), 23 (16.0), 27 (18.8), 63 (43.7) for the ARNI group and 25 (19.2), 22 (16.9), 22 (16.9), 61 (47.0) for the ACEI/ARB group, at baseline, respectively. On follow‐up, these were 75 (52.1), 23 (16.0), 17 (11.8), 29 (20.1) for the ARNI group and 34 (26.2), 14 (10.8), 30 (23.1), 52 (40) for the ACEI/ARB group (Figure 5 ). Therefore, ARNI users have greater reductions in LAVs compared with ACEI/ARB users with HFrEF. Furthermore, the risk of all‐cause mortality was reduced in the mildly abnormal LAVI group (left panel, Figure 6 ; P = 0.044, log‐rank test), but not in the moderately abnormal (middle panel, Figure 6 ; P = 0.571) or severely abnormal LAVI groups (right panel, Figure 6 ; P = 0.609). This suggests that early initiation of ARNI can have a benefit on all‐cause mortality.
Figure 5.
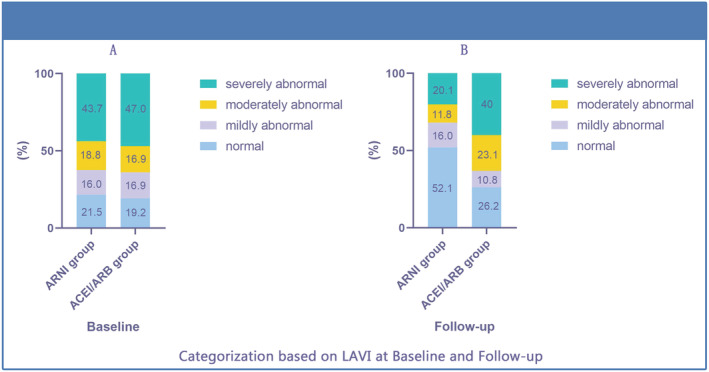
Changes in LAVI between baseline and follow‐up for the ARNI and ACEI/ARB groups.
Figure 6.
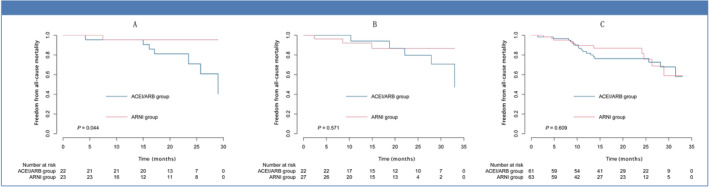
Mortality between the subgroups of left atrial volume index (LAVI): the mildly abnormal group (A), moderately abnormal group (B), and severely abnormal group (C).
Because LARR was defined as a reduction >15% in the LAESV, we stratified the cohort based on LAESV: reduction >15% group vs. reduction ≤15% group. The results indicated that a reduction ≤15% group tended to show a higher all‐cause mortality although no statistical difference was reached [hazard ratio (HR) 1.683, 95% confidence interval (CI), 0.932–3.037, P = 0.084]. There was no significant difference after adjusting for age, gender, ejection fraction, and BNP (HR 1.727, 95% CI, 0.854–3.492, P = 0.129).
Discussion
The main findings of this study are that (i) the ARNI sacubitril/valsartan produced more significant benefits in terms of reversing remodelling of LA and LV, improving cardiac function, and reducing mortality than ACEI/ARB. (ii) Early initiation of sacubitril/valsartan may provide more benefit for HF patients.
The PARADIGM‐HF trial demonstrated that sacubitril/valsartan was superior to enalapril in patients with HFrEF, with significant benefit on reducing the risk of cardiovascular death or first hospitalization with HF by 20%. 21 In our study, patients in the ARNI group showed greater reduction in LV size and had lower mortality compared with those in the ACEI/ARB group. In addition, we found that the difference of mortality between the two groups became more significant with the prolongation of follow‐up.
Recently, there has been increasing recognition of the importance of evaluating the LA before clinical decision making in HF patients. Left atrial remodelling is a time‐dependent response of cardiac myocytes to varying stressors with the expression of structural remodelling, functional remodelling, and electrical remodelling. 22 In 2020, the term atrial failure was proposed as a clinically relevant entity, defined as any atrial dysfunction causing impaired heart performance, symptoms, and worsening quality of life or life expectancy, placing a spotlight on the LA. 23 The LAVI, which reflects the degree of change in LV filling pressure and atrial structural remodelling, is a powerful predictor of prognosis in HF. 24 , 25 , 26 The results of the EVALUATE‐HF trial 27 revealed that greater decreases from baseline in LAVI were seen among HFrEF patients assigned to sacubitril/valsartan compared with those assigned to enalapril. The PROVE‐HF 28 showed that following initiation and titration of sacubitril/valsartan, cross‐sectional improvement in cardiac remodelling parameters was seen in those with and without diabetes mellitus. They also found that, in addition to LVEF improvement, patients with diabetes mellitus showed improved LAVI and diastolic function. In our study, we found that indications for evaluating left atrial volume such as LAD, LASID, LATD, LAV, LAVI, and LASI were significantly decreased after the treatment of sacubitril/valsartan compared with ACEI/ARB. LARR is another important metric of LA reverse remodelling. A prospective study reported a significant increase in left ventricular reverse remodelling (LVRR, an increase in the LVEF ≥ 10 points associated with a decrease ≥10% in indexed LV end‐diastolic diameter) and LARR during treatment with sacubitril//valsartan. Castrichini et al. analysed 77 HFrEF patients and found that, after a median follow‐up of 9 months following initiating sacubitril/valsartan, LVRR was detected in 20 patients (26%) and LARR in 33 patients (43%). In our research, the results also revealed that the extent of LARR was to different extents for ARNI and ACEI/ARB patients, with ARNI being a more effective agent.
The mechanisms of LARR are likely multifactorial. Animal experiments have found that sacubitril/valsartan can reduce interstitial fibrosis and the elevation of left atrial pressure, decrease myolysis, contractile protein loss, and left atrial dysfunction, and shorten the duration of AF, which may partially explain why ARNI can effectively reverse left atrial remodelling. 29
Limitations
Several limitations of this study should be noted. Firstly, considering the single‐centre nature of our study with relatively few subjects, the findings may not be generalizable to other settings and a larger multi‐centre clinical study is needed. Secondly, the indicators of assessing LA remodelling were mainly obtained from 2D echocardiography, and data on 3D echocardiography and cardiac magnetic resonance that can be more precisely characterize the myocardial tissue were only available in a small number of patients. Thirdly, most of the follow‐up were obtained during rehospitalization for worsening HF, with a smaller number of patients at the outpatient clinics. Fourthly, nearly half of HF patients received sacubitril/valsartan at a dose of 50 mg twice daily, with only ~20% taking more than 100 mg twice daily. In clinical practice of Asian populations, few patients could reach the recommended target dose of 200 mg twice daily when taking ARNI. Lastly, new‐onset AF was not examined in this study, but a longer and more detailed study would permit arrhythmic endpoints to be explored in the future.
Conclusions
In this proof‐of‐concept study, we found that the ARNI sacubitril/valsartan provided greater effects on LARR and prognosis compared with ACEI/ARB in HFrEF. Early initiation of ARNI in the HF disease process may produce greater benefit, but this needs to be confirmed in future studies.
Conflict of interest
None declared.
Funding
This research was supported by the National Natural Science Foundation of China (grant numbers U1908209 and 82170385).
Acknowledgements
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Sun, Y. , Song, S. , Zhang, Y. , Mo, W. , Zhang, X. , Wang, N. , Xia, Y. , Tse, G. , and Liu, Y. (2022) Effect of angiotensin receptor neprilysin inhibitors on left atrial remodeling and prognosis in heart failure. ESC Heart Failure, 9: 667–675. 10.1002/ehf2.13691.
Contributor Information
Gary Tse, Email: garytse86@gmail.com.
Ying Liu, Email: yingliu.med@gmail.com.
References
- 1. Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: Mechanisms: Part 1 of 2. Circulation 2013; 128: 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bisbal F, Baranchuk A, Braunwald E, Bayés de Luna A, Bayés‐Genís A. Atrial failure as a clinical entity. J Am Coll Cardiol 2020; 75: 222–232. [DOI] [PubMed] [Google Scholar]
- 3. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the european Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016; 29: 277–314. [DOI] [PubMed] [Google Scholar]
- 4. Jalife J, Kaur K. Atrial remodeling, fibrosis, and atrial fibrillation. Trends Cardiovasc Med 2015; 25: 475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bazoukis G, Stavrakis S, Zhou J, Bollepalli SC, Tse G, Zhang Q, Singh JP, Armoundas AA. Machine learning versus conventional clinical methods in guiding management of heart failure patients‐a systematic review. Heart Fail Rev 2021; 26: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Y, Yuan M, Gong M, Li G, Liu T, Tse G. Associations between prefrailty or frailty components and clinical outcomes in heart failure: A follow‐up meta‐analysis. J Am Med Dir Assoc 2019; 20: 509–510. [DOI] [PubMed] [Google Scholar]
- 7. Tse G, Zhou J, Woo SWD, Ko CH, Lai RWC, Liu T, Liu Y, Leung KSK, Li A, Lee S, Li KHC, Lakhani I, Zhang Q. Multi‐modality machine learning approach for risk stratification in heart failure with left ventricular ejection fraction ≤= 45. ESC Heart Fail 2020; 7: 371–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dickstein K, Cohen‐Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole‐Wilson PA, Stromberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K. Guidelines ESCCfP. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology.Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail. 2008; 10: 933–989. [DOI] [PubMed] [Google Scholar]
- 9. Schroten NF, Gaillard CA, van Veldhuisen DJ, Szymanski MK, Hillege HL, de Boer RA. New roles for renin and prorenin in heart failure and cardiorenal crosstalk. Heart Fail Rev 2012; 17: 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. von Lueder TG, Krum H. RAAS inhibitors and cardiovascular protection in large scale trials. Cardiovasc Drugs Ther 2013; 27: 171–179. [DOI] [PubMed] [Google Scholar]
- 11. Devereux RB, Dahlof B, Gerdts E, Boman K, Nieminen MS, Papademetriou V, Rokkedal J, Harris KE, Edelman JM, Wachtell K. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: The losartan intervention for endpoint reduction in hypertension (LIFE) trial. Circulation 2004; 110: 1456–1462. [DOI] [PubMed] [Google Scholar]
- 12. Klingbeil AU, Schneider M, Martus P, Messerli FH, Schmieder RE. A meta‐analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med 2003; 115: 41–46. [DOI] [PubMed] [Google Scholar]
- 13. Kjeldsen SE, Hedner T, Narkiewicz K, Oparil S. Angiotensin receptor‐‐neprilysin inhibiton (ARNI)‐‐a novel therapeutic concept for management of hypertension and heart failure. Blood Press 2012; 21: 329–330. [DOI] [PubMed] [Google Scholar]
- 14. von Lueder TG, Sangaralingham SJ, Wang BH, Kompa AR, Atar D, Burnett JC Jr, Krum H. Renin‐angiotensin blockade combined with natriuretic peptide system augmentation: Novel therapeutic concepts to combat heart failure. Circ Heart Fail 2013; 6: 594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ruilope LM, Dukat A, Bohm M, Lacourciere Y, Gong J, Lefkowitz MP. Blood‐pressure reduction with LCZ696, a novel dual‐acting inhibitor of the angiotensin II receptor and neprilysin: A randomised, double‐blind, placebo‐controlled, active comparator study. Lancet 2010; 375: 1255–1266. [DOI] [PubMed] [Google Scholar]
- 16. Pathadka S, Yan VKC, Li X, Tse G, Wan EYF, Lau H, Lau WCY, Siu DCW, Chan EW, Wong ICK. Hospitalization and mortality in patients with heart failure treated with Sacubitril/Valsartan vs. Enalapril: A real‐world, population‐based study. Front Cardiovasc Med. 2020; 7: 602363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Y, Tse G, Roever L, Liu T. Sacubitril/valsartan in the treatment of cancer therapy‐related cardiac dysfunction. Int J Cardiol 2020; 1: 130. [DOI] [PubMed] [Google Scholar]
- 18. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Chamber quantification writing G, American Society of Echocardiography's G, standards C, european Association of E. Recommendations for chamber quantification: A report from the American Society of Echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the european Association of Echocardiography, a branch of the european Society of Cardiology. J Am Soc Echocardiogr 2005; 18: 1440–1463. [DOI] [PubMed] [Google Scholar]
- 19. Bisbal F, Guiu E, Calvo N, Marin D, Berruezo A, Arbelo E, Ortiz‐Perez J, de Caralt TM, Tolosana JM, Borras R, Sitges M, Brugada J, Mont L. Left atrial sphericity: A new method to assess atrial remodeling. Impact on the outcome of atrial fibrillation ablation. J Cardiovasc Electrophysiol 2013; 24: 752–759. [DOI] [PubMed] [Google Scholar]
- 20. Castrichini M, Manca P, Nuzzi V, Barbati G, De Luca A, Korcova R, Stolfo D, Lenarda AD, Merlo M, Sinagra G. Sacubitril/Valsartan induces global cardiac reverse remodeling in long‐lasting heart failure with reduced ejection fraction: Standard and advanced echocardiographic evidences. J Clin Med 2020; 9: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mc Causland FR, Lefkowitz MP, Claggett B, Anavekar NS, Senni M, Gori M, Jhund PS, McGrath MM, Packer M, Shi V, Van Veldhuisen DJ, Zannad F, Comin‐Colet J, Pfeffer MA, McMurray JJV, Solomon SD. Angiotensin‐neprilysin inhibition and renal outcomes in heart failure with preserved ejection fraction. Circulation 2020; 142: 1236–1245. [DOI] [PubMed] [Google Scholar]
- 22. Thomas L, Abhayaratna WP. Left atrial reverse remodeling: Mechanisms, evaluation, and clinical significance. JACC Cardiovasc Imaging 2017; 10: 65–77. [DOI] [PubMed] [Google Scholar]
- 23. Bisbal F, Baranchuk A, Braunwald E, Bayes de Luna A, Bayes‐Genis A. Atrial failure as a clinical entity: JACC review topic of the week. J Am Coll Cardiol 2020; 75: 222–232. [DOI] [PubMed] [Google Scholar]
- 24. Møller JE, Hillis GS, Oh JK, Seward JB, Reeder GS, Wright RS, Park SW, Bailey KR, Pellikka PA. Left atrial volume. Circulation 2003; 107: 2207–2212. [DOI] [PubMed] [Google Scholar]
- 25. Andersen OS, Smiseth OA, Dokainish H, Abudiab MM, Schutt RC, Kumar A, Sato K, Harb S, Gude E, Remme EW, Andreassen AK, Ha J‐W, Xu J, Klein AL, Nagueh SF. Estimating left ventricular filling pressure by echocardiography. J Am Coll Cardiol 2017; 69: 1937–1948. [DOI] [PubMed] [Google Scholar]
- 26. Thadani SR, Shaw RE, Fang Q, Whooley MA, Schiller NB. Left atrial end‐diastolic volume index as a predictor of cardiovascular outcomes. Circulation. Cardiovasc Imaging. 2020; 13: e009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Desai AS, Solomon SD, Shah AM, Claggett BL, Fang JC, Izzo J, McCague K, Abbas CA, Rocha R, Mitchell GF, Investigators E‐H. Effect of sacubitril‐valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: A randomized clinical trial. JAMA 2019: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khan MS, Felker GM, Pina IL, Camacho A, Bapat D, Ibrahim NE, Maisel AS, Prescott MF, Ward JH, Solomon SD, Januzzi JL, Butler J. Reverse cardiac remodeling following initiation of Sacubitril/Valsartan in patients with heart failure with and without diabetes. JACC Heart Fail. 2021; 9: 137–145. [DOI] [PubMed] [Google Scholar]
- 29. Li LY, Lou Q, Liu GZ, Lv JC, Yun FX, Li TK, Yang W, Zhao HY, Zhang L, Bai N, Zhan CC, Yu J, Zang YX, Li WM. Sacubitril/valsartan attenuates atrial electrical and structural remodelling in a rabbit model of atrial fibrillation. Eur J Pharmacol 2020; 15: 173120. [DOI] [PubMed] [Google Scholar]


