Abstract
Aims
Control of pulmonary pressures monitored remotely reduced heart failure hospitalizations mainly by lowering filling pressures through the use of loop diuretics. Sacubitril/valsartan improves heart failure outcomes and increases the kidney sensitivity for diuretics. We explored whether sacubitril/valsartan is associated with less utilization of loop diuretics in patients guided with haemodynamic monitoring in the CardioMEMS European Monitoring Study for Heart Failure (MEMS‐HF).
Methods and results
The MEMS‐HF population (n = 239) was separated by the use of sacubitril/valsartan (n = 68) or no use of it (n = 164). Utilization of diuretics and their doses was prespecified in the protocol and was monitored in both groups. Multivariable regression, ANCOVA, and a generalized linear model were used to fit baseline covariates with furosemide equivalents and changes for 12 months. MEMS‐HF participants (n = 239) were grouped in sacubitril/valsartan users [n = 68, 64 ± 11 years, left ventricular ejection fraction (LVEF) 25 ± 9%, cardiac index (CI) 1.89 ± 0.4 L/min/m2] vs. non‐users (n = 164, 70 ± 10 years, LVEF 36 ± 16%, CI 2.11 ± 0.58 L/min/m2, P = 0.0002, P < 0.0001, and P = 0.0015, respectively). In contrast, mean pulmonary artery pressure (PAP) values were comparable between groups (29 ± 11 vs. 31 ± 11 mmHg, P = 0.127). Utilization of loop diuretics was lower in patients taking sacubitril/valsartan compared with those without (P = 0.01). Significant predictor of loop diuretic use was a history of renal failure (P = 0.005) but not age (P = 0.091). After subjects were stratified by sacubitril/valsartan or other diuretic use, PAP was nominally, but not significantly lower in sacubitril/valsartan‐treated patients (baseline: P = 0.52; 6 months: P = 0.07; 12 months: P = 0.53), while there was no difference in outcome or PAP changes. This difference was observed despite lower CI (P = 0.0015). Comparable changes were not observed for other non‐loop diuretics (P = 0.21).
Conclusions
In patients whose treatment was guided by remote PAP monitoring, concomitant use of sacubitril/valsartan was associated with reduced utilization of loop diuretics, which could potentially be relevant for outcomes.
Keywords: Drug therapy, Loop diuretics, Heart failure, Pulmonary artery pressure, Monitor
Introduction
Heart failure is associated with a high rate of hospitalizations and healthcare costs. 1 After recompensation, a high incidence of recurring symptoms leading to readmissions is predictive of cardiovascular death during follow‐up. 2 , 3 As hospitalization for worsening of heart failure is usually associated with volume overload leading to congestion, treatment with diuretics is mandatory to reduce hospitalizations. 4 Pulmonary artery pressure (PAP) rises days and weeks before clinical signs and symptoms develop, 5 and early treatment with diuretics might be a tool to prevent hospitalizations. 6 It has been shown that titration of heart failure medications guided by haemodynamic parameters collected remotely (CardioMEMS HF System) is feasible, 7 reduces hospitalization, and improves quality of life. 8 , 9 Recently, outcomes from studies using the angiotensin receptor blocker/neprilysin inhibitor sacubitril/valsartan and studies with the sodium‐glucose transporter 2 inhibitors (SGLT2i) dapagliflozin 10 and empagliflozin 11 have shown a reduction in cardiovascular death and hospitalization. According to observational studies from the PARADIGM trial 11 and data from longitudinal cohort study in outpatient clinics, 12 sacubitril/valsartan reduces the utilization of loop diuretics, which was not detected with the SGLT‐2 inhibitor dapagliflozin. 13 This is important as loop diuretics can cause neuro‐hormonal activation 14 and electrolyte disturbances 15 associated with arrhythmias, heart failure progression, and death. 16 , 17 , 18 However, the utilization of diuretics has only been studied in a registry and a clinical trial (PARADIGM‐HF) with drug titration left to the discretion of investigators. 12 , 19 Herein, we asked the question whether patients treated with sacubitril/valsartan have a lower diuretic use, less initiation of loop diuretics, and different response to diuretic changes, when PAP is individually adjusted early with diuretics to prevent later congestion and hospitalizations. Data from the CardioMEMS European Monitoring Study for Heart Failure (MEMS‐HF) were utilized, and the patient population studied was stratified according to the use of sacubitril/valsartan compared with patients who were not treated with sacubitril/valsartan in addition to heart failure management guided by haemodynamic data on top of standard of care. The pre‐existing therapy and incident intensification with diuretics was captured as part of the predefined study protocol. 9 , 20
Methods
Trial design
Information on the study design and population of the Cardio‐MEMS European Monitoring Study for Heart Failure (MEMS‐HF, NCT 02693691) has been published previously. 20 In brief, MEMS‐HF is a prospective non‐randomized open‐label multicentre cohort study to evaluate the effect of the CardioMEMS HF systems in real‐world settings. The trial was conducted in 26 German centres, 4 centres in the Netherlands, and 1 centre in Ireland. Patients were enrolled from outpatient centres or while being hospitalized for worsening of heart failure. Patients had a history of New York Heart Association (NYHA) Class III symptoms on optimal, guideline‐directed medical and device therapies. Ejection fraction was not an inclusion criterion, and ejection fraction at the start of treatment with sacubitril/valsartan was not captured. Patients of both sexes were eligible if 18 years of age or older, and if having been hospitalized for worsening of heart failure in the previous 12 months. The CardioMEMS™ PA Sensor estimates PAP using micro‐electromechanical systems technology that does not require batteries or leads as reported previously. 7 , 8 , 21 , 22 Training activities for patients and staff, study flow, data collection, and follow‐up details have been published previously. 20
Medical treatment
Pulmonary artery pressure tracings were reviewed regularly in the individual study centres. Patients needed to be managed clinically to keep PAP within each patient's individually defined range. Frequency of reviewing PAP trends was dependent on the clinical status of the patient. Supervision protocols and treatment algorithms have been published previously. 6 , 7 , 20 , 21 , 22 The majority of medication changes in MEMS‐HF was due to dose increases of diuretics [1068 medication changes compared with 312 changes in angiotensin‐converting enzyme (ACE) inhibitors, 209 changes in beta‐blockers, and 170 changes in mineralocorticoid antagonists]. For diuretic dose equivalents in furosemide (loop diuretics) or metolazone (thiazides), see Table 1 . In this analysis, the total population of 232 patients was separated into those on sacubitril/valsartan (n = 68) or no sacubitril/valsartan (n = 164).
Table 1.
Conversion for diuretics
| Loop diuretics | TD (mg) | Furosemide equivalency conversion |
|---|---|---|
| Furosemide | 40 | × 1.0 |
| Bumetanide | 1 | × 40.0 |
| Torsemide | 20 | × 2.0 |
| Thiazides | TD (mg) | Metolazone equivalency conversion |
|---|---|---|
| Metolazone | 2.5 | × 1.0 |
| Bendroflumethiazide | 2.5 | × 1.0 |
| Hydrochlorothiazide | 2.5 | × 0.1 |
| Indapamide | 2.5 | × 1.0 |
Statistical analysis
Baseline characteristics were summarized according to sacubitril/valsartan use or non‐use (Table 2 ) in those patients with recorded dosage and data for all diuretics. Cumulative changes of baseline and diastolic and systolic and mean PAP were evaluated using an area under the curve (AUC analysis), which quantifies frequency and duration of PAP values below baseline (first week of home readings) using numeric integration. 20 Patients with a complete capturing of diuretics were used for the final analysis (n = 155, on sacubitril/valsartan n = 48, without sacubitril/valsartan n = 107). The likelihood of changing diuretic dose was predicted by average daily change of PAP (30 days prior) = sum of (current reading − baseline reading) ÷ number total readings. The odds of getting an up‐titration of diuretics for sacubitril/valsartan and no sacubitril/valsartan users was calculated for each mmHg increase in average daily PAP change from baseline and for 10 mmHg increase in average daily PAP. A multivariable regression analysis adjusting for baseline pressure was performed. Up‐titration included increased dose, new start, and restart of medications, and down‐titration included decreased dose and stop of medication. ANCOVA was applied to compare the two different groups to determine the group difference for furosemide equivalence and metolazone equivalence for non‐loop diuretics as well as the difference between baseline and follow‐up for all diuretic users at 6 months taking baseline values into consideration. For both of these analyses, a generalized linear model (GLM) was fitted with baseline covariates to evaluate actual dose equivalents and change in dose at 6 months. The least square means between analysis groups was tested in order to assess any differences in diuretic use between groups. Baseline variables were selected for entry into the model based on a P‐value criterion of P < 0.05 indicating variables as different between groups. These variables included age, cardiac index, ejection fraction, and history of renal failure. The model utilized a backward elimination approach, removing covariates one at a time until all of the covariates meet the staying criterion of P < 0.1. This GLM allows for evaluating measures over time and between analysis groups rather than just comparing groups individually at each study visit. In order to assess the diuretic dose utilized at 6 months, values from baseline, 3 months, and 6 months were included in the models testing actually dose and change values from 3 and 6 months were included for the models testing change from baseline dose. A two‐sided P‐value of <0.05 was considered statistically significant. SAS Version 9.4 (SAS Institute Incorporation, Kerry, NC, USA) was used for these analyses.
Table 2.
Demographics and baseline characteristics (as treated population stratified by sacubitril/valsartan, diuretic use)
| Sacubitril/valsartan users (N = 68) | Non‐users (N = 164) | P‐value a | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 63.9 ± 10.9 (68) | 69.9 ± 9.9 (164) | 0.0002 |
| Sex (male) | 82.4% (56/68) | 76.8% (126/164) | 0.3859 |
| Vital signs/lab analyses | |||
| Body mass index (kg/m2) | 28.59 ± 4.64 (68) | 28.86 ± 5.56 (164) | 0.7012 |
| Systolic BP (mmHg) | 116.5 ± 16.6 (68) | 116.5 ± 17.0 (161) | 0.9941 |
| Diastolic BP (mmHg) | 71.0 ± 11.3 (68) | 67.8 ± 10.9 (161) | 0.0532 |
| Heart rate (b.p.m.) | 72.8 ± 12.5 (68) | 70.6 ± 11.8 (164) | 0.2102 |
| NT‐proBNP (pg/mL) | 4157.5 ± 7366.1 (53) | 5473.5 ± 8933.9 (123) | 0.3109 |
| Implant catheter haemodynamics | |||
| PA systolic pressure (mmHg) | 44.5 ± 18.2 (68) | 48.2 ± 16.4 (163) | 0.1539 |
| PA diastolic pressure (mmHg) | 18.7 ± 8.2 (68) | 19.7 ± 8.1 (163) | 0.3782 |
| PA mean pressure (mmHg) | 28.6 ± 11.4 (68) | 31.1 ± 10.5 (163) | 0.127 |
| PA wedge pressure (mmHg) | 18.7 ± 9.2 (67) | 20.1 ± 9.5 (161) | 0.2751 |
| Cardiac output (L/min) | 3.91 ± 0.97 (68) | 4.29 ± 1.29 (163) | 0.0153 |
| Cardiac index (L/min/m2) | 1.89 ± 0.44 (68) | 2.11 ± 0.58 (163) | 0.0015 |
| Pulmonary vascular resistance (mmHg·min/L) | 2.72 ± 1.89 (67) | 2.90 ± 2.32 (161) | 0.537 |
| Medical history | |||
| Primary aetiology of cardiomyopathy | |||
| Ischaemic cardiomyopathy | 57.4% (39/68) | 51.8% (85/164) | 0.4722 |
| Non‐ischaemic cardiomyopathy | 36.8% (25/68) | 36.0% (59/164) | 1 |
| Not determined | 0.0% (0/68) | 8.5% (14/164) | 0.0121 |
| Unknown | 5.9% (4/68) | 3.7% (6/164) | 0.4844 |
| Atrial tachycardia, flutter/fibrillation | 55.9% (38/68) | 64.0% (105/164) | 0.2992 |
| Ventricular arrhythmia | 30.9% (21/68) | 19.5% (32/164) | 0.0849 |
| Diabetes mellitus | 42.6% (29/68) | 47.6% (78/164) | 0.5634 |
| History of renal failure | 42.6% (29/68) | 64.6% (106/164) | 0.0033 |
| Renal failure requiring dialysis | 0.0% (0/68) | 1.2% (2/164) | 1 |
| Cerebrovascular accident | 7.4% (5/68) | 15.2% (25/164) | 0.1327 |
| Chronic obstructive pulmonary disease | 16.2% (11/68) | 22.6% (37/164) | 0.3732 |
| Hyperlipidaemia | 58.8% (40/68) | 60.4% (99/164) | 0.8833 |
| Myocardial infarction | 42.9% (27/63) | 33.3% (51/153) | 0.2134 |
| Pulmonary oedema | 20.6% (14/68) | 15.2% (25/164) | 0.3385 |
| Pulmonary embolus | 4.5% (3/67) | 1.8% (3/163) | 0.3608 |
| CRT, CRT‐P, or CRT‐D | 23.5% (16/68) | 28.0% (46/164) | 0.5183 |
| ICD | 58.8% (40/68) | 30.5% (50/164) | 0.0001 |
| HF medical history | |||
| Ejection fraction (%) | 25.4 ± 9.4 (67) | 36.2 ± 15.9 (162) | <0.0001 |
| NYHA Class I | 0.0% (0/68) | 0.0% (0/164) | 1 |
| NYHA Class II | 0.0% (0/68) | 0.0% (0/164) | 1 |
| NYHA Class III | 100.0% (68/68) | 99.4% (163/164) | 1 |
| NYHA Class IV | 0.0% (0/68) | 0.6% (1/164) | 1 |
| Days since last HFH prior to implant | 68.4 ± 82.6 (67) | 71.1 ± 88.7 (160) | 0.8254 |
| HFH within 6 months before implant | 89.6% (60/67) | 88.1% (141/160) | 0.8237 |
| HFH within 3 months before implant | 74.6% (50/67) | 75.6% (121/160) | 0.8674 |
| History of HF medications | |||
| ACEi/ARB/ARNi | 94.1% (64/68) | 81.7% (134/164) | 0.0143 |
| Beta‐blocker | 94.1% (64/68) | 86.6% (142/164) | 0.1133 |
| Mineralocorticoid antagonist | 91.2% (62/68) | 64.0% (105/164) | <0.0001 |
| Diuretic | 97.1% (66/68) | 96.3% (158/164) | 1 |
| Baseline patient‐reported outcomes | |||
| EQ‐5D‐5L VAS | 55.2 ± 22.4 (68) | 54.0 ± 20.1 (157) | 0.7004 |
| KCCQ Overall Summary Score | 49.88 ± 24.31 (68) | 45.58 ± 23.97 (157) | 0.2237 |
| KCCQ Clinical Summary Score | 54.52 ± 24.72 (68) | 49.61 ± 24.77 (157) | 0.1737 |
| PHQ‐9 Summary Score | 8.4 ± 5.9 (67) | 8.8 ± 6.0 (156) | 0.5932 |
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor‐neprilysin inhibitor; BP, blood pressure; CRT, cardiac resynchronization therapy; CRT‐D, cardiac resynchronization therapy‐defibrillator; CRT‐P, cardiac resynchronization therapy‐pacemaker; HF, heart failure; HFH, heart failure hospitalization; ICD, implantable cardioverter defibrillator; KCCQ, Kansas City Cardiomyopathy Questionnaire; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; PA, pulmonary artery; PHQ‐9, Patient Health Questionnaire‐9.
P‐value from two‐group t‐test with unequal variances for continuous variables and Fisher's exact test for categorical variables.
Results
Between May 2016 and March 2018, 239 patients were enrolled with 232 patients entering this analysis. Seven patients with incomplete medication data or no data on loop diuretic or thiazide therapies were excluded from this analysis. Patients were separated into those on sacubitril/valsartan (N = 68) or those without sacubitril/valsartan (N = 164). In 11 of 68 patients, sacubitril/valsartan was started during the trial, but no patient stopped the drug during follow‐up. Table 2 summarizes the baseline criteria. Patients on sacubitril/valsartan had similar pulmonary wedge pressures, slightly lower cardiac output and cardiac index, had a lower ejection fraction, and were more likely to be treated with mineralocorticoid antagonists. Average mean PA pressures were similar with and without sacubitril/valsartan (28.6 ± 11.4 and 31.1 ± 10.5 mmHg, respectively, P = 0.127). Patient‐reported outcomes at baseline were also similar (Table 2 ).
Diuretic utilization by use of sacubitril/valsartan
Figure 1 summarizes the time course from implantation to 9 months of follow‐up of current diuretic dose by sacubitril/valsartan users for loop diuretics (A) and thiazide diuretics (B). In general, at baseline and over time, the utilization of loop diuretics was lower when patients were on sacubitril/valsartan compared with those without sacubitril/valsartan. There was a statistical difference at 6 of 7 time points [Figure 1 (A) ]. The generalized linear mixed model comparing actual diuretic loop dose up to 12 months revealed a statistical significance between the groups (P = 0.01). The covariate representing a significant predictor in the model was history of renal failure (P = 0.005) but not age (P = 0.091). Figure 1 (B) shows the same for non‐loop diuretics, where the treatment was not statistically different (P = 0.21). Loop diuretic doses, but not thiazide doses, were lower in sacubitril/valsartan users at baseline and 6 months (Supporting Information, Figure S1 ). Next, we studied loop diuretic dose changes comparing sacubitril/valsartan users with non‐sacubitril/valsartan users (Figure 2 ). The change was numerically different but yielded no significant difference in the multivariable statistical models using full adjustment (P = 0.15).
Figure 1.
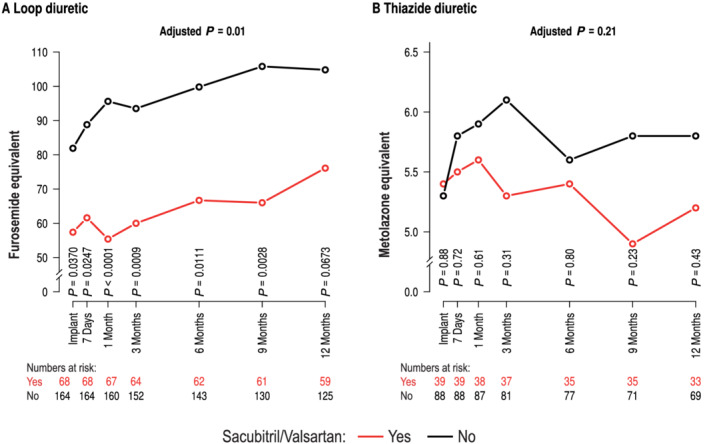
Use of loop diuretic (A) and use of thiazide diuretic (B) over time in patients on sacubitril/valsartan (red) or no sacubitril/valsartan (black). P‐values denote comparison between individual time points and P‐values for a generalized linear model to compare diuretic utilization over time.
Figure 2.
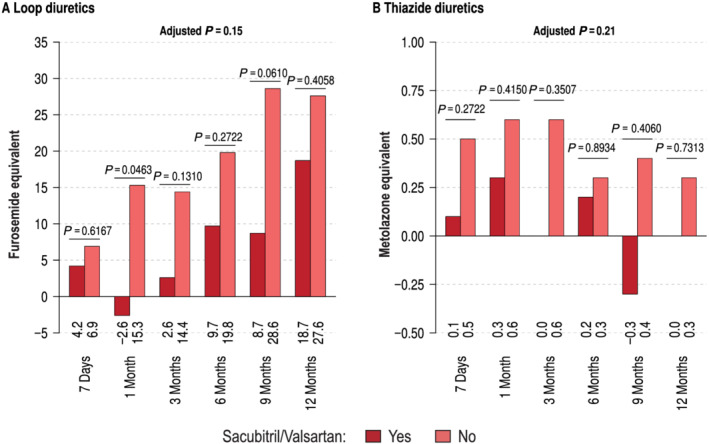
Change from baseline diuretic doses over time for loop diuretics (A) and thiazide diuretics (B) in patients with (dark red) and without (light red) sacubitril/valsartan.
Effect of diuretic up‐titration
With increasing average daily change of PAP from baseline, the odds of being up‐titrated with a diuretic was similar between patients on sacubitril/valsartan [odds ratio (OR) = 1.084, confidence interval (CI) = 0.990–1.187, P = 0.083] and no sacubitril/valsartan (OR = 1.046, CI = 0.994–1.101, P = 0.081). By adjusting for baseline PAP, there was an increase of loop diuretic utilization in non‐users of sacubitril/valsartan (OR = 1.062, CI = 1.003–1.125, P = 0.04). Similar results were obtained for the multivariable regression analysis adjusted for baseline pressures. After changes in loop diuretics, the 7 day average mean PAP achieved was nominally, however, not significantly lower in patients on sacubitril/valsartan compared with no sacubitril/valsartan (Table 3 ). Nominally, but not significantly more patients on sacubitril/valsartan responded with a PAP drop compared with non‐users (Table 4 ), but there were no differences in outcomes (Table 5 ).
Table 3.
Add. mean pulmonary arterial pressure (7 day average) (as treated population stratified by sacubitril/valsartan)
| Mean pulmonary arterial pressure (mmHg) | Sacubitril/valsartan | P‐value a | Full cohort (N = 232) | |
|---|---|---|---|---|
| Yes (N = 68) | No (N = 164) | |||
| Baseline | 0.52 | |||
| Mean ± SD (n) | 35.7 ± 11.9 (67) | 36.8 ± 11.2 (158) | 36.5 ± 11.4 (225) | |
| Median (min, max) | 36.4 (15.0, 71.6) | 35.5 (14.3, 74.0) | 36.0 (14.3, 74.0) | |
| 6 months | 0.07 | |||
| Mean ± SD (n) | 28.7 ± 11.6 (56) | 32.1 ± 11.1 (134) | 31.1 ± 11.3 (190) | |
| Median (min, max) | 26.2 (6.7, 54.6) | 31.2 (12.1, 62.2) | 29.3 (6.7, 62.2) | |
| 12 months | 0.53 | |||
| Mean ± SD (n) | 28.7 ± 11.0 (54) | 29.8 ± 11.0 (116) | 29.5 ± 11.0 (170) | |
| Median (min, max) | 27.4 (7.0, 62.3) | 28.2 (6.4, 61.5) | 28.0 (6.4, 62.3) | |
SD, standard deviation.
Two‐sample t‐test.
Table 4.
Add. mean pulmonary arterial pressure after diuretic change (as treated population stratified by sacubitril/valsartan)
| Average daily change of PAP mean (within 30 days of medication change) | Down‐titrated | Up‐titrated | P‐value a | ||
|---|---|---|---|---|---|
| Sacubitril/valsartan | Sacubitril/valsartan | ||||
| Yes (N = 11) | No (N = 35) | Yes (N = 34) | No (N = 69) | ||
| Decrease | 9 (81.82%) | 26 (74.29%) | 22 (64.71%) | 41 (59.42%) | 0.82 |
| Increase | 2 (18.18%) | 9 (25.71%) | 12 (35.29%) | 28 (40.58%) | |
PAP, pulmonary artery pressure.
Breslow–Day's test for homogeneity of the odds ratios.
Table 5.
Add. heart failure‐related hospitalization (as treated population stratified by sacubitril/valsartan)
| Event | Sacubitril/valsartan | Relative risk (95% CI) | Relative rate (95% CI) | |||
|---|---|---|---|---|---|---|
| Yes (N = 68) | No (N = 164) | |||||
| # Patient (%) | # Events (EPPY) | # Patient (%) | # Events (EPPY) | |||
| Heart failure hospitalizations | 27 (39.7%) | 37 (0.55) | 64 (39.0%) | 100 (0.66) | 1.0 (0.8, 1.2) | 0.8 (0.6, 1.2) |
CI, confidence interval; EPPY, events per patient year.
Patient‐reported outcomes
There was no significant difference in the Kansas City Cardiomyopathy Questionnaire (KCCQ) Overall Summary Score (OSS) or Clinical Summary Score (CSS) between sacubitril/valsartan users and non‐users at baseline or 6 months. However, both the KCCQ OSS and CSS showed significant improvements from baseline to 6 months (P < 0.0001 for both OSS and CSS) and 12 months (P < 0.0001 OSS, P = 0.0004 CSS) for sacubitril/valsartan users and non‐users (P < 0.0001 for both OSS and CSS at 6 and 12 months). Furthermore, we evaluated in patients with or without sacubitril/valsartan who had at least a 5‐point increase in KCCQ over time. The proportion of patients with a 5‐point improvement in KCCQ was not statistically different between the groups (55.7% vs. 50.4% in users and non‐users, P = 0.53).
Discussion
We found that sacubitril/valsartan users compared with non‐users utilized less loop diuretics at baseline and over time, when patients with symptomatic heart failure NYHA Class III underwent diuretic‐based PAP‐guided therapy using the CardioMEMS™ HF system to monitor PAP. This was the case despite similar baseline characteristics concerning drug treatment and comparable quality of life at baseline and at follow‐up and with slightly lower ejection fractions and lower cardiac indices in patients on sacubitril/valsartan.
The use of loop diuretics, in particular at high doses, has been associated with cardiovascular outcomes 15 including arrhythmic death and progressive heart failure. 16 , 17 Mechanisms involved might be an increased secretion of renin due to diuretic actions on sodium‐potassium‐2 cotransporter 23 leading to neurocrine activation such as renin release potentially linked to poor outcomes. 15 , 16 , 17 , 18 , 24 The angiotensin receptor blocker/neprilysin inhibitor sacubitril/valsartan has been shown to reduce cardiovascular death and heart failure hospitalization in the PARADIGM trial. 25 In addition, sacubitril/valsartan reduced loop diuretic utilization in this trial 12 and also in real‐world populations of outpatients treated by family doctors and cardiologists. 19 The latter two studies neither captured PAP nor did they control for quality of life measures. This study extends those findings by showing that in a population of sacubitril/valsartan users compared with non‐users, loop diuretic doses at baseline and over time at follow‐up are approximately 30–40% lower, when PAP‐guided treatment is performed. PAP tended to be lower after diuretic treatment showing that the lower loop diuretic utilization was not due to diuretic under‐treatment in the sacubitril/valsartan group. Guided by PAP values individualized for every patient, 20 the majority of treatment changes were adjustments of diuretic doses in the MEMS‐HF study 9 as in previous studies showing an improved outcome compared with a control group on standard care. 7 , 8 As use of diuretics and reasons for changing diuretic dose were not captured in previous studies on the diuretic saving effects of sacubitril/valsartan, 12 , 19 MEMS‐HF is first to provide an objective orientation of diuretic treatment based on the estimation and close monitoring of mean PAP. 7 , 8 , 9
Neprilysin degrades vasoactive peptides, which might have an important consequence for renal haemodynamics in diabetes and heart failure 26 attenuating vasodilatory effects of circulating natriuretic peptides by impairing generation of cyclic guanosine‐monophosphate, in turn reducing perfusion of the renal parenchyma. 26 , 27 As sacubitril/valsartan compared with enalapril further increases natriuretic peptides such as BNP and presumably also ANP and CNP, 28 a rapid response of kidney perfusion might be one of the mechanisms of the loop diuretic sparing effects of sacubitril/valsartan. Neprilysin inhibition in general exerts beneficial effects on the kidney also when combined with an ACE‐inhibiting moiety integrated in the drug omapatrilate. 29 Taken together, the combination of an improved dilation of the vas afferents as well as the natriuretic effect of augmented natriuretic peptides might be the reason for the diuretic sparing effect of sacubitril/valsartan. 27 , 28 , 29 Direct effects of sacubitril/valsartan on filling pressures as shown within a small patient group with a CardioMEMS™ HF system in place could have contributed to saving diuretics. 30
Strengths and limitations
If the unproven hypothesis is true that diuretics play a causal role in neuroendocrine activation and poor outcome, 15 , 16 , 17 , 18 then the diuretic sparing effect of sacubitril/valsartan could contribute to its beneficial effects on outcomes. Herein, we could not demonstrate fewer endpoints on sacubitril/valsartan. However, the study and this exploratory analysis were not aimed or powered to demonstrate outcome benefits of sacubitril/valsartan. Data in patients with an adjustment of PAP pressures by the CardioMEMS™ HF system might allow detailed analyses of drugs affecting volume homeostasis. These data have to be interpreted with their strengths and limitations. This is a secondary analysis of an open‐label study evaluating the CardioMEMS™ HF system. There was no randomized comparison between users or non‐users of sacubitril/valsartan creating a potential for residual confounding. We did not capture the ejection fraction immediately prior to sacubitril/valsartan treatment and have limited data on the initiation and duration of this therapy if the therapy was initiated before the patient was enrolled in this study. Patients on sacubitril/valsartan were on average 6 years younger and had less prevalence of renal failure. However, medical treatment and health status as assessed by the KCCQ at baseline and follow‐up, which strongly depends on volume status, were similar between the two groups. Furthermore, a lower ejection fraction and lower cardiac output in sacubitril/valsartan users indicate that the difference in utilization of loop diuretics could have been underestimated rather than overestimated. A contribution to improvement of ejection fraction and/or cardiac output by sacubitril/valsartan is possible, but has not been determined in this study.
Conclusions
In summary, the CardioMEMS™ HF system has been shown to allow monitoring of PAP leading to a reduction of hospitalization rate by enabling fluid homeostasis mainly by dose adjustments of diuretics. Patients with these devices can also be studied according to other drugs, which are affecting volume homeostasis. Patients on optimal treatment with sacubitril/valsartan use less loop diuretics despite similar quality of life changes and accompanying drug treatments.
Conflict of interest
M.B. reports personal fees from Abbott, Amgen, Astra‐Zeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cytokinetics, Medtronic, Novartis, Servier, and Vifor. B.A. reports honoraria for Bayer and Novartis, and speaker fees from Abbott, Alnylam, Astra‐Zeneca, Bayer, Boehringer Ingelheim, Novartis, Pfizer, and Vifor; served on the MEMS‐HF steering committee. F.W.A. has no conflicts of interest to report. J.B. reports honoraria for consultancy and speakers fees and scientific support by Abbott, Medtronic, and Biotronik and served on the MEMS‐HF steering committee. M.E.B. is an employee and shareholder of Abbott. J.J.B. was on the steering committee of the Abbott‐sponsored MONITOR‐HF study. G.E. reports personal fees from Astra‐Zeneca, Abbott, Boehringer Ingelheim, Novartis, and Vifor, all outside the submitted work. He further acknowledges non‐financial support from the University Hospital Würzburg, non‐financial support from Comprehensive Heart Failure Center Würzburg, and grant support from German Ministry for Education and Research (BMBF). L.H. has no conflicts of interest to report. A.W. is an employee and shareholder of Abbott. F.K. reports research funding by the German Federal Ministry of Economics and Technology, the European Commission, and the German Federal Ministry of Education and Research and served on the MEMS‐HF steering committee. S.R. reports honoraria for consultancy, speaker fees, and scientific support by Abbott and has received remuneration for lectures and/or consultancy from Actelion, Bayer, BMS, MSD, Novartis, Pfizer, Vifor, and United Therapeutics, and his institution has received research grants from Actelion, Bayer, Novartis, and United Therapeutics and served on the MEMS‐HF steering committee. S.D.A. reports receiving fees from Abbott, Bayer, Boehringer Ingelheim, Cardiac Dimension, Cordio, Impulse Dynamics, Novartis, Occlutech, Servier, and Vifor Pharma and grant support from Abbott and Vifor Pharma. D.M.L. reports lecture fees and research grants from Abbott, Novartis, Vifor Pharma, Boehringer, Astra‐Zeneca, and Bayer. A.A. has no conflicts of interest to report. J.W. has received speaker honoraria from Bristol‐Myers Squibb. Q.Z. reports grants from Boehringer Ingelheim, personal fees from Astra‐Zeneca, grants and personal fees from Abbott, and personal fees from Novartis outside the submitted work. P.B.A. is an employee and stockholder of Abbott. C.E.A. reports grant support, personal fees, and/or non‐financial support from Abbott, Astra‐Zeneca, Boehringer Ingelheim, Medtronic, Novartis, ResMed, Thermo Fisher, and Vifor, all outside of the submitted work. She further acknowledges non‐financial support from the University Hospital Würzburg, non‐financial support from Comprehensive Heart Failure Center Würzburg, and grant support from German Ministry for Education and Research (BMBF).
Funding
Prof. Böhm is supported by the Deutsche Forschungsgemeinschaft (DFG, TRR 219, S‐01, Project ID 322900939). The CardioMEMS European Monitoring Study for Heart Failure (MEMS‐HF) was supported by Abbott.
Open Access funding enabled and organized by Projekt DEAL.
Supporting information
Figure S1. Diuretic doses in furosemide equivalents for loop diuretics (A) and thiazide diuretics (B) in patients with (dark red) and without (light red) sacubitril/valsartan use.
Böhm, M. , Assmus, B. , Anker, S. D. , Asselbergs, F. W. , Brachmann, J. , Brett, M.‐E. , Brugts, J. J. , Ertl, G. , Wang, A. , Hilker, L. , Koehler, F. , Rosenkranz, S. , Leistner, D. M. , Abdin, A. , Wintrich, J. , Zhou, Q. , Adamson, P. B. , and Angermann, C. E. (2022) Less loop diuretic use in patients on sacubitril/valsartan undergoing remote pulmonary artery pressure monitoring. ESC Heart Failure, 9: 155–163. 10.1002/ehf2.13665.
Clinical trial registration: http://clinicaltrials.gov.unique identifier: NCT02693691.
References
- 1. Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Pieper K, Sun JL, Yancy CW, Young JB, OPTIMIZE‐HF investigators and hospitals . Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE‐HF. Arch Intern Med 2008; 168: 847–854. [DOI] [PubMed] [Google Scholar]
- 2. Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J 2007; 154: 260–266. [DOI] [PubMed] [Google Scholar]
- 3. Okumura N, Jhund PS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Swedberg K, Zile MR, Solomon SD, Packer M, McMurray JJ, PARADIGM‐HF investigators and Committees* . Importance of clinical worsening of heart failure treated in the outpatient setting: evidence from the prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM‐HF). Circulation 2016; 133: 2254–2262. [DOI] [PubMed] [Google Scholar]
- 4. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force MembersDocument Reviewers . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 5. Stevenson LW, Zile M, Bennett TD, Kueffer FJ, Jessup ML, Adamson P, Abraham WT, Manda V, Bourge RC. Chronic ambulatory intracardiac pressures and future heart failure events. Circ Heart Fail 2010; 3: 580–587. [DOI] [PubMed] [Google Scholar]
- 6. Zile MR, Bennett TD, El Hajj S, Kueffer FJ, Baicu CF, Abraham WT, Bourge RC, Warner SL. Intracardiac pressures measured using an implantable hemodynamic monitor: relationship to mortality in patients with chronic heart failure. Circ Heart Fail 2017; 10: e003594. [DOI] [PubMed] [Google Scholar]
- 7. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS, CHAMPION trial study group . Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011; 377: 658–666. [DOI] [PubMed] [Google Scholar]
- 8. Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB, CHAMPION trial study group . Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow‐up results from the CHAMPION randomised trial. Lancet 2016; 387: 453–461. [DOI] [PubMed] [Google Scholar]
- 9. Angermann CE, Assmus B, Anker SD, Asselbergs FW, Brachmann J, Brett ME, Brugts JJ, Ertl G, Ginn G, Hilker L, Koehler F, Rosenkranz S, Zhou Q, Adamson PB, Böhm M, MEMS‐HF Investigators . Pulmonary artery pressure‐guided therapy in ambulatory patients with symptomatic heart failure: the CardioMEMS european monitoring study for heart failure (MEMS‐HF). Eur J Heart Fail 2020; 22: 1891–1901. [DOI] [PubMed] [Google Scholar]
- 10. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF investigators and committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 11. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM, DAPA‐HF trial committees and investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 12. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F, EMPEROR‐reduced trial investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 383: 1413–1424. [DOI] [PubMed] [Google Scholar]
- 13. Vardeny O, Claggett B, Kachadourian J, Desai AS, Packer M, Rouleau J, Zile MR, Swedberg K, Lefkowitz M, Shi V, McMurray JJV, Solomon SD. Reduced loop diuretic use in patients taking sacubitril/valsartan compared with enalapril: the PARADIGM‐HF trial. Eur J Heart Fail 2019; 21: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wachter R, Fonseca AF, Balas B, Kap E, Engelhard J, Schlienger R, Klebs S, Wirta SB, Kostev K. Real‐world treatment patterns of sacubitril/valsartan: a longitudinal cohort study in Germany. Eur J Heart Fail 2019; 21: 588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jackson AM, Dewan P, Anand IS, Bělohlávek J, Bengtsson O, de Boer RA, Böhm M, Boulton DW, Chopra VK, DeMets DL, Docherty KF, Dukát A, Greasley PJ, Howlett JG, Inzucchi SE, Katova T, Køber L, Kosiborod MN, Langkilde AM, Lindholm D, Ljungman CEA, Martinez FA, O'Meara E, Sabatine MS, Sjöstrand M, Solomon SD, Tereshchenko S, Verma S, Jhund PS, McMurray JJV. Dapagliflozin and diuretic use in patients with heart failure and reduced ejection fraction in DAPA‐HF. Circulation 2020; 142: 1040–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Francis GS, Siegel RM, Goldsmith SR, Olivari MT, Levine TB, Cohn JN. Acute vasoconstrictor response to intravenous furosemide in patients with chronic congestive heart failure. activation of the neurohumoral axis. Ann Intern Med 1985; 103: 1–6. [DOI] [PubMed] [Google Scholar]
- 17. Damman K, Kjekshus J, Wikstrand J, Cleland JG, Komajda M, Wedel H, Waagstein F, McMurray JJ. Loop diuretics, renal function and clinical outcome in patients with heart failure and reduced ejection fraction. Eur J Heart Fail 2016; 18: 328–336. [DOI] [PubMed] [Google Scholar]
- 18. Cooper HA, Dries DL, Davis CE, Shen YL, Domanski MJ. Diuretics and risk of arrhythmic death in patients with left ventricular dysfunction. Circulation 1999; 100: 1311–1315. [DOI] [PubMed] [Google Scholar]
- 19. Domanski M, Norman J, Pitt B, Haigney M, Hanlon S, Peyster E. Diuretic use, progressive heart failure, and death in patients in the studies of left ventricular dysfunction (SOLVD). J Am Coll Cardiol 2003; 42: 705–708. [DOI] [PubMed] [Google Scholar]
- 20. Domanski M, Tian X, Haigney M, Pitt B. Diuretic use, progressive heart failure, and death in patients in the DIG study. J Card Fail 2006; 12: 327–332. [DOI] [PubMed] [Google Scholar]
- 21. Angermann CE, Assmus B, Anker SD, Brachmann J, Ertl G, Köhler F, Rosenkranz S, Tschöpe C, Adamson PB, Böhm M. Safety and feasibility of pulmonary artery pressure‐guided heart failure therapy: rationale and design of the prospective CardioMEMS monitoring study for heart failure (MEMS‐HF). Clin Res Cardiol 2018; 107: 991–1002. [DOI] [PubMed] [Google Scholar]
- 22. Heywood JT, Jermyn R, Shavelle D, Abraham WT, Bhimaraj A, Bhatt K, Sheikh F, Eichorn E, Lamba S, Bharmi R, Agarwal R, Kumar C, Stevenson LW. Impact of practice‐based management of pulmonary artery pressures in 2000 patients implanted with the CardioMEMS sensor. Circulation 2017; 135: 1509–1517. [DOI] [PubMed] [Google Scholar]
- 23. Desai AS, Bhimaraj A, Bharmi R, Jermyn R, Bhatt K, Shavelle D, Redfield MM, Hull R, Pelzel J, Davis K, Dalal N, Adamson PB, Heywood JT. Ambulatory hemodynamic monitoring reduces heart failure hospitalizations in "real‐world" clinical practice. J Am Coll Cardiol 2017; 69: 2357–2365. [DOI] [PubMed] [Google Scholar]
- 24. Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 2009; 54: 1747–1762. [DOI] [PubMed] [Google Scholar]
- 25. Masson S, Solomon S, Angelici L, Latini R, Anand IS, Prescott M, Maggioni AP, Tognoni G, Cohn JN, Val‐Heft Investigators . Elevated plasma renin activity predicts adverse outcome in chronic heart failure, independently of pharmacologic therapy: data from the valsartan heart failure trial (Val‐HeFT). J Card Fail 2010; 16: 964–970. [DOI] [PubMed] [Google Scholar]
- 26. Sakamoto K, Kikkawa R, Haneda M, Shigeta Y. Prevention of glomerular hyperfiltration in rats with streptozotocin‐induced diabetes by an atrial natriuretic peptide receptor antagonist. Diabetologia 1995; 38: 536–542. [DOI] [PubMed] [Google Scholar]
- 27. Knecht M, Pagel I, Langenickel T, Philipp S, Scheuermann‐Freestone M, Willnow T, Bruemmer D, Graf K, Dietz R, Willenbrock R. Increased expression of renal neutral endopeptidase in severe heart failure. Life Sci 2002; 71: 2701–2712. [DOI] [PubMed] [Google Scholar]
- 28. Zile MR, Claggett BL, Prescott MF, McMurray JJ, Packer M, Rouleau JL, Swedberg K, Desai AS, Gong J, Shi VC, Solomon SD. Prognostic implications of changes in N‐terminal pro‐B‐type natriuretic peptide in patients with heart failure. J Am Coll Cardiol 2016; 68: 2425–2436. [DOI] [PubMed] [Google Scholar]
- 29. Packer M, Califf RM, Konstam MA, Krum H, McMurray JJ, Rouleau JL, Swedberg K. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the omapatrilat versus enalapril randomized trial of utility in reducing events (OVERTURE). Circulation 2002; 106: 920–926. [DOI] [PubMed] [Google Scholar]
- 30. Khan Z, Gholkar G, Tolia S, Kado H, Zughaib M. Effect of sacubitril/valsartan on cardiac filling pressures in patients with left ventricular systolic dysfunction. Int J Cardiol 2018; 271: 169–173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Diuretic doses in furosemide equivalents for loop diuretics (A) and thiazide diuretics (B) in patients with (dark red) and without (light red) sacubitril/valsartan use.


