Abstract
The assessment of both thromboembolic and haemorrhagic risks and their management in systemic amyloidosis have been poorly emphasized so far. This narrative review summarizes main evidence from literature with clinical perspective.
The rate of thromboembolic events is as high as 5–10% amyloidosis patients, at least in patients with cardiac involvement, with deleterious impact on prognosis. The most known pro‐thrombotic factors are heart failure, atrial fibrillation, and atrial myopathy. Atrial fibrillation could occur in 20% to 75% of systemic amyloidosis patients. Cardiac thrombi are frequently observed in patients, particularly in immunoglobulin light chains (AL) amyloidosis, up to 30%, and it is advised to look for them systematically before cardioversion. In AL amyloidosis, nephrotic syndrome and the use of immunomodulatory drugs also favour thrombosis. On the other hand, the bleeding risk increases because of frequent amyloid digestive involvement as well as factor X deficiency, renal failure, and increased risk of dysautonomia‐related fall.
Keywords: Amyloidosis, Thromboembolism, Bleeding, Anticoagulative therapy
Introduction
Systemic amyloidosis is due to amyloid fibril deposits that involve numerous organs. More than 30 proteins are currently considered to be ‘amyloidogenic’, but the two most common forms of amyloidosis are variant (vATTR) or wild‐type transthyretin (wtATTR) and immunoglobulin light‐chain (AL). 1 , 2 The incidence of AL amyloidosis is about 12 cases per million persons per year. 3 TTR amyloidosis is more frequent (prevalence: 13% of heart failure with preserved ejection fraction (HFpeF), 4 5% of all hypertrophic cardiomyopathies). 5 Amyloidosis can involve numerous organs, such as kidney, nerves, heart, digestive tract, liver, bone or joints, each of these leading to specific complications. Among amyloidosis‐related complications are thrombotic and bleeding events 6 , 11 whose prediction and management is particularly challenging. So far, there is no specific guideline about the use of antithrombotic treatment in amyloidosis. The objectives of this narrative review are (i) to specify current epidemiological data on thrombotic and bleeding risks, (ii) to analyse main determinants and predictors of these two risks, and (iii) to review current evidence about antithrombotic treatment in amyloidosis.
Frequency and severity of thromboembolic events in amyloidosis
For 20 years, thrombotic events (TEs) have been described in amyloidosis and more particularly in patients with cardiac amyloidosis (CA). 7 , 8 , 9 , 10 , 11 Indeed, cardiac involvement is particularly frequent (60–70%). TTR CA is estimated to be as high as 13% of patients with HFpEF. 4 Cappelli et al. 12 published the largest cohort including 406 patients with CA (134 AL, 73 vATTR, and 199 wtATTR) with a median follow‐up of 19 months. The incidence of arterial TE was 7.6% (Table 1 ). Most events (29 out of 31) were cerebrovascular events (21 ischaemic strokes and 8 transient ischaemic attacks). TEs were the first disease manifestations in 10 patients. Eleven per cent of patients with CA and atrial fibrillation (AF) had a TE. The incidence rate of TE in patients with CA and AF was 3.2 per 100 patients per year. The only predictor of TE in Cox analysis was CHADS2‐VAsc score ≥3 [hazard ratio 2.84, 95% confidence interval (CI) 1.02–7.92, P = 0.05] especially in patients with sinus rhythm. The rate of events in sinus rhythm without history of AF was 32% (10 events).
Table 1.
Prevalence of thromboembolic events in systemic amyloidosis
| Study, date | Population | Prevalence | Thromboembolic events details | Favouring factors |
|---|---|---|---|---|
| Capelli et al., 2020 12 |
N = 262 134 AL, 73 vTTR, 199 wtTTR |
7.6% Prevalence of clinical events |
21 ischaemic stroke 8 TIA 2 peripheral events: 1 mesenteric and 1 femoral embolism |
AF LVEF <50% CHADS2VASC > 2 Chronic kidney disease |
| Mitrani et al., 2020 21 | N = 290 TTR |
6% Prevalence of clinical events, all had AF |
9 stroke 8 TIA |
AF |
| Donnellan et al., 2020 22 |
N = 382 TTR 111wtTTR, 271 vTTR |
16% 20% with AF vs 9% without AF |
Cerebrovascular events |
Increased CHADS2VASC score No anticoagulation therapy |
| Feng et al., 2007 6 | N = 116 autopsies 55 AL, 55 wtTTR, 4 AA | 33% | Intracardiac thrombi by autopsy |
AL subtype AF |
| Feng et al., 2009 17 |
N = 156 80 AL, 73 TTR, 3 AA |
27% | Intracardiac thrombi by ultrasound |
AL subtype Low systolic pressure Low atrial emptying velocity Diastolic dysfunction |
| Martinez‐Naharro et al., 2019 18 |
N = 324, 166 TTR, 155 AL |
6.2% | Intracardiac thrombi by CMR |
Biventricular systolic dysfunction Atrial dilation Higher ECV AF AL subtype |
| Em Al et al., 2019 24 | N = 58 | 28% | Intracardiac thrombus (TEE) | AF |
AF, atrial fibrillation; AL, immunoglobulin light chain amyloidosis; CMR, cardiac magnetic resonance; TTR: transthyretin; wTTR, wild type transthyretin; VTTR: variant transthyretin; TEE, transesophagal echocardiography; TIA: transient ischaemic attack.
Other types of TE occur in amyloidosis and appear always associated with poor prognosis. In a Mayo Clinic's study 13 involving 40 AL amyloidosis patients with TE, the authors reported that (i) 29 patients had deep vein thrombosis (73%) and 11 had arterial thrombosis (28%); (ii) embolic event preceded the diagnosis of amyloidosis in 11 patients (28%); (iii) TE were associated with a poor prognosis with death occurring in 8 patients 1 month after the event and 18 deaths in the year following the TE.
Stroke as the first manifestation of cardiac amyloidosis
A retrospective Mayo Clinic study 14 carried out in 40 patients with systemic amyloidosis (37 AL and 3 ATTR) and stroke revealed that ischaemic stroke was the initial manifestation in 13 patients (33%). The median survival of patients in whom stroke was the first manifestation was 7 months. Stroke occurred 9.6 months before the diagnosis of amyloidosis. In most cases, the stroke was hemispherical (73%) and in a single vascular territory (68%). Amyloidosis, and particularly CA should therefore be considered in stroke patients, especially in the presence of features suggestive of transthoracic echocardiography (TTE) (left ventricular hypertrophy, dilated left atrium, restrictive filling pattern, pericardial effusion, aortic stenosis, 15 and elevated cardiac biomarkers). 16
Evidence for cardioembolic cause of thromboembolic events and mechanisms
If recurrent cerebral embolism was described in amyloidosis patients even in the absence of AF or symptomatic heart failure, it is admitted that most are cardioembolic events. Feng et al. published 6 a large study involving 116 autopsies of patients who had biopsy‐proven CA (55 AL, 55 wtTTR, 4 AA‐serum amyloid A protein and 2 vTTR). The prevalence of intracardiac thrombi in patients with CA was 33%. Patients with AL type CA were younger and had fewer supraventricular arrhythmias but had more frequent intracardiac thrombi than those with other types of CA (51% vs. 16%, P < 0.001). AF and AL subtype were associated with a very high risk of thromboembolism (odds ratio 55; 95% CI 8–1134). TEs were a significant cause of death in patients with CA. Indeed, 19/23 emboli were considered to be fatal (14/53: 26% in the AL CA subgroup vs 5/59: 8% in the other types of CA, P < 0.001). The same team studied the prevalence of intracardiac thrombi by transthoracic (TTE) and transoesophageal (TEE) echocardiography 17 in all CA patients who underwent cardiac ultrasound in the Mayo Clinic database (from 1999 to 2007). One hundred fifty‐six patients (80 AL, 73 ATTR, and 3 AA type) were studied; 58 thrombi were found in 42 patients (prevalence = 27%). The prevalence of intracardiac thrombi was higher in AL amyloidosis than ATTR or AA: 35% vs. 18%, P = 0.02. The prevalence of intracardiac thrombi assessed by cardiac magnetic resonance is 6.2% in a recent study of Martinez‐Naharro et al. including 324 amyloidosis patients (166 TTR and 155 AL). Favouring factors of TE were biventricular systolic dysfunction, atrial dilation, AF, higher extracellular volume, and AL subtype. 18
Comprehensive cardiac imaging should be considered in all patients with amyloidosis and TE events. Cardiac imaging could start with TTE and use of contrast agent in case of insufficient echogenicity. If there is no evidence of thrombus with this first examination, patients should be considered for TEE and/or computed tomography scan and/or cardiac magnetic resonance.
This high risk of cardiac thrombi and subsequent embolic events can be also related to supraventricular arrhythmia and also to specific involvement of atria by amyloidosis, that has been so‐called atria myopathy.
Supraventricular arrhythmia in amyloidosis
Systemic amyloidosis increases the occurrence of AF or atrial flutter through several mechanisms: amyloid infiltration, impairment of ventricular and atrial compliance and relaxation with subsequent increase in left ventricular filling pressures resulting in LA enlargement and remodelling.
In published cohorts, the reported rate of AF varies between 20% and 75% (Table 2 ). Sanchis et al. 19 reported AF prevalence as up to 44% in 238 patients with amyloid heart disease: 71% in wtATTR, 26% in AL and 19% in vATTR amyloidosis. Longhi et al. 20 reported AF in 15% of 262 patients with CA: 40% in wtTTR, 9% in AL, and 11% in vATTR. Mitrani et al. 21 reported the rate of AF as high as 74% of 290 patients with ATTR cardiomyopathy, higher in the wtATTR group than in the vTTR group (85% and 52%, respectively). In addition, Mitrani et al. reported a rate of 17 embolic events in 15 patients; all embolic events occurred in patients with AF. In a recent study including 382 patients with ATTR cardiomyopathy, Donellan et al. reported AF in 69% cases. 22 Older age, male gender, history of heart failure, and advanced cardiac involvement were strongly associated with the risk of AF. 20 , 22 , 23 AF was not associated with an increased mortality in most studies.
Table 2.
Prevalence of AF in AL and TTR amyloidosis in most recent studies
| Study, date | Population | Overall prevalence | Prevalence in TTR | Prevalence in AL |
|---|---|---|---|---|
| Longhi et al., 2015 20 | N = 262 | |||
| 123 AL, 94 hTTR, 45 wtTTR | 15% | 35% | 9% | |
| Mints et al., 2018 23 | N = 146 wtTTR | 70% | 70% | — |
| Sanchis et al., 2019 19 | N = 238, 115 AL, 97 wtTTR, 26 hTTR | 44% | 60% | 26% |
| Martinez‐Naharro et al., 2019 18 | N = 324, 166 TTR, 155 AL | 46% | 14% | |
| Mitrani et al., 2020 21 | N = 290 TTR | 75% | 75% | — |
| Donellan et al., 2020 22 | N = 265, 205 wt, 60 vTTR | 69% | 69% | — |
AL, immunoglobulin light chain amyloidosis; TTR, transthyretin; vTTR, variant transthyretin; wTTR: wild type transthyretin.
El‐Am et al. 24 studied AF‐related thromboembolic risk through the comparison of TEE data between a cohort of 58 patients with CA and 114 patients without amyloidosis. All patients had AF, and the TEE was planned before electrical cardioversion. After TEE, the electrical cardioversion was cancelled in 16/58 (28%) CA patients vs 8/114 (7%) non‐amyloid patients, P < 0.001. The main cause of cardioversion cancellation was intracardiac thrombus in 13/46 (28%) patients with CA. Among these 13 patients, 2 patients were in AF from less than 48 h, and 4 had had an INR in the therapeutic range for more than 3 weeks. This study suggests that AF in amyloid cardiomyopathy is associated with a higher risk of atrial thrombosis. The authors advised for systematic TEE before cardioversion in patients with amyloidosis. They also observed more complications from cardioversion in patients with CA: 6/42 (14%) vs. 2/106 (2%), P = 0.007 including 2 ventricular arrhythmias and 2 severe brady‐arrhythmias requiring permanent pacemakers.
Evidence of atrial amyloid myopathy
Thirty years ago, Dubrey et al. 25 reported three cases of patients with CA, sinus rhythm and atrial thrombi. These authors suggested that lack of atria mechanical function irrespective of sinus rhythm favoured intra‐atrial thrombi. Indeed, they observed a very low velocity of the A wave suggesting that atrial contraction could be impaired by amyloid tissue infiltration. Other evidence of left atrial dysfunction in patients with CA is reported below.
In a large pathological study, appendages were removed during cardiac surgery in 245 patients (median age 63 years old) and analysed by both Congo red staining and immunohistochemistry: amyloid deposits were found in up to 16% of patients. 26 Such atrial amyloidosis was independently associated with the occurrence of AF.
In their pathological studies, Feng et al. observed that predictors of intracardiac thrombi were severe diastolic dysfunction, low atrial emptying velocity in TEE, low systolic blood pressure, and the AL subtype. 10 , 17 In other words, these studies suggested that the increase in thromboembolic risk was due to amyloid infiltration leading to impairment of atrial contractility and blood stasis, and the strong endothelial cytotoxicity of amyloid light‐chains.
Several studies based on cardiac imaging highlighted the high rate and the role of atrial dysfunction in amyloidosis. Henein et al. showed that the LA strain rate during atrial contraction phase was the only predictor of the occurrence of AF on Holter‐ECG recordings, independently of atrial size, in 46 patients with ATTR amyloidosis. 29 Brand et al. showed that atrial mechanics assessed by strain were decreased in amyloidosis with a high accuracy for the diagnosis of CA in unexplained LV hypertrophy. 27 Mohty et al. showed that impaired atrial function, assessed by TTE strain 3D 28 and cardiac magnetic resonance imaging LA emptying function, 29 worsened the prognosis of patients with AL amyloidosis. Indeed, 2 year survival was significantly reduced in patients with an LA emptying fraction <16% and that was associated with the prognostic classification of the Mayo Clinic.
However, no study has shown the predictive role of atrial dysfunction per se in the occurrence of thromboembolic events so far.
Non‐cardiac factors associated with thromboembolic events in amyloidosis
Besides AF and CA, number of others causes or favouring factors can explain the high risk of TE in amyloidosis: nephrotic syndrome leading to urinary loss of natural anticoagulants factors (antithrombin, protein S) and increased synthesis of procoagulant factors (factor V, VII, fibrinogen), decreased mobility and chemotherapy. Hausfater et al. 30 studied the causes of TE in a small cohort of patients with AL amyloidosis where 9 of the 15 patients had a TE: 3 strokes, 2 transient ischaemic attacks, 1 peripheral arterial embolism, 1 iliac thrombosis, 1 mesenteric ischaemia, and 1 ocular ischaemia. All patients were in sinus rhythm and only one patient had an intracardiac thrombus. The factors associated with TEs were oestrogen contraception, thalidomide, and growth factors. The authors postulated that all the elements of Virchow's triad were implied, specifically the endothelial injury by endocardial amyloid deposits leading to parietal injury and valvular amyloid deposits, the stasis by restrictive cardiomyopathy characterized by slow diastolic filling and arrhythmias, and plasma hypercoagulability by a nephrotic syndrome, thrombocytosis related to hyposplenism, blood viscosity related to circulating monoclonal component. In the study of Haligan et al., 13 the main predictive factors of thrombosis were nephrotic syndrome, immobilization, heart failure, and tobacco use.
In AL amyloidosis, circulating levels of free light‐chains and of β2‐microglobulin were shown to be associated with TE risk. 31 Plasma D‐dimer level was also associated with a worse prognosis. 32 An impairment of the thrombin–antithrombin (AT) pathway has also been evidenced in AL patients, with lower AT activity than AT antigen associated with reduced binding capacity to heparin, thus leading to a hypercoagulable state. 33
The use of immunomodulatory drugs (IMIDs) also increases the risk of venous thromboembolism. Current recommendations advise the prescription of prophylactic antithrombotic treatment (vitamin K antagonist, low molecular weight heparin, or aspirin) in patients receiving IMID‐based chemotherapy. 34 , 35 An increased risk of thalidomide related TE has been shown in multiple myeloma and AL amyloidosis. 36 , 37
Amyloidosis and bleeding risk
The most frequently reported bleedings are ecchymosis and purpura; bleedings from the gastrointestinal tract and the renal tract are also common 38 , 39 , 40 , 41 (Table 3 ). Amyloid deposits can be found in digestive and mucous membranes. Perivascular amyloidosis deposits can lead to amyloid angiopathy that increases capillary fragility and impairs capillary vasomotion. Gastrointestinal bleeding is explained by amyloid deposits causing fragility of the vascular wall, muscle infiltration leading to increased vulnerability of the mucosa and/or intestinal ischaemia. 33 , 42 , 43
Table 3.
Prevalence of bleeding events in systemic amyloidosis
| Study, date | Population | Prevalence of bleeding | Bleeding description | Favouring factors |
|---|---|---|---|---|
| Yood et al., 1983 37 | 100 AL amyloidosis |
41/100 = 41% (3% cause of death) |
23% petechia and ecchymoses 18% gastrointestinal tract bleeding 8% after procedure 3% haematuria 2% haemoptysia |
|
| Mumford et al., 2000 43 | 337 AL amyloidosis | 28% |
18% cutaneous bleeding 5% gastrointestinal bleeding 1% post procedure |
Prolongation of thrombin time (32%) |
| Kumar et al., 2001 40 | 45 AL amyloidosis treated with blood stem cell | 20% |
7% of lower GI tract bleeding 9% of upper |
Multiorgan involvement haemodialysis |
| Choufani et al., 2001 45 | 368 AL amyloidosis | 5%, all with FX deficiency | Frequency and severity worse with the lowest levels of FX | FX deficiency < 50% |
| Mitrani et al., 2020 21 | 290 ATTR | 7% (all had anticoagulant therapy) | Labile INR |
AL, immunoglobulin light chain amyloidosis; GI, gastrointestinal; TTR, transthyretin; wTTR, wild type transthyretin; vTTR, variant transthyretin.
Bleeding complications can also occur after an invasive procedure, such as biopsy. Laboratory characteristics reflect the clinical diversity in cohort studies 44 , 45 , 46 , 47 (Table 4 ). Different clotting abnormalities have been described, as shown in Table 4 . In a study including 337 patients with AL‐amyloidosis, Mumford et al. 44 reported in 51% of patients at least one abnormality in the semi‐global clotting times, namely thrombin time (TT), prothrombin time (PT) or activated partial prothrombin time (aPTT), with substantial variation in the nature and magnitude of the defects. These results were confirmed in further studies. 45 , 46 , 47 TT prolongation was potentially related by Mumford et al. to abnormal glycosylated fibrinogen resulting in dysfibrinogenemia combined with severe hypoalbuminaemia, and subsequent abnormal fibrin polymerization, especially in patients with nephrotic syndrome, who represented 28% of patients in this cohort. 44
Table 4.
Main studies investigating coagulation abnormalities in patients with AL‐amyloidosis since 2000
| Reference | Study design | Number of patients/sex ratio (male/female) | Median age (years) | Coagulation abnormalities a and relationships with clinical features |
|---|---|---|---|---|
| Mumford AD et al., 2000 41 | Retrospective single‐centre cohort study | 337/0.54 | 61.2 |
‐TT prolongation (32% of patients) associated with hepatic amyloid deposits (P < 10−4), 24‐h proteinuria (P < 10−3), and hypoalbuminaemia (P < 10−5) ‐PT prolongation (24% of patients) associated with abnormal bleeding (P 0.0012) ‐aPTT prolongation (14% of patients) ‐FX:C deficiency (<70 IU/dL): 22/154 (14%), of whom 7 (5%) < 20 IU/dL ‐FX:Ag/FX:C 2.5 in patients with FX deficiency vs FX:Ag/FX:C 0.96 in patients without (P < 10−4) ‐Mild FVII:C deficiency in 2 patients (44 and 23 IU/dL) ‐Absence of FX inhibitor |
| Gamba G et al., 2000 42 | Prospective | 36/2.0 | NA |
‐TT prolongation (85% of patients) ‐PT prolongation (22% of patients) ‐aPTT prolongation (65% of patients) ‐ FX:C deficiency (< 65 IU/dL)(27% of patients) |
| Choufani EB et al., 2001 43 | Prospective clinical trial | 368/1.5 b | 58.0 b |
‐FX:C deficiency (< 50 IU/dL): 32/368 (8.7% a ) of whom 12 < 25 IU/dL (9 with bleeding complications) ‐Frequency and severity worse in the patients with the lowest levels of FX |
| Patel G et al., 2019 44 | Retrospective single‐centre cohort study | 104/0.54 | 63.4 | ‐FX:C deficiency (<50 IU/dL): 10/104 (9.6% a ) of whom 2 < 25 IU/dL |
Patients receiving vitamin K antagonist were excluded.
Sex ratio and median age of patients with FX deficiency; TT: thrombin time.
aPTT: activated partial prothrombin time; Ag, antigen; F, factor; FX:C, FX clotting activity; NA, non‐available; PT, prothrombin time.
The PT and aPTT prolongation were associated with acquired FX:C deficiency, more rarely FV deficiency. Overall, FX levels are reduced to below 20 to 50 IU/dL in a relatively small proportion of patients (5% to 10%) with systemic amyloidosis. The frequency of FX deficiency in AL amyloidosis varies across studies. Greipp et al. 48 reported a frequency of FX below 20 IU/dL in 6 patients out of 95 patients (7.6%). Mumford et al. 44 reported a frequency of 14% (< 70 IU/dL), of whom 7 (5%) were 20 IU/dL. In a cohort of 368 patients with AL‐amyloidosis, 46 32 patients (7.8%) had an FX level below 50 IU/dL, of whom 18 (56%) had bleeding complications, mostly in those with an FX level below 25 IU/dL. Notably, aggressive chemotherapy combined with autologous haematopoietic cell transplantation improved the amyloid‐related FX deficiency. 46 More recently, Patel et al. reported an FX deficiency frequency of 9.6% (< 50 IU/dL), with only two patients (2%) with FX < 10 IU/dL. 47 Compared with patients with normal FX activity, patients with FX deficiency were more likely to have a worse disease stage (P = 0.05), elevated higher biomarkers (NT‐proBNP, P = 0.002; cardiac troponin T, cTNT, P = 0.03). Remarkably, all patients with the acquired FX deficiency had cardiac involvement, as compared with 70% in the other group (P = 0.06). 47 No bleeding occurred in the 10 patients with FX deficiency in the first 12 months of follow‐up after FX was measured. (Table 2 ).
One hypothesis to explain FX deficiency is that FX is eliminated from the circulation by selective binding to amyloid deposits, especially in the perivascular tissues in the spleen. The correction of FX deficiency after splenectomy in some case reports supports this hypothesis. 49 However, this simple FX adsorptive model cannot explain the discrepancy between circulating FX antigen and FX coagulant activity observed in some patients in Mumford's cohort. Additional mechanisms may be involved in FX functional impairment. Recently, the group of Christophe et al. have discovered new partners regulating FX circulating levels: they have identified scavenger receptor class A member I (SR‐AI) as a receptor for FX at the macrophage surface and pentraxin‐2 (PTX2), which forms a ternary complex with SR‐AI and FX. 50 PTX2 is essential to prevent internalization of FX by SR‐AI. Like FX, PTX2 may be targeted to amyloid plaques. Because SR‐AI/PTX2/FX complex is necessary to maintain normal plasma levels of FX and PTX2, a disequilibrium in the complex may contribute to a depletion in FX in systemic AL‐amyloidosis.
Antithrombotic drugs in amyloidosis
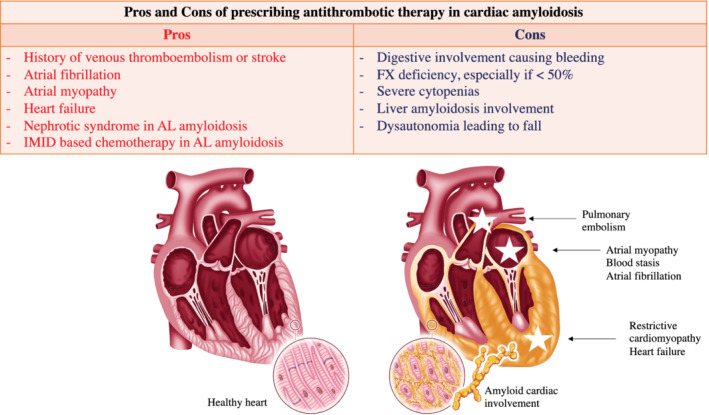
Currently, the main indications of anticoagulation in secondary prevention are AF, regardless of CHADS2‐VaSC score, deep vein thrombosis and/or pulmonary embolism, nephrotic syndrome with hypoalbuminaemia <20 g/L and unexpected intracardiac thrombus diagnosed during cardiac imaging (echocardiography, computed tomography scan, and magnetic resonance imaging) 18 (Figure 1).
Figure 1.
Main determinants of thromboembolism and bleeding in systemic amyloidosis.
The situations that are to be considered for prescribing prophylactic anticoagulation are the prescription of IMIDs, heart failure hospitalization, a history of arterial thrombosis (stroke with presumed embolic origin, TIA) in the absence of a curable cause, a nephrotic syndrome with albuminaemia >20 g/L.
The contraindications to anticoagulant treatment are less well established. The most common are active digestive bleeding requiring blood transfusion (especially if linked to diffuse amyloid digestive involvement), coagulation disorders, especially FX < 50 IU/dL. Other risky situations that do not represent absolute contraindications to anticoagulant therapy are cytopenia, hepatic amyloid damage and renal failure.
There are important differences between AL and TTR amyloidosis (Table 5 ). Indeed, AL amyloidosis seems associated with a greater risk of haemorrhage because digestive involvement is more often present, association with a myeloma can lead to cytopenia, FX deficiency is associated with AL amyloidosis, renal failure is more common. There are also more interactions between anticoagulants and the treatments for AL amyloidosis (corticosteroids, chemotherapy: proteasome inhibitors, IMIDs, daratumumab). Conversely, tafamidis, the only approved treatment for ATTR cardiomyopathy, does not appear to interact with cytochrome CYP3A4.
Table 5.
Specific thrombotic and bleeding risks according to the type of amyloidosis
| Type of amyloidosis | Prothrombotic risk factors | Bleeding risk factors |
|---|---|---|
| AL |
|
|
| TTR |
|
|
AL, immunoglobulin light chain amyloidosis; IMID, immunomodulatory drugs; TTR: transthyretin.
Vitamin K antagonists
Vitamin K antagonist (VKA) response is marked by inter‐patient and intra‐patient variability. Acute illnesses, deterioration in chronic comorbidities or changes in associated drugs may dramatically impact anticoagulation control. Especially, VKAs interact with chemotherapy in AL amyloidosis. Thus, managing patients with amyloidosis may be challenging requiring a close monitoring of international normalized ratio (INR). INR takes the advantage of being widely available and cheap. In addition, there is an easy‐to‐use antidote for overdose. Finally, VKA is an inexpensive treatment that can be used in patients with severe renal failure (Figure 1). In the study by Mitrani et al., which included only TTR cardiomyopathies, INR lability was noted in 87% of patients. The event rate of major bleeds was 3.7 per 100 person years in this study as compared to 2.2–3.9 in the general population. 51
Direct oral anticoagulants (DOACs)
The pharmacokinetics of the 3 most used direct oral anticoagulants (DOACs) are very different. 52 Dabigatran has predominantly renal clearance (80%) and hepatic clearance (20%) and is not metabolized by cytochrome CYP3A4. Rivaroxaban and apixaban have predominantly hepatic clearance (65% and 73%, respectively). They also are both metabolized by CYP3A4.
The main advantages of DOACs are their easy use, rapid efficacy within a few hours after introduction, oral administration, and a wide therapeutic window. The data published in venous thrombosis in cancer patients have shown the efficacy and safety of DOACs. 53 , 54 There are few safety data concerning apixaban use for venous thrombotic prevention in multiple myeloma treated with IMID‐based chemotherapy 55 ; indeed, no signal of ineffectiveness (little or no thrombosis) or higher risk of bleeding has been reported. Mitrani et al. 21 have published the only available data on the use of DOACs in ATTR cardiomyopathy. There was no difference in thrombotic or haemorrhagic events compared to VKA treatment.
One of the main drawbacks of DOACs use is the management of patients with life‐threatening bleedings or in emergency situations. The use of specific antidotes is expensive and may expose patients to an increased risk of thrombotic complications. Secondly, there are drug interactions with chemotherapies used in AL amyloidosis. For example, dexamethasone (CYP3A4 inducer) may decrease DOAC levels and lead to lower levels of anticoagulation. There is also an increased risk of gastrointestinal bleeding especially with dabigatran and rivaroxaban. 56 , 57 However, the Amplify study 53 showed the benefit of apixaban in major bleeding as compared with controls thanks to a decrease in gastrointestinal bleeding in cancer patients. In the general population, the rate of major bleeding in patients treated with DOAC is 2.9 to 4 per 100 person years. 51 , 58 In Mitrani et al. data 21 (TTR anticoagulated amyloidosis), this event rate was 5.2 per 100 patient years. Finally, xabans and dabigatran are contra‐indicated in severe renal failure with creatinine clearance <15 and 30 mL/min, respectively, and 60% of AL amyloidosis patients have renal involvement. Furthermore, 10% to 15% of patients have liver damage which may also limit their prescription.
Direct oral anticoagulants could be used safely in TTR amyloidosis in the absence of severe renal failure. During the administration of chemotherapy containing dexamethasone, anticoagulant therapy with low molecular weight heparin is preferred. On the other hand, once the patient is in remission and no longer receiving chemotherapy, anticoagulation by DOACs may be considered. Apixaban has shown a reduction in digestive bleeding in cancer patients with venous thromboembolism. 53
Finally, the MYELAXAT 59 study has tested the protective effect of apixaban (2.5 mg twice daily) for 6 months in multiple myeloma patients (all with creatinine clearance >30 mL/min) and treated with IMID (thalidomide and/or lenalidomide). In this non‐controlled and non‐randomized study, 104 patients were included. Two patients experienced a TE (the incidence of TE was 0.38 patient‐months in the entire population, 95% CI 0.05–1.4). Neither arterial cardiovascular event, nor pulmonary embolism, and no death due to venous TE was observed. Eleven patients experienced bleeding: 10 clinically relevant non major bleedings [incidence 1.9% patient‐months (95% CI 0.9–3.5)] and one major bleeding requiring blood transfusion [incidence 0.19% patient‐months (95% CI 0.04–1.1)]. These preliminary data suggest the safety of this DOAC in myeloma patients.
Low molecular weight heparins
Low molecular weight heparin (enoxaparin and tinzaparin) are widely used. These treatments are easy to handle with a short half‐life making it possible to stop them in case of bleeding. There are few drug interactions, especially with chemotherapy. However, low molecular weight heparin need subcutaneous injection and are contraindicated if creatinine renal clearance is below 15–20 mL/min. Moreover, a substantial decrease of AT activity may lead to a suboptimal effect of heparin derivatives.
Left atrial appendage closure
Some CA patients with AF with or without previous cardioembolic event have a history of severe bleeding, such as diffuse digestive bleeding. In these cases, a left atrial appendage closure has to be considered in order to stop or to avoid anticoagulation.
Conclusions
Thromboembolic and haemorrhagic events are frequent and major issues in cardiac AL and TTR amyloidosis. Physicians should systematically look for factors favouring thromboembolism such as heart failure, atriopathy, supraventricular arrhythmias, history of a previous thromboembolic event, nephrotic syndrome, and thrombotic risk related to chemotherapy. The thrombotic risk has to be weighed with the haemorrhagic risk that is increased by amyloid digestive or liver involvement, coagulation disorders such as FX deficiency, renal failure, thrombopathy. Prospective studies are needed to specify when and how to prescribe preventive antithrombotic therapy in this specific population.
Conflict of interest
Nothing to declare.
Nicol, M. , Siguret, V. , Vergaro, G. , Aimo, A. , Emdin, M. , Dillinger, J. G. , Baudet, M. , Cohen‐Solal, A. , Villesuzanne, C. , Harel, S. , Royer, B. , Arnulf, B. , and Logeart, D. (2022) Thromboembolism and bleeding in systemic amyloidosis: a review. ESC Heart Failure, 9: 11–20. 10.1002/ehf2.13701.
References
- 1. Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation 2005; 112: 2047–2060. [DOI] [PubMed] [Google Scholar]
- 2. Rapezzi C, Merlini G, Quarta CC, Riva L, Longhi S, Leone O, Salvi F, Ciliberti P, Pastorelli F, Biagini E, Coccolo F, Cooke RMT, Bacchi‐Reggiani L, Sangiorgi D, Ferlini A, Cavo M, Zamagni E, Fonte ML, Palladini G, Salinaro F, Musca F, Obici L, Branzi A, Perlini S. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation 2009; 120: 1203–1212. [DOI] [PubMed] [Google Scholar]
- 3. Gertz MA, Dispenzieri A. Systemic amyloidosis recognition, prognosis, and therapy: a systematic review. JAMA 2020. 07; 324: 79–89. [DOI] [PubMed] [Google Scholar]
- 4. González‐López E, Gallego‐Delgado M, Guzzo‐Merello G, de Haro‐Del Moral FJ, Cobo‐Marcos M, Robles C, Bornstein B, Salas C, Lara‐Pezzi E, Alonso‐Pulpon L, Garcia‐Pavia P. Wild‐type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J 2015; 36: 2585–2594. [DOI] [PubMed] [Google Scholar]
- 5. Damy T, Costes B, Hagège AA, Donal E, Eicher J‐C, Slama M, Guellich A, Rappeneau S, Gueffet JP, Logeart D, Planté‐Bordeneuve V. Prevalence and clinical phenotype of hereditary transthyretin amyloid cardiomyopathy in patients with increased left ventricular wall thickness. Eur Heart J 2016. 14; 37: 1826–1834. [DOI] [PubMed] [Google Scholar]
- 6. Feng D, Edwards WD, Oh JK, Chandrasekaran K, Grogan M, Martinez MW, Syed II, Hughes DA, Lust JA, Jaffe AS, Gertz MA, Klarich KW. Intracardiac thrombosis and embolism in patients with cardiac amyloidosis. Circulation 2007; 116: 2420–2426. [DOI] [PubMed] [Google Scholar]
- 7. Bøtker HE, Rasmussen OB. Recurrent cerebral embolism in cardiac amyloidosis. Int J Cardiol 1986; 13: 81–83. [DOI] [PubMed] [Google Scholar]
- 8. Santarone M, Corrado G, Tagliagambe LM, Manzillo GF, Tadeo G, Spata M, Longhi M. Atrial thrombosis in cardiac amyloidosis: diagnostic contribution of transesophageal echocardiography. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 1999; 12: 533–536. [DOI] [PubMed] [Google Scholar]
- 9. Cools FJ, Kockx MM, Boeckxstaens GE, Heuvel PV, Cuykens JJ. Primary systemic amyloidosis complicated by massive thrombosis. Chest 1996; 110: 282–284. [DOI] [PubMed] [Google Scholar]
- 10. Willens HJ, Levy R, Kessler KM. Thromboembolic complications in cardiac amyloidosis detected by transesophageal echocardiography. Am Heart J 1995; 129: 405–406. [DOI] [PubMed] [Google Scholar]
- 11. Browne RS, Schneiderman H, Kayani N, Radford MJ, Hager WD. Amyloid heart disease manifested by systemic arterial thromboemboli. Chest 1992; 102: 304–307. [DOI] [PubMed] [Google Scholar]
- 12. Cappelli F, Tini G, Russo D, Emdin M, Del Franco A, Vergaro G, Di Bella G, Mazzeo A, Canepa M, Volpe M, Perfetto F. Arterial thrombo‐embolic events in cardiac amyloidosis: a look beyond atrial fibrillation. Amyloid Int J Exp Clin Investig Off J Int Soc Amyloidosis 2020; 28: 1–7. [DOI] [PubMed] [Google Scholar]
- 13. Halligan CS, Lacy MQ, Vincent Rajkumar S, Dispenzieri A, Witzig TE, Lust JA, Fonseca R, Gertz MA, Kyle RA, Pruthi RK. Natural history of thromboembolism in AL amyloidosis. Amyloid Int J Exp Clin Investig Off J Int Soc Amyloidosis 2006; 13: 31–36. [DOI] [PubMed] [Google Scholar]
- 14. Zubkov AY, Rabinstein AA, Dispenzieri A, Wijdicks EFM. Primary systemic amyloidosis with ischemic stroke as a presenting complication. Neurology 2007; 69: 1136–1141. [DOI] [PubMed] [Google Scholar]
- 15. Nitsche C, Scully PR, Patel KP, Kammerlander AA, Koschutnik M, Dona C, Wollenweber T, Ahmed N, Thornton GD, Kelion AD, Sabharwal N, Newton JD, Ozkor M, Kennon S, Mullen M, Lloyd G, Fontana M, Hawkins PN, Pugliese F, Menezes LJ, Moon JC, Mascherbauer J, Treibel TA. Prevalence and outcomes of concomitant aortic stenosis and cardiac amyloidosis. J Am Coll Cardiol 2021; 77: 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vergaro G, Aimo A, Barison A, Genovesi D, Buda G, Passino C, Emdin M. Keys to early diagnosis of cardiac amyloidosis: red flags from clinical, laboratory and imaging findings. Eur J Prev Cardiol 2020; 27: 1806–1815. [DOI] [PubMed] [Google Scholar]
- 17. Feng D, Syed IS, Martinez M, Oh JK, Jaffe AS, Grogan M, Edwards WD, Gertz MA, Klarich KW. Intracardiac thrombosis and anticoagulation therapy in cardiac amyloidosis. Circulation 2009; 119: 2490–2497. [DOI] [PubMed] [Google Scholar]
- 18. Martinez‐Naharro A, Gonzalez‐Lopez E, Corovic A, Mirelis JG, Baksi AJ, Moon JC, Garcia‐Pavia P, Gillmore JD, Hawkins PN, Fontana M. High prevalence of intracardiac thrombi in cardiac amyloidosis. J Am Coll Cardiol 2019. 09; 73: 1733–1734. [DOI] [PubMed] [Google Scholar]
- 19. Sanchis K, Cariou E, Colombat M, Ribes D, Huart A, Cintas P, Fournier P, Rollin A, Carrié D, Galinier M, Maury P, Duparc A, Lairez O, Toulouse Amyloidosis Research Network collaborators . Atrial fibrillation and subtype of atrial fibrillation in cardiac amyloidosis: clinical and echocardiographic features, impact on mortality. Amyloid Int J Exp Clin Investig Off J Int Soc Amyloidosis 2019; 26: 128–138. [DOI] [PubMed] [Google Scholar]
- 20. Longhi S, Quarta CC, Milandri A, Lorenzini M, Gagliardi C, Manuzzi L, Bacchi‐Reggiani ML, Leone O, Ferlini A, Russo A, Gallelli I, Rapezzi C. Atrial fibrillation in amyloidotic cardiomyopathy: prevalence, incidence, risk factors and prognostic role. Amyloid Int J Exp Clin Investig Off J Int Soc Amyloidosis 2015; 22: 147–155. [DOI] [PubMed] [Google Scholar]
- 21. Mitrani LR, De Los Santos J, Driggin E, Kogan R, Helmke S, Goldsmith J, Biviano AB, Maurer MS. Anticoagulation with warfarin compared to novel oral anticoagulants for atrial fibrillation in adults with transthyretin cardiac amyloidosis: comparison of thromboembolic events and major bleeding. Amyloid Int J Exp Clin Investig Off J Int Soc Amyloidosis 2020; 28: 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Donnellan E, Wazni OM, Hanna M, Elshazly MB, Puri R, Saliba W, Kanj M, Vakamudi S, Patel DR, Baranowski B, Cantillon D, Dresing T, Jaber WA. Atrial fibrillation in transthyretin cardiac amyloidosis: predictors, prevalence, and efficacy of rhythm control strategies. JACC Clin Electrophysiol 2020; 6: 1118–1127. [DOI] [PubMed] [Google Scholar]
- 23. Mints YY, Doros G, Berk JL, Connors LH, Ruberg FL. Features of atrial fibrillation in wild‐type transthyretin cardiac amyloidosis: a systematic review and clinical experience. ESC Heart Fail 2018; 5: 772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. El‐Am EA, Dispenzieri A, Melduni RM, Ammash NM, White RD, Hodge DO, Noseworthy PA, Lin G, Pislaru SV, Egbe AC, Grogan M. Direct current cardioversion of atrial arrhythmias in adults with cardiac amyloidosis. J Am Coll Cardiol 2019. 12; 73: 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dubrey S, Pollak A, Skinner M, Falk RH. Atrial thrombi occurring during sinus rhythm in cardiac amyloidosis: evidence for atrial electromechanical dissociation. Br Heart J 1995; 74: 541–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Röcken C, Peters B, Juenemann G, Saeger W, Klein HU, Huth C, Roessner A, Goette A. Atrial amyloidosis: An arrhythmogenic substrate for persistent atrial fibrillation. Circulation 2002; 106: 2091–2097. [DOI] [PubMed] [Google Scholar]
- 27. Brand A, Frumkin D, Hübscher A, Dreger H, Stangl K, Baldenhofer G, Knebel F. Phasic left atrial strain analysis to discriminate cardiac amyloidosis in patients with unclear thick heart pathology. Eur Heart J Cardiovasc Imaging 2021; 22: 680–687. [DOI] [PubMed] [Google Scholar]
- 28. Mohty D, Petitalot V, Magne J, Fadel BM, Boulogne C, Rouabhia D, el Hamel C, Lavergne D, Damy T, Aboyans V, Jaccard A. Left atrial function in patients with light chain amyloidosis: a transthoracic 3D speckle tracking imaging study. J Cardiol 2018; 71: 419–427. [DOI] [PubMed] [Google Scholar]
- 29. Mohty D, Boulogne C, Magne J, Varroud‐Vial N, Martin S, Ettaif H, Fadel BM, Bridoux F, Aboyans V, Damy T, Jaccard A. Prognostic value of left atrial function in systemic light‐chain amyloidosis: a cardiac magnetic resonance study. Eur Heart J Cardiovasc Imaging 2016; 17: 961–969. [DOI] [PubMed] [Google Scholar]
- 30. Hausfater P, Costedoat‐Chalumeau N, Amoura Z, Cacoub P, Papo T, Grateau G, Leblond V, Godeau P, Piette JC. AL cardiac amyloidosis and arterial thromboembolic events. Scand J Rheumatol 2005; 34: 315–319. [DOI] [PubMed] [Google Scholar]
- 31. Park H, Kim J‐W, Youk J, Koh Y, Lee J‐O, Kim KH, Bang SM, Kim I, Park S, Yoon SS. Serum free light chain difference and β2 microglobulin levels are risk factors for thromboembolic events in patients with AL amyloidosis. Clin Lymphoma Myeloma Leuk 2018; 18: 408–414. [DOI] [PubMed] [Google Scholar]
- 32. Pudusseri A, Sanchorawala V, Sloan JM, Bever KM, Doros G, Kataria S, Sarosiek S. Prevalence and prognostic value of D‐dimer elevation in patients with AL amyloidosis. Am J Hematol 2019; 94: 1098–1103. [DOI] [PubMed] [Google Scholar]
- 33. Sattianayagam PT, Hawkins PN, Gillmore JD. Systemic amyloidosis and the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 2009; 6: 608–617. [DOI] [PubMed] [Google Scholar]
- 34. Larocca A, Cavallo F, Bringhen S, Di Raimondo F, Falanga A, Evangelista A, Cavalli M, Stanevsky A, Corradini P, Pezzatti S, Patriarca F. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood 2012; 119: 933–939 quiz 1093. [DOI] [PubMed] [Google Scholar]
- 35. Palumbo A, Cavo M, Bringhen S, Zamagni E, Romano A, Patriarca F, Rossi D, Gentilini F, Crippa C, Galli M, Nozzoli C, Ria R, Marasca R, Montefusco V, Baldini L, Elice F, Callea V, Pulini S, Carella AM, Zambello R, Benevolo G, Magarotto V, Tacchetti P, Pescosta N, Cellini C, Polloni C, Evangelista A, Caravita T, Morabito F, Offidani M, Tosi P, Boccadoro M. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a phase III, open‐label, randomized trial. J Clin Oncol Off J Am Soc Clin Oncol 2011; 29: 986–993. [DOI] [PubMed] [Google Scholar]
- 36. Catovsky D, Ikoku NB, Pitney WR, Galton DA. Thromboembolic complications in myelomatosis. Br Med J 1970; 3: 438–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Osman K, Comenzo R, Rajkumar SV. Deep venous thrombosis and thalidomide therapy for multiple myeloma. N Engl J Med 2001; 344: 1951–1952. [DOI] [PubMed] [Google Scholar]
- 38. Yood RA, Skinner M, Rubinow A, Talarico L, Cohen AS. Bleeding manifestations in 100 patients with amyloidosis. JAMA 1983; 249: 1322–1324. [PubMed] [Google Scholar]
- 39. Tanaka Y, Hosotani K, Fukushima M. A case of amyloid light‐chain amyloidosis presenting with submucosal hematoma and bleeding in the upper gastrointestinal tract. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 2020; 18: e158–e159. [DOI] [PubMed] [Google Scholar]
- 40. Sundaram S, Rathod R. Gastric amyloidosis causing nonvariceal upper gastrointestinal bleeding. ACG Case Rep J 2019; 6: 3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kumar S, Dispenzieri A, Lacy MQ, Litzow MR, Gertz MA. High incidence of gastrointestinal tract bleeding after autologous stem cell transplant for primary systemic amyloidosis. Bone Marrow Transplant 2001; 28: 381–385. [DOI] [PubMed] [Google Scholar]
- 42. Gould M, Zarrin‐Khameh N, Sellin J. Small bowel amyloidosis. Curr Gastroenterol Rep 2013; 15: 350. [DOI] [PubMed] [Google Scholar]
- 43. Cowan AJ, Skinner M, Seldin DC, Berk JL, Lichtenstein DR, O'Hara CJ, Doros G, Sanchorawala V. Amyloidosis of the gastrointestinal tract: a 13‐year, single‐center, referral experience. Haematologica 2013; 98: 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mumford AD, O'Donnell J, Gillmore JD, Manning RA, Hawkins PN, Laffan M. Bleeding symptoms and coagulation abnormalities in 337 patients with AL‐amyloidosis. Br J Haematol 2000; 110: 454–460. [DOI] [PubMed] [Google Scholar]
- 45. Gamba G, Montani N, Anesi E, Palladini G, Lorenzutti F, Perfetti V, Merlini G. Abnormalities in thrombin‐antithrombin pathway in AL amyloidosis. Amyloid Int J Exp Clin Investig Off J Int Soc Amyloidosis 1999; 6: 273–277. [DOI] [PubMed] [Google Scholar]
- 46. Choufani EB, Sanchorawala V, Ernst T, Quillen K, Skinner M, Wright DG, Seldin DC. Acquired factor X deficiency in patients with amyloid light‐chain amyloidosis: incidence, bleeding manifestations, and response to high‐dose chemotherapy. Blood 2001; 97: 1885–1887. [DOI] [PubMed] [Google Scholar]
- 47. Patel G, Hari P, Szabo A, Rein L, Kreuziger LB, Chhabra S, Dhakal B, D'Souza A. Acquired factor X deficiency in light‐chain (AL) amyloidosis is rare and associated with advanced disease. Hematol Oncol Stem Cell Ther 2019; 12: 10–14. [DOI] [PubMed] [Google Scholar]
- 48. Greipp PR, Kyle RA, Bowie EJ. Factor‐X deficiency in amyloidosis: a critical review. Am J Hematol 1981; 11: 443–450. [DOI] [PubMed] [Google Scholar]
- 49. Rosenstein ED, Itzkowitz SH, Penziner AS, Cohen JI, Mornaghi RA. Resolution of factor X deficiency in primary amyloidosis following splenectomy. Arch Intern Med 1983; 143: 597–599. [PubMed] [Google Scholar]
- 50. Muczynski V, Aymé G, Regnault V, Vasse M, Borgel D, Legendre P, Bazaa A, Harel A, Loubière C, Lenting PJ, Denis CV, Christophe OD. Complex formation with pentraxin‐2 regulates factor X plasma levels and macrophage interactions. Blood 2017; 129: 2443–2454. [DOI] [PubMed] [Google Scholar]
- 51. van Walraven C, Hart RG, Singer DE, Laupacis A, Connolly S, Petersen P, Koudstaal PJ, Chang Y, Hellemons B. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta‐analysis. JAMA 2002; 288: 2441–2448. [DOI] [PubMed] [Google Scholar]
- 52. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan‐Schilling V, Rowell N, Sinnaeve P, Collins R, Camm AJ, Heidbüchel H, ESC Scientific Document Group , Lip GYH, Weitz J, Fauchier L, Lane D, Boriani G, Goette A, Keegan R, MacFadyen R, Chiang CE, Joung B, Shimizu W. The 2018 European Heart Rhythm Association practical guide on the use of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 2018; 39: 1330–1393. [DOI] [PubMed] [Google Scholar]
- 53. Agnelli G, Becattini C, Meyer G, Muñoz A, Huisman MV, Connors JM, Cohen A, Bauersachs R, Brenner B, Torbicki A, Sueiro MR, Lambert C, Gussoni G, Campanini M, Fontanella A, Vescovo G, Verso M. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med 2020; 382: 1599–1607. [DOI] [PubMed] [Google Scholar]
- 54. Khorana AA, Soff GA, Kakkar AK, Vadhan‐Raj S, Riess H, Wun T, Streiff MB, Garcia DA, Liebman HA, Belani CP, O'Reilly EM, Patel JN, Yimer HA, Wildgoose P, Burton P, Vijapurkar U, Kaul S, Eikelboom J, McBane R, Bauer KA, Kuderer NM, Lyman GH. Rivaroxaban for thromboprophylaxis in high‐risk ambulatory patients with cancer. N Engl J Med 2019; 380: 720–728. [DOI] [PubMed] [Google Scholar]
- 55. Cornell RF, Goldhaber SZ, Engelhardt BG, Moslehi J, Jagasia M, Harrell S, Rubinstein SM, Hall R, Wyatt H, Piazza G. Primary prevention of venous thromboembolism with apixaban for multiple myeloma patients receiving immunomodulatory agents. Br J Haematol 2020; 190: 555–561. [DOI] [PubMed] [Google Scholar]
- 56. Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, Baanstra D, Schnee J, Goldhaber SZ. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361: 2342–2352. [DOI] [PubMed] [Google Scholar]
- 57. EINSTEIN–PE Investigators , Büller HR, Prins MH, Lensin AWA, Decousus H, Jacobson BF, Minar E, Chlumsky J, Verhamme P, Wells P, Agnelli G, Cohen A. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366: 1287–1297. [DOI] [PubMed] [Google Scholar]
- 58. Lamberts M, Staerk L, Olesen JB, Fosbøl EL, Hansen ML, Harboe L, Lefevre C, Evans D, Gislason GH. Major bleeding complications and persistence with oral anticoagulation in non‐valvular atrial fibrillation: contemporary findings in real‐life Danish patients. J Am Heart Assoc 2017; 6: e004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pegourie B, Karlin L, Benboubker L, Orsini‐Piocelle F, Tiab M, Auger‐Quittet S, Rodon P, Royer B, Leleu X, Bareau B, Cliquennois M, Fuzibet JG, Voog E, Belhadj‐Merzoug K, Decaux O, Rey P, Slama B, Leyronnas C, Zarnitsky C, Boyle E, Bosson JL, Pernod G, for the IFM Group . Apixaban for the prevention of thromboembolism in immunomodulatory‐treated myeloma patients: Myelaxat, a phase 2 pilot study. Am J Hematol 2019; 94: 635–640. [DOI] [PubMed] [Google Scholar]


