Abstract
Aims
Although the prevalence of coronary artery disease (CAD) is high among patients with atrial fibrillation (AF), studies on stress perfusion cardiac magnetic resonance (CMR) imaging frequently exclude patients with AF, and its prognostic and diagnostic value in high‐risk patients with suspected or known CAD remains unclear.
Methods and results
In this longitudinal cohort study, we included 164 consecutive patients with AF during vasodilator perfusion CMR. Diagnostic value was evaluated regarding invasive coronary angiography in a subset of patients. We targeted a follow‐up of >5 years and used CMR results as stratification, and the primary outcome was major adverse cardiac events [MACE, cardiovascular (CV) death and myocardial infarction (MI)]. Secondary outcomes included late coronary revascularization or stroke and the components of the primary outcome. Of the whole cohort (73.8% male, mean age 72.2 years ± 7.8 SD), 99.4% were successfully scanned (163/164 patients). Median CHA2DS2‐VASc score was 4 [interquartile range (IQR) 3–5], and median 10‐year risk for CV events based on SMART risk score was high (24%, IQR 16–32%). Thirty‐two patients (19.6%) presented with ischaemia and 52 patients (31.9%) with late gadolinium enhancement (LGE). A combination of LGE and inducible ischaemia was present in 20 patients (12.3%). Diagnostic accuracy was 86.2% [confidence interval (CI) 68.3–96.1%]. The median follow‐up was 6.6 years (IQR 3.6–7.8). Ischaemia in vasodilator perfusion CMR was significantly associated with the occurrence of MACE [P < 0.01; hazard ratio (HR) 2.65, CI 1.39–5.08], as well as LGE (P = 0.03; 1.74, CI 1.07–3.64) and the combination of both (P < 0.01; HR 2.67, CI 1.59–5.62). After adjustment by age, left ventricular ejection fraction, and the presence of diabetes, ischaemia in vasodilator perfusion CMR remained significantly associated with the occurrence of MACE (2.10, CI 1.08–4.10; P = 0.03). In secondary endpoint analysis, there was a significant association of ischaemia in CMR with CV death (P < 0.05; HR 1.93, CI 0.95–3.9) and MI (P < 0.01; HR 13, CI 1.35–125.4), while no significant association was found regarding the occurrence of revascularization (P = 0.45; HR 1.43, CI 0.57–3.58) or stroke (P = 0.99; HR 0.99, CI 0.21–2.59).
Conclusions
Vasodilator stress perfusion CMR demonstrated an excellent diagnostic and significant prognostic value at long‐term follow‐up in high‐risk patients with persistent AF and suspected or known CAD.
Keywords: Atrial fibrillation, Perfusion, Ischaemia, Prognosis, Cardiac magnetic resonance, Late gadolinium enhancement
Introduction
As the population ages and the number of patients with atrial fibrillation (AF) constantly grows, 1 the concomitant prevalence of coronary artery disease (CAD) and atrial arrhythmias is an increasingly common finding. 2 Patients with AF are at high risk not only for directly related adverse events such as stroke but also for other cardiac events such as myocardial infarction (MI). 1 Likewise, the presence of AF in patients with cardiac events potentially worsens prognosis. 3 , 4 However, the prognostic and diagnostic value of non‐invasive methods such as stress perfusion cardiac magnetic resonance (CMR) to assess relevant CAD in patients with AF is unclear.
Traditional methods to evaluate the presence of potentially relevant CAD such as electrocardiography (ECG) suffer from an impaired diagnostic accuracy in patients with AF, 5 emphasizing the need for adequate imaging techniques in these patients. In the wake of the ISCHEMIA trial, the role of invasive diagnostic and therapeutic approaches is further questioned, 6 yet patients with arrhythmias are often excluded from studies on non‐invasive imaging techniques 7 , 8 and not reported on 9 or included only in small proportions. 10 While computed tomography (CT) angiography is reserved for patients at low to intermediate probability of CAD, 11 current guidelines recommend the use of functional ischaemia assessment in patients with a moderate to high pre‐test probability. 12 Myocardial perfusion single‐photon emission computed tomography (SPECT), a well‐established functional diagnostic tool for the detection of ischaemia, often fails to reliably identify CAD in patients with AF. 13
Adenosine‐stress perfusion CMR has good accuracy for the diagnosis of relevant CAD and is a valuable tool for the identification of patients at high risk for major adverse cardiac events (MACE). 9 , 14 , 15 However, the presence of AF poses substantial challenges to image acquisition and interpretation of the results. 16 , 17 Prior studies showed good short‐term follow‐up 18 , 19 and good prognostic implications in low‐risk to intermediate‐risk cohorts, 20 , 21 but long‐term implications in patients at high risk for cardiac events remain insufficiently investigated. The purpose of this study was hence to investigate the diagnostic accuracy and long‐term prognostic value of vasodilator stress perfusion CMR in patients with AF and high cardiovascular (CV) risk at a targeted follow‐up of 5 years.
Methods
Study population
Between August 2009 and March 2015, we retrospectively included patients referred to vasodilator stress CMR. Patients were eligible if they had AF during the CMR procedure. The diagnosis of AF was made in consensus by two experienced cardiologists based on a 12‐lead ECG before and after the test. Patients with contraindications to CMR imaging, such as incompatible metallic implants (e.g. incompatible pacemakers) or claustrophobia, and patients with contraindications to adenosine stressor agent such as hypersensitivity to the drug, second‐degree or third‐degree atrioventricular block, or severe aortic stenosis were excluded. In patients with chronic lung disease, CMR was performed with Regadenoson at the discretion of the clinician. Patients with known allergy to gadolinium‐based contrast medium or a glomerular filtration rate < 30 mL/min/1.73 m2 were excluded.
The study complies with the local institutional standards and the Declaration of Helsinki. The locally appointed ethics committee of Charité – Universitätsmedizin Berlin approved the research protocol. Informed consent has been obtained from the subjects or their guardians.
Cardiac magnetic resonance protocol
All patients were instructed to refrain from caffeine and smoking 24 h before vasodilator stress CMR. After informed consent, CMR was performed on a Philips Ingenia 3.0 Tesla (T) scanner with a 70‐cm‐wide bore system using phased array receiver coils (16 elements anterior, 12 elements posterior) and on a 1.5 T MRI scanner (Achieva, Philips Healthcare, Best, the Netherlands) using a five‐channel phased‐array receiver coil. Patients were placed in the supine position, and images were acquired during breath‐holds of 10 to 15 s by using vector electrocardiogram gating. A rapid balanced steady‐state free precession (SSFP) sequence allowed for localization of the heart in the three standard planes with a repetition time (TR) = 3.4 ms, an echo time (TE) = 1.7 ms, flip angle = 60° at 1.5 T and TR = 3.1 ms, TE = 1.55 ms, flip angle = 45° at 3 T. Cine images were derived using a balanced SSFP sequence in three left ventricular (LV) long‐axis planes and short‐axis cine images covering the entire LV myocardium. The acquisition voxel size was 1.8 × 1.9 × 8.0 mm3, and 30 phases per cardiac cycle were acquired for both field strengths.
First‐pass stress perfusion was begun after 3 min after injection of intravenous adenosine infusion (140 μg/kg/min) or after a bolus 400 μg Regadenoson and a peripheral contrast bolus of 0.075 mmol/kg (at 1.5 T) and 0.0375 mmol/kg (at 3.0 T) Gadovist (Bayer AG, Leverkusen, Germany). The imaging parameters were at 1.5 T: balanced SSFP, TR/TE/flip angle 2 ms/1 ms/50°, spatial resolution 2.8 × 2.9 × 10.0 mm3 and at 3 T: spoiled gradient echo sequence, TR/TE/flip angle 2.6 ms/1.1 ms/15°, spatial resolution 2.9 × 2.9 × 8.0 mm3. For both field strengths, acquisition time was ~150 ms per slice, 1 saturation prepulse per slice, prepulse delay 100 ms, 3 slices per heartbeat, and parallel imaging with acceleration factor 2. Late gadolinium enhancement (LGE) images were acquired around 10 min after an additional bolus of Gadovist (total amount of contrast per scan: 0.15 mmol/kg) with an inversion‐recovery 3D spoiled gradient‐echo sequence. Typical parameters (both field strength) were voxel size 1.8 × 1.8 × 5 mm3, TR/TE = 3.4 ms/1.6 ms, and flip angle of 15°. Inversion time was assessed individually with the use of a Look‐Locker sequence using an individually adapted prepulse delay sequence. Short‐axis LGE views of the entire LV myocardium and two‐chamber, three‐chamber, and four‐chamber LGE views were obtained. In addition, a respiratory and cardiac triggered single‐shot version for LGE imaging was used. For 1.5 T, an inversion prepared balanced SSFP sequence with TR/TE/flip angle = 2.9 ms/1.45 ms/50°, voxel size 1.8 × 1.9 × 10 mm, and acquisition time 160 ms per slice was used. For 3 T, we used an inversion prepared gradient echo sequence with TR/TE/flip angle = 3 ms/1.4 ms/20°, voxel size 1.8 × 1.9 × 10 mm, and acquisition time 190 ms per slice.
Image analysis
Short‐axis cine images were used to calculate LV volumes, mass, and ejection fraction. Regarding LGE and stress perfusion, scans were evaluated based on the 17‐segment model of the American Heart Association by two experienced readers. Inducible ischaemia was defined as stress perfusion deficits, that is, regional hypo‐enhancements (i) persisting over at least three phases after peak contrast enhancement and (ii) consistent with coronary distribution with (iii) at least 25% subendocardial or complete transmural expansion. In the presence of LGE, the extend of the perfusion deficit was required to exceed scar size for the diagnosis of inducible ischaemia. 22 Additionally, the total number of ischaemic segments was quantified. Image quality was assessed by both readers, and any limitations to image quality were denoted.
Follow‐up
A target follow‐up duration was set at 5 years or more. Follow‐up data were collected using a standardized questionnaire. The standardized follow‐up questionnaire contained questions regarding the current status of patients as well as asking about past events such as the clinical endpoints, as well as whether a repeat CMR had been performed outside of our clinical compound. Patients were called at least three times, and, when not directly reachable, their doctors were contacted. Upon oral confirmation, all participants agreeing to have their data collected were sent a letter including the identical questionnaire together with a consent form. Additionally, we asked patients to provide additional medical reports and collated them with our institutional medical data by revision of medical files and clinical visits (83%). Data were confirmed with the general practitioner or referring cardiologist when necessary. Survival data were additionally verified by consultation of the German National Death Registry. Patients who did not respond were correlated with data collected from the German National Death Registry and ultimately considered lost to follow‐up.
The primary endpoint was the occurrence of MACE, defined as CV death or nonfatal MI. Secondary endpoints were the components of the primary endpoint and nonfatal ischaemic stroke or any coronary revascularization procedure. All events were categorized following standard definitions. 23 CV death was defined as sudden cardiac death, any death preceded by MI, fatal decompensation of heart failure, or from any cardiac cause. MI was defined according to the fourth Universal Definition of Myocardial Infarction as dynamic cardiac troponin levels with at least one value above the 99th percentile and clinical signs suggestive of MI. 24 Coronary revascularization was defined as either coronary artery bypass grafting or percutaneous coronary intervention. For patients with a positive stress CMR, early coronary revascularization, MI, and CV death were excluded from analysis if occurring within a 90 day blanking period after CMR. The CHA2DS2‐VASc [congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke or transient ischaemic attack (TIA), vascular disease, age 65 to 74 years, and gender] score and the SMART (Secondary Manifestations of ARTerial disease) score were calculated according to the published formulas. 25 , 26
Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD) or median and interquartile range [IQR, represented by reporting the 25th and 75th quartiles (Q1–Q3); single value range would be given by subtracting Q3 − Q1] after assessing for normal distribution by the Shapiro–Wilk test. Categorical variables are expressed as numbers and proportions. Patient and CMR characteristics were compared using Student's t‐test or the Mann–Whitney U test for continuous variables and the χ 2 or Fisher exact test for categorical and ordinal variables. Sensitivity, specificity, as well as positive and negative likelihood ratios, were calculated to assess the diagnostic performance of stress CMR. Kaplan–Meier estimator was used to estimate event‐free survival and cumulative incidence rates of composite and individual outcomes and compared with the log‐rank test. To assess confounding and calculate hazard ratios (HRs) including confidence intervals (CIs), univariate Cox proportional hazards regressions were performed on patient and CMR characteristics, pre‐selected by clinical reasoning. All variables significantly associated with MACE (P < 0.10) were entered in a multivariate model. Stepwise backward selection using the Wald statistic was used to build a multivariate model, with exit criteria set at P < 0.10. Schoenfeld‐type residuals were examined to test the assumption of the proportional hazards ratio. Harrell's concordance index was calculated to assess goodness of fit for the final model. Logistic and linear regression was used to calculate odds ratios (ORs). If not specified otherwise, a two‐tailed value of P < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS Version 27.0 (IBM Corp., Armonk, NY, USA) and R Version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Demographics
A total of 164 patients were enrolled between August 2009 and March 2015. The final study cohort consisted of 163 patients in whom CMR protocol was successfully completed. One patient developed high‐grade atrioventricular block after adenosine infusion and was excluded from the study. Detailed baseline patient characteristics for patients with and without inducible ischaemia are presented in Table 1 . Patients with inducible perfusion deficits were older (75.1 ± 7.4 SD vs. 71.4 years ± 7.8 SD; P = 0.02) and had a higher CHA2DS2‐VASc score [4 points (IQR 4–5) for patients with ischaemia vs. 4 points (IQR 2–4) for patients without inducible ischaemia; P = 0.07], although a history of prior stroke was denoted for equal proportions of both groups. Of note, a history of stroke and a higher CHA2DS2‐VASc score were both significantly associated with stroke or TIA during follow‐up (P = 0.002 and P < 0.001). The prevalence of CAD was higher for patients with inducible ischaemia (87.5% vs. 53.4%; P < 0.001), and significantly more patients with inducible ischaemia had had revascularization (71.9% vs. 37.4%; P < 0.001) or prior MI (31.3% vs. 15.3%; P = 0.04).
Table 1.
Baseline characteristics of patients with and without ischaemia in cardiac magnetic resonance
| All patients (n = 163) | No ischaemia (n = 131) | Ischaemia (n = 32) | P‐value | ||
|---|---|---|---|---|---|
| Age, years | 72.2 ± 7.8 | 71.4 ± 7.8 | 75.1 ± 7.4 | 0.02 | |
| Male | 121 (74.2) | 95 (72.5) | 26 (81.3) | 0.31 | |
| BMI, kg/m2 | 27 (25–31) | 27 (25–31) | 26 (23–30) | 0.20 | |
| Body surface area (Du Bois), m2 | 2 ± 0.2 | 2 ± 0.2 | 1.9 ± 0.2 | 0.08 | |
| Diabetes mellitus | 51 (31.3) | 37 (28.2) | 13 (40.6) | 0.17 | |
| Hypertension | 135 (82.8) | 105 (80.2) | 29 (90.6) | 0.17 | |
| Hypercholesterinaemia | 109 (66.9) | 81 (61.8) | 27 (84.4) | 0.02 | |
| Current smoking | 16 (9.8) | 15 (11.5) | 1 (3.1) | 0.16 | |
| Family history of CAD | 2 (1.2) | 2 (1.5) | 0 (0) | 0.48 | |
| Angina pectoris | No angina | 63 (38.7) | 49 (37.4) | 13 (40.6) | 0.47 |
| Atypical angina | 87 (53.4) | 75 (57.3) | 12 (37.5) | ||
| Typical angina | 14 (8.6) | 7 (5.3) | 7 (21.9) | ||
| NYHA class | 0 | 63 (38.7) | 49 (37.4) | 13 (40.6) | 0.91 |
| I | 47 (28.8) | 38 (29) | 9 (28.1) | ||
| II | 37 (22.7) | 32 (24.4) | 5 (15.6) | ||
| III–IV | 17 (10.4) | 12 (9.2) | 5 (15.6) | ||
| CHA2DS2‐VASc score | 4 (3–5) | 4 (2–4) | 4 (4–5) | 0.07 | |
| Known CAD | 98 (60.1) | 70 (53.4) | 28 (87.5) | <0.001 | |
| Prior MI | 30 (18.4) | 20 (15.3) | 10 (31.3) | 0.04 | |
| Prior revascularization (PCI or CABG) | 72 (44.2) | 49 (37.4) | 23 (71.9) | <0.001 | |
| Stroke or systemic embolism | 27 (16.6) | 22 (16.8) | 5 (15.6) | 0.87 | |
| Peripheral artery disease | 19 (11.7) | 12 (9.2) | 7 (21.9) | 0.05 | |
| Cerebrovascular artery disease | 7 (4.3) | 4 (3.1) | 3 (9.4) | 0.11 | |
| Abdominal artery disease | 10 (6.1) | 7 (5.3) | 3 (9.4) | 0.39 | |
| Oral anticoagulant drugs | 141 (86.5) | 115 (87.8) | 26 (81.3) | 0.33 | |
| Beta‐blockers | 135 (82.3) | 106 (80.9) | 28 (87.5) | 0.38 | |
| Statins | 106 (65.0) | 79 (60.3) | 26 (81.3) | 0.03 | |
| ACE inhibitors or ARBs | 134 (82.2) | 108 (82.4) | 25 (78.1) | 0.57 | |
| Calcium channel blocker | 52 (31.9) | 40 (30.5) | 12 (37.5) | 0.45 | |
| Diuretics | 84 (51.5) | 65 (49.6) | 19 (59.4) | 0.32 | |
| Nitrates | 2 (1.2) | 1 (0.8) | 1 (3.1) | 0.28 | |
| 10 year risk, % a | 24 (16–36) | 21 (14–32) | 32 (24–40) | <0.001 | |
ACE, angiotensin‐converting enzyme; ARB, angiotensin II receptor blockers; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; MI, myocardial infarction; NYHA, New York Heart Association classification; PCI, percutaneous coronary intervention.
Values are mean ± standard deviation, n (%), or median (interquartile range represented by Q1–Q3).
Estimated 10‐year risk for recurrent vascular events based on the SMART risk score (http://www.escardio.org/Education/ESC‐Prevention‐of‐CVD‐Programme/Risk‐assessment/SMART‐Risk‐Score).
Image analysis
Of 164 patients with AF, 163 were successfully scanned (99.4%). Details of patient flow and reasons for invasive coronary angiography (ICA) are presented in Figure 1 .
Figure 1.
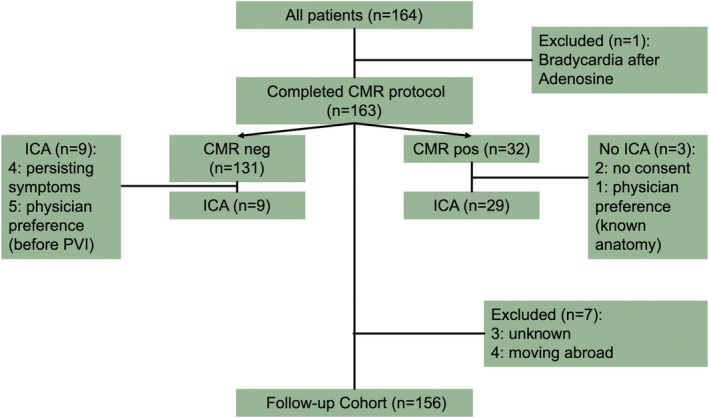
The middle column indicates patient flow during the study with one patient being excluded due to bradycardia after adenosine and seven patients being excluded from the follow‐up cohort. Smaller boxes demonstrate results of vasodilator cardiac magnetic resonance (CMR) and invasive coronary angiography (ICA).
In 8 of 163 patients (4.9%), diminished image quality was denoted while still being diagnostic, due to ECG‐gating problems. No patient or CMR characteristic was predictive of impaired image quality. Fifteen patients (9.2%) received Regadenoson. The mean heart rate was 74 ± 15 b.p.m. Vasodilator perfusion CMR identified 32 patients (19.6%) with stress‐induced perfusion deficits and 52 patients (31.9%) with LGE. A combination of LGE and inducible ischaemia was present in 20 patients (12.3%). Ischaemia in CMR was associated with a higher prevalence of resting wall motion abnormalities (64.5% vs. 33.1%; P < 0.001) and LGE (56.3% vs. 26.4%; P < 0.001), but the number of segments with scar tissue in patients with LGE was similarly distributed over both groups. Detailed examination characteristics are presented in Table 2 . A total of 29 (90.6%) of the 32 patients with perfusion deficits in CMR proceeded to ICA, of which 25 were diagnosed with relevant CAD, while 4 patients showed no signs of the disease. This results in a sensitivity of 86.3% (CI 86.3–100%) and overall diagnostic accuracy of 86.2% (CI 68.3–96.1%). A case example is shown in Figure 2 . Of note, from 131 patients with non‐pathological stress perfusion results, 9 still had ICA, of which 4 patients presented with significant CAD. Considering that 44.4% (n = 4) of these patients presented with persisting or aggravating symptoms, they are most probably not representative of the entire cohort. Patients were included in the follow‐up cohort, regardless of ICA results.
Table 2.
Characteristics of cardiovascular magnetic resonance examination of patients with and without ischaemia in cardiac magnetic resonance
| All patients (n = 163) | No ischaemia (n = 131) | Ischaemia (n = 32) | P‐value | ||
|---|---|---|---|---|---|
| LV ejection fraction, % | 53 ± 9 | 53 ± 9 | 52 ± 9 | 0.74 | |
| LV end‐diastolic volume index, mL/m2 | 74.5 ± 21.9 | 74 ± 22.4 | 75.9 ± 20.1 | 0.67 | |
| LV end‐systolic volume index, mL/m2 | 36.5 ± 17.3 | 36.2 ± 17.8 | 37.3 ± 15.7 | 0.73 | |
| LV end‐diastolic diameter, mm | 51 (48–55) | 51 (48–55) | 52 (47–55) | 0.64 | |
| LV end‐diastolic septum diameter, mm | 12 (10–14) | 12 (10–14) | 12 (11–15) | 0.56 | |
| HR at rest, b.p.m. | 74 ± 15 | 73 ± 14 | 78 ± 18 | 0.15 | |
| Systolic BP at rest, mmHg | 129 ± 24 | 129 ± 23 | 129 ± 28 | 0.97 | |
| HR at stress, b.p.m. | 81 ± 18 | 81 ± 17 | 83 ± 17 | 0.55 | |
| Systolic BP at rest, mmHg | 131 ± 25 | 131 ± 25 | 133 ± 25 | 0.65 | |
| RPP at rest, mmHg × b.p.m. a | 9.55 (7.62–11.34) | 9.45 (7.56–11.24) | 9.87 (8.29–11.82) | 0.31 | |
| RPP at rest, mmHg × b.p.m. a | 10 (8.34–12.14) | 10 (8.34–12) | 10.15 (8.81–12.75) | 0.53 | |
| Symptoms at stress | No symptoms | 72 (44.2) | 58 (44.3) | 13 (40.6) | 0.28 |
| Dyspnoea | 48 (29.4) | 39 (29.8) | 10 (30.3) | ||
| Angina pectoris | 44 (27.0) | 23 (17.6) | 9 (27.3) | ||
| Resting wall motion abnormality | 63 (38.7) | 43 (33.1) | 20 (64.5) | <0.001 | |
| Presence of LGE | 52 (31.9) | 34 (26.4) | 18 (56.3) | <0.001 | |
| Number of segments with LGE | 3 ± 2 | 3 ± 2 | 3 ± 2 | 0.51 | |
| Field strength | 1.5 T | 132 (81.0) | 102 (77.9) | 29 (90.6) | 0.10 |
| 3 T | 32 (19.0) | 29 (22.1) | 3 (9.4) | ||
| Type of vasodilator | Adenosine | 148 (90.8) | 117 (89.3) | 31 (96.9) | 0.19 |
| Regadenoson | 15 (9.2) | 14 (10.7) | 1 (3.1) | ||
| Adenosine dose, mg/kg | 0.2 ± 0 | 0.2 ± 0 | 0.2 ± 0 | 0.19 | |
| Regadenoson, μg | 400 ± 0 | 400 ± 0 | 400 ± 0 | ||
| Contrast agent dose, mL | 23 ± 11 | 24 ± 11 | 23 ± 11 | 0.77 | |
| GFR (MDRD), mL | 74 ± 19 | 76 ± 17 | 70 ± 25 | 0.25 | |
BP, blood pressure; GFR, glomerular filtration rate; HR, heart rate; LGE, late gadolinium enhancement; LV, left ventricular; MDRD, Modification of Diet in Renal Disease formula; RPP, rate‐pressure product.
Values are mean ± standard deviation, n (%), or median (interquartile range represented by Q1–Q3).
In thousands.
Figure 2.
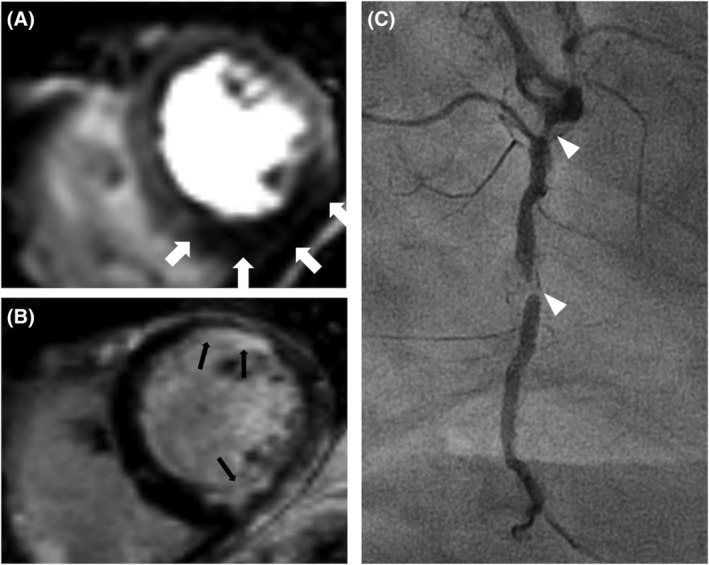
Case example of a patient with typical angina pectoris and a history of myocardial infarction with percutaneous intervention of the LAD. (A) Mid‐ventricle short‐axis view of vasodilator CMR shows induced hypoperfusion inferoseptal, inferior, and inferolateral (white arrows). (B) Short‐axis LGE imaging shows a subendocardial scar tissue inferior, anterior, and anterolateral (black arrows). Of note, the inferior perfusion deficit in image (A) exceeds the scar tissue seen in the relevant region in image (B), thus fulfilling the criteria of relevant ischaemia in this patient. (C) shows a coronary angiogram of the right coronary artery at a 45° right anterior‐oblique rotation in the same patient, revealing relevant stenosis in Segments 1 and 2 (white arrowheads).
Prognostic performance of stress cardiac magnetic resonance
A total of seven patients (4.3%) were lost to follow‐up and not included in the follow‐up cohort (n = 156). Reasons for loss to follow‐up were moving abroad (n = 4) or withdrawn consent (n = 3). The median follow‐up duration was 6.6 years (IQR 3.6–7.8) years, during which 38 (24.4%) CV deaths and 4 (2.6%) MIs occurred.
The presence of LGE in CMR was associated with an event‐free survival rate of 65.4% in contrast to 76.9% in patients without LGE (P = 0.11). Event‐free survival at the end of follow‐up was 56.3% in patients with inducible ischaemia in CMR and 77.4% in the cohort without inducible ischaemia (P = 0.02). For patients with both LGE and inducible perfusion deficits at baseline CMR, the total event‐free survival rate was 55% vs. 75.7% in those without the combined finding (P = 0.05). Event rates based on CMR findings as well as a corresponding Cox regression analysis (HR 1.53, CI 1.19–1.98; P < 0.001) are presented in Figure 3 . Survival analysis based on Kaplan–Meier estimates and Cox regression analysis showed a significant association of ischaemia (P < 0.01; HR 2.65, CI 1.39–5.08) in vasodilator perfusion CMR, LGE (P = 0.03; 1.97, CI 1.07–3.64), and the combination of both (P < 0.01; HR 2.67, CI 1.59–5.62) with lower survival probability in our cohort. The results of the Kaplan–Meier analysis are presented in Figure 4 .
Figure 3.
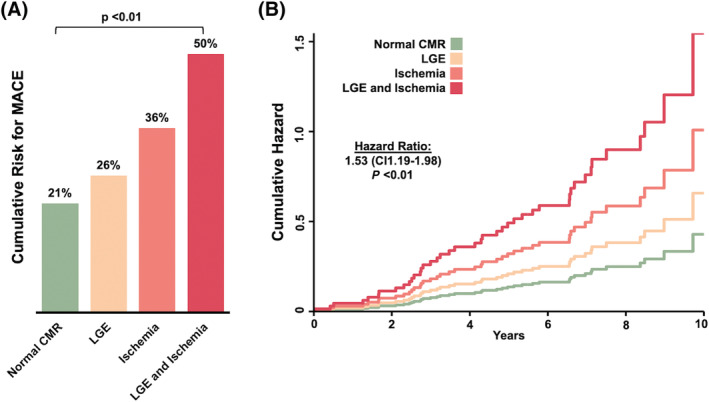
(A) Proportion of cumulative incidence of MACE in cohorts stratified by CMR result: without ischaemia and late gadolinium enhancement (LGE), without ischaemia and with LGE, with ischaemia and without LGE, and with both ischaemia and LGE. The P‐value for trend is given, assuming a linear trend for odds ratio (OR: 1.5; confidence interval 1.1–2.1). (B) Cumulative hazard for MACE in cohorts stratified by CMR result. The hazard ratio is calculated using Cox regression analysis.
Figure 4.
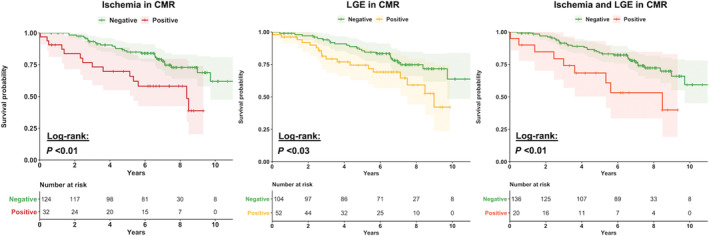
Kaplan–Meier analysis on ischaemia, LGE, or the combination of both for the occurrence of MACE. P‐value is calculated using the log‐rank test. The shaded area behind the graphs illustrates confidence intervals. (A) Kaplan–Meier curves for MACE in vasodilator perfusion CMR for patients with a positive result for ischaemia (red line) or a negative CMR result (green line). (B) Kaplan–Meier curve for MACE for patients with a presence of LGE (yellow line) or without LGE (green line). (C) Kaplan–Meier curve for MACE for patients with a combination of LGE and ischaemia in vasodilator perfusion CMR (orange line) and a normal CMR result (green line).
In univariate analysis, older age, diabetes mellitus, a history of prior coronary revascularization, a higher CHA2DS2‐VASc score, a lower left ventricular ejection fraction (LVEF), and the presence of LGE or ischaemia were significantly (P < 0.1) associated with the occurrence of MACE. After performing backwards stepwise multivariate Cox regression analysis (selection criterion P < 0.10), ischaemia, older age, diabetes, and a lower LVEF remained independent predictors of MACE. The detailed results of univariate and multivariate Cox regression analysis are presented in Table 3 . Accordingly, the HR of ischaemia in CMR on the occurrence of MACE adjusted by age, LVEF, and the presence of diabetes was 2.10 (CI 1.08–4.10; P = 0.03).
Table 3.
Univariate and multivariate Cox regression analysis for major adverse cardiac events (n = 156)
| Univariate comparison | Multivariate comparison | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | |
| Age, years | 1.10 (1.05–1.15) | <0.01 | 1.10 (1.05–1.15) | <0.01 |
| Male | 1.24 (0.63–2.42) | 0.54 | ‐ | |
| BMI, kg/m2 | 1.04 (0.99–1.10) | 0.14 | ‐ | |
| Known CAD | 1.70 (0.88–3.27) | 0.11 | ‐ | |
| Prior MI | 1.55 (0.78–3.08) | 0.21 | ‐ | |
| Prior revascularization (PCI or CABG) | 1.92 (1.04–3.54) | 0.04 | ‐ | |
| Stroke or systemic embolism | 0.61 (0.24–1.54) | 0.29 | ‐ | |
| Diabetes mellitus | 2.32 (1.25–4.28) | <0.01 | 2.61 (1.39–4.91) | <0.01 |
| Hypertension | 2.38 (0.85–6.67) | 0.10 | ‐ | |
| Hypercholesterinaemia | 1.51 (0.77–2.96) | 0.23 | ‐ | |
| Current smoking | 0.6 (0.18–1.95) | 0.39 | ‐ | |
| CHA2DS2‐VASc score | 1.35 (1.16–1.65) | 0.02 | ‐ | |
| 10 year risk group a | 1.71 (1.32–2.21) | <0.01 | ‐ | |
| LV ejection fraction, % | 0.96 (0.94–0.99) | 0.01 | 0.96 (0.93–0.99) | <0.01 |
| LV end‐diastolic volume index, mL/m2 | 1.01 (0.99–1.02) | 0.11 | ‐ | |
| Presence of LGE | 1.74 (1.07–3.64) | 0.03 | ‐ | |
| Ischaemia | 2.65 (1.39–5.08) | <0.01 | 2.10 (1.08–4.10) | 0.03 |
BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CI, confidence interval; LGE, late gadolinium enhancement; LV, left ventricular; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Patients were stratified according to low (<10%), moderate (10 to <20%), or high to extremely high risk (>20%) for vascular events. Covariates for the final multivariate Cox model were selected by stepwise variable selection with exit criteria set at the P < 0.10 level. The area under the curve for the multivariate model is 0.72 (CI 0.63–0.81).
Estimated 10‐year risk for recurrent vascular events based on the SMART risk score.
Regarding secondary endpoints, an additional 11 (7.1%) ischaemic strokes and 25 (16.0%) coronary revascularizations occurred, resulting in a total number of 78 (50.0%) events. Competing‐risk analysis as proposed by Fine and Gray revealed CV death (P = 0.04) and MI (P < 0.01) to be independently associated with ischaemia in CMR, while the distribution of ischaemic strokes (P = 0.08) and revascularization (P = 0.07) did not differ significantly among groups.
Similarly, Cox regression survival analysis showed significant association of ischaemia in CMR for CV death (P = 0.06; HR 1.93, CI 0.95–3.9) and MI (P < 0.01; HR 13, CI 1.35–125.4), while revascularization and stroke were not significantly associated with ischaemia in CMR (P = 0.45; HR 1.43, CI 0.57–3.58 and P = 0.99; HR 0.99, CI 0.21–2.59). Further analysis (Figure 5 ) revealed a higher discriminatory power for inducible ischaemia in patients with a lower risk for recurrent vascular events (SMART score, Figure 5 A ), while the CHA2DS2‐VASc score shows a modest possible influence towards higher HRs in patients with high risk for stroke (Figure 5 C ). The two continuous variables that were identified as independent predictors in multivariate comparison, age and LVEF, were both inversely correlated with the HRs of ischaemia in CMR (Figure 5 B and 5 D ).
Figure 5.
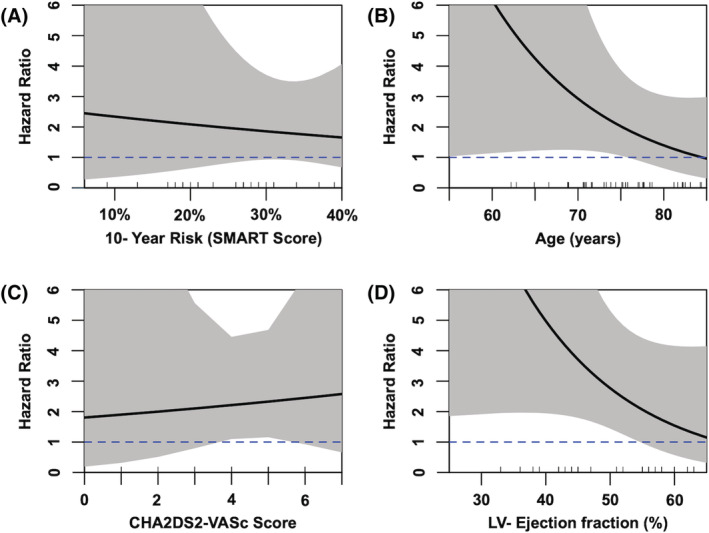
Hazard ratios for patients with inducible ischaemia in vasodilator CMR according to CHA2DS2‐VASc score and SMART score, LVEF, and age. Panel (A) shows the hazard ratio in patients in comparison with their 10% year risk for recurrent vascular events (as assessed by SMART score), and panel (B) shows the hazard ratio for inducible ischaemia in patients in relation to their age at baseline. Panel (C) shows the correlation with the CHA2DS2‐VASc score, while panel (D) shows the correlation with LVEF at rest (baseline). Grey area indicates 95% confidence interval; blue dashed is drawn at a hazard ratio of 1 for orientation. Small lines at the top of the x‐axis indicate the actual cases.
Discussion
Vasodilator stress perfusion CMR is feasible in patients with AF and has good discriminative power as a diagnostic tool. Over a median of almost 7 years, the longest follow‐up published to date, CMR findings were important predictors of MACE in this high‐risk population.
Our findings showed an excellent diagnostic accuracy of stress perfusion CMR (86.2%) in line with previous findings, 15 , 17 , 18 although a slight decrease compared with patients with sinus rhythm has to be accepted. 7 Of note, we believe that the true discriminative power of stress CMR is underestimated in our study, as most patients with an inconspicuous result for stress perfusion never had coronary angiography. The subset of patients that did undergo invasive angiography possibly had a higher clinical burden including persistent or aggravating symptoms to warrant an invasive approach despite a negative perfusion CMR. The findings on Regadenoson stress CMR show a promising potential to further improve specificity and accuracy in this challenging patient group. 16 Considering the poor performance of SPECT in patients with AF, 13 and the lack of reliable data on CT‐FFR regarding prognosis and especially in patients with AF, 27 vasodilator perfusion CMR constitutes a valuable diagnostic tool in patients with known or suspected CAD and AF.
In line with previous findings in patients with AF, our study highlights the long‐term prognostic importance of both LGE and perfusion deficits in vasodilator CMR with a long median follow‐up and a low number of patients lost to follow‐up. 9 , 10 Even in our high‐risk group with a high overall occurrence of MACE and a high median risk for recurrent CV events, 28 perfusion deficits in CMR were associated with a worse prognosis regarding the occurrence of MACE. While the short‐term implication of a perfusion CMR in patients with AF has been extensively studied 18 , 19 , 21 and while large outcome studies are awaited, 29 the data on long‐term prognosis are relatively scarce. A recently published cohort focusing on the influence of perfusion CMR using dipyridamole 20 similarly found a strong association with a positive stress test. Analogously, LGE was identified as an additional important predictor of MACE. Their total number of MACE was however lower. In addition to the slightly shorter median follow‐up time, the cohort in our study suffered from markedly higher morbidity, such as a more common history of CAD (59.8 vs. 24.7%), prior MI (18.3 vs. 10.8%), and ischaemic stroke (16.6 vs. 5.9%). Although not explicitly denoted by our colleagues, this allows for the assumption of a higher mean SMART score in our cohort, given the similar structures of the cohorts regarding ejection fraction, age, and gender. This hypothesis is supported by subgroup analysis that shows the importance of traditional risk factors and established scoring systems in our population, diminishing the discriminative meaning of inducible ischaemia in patients at very high baseline risk or very old age. When looking at the CIs in Figure 5 B , the CHA2DS2‐VASc score most probably has a rather modest or neglectable influence on the discriminatory power of stress CMR in patients with AF. Of note, in this ‘real‐world’ cohort, not all patients received oral anticoagulation as medical therapy was left at the treating physician's discretion, but the distribution of patients was similar among the two groups (86% of patients without ischaemia vs. 81% of patients with inducible ischaemia in CMR; P = 0.33; Table 1 ). The inverse correlation of the LVEF and ischaemia in CMR is less intuitively understood, indicating possibly an increased vulnerability by ischaemia in patients with low LVEF, which is in line with current indications for revascularization in these patients.
These subgroup analyses have to be judged with due precaution as the study population size produces broad CIs (grey areas in Figure 5 ) and further research including meta‐analyses of existing populations is necessary to truly assess the influence of these possible confounders on the discriminatory ability of CMR. In our opinion, the strong correlation of the CHA2DS2‐VASc score and a history of stroke as a predictor of future embolic events support the validity of our data. In line with previous findings, secondary endpoint analysis revealed CV death and MI as crucial elements of our composite endpoint. It should be mentioned that ischaemia in vasodilator perfusion CMR is not exclusively caused by macroscopically assessable coronary stenosis in the epicardial coronary arteries, but possibly as well by microvascular dysfunction. 30 Additionally, even if patients have macroscopic stenosis and receive adequate treatment, AF remains an important predictor of MACE. 31 In line with the ISCHEMIA and COURAGE trials, this might lead to speculation whether the importance of the presence of pathological CMR findings supersedes the fact whether these patients are subsequently treated by ICA or not, although our study is not designed to elaborate on this question. 6 , 32
Limitations
The present study has some limitations. First, qualitative visual analysis was the method used for interpreting perfusion images, so we cannot rule out different results, should a quantitative approach be used. However, we believe visual analysis may be more applicable to routine everyday clinical practice. Furthermore, the fact that we employed two MRI systems with different field strengths (1.5 and 3 T), while evenly distributed in our cohort, could potentially influence the diagnostic accuracy even when adhering to renowned standards of image acquisition and interpretation. 15 , 22 Second, the diagnosis of relevant CAD was solely at the treating clinicians' discretions without functional assessment by flow‐reserve measurements. Optimally, all patients should have been subjected to coronary angiography for a more accurate assessment of false‐negative results. However, this was clinically not warranted. We did not investigate whether performing revascularization in patients with ischaemia in CMR had a prognostic impact. We hope that, in the wake of the aforementioned ISCHEMIA and COURAGE trials, future research will be able to further address this interesting research question. 6 , 32
The choice of the SMART score to assess patients' risk of events is disputable. We followed recommendations from the European Society of Cardiology and decided to employ a score originally designed to assess the risk in patients with an established diagnosis of CAD. Given the high prevalence of CAD in our cohort (59.8% overall, 87.5% in those with inducible ischaemia), we believe this is the most adequate choice for a uniform assessment in our cohort. Considering the high‐risk nature of our cohort, our results might not be generalizable to cohorts with lower overall CV risk. Regarding prognostic evaluation, the retrospective inclusion of patients is possibly more prone to bias than a prospective approach due to a potential bias by loss to follow‐up. We sought to compensate for this by thorough investigation including phone calls, mailed questionnaires, as well as validation by the national death registry. Owing to the retrospective nature of our study, no data on possible changes of risk factors during follow‐up was available, which would enable the inclusion of time‐varying variables in our survival models. Lastly, necropsy data were not routinely available and the determining CV diseases as the cause of death relied on a thorough assessment of medical reports, consultation of the treating physicians, and the official death certificates from the German National Death Registry.
In conclusion, vasodilator stress CMR is a reliable non‐invasive stress test in patients with AF with excellent diagnostic accuracy. Even in high‐risk cohorts, it can accurately identify patients at an increased risk for CV death and MI, while the importance of revascularization vs. optimal medical therapy cannot be answered by our trial. Patients with a negative CMR stress test have a significantly lower risk for future cardiac events.
Conflict of interest
Dr Anker reports personal fees from Servier, outside the submitted work. All other authors have no conflicts of interest to declare that are relevant to the content of this article.
Funding
No funding was received for conducting this study.
Acknowledgements
We thank Corinna Else, Sarah Al‐Tabatabaee, Victoria Zieschang, and Collin Götze for their assistance in follow‐up.
Open Access funding enabled and organized by Projekt DEAL.
Weiss K. J., Nasser S. B., Bigvava T., Doltra A., Schnackenburg B., Berger A., Anker M. S., Stehning C., Doeblin P., Abdelmeguid M., Talat M., Gebker R., E‐Naggar W., Pieske B., and Kelle S. (2022) Long‐term prognostic value of vasodilator stress cardiac magnetic resonance in patients with atrial fibrillation. ESC Heart Failure, 9: 110–121. 10.1002/ehf2.13736
References
- 1. Virani Salim S, Alvaro A, Benjamin Emelia J, Bittencourt Marcio S, Callaway Clifton W, Carson April P, Chamberlain Alanna M, Chang Alexander R, Susan C, Delling Francesca N, Luc D, Elkind Mitchell SV, Ferguson Jane F, Myriam F, Khan Sadiya S, Kissela Brett M, Knutson Kristen L, Kwan Tak W, Lackland Daniel T, Lewis Tené T, Lichtman Judith H, Longenecker Chris T, Shane LM, Lutsey Pamela L, Martin Seth S, Kunihiro M, Moran Andrew E, Mussolino Michael E, Marma PA, Rosamond Wayne D, Roth Gregory A, Sampson Uchechukwu KA, Satou Gary M, Schroeder Emily B, Shah Svati H, Shay Christina M, Spartano Nicole L, Andrew S, Tirschwell David L, VanWagner Lisa B, Tsao Connie W. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation 2020; 141: e139–e596. [DOI] [PubMed] [Google Scholar]
- 2. Michniewicz E, Mlodawska E, Lopatowska P, Tomaszuk‐Kazberuk A, Malyszko J. Patients with atrial fibrillation and coronary artery disease—double trouble. Adv Med Sci 2018; 63: 30–35. [DOI] [PubMed] [Google Scholar]
- 3. Reinstadler SJ, Stiermaier T, Eitel C, Fuernau G, Saad M, Pöss J, de Waha S, Mende M, Desch S, Metzler B, Thiele H, Eitel I. Impact of atrial fibrillation during ST‐segment‐elevation myocardial infarction on infarct characteristics and prognosis. Circ Cardiovasc Imaging 2018; 11: e006955. [DOI] [PubMed] [Google Scholar]
- 4. Fauchier L, Bisson A, Bodin A, Herbert J, Angoulvant D, Danchin N, Cottin Y. Outcomes in patients with acute myocardial infarction and new atrial fibrillation: a nationwide analysis. Clin Res Cardiol 2021; 110: 1431–1438. [DOI] [PubMed] [Google Scholar]
- 5. Androulakis A, Aznaouridis KA, Aggeli CJ, Roussakis GN, Michaelides AP, Kartalis AN, Stougiannos PN, Dilaveris PE, Misovoulos PI, Stefanadis CI, Kallikazaros IE. Transient ST‐segment depression during paroxysms of atrial fibrillation in otherwise normal individuals: relation with underlying coronary artery disease. J Am Coll Cardiol 2007; 50: 1909–1911. [DOI] [PubMed] [Google Scholar]
- 6. Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O'Brien SM, Boden WE, Chaitman BR, Senior R, López‐Sendón J, Alexander KP, Lopes RD, Shaw LJ, Berger JS, Newman JD, Sidhu MS, Goodman SG, Ruzyllo W, Gosselin G, Maggioni AP, White HD, Bhargava B, Min JK, Mancini GBJ, Berman DS, Picard MH, Kwong RY, Ali ZA, Mark DB, Spertus JA, Krishnan MN, Elghamaz A, Moorthy N, Hueb WA, Demkow M, Mavromatis K, Bockeria O, Peteiro J, Miller TD, Szwed H, Doerr R, Keltai M, Selvanayagam JB, Steg PG, Held C, Kohsaka S, Mavromichalis S, Kirby R, Jeffries NO, Harrell FE, Rockhold FW, Broderick S, Ferguson TB, Williams DO, Harrington RA, Stone GW, Rosenberg Y, ISCHEMIA Research Group . Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 2020; 382: 1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamon M, Fau G, Née G, Ehtisham J, Morello R, Hamon M. Meta‐analysis of the diagnostic performance of stress perfusion cardiovascular magnetic resonance for detection of coronary artery disease. J Cardiovasc Magn Reson 2010; 12: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nandalur KR, Dwamena BA, Choudhri AF, Nandalur MR, Carlos RC. Diagnostic performance of stress cardiac magnetic resonance imaging in the detection of coronary artery disease. J Am Coll Cardiol 2007; 50: 1343–1353. [DOI] [PubMed] [Google Scholar]
- 9. Heitner JF, Kim RJ, Kim HW, Klem I, Shah DJ, Debs D, Farzaneh‐Far A, Polsani V, Kim J, Weinsaft J, Shenoy C, Hughes A, Cargile P, Ho J, Bonow RO, Jenista E, Parker M, Judd RM. Prognostic value of vasodilator stress cardiac magnetic resonance imaging: a multicenter study with 48 000 patient‐years of follow‐up. JAMA Cardiol 2019; 4: 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kwong Raymond Y, Yin G, Kevin S, Scott B, Shuaib A, Kana F, Wei W, Ankur P, Yi‐Yun C, Ronald MJ, Sebastian B, Arai Andrew E, Patricia BW, Shanbhag Sujata M, Patel Amit R, Akhil N, Afshin F‐F, Benjamin R, Heitner John F, Ho Jean Y, Jaspal S, Chetan S, Andrew H, Leung Steve W, Meera M, Gonzalez Jorge A, Sandeep M, Shah Dipan J, Dany D, Raman Subha V, Avirup G, Ferrari Victor A, Jeanette S‐M, Rory H, Matthias S, Simonetti Orlando P. Cardiac magnetic resonance stress perfusion imaging for evaluation of patients with chest pain. J Am Coll Cardiol 2019; 74: 1741–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arbab‐Zadeh A, Miller JM, Rochitte CE, Dewey M, Niinuma H, Gottlieb I, Paul N, Clouse ME, Shapiro EP, Hoe J, Lardo AC, Bush DE, de Roos A, Cox C, Brinker J, Lima JAC. Diagnostic accuracy of CT coronary angiography according to pretest probability of coronary artery disease and severity of coronary arterial calcification: the CorE‐64 international, multicenter study. J Am Coll Cardiol 2012; 59: 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck‐Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ, ESC Scientific Document Group . ESC Guidelines for the diagnosis and management of chronic coronary syndromes: the Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J 2019; 2020: 407–477. [DOI] [PubMed] [Google Scholar]
- 13. Smit MD, Tio RA, Slart RHJA, Zijlstra F, Van Gelder IC. Myocardial perfusion imaging does not adequately assess the risk of coronary artery disease in patients with atrial fibrillation. Eur Eur Pacing Arrhythm Card Electrophysiol J Work Groups Card Pacing Arrhythm Card Cell Electrophysiol Eur Soc Cardiol 2010; 12: 643–648. [DOI] [PubMed] [Google Scholar]
- 14. Doltra A, Schneeweis C, Fleck E, Kelle S. Cardiac magnetic resonance for prognostic assessment: present applications and future directions. Expert Rev Cardiovasc Ther 2014; 12: 771–782. [DOI] [PubMed] [Google Scholar]
- 15. Yang K, Yu S‐Q, Lu M‐J, Zhao S‐H. Comparison of diagnostic accuracy of stress myocardial perfusion imaging for detecting hemodynamically significant coronary artery disease between cardiac magnetic resonance and nuclear medical imaging: a meta‐analysis. Int J Cardiol 2019; 293: 278–285. [DOI] [PubMed] [Google Scholar]
- 16. Bieging ET, Haider I, Adluru G, Chang L, Suksaranjit P, Likhite D, Shaaban A, Jensen L, Wilson BD, McGann CJ, DiBella E. Rapid rest/stress regadenoson ungated perfusion CMR for detection of coronary artery disease in patients with atrial fibrillation. Int J Cardiovasc Imaging 2017; 33: 1781–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greulich S, Steubing H, Birkmeier S, Grün S, Bentz K, Sechtem U, Mahrholdt H. Impact of arrhythmia on diagnostic performance of adenosine stress CMR in patients with suspected or known coronary artery disease. J Cardiovasc Magn Reson 2015; 17: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oebel S, Paetsch I, Stegmann C, Kircher S, Sommer P, Arya A, Lindemann F, Bollmann A, Hindricks G, Jahnke C. Combined single‐session cardiovascular magnetic resonance: stress perfusion and three‐dimensional pulmonary vein angiography for stratification of atrial fibrillation patients with chest pain syndromes prior to catheter ablation. EP Eur 2019; 21: 1809–1816. [DOI] [PubMed] [Google Scholar]
- 19. Ingkanisorn WP, Kwong RY, Bohme NS, Geller NL, Rhoads KL, Dyke CK, Paterson DI, Syed MA, Aletras AH, Arai AE. Prognosis of negative adenosine stress magnetic resonance in patients presenting to an emergency department with chest pain. J Am Coll Cardiol 2006; 47: 1427–1432. [DOI] [PubMed] [Google Scholar]
- 20. Pezel T, Sanguineti F, Kinnel M, Landon V, Toupin S, Unterseeh T, Louvard Y, Champagne S, Morice MC, Hovasse T, Garot P, Garot J. Feasibility and prognostic value of vasodilator stress perfusion CMR in patients with atrial fibrillation. JACC Cardiovasc Imaging 2021; 14: 379–389. [DOI] [PubMed] [Google Scholar]
- 21. Lerakis S, McLean DS, Anadiotis AV, Janik M, Oshinski JN, Alexopoulos N, Zaragoza‐Macias E, Veledar E, Stillman AE. Prognostic value of adenosine stress cardiovascular magnetic resonance in patients with low‐risk chest pain. J Cardiovasc Magn Reson 2009; 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schulz‐Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff‐Brenkenhoff F, Kramer CM, Pennell DJ, Plein S, Nagel E. Standardized image interpretation and post‐processing in cardiovascular magnetic resonance—2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post‐Processing. J Cardiovasc Magn Reson Off J Soc Cardiovasc Magn Reson 2020; 22: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, Limacher MC, Mahaffey KW, Mehran R, Nissen SE, Smith EE, Targum SL. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol 2015; 66: 403–469. [DOI] [PubMed] [Google Scholar]
- 24. Kristian T, Alpert Joseph S, Jaffe Allan S, Chaitman Bernard R, Bax Jeroen J, Morrow David A, White Harvey D. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 2018; 72: 2231–2264. [DOI] [PubMed] [Google Scholar]
- 25. Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest 2010; 137: 263–272. [DOI] [PubMed] [Google Scholar]
- 26. Dorresteijn JAN, Visseren FLJ, Wassink AMJ, Gondrie MJA, Steyerberg EW, Ridker PM, Cook NR, van der Graaf Y, SMART Study Group . Development and validation of a prediction rule for recurrent vascular events based on a cohort study of patients with arterial disease: the SMART risk score. Heart 2013; 99: 866–872. [DOI] [PubMed] [Google Scholar]
- 27. Taron J, Foldyna B, Eslami P, Hoffmann U, Nikolaou K, Bamberg F. Cardiac computed tomography—more than coronary arteries? A clinical update. ROFO Fortschr Geb Rontgenstr Nuklearmed 2019; 191: 817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dorresteijn JAN, Visseren FLJ, Wassink AMJ, Gondrie MJA, Steyerberg EW, Ridker PM, Cook NR, van der Graaf Y, SMART Study Group . Development and validation of a prediction rule for recurrent vascular events based on a cohort study of patients with arterial disease: the SMART risk score. Heart Br Card Soc 2013; 99: 866–872. [DOI] [PubMed] [Google Scholar]
- 29. Bohnen S, Avanesov M, Jagodzinski A, Schnabel RB, Zeller T, Karakas M, Schneider J, Tahir E, Cavus E, Spink C, Radunski UK, Ojeda F, Adam G, Blankenberg S, Lund GK, Muellerleile K. Cardiovascular magnetic resonance imaging in the prospective, population‐based, Hamburg City Health cohort study: objectives and design. J Cardiovasc Magn Reson Off J Soc Cardiovasc Magn Reson 2018; 20: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wijesurendra RS, Alexander L, Francesco N, Ntusi Ntobeko AB, Karamitsos TD, Yaver B, Matthew G, Kim R, Betts TR, Michael J‐H, Ferreira VM, Stefan N, Barbara C. Myocardial perfusion is impaired and relates to cardiac dysfunction in patients with atrial fibrillation both before and after successful catheter ablation. J Am Heart Assoc 2018; 7: e009218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bramlage P, Cuneo A, Zeymer U, Hochadel M, Richardt G, Silber S, Senges J, Nienaber CA, Tebbe U, Kuck K‐H. Prognosis of patients with atrial fibrillation undergoing percutaneous coronary intervention receiving drug eluting stents. Clin Res Cardiol 2013; 102: 289–297. [DOI] [PubMed] [Google Scholar]
- 32. Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GBJ, Weintraub WS. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356: 1503–1516. [DOI] [PubMed] [Google Scholar]


