Abstract
Aims
Recent trials evaluating the effect of aspirin in the primary prevention of cardiovascular disease showed little or no benefit. However, the role of aspirin on the risk of incident heart failure (HF) remains elusive. This study aimed to evaluate the role of aspirin use on HF incidence in primary and secondary prevention and whether aspirin use increases the risk of incident HF in patients at risk.
Methods and results
Data from 30 827 patients at risk for HF enrolled in six observational studies were analysed [women 33.9%, mean age (±standard deviation) 66.8 ± 9.2 years]. Cardiovascular risk factors and aspirin use were recorded at baseline, and patients were followed up for the first incident of fatal or non‐fatal HF. The association of incident HF with aspirin use was assessed using multivariable‐adjusted proportional hazard regression, which accounted for study and cardiovascular risk factors. Over 5.3 years (median; 5th–95th percentile interval, 2.1–11.7 years), 1330 patients experienced HF. The fully adjusted hazard ratio (HR) associated with aspirin use was 1.26 [95% confidence interval (CI) 1.12–1.41; P ≤ 0.001]. Further, in a propensity‐score‐matched analysis, the HR was 1.26 (95% CI 1.10–1.44; P ≤ 0.001). In 22 690 patients (73.6%) without history of cardiovascular disease, the HR was 1.27 (95% CI 1.10–1.46; P = 0.001).
Conclusions
In patients, at risk, aspirin use was associated with incident HF, independent of other risk factors. In the absence of conclusive trial evidence, our observations suggest that aspirins should be prescribed with caution in patients at risk of HF or having HF.
Keywords: Aspirin use, Heart failure, Primary prevention, Secondary prevention, Cardiovascular diseases
Introduction
Heart failure (HF) is a clinical syndrome with abnormal cardiac function and structure. 1 , 2 Due to comorbidities and diminished cardiac output, HF is qualified as a pro‐thrombotic condition, which might indicate the initiation of antithrombotic treatment. 3 On the other hand, antithrombotic treatment, particularly aspirin, is prescribed in the prevention of cardiovascular events [cardiovascular disease (CVD)], 4 which may consequently lead to HF. Recent trials showed a little benefit of aspirin use in primary prevention, 5 , 6 , 7 and the effect of aspirin in the secondary prevention of CVD events 8 has been well established. However, the use of aspirin in HF is still controversial. Earlier prospective randomized trials WATCH 9 and WARCEF 3 reported no beneficial effect of aspirin in HF patients compared with other antithrombotic therapy. The WASH trial, 10 which did not include an antithrombotic arm, found no evidence that aspirin is effective or safe in patients with HF. In contrast, a retrospective HF patients cohort study reported that low‐dose aspirin of 75 mg/day was associated with reduced mortality risk. 11 Also, a more recent study 12 with Danish residents, included 12 277 patients with new‐onset HF, was unable to detect an association between low‐dose aspirin use and the composite outcome of all‐cause mortality, admission for myocardial infarction, and admission for stroke in HF patients. Interestingly, this study reported that aspirin use was associated with an increased risk of re‐admissions for HF. Again, in context to recent trials, 5 , 6 , 7 the effect of aspirin on the HF remained understudied and generated uncertainty. 8 , 13 To address these knowledge gaps, we investigated the association between aspirin use and incident HF in a large sample of participants free of HF enrolled in the HOMAGE study.
Methods
Heart ‘Omics’ in AGEing (HOMAGE) database is constructed and stored at the Studies Coordinating Centre in Leuven, Belgium. The HOMAGE partners contributed with anonymized data and confirmed that their studies complied with the Helsinki Declaration, 14 and all participants provided written informed consent. The HOMAGE database has been described in detail elsewhere, 2 , 15 and the data were locked on 14 March 2017.
Study population
The HOMAGE database consisted of subject‐level data of 46 437 participants from 21 studies. Studies were eligible for inclusion in this analysis if the information on aspirin use and the incidence of fatal and/or non‐fatal HF was available, with at least 20 HF events. We excluded 3 HF studies (n = 1073) and 12 studies (n = 11 160) without information on HF incidence. We included the participants that were enrolled in six studies: The Anglo‐Scandinavian Cardiac Outcomes Trial (ASCOT), 16 The Flemish Study on Environment, Genes and Health Outcome (FLEMENGHO), 17 The Health Aging and Body Composition study (HEALTH ABC), 18 The HULL LIFELAB patient study, 15 Valutazione Della PREvalenza di DIsfunzione Cardiaca asinTOmatica e di scompenso cardiaco (PREDICTOR), 19 and The Prospective Study of Pravastatin in the Elderly at Risk (PROSPER). 20 Of 34 204 participants with a history of HF (n = 2437), no information on aspirin use at baseline (n = 88), history of valvular heart disease (n = 8) or history of coronary artery bypass grafting (n = 51), anticoagulant use at baseline (n = 333), below the age of 40 years (n = 187), and lost to follow‐up (n = 273) were excluded. In total, 30 827 participants were included in the analyses (Supporting Information, Figure S1 ).
Derivation set
The ASCOT, a prospective, randomized, open, blinded endpoint trial with high‐risk hypertensive patients, recruited in Scandinavian countries, the UK, and Ireland (1998–2000), was used as the derivation set. 16
Validation set
The validation set consisted of the FLEMENGHO, 17 HEALTH ABC, 18 HULL LIFE LAB, 15 PREDICTOR, 19 and PROSPER 20 studies.
The FLEMENGHO is a population study of a random sample of households living in Northern Belgium, recruited between 1985 and 1999. From 2005 until 2010, participants were invited for a follow‐up examination at the field centre, including echocardiography. 17 The HEALTH ABC is a cohort of women and men (aged ≥70 years), randomly recruited among Medicare beneficiaries residing in Pittsburg, Pennsylvania, and Memphis, Tennessee, USA, 18 between April 1997 and June 1998. The HULL LIFE LAB patient cohort consists of consecutive patients referred to a community HF clinic at the Hull Royal Infirmary Hospital, UK, for investigation of suspected HF between 2001 and 2014. 15 The PREDICTOR is a population study in older participants (65–84 years) randomly selected from the regional population registry of four cities in the Lazio region in central Italy between 2007 and 2010. 19 The PROSPER is a randomized, double‐blinded, placebo‐controlled trial of women and men older than 70 years, with a history of vascular disease or at high risk for developing vascular disease. From 1997 until 1999, patients were enrolled in Scotland, Ireland, and the Netherlands. 20
Ascertainment of heart failure
At baseline, all participants were free of HF. The diagnosis of first incident HF required hospitalization. Hospital records were examined, and HF was defined based on a combination of signs and symptoms, including chest radiography with fluid congestion or echocardiogram. 1 Further confirmation was made by reviewing medical records. More detailed information on each study is provided in the supporting information. The primary outcome of the study was a composite of fatal and non‐fatal HF. The study population was followed up until death, HF incident, or end of the study.
Assessment of aspirin use
The information for the use of aspirin [acetylsalicylic acid (ATC code B01AC06)] at baseline was recorded from all participants. At the time of the enrolment, participants were either aspirin users or non‐users and had no other antithrombotic treatment. Among the aspirin users, we registered the main therapeutic indications, such as a history of hypertension, diabetes mellitus, myocardial infarction, coronary heart disease, atrial fibrillation, or a history of cerebrovascular events.
Other information
Other baseline information included smoking status, alcohol use, anthropometric characteristics, co‐medication use, routine haematological and biochemical measurements including total cholesterol, high‐density cholesterol (HDL), and creatinine. Body mass index (BMI) was calculated as weight in kilograms divided by height in metres squared. Diabetes mellitus was a fasting glucose concentration of ≥7.0 mmol/L or non‐fasting blood glucose concentration of ≥11.1 mmol/L or the use of anti‐diabetic medication. Hypertension was an office blood pressure ≥ 140/90 mmHg or the use of antihypertensive treatment. The previous history of CVDs was verified based on medical records.
Statistical analysis
SAS software Version 9.4 (SAS Institute, Cary; NC, USA) was used for database management and statistical analysis. Continuous variables were presented as mean ± standard deviation and categorical variables as proportions. For comparison of means and proportions, we applied the large‐sample z‐test and χ 2 test, respectively. Missing values for the covariates such as BMI (n = 14), systolic (n = 9) and diastolic (n = 10) blood pressure, heart rate (n = 136), total cholesterol (n = 236), HDL‐cholesterol (n = 277), and creatinine (n = 6392) were interpolated from the regression slope on age after stratification for study and sex. To compute 95% confidence intervals (CIs) of rates, we applied the formula as , where R and T are the rates and the number of individuals used to compute the rate. A two‐sided P‐value of <0.05 was used as statistical significance.
We assessed the aspirin use in multivariable‐adjusted Cox proportional hazards regression. The proportional hazards assumption was checked by the Kolmogorov‐type supremum test and by testing the interaction between follow‐up duration and aspirin treatment. We implemented four levels of adjustment. The first model adjusted for sex and age. In the second model, we additionally adjusted for BMI, smoking and alcohol drinking, systolic and diastolic blood pressure, heart rate, total cholesterol/HDL ratio, and creatinine. In the third model, we added treatment with renin‐angiotensin‐aldosterone‐system (RAAS) inhibitors, calcium channel blockers, diuretics, beta‐blockers, and lipid‐lowering treatment. And finally, in the fourth model, we additionally adjusted for history of hypertension, diabetes mellitus, and history of CVD. In all models, we accounted for the clustering of participants within studies by including cohorts as a random effect.
The consistencies of the results were assessed in a four‐step approach. First, we used the propensity‐score matching method 21 to match aspirin users with non‐users. Using logistic regression, we calculated the propensity of aspirin use using all covariables as listed in Supporting Information, Table S3 . Second, we performed sensitivity analyses by excluding all participants with a known history of CVD. Third, to minimize the influence of the reverse causation, we excluded the participants who developed HF within the first 2 years of the follow‐up. Finally, we performed stratified analyses to check the consistency of the results according to sex, study‐specific medians for age and body weight, 22 and categories of systolic and diastolic blood pressure. Furthermore, using stratified analyses, we also investigated drug interactions.
Results
Baseline characteristics
The baseline characteristics of the participants in the derivation and validation sets, and in the HOMAGE set, are summarized in Table 1 . The total study population (n = 30 827) included 10 451 (33.9%) women, 6640 (21.5%) diabetics, 8144 (26.4%) current smokers, and 20 561 (66.7%) participants reporting alcohol consumption. Age averaged 66.8 (standard deviation 9.2) years. Of the 26 453 (85.5%) hypertensive patients, 21 633 (81.7%) were taking antihypertensive drugs. The number of participants reporting a history of myocardial infarction, coronary heart disease, stroke, or atrial fibrillation was 874 (2.8%), 8137 (26.4%), 2987 (9.6%), and 343 (1.1%), respectively. At baseline, a total of 7698 (24.9%) participants were treated with aspirin.
TABLE 1.
Baseline characteristics
| Characteristic | All | Derivation set | Validation set | P |
|---|---|---|---|---|
| Number patients | 30 827 | 19 257 | 11 570 | |
| Number with characteristics, (%) | ||||
| Woman | 10 451 (33.9) | 4515 (23.4) | 5936 (51.3) | <0.001 |
| Smoking | 8144 (26.4) | 5893 (30.6) | 2251 (19.4) | <0.001 |
| Alcohol intake | 20 561 (66.7) | 14 294 (74.2) | 6267 (54.1) | <0.001 |
| Hypertension | 26 453 (85.8) | 19 257 (100) | 7196 (62.2) | <0.001 |
| Diabetes mellitus | 6640 (21.5) | 5145 (26.7) | 1495 (12.9) | <0.001 |
| History of myocardial infarction | 874 (2.8) | 0 (0) | 874 (7.5) | <0.001 |
| History of coronary artery disease | 8137 (26.4) | 5284 (27.4) | 2853 (24.6) | <0.001 |
| History of atrial fibrillation | 343 (1.1) | 230 (1.2) | 113 (1.0) | 0.08 |
| History of cerebrovascular incident | 2987 (9.6) | 2113 (10.9) | 874 (7.5) | <0.001 |
| Antihypertensive treatment | 21 633 (81.7) | 15 591 (80.9) | 6042 (52.2) | <0.001 |
| Use of RAAS | 8750 (28.3) | 6129 (31.8) | 2621 (22.6) | <0.001 |
| Use of calcium channel blockers | 7918 (25.7) | 5515 (28.6) | 2403 (20.7) | <0.001 |
| Use of diuretics | 9220 (29.9) | 5490 (28.5) | 3730 (32.2) | <0.001 |
| Use of beta‐blockers | 8658 (28.1) | 6158 (31.9) | 2500 (21.6) | <0.001 |
| Use of statins | 9158 (29.7) | 5134 (26.6) | 4024 (34.7) | <0.001 |
| Use of aspirin | 7698 (24.9) | 3688 (19.1) | 4010 (34.6) | <0.001 |
| Mean of characteristic ± SD | ||||
| Age, years | 66.8 ± 9.2 | 62.9 ± 8.4 | 73.3 ± 6.3 | <0.001 |
| Systolic blood pressure, mmHg | 157.1 ± 21.8 | 164.0 ± 18.0 | 145.7 ± 22.7 | <0.001 |
| Diastolic blood pressure, mmHg | 89.1 ± 13.2 | 94.6 ± 10.3 | 79.8 ± 12.2 | <0.001 |
| Heart rate, beats per min | 70.0 ± 12.5 | 71.1 ± 12.6 | 66.8 ± 11.8 | <0.001 |
| Body mass index, kg/m2 | 28.0 ± 4.6 | 28.7 ± 4.7 | 27.0 ± 4.5 | <0.001 |
| Total cholesterol/HDL ratio | 4.6 ± 1.3 | 4.8 ± 1.3 | 4.3 ± 1.2 | <0.001 |
| Serum creatinine, μmol/L | 96.9 ± 18.3 | 98.1 ± 14.0 | 95.0 ± 23.8 | <0.001 |
HDL, high‐density lipoprotein; RAAS, angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker; SD, standard deviation.
Average values are arithmetic mean (SD) for continuous variables or numbers (percentage) for categorical variables. P‐values were derived by the large‐sample z‐test and the χ 2 statistic.
Incidence of heart failure in aspirin users and non‐users
Median follow‐up was 5.3 years (5th to 95th percentile interval, 2.1 to 11.7 years). In the HOMAGE set during 164 913 person‐years of follow‐up, 1330 participants experienced fatal or non‐fatal HF with an incident rate of 14.5 (95% CI 13.4–15.7) per 1000 person‐years in the aspirin group versus 5.9 (95% CI 5.5–6.4) per 1000 person‐years in the non‐aspirin group. In the discovery set during 105 623 person‐years of follow‐up, 293 incident cases of HF occurred, with a rate of 4.3 (95% CI 3.5–5.3) per 1000 person‐years in the aspirin group versus 2.4 (95% CI 2.1–2.7) per 1000 person‐years in the non‐aspirin group. In the validation set (Figure 1 ), during 59 289 person‐years of follow‐up, 1037 incident HF occurred, an incident rate of 24.4 (95% CI 22.4–26.7) per 1000 person‐years in the aspirin group versus 13.8 (95% CI 12.7–15.0) per 1000 person‐years in the non‐aspirin group. Supporting Information, Table S6 lists the number of events per individual study.
FIGURE 1.
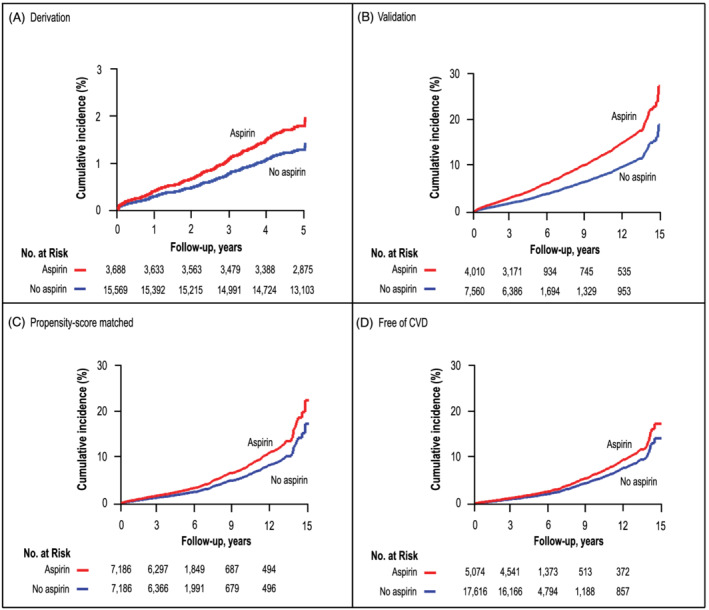
Panels (A), (B), (C), and (D) show study, sex‐standardized, and age‐standardized cumulative incidence of heart failure in participants using aspirin use (red line) and non‐using aspirin (blue line). The tabulated numbers represent the participants at risk with intervals in years. P‐value is for the significance of the difference between aspirin use and non‐use.
Association between incident heart failure and aspirin use
Table 2 shows the hazard ratios (HRs) associated with aspirin intake in the derivation and validation sets, as well as in the whole analysis data. The proportional hazard assumption was met for all models. Irrespective of the data set used and the level of adjustment, the risk of HF was consistently and positively associated with aspirin use. As expected, the HRs tended to weaken with tighter adjustment ranging from 1.43 (P = 0.006) to 1.32 (P = 0.03) in the derivation cohort, from 1.52 (P < 0.001) to 1.17 (P = 0.02) for the validation cohort, and from 1.52 (P < 0.001) to 1.26 (P < 0.001) in all study participants (Table 2 ).
TABLE 2.
Association between heart failure and aspirin use
| Aspirin use | Yes | No | Hazard ratio (95% CI) | P‐value |
|---|---|---|---|---|
| Derivation | HF events/at risk | HF events/at risk | HF events/at risk | |
| Model 1 | 86/3688 | 207/15 569 | 1.40 (1.09–1.81) | 0.009 |
| Model 2 | 86/3688 | 207/15 569 | 1.43 (1.11–1.84) | 0.006 |
| Model 3 | 86/3688 | 207/15 569 | 1.32 (1.02–1.71) | 0.03 |
| Model 4 | 86/3688 | 207/15 569 | 1.33 (1.03–1.72) | 0.03 |
| Validation | ||||
| Model 1 | 497/4010 | 540/7560 | 1.47 (1.31–1.68) | <0.001 |
| Model 2 | 497/4010 | 540/7560 | 1.52 (1.34–1.72) | <0.001 |
| Model 3 | 497/4010 | 540/7560 | 1.39 (1.22–1.57) | <0.001 |
| Model 4 | 497/4010 | 540/7560 | 1.17 (1.03–1.34) | 0.02 |
| HOMAGE | ||||
| Model 1 | 583/7698 | 747/23 129 | 1.47 (1.32–1.65) | <0.001 |
| Model 2 | 583/7698 | 747/23 129 | 1.52 (1.36–1.69) | <0.001 |
| Model 3 | 583/7698 | 747/23 129 | 1.39 (1.24–1.55) | <0.001 |
| Model 4 | 583/7698 | 747/23 129 | 1.26 (1.12–1.41) | <0.001 |
CI, confidence interval; HF, heart failure; HR, hazard ratio.
Estimates (HR), given with a 95% confidence interval, represent the risk of heart failure on exposure to the aspirin. Model 1—adjusted for study, sex, and age; Model 2—Model 1 + body mass index, smoking and drinking, systolic and diastolic blood pressure, heart rate, total cholesterol/high‐density lipoprotein ratio, and creatinine; Model 3—Model 2 + treatment with renin‐angiotensin‐aldosterone inhibitors, calcium channel blockers, diuretics, beta‐blockers, and statins; Model 4—Model 3 + history of cardiovascular diseases.
Sensitivity analyses
As shown in Table 3 , sensitivity analyses produced confirmatory results, if participants were matched based on a propensity score including all covariables (HR 1.26; P < 0.001), if analyses were limited to participants without a history of CVD at baseline (HRs in Model 3, 1.27; P = 0.001), and if participants with incident HF within 2 years of enrolment were excluded (HR in Model 4, 1.23; P = 0.004).
TABLE 3.
Association between heart failure and aspirin use in participants in propensity‐score‐matched participants, participants without a history of cardiovascular diseases, participants without incident heart failure within the first 2 years of follow‐up
| Aspirin use | Yes | No | Hazard ratio (95% CI) | P‐value |
|---|---|---|---|---|
| Propensity score | HF events/at risk | HF events/at risk | HF events/at risk | |
| Model | 496/7186 | 396/7186 | 1.26 (1.10–1.44) | <0.001 |
| Free of CVD | ||||
| Model 1 | 283/5074 | 577/17 616 | 1.29 (1.12–1.49) | <0.001 |
| Model 2 | 283/5074 | 577/17 616 | 1.33 (1.15–1.53) | <0.001 |
| Model 3 | 283/5074 | 577/17 616 | 1.27 (1.10–1.46) | 0.001 |
| 2 years excluded | ||||
| Model 1 | 371/7486 | 515/22 897 | 1.40 (1.22–1.61) | <0.001 |
| Model 2 | 371/7486 | 515/22 897 | 1.44 (1.25–1.65) | <0.001 |
| Model 3 | 371/7486 | 515/22 897 | 1.33 (1.16–1.53) | <0.001 |
| Model 4 | 371/7486 | 515/22 897 | 1.23 (1.06–1.41) | 0.004 |
CI, confidence interval; CVD, cardiovascular disease; HF, heart failure.
Hazard ratio (HR), given with a 95% confidence interval, represents the risk of heart failure in exposure to the aspirin. Model 1—adjusted for study, sex, and age; Model 2—Model 1 + body mass index, smoking and drinking, systolic and diastolic blood pressure, heart rate, total cholesterol/high‐density lipoprotein ratio, and creatinine; Model 3—Model 2 + treatment with renin‐angiotensin‐aldosterone inhibitors, calcium channel blockers, diuretics, beta‐blockers, and statins; Model 4—Model 3 + history of cardiovascular diseases.
The association between the HF risk and aspirin use was consistent across strata of systolic (Figure 2 A ) and diastolic (Figure 2 B ) blood pressure at entry. Interaction analyses (Figure 2 C ) suggested that the HF risk was associated with slightly greater in patients not taking diuretics versus those on diuretics at entry (multivariable‐adjusted HRs, 1.38 vs. 1.14; P‐value for interaction, 0.02) and in patients taking statins versus those not on statin treatment (multivariable‐adjusted HRs, 1.50 vs. 1.19; P‐value for interaction, 0.04).
FIGURE 2.
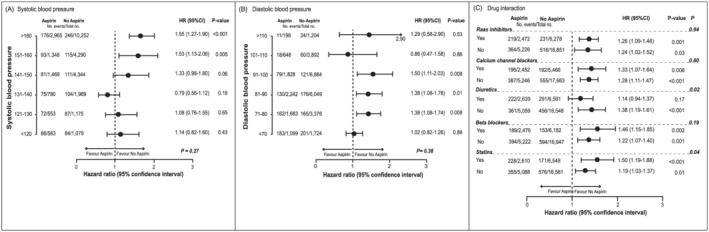
Panels (A) and (B) represent the plotted hazard ratio of systolic and diastolic blood pressure categories in stratified analyses fully adjusted for all risk factors presented in Table 1 . Panel (C) represents the risk of heart failure based on the concomitant use of antihypertensive drugs by class and statin medication for participants treated and not treated with aspirin and plotted as a hazard ratio and 95% confidence interval. P‐value is for the significance of the difference between aspirin use and non‐use. P‐interaction represents the P‐value for the interaction.
Stratified analyses
Stratified analyses were conducted to assess the differences between the categories of sex, age, and body weight in three sets, using a study‐specific median. In the HOMAGE set, we found an association of increased risk for incident HF in men (HR 1.37, 95% CI 1.17–1.59; P‐interaction = 0.04) and an association of increased risk for incident HF among the participants aged >69 years (P‐interaction = 0.03) (Supporting Information, Table S2.2 ).
Discussion
In this large‐scale study with participants free of HF at enrolment, aspirin use was associated with a higher risk of HF. The results of the present study with aspirin use are the first to report an increased risk of HF, among participants at risk for HF.
Uncertainty on aspirin use has been reflected in current guideline recommendations 4 , 23 and aspirin use for primary prevention has been controversial even when compared with aspirin use in patients with established atherosclerotic CVD. 22 , 24 Considering the evidence on the risk of HF, the current study demonstrates that cardiovascular benefits associated with aspirin use on HF events require further clarity. Further, recent published clinical trials assessed the effect of aspirin in primary prevention, ASPREE reported 88 HF hospitalizations (excluding fatal HF) with an HR 1.07 (95% CI 0.79–1.44), 7 ASCEND reported 95 HF incidents with a rate ratio of 0.84 (95% CI 0.64–1.10), 5 and ARRIVE did not report HF. 6 Similar HF incidents among aspirin‐treated patients were observed also in ASCOT and PROSPER trials with 86 and 113, respectively. When we focused on participants with low cardiovascular risk in primary prevention, similar to recent trials with few CVD risk factors, aspirin use was associated with increased risk for HF and the results were confirmatory. Although the differences between studies exist, as trials with primary prevention recruited the healthier participants with fewer risk factors and lower cardiovascular risk in general, yet in ASPREE, a positive trend between aspirin use and HF hospitalizations was present. Given that ASCOT and PROSPER primarily tested the effect of statins, we have observed a significant interaction between aspirin and statins in concomitant use. Therefore, the observed trend in ASPREE might be explained based on the interaction between these drugs. Alternatively, in ASCEND, 19% of aspirin users were on concomitant use of diuretics. When we assessed the role of the diuretics, we found that the use of diuretics is associated with a reduced risk of HF in aspirin users, which might influence into lower rate ratio within ASCEND.
Aspirin use remains controversial in secondary prevention. 8 , 25 , 26 , 27 In this regard, we elaborated on the role of aspirin in secondary prevention, among patients with overt CVD, and findings showed a similar trend with primary prevention, where aspirin was associated with increased risk for HF. Previous studies have assessed the effects of aspirin use in cardiovascular events or secondary endpoints among HF patients including HF hospitalizations as well. An observational retrospective study investigated aspirin use in 1476 HF patients and reported that low‐dose aspirin of 75 mg/day was associated with a reduction of deaths in HF patients but did not reduce HF hospitalizations. 11 Another recent study that included 12 277 patients with new‐onset of HF, using propensity‐matched analysis, reported no association between low‐dose aspirin and composite outcomes of all‐cause mortality, myocardial infarction, or stroke. This study also reported that aspirin use was associated with an increased risk of re‐hospitalizations for HF. 12 Earlier, the WASH trial randomized 279 HF patients using aspirin versus warfarin or no antithrombotic treatment, and the trial reported an increased risk for HF hospitalizations in the aspirin arm. 10 In Addition, the WATCH trial compared aspirin versus warfarin in terms of safety and effectiveness in patients with chronic HF, and the trial was prematurely terminated due to a non‐significant difference between tested drugs, yet the trial reported significantly higher hospitalization events in aspirin arm 218 vs. 155 in warfarin arm (P < 0.001). 9 Therefore, both studies suggested that an increased risk for HF hospitalization was observed among patients receiving aspirin. Also, the WARCEF trial 3 included 2305 HF patients and reported no significant difference in HF hospitalization between the warfarin group and the aspirin group, but yet comparable event rates for HF hospitalizations (warfarin 6.7 vs. aspirin 5.6). 3 And more recently, a COMPASS trial, compared with aspirin alone vs. rivaroxaban‐plus‐aspirin in patients with a history of stable atherosclerotic vascular disease and reported 192 incident HF in the aspirin‐alone arm versus 197 in the rivaroxaban‐plus‐aspirin arm compared and 191 in the rivaroxaban‐alone arm. 28
Accounting for the risk factors, given that hypertension is a major risk factor for cardiovascular outcomes, 29 , 30 , 31 , 32 including HF, 33 we investigated the interaction of systolic and diastolic blood pressure and the association of aspirin use with the risk of HF. In participants with a systolic blood pressure of 131 mmHg or higher, aspirin use was associated with increased risk of HF; likewise, diastolic blood pressure of 71 mmHg or higher was significantly associated with increased risk of HF. There was no significant interaction between systolic or diastolic blood pressure with aspirin use. In the context of blood pressure, patients on diuretic use potentially benefited from diuretics that contribute to better control of blood pressure 34 while non‐users were harmed due to renal function impairment, caused by aspirin. 12 , 35 , 36 Aspirin acts in kidneys through water and salt retention mechanisms while our data show that aspirin interacts with diuretics in concomitant use. Diuretics provide a beneficial role and may attenuate risk for incident HF in effect‐modification fashion, through accelerated diuresis and increased excretion of aspirin. 34 Another biological plausible explanation is aspirin has been reported to induce angiogenesis and neovascularization within atherosclerotic plaque and promote the presence of intraplaque haemorrhage. 37 Further, plaque progression mechanisms are involved in coronary ischaemia, given that intraplaque haemorrhage is the driver of atherosclerotic plaque progression and worsening of ischaemia in coronaries, which at a later stage might translate into a higher incident of HF. 38 Alternatively, other mechanisms linking the aspirin effect with kidney function and iron deficiency may indirectly elucidate HF incidents. 39 , 40 In addition, aspirin interacted with statins, and this interaction may contribute to improved platelet responsiveness to aspirin 41 and further reduced platelet adhesion, 42 which may contribute to extended leakage from neovascular vessels. 43
Strength and limitations
The strengths of the current study include the large sample size with free HF participants with long follow‐ups. To our knowledge, this is the first large study to investigate the role of aspirin on the risk of HF in primary prevention as well as secondary prevention. Also, replication of the findings in discovery and replication set along with corroboration or results through sensitivity analysis should be mentioned. Moreover, taking into account the combination of individual data of a large number of participants from 6 studies in 12 countries and 2 continents simplifies the generalizability of findings on a large scale. Nevertheless, our study has also limitations. First, we had information on the use of aspirin and other drugs at enrolment but could not analyse the intake of medication as a time‐dependent covariable. Second, because of dichotomous information on aspirin use, we could not assess the dose effect. Third, we have no information on adherence to prescribed medications and non‐hospitalized HF incidents. Fourth, although the ejection fraction (EF) is considered an HF diagnostic parameter and guidance for HF treatment after diagnosis, we lacked the data on EF to better characterize the incident HF according to EF subgroups of HF and lacked data to distinguish HF between ischaemic and non‐ischaemic. Although we performed a sensitivity analysis to minimize the effect of reverse causality, yet we cannot exclude reverse causality with absolute certainty. Finally, the study suffers residual confounding that cannot be ruled out due to observational design; however, in the absence of a conclusive trial, our data provide important scientific evidence that may contribute to clinical practice.
Conclusions
Aspirin use is associated with an increased risk of HF in patients receiving aspirin with or without a previous history of CVDs. In the absence of conclusive trial evidence, our observations suggest that aspirin should be prescribed with caution in patients at risk of HF or having HF.
Conflict of interest
None declared.
Funding
The European Union (HEALTH‐F7‐305507 HOMAGE), the European Research Council (Advanced Researcher Grant 2011‐294713‐EPLORE), the European Research Council (Proof‐of‐Concept Grant 713601‐uPROPHET), and the European Research Area Net for Cardiovascular Diseases (JTC2017‐046‐PROACT) supported the Research Unit Hypertension and Cardiovascular Research. The Non‐Profit Association Alliance for the Promotion of Preventive Medicine (APPREMED; URL, http://www.appremed.org), Mechelen, Belgium, received a nonbinding grant from OMRON Healthcare, Co, Ltd, Kyoto, Japan. The sponsors had no role in the preparation of this report.
Supporting information
Figure S1. The flow chart of the study population.
Figure S2. The cumulative incidence in HOMAGE.
Table S1. Baseline characteristics per study.
Table S1.1 Baseline characteristics per study (continuation).
Table S2. Effect of aspirin on the risk of heart failure in stratified analysis.
Table S2.1 Effect of aspirin in the risk of heart failure in stratified analysis.
Table S2.2 Effect of aspirin in the risk of heart failure in stratified analysis.
Table S3. Aspirin use in subjects with history of cardiovascular diseases.
Table S4. Comparison of characteristics propensity score sample.
Table S5. Description of studies.
Table S6. Incidence and number of events.
Acknowledgements
The authors gratefully acknowledge the expert clerical assistance of Vera De Leebeeck and Renilde Wolfs at the Studies Coordinating Centre in Leuven, Belgium.
Open Access funding enabled and organized by Projekt DEAL.
Mujaj, B. , Zhang, Z.‐Y. , Yang, W.‐Y. , Thijs, L. , Wei, F.‐F. , Verhamme, P. , Delles, C. , Butler, J. , Sever, P. , Latini, R. , GF Cleland, J. , Zannad, F. , Staessen, J. A. , and Heart Omics in Ageing Investigators (2022) Aspirin use is associated with increased risk for incident heart failure: a patient‐level pooled analysis. ESC Heart Failure, 9: 685–694. 10.1002/ehf2.13688.
Address reprint requests to Dr. Staessen.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failureThe task force for the diagnosis and treatment of acute and chronic heart failure of the european Society of Cardiology (ESC)Developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 2. Jacobs L, Efremov L, Ferreira JP, Thijs L, Yang WY, Zhang ZY, Latini R, Masson S, Agabiti N, Sever P, Delles C, Sattar N, Butler J, Cleland JGF, Kuznetsova T, Staessen JA, Zannad F, the Heart “OMics” in AGEing (HOMAGE) investigators , Pinet F, Pizard A, Rouet P, Leenders J, Diez J, Odili A, Wei FF, Newman A, Papadimitrious L, Davoli M, Mureddu GF, Ford I, Jukema W, Stott DJ, Poulter N. Risk for incident heart failure: A subject‐level meta‐analysis from the heart "OMics" in AGEing (HOMAGE) study. J Am Heart Assoc 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, Ammon SE, Graham S, Sacco RL, Mann DL, Mohr JP, Massie BM, Labovitz AJ, Anker SD, Lok DJ, Ponikowski P, Estol CJ, Lip GY, di Tullio MR, Sanford AR, Mejia V, Gabriel AP, del Valle M, Buchsbaum R, WARCEF Investigators. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med 2012; 366: 1859–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen ML, Löllgen H, Marques‐Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S, ESC Scientific Document Group . 2016 European Guidelines on cardiovascular disease prevention in clinical practiceThe Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016; 37: 2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, Murawska A, Young A, Lay M, Chen F, Sammons E, Waters E, Adler A, Bodansky J, Farmer A, McPherson R, Neil A, Simpson D, Peto R, Baigent C, Collins R, Parish S, Armitage J. Effects of aspirin for primary prevention in persons with diabetes mellitus. New England J Med. 2018; 379: 1529–1539. [DOI] [PubMed] [Google Scholar]
- 6. Gaziano JM, Brotons C, Coppolecchia R, Cricelli C, Darius H, Gorelick PB, Howard G, Pearson TA, Rothwell PM, Ruilope LM, Tendera M, Tognoni G, ARRIVE Executive Committee. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): A randomised, double‐blind, placebo‐controlled trial. Lancet (London, England). 2018; 392: 1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McNeil JJ, Wolfe R, Woods RL, Tonkin AM, Donnan GA, Nelson MR, Reid CM, Lockery JE, Kirpach B, Storey E, Shah RC, Williamson JD, Margolis KL, Ernst ME, Abhayaratna WP, Stocks N, Fitzgerald SM, Orchard SG, Trevaks RE, Beilin LJ, Johnston CI, Ryan J, Radziszewska B, Jelinek M, Malik M, Eaton CB, Brauer D, Cloud G, Wood EM, Mahady SE, Satterfield S, Grimm R, Murray AM, ASPREE Investigator Group. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med 2018; 379: 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta‐analysis of individual participant data from randomised trials. Lancet. 2009; 373: 1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Massie BM, Collins JF, Ammon SE, Armstrong PW, Cleland JG, Ezekowitz M, Jafri SM, Krol WF, O'Connor CM, Schulman KA, Teo K, Warren SR, WATCH Trial Investigators. Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure: The warfarin and antiplatelet therapy in chronic heart failure (WATCH) trial. Circulation 2009; 119: 1616–1624. [DOI] [PubMed] [Google Scholar]
- 10. Cleland JG, Findlay I, Jafri S, Sutton G, Falk R, Bulpitt C, Prentice C, Ford I, Trainer A, Poole‐Wilson PA. The Warfarin/Aspirin Study in Heart failure (WASH): a randomized trial comparing antithrombotic strategies for patients with heart failure. Am Heart J. 2004; 148: 157–164. [DOI] [PubMed] [Google Scholar]
- 11. Bermingham M, Shanahan MK, O'Connell E, Dawkins I, Miwa S, O'Hanlon R, O'Connell E, Dawkins I, Miwa S, O'Hanlon R, Gilmer J, McDonald K, Ledwidge M. Aspirin use in heart failure: Is low‐dose therapy associated with mortality and morbidity benefits in a large community population? Circ Heart Fail 2014; 7: 243–250. [DOI] [PubMed] [Google Scholar]
- 12. Madelaire C, Gislason G, Kristensen SL, Fosbol EL, Bjerre J, D'Souza M, Fosbøl EL, Bjerre J, D'Souza M, Gustafsson F, Kober L, Torp‐Pedersen C, Schou M. Low‐dose aspirin in heart failure not complicated by atrial fibrillation: A Nationwide propensity‐matched study. JACC Heart Fail. 2018; 6: 156–167. [DOI] [PubMed] [Google Scholar]
- 13. Cleland JG. Is aspirin useful in primary prevention? Eur Heart J 2013; 34: 3412–3418. [DOI] [PubMed] [Google Scholar]
- 14. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013; 310: 2191–2194. [DOI] [PubMed] [Google Scholar]
- 15. Jacobs L, Thijs L, Jin Y, Zannad F, Mebazaa A, Rouet P, Pinet F, Bauters C, Pieske B, Tomaschitz A, Mamas M, Diez J, McDonald K, Cleland JG, Brunner‐la Rocca HP, Heymans S, Latini R, Masson S, Sever P, Delles C, Pocock S, Collier T, Kuznetsova T, Staessen JA. Heart 'omics' in AGEing (HOMAGE): Design, research objectives and characteristics of the common database. J Biomed Res 2014; 28: 349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J. Rationale, design, methods and baseline demography of participants of the anglo‐scandinavian cardiac outcomes trial. ASCOT investigators. J Hypertens. 2001; 19: 1139–1147. [DOI] [PubMed] [Google Scholar]
- 17. Staessen JA, Wang JG, Brand E, Barlassina C, Birkenhager WH, Herrmann SM, Birkenhäger WH, Herrmann SM, Fagard R, Tizzoni L, Bianchi G. Effects of three candidate genes on prevalence and incidence of hypertension in a caucasian population. J Hypertens 2001; 19: 1349–1358. [DOI] [PubMed] [Google Scholar]
- 18. Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, Psaty BM, Smith NL, Newman AB, Rodondi N, Satterfield S, Bauer DC, Bibbins‐Domingo K, Smith AL, Wilson PWF, Vasan RS, Harris TB, Butler J. Epidemiology of incident heart failure in a contemporary elderly cohort: The health, aging, and body composition study. Arch Intern Med 2009; 169: 708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mureddu GF, Tarantini L, Agabiti N, Faggiano P, Masson S, Latini R, Cesaroni G, Miceli M, Forastiere F, Scardovi AB, Uguccioni M, Boccanelli A. Evaluation of different strategies for identifying asymptomatic left ventricular dysfunction and pre‐clinical (stage B) heart failure in the elderly. Results from 'PREDICTOR', a population based‐study in Central Italy. Eur J Heart Fail 2013; 15: 1102–1112. [DOI] [PubMed] [Google Scholar]
- 20. Shepherd J, Blauw GJ, Murphy MB, Cobbe SM, Bollen EL, Buckley BM, Ford I, Jukema JW, Hyland M, Gaw A, Lagaay AM. The design of a prospective study of Pravastatin in the Elderly at Risk (PROSPER). PROSPER Study Group. PROspective Study of Pravastatin in the Elderly at Risk. Am J Cardiol. 1999; 84: 1192–1197. [DOI] [PubMed] [Google Scholar]
- 21. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med 2009; 28: 3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rothwell PM, Cook NR, Gaziano JM, Price JF, Belch JFF, Roncaglioni MC, Morimoto T, Mehta Z. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: Analysis of individual patient data from randomised trials. Lancet (London, England). 2018; 392: 387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy J, Michos ED, Miedema MD, Muñoz D, Smith SC Jr, Virani SS, Williams KA Sr, Yeboah J, Ziaeian B. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the american College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation 2019; 140: e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zheng SL, Roddick AJ. Association of Aspirin use for primary prevention with cardiovascular events and bleeding events: A systematic review and meta‐analysis. JAMA 2019; 321: 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raber I, McCarthy CP, Vaduganathan M, Bhatt DL, Wood DA, Cleland JGF, Blumenthal RS, McEvoy JW. The rise and fall of aspirin in the primary prevention of cardiovascular disease. Lancet. 2019; 393: 2155–2167. [DOI] [PubMed] [Google Scholar]
- 26. Jacobsen AP, Raber I, McCarthy CP, Blumenthal RS, Bhatt DL, Cusack RW, Serruys PW, Wijns W, McEvoy JW. Lifelong aspirin for all in the secondary prevention of chronic coronary syndrome: still sacrosanct or is reappraisal warranted? Circulation. 2020; 142: 1579–1590. [DOI] [PubMed] [Google Scholar]
- 27. Jacobsen AP, Raber I, McCarthy CP, Blumenthal RS, Bhatt DL, Cusack RW, Serruys PW, Wijns W, McEvoy JW. Lifelong aspirin for all in the secondary prevention of chronic coronary syndrome. Circulation. 2020; 142: 1579–1590. [DOI] [PubMed] [Google Scholar]
- 28. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, Widimsky P, Hori M, Avezum A, Piegas LS, Branch KRH, Probstfield J, Bhatt DL, Zhu J, Liang Y, Maggioni AP, Lopez‐Jaramillo P, O'Donnell M, Kakkar AK, Fox KAA, Parkhomenko AN, Ertl G, Störk S, Keltai M, Ryden L, Pogosova N, Dans AL, Lanas F, Commerford PJ, Torp‐Pedersen C, Guzik TJ, Verhamme PB, Vinereanu D, Kim JH, Tonkin AM, Lewis BS, Felix C, Yusoff K, Steg PG, Metsarinne KP, Cook Bruns N, Misselwitz F, Chen E, Leong D, Yusuf S. Rivaroxaban with or without aspirin in stable cardiovascular disease. New England Journal of Medicine. 2017; 377: 1319–1330. [DOI] [PubMed] [Google Scholar]
- 29. Yang W‐Y, Melgarejo JD, Thijs L, Zhang Z‐Y, Boggia J, Wei F‐F, Hansen TW, Asayama K, Ohkubo T, Jeppesen J, Dolan E, Stolarz‐Skrzypek K, Malyutina S, Casiglia E, Lind L, Filipovský J, Maestre GE, Li Y, Wang JG, Imai Y, Kawecka‐Jaszcz K, Sandoya E, Narkiewicz K, O'Brien E, Verhamme P, Staessen JA, for The International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes (IDACO) Investigators . Association of Office and Ambulatory Blood Pressure with Mortality and cardiovascular outcomes. JAMA 2019; 322: 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yusuf S, Lonn E, Pais P, Bosch J, López‐Jaramillo P, Zhu J, Xavier D, Avezum A, Leiter LA, Piegas LS, Parkhomenko A, Keltai M, Keltai K, Sliwa K, Chazova I, Peters RJG, Held C, Yusoff K, Lewis BS, Jansky P, Khunti K, Toff WD, Reid CM, Varigos J, Accini JL, McKelvie R, Pogue J, Jung H, Liu L, Diaz R, Dans A, Dagenais G. Blood‐pressure and cholesterol lowering in persons without cardiovascular disease. New England Journal of Medicine. 2016; 374: 2032–2043. [DOI] [PubMed] [Google Scholar]
- 31. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, ESC Scientific Document Group , de Backer G, Heagerty AM, Agewall S, Bochud M, Borghi C, Boutouyrie P, Brguljan J, Bueno H, Caiani EG, Carlberg B, Chapman N, Cífková R, Cleland JGF, Collet JP, Coman IM, de Leeuw PW, Delgado V, Dendale P, Diener HC, Dorobantu M, Fagard R, Farsang C, Ferrini M, Graham IM, Grassi G, Haller H, Hobbs FDR, Jelakovic B, Jennings C, Katus HA, Kroon AA, Leclercq C, Lovic D, Lurbe E, Manolis AJ, McDonagh TA, Messerli F, Muiesan ML, Nixdorff U, Olsen MH, Parati G, Perk J, Piepoli MF, Polonia J, Ponikowski P, Richter DJ, Rimoldi SF, Roffi M, Sattar N, Seferovic PM, Simpson IA, Sousa‐Uva M, Stanton AV, van de Borne P, Vardas P, Volpe M, Wassmann S, Windecker S, Zamorano JL, Windecker S, Aboyans V, Agewall S, Barbato E, Bueno H, Coca A, Collet JP, Coman IM, Dean V, Delgado V, Fitzsimons D, Gaemperli O, Hindricks G, Iung B, Jüni P, Katus HA, Knuuti J, Lancellotti P, Leclercq C, McDonagh TA, Piepoli MF, Ponikowski P, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Sousa‐Uva M, Zamorano JL, Tsioufis C, Lurbe E, Kreutz R, Bochud M, Rosei EA, Jelakovic B, Azizi M, Januszewics A, Kahan T, Polonia J, van de Borne P, Williams B, Borghi C, Mancia G, Parati G, Clement DL, Coca A, Manolis A, Lovic D, Benkhedda S, Zelveian P, Siostrzonek P, Najafov R, Pavlova O, de Pauw M, Dizdarevic‐Hudic L, Raev D, Karpettas N, Linhart A, Olsen MH, Shaker AF, Viigimaa M, Metsärinne K, Vavlukis M, Halimi JM, Pagava Z, Schunkert H, Thomopoulos C, Páll D, Andersen K, Shechter M, Mercuro G, Bajraktari G, Romanova T, Trušinskis K, Saade GA, Sakalyte G, Noppe S, DeMarco DC, Caraus A, Wittekoek J, Aksnes TA, Jankowski P, Polonia J, Vinereanu D, Baranova EI, Foscoli M, Dikic AD, Filipova S, Fras Z, Bertomeu‐Martínez V, Carlberg B, Burkard T, Sdiri W, Aydogdu S, Sirenko Y, Brady A, Weber T, Lazareva I, Backer TD, Sokolovic S, Jelakovic B, Widimsky J, Viigimaa M, Pörsti I, Denolle T, Krämer BK, Stergiou GS, Parati G, Trušinskis K, Miglinas M, Gerdts E, Tykarski A, de Carvalho Rodrigues M, Dorobantu M, Chazova I, Lovic D, Filipova S, Brguljan J, Segura J, Gottsäter A, Pechère‐Bertschi A, Erdine S, Sirenko Y, Brady A. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 2018; 39: 3021–3104. [DOI] [PubMed] [Google Scholar]
- 32. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018; 71: 1269–1324. [DOI] [PubMed] [Google Scholar]
- 33. Kannan A, Janardhanan R. Hypertension as a risk factor for heart failure. Curr Hypertens Rep 2014; 16: 447. [DOI] [PubMed] [Google Scholar]
- 34. Duarte JD, Cooper‐DeHoff RM. Mechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide‐like diuretics. Expert Rev Cardiovasc Ther 2010; 8: 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Segal R, Lubart E, Leibovitz A, Berkovitch M, Habot B, Yaron M, Caspi D. Early and late effects of low‐dose aspirin on renal function in elderly patients. Am J Med 2003; 115: 462–466. [DOI] [PubMed] [Google Scholar]
- 36. Segal R, Lubart E, Leibovitz A, Iaina A, Caspi D. Renal effects of low dose aspirin in elderly patients. Isr Med Assoc J 2006; 8: 679–682. [PubMed] [Google Scholar]
- 37. Mujaj B, Bos D, Muka T, Lugt AV, Ikram MA, Vernooij MW, Stricker BH, Franco OH. Antithrombotic treatment is associated with intraplaque haemorrhage in the atherosclerotic carotid artery: a cross‐sectional analysis of The Rotterdam Study. Eur Heart J. 2018; 39: 3369–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bos D, Arshi B, van den Bouwhuijsen QJ, Ikram MK, Selwaness M, Vernooij MW, Kavousi M, van der Lugt A. Atherosclerotic carotid plaque composition and incident stroke and coronary events. J Am Coll Cardiol. 2021; 77: 1426–1435. [DOI] [PubMed] [Google Scholar]
- 39. Cleland JGF, Parsons S. Aspirin for heart failure. Circulation: Heart Fail 2014; 7: 237–238. [DOI] [PubMed] [Google Scholar]
- 40. de Silva R, Rigby AS, Witte KKA, Nikitin NP, Tin L, Goode K, Bhandari S, Clark AL, Cleland JGF. Anemia, renal dysfunction, and their interaction in patients with chronic heart failure. Am J Cardiol 2006; 98: 391–398. [DOI] [PubMed] [Google Scholar]
- 41. Friend M, Vucenik I, Miller M. Platelet responsiveness to aspirin in patients with hyperlipidaemia. BMJ 2003; 326: 82–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liao JK. Beyond lipid lowering: The role of statins in vascular protection. Int J Cardiol 2002; 86: 5–18. [DOI] [PubMed] [Google Scholar]
- 43. De Berardis G, Sacco M, Evangelista V, Filippi A, Giorda CB, Tognoni G, de Berardis G, Sacco M, Evangelista V, Filippi A, Giorda CB, Tognoni G, Valentini U, Nicolucci A, ACCEPT‐D Study Group . Aspirin and simvastatin combination for cardiovascular events prevention trial in diabetes (ACCEPT‐D): Design of a randomized study of the efficacy of low‐dose aspirin in the prevention of cardiovascular events in subjects with diabetes mellitus treated with statins. Trials 2007; 8: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The flow chart of the study population.
Figure S2. The cumulative incidence in HOMAGE.
Table S1. Baseline characteristics per study.
Table S1.1 Baseline characteristics per study (continuation).
Table S2. Effect of aspirin on the risk of heart failure in stratified analysis.
Table S2.1 Effect of aspirin in the risk of heart failure in stratified analysis.
Table S2.2 Effect of aspirin in the risk of heart failure in stratified analysis.
Table S3. Aspirin use in subjects with history of cardiovascular diseases.
Table S4. Comparison of characteristics propensity score sample.
Table S5. Description of studies.
Table S6. Incidence and number of events.


