Abstract
Objective
The purpose of this study was to provide contemporary estimates of the prevalence of lower extremity motor impairment and walking limitation after first-ever stroke and to characterize the predictive nature of early walking ability for being discharged home after acute hospitalization.
Methods
In this cohort study, data were collected from a metropolitan acute care hospital in Canada at admission for 487 adults with first-ever acute ischemic or hemorrhagic stroke. Lower extremity motor impairment and walking limitation were measured using the National Institutes of Health Stroke Scale and AlphaFIM, respectively. Parallel multivariable logistic regression models were built to predict discharge home after acute hospitalization compared with further hospitalization.
Results
For patients surviving a first-ever stroke, 44.1% presented with some degree of lower extremity motor impairment and 46.0% were unable to walk. In a multivariable model built around a binary classification of walking (Nagelkerke R2 = 0.41), those with any ability to walk at admission (with or without therapist assistance) had 9.48 times greater odds of being discharged home (odds ratio = 9.48, 95% CI = 6.11–14.92) than those who were unable. In a parallel multivariable model built around an ordinal classification of walking (Nagelkerke R2 = 0.49), patients had 2.07 times greater odds (odds ratio = 2.07, 95% CI = 1.82–2.38) of being discharged home for each increment on a 6-point walking scale (total dependence to complete independence) assessed at acute admission.
Conclusion
Approximately one-half of patients with first-ever stroke present with lower extremity weakness and walking limitation. Early walking ability is a significant predictor of returning home after acute hospitalization, independent of stroke severity. Discharge planning may be facilitated early after stroke with the familiar assessment of walking ability.
Impact
An early assessment of walking function within days of stroke admission can help to streamline discharge planning.
Lay Summary
Nearly one-half of all individuals who experience a first-time stroke have walking difficulty when they arrive at the hospital. The severity of the walking limitation can predict whether a patient will eventually be discharged home or go on to further hospitalization.
Keywords: Lower Extremity, Prognosis, Stroke, Walking
Introduction
Stroke is the second leading cause of adult mortality and disability-related disease burden worldwide,1 often resulting in mental and physical impairments.2 Of particular concern to individuals who survive stroke is the presence of lower extremity weakness and limitations in walking activity, often cited as the highest priority for rehabilitation and research efforts.3,4 Lower extremity strength and the ability to walk after stroke have been routinely linked to important outcomes, including greater functional independence,5,6 community mobility,7,8 and improved quality of life in the chronic stage of stroke.9,10 Furthermore, walking ability has been associated with lower health care utilization and higher exercise adherence after stroke.11,12 Thus, it is clear that the ability to walk is a key determinant of long-term stroke outcomes.
Given the relation of post-stroke lower extremity motor impairment and walking limitation to individual prognosis and health care burden,11,13,14 it may be useful to estimate their prevalence as early as possible after stroke. Although conducted more than 20 years ago, the Copenhagen Stroke Study is one of the most frequently cited population studies, having documented the prevalence of various impairments from early on after stroke.15 From consecutive hospital admissions for acute stroke between 1992 and 1993, the investigators found that 51% of patients were unable to walk immediately after stroke, 12% could walk with assistance, and 37% could walk independently.16 However, recent global and national reports have shown a downward trend in stroke mortality and disability,17,18 likely due to various factors such as earlier detection, improved medical care (thrombolysis and endovascular thrombectomy), and increasing incidence in younger adults.19–22 An update of the prevalence of lower extremity motor impairment and walking limitation after stroke is warranted to ensure health care is optimized for current trends and to facilitate resource planning.
With rising admissions for stroke and accompanying concerns surrounding health care and resource allocation, it is also critical to predict the trajectory of patients as early and accurately as possible. Numerous studies have established the predictive ability of different functional measures to predict walking outcomes and discharge disposition after inpatient rehabilitation.23–26 However, studies predicting discharge disposition after acute hospitalization have largely focused on measures of stroke severity.27–29 Though walking ability is often considered a downstream outcome in stroke prediction models, it may also serve as a useful predictor of discharge disposition after acute care when assessed early for individuals surviving stroke. It is routinely and quickly assessed by physical therapists and represents a functional summary of many factors, such as physical impairment, confidence, motivation, and balance.
Hence, the primary objective of this study was to provide contemporary estimates of the prevalence of lower extremity motor impairment and walking limitation after first-ever stroke, focusing on differences between patients who are able to walk immediately after stroke and those who are not. A secondary objective was to characterize the predictive nature of early walking ability for being discharged home after acute hospitalization. Based on previous literature and emerging trends in stroke incidence, we hypothesized that between 40% and 50% of patients with stroke would experience walking limitations immediately after stroke, which would significantly impact their discharge outcome.
Methods
This observational study used a convenience sample comprised of screening data from a consecutive sample longitudinal cohort study examining recovery post stroke, for which ethics and operational approval was obtained from the local university and hospital review board. The requirement for informed consent was waived by a subsequent ethical approval for retrospective data collection. This analysis was reported in accordance with the Strengthening the Reporting of Observational studies in Epidemiology guidelines.30
Participants
All individuals admitted between February 28, 2016, and August 31, 2017, to the stroke unit of the Vancouver General Hospital in British Columbia, Canada, were prospectively screened for the longitudinal cohort study. This quaternary hospital serves a population of approximately 2.8 million people and is 1 of only 2 comprehensive stroke centers in its province.31 As such, patients with suspected stroke are frequently transferred from other areas to this center for a higher level of care.
To answer the proposed research questions, individuals over the age of 18 years who experienced a first-ever ischemic (including lacunar stroke) or hemorrhagic stroke, confirmed by CT or MRI, were included in this analysis. Individuals hospitalized for a transient ischemic attack, subarachnoid hemorrhage, or cerebral venous thrombosis were excluded, given their unique pathophysiology, prognosis, and management compared with arterial stroke.32–35 Individuals already in hospital at the time of their stroke, admitted more than 48 hours after their stroke, or who died during the acute hospitalization period were also excluded.
Data Collection and Variables
A trained research assistant extracted demographic information (age, sex), stroke characteristics (side, type, location, interventions, severity), and functional characteristics (lower extremity motor function, walking ability) from medical records while prospectively screening patients on the stroke unit. Discharge details (length of stay and discharge location) were abstracted from the medical records by a second research assistant.
Primary Objective: Lower Extremity Motor Impairment and Walking Limitation After Stroke
Lower extremity motor impairment was assessed on hospital admission by the consulting neurologist using the National Institutes of Health Stroke Scale (NIHSS) lower extremity motor score.36–38 The 5-point motor score is rated from 0 (no weakness, indicated by no drift when held up against gravity) to 4 (no movement in the limb at all). The score for the paretic lower extremity was recorded in addition to the total NIHSS score. Overall, the NIHSS measures stroke severity across 15 domains potentially affected by stroke, including level of consciousness, vision, speech and language, motor function, sensation, and coordination. Higher scores on the NIHSS, scored out of 42, indicate greater severity of stroke.
Early walking ability was extracted from the AlphaFIM outcome.39 The AlphaFIM is an abbreviated version of the Functional Independence Measure (FIM)40 and has been shown to be reliable and valid in the stroke population.26 Unlike the FIM, walking is only graded on the AlphaFIM if a patient is deemed able to ambulate over 150 feet (with or without assistance); otherwise a sedentary task is graded instead (ie, eating and grooming). For the primary objective, the AlphaFIM therefore provided 2 variables relating to walking ability: a binary variable of being able to walk in any capacity (ie, with or without assistance; can walk: yes) or not (can walk: no), and an ordinal variable of walking ability (ranging from total dependence to complete independence). The ordinal variable was revised from a 7-point to a 6-point scale such that those requiring total or maximal assistance were collapsed into a single (non-functional) value, given difficulties in discerning the 2 categories from a non-ambulatory status; this only affected 6 participants. Those deemed non-ambulatory (can walk: no) who did not receive an ordinal rating were assigned the non-functional ordinal value, and vice versa. The walking and mobility components of the AlphaFIM were completed by the treating physical therapist within 3 days of ischemic stroke or 5 days of hemorrhagic stroke.
Secondary Objective: Predicting Home Discharge
The dependent variable for the secondary objective was the binary outcome of being discharged home or elsewhere after the acute hospitalization period. A home discharge without the need for further institutionalization is considered the optimal trajectory after stroke, and so for this analysis, discharge to another hospital, inpatient rehabilitation, or long-term care were grouped together. Given previous research highlighting the potential association of age, sex, and various stroke characteristics with home discharge after acute hospitalization,41 the independent variables were comprised of the aforementioned demographic information, stroke characteristics, lower extremity motor impairment, and walking ability.
Statistical Analysis
Descriptive statistics were used to summarize the sample. Mean and SD were reported for continuous data; median and interquartile range (IQR) were reported for ordinal data and continuous data that were not normally distributed. To address the primary objective, counts and percentages were used to report prevalence of lower extremity motor impairment and walking limitation. To further characterize the population relative to walking ability, demographics and stroke characteristics were compared between ambulatory and non-ambulatory participants using independent t test, Mann Whitney U, and χ2 analyses. A complete case analysis was performed.
For the secondary objective, logistic regression was performed to investigate the role of early walking ability in predicting discharge home from acute care in relation to patient demographics and stroke characteristics. Univariable logistic regression was first performed for each independent variable and the binary outcome of being discharged home or elsewhere. Multivariable logistic regression was then performed by purposeful selection,42 including all independent variables that were associated with the outcome variable at the P < .10 level. Because lower extremity motor impairment and walking score are broadly captured within the total NIHSS score and binary ambulatory status, respectively, parallel multivariable models were built to avoid variable collinearity and conceptual overlap; collinearity between non-categorical independent variables was checked using Spearman’s rank-order correlation (rho ≥0.7). Model A was built around the 6-point walking score and the 5-point lower extremity motor impairment score, and Model B was built around the binary ambulatory status (can walk: yes/no) and total NIHSS score. Variables that did not contribute to the multivariable model (ie, P > .05) were eliminated iteratively, until a final model containing only statistically significant variables was obtained. Assumptions of logistic regression and interactions between significant independent variables were assessed. The Hosmer-Lemeshow test, Nagelkerke R2, and area under the receiver operating characteristic curve were used to assess model fit and predictive capacity of the final logistic regression models.42–44 An area under the curve (AUC) of 0.9 or greater indicates outstanding discriminative ability, 0.8 to 0.9 indicates excellent discriminative ability, and 0.7 to 0.8 indicates acceptable discriminative ability.45,46 Finally, an optimal cut-off score for the 6-point walking score was explored, by maximizing Youden Index,47 to predict home discharge. Validity indexes, including sensitivity and specificity, of this cut-off were assessed.
All analyses were performed using RStudio version 1.3.959 (RStudio, Boston, MA, USA) running on R version 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria). P < .05 was considered statistically significant.
Role of the Funding Source
The funders played no role in the design, conduct, or reporting of this study.
Results
Of 819 admissions to the stroke unit, 669 patients were admitted to the hospital for a first-time stroke within 48 hours of onset, 42 of whom (6.3%) died during acute hospitalization. Of those who survived, 487 patients had a complete initial NIHSS score and had been assessed for early walking ability, forming the primary population. A total 140 patients were excluded from the analysis because of missing initial NIHSS score or early walking ability (22.3%), but they did not differ from the primary population by age, sex, stroke characteristics (side, type, location), or discharge disposition.
Lower Extremity Motor Impairment and Walking Limitation After Stroke
Patient demographics, stroke characteristics, and discharge disposition for the sample (n = 487) are displayed in Table 1, grouped into those unable to walk (including total or maximal dependence) and those with some ambulatory capacity (moderate assist to complete independence). Less than one-half (44.1%) of patients admitted for stroke presented initially with some level of lower extremity weakness. The distribution of walking scores is shown in Table 2, indicating that 46.0% of patients were completely dependent, and up to 57.9% of patients were unable to walk without any form of physical assistance within the first 3 to 5 days of stroke. A significantly greater proportion of non-ambulators had a hemorrhagic stroke (15.2% vs 9.1%); non-ambulators also had significantly greater stroke severity and lower extremity motor impairment scores than ambulatory participants. Those who retained or regained some walking function in the 3 to 5 days after stroke had a shorter length of stay than those who did not (P < .001), and a greater proportion returned home after acute hospitalization. Early medical intervention for ischemic stroke, either thrombolysis or thrombectomy, was not significantly associated with early walking ability.
Table 1.
Patient Characteristics in Relation to Binary Ability to Walk in the Total Cohorta
| Variable |
Total
N = 487 |
Can Walk: Yes
n = 263 |
Can Walk: No
n = 224 |
P |
|---|---|---|---|---|
| Age, y, mean (SD) | 68.5 (14.9) | 67.4 (13.9) | 69.7 (15.9) | .09b |
| Sex, female, n (%) | 206 (42.3%) | 106 (40.3%) | 100 (44.6%) | .33c |
| Side affected, n (%) | .31c | |||
| Left | 240 (49.3%) | 124 (47.1%) | 116 (51.8%) | |
| Right | 214 (42.1%) | 112 (42.6%) | 93 (41.5%) | |
| Both | 45 (8.6%) | 27 (10.3%) | 15 (6.7%) | |
| Type of stroke | .04c | |||
| Ischemic, n (%) | 429 (88.1%) | 239 (90.9%) | 190 (84.8%) | |
| Hemorrhagic, n (%) | 58 (11.9%) | 24 (9.1%) | 34 (15.2%) | |
| Location of stroke, n (%) | .08c | |||
| Cortical | 175 (35.9%) | 106 (40.3%) | 69 (30.8%) | |
| Subcorticald | 248 (50.9%) | 127 (48.3%) | 121 (54.0%) | |
| Both | 64 (13.1%) | 30 (11.4%) | 34 (15.2%) | |
| Received thrombolysis, n (% of ischemic) | 137 (31.9%) | 72 (30.1%) | 65 (34.2%) | .37c,e |
| Received endovascular thrombectomy, n (% of ischemic) | 91 (21.2%) | 50 (20.9%) | 41 (21.6%) | .87c,e |
| Initial NIHSS, median (IQR) | 6 (3–13) | 4 (2–8) | 9 (5–16) | <.001f |
| Lower extremity motor impairment (NIHSS motor score), n (%) | <.001f | |||
| 0 | 272 (55.9%) | 189 (71.9%) | 83 (37.0%) | |
| 1 | 64 (13.1%) | 34 (12.9%) | 30 (13.4%) | |
| 2 | 41 (8.4%) | 13 (4.9%) | 28 (12.5%) | |
| 3 | 57 (11.7%) | 17 (6.5%) | 40 (17.9%) | |
| 4 | 53 (10.9%) | 10 (3.8%) | 43 (19.2%) | |
| Discharge destination, n (%) | <.001c | |||
| Home | 248 (50.9%) | 201 (76.4%) | 47 (21.0%) | |
| Rehab | 113 (23.2%) | 32 (12.2%) | 81 (36.2%) | |
| Long term care | 31 (6.4%) | 5 (1.9%) | 26 (11.6%) | |
| Repatriated to another hospital | 95 (19.5%) | 25 (9.5%) | 70 (31.2%) | |
| Length of stay, d, median (IQR) | 8 (4–17.5) |
6 (3–10) |
14.5 (7.75–31.25) |
<.001c |
a IQR = interquartile range; NIHSS = National Institutes of Health Stroke Scale.
b Independent t test.
c Comparison made only between those with ischemic stroke.
d Includes midbrain and brainstem strokes.
e χ2 test.
f Mann-Whitney U test.
Table 2.
Walking Ability of Whole Sample (N = 487)
| Walking Score | Description of Walking Classification | n (%) |
|---|---|---|
| 0 | Non-functional (can walk: no, AlphaFIM walking rating 1–2) Unable/patient performs <25% of effort/25–49% of effort |
224a (46.0%) |
| 1 | Moderate assist (AlphaFIM walking rating 3) Patient performs 50–74% of effort |
14 (2.9%) |
| 2 | Minimal assist (AlphaFIM walking rating 4) Patient performs ≥75% of effort |
44 (9.0%) |
| 3 | Supervision (AlphaFIM walking rating 5) No hands on, patient performs 100% of effort |
61 (12.5%) |
| 4 | Modified independence (AlphaFIM walking rating 6) Requires device, or not timely or safely |
32 (6.6%) |
| 5 | Complete independence (AlphaFIM walking rating 7) No device required, timely, safely |
112 (23.0%) |
a Initial ratings of unable = 218; total assistance = 3; maximal assistance = 3.
Predicting Home Discharge
In this cohort, 50.9% of patients admitted to hospital for acute first-ever stroke were discharged home. Only 21.0% of non-ambulators were discharged home compared with 76.4% of those with some ambulatory function. Table 3 shows the association of each independent variable, on univariable logistic regression, with being discharged home after stroke for the whole sample. Those discharged home stayed in the hospital for a median of 5.5 days (IQR = 3–10), whereas those discharged elsewhere remained in-hospital for a median of 14 days (IQR = 7.5–26.5).
Table 3.
Association of Patient Characteristics With Home Dischargea
| Variable |
Discharged Home
n = 248 |
Discharged Elsewhere
n = 239 |
OR
(95% CI) |
P |
|---|---|---|---|---|
| Age, y, mean (SD) | 67.0 (14.4) | 70.0 (15.2) | 0.99 (0.97–1.00) |
.03 |
| Sex, female (reference), n (%) | 103 (41.5%) | 103 (43.1%) | 1.07 (0.74–1.52) | .73 |
| Side affected, n (%) | ||||
| Left (reference) | 117 (47.2%) | 123 (51.5%) | ||
| Right | 108 (43.5%) | 97 (40.6%) | 1.17 (0.81–1.70) | .41 |
| Both | 23 (9.3%) | 19 (7.9%) | 1.27 (0.66–2.48) | .47 |
| Type of stroke, ischemic (reference), n (%) | 227 (91.5%) | 202 (84.5%) | 0.51 (0.28–0.88) | .02 |
| Location of stroke, n (%) | ||||
| Cortical (reference) | 105 (42.3%) | 70 (29.3%) | ||
| Subcortical | 114 (46.0%) | 134 (56.1%) | 0.58 (0.38–0.84) | .005 |
| Both | 29 (11.7%) | 35 (14.6%) | 0.55 (0.31–0.98) | .04 |
| Initial NIHSS, median (IQR) | 4 (2–7) | 9 (5–15.5) | 0.90 (0.87–0.93) | <.001 |
| Lower extremity motor impairment, median (IQR) | 0 (0–1) | 1 (0–3) | 0.60 (0.51–0.68) | <.001 |
| Can walk, yes, n (%) (reference: No) |
201 (81.1%) | 62 (25.9%) | 12.21 (8.01–18.93) | <.001 |
| Walking score, median (IQR) | 4 (2–5) | 0 (0–1) | 2.19 (1.94–2.51) | <.001 |
a IQR = interquartile range; NIHSS = National Institutes of Health Stroke Scale; OR = odds ratio; ref = reference.
Age, stroke type, stroke location, stroke severity, lower extremity motor impairment, and walking ability (both binary and ordinal scores) were identified as relevant predictors of being discharged home. Only stroke severity and motor impairment demonstrated strong correlation (rho = 0.75). Initial and final parallel multivariable models are presented in Table 4 showing an ordinal walking Model A (containing lower extremity motor impairment and 6-point walking score) and a binary walking Model B (containing overall stroke severity and binary ambulatory status). Goodness-of-fit was acceptable for both final models by either Nagelkerke R2 or Hosmer-Lemeshow test; no significant interactions were found in either final model. On comparison of area under the receiver operating characteristic curves, Model A had slightly greater accuracy than Model B in predicting home discharge. However, both Model A (AUC = 0.86, 95% CI = 0.82 to 0.89) and Model B (AUC = 0.83, 95% CI = 0.80 to 0.87) were considered to have excellent discriminative ability (Figure).
Table 4.
Final Multivariable Regression Models for Discharge Home (vs Other Institutions)a
| Model | Initial Model | Final Model | ||||||
|---|---|---|---|---|---|---|---|---|
| B | OR | 95% CI | P | B | OR | 95% CI | P | |
| Model Ab | ||||||||
| Age | −0.008 | 0.99 | 0.98–1.01 | .33 | ||||
| Hemorrhagic (ref = ischemic) | −0.42 | 0.65 | 0.31–1.36 | .26 | ||||
| Location (ref = cortical) | ||||||||
| Subcortical | −0.43 | 0.65 | 0.38–1.10 | .11 | ||||
| Both | −0.23 | 0.79 | 0.37–1.68 | .55 | ||||
| Lower extremity motor impairment | −0.25 | 0.78 | 0.65–0.92 | .005 | −0.24 | 0.79 | 0.66–0.93 | .006 |
| Walking score (ordinal) | 0.71 | 2.04 | 1.79–2.35 | <.001 | 0.73 | 2.07 | 1.82–2.38 | <.001 |
| Model Bc | ||||||||
| Age | −0.01 | 0.99 | 0.97–1.00 | .08 | ||||
| Hemorrhagic (ref = ischemic) | −0.48 | 0.62 | 0.30–1.26 | .19 | ||||
| Location (ref = cortical) | ||||||||
| Subcortical | −0.64 | 0.53 | 0.32–0.86 | .01 | −0.62 | 0.54 | 0.33–0.87 | .01 |
| Both | −0.27 | 0.76 | 0.37–1.56 | .45 | −0.22 | 0.81 | 0.39–1.65 | .55 |
| Initial NIHSS | −0.07 | 0.93 | 0.90–0.96 | <.001 | −0.07 | 0.93 | 0.90–0.96 | <.001 |
| Walking score (binary, ref = no) | 2.23 | 9.29 | 5.98–14.66 | <.001 | 2.25 | 9.48 | 6.11–14.92 | <.001 |
a B = variable coefficient in model; OR = odds ratio.
b Model A (final): Nagelkerke Pseudo-R2: 0.49; Hosmer-Lemeshow χ2 = 5.67, df = 8, P = .68.
c Model B (final): Nagelkerke Pseudo-R2: 0.41; Hosmer-Lemeshow: χ2 = 9.70, df = 8, P = .29.
Figure.
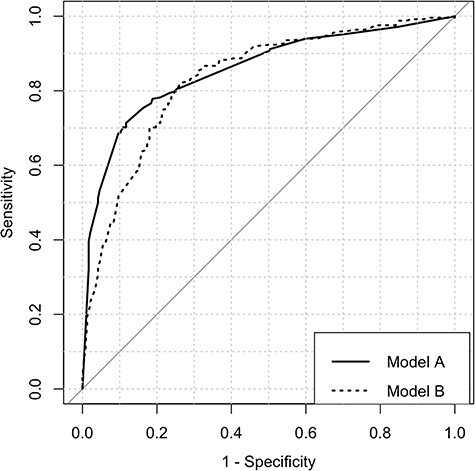
Receiver operating characteristic curves for predicting home discharge in parallel multivariable models centered on an ordinal walking classification (Model A) and binary (Model B) walking classification.
Early walking ability, whether quantified on a binary or ordinal scale of dependence, was an independent predictor of being discharged home. Those with some ambulatory function at admission had 9.48 times greater odds (95% CI = 6.11 to 14.92, P < .001) than non-ambulators for being discharged home after acute hospitalization. Similarly, higher admission scores on the 6-point walking scale were associated with greater odds of being discharged home by a factor of 2.07 (95% CI = 1.82 to 2.38, P < .001) for each increment in the scale. However, the odds of returning home decreased with more severe motor impairment, with a 21% (odds ratio = 0.79, 95% CI = 0.66 to 0.93, P = .006) reduction for each increment of the 5-point lower extremity motor impairment classification at acute admission. In Model B (Tab. 4), in addition to walking ability and stroke severity, a stroke affecting subcortical structures of the brain was also associated with lower odds of returning home, relative to a cortical stroke (odds ratio = 0.54, 95% CI = 0.33 to 0.87, P = .01).
The utility of the ordinal walking scale for predicting home discharge was further explored. Analyzed alone, the 6-point walking scale had excellent discriminative ability (AUC = 0.84, 95% CI = 0.81 to 0.87). An optimal cut-off walking score of 3 (ie, requiring supervision to walk) was identified for predicting those who return home after acute hospitalization, which yielded a sensitivity of 0.71 and specificity of 0.88 (Tab. 5).
Table 5.
Accuracy of Ordinal Walking Score for Predicting Home Discharge
| Validity Index | Walking Score Cut-Off of 3 (Supervision) |
|---|---|
| Area under the curve (95% CI) | 0.84 (0.81–0.87) |
| Sensitivity (95% CI) | 0.71 (0.66–0.83) |
| Specificity (95% CI) | 0.88 (0.77–0.92) |
| Positive predictive value | 0.86 |
| Negative predictive value | 0.75 |
| Positive likelihood ratio | 6.09 |
| Negative likelihood ratio | 0.32 |
Discussion
This study aimed to provide an updated prevalence of lower extremity motor impairment and walking limitation for patients hospitalized with a first-ever stroke as well as to characterize the predictive nature of early walking ability for being discharged home. In total, 44.1% of patients presented with some degree of lower extremity motor impairment, as assessed using the NIHSS scale, when first admitted to hospital with acute stroke. Similarly, 46.0% to 57.9% were unable to walk without varying levels of physical assistance. Just over one-half (50.9%) of patients were discharged home, a large percentage (81.1%) of whom had some early walking ability. Whether quantified on a binary or ordinal scale of dependence, walking ability was a significant and independent predictor of being discharged home.
The prevalence findings in this study are slightly lower for lower extremity motor impairment (44.1%) and walking limitation (57.9%) compared with those presented in the Copenhagen Stroke Study (65% for lower extremity impairment and 63% for walking limitation).16 However, the Copenhagen Stroke Study included participants who died during acute hospitalization,16 which may have inflated their prevalence estimates relative to ours; assuming those who died presented with limitations in walking activity, exclusion of these individuals would have resulted in an adjusted walking limitation prevalence of 54%. Furthermore, our prevalence estimates aligned with reported baseline walking limitation (56%–60%) in recent large-scale acute intervention studies,48,49 suggesting that rates of walking dysfunction after stroke have remained stable despite changes in medical intervention, stroke incidence patterns, and mortality rates.
The finding that lower extremity motor impairment, early walking ability, and initial stroke severity are predictive of home discharge after acute hospitalization may seem intuitive. Indeed, several studies have shown that the NIHSS, taken at admission, can predict discharge to rehabilitation or home.29,50,51 However, recent research has posited that admission NIHSS is less useful in an era of modern thrombolytic interventions.28 Our model using a binary variable of walking ability and initial NIHSS yielded excellent discriminative ability of home discharge, indicating that inclusion of early walking ability may be sufficient to account for potential postintervention changes in stroke severity. We also showed that initial lower extremity motor impairment alone independently predicts discharge home, even after accounting for walking ability in a multivariable model. This is supported by a previous study that found that certain individual items on the NIHSS can predict discharge disposition in minor stroke52; the authors of that study also suggested that pairing ambulation with the NIHSS could aid in making treatment decisions. Our study emphasizes the importance of walking at the beginning of the overall trajectory of stroke recovery.
The present study reinforces the utility of completing a walking assessment as early as possible after stroke. Although the AlphaFIM taken during acute care has been shown to predict functional outcomes after inpatient rehabilitation,26,53 our study shows that the walking domain of the measure alone can be used to predict discharge to home after acute hospitalization. Physical therapists regularly assess walking ability and can do so quickly; knowing that the odds for being discharged home are 2.07 times greater for every increment in early walking ability, or that patients requiring only supervision to walk are likely to go home, may help clinicians manage expectations and begin discharge planning from an earlier standpoint. It is important to note that this analysis does not necessarily convey causality; it should not be interpreted that attempting to improve walking ability during early therapy will lead to better discharge outcomes. Indeed, this was proven otherwise by a large multi-site randomized controlled trial in which very early mobilization led to poorer outcomes.48
This analysis adds to the existing literature in predicting home discharge after acute hospitalization. Though it may seem obvious that early walking ability is predictive of being discharged home after stroke, it cannot be understated that accurately streamlining patients to the home setting after stroke is of utmost importance. Home-time is defined as the number of days spent at home outside of the acute care setting in the first 90 days poststroke and has been shown to be associated with improved functional outcomes in the subacute and chronic phase of stroke.54,55 No matter how it is predicted, accurately preparing patients to return home from the acute hospitalization period without further institutionalization may reduce health care costs and optimize resource utilization in the long term.56 Furthermore, being able to predict discharge home as early and accurately as possible is increasingly important as the push for social distancing and at-home telerehabilitation grows.57
A strength of this study is the high discriminative power of a predictive model centered on early lower extremity motor impairment and walking limitation in a large cohort of patients with stroke. Previous research has identified ethnicity, spousal support, and presence of other comorbidities as key determinants of discharge disposition during the acute hospitalization period.51,58,59 Other impairment outcomes not accounted for in this analysis, such as cognition and mood, have also been identified as predictors of discharge disposition.60,61 However, without these additional variables, the predictive ability of our model still falls within the range of reported Nagelkerke R2 in other studies (0.11–0.63).26,51 We suggest that future studies predicting discharge disposition should focus explicitly on those with more severe walking deficits after stroke, because those patients will require the most support whether at home or in another institution.
Limitations
This study has several limitations. Generalizability to a more diverse stroke population is reduced by the exclusion of those patients who died from stroke, experienced recurrent stroke, were already in hospital for other medical reasons, or were admitted more than 48 hours after their stroke. A moderate proportion of admissions had missing data and were excluded; although the patients who were excluded did not differ from the analyzed sample in demographics or stroke characteristics, it is possible that they may have differed in stroke severity or walking ability, and a complete dataset would have resulted in different findings. Finally, data were collected from a large hospital with a specialized stroke unit that admits patients from surrounding geographical regions. As such, the analyzed sample may not be representative of the typical stroke admissions to a local acute care hospital.
In conclusion, approximately one-half of patients experiencing a first-ever stroke will have lower extremity weakness and experience walking limitations. Early walking ability is a significant predictor of returning home after acute hospitalization, independent of stroke severity. Knowing this may allow physical therapists to begin discharge planning early after stroke with the simple and familiar assessment of walking function.
Author Contributions
Concept/idea/research design: D.R. Louie, L.A. Simpson, W.B. Mortenson, J. Yao, J.J. Eng
Writing: D.R. Louie, L.A. Simpson, J. Yao, J.J. Eng
Data collection: D.R. Louie, L.A. Simpson
Data analysis: D.R. Louie, W.B. Mortenson, J.J. Eng
Fund procurement: J.J. Eng
Providing facilities/equipment: J. Yao
Providing institutional liaisons: J. Yao
Consultation (including review of manuscript before submitting): L.A. Simpson, W.B. Mortenson, T.S. Field, J. Yao, J.J. Eng
Ethics Approval
Ethics and operational approval was obtained from the local university and hospital review boards.
Funding
This research was supported by the Canadian Institutes of Health Research (FND-143340). J.J. Eng is supported by the Canada Research Chairs Program. D.R. Louie is supported by the Canada Graduate Scholarships Vanier Program.
Disclosures
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
A version of this manuscript forms part of a PhD dissertation (D.R.L.), which was published at the University of British Columbia in March 2021.
References
- 1. World Health Organization . Global Health Estimates: Life expectancy and leading causes of death and disability. 2021. Accessed November 14, 2021. https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates.
- 2. Hankey GJ. Stroke. Lancet. 2017;389:641–654. [DOI] [PubMed] [Google Scholar]
- 3. Harris JE, Eng JJ. Goal priorities identified through client-centred measurement in individuals with chronic stroke. Physiother Can. 2004;56:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rudberg AS, Berge E, Laska AC, et al. Stroke survivors’ priorities for research related to life after stroke. Top Stroke Rehabil. 2021;28:153–158. [DOI] [PubMed] [Google Scholar]
- 5. Pundik S, Holcomb J, Mccabe J, Daly JJ. Enhanced life-role participation in response to comprehensive gait training in chronic stroke survivors. Disabil Rehabil. 2012;34:1535–1539. [DOI] [PubMed] [Google Scholar]
- 6. Faria-Fortini I, Basílio ML, Polese JC, et al. Strength deficits of the paretic lower extremity muscles were the impairment variables that best explained restrictions in participation after stroke. Disabil Rehabil. 2017;39:2158–2163. [DOI] [PubMed] [Google Scholar]
- 7. Bijleveld-Uitman M, Van De Port I, Kwakkel G. Is gait speed or walking distance a better predictor for community walking after stroke? J Rehabil Med. 2013;45:535–540. [DOI] [PubMed] [Google Scholar]
- 8. Taylor-Piliae RE, Latt LD, Hepworth JT, Coull BM. Predictors of gait velocity among community-dwelling stroke survivors. Gait Posture. 2012;35:395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muren MA, Hütler M, Hooper J. Functional capacity and health-related quality of life in individuals post stroke. Top Stroke Rehabil. 2008;15:51–58. [DOI] [PubMed] [Google Scholar]
- 10. Wyller TB, Sveen U, Sedring KM, Pettersen AM, Bautz-Holter E. Subjective well-being one year after stroke. Clin Rehabil. 1997;11:139–145. [DOI] [PubMed] [Google Scholar]
- 11. Minet LR, Peterson E, Koch L, Ytterberg C. Healthcare utilization after stroke: a 1-year prospective study. J Am Med Dir Assoc. 2020;21:1684–1688. [DOI] [PubMed] [Google Scholar]
- 12. Caetano LCG, Pacheco BD, Samora GAR, Teixeira-Salmela LF, Scianni AA. Self-efficacy to engage in physical exercise and walking ability best predicted exercise adherence after stroke. Stroke Res Treat. 2020;2020:2957623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burke E, Dobkin BH, Noser EA, Enney LA, Cramer SC. Predictors and biomarkers of treatment gains in a clinical stroke trial targeting the lower extremity. Stroke. 2014;45:2379–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Franceschini M, La Porta F, Agosti M, Massucci M. Is health-related-quality of life of stroke patients influenced by neurological impairments at one year after stroke? Eur J Phys Rehabil Med. 2010;46:389–399. [PubMed] [Google Scholar]
- 15. Jørgensen HS. The Copenhagen Stroke Study experience. J Stroke Cerebrovasc Dis. 1996;6:5–16. [DOI] [PubMed] [Google Scholar]
- 16. Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of walking function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:27–32. [DOI] [PubMed] [Google Scholar]
- 17. Wafa HA, Wolfe CDA, Bhalla A, Wang Y. Long-term trends in death and dependence after ischaemic strokes: a retrospective cohort study using the South London Stroke Register (SLSR). PLoS Med. 2020;17:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson CO, Nguyen M, Roth GA, et al. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:439–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics - 2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lackland DT, Roccella EJ, Deutsch AF, et al. Factors influencing the decline in stroke mortality a statement from the American Heart Association/American Stroke Association. Stroke. 2014;45:315–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hathidara MY, Saini V, Malik AM. Stroke in the young: a global update. Curr Neurol Neurosci Rep. 2019;19:91. [DOI] [PubMed] [Google Scholar]
- 22. Kamal N, Lindsay MP, Côté R, Fang J, Kapral MK, Hill MD. Ten-year trends in stroke admissions and outcomes in Canada. Can J Neurol Sci. 2015;42:168–175. [DOI] [PubMed] [Google Scholar]
- 23. Bland MD, Sturmoski A, Whitson M, et al. Prediction of discharge walking ability from initial assessment in a stroke inpatient rehabilitation facility population. Arch Phys Med Rehabil. 2012;93:1441–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vluggen TPMM, Van Haastregt JCM, Tan FES, Kempen GIJM, Schols JMGA, Verbunt JA. Factors associated with successful home discharge after inpatient rehabilitation in frail older stroke patients. BMC Geriatr. 2020;20:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown AW, Therneau TM, Schultz BA, Niewczyk PM, Granger CV. Measure of functional independence dominates discharge outcome prediction after inpatient rehabilitation for stroke. Stroke. 2015;46:1038–1044. [DOI] [PubMed] [Google Scholar]
- 26. Stillman G, Granger C, Niewczyk P. Projecting function of stroke patients in rehabilitation using the AlphaFIM instrument in acute care. PM R. 2009;1:234–239. [DOI] [PubMed] [Google Scholar]
- 27. Schlegel D, Kolb SJ, Luciano JM, et al. Utility of the NIH stroke scale as a predictor of hospital disposition. Stroke. 2003;34:134–137. [DOI] [PubMed] [Google Scholar]
- 28. Reznik ME, Yaghi S, Jayaraman MV, et al. Baseline NIH stroke scale is an inferior predictor of functional outcome in the era of acute stroke intervention. Int J Stroke. 2018;13:806–810. [DOI] [PubMed] [Google Scholar]
- 29. Rundek T, Mast H, Hartmann A, et al. Predictors of resource use after acute hospitalization: the Northern Manhattan Stroke Study. Neurology. 2000;55:1180–1187. [DOI] [PubMed] [Google Scholar]
- 30. Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 31. Statistics Canada . Lower mainland--southwest [economic region], British Columbia and British Columbia [province] (table). Census profile. 2016Census. 2017. Accessed September 22, 2021. https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/details/page.cfm?Lang=E&Geo1=ER&Code1=5920&Geo2=PR&Code2=59&SearchText=LowerMainland--Southwest&SearchType=Begins&SearchPR=01&B1=All&GeoLevel=PR&GeoCode=5920&TABID=1&type=0. [Google Scholar]
- 32. Zuurbier SM, Coutinho JM. Cerebral venous thrombosis. Adv Exp Med Biol. 2017;906:183–193. [DOI] [PubMed] [Google Scholar]
- 33. Dmytriw AA, Song JSA, Yu E, Poon CS. Cerebral venous thrombosis: state of the art diagnosis and management. Neuroradiology. 2018;60:669–685. [DOI] [PubMed] [Google Scholar]
- 34. Pace A, Mitchell S, Casselden E, et al. A subarachnoid haemorrhage-specific outcome tool. Brain. 2018;141:1111–1121. [DOI] [PubMed] [Google Scholar]
- 35. Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306–318. [DOI] [PubMed] [Google Scholar]
- 36. Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. 10.1161/01.STR.20.7.864. [DOI] [PubMed] [Google Scholar]
- 37. Lyden P, Brott T, Tilley B, et al. Improved reliability of the NIH stroke scale using video training. Stroke. 1994;25:2220–2226. [DOI] [PubMed] [Google Scholar]
- 38. Lyden P. Using the National Institutes of Health stroke scale. Stroke. 2017;48:513–519. [DOI] [PubMed] [Google Scholar]
- 39. Uniform Data System for Medical Rehabilitation . The AlphaFIM Instrument Guide, Version 4.01. Buffalo, NY, USA: UDSMR; 2009. [Google Scholar]
- 40. Uniform Data System for Medical Rehabilitation . Guide for the Uniform Data Set for Medical Rehabilitation (Including the FIM(TM) Instrument), Version 5.1. Buffalo, NY, USA: UDSMR; 1997. [Google Scholar]
- 41. Mees M, Klein J, Yperzeele L, Vanacker P, Cras P. Predicting discharge destination after stroke: a systematic review. Clin Neurol Neurosurg. 2016;142:15–21. [DOI] [PubMed] [Google Scholar]
- 42. Zhang Z. Model building strategy for logistic regression: purposeful selection. Ann Transl Med. 2016;4:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bewick V, Cheek L, Ball J. Statistics review 14: logistic regression. Crit Care. 2005;9:112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smith TJ, McKenna CM. A comparison of logistic regression pseudo R2 indices. Mult Linear Regres Viewpoints. 2013;39:17–26. [Google Scholar]
- 45. Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5:1315–1316. [DOI] [PubMed] [Google Scholar]
- 46. Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. New York, NY: Wiley-Interscience; 2000. [Google Scholar]
- 47. Fluss R, Faraggi D, Reiser B. Estimation of the Youden index and its associated cutoff point. Biom J. 2005;47:458–472. [DOI] [PubMed] [Google Scholar]
- 48. AVERT Trial Collaboration group, Bernhardt J, Langhorne P, et al. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. Lancet. 2015;386:46–55. [DOI] [PubMed] [Google Scholar]
- 49. Hankey GJ, Hackett ML, Almeida OP, et al. Safety and efficacy of fluoxetine on functional outcome after acute stroke (AFFINITY): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19:651–660. [DOI] [PubMed] [Google Scholar]
- 50. Schlegel DJ, Tanne D, Demchuk AM, Levine SR, Kasner SE. Prediction of hospital disposition after thrombolysis for acute ischemic stroke using the National Institutes of Health stroke scale. Arch Neurol. 2004;61:1061–1064. [DOI] [PubMed] [Google Scholar]
- 51. Dutrieux RD, Eijk M, Mierlo ML, Heugten CM, Visser-Meily JMA, Achterberg WP. Discharge home after acute stroke: differences between older and younger patients. J Rehabil Med. 2016;48:14–18. [DOI] [PubMed] [Google Scholar]
- 52. Yaghi S, Willey JZ, Andrews H, Boehme AK, Marshall RS, Boden-Albala B. The itemized NIHSS scores are associated with discharge disposition in patients with minor stroke. The Neurohospitalist. 2016;6:102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lo A, Tahair N, Sharp S, Bayley MT. Clinical utility of the AlphaFIM® instrument in stroke rehabilitation. Int J Stroke. 2012;7:118–124. [DOI] [PubMed] [Google Scholar]
- 54. Quinn TJ, Dawson J, Lees JS, Chang TP, Walters MR, Lees KR. Time spent at home poststroke: “home-time” a meaningful and robust outcome measure for stroke trials. Stroke. 2008;39:231–233. [DOI] [PubMed] [Google Scholar]
- 55. Sung SF, Su CC, Hsieh CY, et al. Home-time as a surrogate measure for functional outcome after stroke: a validation study. Clin Epidemiol. 2020;12:617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dewilde S, Annemans L, Peeters A, et al. The relationship between home-time, quality of life and costs after ischemic stroke: the impact of the need for mobility aids, home and car modifications on home-time. Disabil Rehabil. 2020;42:419–425. [DOI] [PubMed] [Google Scholar]
- 57. Bashir A. Stroke and telerehabilitation: a brief communication (preprint). JMIR Rehabil Assist Technol. 2020;7:e18919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Béjot Y, Troisgros O, Gremeaux V, et al. Poststroke disposition and associated factors in a population-based study: the Dijon Stroke Registry. Stroke. 2012;43:2071–2077. [DOI] [PubMed] [Google Scholar]
- 59. Tanwir S, Montgomery K, Chari V, Nesathurai S. Stroke rehabilitation: availability of a family member as caregiver and discharge destination. Eur J Phys Rehabil Med. 2014;50:355–362. [PubMed] [Google Scholar]
- 60. Geubbels HJB, Nusselein BAM, Van Heugten CM, Valentijn SAM, Rasquin SMC. Can the Montreal Cognitive Assessment predict discharge destination in a stroke population in the hospital? J Stroke Cerebrovasc Dis. 2015;24:1094–1099. [DOI] [PubMed] [Google Scholar]
- 61. Cho JS, Hu Z, Fell N, Heath GW, Qayyum R, Sartipi M. Hospital discharge disposition of stroke patients in Tennessee. South Med J. 2017;110:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]


