Abstract
Aims
Cardiac resynchronization therapy (CRT) is highly effective in dilated cardiomyopathy (DCM) patients with impaired left ventricular ejection fraction (LVEF) and left bundle block branch. In cardiac amyloidosis (CA) patients, left ventricular dysfunction and conduction defects are common, but the potential of CRT to improve cardiac remodelling and survival in this particular setting remains undefined. We investigated cardiovascular outcomes in CA patients after CRT implantation in terms of CRT echocardiographic response and major cardiovascular events (MACEs).
Methods and results
Our retrospective study included 47 CA patients implanted with CRT devices from January 2012 to February 2020, in nine French university hospitals (77 ± 6 years old, baseline LVEF 30 ± 8%) compared with propensity‐matched (1:1 for age, LVEF at implantation, and CRT indication) DCM patients with a CRT device. CA patients had lower rates of CRT response (absolute delta LVEF ≥ 10%) compared with DCM patients (36% vs. 70%, P = 0.002). After multivariate Cox analysis, CA was independently associated with MACE (hospitalization for heart failure/cardiovascular death) [hazard ratio (HR) 3.73, 95% confidence interval (CI) 1.85–7.54, P < 0.001], along with the absence of CRT response (HR 3.01, 95% CI 1.56–5.79, P = 0.001). The presence of echocardiographic CRT response (absolute delta LVEF ≥ 10%) was the only predictive factor of MACE‐free survival in CA patients (HR 0.36, 95% CI 0.15–0.86, P = 0.002).
Conclusion
Compared with a matched cohort of DCM patients, CA patients had a lower rate of CRT response and consequently a worse cardiovascular prognosis after CRT implantation. However, CRT could be beneficial even in CA patients given that CRT response was associated with better cardiac outcomes in this population.
Keywords: Cardiac amyloidosis, Cardiac resynchronization therapy, Heart failure, Pacemaker, Implantable cardioverter defibrillator
Introduction
Amyloidosis is a systemic disease due to an abnormal accumulation of protein in the tissues. 1 The prognosis is poor, with a median survival <3 years after the onset of heart failure symptoms. 2 Cardiac involvement may occur in three main types of amyloidosis: amyloidosis with immunoglobulin light chains (AL), wild‐type transthyretin amyloidosis (ATTRwt), and hereditary transthyretin amyloidosis caused by TTR gene variants (ATTRv). Cardiac amyloidosis (CA) involvement is due to the accumulation of amyloid fibrils with an increase in ventricular wall thickening and myocardium stiffness that is frequently complicated by electrical conduction defects requiring a permanent pacemaker 3 and impaired left ventricular ejection fraction (LVEF) at the late stage of the disease.
Cardiac resynchronization therapy (CRT) is highly effective in dilated cardiomyopathy (DCM) patients with impaired LVEF and left bundle branch block (LBBB). The European Society of Cardiology guidelines recommend the implantation of a CRT device in patients with LBBB and LVEF ≤ 35%, but device upgrading to CRT is also recommended when systolic left ventricular (LV) dysfunction is induced by right ventricular (RV) pacing 4 and for atrioventricular (AV) block with impaired LV function. 5
In CA patients, the potential of CRT to improve cardiac remodelling and cardiovascular (CV) survival remains undefined. By extension of the results in DCM patients, several centres tend to implant CRT devices in infiltrative cardiomyopathies, especially in CA patients who develop high‐grade conduction disorders or to upgrade patients with a high rate of RV pacing and heart failure symptoms. However, given the specific pathophysiology of CA, the results from non‐CA cohorts cannot be extrapolated to CA patients, and it is currently unclear whether CRT could be effective in this population. A recent study on 30 CA patients suggested low rates of CRT response but improved survival compared with matched non‐CRT CA patients. 6
Thus, we aimed to assess echocardiographic response and major CV event (MACE) rates after CRT implantation in CA patients compared with matched DCM patients.
Methods
Study design
This retrospective case–control observational study was conducted in nine French university hospitals: Besançon, Créteil Henri Mondor, Dijon, Nancy, Poitiers, Reims, Rennes, Saint‐Étienne, and Tours.
Cardiac amyloidosis diagnosis was established by the treating physicians. However, every patient's medical records were checked by the principal investigator of the study (K. F.), and only patients whose diagnosis criteria met consensus expert guidelines 7 were included in the analysis. CA criteria were based on morphological characteristics using transthoracic echocardiography (diastolic cardiac septum thickness >12 mm with no other cause of hypertrophy 8 ), magnetic resonance imaging, and bisphosphonate scintigraphy. Biological tests were also used to confirm the diagnosis and included genetic transthyretin (TTR) screening, serum electrophoresis, immuno‐fixation on serum or urine, and biopsy in the presence of a gammopathy for immunohistochemistry for lambda or kappa immunostaining to distinguish AL type from ATTR. We also collected high‐sensitivity troponin, N‐terminal pro‐BNP, and creatinine plasma levels to confirm the stage of CA before CRT implantation (Supporting Information, Table S1 ).
Inclusion criteria were as follows:
patients with CA (AL, ATTRv, and ATTRwt),
patients implanted with a CRT‐P (pacemaker)/CRT‐D (defibrillator) device after the diagnosis of CA, and
patients with a minimum of 6 months of follow‐up after CRT implantation in the centre where the CRT was implanted.
Dilated cardiomyopathy was diagnosed by transthoracic echocardiography, magnetic resonance imaging, and/or after coronary angiography. Patients with previous coronary artery disease, coronary angioplasty, or coronary artery bypass graft were excluded from the comparison cohort.
Screening methodology
Patients from Besançon, Dijon, Poitiers, Reims, Rennes, Saint‐Étienne, and Tours were recruited through the hospital's department of medical information using the International Classification of Diseases (ICD‐10) diagnostic codes for CA and the Common Classification of Medical Procedures codes for CRT implant (Figure 1 ). For the other centres, patients were identified from pre‐existing internal registries (Nancy and Créteil Henri Mondor).
Figure 1.
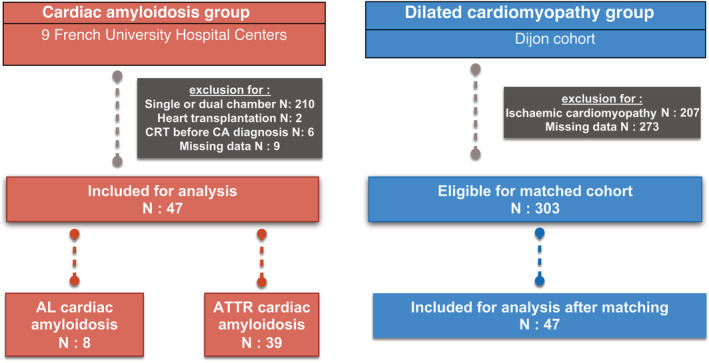
Flow chart of the study. AL, amyloidosis with immunoglobulin light chains; ATTR, transthyretin amyloidosis; CA, cardiac amyloidosis; CRT, cardiac resynchronization therapy.
For the non‐ischaemic DCM‐matched cohort, a total of 783 patients who had a CRT device implanted between 2012 and 2019 were retrospectively screened. Baseline or follow‐up data were incomplete in 273 patients (mainly due to lack of echocardiography data at baseline and follow‐up in patients implanted but not followed in the centre), and 207 patients had ischaemic cardiomyopathy or previous coronary artery disease. Thus, among the remaining 303 patients, a 1:1 propensity score matching with CA patients was performed (on age, LVEF at baseline, and CRT indication), as well as a case–control matching for sensitivity analysis purposes.
Data collection
Patient characteristics were collected from the ICD‐10, CCAM, and the electronic medical records: CV risk factors, clinical data, previous CV history, characteristics of cardiac resynchronization (position of the LV lead and indication of CRT), type of bundle branch block, type of amyloidosis and date of the diagnosis, acute and discharge medications (amyloidosis treatment was defined as tafamidis for ATTR patients and chemotherapy for AL patients), biological data, echocardiographic data (LVEF using Simpson's biplane method and longitudinal strain deformation when available 9 ) at baseline and at follow‐up after CRT implantation (at least 3 months after CRT), biventricular pacing rate on the last device interrogation, and sustained ventricular tachycardia (VT) (defined as >30 s)/ventricular fibrillation (VF) treated by ATP or shock on device download or remote monitoring. Follow‐up data for CV outcomes including death (and its cause), CV death, and hospitalization for heart failure were collected using the medical records and the local registry if present. According to institutional policy, we did not require institutional review board approval given the retrospective nature of the work and the use of anonymized data sheets. This study was approved by the Clinical Research Department of Dijon University Hospital and complied with the Declaration of Helsinki.
Cardiac resynchronization therapy—pacemaker or cardiac resynchronization therapy—defibrillator indications
We categorized implantation indications as follows:
LBBB + LVEF ≤ 35%,
non‐LBBB enlarged QRS + LVEF ≤ 35% (including right bundle branch block and non‐specific intraventricular conduction delay),
upgrading from VVI or DDD to CRT due low LVEF induced by RV pacing, and
high‐degree AV block + LVEF < 50% ‘BLOCK‐HF like indication’.
Study outcomes
The main outcomes were echocardiographic CRT response (defined according to previous publications: absolute increased delta in LVEF ≥ 10% between pre‐implantation and at follow‐up echocardiography 10 ) and MACE (defined as hospitalization for heart failure or CV death) at follow‐up after CRT implantation.
Statistical analysis
The statistical results of the continuous variables are presented as means ± standard deviation for Gaussian distribution, medians (first quartile to third quartile) for non‐Gaussian distribution after the Kolmogorov–Smirnov test, and the results of the dichotomous variables as numbers (%). For categorical data, χ 2 or Fisher's exact test was used, while Student's t‐test was used for the comparison of continuous data with normal distribution variable or Mann–Whitney U test for non‐parametric variables. CA patients were matched 1:1 with DCM patients using nearest‐neighbour matching on the linear propensity score with a tolerance of 0.10 on age, LVEF at implantation, and CRT indication. Moreover, a second 1:1 matching was performed using case–control method with no tolerance on CRT indication and a tolerance of 0.10 on age and LVEF, for sensitivity analysis of the matching. The Kaplan–Meier survival curves were performed to study the occurrence of MACE at follow‐up and compared with the log‐rank test. Data were censored at the date of the MACE episode, the last date of follow‐up, or at 2500 days.
Before the construction of the multivariate models, collinearity between variables was excluded. Variables entered into the multivariate model were chosen according to their univariate relationship with an inclusion cut‐off at 5% and exclusion cut‐off at 5% for the first model including both CA and DCM patients, and 20% of inclusion cut‐off in the CA cohort analysis due to limited sample size. Cox multivariate stepwise backward conditional regression analyses were performed to identify predictors of MACE in the two groups and then specifically in the CA group to test MACE and CRT response predictors. The effect of the variables was adjusted on the delay between CRT and echocardiography, using various cut‐offs classically described in the literature (6, 9, and 12 months after implantation), because the log‐linearity assumption was not verified. We thus adjusted the group effect on the delay using 6 months for the primary analysis. The aim of the sensitivity analysis was to check whether the group effect was consistent when changing the cut‐off of the delay used as a dependent variable in the model.
Because of missing data, biological variables at implantation were not included in multivariable models.
All of the tests were two sided, and a P value <0.05 was considered significant. The statistical tests were performed with SPSS software Version 26 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
Between January 2012 and February 2020, among the 274 patients screened in our nine centres, 47 were included in the CA cohort (Figure 1 ). The mean age of CA patients was 77 (±6), 43 (92%) were male, and median baseline LVEF was 30% (25–39.8). CRT indication was LVEF ≤ 35% + LBBB in 27 (57%) patients, upgrading for low LVEF due to RV pacing in 10 (21%) patients, LVEF ≤ 35% + non‐LBBB enlarged QRS in 6 (13%) patients, and complete AV block with impaired LVEF in the remaining 4 (9%) patients.
After propensity score matching, DCM patients had similar age, New York Heart Association stage, baseline LVEF, and indication for CRT implantation as the CA cohort. However, the two groups differed on several baseline parameters: CA patients were more often male than DCM patients (92% of CA vs. 66% of DCM, P = 0.002) and had more previous hospitalizations for heart failure (87% vs. 47%, P = 0.004) (Table 1 ). CA patients had lower rates of CRT response (absolute delta LVEF ≥ 10%) compared with DCM patients (36% vs. 70%) (P = 0.002) (Table 1 ).
Table 1.
Patient characteristics [n (%), mean ± standard deviation, or median (inter‐quartile range)] of the two groups: cardiac amyloidosis and matched dilated cardiomyopathy patients
| Cardiac amyloidosis | DCM | ||
|---|---|---|---|
| N = 47 | N = 47 | P | |
| Population | |||
| Age at implantation (years) | 77.2 ± 5.9 | 76.3 ± 5.3 | 0.64 |
| Age at implantation >75 years | 29 (62) | 28 (60) | 1 |
| Male sex | 43 (92) | 31 (66) | 0.002 |
| NYHA stage | 0.73 | ||
| I | 5 (11) | 5 (11) | |
| II | 26 (55) | 29 (62) | |
| III | 15 (32) | 13 (28) | |
| IV | 1 (2) | 0 | |
| Cardiovascular risk factors | |||
| Current smoking | 10 (21) | 9 (19) | 1 |
| Hypertension | 27 (54) | 27 (54) | 1 |
| Hypercholesterolaemia | 23 (49) | 16 (34) | 0.21 |
| Diabetes | 5 (11) | 9 (19) | 0.39 |
| Family history of coronary artery disease | 6 (13) | 0 | 0.03 |
| Cardiovascular history | |||
| Coronary artery disease | 10 (21) | 0 | <0.001 |
| Previous atrial fibrillation | 32 (68) | 21 (45) | 0.04 |
| Previous hospitalization for heart failure | 41 (87) | 22 (47) | 0.004 |
| Chronic medications | |||
| Beta‐blocker | 8 (17) | 46 (98) | <0.001 |
| ACE inhibitor/ARBs | 15 (32) | 41 (87) | <0.001 |
| Diuretic | 42 (89) | 35 (75) | 0.11 |
| MRA | 22 (47) | 19 (40) | 0.68 |
| Valsartan/sacubitril | 2 (4) | 3 (6) | 1 |
| Digoxin | 2 (4) | 5 (11) | 0.44 |
| Calcium channel blocker | 0 | 1 (2) | 1 |
| Amiodarone | 14 (30) | 6 (13) | 0.08 |
| Anticoagulation therapy | 43 (92) | 24 (51) | 0.004 |
| Amyloidosis treatment | |||
| Tafamidis | 15 (32%) | ||
| Chemotherapy for AL | 7 (15%) | ||
| Echocardiography | |||
| LVEF at baseline (%) | 30 (25–35) | 30 (25–34) | 0.89 |
| LVEF at follow‐up after implantation (%) | 37 (31–43) | 45 (40–50) | <0.001 |
| Time between CRT and TTE (days) | 273 (182–365) | 306 (182–458) | 0.20 |
| Absolute delta LVEF ≥ 10% | 17 (36) | 33 (70) | 0.002 |
| Indication for implantation | 0.20 | ||
| LBBB + LVEF ≤ 35% | 27 (54) | 32 (68) | |
| Non‐LBBB enlarged QRS + LVEF ≤ 35% | 6 (13) | 1 (2) | |
| Upgrading | 10 (21) | 8 (17) | |
| ‘BLOCK‐HF like’ indication | 4 (12) | 6 (13) | |
| Implantation characteristics and follow‐up | |||
| CRT device | 0.006 | ||
| CRT‐P | 27 (57) | 13 (28) | |
| CRT‐D | 20 (43) | 34 (72) | |
| LV lead type | 0.013 | ||
| Bipolar | (60) | (32) | |
| Quadripolar | 19 (40) | 32 (68) | |
| LV lead position | 1 | ||
| Endovascular (lateral) | 45 (96) | 44 (93) | |
| Epicardial (surgical) | 2 (4) | 3 (6) | |
| Atrioventricular node ablation | 9 (19) | 5 (11) | 0.39 |
| Biventricular stimulation rate (%) | 98 (95–99) | 99 (97–99) | 0.002 |
| Biventricular stimulation rate >95% | 36 (77) | 43 (92) | 0.09 |
ACE, angiotensin‐converting enzyme; AL, amyloidosis with immunoglobulin light chains; ARBs, angiotensin II receptor blockers; CRT, cardiac resynchronization therapy; CRT‐D, cardiac resynchronization therapy—defibrillator; CRT‐P, cardiac resynchronization therapy—pacemaker; DCM, dilated cardiomyopathy; LBBB, left bundle block branch; LV, left ventricular; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; TTE, transthoracic echocardiography.
Cardiovascular outcomes in cardiac amyloidosis patients compared with matched dilated cardiomyopathy patients
Regarding ventricular arrhythmias, five patients in the CA group experienced sustained VT/VF on their device. Among them, four were ICD carriers and one was a PM carrier. No difference was observed between the two groups for sustained VT or VF episodes on CRT devices during follow‐up (Table 5 ). After a median follow‐up of 518 (274–851) days and 1279 (608–2375) days in the CA and DCM groups, respectively (P < 0.001), MACE occurred in 70% of CA patients compared with 34% of DCM patients. MACE rate at 1 and 2 years of follow‐up after CRT was 48% and 67% for the CA group and 11% and 23% for the DCM group (log‐rank P < 0.001) (Figure 2 A ). Additional survival curves for HF hospitalization, CV death, and all‐cause death are provided in Supporting Information, Figure S1 . In deceased patients, there were no references in the medical files to ventricular arrhythmias on the devices (CRT‐P or CRT‐D) or to sudden cardiac arrest. All CV deaths were related to terminal HF, and the non‐CV deaths were related mostly to the underlying condition responsible for amyloidosis.
Table 5.
Outcomes in the two groups: cardiac amyloidosis and matched dilated cardiomyopathy patients, n (%)
| Cardiac amyloidosis | DCM | ||
|---|---|---|---|
| N = 47 | N = 47 | P | |
| Outcomes at follow‐up | |||
| New‐onset atrial fibrillation | 14 (30) | 9 (19) | 0.33 |
| CRT complications | 3 (6) | 1 (2) | 0.62 |
| Hospitalization for heart failure | 31 (66) | 13 (28) | 0.001 |
| Cardiovascular death | 17 (36) | 6 (13) | 0.001 |
| Death | 21 (45) | 9 (19) | 0.02 |
| MACE | 33 (70) | 16 (34) | 0.001 |
| Sustained VT/VF episode on CRT device | 5 (11) | 3 (6) | 0.71 |
| Cause of death | 0.02 | ||
| Terminal heart failure | 17 (36) | 6 (13) | |
| VT/VF | 0 | 0 | |
| Acute coronary syndrome | 0 | 0 | |
| Non cardiac cause | 4 (9) | 3 (6) | |
| Follow‐up (days) | 518 (274–851) | 1279 (608–2375) | <0.001 |
CRT, cardiac resynchronization therapy; DCM, dilated cardiomyopathy; MACE, major cardiovascular event; VF, ventricular fibrillation; VT, ventricular tachycardia.
Figure 2.
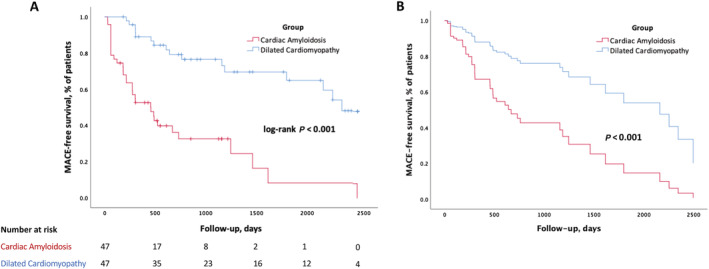
Estimated major cardiovascular event (MACE)‐free survival rates after cardiac resynchronization therapy implantation in cardiac amyloidosis and matched dilated cardiomyopathy patients. (A) Unadjusted data. (B) Adjusted for differences in clinical characteristics and concomitant diseases by Cox multivariate hazard regression. P value refers to log‐rank test for (A) and Cox model for (B).
After multivariate Cox analysis adjusted on the delay between CRT implantation and follow‐up echocardiography, CA was independently associated with MACE [hazard ratio (HR) 3.73, 95% confidence interval (CI) 1.85–7.54, P < 0.001], along with the absence of CRT response (delta LVEF < 10%, HR 3.01, 95% CI 1.56–5.79, P = 0.001), male sex (HR 2.68, 95% CI 1.04–6.91, P = 0.041), and biventricular pacing rate <95% (HR 2.27, 95% CI 1.07–4.80, P = 0.032) (Table 2 and Figure 2 B ). As sensitivity analysis, we computed the model with several cut‐offs of the delay and retained 6 months as the reference, but the estimated HR of the variables remained significant in all analyses and did not change substantially. Moreover, when the matching was performed on a case–control pattern rather than propensity score as sensitivity analysis of our matching strategy, the multivariate results remained almost identical: CA remained independently associated with MACE (HR 4.27, 95% CI 1.98–9.19, P < 0.001), along with the absence of CRT response (delta LVEF < 10%, HR 2.75, 95% CI 1.37–5.53, P = 0.004).
Table 2.
Univariate and multivariate Cox regression analysis to estimate predictors of MACE in cardiac amyloidosis and matched dilated cardiomyopathy patients
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Male sex | 3.68 | 1.45–9.33 | 0.006 | 2.68 | 1.04–6.91 | 0.041 |
| Age at implantation | 1.05 | 0.99–1.11 | 0.062 | |||
| Hypertension | 1.15 | 0.65–2.05 | 0.63 | |||
| Dyslipidaemia | 0.99 | 0.56–1.79 | 0.99 | |||
| Diabetes | 0.87 | 0.39–1.94 | 0.74 | |||
| Current smoking | 1.20 | 0.61–2.35 | 0.600 | |||
| Previous atrial fibrillation a | 2.39 | 1.31–4.38 | 0.005 | |||
| Previous coronary artery disease | 2.37 | 1.03–5.42 | 0.041 | |||
| Previous hospitalization for heart failure | 2.33 | 1.21–4.51 | 0.008 | |||
| Beta‐blocker a | 0.26 | 0.14–0.46 | <0.001 | |||
| ACE inhibitor/ARBs a | 0.38 | 0.21–0.67 | 0.001 | |||
| Creatinine b | 1.01 | 0.999–1.004 | 0.224 | |||
| Log NT‐proBNP b | 1.62 | 1.20–2.18 | 0.002 | |||
| Troponin b | 4.84 | 1.53–15.29 | 0.007 | |||
| LBBB + LVEF ≤ 35% a | 0.63 | 0.35–1.12 | 0.117 | |||
| CRT‐P (vs. CRT‐D) a | 2.61 | 1.48–4.62 | 0.001 | |||
| Biventricular stimulation rate <95% | 2.84 | 1.37–5.89 | 0.005 | 2.27 | 1.07–4.80 | 0.032 |
| Atrioventricular node ablation | 1.46 | 0.67–3.16 | 0.33 | |||
| Delta LVEF < 10% | 3.81 | 2.06–7.05 | <0.001 | 3.01 | 1.56–7.79 | 0.001 |
| Cardiac amyloidosis group | 4.70 | 2.50–8.87 | <0.001 | 3.73 | 1.85–7.54 | <0.001 |
ACE, angiotensin‐converting enzyme; ARBs, angiotensin II receptor blockers; CI, confidence interval; CRT‐D, cardiac resynchronization therapy—defibrillator; CRT‐P, cardiac resynchronization therapy—pacemaker; HR, hazard ratio; LBBB, left bundle block branch; LVEF, left ventricular ejection fraction; MACE, major cardiovascular event; NT‐proBNP, N‐terminal pro‐BNP.
Multivariate analysis adjusted on the delay between cardiac resynchronization therapy and follow‐up echocardiography.
Not included in the multivariable analysis due to collinearity.
Not included in the multivariable analysis due to missing data.
Predictors of major cardiovascular event and cardiac resynchronization therapy response in cardiac amyloidosis cohort
In the CA cohort, MACE patients did not differ from MACE‐free patients in terms of baseline cardiovascular status (including baseline LVEF), type of CA, treatments for heart failure, biological data, and indication for CRT (data not shown). After Cox multivariate analysis adjusted on the delay between CRT implantation and follow‐up echocardiography, the presence of echocardiographic CRT response (absolute delta LVEF ≥ 10%) was the only predictive factor for the absence of MACE in CA patients (HR 0.36, 95% CI 0.15–0.86, P = 0.002) (Table 3 and Supporting Information, Figure S2 ). Moreover, CRT response was only associated with younger age in CA patients and not with neither CRT indication nor amyloidosis type (Table 4 and Supporting Information, Table S2 ).
Table 3.
Univariate and multivariate Cox regression analysis to estimate predictors of MACE in cardiac amyloidosis group
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Male sex | 3.99 | 0.54–29.47 | 0.17 | |||
| Age at implantation | 1.04 | 0.98–1.11 | 0.23 | |||
| Previous atrial fibrillation | 1.29 | 0.59–2.82 | 0.53 | |||
| Previous hospitalization for heart failure | 2.51 | 0.75–8.40 | 0.14 | 2.79 | 0.984–9.33 | 0.095 |
| LBBB + LVEF ≤ 35% | 0.78 | 0.36–1.64 | 0.52 | |||
| Amyloidosis treatment | 0.69 | 0.24–1.40 | 0.30 | |||
| CRT‐P (vs. CRT‐D) | 1.40 | 0.68–2.89 | 0.37 | |||
| Biventricular stimulation rate <95% | 2.02 | 0.87–4.66 | 0.10 | |||
| Delta LVEF ≥ 10% | 0.43 | 0.18–1.01 | 0.05 | 0.36 | 0.15–0.86 | 0.002 |
CI, confidence interval; CRT‐D, cardiac resynchronization therapy—defibrillator; CRT‐P, cardiac resynchronization therapy—pacemaker; HR, hazard ratio; LBBB, left bundle block branch; LVEF, left ventricular ejection fraction; MACE, major cardiovascular event.
Multivariate analysis adjusted on the delay between cardiac resynchronization therapy and follow‐up echocardiography.
Table 4.
Univariate and multivariate regression analysis to estimate predictors of CRT response in CA group
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Male sex | 0.54 | 0.07–4.19 | 0.55 | |||
| Age at implantation >75 years a | 0.33 | 0.10–1.08 | 0.068 | 0.45 | 0.21–0.99 | 0.047 |
| Previous atrial fibrillation | 1.20 | 0.33–4.36 | 0.78 | |||
| LVEF at implantation ≤35% | 0.68 | 0.34–1.39 | 0.29 | |||
| Creatinine at implantation | 0.997 | 0.991–1.003 | 0.36 | |||
| Previous hospitalization for heart failure | 0.93 | 0.19–4.50 | 0.93 | |||
| LBBB | 1.60 | 0.47–5.47 | 0.45 | |||
| Amyloidosis treatment | 1.02 | 0.31–3.35 | 0.98 | |||
| AL | 1.07 | 0.22–5.17 | 0.93 | |||
| Biventricular stimulation rate <95% a | 0.64 | 0.33–1.24 | 0.19 | |||
AL, amyloidosis with immunoglobulin light chains; CA, cardiac amyloidosis; CI, confidence interval; CRT, cardiac resynchronization therapy; HR, hazard ratio; LBBB, left bundle block branch; LVEF, left ventricular ejection fraction.
Included in the bivariate analysis.
Discussion
The present work is the largest study to investigate the impact of CRT on cardiac outcomes in patients with CA (Table 5 ). The main findings of this work are as follows:
Compared with a matched cohort of non‐ischaemic DCM patients implanted with a CRT, CA patients had lower rates of echocardiographic CRT response and worse cardiovascular prognoses.
In CA patients implanted with a CRT device, CRT echocardiographic response was associated with better CV outcomes.
Cardiac resynchronization therapy response in cardiac amyloidosis compared with matched dilated cardiomyopathy patients
One year after CRT, compared with the DCM‐matched cohort, CA patients had lower rates of echocardiographic CRT response (both using the percentage of changes between baseline and follow‐up as well as the definition of absolute delta LVEF ≥ 10%).
Currently, there is no precise, consensual definition of the echocardiographic response to CRT in the literature. In MADIT‐CRT, 11 which had a 2 year follow‐up period, the authors defined CRT response with absolute delta LVEF: >14.5% (super‐responders), 7.9–14.4% (responders), and <7.9% (hypo‐responders). In this cohort, super‐responders were associated with fewer non‐fatal heart failure events and deaths. At the same time, they found that the criterion of LV end‐systolic volume index changes had no impact on heart failure or survival. 12
In our work, we chose to use the absolute delta LVEF ≥ 10% value to define echocardiographic response to CRT. We observed an echocardiographic CRT response in 70% patients in the DCM group and 36% in the CA group. Using the same criterion, Steffel et al. 10 observed a clinical benefit in 47% of CRT responder patients on survival and hospitalization for heart failure over 3 years of follow‐up. More recently, Choi et al., 13 using the same definition, described a CRT response in 75% patients, which was also associated with a reduction in MACE. The echocardiographic CRT response rate in our DCM cohort is consistent with these previous studies, and our CA patients appeared to be ‘hypo‐responders’, with half the rate of CRT response observed in DCM patients. Recently, Donnellan et al. also described that 33% of 30 CA patients implanted with a CRT device had a delta LVEF ≧ 10% 6 While the exact pathophysiological mechanism of this weak CRT response remains unclear, it appears that CRT may not improve very severely decreased longitudinal function. We can also suppose that LVEF might not be the best marker to measure LV dysfunction in CA, and thus, LV strain might be a better marker to measure the amyloid burden. 9 Myocardial infiltration and impaired relaxation being preponderant, the impairment of diastolic function possibly gives way to systolic dysfunction at a later stage where the prognosis is already advanced. 14 , 15 Moreover, contrary to CA patients, DCM patients are treated with powerful heart failure drugs such as beta‐blockers or angiotensin‐converting enzyme inhibitors.
Unfortunately, current data on the efficacy of CRT in CA and more generally in infiltrative cardiomyopathies are limited. Patel et al. 16 compared the outcomes of biventricular pacing in patients with cardiac sarcoidosis (CS) or DCM. They found a significant average improvement in LVEF in both CS (28.8–35.9%) and DCM patients (25–36.6%). Echocardiographic CRT response was based on absolute delta LVEF > 5% and was present in 61% of CS patients vs. 71% of DCM patients. In our study, the only predictor of CRT echocardiographic response in CA patients was age ≦75 years. Taking into account the relatively low rate of CRT response in CA patients, our results suggest that CRT may be indicated in younger CA patients.
Association between cardiac resynchronization therapy response and major cardiovascular event in cardiac amyloidosis patients
According to our results, an echocardiographic response to CRT was associated with better CV outcomes in CA patients. Ruberg et al. showed an association between MACE and LVEF dysfunction, particularly with LVEF < 50%. 17 In the recent paper of Donnellan et al. comparing 30 CRT and 30 non‐CRT CA‐matched patients, CRT was also associated with improved survival, even after adjusting for age, LVEF, and New York Heart Association functional class. 6 These findings are consistent with our results and the potential benefit of the CRT.
However, in our cohort, the occurrence of MACE was associated neither with baseline LVEF nor with CRT indication. Indeed, it is commonly accepted that LBBB + LVEF ≤ 35% is the main indication associated with better outcomes after CRT in DCM patients. However, in CA patients, some physicians tend to implant CRT devices in patients with high rate of RV pacing and mild impairment in LVEF in order to prevent heart failure progression. A retrospective study 18 reported the effectiveness of upgrading in CA comparing the outcomes in 78 CA patients according to the type of implanted device (RV pacing vs. CRT). They found a worsening in LVEF after implantation in 89% of patients with RV pacing rate >40%. They also showed a LVEF improvement in 78% of CRT patients at 42 months. Taken together with our results, this suggests that CRT could prevent LVEF worsening in CA patients implanted from a pacemaker and that CRT should be discussed in patients with an expected high rate of RV pacing and mild/moderate LVEF impairment, as described in the BLOCK‐HF study. 5
Poor cardiovascular prognosis
In our analysis, as expected, after a median follow‐up of 518 days, CA patients had a poorer prognosis than matched DCM patients, with terminal heart failure as the main cause of death. Despite the recently development of treatments for CA, it is well known that these patients have rather poor prognosis, with a median survival of 6 months for the AL form 19 and 60 months for the ATTR form. 20
The rest of the current literature has focused on the effect of ICD on the survival of patients with CA. The results of a meta‐analysis from Rezk et al. 21 confirm an overall survival rate of 49% with a median follow‐up of 4.9 months. Hamon et al. 22 showed, in 45 CA patients with a median follow‐up of 17 months, a mortality rate of 26% due to terminal heart failure (50%) and pulseless electrical activity (17%).
More recently, in a CA cohort with ICD and a follow‐up of 60 months (compared with non‐CA patients with ICD and CA patients without ICD), Kim et al. 23 described a higher mortality rate in CA (39%) vs. non‐CA (18%) patients. Moreover, the presence of an ICD was not associated with an improvement in survival rate compared with CA patients without ICDs (46%). They observed higher rates of treated VT/VF episodes in the CA (26%) and DCM groups (26%) than we did in our cohort (11% and 6%, respectively), probably because we had a higher mortality rate and more CRT‐P in our population. However, the role of ICD in CA patients implanted from a CRT device remains to be prospectively studied.
Limitations
We acknowledge several limitations in this study. Firstly, we conducted a retrospective study with a potential for selection and information biases. However, registry data, and CCAM and ICD‐10 codes have been successfully used in this setting. 24 We did not have the access to full medical records for some patients followed‐up in another centre. Several patients were therefore excluded from the analysis, which was limited to the variables available for the entire cohort. Moreover, echocardiographic response was only based on LVEF, as strain was not available or measured with a different device, whereas more recent studies used LV end‐systolic volume to estimate CRT response due to LVEF variability. As regards electrocardiographic parameters, we were not able to precisely assess the changes in QRS duration before and after CRT even though these changes may be associated with CRT response. 25 DCM and CA populations had significant differences in terms of previous medical history and treatments, which could also explain the better prognosis observed in the DCM group. However, given the specific clinical profile of CA patients, it was not possible to obtain perfect matching on these parameters, despite a large DCM cohort of 303 patients.
Also, we did not compare outcomes in CA patients with decreased LVEF and dyssynchrony who did not undergo CRT. We did not have the complete set of data for this criterion and were therefore not able to compare its predictive performance with our LVEF‐based definition. However, the 10% absolute improvement in LVEF after CRT has already been used in many studies and was proven to be associated with a reduction in MACE during follow‐up.
Finally, although this is a multicentre study, the sample size limits the power of our statistical analysis of the CA cohort and particularly for the interaction of CRT response with amyloidosis treatment such as tafamidis. However, to our knowledge, this is currently the largest series of CA patients implanted with CRT in the literature.
Conclusions
After CRT implantation, compared with a matched cohort of DCM patients, CA patients had lower rates of CRT response and worse CV outcomes with significantly higher rates of hospitalization for heart failure and cardiovascular death. However, CRT therapy may still be of interest in CA patients (especially younger ones) given that CRT response was associated with lower rates of cardiac events in this population.
In the light of these results, the potential benefit of CRT in CA patients needs further investigation.
Conflict of interest
T.D. reports grants and personal fees from Pfizer and Ionis‐Akcea and personal fees from Alnylam and Neurimmune, during the conduct of the study. G.L. reports personal fees from Abbott, Biosense Webster, MicroPort CRM, Boston Scientific, Medtronic, and Biotronik, outside the submitted work. C.G. reports personal fees from MicroPort CRM, Boston Scientific, and Medtronic, outside the submitted work. J.‐B.G. reports personal fees from Abbott and non‐financial support from Bayer, outside the submitted work. N.C. reports personal fees from Medtronic, outside the submitted work. F.L. reports non‐financial support from MicroPort and Johnson & Johnson and personal fees from Meda Pharma, Sanofi, Bayer, and Pfizer, outside the submitted work. The other authors have nothing to declare.
Funding
This research received no external funding.
Supporting information
Table S1. Supporting Information.
Table S2. Patient characteristics (n (%), mean ± standard deviation or median (interquartile range)) in cardiac amyloidosis patients according to CRT response.
Figure S1. Supporting Information.
Figure S2. Supporting Information.
Acknowledgement
The authors thank Suzanne Rankin for English revision of the paper.
Fischer, K. , Lellouche, N. , Damy, T. , Martins, R. , Clementy, N. , Bisson, A. , Lesaffre, F. , Espinosa, M. , Garcia, R. , Degand, B. , Serzian, G. , Jourda, F. , Huttin, O. , Guichard, J.‐B. , Devilliers, H. , Eicher, J.‐C. , Laurent, G. , and Guenancia, C. (2022) Cardiovascular outcomes after cardiac resynchronization therapy in cardiac amyloidosis. ESC Heart Failure, 9: 740–750. 10.1002/ehf2.13663.
References
- 1. Gertz MA, Dispenzieri A, Sher T. Pathophysiology and treatment of cardiac amyloidosis. Nat Rev Cardiol 2015; 12: 91–102. [DOI] [PubMed] [Google Scholar]
- 2. Damy T, Jaccard A, Guellich A, Lavergne D, Galat A, Deux J‐F, Hittinger L, Dupuis J, Frenkel V, Rigaud C, Plante‐Bordeneuve V, Bodez D, Mohty D. Identification of prognostic markers in transthyretin and AL cardiac amyloidosis. Amyloid Int J Exp Clin Investig Off J Int Soc Amyloidosis 2016; 23: 194–202. [DOI] [PubMed] [Google Scholar]
- 3. Algalarrondo V, Dinanian S, Juin C, Chemla D, Bennani SL, Sebag C, Planté V, Le Guludec D, Samuel D, Adams D, Slama MS. Prophylactic pacemaker implantation in familial amyloid polyneuropathy. Heart Rhythm 2012; 9: 1069–1075. [DOI] [PubMed] [Google Scholar]
- 4. Bogale N, Witte K, Priori S, Cleland J, Auricchio A, Gadler F, Gitt A, Limbourg T, Linde C, Dickstein K, the Scientific Committee, National coordinators and the investigators . The European Cardiac Resynchronization Therapy Survey: comparison of outcomes between de novo cardiac resynchronization therapy implantations and upgrades. Eur J Heart Fail 2011; 13: 974–983. [DOI] [PubMed] [Google Scholar]
- 5. Curtis AB, Worley SJ, Chung ES, Li P, Christman SA, St. John Sutton M. Improvement in clinical outcomes with biventricular versus right ventricular pacing: the BLOCK HF study. J Am Coll Cardiol American College of Cardiology Foundation 2016; 67: 2148–2157. [DOI] [PubMed] [Google Scholar]
- 6. Donnellan E, Wazni OM, Hanna M, Kanj M, Saliba WI, Jaber WA. Cardiac resynchronization therapy for transthyretin cardiac amyloidosis. J Am Heart Assoc American Heart Association 2020; 9: e017335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dorbala S, Ando Y, Bokhari S, Dispenzieri A, Falk RH, Ferrari VA, Fontana M, Gheysens O, Gillmore JD, Glaudemans AWJM, Hanna MA, Hazenberg BPC, Kristen AV, Kwong RY, Maurer MS, Merlini G, Miller EJ, Moon JC, Murthy VL, Quarta CC, Rapezzi C, Ruberg FL, Shah SJ, Slart RHJA, Verberne HJ, Bourque JM. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: part 2 of 2—diagnostic criteria and appropriate utilization. J Card Fail 2019; 25: 854–865. [DOI] [PubMed] [Google Scholar]
- 8. Damy T, Maurer MS, Rapezzi C, Planté‐Bordeneuve V, Karayal ON, Mundayat R, Suhr OB, Kristen AV. Clinical, ECG and echocardiographic clues to the diagnosis of TTR‐related cardiomyopathy. Open Heart 2016; 3: e000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ternacle J, Bodez D, Guellich A, Audureau E, Rappeneau S, Lim P, Radu C, Guendouz S, Couetil J‐P, Benhaiem N, Hittinger L, Dubois‐Randé J‐L, Plante‐Bordeneuve V, Mohty D, Deux J‐F, Damy T. Causes and consequences of longitudinal LV dysfunction assessed by 2D strain echocardiography in cardiac amyloidosis. JACC Cardiovasc Imaging 2016; 9: 126–138. [DOI] [PubMed] [Google Scholar]
- 10. Steffel J, Milosevic G, Hürlimann A, Krasniqi N, Namdar M, Ruschitzka F, Lüscher TF, Duru F, Holzmeister J, Hürlimann D. Characteristics and long‐term outcome of echocardiographic super‐responders to cardiac resynchronisation therapy: ‘real world’ experience from a single tertiary care centre. Heart Br Card Soc 2011; 97: 1668–1674. [DOI] [PubMed] [Google Scholar]
- 11. Hsu JC, Solomon SD, Bourgoun M, McNitt S, Goldenberg I, Klein H, Moss AJ, Foster E. Predictors of super‐response to cardiac resynchronization therapy and associated improvement in clinical outcome. J Am Coll Cardiol 2012; 59: 2366–2373. [DOI] [PubMed] [Google Scholar]
- 12. Skaf S, Thibault B, Khairy P, O'Meara E, Fortier A, Vakulenko HV, Pitre C, White M, Ducharme A. Impact of left ventricular vs biventricular pacing on reverse remodelling: insights from the Evaluation of Resynchronization Therapy for Heart Failure (EARTH) trial. Can J Cardiol 2017; 33: 1274–1282. [DOI] [PubMed] [Google Scholar]
- 13. Choi Y, Byeon J, Jung M‐H, Jung HO, Youn H‐J. Echocardiographic, electrocardiographic changes and clinical outcomes of patients who respond to cardiac resynchronization therapy after one year. J Cardiovasc Ultrasound 2017; 25: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giancaterino S, Urey MA, Darden D, Hsu JC. Management of arrhythmias in cardiac amyloidosis. JACC Clin Electrophysiol 2020; 6: 351–361. [DOI] [PubMed] [Google Scholar]
- 15. Falk RH. Pondering the prognosis and pathology of cardiac amyloidosis: answers breed questions. JACC Cardiovasc Imaging 2016; 9: 139–141. [DOI] [PubMed] [Google Scholar]
- 16. Patel D, Trulock KM, Toro S, Grimaldi A, Gonzalez M, Moennich LA, Gorodeski EZ, Joyce E, Niebauer M, Wilkoff BL, Varma N, Rickard JW. Effect of cardiac resynchronization therapy on left ventricular remodeling in patients with cardiac sarcoidosis. Am J Cardiol 2019; 123: 329–333. [DOI] [PubMed] [Google Scholar]
- 17. Ruberg FL, Maurer MS, Judge DP, Zeldenrust S, Skinner M, Kim AY, Falk RH, Cheung KN, Patel AR, Pano A, Packman J, Grogan DR. Prospective evaluation of the morbidity and mortality of wild‐type and V122I mutant transthyretin amyloid cardiomyopathy: the Transthyretin Amyloidosis Cardiac Study (TRACS). Am Heart J 2012; 164: 222–228.e1. [DOI] [PubMed] [Google Scholar]
- 18. Donnellan E, Wazni OM, Saliba WI, Baranowski B, Hanna M, Martyn M, Patel D, Trulock K, Menon V, Hussein A, Aagaard P, Jaber W, Kanj M. Cardiac devices in patients with transthyretin amyloidosis: impact on functional class, left ventricular function, mitral regurgitation, and mortality. J Cardiovasc Electrophysiol 2019; 30: 2427–2432. [DOI] [PubMed] [Google Scholar]
- 19. Grogan M, Gertz MA, Kyle RA, Tajik AJ. Five or more years of survival in patients with primary systemic amyloidosis and biopsy‐proven cardiac involvement. Am J Cardiol 2000; 85: 664–665. [DOI] [PubMed] [Google Scholar]
- 20. Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state‐of‐the‐art review. J Am Coll Cardiol 2019; 73: 2872–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rezk T, Whelan CJ, Lachmann HJ, Fontana M, Sachchithanantham S, Mahmood S, Khan F, Khiani R, Tomson J, Youngstein T, Gillmore JD, Hawkins PN, Wechalekar AD. Role of implantable intracardiac defibrillators in patients with cardiac immunoglobulin light chain amyloidosis. Br J Haematol 2018; 182: 145–148. [DOI] [PubMed] [Google Scholar]
- 22. Hamon D, Algalarrondo V, Gandjbakhch E, Extramiana F, Marijon E, Elbaz N, Selhane D, Dubois‐Rande J‐L, Teiger E, Plante‐Bordeneuve V, Damy T, Lellouche N. Outcome and incidence of appropriate implantable cardioverter‐defibrillator therapy in patients with cardiac amyloidosis. Int J Cardiol 2016; 222: 562–568. [DOI] [PubMed] [Google Scholar]
- 23. Kim E‐J, Holmes BB, Huang S, Lugo R, Al Aboud A, Goodman S, Hung RR, Slosky D, Stevenson WG, Michaud GF, John RM. Outcomes in patients with cardiac amyloidosis and implantable cardioverter‐defibrillator. Europace 2020; 22: 1216–1223. [DOI] [PubMed] [Google Scholar]
- 24. Rosier L, Zouaghi A, Barré V, Martins R, Probst V, Marijon E, Sadoul N, Chauveau S, Da Costa A, Badoz M, Peyrol M, Barraud J, Massoullie G, Eschalier R, Espinosa M, Lesaffre F, Garcia R, Degand B, Noël A, Mansourati J, Extramiana F, Algalarrondo V, Devilliers H, Cottin Y, Gandjbakhch E, Guenancia C. High risk of sustained ventricular arrhythmia recurrence after acute myocarditis. J Clin Med 2020; 9: 848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bazoukis G, Naka KK, Alsheikh‐Ali A, Tse G, Letsas KP, Korantzopoulos P, Liu T, Yeung C, Efremidis M, Tsioufis K, Baranchuk A, Stavrakis S. Association of QRS narrowing with response to cardiac resynchronization therapy—a systematic review and meta‐analysis of observational studies. Heart Fail Rev 2020; 25: 745–756. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Supporting Information.
Table S2. Patient characteristics (n (%), mean ± standard deviation or median (interquartile range)) in cardiac amyloidosis patients according to CRT response.
Figure S1. Supporting Information.
Figure S2. Supporting Information.


