Abstract
In this report we describe the cultivation of two isolates of microsporidia, one from urine and the other from sputum samples from a Spanish AIDS patient. We identified them as Encephalitozoon cuniculi, type strain III (the dog genotype), based on ultrastructure, antigenic characteristics, PCR, and the sequence of the ribosomal DNA internal transcribed spacer region.
Encephalitozoon cuniculi is known to infect different tissues, including the urinary system and the central nervous system (CNS), of laboratory animals and cause widespread disease (2). Reports of CNS infection in immunocompetent humans thought to be caused by E. cuniculi have also been decribed for a Japanese boy (17) and a Swedish child (1) as well as for the liver (22) and peritoneum (30) of AIDS patients. Further, recent studies based on in vitro culture and molecular and antigenic characterization of several isolates (3, 6, 8, 9, 11, 13–16, 18, 20, 28) have identified E. cuniculi as an agent of respiratory, urinary, and CNS infections in AIDS patients. We describe here the isolation, in vitro cultivation, and ultrastructural, antigenic, and molecular characterization of E. cuniculi isolated from the sputum and urine of a Spanish patient with AIDS.
CASE REPORT
A 35-year-old Spanish injection drug user known to be HIV positive since 1985 was admitted to the Ramon y Cajal Hospital in Madrid (Spain) in August 1992 because of an 8-month history of fever, progressive weight loss (10 kg), asthenia, epigastric abdominal pain, and diarrhea. He had a CD4 count of 34/mm3 and was negative for toxoplasmosis, leishmaniasis, cryptococcosis, brucellosis, tuberculosis, and Mycobacterium avium complex and other pathogenic bacteria. Biopsies of bone marrow, stomach, and duodenum as well as feces were negative for parasites (including Cryptosporidium but not microsporidia), fungi, and bacteria, although he was culture positive for cytomegalovirus (CMV). The patient was enrolled in a prospective study of human microsporidiosis. Smears made from seven stool samples obtained during a period of 10 months, sputum, and two urine samples when stained with modified trichrome (26) revealed microsporidial spores. Although the patient was treated for multiple opportunistic infections, his condition deteriorated gradually, resulting in his death.
MATERIALS AND METHODS
In vitro culture, electron microscopy, and serologic and molecular studies.
Urine and sputum samples were processed for culture as described previously (24), and the resultant cultures were designated USP-A1 (established from the urine sample) and USP-A2 (resulting from the sputum sample). Reference strains Encephalitozoon intestinalis CDC:V297 (23), Encephalitozoon hellem CDC:V213 (25), and E. cuniculi CDC:V282 (6) were also cultured. Spores from test isolates and from the reference strains were harvested periodically, pooled, and purified separately as described before (7). Smears of culture-derived spores were also stained with either the Gram chromotrope technique (19) or with Calcofluor white reagent (10). Actively growing cultures were scraped and fixed in 2.5% glutaraldehyde in cacodylate buffer and processed for transmission electron microscopy (7). An indirect immunofluorescence test was performed on smears from stool, sputum, and urine samples, as well as on culture-derived spores by using rabbit polyclonal antibodies made against the three reference strains (25). DNA was extracted from patient feces, urine, and sputum specimens, uninfected E6 cell culture, E6 cell cultures infected with the different test isolates, and E6 cell culture infected with the three reference strains (7). PCR was performed using four different diagnostic primer pairs that are specific for Encephalitozoon bieneusi, E. intestinalis, E. hellem, and E. cuniculi (4, 5, 6, 25). PCR amplification was made with a GenAmp kit (Perkin-Elmer Cetus, Norwalk, Conn.) according to the manufacturer's directions, and amplification products were analyzed after electrophoresis in 2% agarose gel and were visualized by staining with ethidium bromide. The USP-A1 and USP-A2 isolates were genotyped by sequence analysis of the internal transcribed spacer (ITS) of the rRNA gene. Briefly, the 3′ end of the small subunit rRNA and the ITS were amplified from extracted DNA by PCR using primers ss530f (5′-GTGCCAGC(C/A)GCTGGCAC-3′) and 1s212r1 (5′-GTT(G/A)GTTTCTTTTCCTC-3′) (12, 29). The PCR product was sequenced in both directions on an AB1377 autosequencer (Applied Biosystems, Foster City, Calif.). The E. cuniculi genotype was determined by the number of the GTTT repeats present in the ITS region: i.e., two repeats for the mouse genotype or strain type II, three repeats for the rabbit genotype or strain I, and four repeats for the dog genotype or strain type III (12).
RESULTS
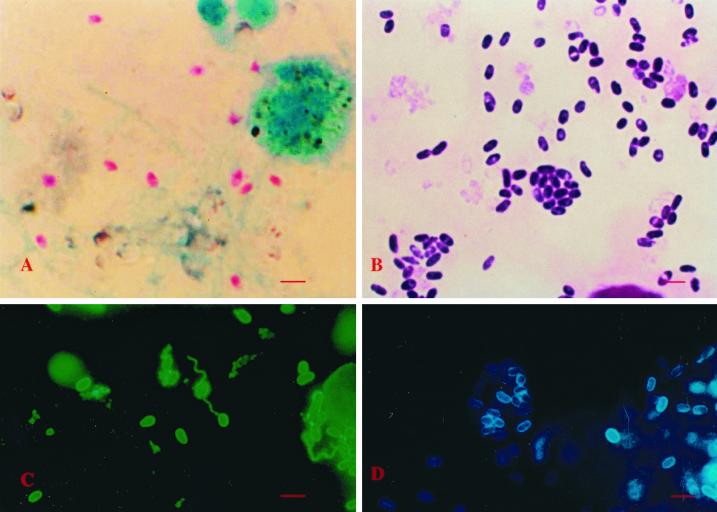
Spores in the chromotrope-stained smears of feces, sputum, and urine samples appeared pinkish red and measured 1.8 to 3.0 μm. Many spores exhibited the characteristic posterior vacuole and beltlike stripe in the middle (Fig. 1A). In the indirect immunofluorescence test, microsporidial spores present in the feces, urine, and sputum samples reacted only with the anti-E. cuniculi serum at a dilution of 1:400 and produced apple-green fluorescence.
FIG. 1.
Optical microscopic images of microsporidial spores after treatment with various procedures. (A) A sputum smear stained with the chromotrope technique (bar = 10 μm). (B) A smear of the cell culture inoculated with the patient's urine sample stained with the “quick-hot” Gram chromotrope technique. (C) A smear made from the cell culture supernatant reacted with the anti-E. cuniculi serum (bar = 10 μm). (D) A smear of the culture supernatant from the same flask as above but stained with the Calcofluor white reagent (bar = 10 μm).
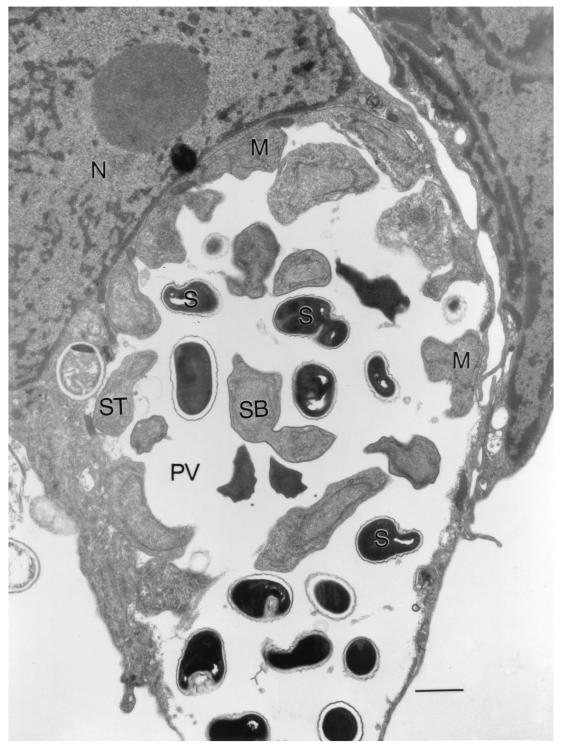
Cell cultures inoculated with the urine and sputum samples showed foci of infected cells within 2 to 4 weeks. Spores that appeared in the culture supernatants measured 1.8 to 3.0 μm, stained dark violet with the Gram chromotrope technique, and showed the characteristic posterior vacuole and belt-like stripe in the middle and the presence of gram-positive granules (19; Fig. 1B). Culture-derived spores reacted brightly with the anti-E. cuniculi serum and to the same extent (>1:4,096) as the spores of CDC:V282, the positive control, and produced bright apple-green fluorescence. When reacted with the rabbit anti-E. hellem and -E. intestinalis sera, the spores reacted moderately at a 1:100 dilution but failed to react at a dilution of 1:800 (Fig. 1C). Smears of culture-derived spores also reacted with the Calcofluor white and produced bluish white fluorescence (Fig. 1D). Transmission electron microscopy revealed that all stages including spores developed within an unseptated parasitophorous vacuole (PV) (Fig. 2). Developing stages consisted of meronts, some with two nuclei, which were always found attached to the PV membrane. Sporogonial stages consisted of sporonts, di- and tetrasporoblastic stages, and mature spores. The spores had approximately five coils of the polar tube, a thin electron-dense exospore, a thick electron-lucent endospore, and a thin cell membrane surrounding the spore contents. These morphological features were consistent with those of Encephalitozoon.
FIG. 2.
Ultrastructure of E. cuniculi (USP-A1) within an infected E6 cell showing the PV filled with spores (S) and developing stages. M, meront; SB, sporoblast; St, sporont; N, host cell nucleus. (Bar = 2 μm.)
Electrophoretically separated proteins extracted from the reference strain CDC:V282 of E. cuniculi and the test isolates, when stained with the silver reagent, exhibited a complex pattern producing more than 50 bands ranging from 14 to 224 kDa. The protein banding patterns of the USP-A1 and USP A-2 isolates were similar to that of the reference strain. Western blot analysis of the separated proteins reacted extensively with the anti-E. cuniculi serum and produced a similar banding pattern, and the bands ranged from 14 to 224 kDa. The protein extract from the uninfected E6 cells showed no visible reactivity.
The four species-specific PCR primers targeting small-subunit rRNA coding sequences selectively amplified E. intestinalis, E. hellem, E. cuniculi, and E. bieneusi diagnostic fragments, respectively, with no background from mammalian cells. Only DNA isolated from the patient specimens (feces, urine, and sputum) and cell culture-infected test isolates (USP-A1 and USP-A2) and the reference strain (CDC:V282) reacted with E. cuniculi-specific primers only, and a diagnostic band of 549 bp was detected in the agarose. Nucleotide sequence analysis of PCR-amplified product from the isolates revealed the presence of four GTTT repeats in the ITS region, indicating that the patient was infected with the E. cuniculi dog genotype (strain type III).
DISCUSSION
Among the 14 species of microsporidia that infect humans, E. bieneusi is known to infect the small intestine and spread into the hepatobiliary tree in patients with AIDS (21, 27). E. intestinalis, on the other hand, can cause disseminated microsporidiosis, including in the gastrointestinal (GI) tract. E. cuniculi and E. hellem are also known to cause infections of the urogenital, respiratory, and ocular organs. E. hellem has not been identified in the GI tract, but reports of E. cuniculi in the GI tract as well as disseminated to other organs, including the brain, have been published (18, 28).
Because our patient had complained of chronic diarrhea and abdominal pain, and since he tested positive only for CMV, it was thought that CMV was the agent of chronic diarrhea. But when the diarrhea did not abate, he was tested for microsporidia and was found positive only for microsporidial spores and no other intestinal pathogens. Subsequently, microsporidial spores were also identified in sputum, urine, and fecal samples. Detailed studies including culture, antigenic analysis, and PCR were done to identify the parasite as E. cuniculi. Currently, 13 cases of infections with E. cuniculi have been described in the literature. Of these 13 cases, 1 each has occurred in Germany (13), Italy (20), Mexico (8, 9), and the United Kingdom (14, 15), 2 in the United States (6, 18), and 7 in Switzerland (8, 9, 16, 28). The present case represents the 14th and the first case of E. cuniculi infection from Spain. E. cuniculi strains have been shown to belong to one of three types based on the DNA sequencing of the ITS region (12). The three types differ from one another by a small repetitive sequence consisting of 5′-GTTT-3′. In type I this sequence is repeated three times as in rabbit isolates; in type II this sequence is repeated twice as in most mouse isolates; and in type III, as seen in most dogs, the sequence is repeated four times. Some of the human E. cuniculi isolates have been typed as type III (8, 11, 18) and others as type I (8, 9, 20). We identified four repeats in the ITS region of our isolates, thus classifying them as type III. Development of antigenic and molecular markers of the isolates may be useful in molecular epidemiology, particularly in tracing the sources of the causal agent and thereby helping to formulate preventive strategies.
ACKNOWLEDGMENTS
We are grateful to L. Hamalainen for help in the preparation of this manuscript.
This work was supported in part by grants (06/97, 09/98, 01/99) from the Fundación San Pablo-CEU and from Fundación Caja Madrid. Gordon J. Leitch was supported in part by Public Health Service grant RR03034.
REFERENCES
- 1.Bergquist N R, Stintzing G, Smedman L T, Waller T, Andersson T. Diagnosis of encephalitozoonosis in man by serological tests. Br Med J. 1984;288:902. doi: 10.1136/bmj.288.6421.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canning E U, Lom J, Dykova I. The microsporidia of vertebrates. New York, N.Y: Academic Press; 1986. [Google Scholar]
- 3.Croppo G P, Visvesvara G S, Leitch G J, Wallace S, DeGroote M A. Western blot and immunofluorescence analysis of a human isolate of Encephalitozoon cuniculi established in culture from the urine of a patient with AIDS. J Parasitol. 1997;83:66–69. [PubMed] [Google Scholar]
- 4.Da Silva A J, Schwartz D A, Visvesvara G S, Moura H, Slemenda S B, Pieniazek N J. Sensitive PCR diagnosis of infections by Enterocytozoon bieneusi (microsporidia) using primers based on the region coding for small-subunit rRNA. J Clin Microbiol. 1996;34:986–987. doi: 10.1128/jcm.34.4.986-987.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Da Silva A J, Slemenda S B, Visvesvara G S, Schwartz D A, Wilcox C M, Wallace S, Pieniazek N J. Detection of Septata intestinalis (Microsporidia) Cali et al. 1993 using polymerase chain reaction primers targeting the small subunit ribosomal RNA coding region. Mol Diagn. 1997;2:47–52. doi: 10.1054/MODI00200047. [DOI] [PubMed] [Google Scholar]
- 6.DeGroote M A, Visvesvara G S, Wilson M L, Pieniazek N J, Slemenda S B, Da Silva A J, Leitch G J, Bryan R T, Reves R. Polymerase chain reaction and culture confirmation of disseminated Encephalitozoon cuniculi in a patient with AIDS: successful therapy with albendazole. J Infect Dis. 1995;171:1375–1378. doi: 10.1093/infdis/171.5.1375. [DOI] [PubMed] [Google Scholar]
- 7.Del Aguila C, Croppo G P, Moura H, Da Silva A J, Leitch G J, Moss D M, Wallace S, Slemenda S B, Pieniazek N J, Visvesvara G S. Ultrastructure, immunofluorescence, Western blot, and PCR analysis of eight isolates of Encephalitozoon (Septata) intestinalis established in culture from sputum and urine samples and duodenal aspirates of five patients with AIDS. J Clin Microbiol. 1998;36:1201–1208. doi: 10.1128/jcm.36.5.1201-1208.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deplazes P, Mathis A, Baumgartner R, Tanner I, Weber R. Immunologic and molecular characteristics of Encephalitozoon-like microsporidia isolated from humans and rabbits indicate that Encephalitozoon cuniculi is a zoonotic parasite. Clin Infect Dis. 1996;22:557–559. doi: 10.1093/clinids/22.3.557. [DOI] [PubMed] [Google Scholar]
- 9.Deplazes P, Mathis A, Muller C, Weber R. Molecular epidemiology of Encephalitozoon cuniculi and first detection of Enterocytozoon bieneusi in fecal samples of pigs. J Euk Microbiol. 1996;43:93S. doi: 10.1111/j.1550-7408.1996.tb05018.x. [DOI] [PubMed] [Google Scholar]
- 10.Didier E S, Orenstein J M, Aldras A, Bertucci D, Rogers L B, Janney F A. Comparison of three staining methods for detecting microsporidia in fluids. J Clin Microbiol. 1995;33:3138–3145. doi: 10.1128/jcm.33.12.3138-3145.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Didier E S, Visvesvara G S, Baker M D, Rogers L B, Bertucci D C, DeGrotte M A, Vossbrinck C R. A microsporidian isolated from an AIDS patient corresponds to Encephalitozoon cuniculi III, originally isolated from domestic dogs. J Clin Microbiol. 1996;34:2835–2837. doi: 10.1128/jcm.34.11.2835-2837.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Didier E S, Vossbrinck C R, Baker M D, Rogers L B, Bertucci D C, Shadduck J A. Identification and characterization of three Encephalitozoon cuniculi strains. Parasitology. 1995;111:411–422. doi: 10.1017/s0031182000065914. [DOI] [PubMed] [Google Scholar]
- 13.Franzen C, Schwartz D A, Visvesvara G S, Müller A, Schwenk A, Salzberger B, Fätkenheuer G, Hartmann P, Mahrle G, Diehl V, Schrappe M. Immunologically confirmed disseminated, asymptomatic Encephalitozoon cuniculi infection of the gastrointestinal tract in a patient with AIDS. Clin Infect Dis. 1995;21:1480–1484. doi: 10.1093/clinids/21.6.1480. [DOI] [PubMed] [Google Scholar]
- 14.Hollister W S, Canning E U, Colbourn N I, Aarons E J. Encephalitozoon cuniculi isolated from the urine of an AIDS patient, which differs from canine and murine isolates. J Euk Microbiol. 1995;42:367–372. doi: 10.1111/j.1550-7408.1995.tb01595.x. [DOI] [PubMed] [Google Scholar]
- 15.Hollister W S, Canning E U, Colbourn N I. A species of Encephalitozoon isolated from an AIDS patient: criteria for species differentiation. Folia Parasitol. 1993;40:293–295. [PubMed] [Google Scholar]
- 16.Mathis A, Michel M, Kuster H, Muller C, Weber R, Deplazes P. Two Encephalitozoon cuniculi strains of human origin are infectious to rabbits. Parasitology. 1997;114:29–35. doi: 10.1017/s0031182096008177. [DOI] [PubMed] [Google Scholar]
- 17.Matsubayashi H, Koike T, Mikata I, Takei H, Hagiwara S. A case of Encephalitozoon-like body in man. Arch Pathol. 1959;67:181–187. [PubMed] [Google Scholar]
- 18.Mertens R B, Didier E S, Fishbein M C, Bertucci D C, Rogers L B, Orenstein J M. Encephalitozoon cuniculi microsporidiosis: infection of the brain, heart, kidneys, trachea, adrenal glands, and urinary bladder in a patient with AIDS. Modern Pathol. 1997;10:68–77. [PubMed] [Google Scholar]
- 19.Moura H, Schwartz D A, Bornay-Llinares F, Sodré F C, Wallace S, Visvesvara G S. A new and improved “quick-hot Gram-chromotrope” technique that differentially stains microsporidian spores in clinical samples, including paraffin-embedded tissue sections. Arch Pathol Lab Med. 1997;121:888–893. [PubMed] [Google Scholar]
- 20.Rossi P, la Rosa G, Ludovisi A, Tamburrini A, Gomez Morales M A, Pozzio E. Identification of a human isolate of Encephalitozoon cuniculi type I from Italy. Intern J Parasitol. 1998;28:1361–1366. doi: 10.1016/s0020-7519(98)00122-2. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz D A, Bryan R T. Microsporidia. In: Horsburgh C R, Nelson A M, editors. Emerging infections: clinical and pathologic update. Washington, D.C.: American Society for Microbiology; 1997. pp. 61–93. [Google Scholar]
- 22.Terada S, Reddy R, Jeffers L J, Cali A, Schiff E R. Microsporidian hepatitis in the acquired immunodeficiency syndrome. Ann Intern Med. 1987;107:61–62. doi: 10.7326/0003-4819-107-1-61. [DOI] [PubMed] [Google Scholar]
- 23.Visvesvara G S, Da Silva A J, Croppo G P, Pieniazek N J, Ferguson D, Moura H, Wallace S, Slemenda S B, Tyrrel I, Moore D F, Meador J. In vitro culture and serologic and molecular identification of Septata intestinalis isolated from urine of a patient with AIDS. J Clin Microbiol. 1995;33:930–936. doi: 10.1128/jcm.33.4.930-936.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visvesvara G S, Moura H, Leitch G J, Schwartz D A. Culture and propagation of microsporidia. In: Wittner M, Weiss L M, editors. The microsporidia and microsporidiosis. Washington, D.C.: ASM Press; 1999. pp. 363–392. [Google Scholar]
- 25.Visvesvara G S, Leitch G J, Da Silva A J, Croppo G P, Moura H, Wallace S, Slemenda S B, Schwartz D A, Moss D M, Bryan R T, Pieniazek N J. Polyclonal and monoclonal antibody and PCR-amplified small-subunit RNA identification of a microsporidian, Encephalitozoon hellem, isolated from an AIDS patient with disseminated infection. J Clin Microbiol. 1994;32:2760–2768. doi: 10.1128/jcm.32.11.2760-2768.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber R, Bryan R T, Owen R L, Wilcox C M, Gorelkin L, Visvesvara G S. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. N Engl J Med. 1992;326:161–166. doi: 10.1056/NEJM199201163260304. [DOI] [PubMed] [Google Scholar]
- 27.Weber R, Bryan R T, Schwartz D A, Owen R L. Human microsporidial infections. Clin Microbiol Rev. 1994;7:426–461. doi: 10.1128/cmr.7.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber R, Deplazes P, Flepp M, Mathis A, Baumann R, Sauer B, Kuster H, Lüthy R. Cerebral microsporidiosis due to Encephalitozoon cuniculi in a patient with human immunodeficiency virus syndrome. N Engl J Med. 1997;336:474–478. doi: 10.1056/NEJM199702133360704. [DOI] [PubMed] [Google Scholar]
- 29.Weiss L M, Vossbrinck C R. Molecular biology, molecular phylogeny, and molecular diagnostic approaches to the microsporidia. In: Wittner M, Weiss L M, editors. The microsporidia and microsporidiosis. Washington, D.C.: ASM Press; 1999. pp. 129–171. [Google Scholar]
- 30.Zender H O, Arrigoni E, Eckert J, Kapanci Y. A case of Encephalitozoon cuniculi peritonitis in a patient with AIDS. Am J Clin Pathol. 1989;92:352–356. doi: 10.1093/ajcp/92.3.352. [DOI] [PubMed] [Google Scholar]