Abstract
Aims
Heart failure (HF) and atrial fibrillation (AF) frequently coexist and are both associated with increased levels of N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP). It is known that AF impairs the diagnostic accuracy of NT‐proBNP for HF. The aim of the present study was to compare the diagnostic and predictive accuracy of NT‐proBNP for HF and AF in stable outpatients with cardiovascular risk factors.
Methods and results
Data were obtained from the DIAST‐CHF trial, a prospective cohort study that recruited individuals with cardiovascular risk factors and followed them up for 12 years. Data were validated in three independent population‐based cohorts using the same inclusion/exclusion criteria: LIFE‐Adult (n = 2869), SHIP (n = 2013), and SHIP‐TREND (n = 2408). Serum levels of NT‐proBNP were taken once at baseline. The DIAST‐CHF study enrolled 1727 study participants (47.7% female, mean age 66.9 ± 8.1 years). At baseline, patients without AF or HF (n = 1375) had a median NT‐proBNP of 94 pg/mL (interquartile range 51;181). In patients with AF (n = 93), NT‐proBNP amounted to 667 (215;1130) pg/mL. It was significantly higher than in the first group (P < 0.001) and compared with those with only HF [n = 201; 158 (66;363) pg/mL; P < 0.001]. The highest levels of NT‐proBNP [868 (213;1397) pg/mL] were measured in patients with concomitant HF and AF (n = 58; P < 0.001 vs. control and vs. HF, P = 1.0 vs. AF). In patients with AF, NT‐proBNP levels did not differ between those with HF and preserved ejection fraction (EF) > 50% [n = 38; 603 (175;1070) pg/mL] and those without HF (P = 1.0). Receiver‐operating characteristic curves of NT‐proBNP showed a similar area under the curve (AUC) for the detection of AF at baseline (0.84, 95% CI [0.79–0.88]) and for HF with EF < 50% (0.78 [0.72–0.85]; P = 0.18). The AUC for HF with EF > 50% was significantly lower (0.61 [0.56–0.65]) than for AF (P = 0.001). During follow‐up, AF was newly diagnosed in 157 (9.1%) and HF in 141 (9.6%) study participants. NT‐proBNP was a better predictor of incident AF during the first 2 years (AUC: 0.79 [0.75–0.83]) than of newly diagnosed HF (0.59 [0.55–0.63]; P < 0.001). Data were validated in three independent population‐based cohorts (LIFE‐Adult, n = 2869; SHIP, n = 2013; and SHIP‐TREND, n = 2408).
Conclusions
In stable outpatients, NT‐proBNP is a better marker for prevalent and incident AF than for HF. In AF patients, the diagnostic value of NT‐proBNP for HF with EF > 50% is very limited.
Keywords: Atrial fibrillation, Heart failure, Brain natriuretic peptide, Biomarker, Population‐based cohort studies
Introduction
B‐type natriuretic peptide (BNP) and the biologically inactive cleavage product of its pro‐hormone, N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), are established biomarkers of cardiovascular diseases and subclinical cardiac injury. The most important trigger for their release from the atrial and ventricular myocardium is increased end‐diastolic wall stress, which occurs, for instance, in case of volume expansion or pressure overload. 1
Among the cardiovascular conditions associated with increased BNPs, their disease‐related changes are most comprehensively studied in heart failure (HF). The European Society of Cardiology (ESC) guidelines recommend the measurement of BNP and NT‐proBNP to diagnose and to rule out HF. 2 , 3 BNPs correlate with the clinical severity of HF and are of high prognostic value for future cardiovascular events, re‐hospitalization, and mortality in HF patients. 4 , 5 , 6 , 7
Plasma levels of BNP and NT‐proBNP are also elevated in patients with atrial fibrillation (AF). 8 , 9 , 10 , 11 Several studies demonstrated that they are markers of prevalent AF 4 , 12 and useful tools in detecting paroxysmal AF. 13 , 14 Furthermore, BNPs improve the risk prediction of incident AF, both as independent risk factors and in addition to established clinical risk factors. 8 , 10 , 15 , 16
Heart failure and AF are common chronic diseases with very high overlap. 17 The presence of AF impairs the diagnostic utility of BNPs for the diagnosis of acute HF, 18 but is not thoroughly investigated for the diagnosis of chronic HF. However, current guidelines recommend their use in the diagnosis of all HF entities. 2 , 3 We hypothesized that in stable outpatients, NT‐proBNP is a superior biomarker for the diagnosis and prediction of AF compared with HF and that its diagnostic value for HF is limited in the presence of concomitant AF.
Methods
DIAST‐CHF study
The multicentre, observational Diagnostic Trial on prevalence and clinical course of diastolic dysfunction and diastolic Chronic Heart Failure (DIAST‐CHF; n = 1735) included outpatients aged 50–85 years with either ≥1 risk factor(s) for HF (hypertension, diabetes mellitus, sleep apnoea syndrome, or atherosclerotic disease) or established congestive HF (see definition below). 19 Patients were recruited from general practitioners by computer‐based search of eligible patients (2004–2006). The only exclusion criteria were unwillingness to participate or inability for logistic reasons. The DIAST‐CHF trial complied with the Declaration of Helsinki, the protocol was approved by the responsible ethics committee, and all patients gave written informed consent. At baseline, the diagnostic workup included medical history and physical examination, laboratory analyses, transthoracic echocardiography, and a 12‐channel electrocardiogram (ECG). Participants were followed up for 10 years in person and by telephone. All clinical events (death, hospitalizations, AF, and HF) during follow‐up were confirmed by written medical reports. The definition of HF was met if HF was either stated in the medical record or if the clinical diagnosis of HF was made during physical examination based on the Framingham Diagnostic criteria for congestive HF. 20 In this retrospective analysis, the current HF classification according to the ESC guidelines of 2016 21 could not be applied in detail because of incomplete echocardiographic data in parts of the study population. Still, we classified HF according to their left ventricular ejection fraction (LVEF) in HF with reduced LVEF (HFrEF; LVEF < 40%), HF with medium reduced LVEF (HFmrEF; LVEF 40–49%), and HF with preserved LVEF (HFpEF; LVEF ≥ 50%). The diagnosis of AF was based on the patients' medical records and on a 12‐channel ECG. ECGs were analysed by skilled physicians and the depicted rhythm classified along the following categories: sinus rhythm, AF, and other (i.e. atrial flutter and pacemaker). Assessment of HF and AF was performed by investigators blinded to values of NT‐proBNP. NT‐proBNP was measured by electrochemoluminescence immunoassay on an Elecsys analyser (Roche Diagnostics, Mannheim, Germany).
LIFE‐Adult study
The LIFE‐Adult study is a population‐based cohort study with 10 000 randomly selected participants from the city of Leipzig, Germany. The recruitment period was from August 2011 to November 2014. Study participants underwent an extensive assessment programme including structured interviews, and physical and medical examinations. 22 To make study populations more consistent, only participants with hypertension and/or diabetes mellitus were included in the present analysis. Of those, analysed ECG data were available for n = 2869. The criteria of hypertension, diabetes mellitus, and HF were met if self‐reported medical history included the respective diagnosis. To investigate AF or other cardiac arrhythmias, a 10 s 12‐lead ECG was recorded using the PageWriter TC50® ECG system (Philips Medical Systems DMC GmbH, Hamburg, Germany) after a supine resting period of at least 10 min. The ECG was evaluated by means of the software ECGVue C.03.01.02 (Philips Medical Systems DMC GmbH, Hamburg, Germany). NT‐pro‐BNP was measured once in the baseline blood sample.
SHIP/SHIP‐TREND
The Study of Health in Pomerania is a population‐based project, which consists of two independent cohorts (SHIP and SHIP‐TREND). SHIP recruited participants from 1997 to 2001 and SHIP‐TREND from 2008 to 2011. The first SHIP cohort including 4308 individuals aged 20–75 years at baseline (SHIP‐0) was followed up after 5 (SHIP‐1; n = 3300) and 11 years (SHIP‐2; n = 2333). A second, independent cohort (SHIP‐TREND) included 4420 study participants. For the present study, only study participants of SHIP and SHIP‐TREND with hypertension (systolic blood pressure > 140 mmHg or diastolic blood pressure > 90 mmHg or anti‐hypertensive medication) or diabetes mellitus (medical history or HbA1C > 6.5% or insulin therapy) were included for the present analysis. Complete data sets were available for 2103 study participants in SHIP‐0, 1361 in SHIP‐1, 863 in SHIP‐2, and 2408 in SHIP‐TREND. HF was assessed at all clinical visits following modified criteria of the Rotterdam Study that relied on signs and symptoms of HF as well as on the presence of underlying cardiovascular disease. 23 AF was defined as either AF or atrial flutter on surface ECG that was recorded at each clinical visit. NT‐proBNP was measured at baseline (SHIP‐0 and SHIP‐TREND). More details on study design have been published. 24
Statistical analyses
Statistical analyses were performed with IBM SPSS 24.0 software (IBM 1989, 2016) and R 3.4.1 including the package pROC. Continuous variables of baseline characteristics were presented as mean ± standard deviation (SD) and categorical variables as numbers and percentages. For NT‐proBNP, we show median (quartiles) and log‐transformed the values for analysis. Groups were compared by one‐factorial ANOVA. For pairwise comparisons, Bonferroni correction for multiple testing was applied. Discriminatory and predictive abilities of NT‐proBNP and echocardiographic data for HF and AF were checked by receiver‐operating characteristic (ROC) analyses. The area under the curve (AUC) was calculated and tested. Cut‐off values were generated by maximizing the Youden index (sensitivity + specificity −1). The risk was quantified by odds ratios (ORs). Linear regression analyses were performed to determine risk indicators of NT‐proBNP levels. All patient characteristics were included. Significance level was determined 5% for two‐tailed testing. In all studies, personal doing the NT‐proBNP analysis was blinded to all clinical data and personal doing the baseline and follow‐up visits was blinded to NT‐proBNP data. Patients lost to follow‐up were censored at the last visit.
Results
Descriptive statistics
A total of 1735 participants were enrolled in the DIAST‐CHF study. Eight patients were excluded because of missing information on gender or age. Baseline characteristics are shown in Table 1 . At baseline, AF was present in 151 (8.7%) and HF in 259 (15.0%) participants. Of the participants with HF, the LVEF was <40% in 28 (10.8%; HFrEF), between 40% and 49% in 35 (13.5%; HFmrEF), and ≥50% in 196 (75.7%; HFpEF) participants. The most frequent clinical sign in participants with HF was peripheral oedema (48.3%), and the most frequent symptoms dyspnoea on exertion (73.0%) and nycturia (73.8%). Signs and symptoms of HF were similar in HFrEF, HFmrEF, and HFpEF (Supporting Information, Table S1 ). HF patients suffered of concomitant AF in 22.4%; 38.4% of the patients with AF were also affected by HF. During the study, 157 (9.1%) of study participants were newly diagnosed with AF and 141 (9.6%) patients with HF. Details of follow‐up are presented in Figure S1 .
Table 1.
Baseline characteristics of the DIAST‐CHF cohort
| Variable | Groups | ||||||
|---|---|---|---|---|---|---|---|
| No. of patients (%) | No AF | AF | Total | ||||
| 1576 (91.3%) | 151 (8.7%) | 1727 (100%) | |||||
| No HF | HFr/mrEF | HFpEF | No HF | HFr/mrEF | HFpEF | ||
| 1375 (79.6%) | 43 (2.5%) | 158 (9.2%) | 93 (5.4%) | 20 (1.2%) | 38 (2.2%) | 1727 (100%) | |
| Demographics, n (%) | |||||||
| Female | 675 (49.1%) | 8 (18.6%) | 94 (59.5%) | 29 (31.2%) | 4 (20%) | 14 (36.8%) | 824 (47.7%) |
| Age, mean ± SD, years | 66.3 ± 7.9 | 68.3 ± 8.8 | 68.8 ± 8.8 | 70.2 ± 8.4 | 72.3 ± 5 | 70.8 ± 8.4 | 66.9 ± 8.1 |
| Cardiovascular risk factors, n (%) | |||||||
| Diabetes mellitus | 355 (25.8%) | 16 (37.2%) | 53 (33.5%) | 18 (19.4%) | 9 (45%) | 14 (36.8%) | 465 (26.9%) |
| Arterial hypertension | 1218 (88.6%) | 40 (93%) | 141 (89.2%) | 88 (94.6%) | 18 (90%) | 35 (92.1%) | 1540 (89.2%) |
| Hyperlipidaemia | 596 (43.3%) | 28 (65.1%) | 81 (51.3%) | 40 (43%) | 10 (50%) | 16 (42.1%) | 771 (44.6%) |
| Hyperuricaemia | 199 (14.5%) | 10 (23.3%) | 30 (19%) | 21 (22.6%) | 5 (25%) | 11 (28.9%) | 276 (16.0%) |
| Smoker | 152 (11.1%) | 8 (18.6%) | 13 (8.3%) | 5 (5.4%) | 1 (5%) | 9 (23.7%) | 188 (10.9%) |
| Ex‐smoker | 550 (40%) | 28 (65.1%) | 57 (36.3%) | 47 (50.5%) | 14 (70%) | 16 (42.1%) | 712 (41.2%) |
| Non‐smoker | 672 (48.9%) | 7 (16.3%) | 87 (55.4%) | 41 (44.1%) | 5 (25%) | 13 (34.2%) | 825 (47.8%) |
| Physical investigation, mean ± SD | |||||||
| BMI (kg/m2) | 29.1 ± 4.8 | 31.3 ± 6.1 | 30.1 ± 5.3 | 29.6 ± 4.7 | 27.7 ± 5.1 | 29.7 ± 5.5 | 29.3 ± 4.9 |
| Systolic BP (mmHg) | 150 ± 21 | 139 ± 20 | 145 ± 24 | 148 ± 22 | 123 ± 23 | 147 ± 19 | 148.6 ± 21.5 |
| Diastolic BP (mmHg) | 84 ± 12 | 78 ± 10 | 82 ± 12 | 85 ± 13 | 73 ± 11 | 81 ± 14 | 83.8 ± 11.9 |
| Heart rate at rest (1/min) | 71 ± 12 | 69 ± 11 | 68 ± 11 | 71 ± 15 | 72 ± 13 | 71 ± 12 | 70.4 ± 12.0 |
| TTE, mean ± SD | |||||||
| LVEF (%) | 60.5 ± 7.1 | 39.1 ± 7.6 | 61.5 ± 6.7 | 58 ± 9.5 | 38 ± 8.2 | 59.4 ± 6.1 | 59.7 ± 8.3 |
| LAVI (mL/m2) | 46.8 ± 16.3 | 62.4 ± 27.6 | 49.9 ± 17.7 | 80.2 ± 27.7 | 89.1 ± 30.8 | 76.9 ± 35.5 | 50.6 ± 20.9 |
| Laboratory, mean ± SD | |||||||
| Cholesterol (mmol/L) | 5.26 ± 1.07 | 4.83 ± 0.94 | 5.01 ± 1.02 | 4.89 ± 0.94 | 4.65 ± 1.34 | 4.88 ± 1.1 | 5.19 ± 1.07 |
| LDL (mmol/L) | 3.29 ± 0.83 | 2.95 ± 0.69 | 3.02 ± 0.8 | 3.06 ± 0.77 | 2.74 ± 0.96 | 3.03 ± 0.79 | 3.23 ± 0.83 |
| HDL (mmol/L) | 1.4 ± 0.43 | 1.25 ± 0.41 | 1.33 ± 0.37 | 1.27 ± 0.38 | 1.08 ± 0.37 | 1.21 ± 0.35 | 1.38 ± 0.42 |
| Haemoglobin (mmol/L) | 8.76 ± 0.76 | 8.92 ± 0.86 | 8.55 ± 0.85 | 8.85 ± 0.83 | 8.63 ± 0.97 | 8.65 ± 0.85 | 8.74 ± 0.78 |
|
Creatinine clearance (Cockroft–Gault; mL/min) |
72.3 ± 18.9 | 68.1 ± 19.6 | 67.4 ± 20.7 | 68.1 ± 24 | 49.4 ± 17.2 | 61.6 ± 20.8 | 71.0 ± 19.7 |
| NT‐proBNP | |||||||
| Median (25%;75%) (pg/mL) | 94 (50;181) | 323 (129;616) | 147 (64;277) | 716 (226;1231) | 1345 (627;3027) | 603 (175;1070) | 108 (55;228) |
AF, atrial fibrillation; BMI, body mass index; HDL, high‐density lipoprotein; HF, heart failure; HFpEF, HF with preserved LVEF (LVEF ≥ 50%); HFr/mrEF, HF with medium reduced (LVEF 40–49%) or reduced (LVEF ≤ 40%); LAVI, left atrial volume index; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; SD, standard deviation; TTE, transthoracic echocardiography.
Baseline characteristics of the LIFE‐Adult study and SHIP‐0/SHIP‐TREND are shown in Tables S2 and S3 , respectively. Follow‐up information was only available in SHIP‐0. AF was newly diagnosed in 26 (1.9%) out of 1361 study participants at the 5 year follow‐up (SHIP‐1) and in 36 (4.2%) out of 863 at the 11 year follow‐up (SHIP‐2). The incidence of HF was 103 (7.6%) in SHIP‐1 and 120 (13.9%) in SHIP‐2.
Cross‐sectional analysis of N‐terminal pro‐B‐type natriuretic peptide plasma levels
Baseline plasma concentrations of NT‐proBNP in DIAST‐CHF are presented in Table 1 . The lowest median values of NT‐proBNP were measured in patients presenting without AF and HF and the highest in patients with both AF and HF. Compared with patients without AF, the mean NT‐proBNP level was estimated 3.2‐fold higher in patients with AF in a two‐factorial model (AF and HF) for log NT‐proBNP (P < 0.001). NT‐proBNP was on average increased by a factor of 2.3 in patients with history of AF, but no AF in baseline ECG compared with participants without AF (median 235 pg/mL vs. 101 pg/mL, P < 0.001). The highest plasma levels were observed in patients with current AF (documented on ECG, median 1109 pg/mL, P < 0.001 vs. no AF on ECG). Patients with HFpEF displayed lower median NT‐proBNP levels (without AF: 147 pg/mL; with AF: 607 pg/mL) than those with HFrEF or HFmrEF (without AF: 323 pg/mL; with AF: 1345 pg/mL, P < 0.001 for all comparisons). In patients with AF, NT‐proBNP did not differ significantly between patients with HFpEF and those without HF [median 603 pg/mL (175;1070) vs. 716 pg/mL (226;1231), P = 1.0; Figure 1 ].
Figure 1.
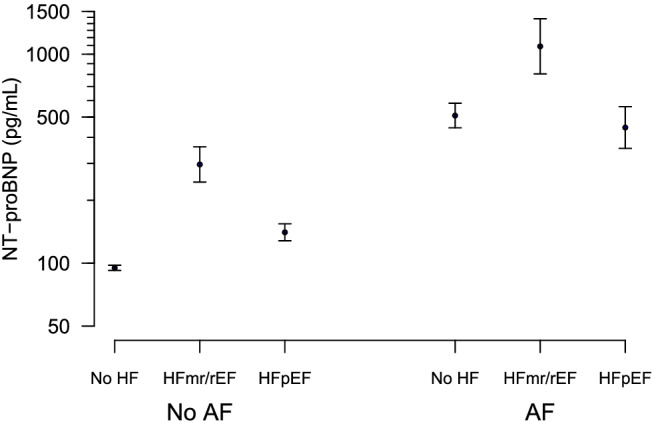
Baseline plasma concentrations of NT‐proBNP (logarithmic scale) in patients of the DIAST‐CHF trial, grouped according to the presence or absence of AF. Values are presented as geometric mean with 95% confidence interval. Patients with HF are divided into patients with LVEF < 50% (HFr/mrEF) and patients with LVEF ≥ 50% (HFpEF). Statistics are given in the text.
Plasma levels of NT‐proBNP in LIFE‐Adult and SHIP/SHIP‐TREND are depicted in Table 2 . In all three cohorts, NT‐proBNP was higher in participants with HF than in those without and higher in patients with AF than in patients with HF (P < 0.001 for all comparisons). NT‐proBNP levels in participants with HF and AF were not different from levels in participants with AF, but without HF.
Table 2.
Baseline values of NT‐proBNP in DIAST‐CHF, LIFE‐Adult, SHIP‐0, and SHIP‐TREND
| Variables | Groups | P‐values (corr. for multiple testing)—Post hoc values | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (25%;75%) | No HF or AF (0) | AF (1) | HF (2) | HF and AF (3) | Total | Global | 1 vs. 0 | 2 vs. 0 | 3 vs. 0 | 2 vs. 1 | 3 vs. 1 | 3 vs. 2 |
| DIAST‐CHF study | ||||||||||||
| Number of patients (%) | 1375 (79.6%) | 93 (5.4%) | 201 (11.6%) | 58 (3.4%) | 1727 (100%) | |||||||
| NT‐proBNP (pg/mL) | 94 (51;181) | 667 (215;1130) | 158 (70, 363) | 868 (213;1397) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.996 | <0.001 | |
| LIFE‐Adult study | ||||||||||||
| Number of patients (%) | 2716 (94.7%) | 77 (2.7%) | 60 (2.1%) | 16 (0.6%) | 2869 (100.0%) | |||||||
| NT‐proBNP (pg/mL) | 82 (47;152) | 1009 (690;1345) | 124 (71;280) | 967 (680;1930) | 86 (48;162) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.415 | <0.001 |
| SHIP‐0 | ||||||||||||
| Number of patients (%) | 1812 (86.2%) | 13 (0.6%) | 254 (12.1%) | 24 (1.1%) | 2103 (100%) | |||||||
| NT‐proBNP (pg/mL) | 62 (31;126) | 831 (419;1468) | 99 (46;204) | 934 (856;1722) | 67 (32;143) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.626 | <0.001 |
| SHIP‐TREND | ||||||||||||
| Number of patients (%) | 1846 (76.7%) | 31 (1.3%) | 500 (20.8%) | 31 (1.3%) | 2408 (100%) | |||||||
| NT‐proBNP (pg/mL) | 73 (37;146) | 862 (457;1311) | 84 (45;180) | 1202 (901;1930) | 78 (40;162) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.489 | <0.001 |
AF, atrial fibrillation; HF, heart failure; NT‐proBNP, amino‐terminal pro‐B‐type natriuretic peptide; SD, standard deviation.
Median with 25% and 75% percentile.
Diagnostic accuracy of N‐terminal pro‐B‐type natriuretic peptide for prevalent heart failure and prevalent atrial fibrillation
We performed ROC curve analyses of NT‐proBNP for prevalent AF and prevalent HF at baseline in DIAST‐CHF (Table 3 and Figure 2 ). For the detection of AF, the optimal cut‐off value was calculated as 338 pg/mL NT‐proBNP (sensitivity 68% and specificity 88% using the observed prevalence). For patients with NT‐proBNP above the aforementioned threshold level, the OR for having AF was 16.1 (95% CI [10.8–24.0]). At baseline, 151 had a history of AF, but only 80 showed AF on the baseline ECG. Even in patients with a history of AF but sinus rhythm on baseline ECG, NT‐proBNP yielded good values for the diagnosis of AF. In a direct comparison, the AUC of NT‐proBNP for the detection of any AF (AUC 0.84; 95% CI [0.79–0.88]) tended to be higher than the AUC for HFrEF/HFmrEF (AUC 0.78; 95% CI [0.72–0.85]; P = 0.18) and was significantly higher than the AUC for HFpEF (AUC 0.61; 95% CI [0.56–0.65]; P < 0.001 vs. AF). In patients with AF, the diagnostic ability of NT‐proBNP to discriminate between those with or without HF was not better than chance (AUC 0.54; 95% CI [0.44–0.65]).
Table 3.
Areas under the curve of N‐terminal pro‐B‐type natriuretic peptide plasma levels in receiver‐operating characteristic curve analysis for the diagnosis of prevalent heart failure and prevalent atrial fibrillation
| DIAST‐CHF study | ||
|---|---|---|
| AUC for the diagnosis of | ||
| HFr/mrEF | Any AF | P |
| 0.78 [0.72–0.85] | 0.84 [0.79–0.88] | 0.18 |
| AUC for the diagnosis of | ||
| HFpEF | Any AF | P |
| 0.61 [0.56–0.65] | 0.84 [0.79–0.88] | <0.001 |
| AUC for the diagnosis of | ||
| AF on ECG | AF known, but not on ECG | P |
| 0.95 [0.92–0.97] | 0.84 [0.79–0.88] | <0.001 |
| LIFE‐Adult study | ||
| AUC for the diagnosis of | ||
| HF | AF on ECG | P |
| 0.67 [0.66–0.74] | 0.97 [0.95–0.99] | <0.001 |
| SHIP | ||
| AUC for the diagnosis of | ||
| HF | AF on ECG | P |
| 0.64 [0.62–0.66] | 0.95 [0.94–0.96] | <0.001 |
| SHIP‐TREND | ||
| AUC for the diagnosis of | ||
| HF | AF | P |
| 0.57 [0.55–0.59] | 0.94 [0.93–0.95] | <0.001 |
AF, atrial fibrillation; AUC, area under the curve presented with 95% confidence interval; ECG, electrocardiogram; HF, heart failure; HFpEF, HF with preserved ejection fraction; HFr/mrEF, HF with reduced/medium reduced ejection fraction.
Figure 2.
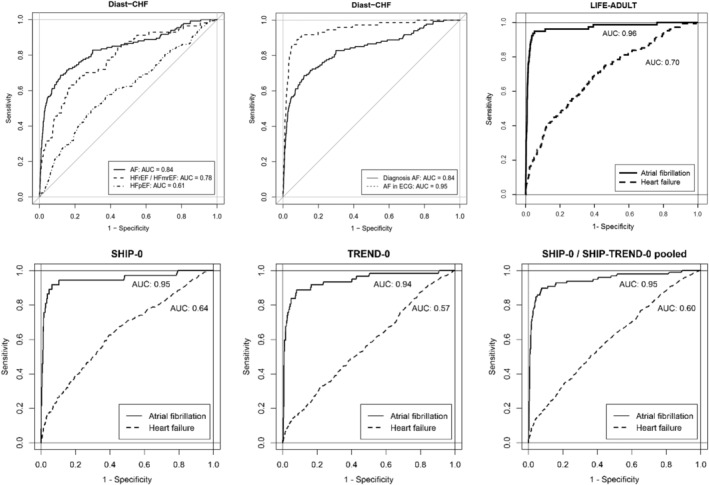
Receiver‐operating characteristic curves with AUC of NT‐proBNP for the diagnosis of HF and AF in DIAST‐CHF, LIFE‐Adult, SHIP‐0, and TREND‐0. In the DIAST‐CHF study, patients with HF were grouped according to their LVEF in HFr/mrEF (LVEF < 50%) and HFpEF (LVEF ≥ 50%). The criterion of AF included the diagnosis of AF and current AF on electrocardiogram (ECG). The second figure illustrates the difference of NT‐proBNP for the diagnosis of current AF and known AF that was not present at timepoint of examination. Statistics are given in the text.
In LIFE‐Adult, the AUC of NT‐proBNP for the diagnosis of AF was excellent (AUC: 0.97; 95% CI [0.946–0.991]). In contrast, the AUC of NT‐proBNP for the diagnosis of HF was low (AUC: 0.67; 95% CI [0.655–0.743]) and significantly lower than the AUC for the detection of AF (P < 0.001) (Table 3 and Figure 2 ).
In SHIP‐0 and SHIP‐TREND, the AUC of NT‐proBNP for the diagnosis of HF was low (0.64 and 0.57, respectively) but was excellent for AF (AUC 0.95 and 0.94 for SHIP and SHIP‐TREND, respectively) (Table 3 and Figure 2 ).
Predictive accuracy of N‐terminal pro‐B‐type natriuretic peptide for atrial fibrillation and heart failure in DIAST‐CHF and SHIP‐0
The ability of NT‐proBNP to predict incident AF during the follow‐up period of 2 years was moderate and low for 12 years of follow‐up (Table 4 ). In comparison, the ability of NT‐proBNP to predict incident HF up to 2 and 12 years was significantly lower. In SHIP‐0, the ability of NT‐proBNP to predict incident AF and HF within 5 and 11 years was low, but the AUC for incident HF was significantly lower than for incident AF (Table 4 and Figure S2 ).
Table 4.
Areas under the curve of N‐terminal pro‐B‐type natriuretic peptide plasma levels in receiver‐operating characteristic curve analysis for the prediction of incident heart failure and incident atrial fibrillation
| DIAST‐CHF study | ||
|---|---|---|
| AUC for the prediction of | ||
| HF (within 2 years) | AF (within 2 years) | P |
| 0.59 [0.55–0.63] | 0.79 [0.75–0.83] | <0.001 |
| AUC for the prediction of | ||
| HF (within 12 years) | AF (within 12 years) | P |
| 0.56 [0.52–0.60] | 0.71 [0.67–0.75] | <0.001 |
| SHIP‐0 | ||
| AUC for the prediction of | ||
| HF (within 5 years) | AF (within 5 years) | P |
| 0.59 [0.57–0.62] | 0.73 [0.70–0.75] | 0.029 |
| AUC for the prediction of | ||
| HF (within 11 years) | AF (within 11 years) | P |
| 0.58 [0.55–0.62] | 0.73 [0.70–0.76] | 0.006 |
AF, atrial fibrillation; AUC, area under the curve presented with 95% confidence interval; HF, heart failure.
Determinants of N‐terminal pro‐B‐type natriuretic peptide in DIAST‐CHF
Linear multivariate regression analyses were performed to analyse the determinants of NT‐proBNP in the DIAST‐CHF trial (Table S4 ). Age, female sex, coronary artery disease, and HDL cholesterol were associated with increased NT‐proBNP plasma levels while diabetes, cholesterol, haemoglobin, glomerular filtration rate, and ejection fraction were associated with reduced NT‐proBNP plasma levels. AF had the highest OR of all analysed parameters (OR 2.43 [1.90–3.11]), followed by HFrEF (OR 2.04 [1.18–3.53]). There was no independent effect of HFmrEF (OR 1.43 [0.93–2.22]) or HFpEF (OR 1.11 [0.93–1.32]) on NT‐proBNP plasma levels.
Discussion
In the DIAST‐CHF trial, NT‐proBNP was increased in both HF and AF, higher in patients with AF than in patients with HF, and a superior biomarker for the diagnosis and prediction of AF compared with HF. These findings were confirmed in three independent cohorts (LIFE‐Adult Study, SHIP‐0, and SHIP‐TREND). In patients with AF, levels of NT‐proBNP did not differ between those with HFpEF and those without HF.
Our findings challenge current recommendations for the use of NT‐proBNP in the diagnosis of chronic HF in patients with AF. The measurement of BNP plasma concentrations is part of the diagnostic algorithm for the diagnosis of HF stated by the ESC guidelines, 2 , 3 but not integrated in those for AF. 25 The diagnostic guideline recommendations for HF are in part based on studies that did not thoroughly investigate HFpEF 26 or analyse the impact of AF on biomarkers in depth. 27 , 28 Although an impaired diagnostic performance of BNPs in acute HF with AF has been reported, 18 no direct comparison of the diagnostic accuracy of NT‐proBNP for chronic HF and AF is available.
Currently, the diagnostic workup of individuals with elevated levels of BNPs focuses on HF and not on potential (paroxysmal) AF. However, literature shows that elevated NT‐proBNP is a strong marker of both HF and AF, which additionally share a high overlap. 5 , 29 , 30 In stroke patients, in whom the detection of AF changes secondary prevention therapy, the predictive accuracy of BNPs has been more thoroughly investigated. 8 , 30 , 31 , 32 Data imply that subsequent to ischaemic stroke, an elevation of BNPs in the absence of HF should trigger extended rhythm monitoring for unknown AF. 14 Our study suggests that likewise outpatients with marked elevation of NT‐proBNP might profit from AF screening if HF was ruled out.
Our data also show that in stable outpatients with cardiovascular risk factors, NT‐proBNP is a better predictor for incident AF than for incident HF. Current literature only analyses both diseases in parallel, but not in comparison. The Cardiovascular Health Study showed an association of HF and AF incidence with quintiles of NT‐proBNP serum levels. 8 In the Framingham Offspring Study, BNP levels predicted the risk of incident HF, 11 whereas the AUC of NT‐proBNP for incident HFNT‐proBNP was low in DIAST‐CHF and SHIP‐0. Because BNPs are elevated in the presence of cardiac structural alterations and early stages of HF, 33 the predictive performance of NT‐proBNP for HF could have been impaired by the patient cohort investigated (all participants had cardiovascular risk factors). Moreover, the majority of HF patients in DIAST‐CHF suffered from HFpEF, which is associated with lower median levels of NT‐proBNP than HFrEF. 21
Recent HFpEF trials recruited patients using elevated BNPs as an inclusion criterion for establishing HF. 34 , 35 , 36 It is probable that in addition to patients with chronic HF, BNPs likely selected a population with diagnosed (or undiagnosed) AF. Treatments that are beneficial in patients with AF (e.g. aldosterone receptor blockade) may therefore be beneficial in patients with slightly elevated BNPs in general. 12 , 37 This finding is corroborated by a recent analysis of BNPs as a predictor of treatment response in HF trials, which found a predictive value mostly in trials with renin–angiotensin–aldosterone system (RAAS) inhibitors and only a modest correlation with risk reduction for cardiovascular hospitalizations, but none with mortality. 36 Some authors have proposed higher study inclusion thresholds for BNPs in HF patients with concomitant AF, 38 but based on our findings, we discourage using BNPs as a diagnostic inclusion criterion in HF patients with AF. This recommendation is supported by the observation that BNPs have no incremental diagnostic value for the diagnosis of HFpEF (assessed with the gold standard of exercise right heart catherization) in patients with suspected HF while the presence of AF is the strongest predictor. 39
Some limitations have to be acknowledged when interpreting our results. Firstly, this was an explorative analysis of DIAST‐CHF. However, the data were prospectively validated in three independent cohorts. Secondly, for better comparison, we only selected patients with cardiovascular risk factors. Diagnostic characteristics of NT‐proBNP may be different in individuals without risk factors or other settings. Thirdly, the definitions for AF and HF slightly differed between studies. No information was available on the types of AF (paroxysmal, permanent, and persistent) that have a different impact on the NT‐proBNP levels themselves. However, NT‐proBNP was consistently higher in AF than in HF in all studies. Moreover, the high AUC of >0.9 for NT‐proBNP in patients with AF on ECG (irrespective of HF status) nearly precludes any additive diagnostic values for HF in these patients. Fourthly, HF may have been underdiagnosed in our AF patients. Still, overt HF was excluded by a thorough clinical investigation during study inclusion. In addition, median NT‐proBNP levels in patients with HFpEF and AF were slightly lower than in those without HF. Even if HF was undiagnosed in some patients in the AF patient group, this is unlikely to have altered our results. Lastly, all studies were performed in Germany with a mostly Caucasian population and must not be extrapolated to other ethnicities.
Conclusions
N‐terminal pro‐B‐type natriuretic peptide was a better biomarker for prevalent and incident AF than for HF in stable outpatients with cardiovascular risk factors. In patients with AF, it should not be used for the diagnosis of chronic HF, especially not in HFpEF. In the absence of HF, screening for AF should be considered in case of elevated NT‐proBNP levels.
Conflict of interest
Stefanie M. Werhahn, Christian Becker, Meinhard Mende, Helge Haarmann, Kathleen Nolte, Ulrich Laufs, Samira Zeynalova, Markus Löffler, Nikolaos Dagres, Daniela Husser, Marcus Dörr, Stefan Gross, Stephan B. Felix, Astrid Petersmann, Christoph Herrmann‐Lingen, Lutz Binder, Martin Scherer, Gerd Hasenfuß, Burkert Pieske, Frank Edelmann, and Rolf Wachter declare that they have no conflict of interest.
Funding
DIAST‐CHF is funded by the Bundesministerium für Bildung und Forschung within the Competence Network Heart Failure. LIFE‐Adult is supported by LIFE—Leipzig Research Center for Civilization Diseases, an organizational unit affiliated to the Medical Faculty of the University Leipzig. LIFE is funded by means of the European Union, by the European Regional Development Fund (ERDF), and by funds from the Free State of Saxony within the framework of the excellence initiative. SHIP is part of the research Network Community Medicine of the University Medicine Greifswald, which is supported by the German Federal State of Mecklenburg‐West Pomerania. The sponsors did not take part in data analysis and did neither see nor influence the current publication.
Supporting information
Figure S1. Flow of study participants in DIAST‐CHF.
Figure S2. ROC curves for the prediction of heart failure and atrial fibrillation in DIAST‐CHF and SHIP‐1.
Table S1. Symptoms and Signs in patients with Heart Failure in DIAST‐CHF.
Table S2. Baseline Characteristics in the LIFE‐Adult study.
Table S3. Baseline Characteristics in SHIP‐0, SHIP‐TREND and SHIP‐0/SHIP‐TREND.
Table S4. Multivariate analysis for determinants of NT‐proBNP in DIAST‐CHF.
Werhahn, S. M. , Becker, C. , Mende, M. , Haarmann, H. , Nolte, K. , Laufs, U. , Zeynalova, S. , Löffler, M. , Dagres, N. , Husser, D. , Dörr, M. , Gross, S. , Felix, S. B. , Petersmann, A. , Herrmann‐Lingen, C. , Binder, L. , Scherer, M. , Hasenfuß, G. , Pieske, B. , Edelmann, F. , and Wachter, R. (2022) NT‐proBNP as a marker for atrial fibrillation and heart failure in four observational outpatient trials. ESC Heart Failure, 9: 100–109. 10.1002/ehf2.13703.
References
- 1. Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, Coats AJS, Metra M, Mebazaa A, Ruschitzka F, Lainscak M, Filippatos G, Seferovic PM, Meijers WC, Bayes‐Genis A, Mueller T, Richards M, Januzzi JL Jr, Heart Failure Association of the European Society of C . Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail 2019; 21: 715–731. [DOI] [PubMed] [Google Scholar]
- 2. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, Group ESCSD . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 3. Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske‐Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P, Filippatos G. How to diagnose heart failure with preserved ejection fraction: the HFA‐PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2020; 22: 391–412. [DOI] [PubMed] [Google Scholar]
- 4. Bettencourt P. NT‐proBNP and BNP: biomarkers for heart failure management. Eur J Heart Fail 2004; 6: 359–363. [DOI] [PubMed] [Google Scholar]
- 5. Khan MS, Kristensen SL, Vaduganathan M, Kober L, Abraham WT, Desai AS, Solomon SD, Swedberg K, Dickstein K, Zile MR, Packer M, McMurray JJ, Butler J. Natriuretic peptide plasma concentrations and risk of cardiovascular versus non‐cardiovascular events in heart failure with reduced ejection fraction: insights from the PARADIGM‐HF and ATMOSPHERE trials. Am Heart J 2021; 237: 45–53. [DOI] [PubMed] [Google Scholar]
- 6. Ledwidge M, Gallagher J, Conlon C, Tallon E, O'Connell E, Dawkins I, Watson C, O'Hanlon R, Bermingham M, Patle A, Badabhagni MR, Murtagh G, Voon V, Tilson L, Barry M, McDonald L, Maurer B, McDonald K. Natriuretic peptide‐based screening and collaborative care for heart failure: the STOP‐HF randomized trial. JAMA 2013; 310: 66–74. [DOI] [PubMed] [Google Scholar]
- 7. Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, Coats AJS, Metra M, Mebazaa A, Ruschitzka F, Lainscak M, Filippatos G, Seferovic PM, Meijers WC, Bayes‐Genis A, Mueller T, Richards M, Januzzi JL Jr, Cardiology obotHFAotESo . Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail 2019; 21: 715–731. [DOI] [PubMed] [Google Scholar]
- 8. Patton KK, Ellinor PT, Heckbert SR, Christenson RH, DeFilippi C, Gottdiener JS, Kronmal RA. N‐terminal pro‐B‐type natriuretic peptide is a major predictor of the development of atrial fibrillation: the Cardiovascular Health Study. Circulation 2009; 120: 1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB Sr, Newton‐Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, Benjamin EJ. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community‐based cohort study. Lancet 2009; 373: 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sinner MF, Stepas KA, Moser CB, Krijthe BP, Aspelund T, Sotoodehnia N, Fontes JD, Janssens AC, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Vasan RS, Wang TJ, Agarwal SK, McManus DD, Franco OH, Yin X, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kaab S, Couper D, Harris TB, Astor BC, Ballantyne CM, Hoogeveen RC, Arai AE, Soliman EZ, Ellinor PT, Stricker BH, Gudnason V, Heckbert SR, Pencina MJ, Benjamin EJ, Alonso A. B‐type natriuretic peptide and C‐reactive protein in the prediction of atrial fibrillation risk: the CHARGE‐AF Consortium of community‐based cohort studies. Europace 2014; 16: 1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 2004; 350: 655–663. [DOI] [PubMed] [Google Scholar]
- 12. Edelmann F, Wachter R, Schmidt AG, Kraigher‐Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Loffler M, Dungen HD, Tschope C, Herrmann‐Lingen C, Halle M, Hasenfuss G, Gelbrich G, Pieske B, Aldo DHFI. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo‐DHF randomized controlled trial. JAMA 2013; 309: 781–791. [DOI] [PubMed] [Google Scholar]
- 13. Badran HM, Eid MA, Michael A. Doppler‐derived indexes and B‐type natriuretic peptide in prediction of paroxysmal atrial fibrillation in essential hypertension: a prospective study. Echocardiography 2007; 24: 911–922. [DOI] [PubMed] [Google Scholar]
- 14. Seegers J, Zabel M, Gruter T, Ammermann A, Weber‐Kruger M, Edelmann F, Gelbrich G, Binder L, Herrmann‐Lingen C, Groschel K, Hasenfuss G, Feltgen N, Pieske B, Wachter R. Natriuretic peptides for the detection of paroxysmal atrial fibrillation. Open Heart 2015; 2: e000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schnabel RB, Wild PS, Wilde S, Ojeda FM, Schulz A, Zeller T, Sinning CR, Kunde J, Lackner KJ, Munzel T, Blankenberg S. Multiple biomarkers and atrial fibrillation in the general population. PLoS One 2014; 9: e112486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weiss NS, Perez Trejo E, Kronmal R, Lima J, Heckbert SR. Incidence of atrial fibrillation in persons with very high serum levels of N‐terminal pro‐B‐type natriuretic peptide: the Multi‐Ethnic Study of Atherosclerosis. Clin Epidemiol 2021; 13: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, Wang TJ, Levy D, Benjamin EJ, Ho JE. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation 2016; 133: 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Richards M, Di Somma S, Mueller C, Nowak R, Peacock WF, Ponikowski P, Mockel M, Hogan C, Wu AH, Clopton P, Filippatos GS, Anand I, Ng L, Daniels LB, Neath SX, Shah K, Christenson R, Hartmann O, Anker SD, Maisel A. Atrial fibrillation impairs the diagnostic performance of cardiac natriuretic peptides in dyspneic patients: results from the BACH Study (Biomarkers in ACute Heart Failure). JACC Heart Fail 2013; 1: 192–199. [DOI] [PubMed] [Google Scholar]
- 19. Stahrenberg R, Edelmann F, Mende M, Kockskamper A, Dungen HD, Luers C, Binder L, Herrmann‐Lingen C, Gelbrich G, Hasenfuss G, Pieske B, Wachter R. The novel biomarker growth differentiation factor 15 in heart failure with normal ejection fraction. Eur J Heart Fail 2010; 12: 1309–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 21. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force M . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 22. Loeffler M, Engel C, Ahnert P, Alfermann D, Arelin K, Baber R, Beutner F, Binder H, Brahler E, Burkhardt R, Ceglarek U, Enzenbach C, Fuchs M, Glaesmer H, Girlich F, Hagendorff A, Hantzsch M, Hegerl U, Henger S, Hensch T, Hinz A, Holzendorf V, Husser D, Kersting A, Kiel A, Kirsten T, Kratzsch J, Krohn K, Luck T, Melzer S, Netto J, Nuchter M, Raschpichler M, Rauscher FG, Riedel‐Heller SG, Sander C, Scholz M, Schonknecht P, Schroeter ML, Simon JC, Speer R, Staker J, Stein R, Stobel‐Richter Y, Stumvoll M, Tarnok A, Teren A, Teupser D, Then FS, Tonjes A, Treudler R, Villringer A, Weissgerber A, Wiedemann P, Zachariae S, Wirkner K, Thiery J. The LIFE‐Adult‐Study: objectives and design of a population‐based cohort study with 10,000 deeply phenotyped adults in Germany. BMC Public Health 2015; 15: 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, Deckers JW, Witteman JC, Stricker BH. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure: the Rotterdam Study. Eur Heart J 2004; 25: 1614–1619. [DOI] [PubMed] [Google Scholar]
- 24. Volzke H, Alte D, Schmidt CO, Radke D, Lorbeer R, Friedrich N, Aumann N, Lau K, Piontek M, Born G, Havemann C, Ittermann T, Schipf S, Haring R, Baumeister SE, Wallaschofski H, Nauck M, Frick S, Arnold A, Junger M, Mayerle J, Kraft M, Lerch MM, Dorr M, Reffelmann T, Empen K, Felix SB, Obst A, Koch B, Glaser S, Ewert R, Fietze I, Penzel T, Doren M, Rathmann W, Haerting J, Hannemann M, Ropcke J, Schminke U, Jurgens C, Tost F, Rettig R, Kors JA, Ungerer S, Hegenscheid K, Kuhn JP, Kuhn J, Hosten N, Puls R, Henke J, Gloger O, Teumer A, Homuth G, Volker U, Schwahn C, Holtfreter B, Polzer I, Kohlmann T, Grabe HJ, Rosskopf D, Kroemer HK, Kocher T, Biffar R, John U, Hoffmann W. Cohort profile: the study of health in Pomerania. Int J Epidemiol 2011; 40: 294–307. [DOI] [PubMed] [Google Scholar]
- 25. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL, Group ESCSD . 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio‐Thoracic Surgery (EACTS). Eur Heart J 2020; 74: 437. [Google Scholar]
- 26. Yamamoto K, Burnett JC Jr, Bermudez EA, Jougasaki M, Bailey KR, Redfield MM. Clinical criteria and biochemical markers for the detection of systolic dysfunction. J Card Fail 2000; 6: 194–200. [DOI] [PubMed] [Google Scholar]
- 27. Fuat A, Murphy JJ, Hungin APS, Curry J, Mehrzad AA, Hetherington A, Johnston JI, Smellie WSA, Duffy V, Cawley P. The diagnostic accuracy and utility of a B‐type natriuretic peptide test in a community population of patients with suspected heart failure. Br J Gen Pract 2006; 56: 327–333. [PMC free article] [PubMed] [Google Scholar]
- 28. Zaphiriou A, Robb S, Murray‐Thomas T, Mendez G, Fox K, McDonagh T, Hardman SM, Dargie HJ, Cowie MR. The diagnostic accuracy of plasma BNP and NTproBNP in patients referred from primary care with suspected heart failure: results of the UK natriuretic peptide study. Eur J Heart Fail 2005; 7: 537–541. [DOI] [PubMed] [Google Scholar]
- 29. Bansal N, Zelnick LR, Soliman EZ, Anderson A, Christenson R, DeFilippi C, Deo R, Feldman HI, He J, Ky B, Kusek J, Lash J, Seliger S, Shafi T, Wolf M, Go AS, Shlipak MG. Change in cardiac biomarkers and risk of incident heart failure and atrial fibrillation in CKD: the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis Off J National Kidney Foundation 2021; 77: 907–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patton KK, Heckbert SR, Alonso A, Bahrami H, Lima JA, Burke G, Kronmal RA. N‐terminal pro‐B‐type natriuretic peptide as a predictor of incident atrial fibrillation in the Multi‐Ethnic Study of Atherosclerosis: the effects of age, sex and ethnicity. Heart 2013; 99: 1832–1836. [DOI] [PubMed] [Google Scholar]
- 31. Wachter R, Lahno R, Haase B, Weber‐Kruger M, Seegers J, Edelmann F, Wohlfahrt J, Gelbrich G, Gorlitz A, Kermer P, Vollmann D, Hasenfuss G, Groschel K, Stahrenberg R. Natriuretic peptides for the detection of paroxysmal atrial fibrillation in patients with cerebral ischemia—the Find‐AF study. PLoS One 2012; 7: e34351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palà E, Pagola J, Juega J, Francisco‐Pascual J, Bustamante A, Penalba A, Comas I, Rodriguez M, De Lera AM, Arenillas JF, de Torres R, Pérez‐Sánchez S, Cabezas JA, Moniche F, González‐Alujas T, Molina CA, Montaner J. B‐type natriuretic peptide over N‐terminal pro‐brain natriuretic peptide to predict incident atrial fibrillation after cryptogenic stroke. Eur J Neurol 2021; 28: 540–547. [DOI] [PubMed] [Google Scholar]
- 33. Luers C, Wachter R, Kleta S, Uhlir M, Koschack J, Scherer M, Binder L, Herrmann‐Lingen C, Zapf A, Kulle B, Kochen MM, Pieske B. Natriuretic peptides in the detection of preclinical diastolic or systolic dysfunction. Clin Res Cardiol 2010; 99: 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anker SD, Butler J, Filippatos G, Shahzeb Khan M, Ferreira JP, Bocchi E, Böhm M, Brunner‐La Rocca HP, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Gomez‐Mesa JE, Janssens S, Januzzi JL, Gonzalez‐Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Seronde MF, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M. Baseline characteristics of patients with heart failure with preserved ejection fraction in the EMPEROR‐Preserved trial. Eur J Heart Fail 2020; 22: 2383–2392. [DOI] [PubMed] [Google Scholar]
- 35. Solomon SD, Rizkala AR, Gong J, Wang W, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, Van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Shi VC, Lefkowitz MP, McMurray JJV. Angiotensin receptor neprilysin inhibition in heart failure with preserved ejection fraction: rationale and design of the PARAGON‐HF trial. JACC Heart Fail 2017; 5: 471–482. [DOI] [PubMed] [Google Scholar]
- 36. Vaduganathan M, Claggett B, Packer M, McMurray JJV, Rouleau JL, Zile MR, Swedberg K, Solomon SD. Natriuretic peptides as biomarkers of treatment response in clinical trials of heart failure. JACC Heart Fail 2018; 6: 564–569. [DOI] [PubMed] [Google Scholar]
- 37. Anand IS, Claggett B, Liu J, Shah AM, Rector TS, Shah SJ, Desai AS, O'Meara E, Fleg JL, Pfeffer MA, Pitt B, Solomon SD. Interaction between spironolactone and natriuretic peptides in patients with heart failure and preserved ejection fraction: from the TOPCAT trial. JACC Heart Fail 2017; 5: 241–252. [DOI] [PubMed] [Google Scholar]
- 38. Kristensen SL, Jhund PS, Mogensen UM, Rorth R, Abraham WT, Desai A, Dickstein K, Rouleau JL, Zile MR, Swedberg K, Packer M, Solomon SD, Kober L, McMurray JJV, Paradigm HF, Committees A , Investigators . Prognostic value of N‐terminal pro‐B‐type natriuretic peptide levels in heart failure patients with and without atrial fibrillation. Circ Heart Fail 2017; 10. [DOI] [PubMed] [Google Scholar]
- 39. Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence‐based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018; 138: 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow of study participants in DIAST‐CHF.
Figure S2. ROC curves for the prediction of heart failure and atrial fibrillation in DIAST‐CHF and SHIP‐1.
Table S1. Symptoms and Signs in patients with Heart Failure in DIAST‐CHF.
Table S2. Baseline Characteristics in the LIFE‐Adult study.
Table S3. Baseline Characteristics in SHIP‐0, SHIP‐TREND and SHIP‐0/SHIP‐TREND.
Table S4. Multivariate analysis for determinants of NT‐proBNP in DIAST‐CHF.


