Abstract
Objective
This study investigated the effect of cervical and lumbar transcutaneous spinal cord stimulation (tSCS) combined with intensive training to improve walking and autonomic function after chronic spinal cord injury (SCI).
Methods
Two 64-year-old men with chronic motor incomplete cervical SCI participated in this single-subject design study. They each underwent 2 months of intensive locomotor training and 2 months of multisite cervical and lumbosacral tSCS paired with intensive locomotor training.
Results
The improvement in 6-Minute Walk Test distance after 2 months of tSCS with intensive training was threefold greater than after locomotor training alone. Both participants improved balance ability measured by the Berg Balance Scale and increased their ability to engage in daily home exercises. Gait analysis demonstrated increased step length for each individual. Both participants experienced improved sensation and bowel function, and 1 participant eliminated the need for intermittent catheterization after the stimulation phase of the study.
Conclusion
These results suggest that noninvasive spinal cord stimulation might promote recovery of locomotor and autonomic functions beyond traditional gait training in people with chronic incomplete cervical SCI.
Impact
Multisite transcutaneous spinal stimulation may induce neuroplasticity of the spinal networks and confer functional benefits following chronic cervical SCI.
Keywords: Autonomic Function, Chronic Cervical Spinal Cord Injury, Locomotor Training, Noninvasive Electrical Spinal Stimulation
Introduction
Spinal cord injury (SCI) results in prolonged neurological impairments limiting participation in essential activities of daily living. More than 0.6 million people worldwide have a new SCI each year,1 and nearly one-half (47.2%) of people with SCI have incomplete cervical injury in the United States.2 People with cervical SCI prioritize restoration of arm and hand function, walking, pain relief, bowel/bladder control, and sexual function to improve their quality of life.3,4 SCI can also induce secondary complications such as osteoprosis and constipation that can be partly attributed to a lack of standing and walking activities.5
Activity-based training, including overground and treadmill training, is the most common clinical approach to improve locomotion following SCI.6,7 Locomotor training can also improve autonomic function.8 Nevertheless, the level of recovery in both locomotion and autonomic systems has yet to approach the functional goals of people with SCI.9
Fortunately, spinal cord stimulation is showing potential for promoting functional recovery after SCI.10 Over the last 4 decades, research has demonstrated that dormant connectivity in the damaged spinal cord can be activated by combining exercise and spinal cord electrical stimulation to recover some physical function.11,12 Locomotor training with epidural stimulation improved walking function for individuals with chronic incomplete SCI.13,14 Recently, several independent research groups have demonstrated the benefits of epidural stimulation on functional recovery after chronic and even motor-complete SCI.15–17 Furthermore, there is growing evidence for the efficacy of epidural stimulation on the autonomic system, including cardiovascular,18,19 bladder,20 bowel,21 and sexual function.19 However, epidural stimulation is invasive, with the associated risks of surgical and implanted device complications.22 The implantation can also be costly.23
Non-invasive electrical stimulation of the spinal cord applied at the skin surface, termed transcutaneous spinal cord stimulation (tSCS), activates similar spinal circuits as epidural stimulation.24,25 Lumbosacral tSCS facilitates lower extremity function in people with chronic complete and incomplete SCI.26–29 Recent studies also revealed immediate improvements in blood pressure control through thoracic tSCS and bladder function through lumbosacral tSCS.30–32 Most studies to date have focused exclusively on lumbosacral tSCS to improve lower extremity function.
There is emerging evidence showing the importance of connections between the cervical and lumbosacral spinal cord through propriospinal interneurons for locomotor function following neurological injury.33,34 Two studies designed to improve hand and arm function via cervical tSCS reported that although cervical tSCS did not evoke direct activation of leg muscles, lower extremity function improved in 4 out of 7 participants.35,36 Furthermore, cervical tSCS may amplify the patterned stepping movements induced by lumbosacral tSCS through the convergence of ascending and descending pathways when delivered at the same time.37 Thus, we postulated that combining cervical tSCS with lumbosacral tSCS might contribute to locomotor recovery in people with incomplete cervical SCI.
The present study tested the effect of stimulating the cervical and lumbosacral spinal cord simultaneously via tSCS during intensive locomotor training. We tested the hypothesis that tSCS paired with intensive locomotor training would provide greater functional gains in locomotor and autonomic function than intensive locomotor training only for people with chronic incomplete cervical SCI.
Role of the Funding Source
The funder played no role in the design, conduct, or reporting of this study.
Methods
All study procedures were approved by the University of Washington Institutional Review Board. The study protocol was registered with ClinicalTrials.gov (NCT03509558).
Participants
Two people with chronic SCI who met all inclusion and exclusion criteria were recruited (Suppl. Tab. 1). They provided informed consent prior to enrolling in the study. Participant 1 (P1) was a 64-year-old male with a C4 American Spinal Injury Association Impairment Scale D injury 3.5 years prior. At baseline, P1 was a wheelchair user and required maximum physical assist (50%–75% of physical assist) for daily activities.38 P1 was taking 40 mg/d Baclofen throughout the study. P1 joined the current study more than 1 year after completing a cervical tSCS study for upper extremity function.35
Participant 2 (P2) was also 64 years of age with a C6 American Spinal Injury Association Impairment Scale D injury 4.5 years prior. P2 was ambulatory with 1 forearm crutch and contact guard assist in the community. He had a history of frequent falls. P2 did not take any medications during the study, and there were no side effects related to tSCS in either participant. However, P2 had a fall during the second week of locomotor training alone, before any application of the stimulation. The fall caused a repeat nasal bone fracture. P2 rested for 1.5 months, and the study was resumed after receiving clearance from his primary care physician and the institutional review board.
Experimental Design
This single-subject study design enrolled 2 participants. Baseline measurements were performed once per week for 4 weeks to account for functional variability over time and learning of the assessments. We collected the 6-Minute Walk Test (6MWT) 3 times, Berg Balance Scale (BBS), and Walk Index for SCI twice over the 1-month baseline period, and we used the last measurements as the baseline to provide a fair comparison with all remaining tests that used the same assistance level. To evaluate any benefit of tSCS on top of a consistent locomotor training program, treatment order was alternated between 4 weeks of intensive locomotor training (A) and 4 weeks of tSCS with locomotor training (B) for P1 (A-B-A-B study design; Fig. 1). P1 demonstrated sustained improvement after the application of tSCS that lasted throughout the early follow-up phase, revealing a potential carry-over effect of tSCS. To control this carry-over effect for P2, we employed an A-A-B-B study design to extend the locomotor training alone period to 2 months and reach a plateau in function prior to beginning stimulation (Fig. 1). No blinding was used for functional measures or stimulation phases.
Figure 1.
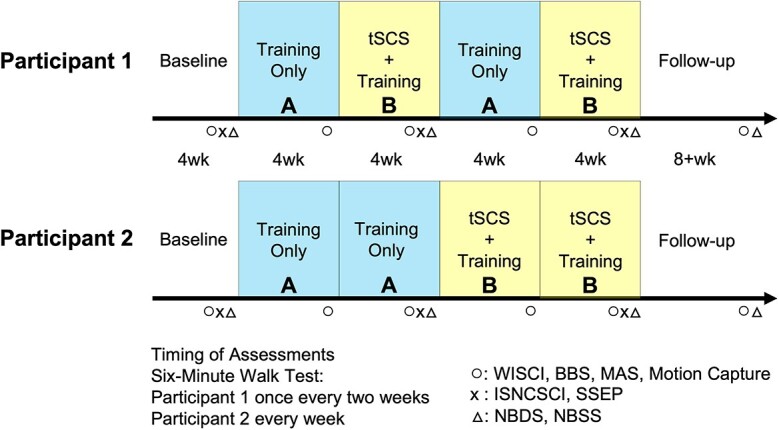
Experimental design and timeline. Study timeline illustrating 1-month baseline testing (left) followed by 4-week intensive locomotor training in both participants (Block “A”). After 4 weeks of training, Participant 1 received transcutaneous spinal cord stimulation (tSCS) with intensive locomotor training for 4 weeks (Block “B”). Subsequently, Participant 1 repeated the same order of the interventions (A-B-A-B design). Participant 2 continued the intensive locomotor training alone for 4 more weeks before tSCS combined with locomotor training for 8 weeks (A-A-B-B design). Participants were followed for at least 2 months without any stimulation or supervised exercise to examine sustained benefits of the intervention. Training: Locomotor training; 6-Minute Walk Test was conducted once every 2 weeks in Participant 1 and every week in Participant 2. The Walking Index for SCI II (WISCI), Berg Balance Scale (BBS), Modified Ashworth Scale (MAS), and Motion Capture were measured every month (o); The International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) and Sensory Evoked Potentials (SSEP) were measured every 2 months (x); Neurogenic Bowel Dysfunction Scale (NBDS) and Neurogenic Bladder Symptom Scale (NBSS) were measured every 2 months (△).
After completing 4 months of interventions, both participants returned monthly for follow-up assessments. During the follow-up period, both participants performed 30 minutes of walking at least 3 times per week at home. Physical assistance from caregivers and assistive devices was provided as needed.
Locomotor Training
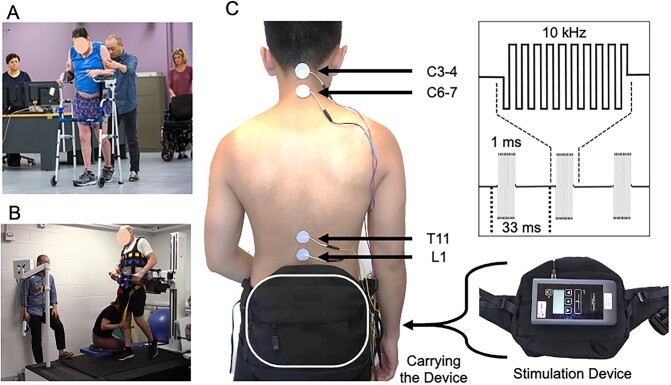
Participants received intensive locomotor training, including overground walking and treadmill training with a safety harness. Overground training focused on walking faster and over longer distances, with reduced weight-bearing on arms and trunk (Fig. 2A).6,7 We provided sensorimotor feedback with manual assistance during treadmill training using a robotic bodyweight supported harness system (KineAssist, Woodway Inc, Waukesha, WI, USA; Fig. 2B).39 The treadmill training focused on increasing speed and providing less bodyweight support (10%–26% bodyweight) with and without manual assistance (Suppl. Video 1). All training sessions were conducted by experienced physical therapists for 1.5 to 2 h/d, 3 to 4 times per week throughout all intervention phases of the study.
Figure 2.
Combined cervical and lumbosacral transcutaneous spinal cord stimulation with intensive locomotor training. A: Intensive overground locomotion training with platform walker. B: Intensive treadmill training with bodyweight support and manual assistance of lower extremity swing at maximum walking speed. C: Electrode locations: 4 active electrodes were placed above and below the cervical spinal cord injury (C3-4, C6-7) and at the T11 and L1 vertebral levels (left). The common ground electrodes were placed at bilateral iliac crests (not shown). Participants wore a fanny pack to carry the stimulation device during locomotor training. Stimulation used biphasic, rectangular, 1-ms pulses at a frequency of 30 Hz filled with a carrier frequency of 10 kHz (right top).
Transcutaneous Spinal Cord Stimulation
The stimulation waveform used biphasic, rectangular, 1-ms pulses at a frequency of 30 Hz filled with a carrier frequency of 10 kHz. The parameters were determined based on prior studies of tSCS for motor function28,29,35,36 (Fig. 2C). Stimulation was delivered via 4 2.5-cm round electrodes (Axelgaard, ValuTrode Cloth, Axelgaard Manufacturing Co Ltd, Fallbrook, CA, USA) placed midline at C3 to 4, C6 to 7, T11, and L1 spinous processes as active electrodes. Two 5- × 10-cm rectangular electrodes (Axelgaard, ValuTrode Cloth) were placed symmetrically over the iliac crests as the common grounds for all active electrodes.
The stimulator (ONWARD Medical Inc, Lexington, MA, USA; Fig. 2C) was portable and carried in a fanny pack during training. Participants received tSCS for 1.5 to 2 hours per session during the same intensive locomotor training described above.
We set the current amplitude of T11 and L1 electrodes first and added current to the C3 to 4 and C6 to 7 electrodes to achieve maximum walking performance without any discomfort.40 The stimulation was below motor threshold as confirmed by observing no direct muscle activation during 2-Hz spinal stimulation from each stimulation electrode recorded on the leg muscle surface electromyograms (Delsys Trigno system, Delsys Inc, Natick, MA, USA) on 7 bilateral muscles: gluteus medius, rectus femoris, vastus medialis, medial hamstrings, tibialis anterior, medial gastrocnemius, and soleus.
The current amplitude of each electrode was increased at the stimulation frequency of 30 Hz to an intensity that enabled maximum voluntary movement leading to maximum treadmill walking speed while remaining below the motor threshold. When the current amplitude was too high, we observed increased muscle tone, which could interfere with voluntary movement. We adjusted the stimulation intensity in each session. The current amplitude ranges were 35 to 75 mA at T11 and L1 electrodes and 5 to 40 mA at C3 to 4 and C6 to 7 electrodes. The current intensity changes throughout the protocol are presented in Supplementary Figure 3. Neither participant reported any discomfort, only an occasional warm sensation of the limbs.
Functional Measures
Details of functional outcomes and the interpretations with evidence are shown in Supplementary Table 5. The primary outcome was the 6MWT, which was evaluated 3 times in both participants in the baseline phase. We used the last data point in the baseline period to document their walking function and endurance. For P1, the platform walker that was used for the 6MWT throughout the remainder of the protocol was not available during the first 2 baseline measurements, so we used only the final baseline data point for P1. This provided the most accurate comparison with all subsequent tests where we used the same testing conditions throughout the protocol for each participant. Specifically, we used a platform walker for P1 and no assistive device for P2 to eliminate confounding effects of changing assistance level.
The secondary outcomes were the Walking Index for SCI II (WISCI) and BBS to assess mobility level and balance ability, respectively. The International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) examination was performed to document upper extremity motor score, lower extremity motor score, and pinprick and light touch sensation.
Lower extremity muscle spasticity was assessed using the Modified Ashworth Scale (MAS) in a supine position. The MAS was performed for knee flexion, knee extension, and ankle plantar/dorsiflexion with the knee in an extended position. We used a scale of 0 to 4 for each movement, with a 1+ converted into a score of 1.5. The scores were summed across the 3 movements per lower extremity, with a maximum score of 24.
The WISCI, BBS, and MAS were conducted by experienced physical therapists every month. The ISNCSCI examinations and classifications were performed by a neurosurgeon every 2 months. The neurosurgeon is a spine surgeon and clinical researcher with 12 years of experience using the ISNCSCI for both clinical use and research studies. He also supervised the participant safety and study protocol as a co-investigator. Figure 1 presents the timing of the assessments. All functional measures were tested without any stimulation to measure changes that persisted beyond the application of stimulation.
To assess the state of sensory pathways, we studied the somatosensory evoked potentials every 2 months (SSEP; Cadwell Sierra Summit, Cadwell Inc, Kennewick, WA, USA). We stimulated the tibial nerve posterior to the medial malleolus and recorded cortical potentials between Cz’ (2 cm posterior to Cz) and Fz.41 We began stimulation at 3 Hz with 400-μs pulses and intensity just below the sensory threshold. We then increased the amplitude in 5-mA increments to 40 mA to recruit all sensory nerves. P40 latencies were calculated from an average of 300 responses for each stimulation intensity after processing with a 10- to 500-Hz bandpass filter.
Motion Capture
Three-dimensional motion capture data were collected at 120 Hz using a 10-camera Qualysis Oqus system over an 8-m walkway (Qualysis AB, Göteborg, Sweden). All recordings were performed without stimulation once per month. Participants performed at least 5 walking trials at a self-selected speed in each session, with rest breaks provided as needed. We employed a lower extremity–modified Conventional Gait Model.42
Gait data were analyzed using custom MATLAB scripts (MathWorks Inc, Natick, MA, USA). Kinematics, including joint excursions, were calculated using the inverse kinematics algorithm in OpenSim.43 We used a generic model with 23 degrees of freedom, including 12 segments scaled to each participant. A gait cycle was defined as the period from heel strike to subsequent ground contact of the same limb. Toe-off events were used to define the transition between the swing phase and the stance phase. These events were extracted based on the velocity of the toe marker.
Data were normalized to percent gait cycle, heel-strike to next heel-strike by linear interpolation with at least 5 gait cycles per lower extremity. Spatiotemporal parameters included step length, stride length, cadence, and step time. Interlimb coordination was assessed using spatiotemporal symmetry metrics.44 Spatial and temporal asymmetry indices were computed for all strides in each session. These measures range from 0 to 1, where lower scores reflect a more symmetric gait.
Bowel and Bladder Assessments
We quantified changes in bladder and bowel function every 2 months using validated patient-reported outcome measures. We used the Neurogenic Bowel Dysfunction Score for bowel function45 and the Neurogenic Bladder Symptom Score for bladder function following SCI.46
Statistical Methods
The current study used a single-subject design; thus, statistical analyses were not employed. The outcome measures are presented as raw values and changes among phases in each case unless otherwise noted. When possible, we provide comparisons with available data such as minimally detectable change.
Role of the Funding Source
The funders played no role in the design, conduct, or reporting of this study.
Results
Locomotor Recovery and Rehabilitative Effect of Stimulation
Both participants improved walking ability during tSCS compared with the locomotor training alone (Fig. 3). Specifically, distance walked during the 6MWT increased threefold more during tSCS compared with locomotor training alone (Fig. 3, right). Nearly all improvements were maintained for at least 2 months without further stimulation (Fig. 3, left).
Figure 3.
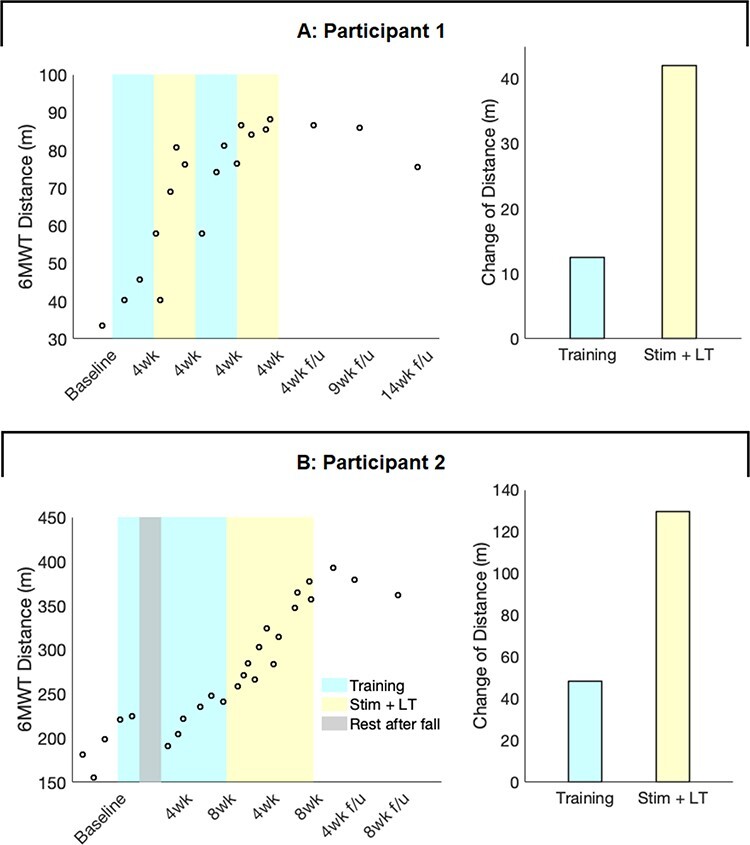
Sustained improvement of 6-minute walk distance. Transcutaneous spinal cord stimulation (tSCS) combined with intensive locomotor training enabled 2 participants to improve their 6-Minute Walk Test (6MWT) distance threefold more than during training alone. A: Participant 1 improved walking distance during the first 4 weeks of tSCS and maintained this improved function for at least 9 weeks without any further intervention (left). The bar plots show the change in walking distance that occurred during a total of 2 months of training alone (blue) and during 2 months of stimulation with training (yellow). The tSCS increased walking distance approximately threefold compared with training alone (+37% of baseline during training vs +124% of baseline during stimulation + training; right). B: Participant 2 demonstrated improvement of the 6MWT distance at a much faster rate during tSCS than locomotor training alone (left). The tSCS increased walking distance approximately threefold compared with training alone (+24% of baseline vs +65% of baseline; right). LT = locomotor training; Stim = transcutaneous spinal cord stimulation. Empty circles present the individual data points. Bar plots show the distance change in the phase of locomotor training alone (blue) and tSCS with locomotor training (yellow). Participant 2 had a fall during the second week of training alone phase and took a 1.5-month rest for recovery (gray). The distance change is the differences between the maximum distance in the previous phase and the maximum distance in the following phase. 6MWT minimally detectable change (MDC): 22% from baseline.75
Both participants required less assistance during walking and improved balance as measured by WISCI and BBS after 2 months of tSCS with intensive training (Suppl. Tab. 2). P1 began the study walking with a platform walker and maximum physical assist at baseline testing. By the end of 8 weeks of stimulation, P1 could walk with a quad-cane and contact guard assist, but a platform walker was still the preferred assistive device while walking (Suppl. Fig. 1 and Suppl. Video 2). P2 improved his balance based on a BBS score of 37 at baseline to 52 at the end of stimulation, a substantial improvement in balance ability.47,48 Better balance was also reflected in sustained improvement of stair management ability (Suppl. Video 3).
Recovery of Autonomic Function With tSCS
Both participants presented clinically meaningful improvements in autonomic function after tSCS with locomotor training. The Neurogenic Bowel Dysfunction Score indicated notable improvement, including the category of the impairment severity from severe to moderate (−4 points) and severe to very minor (−9 points) in P1 and P2, respectively (Suppl. Tab. 3). Both participants decreased bowel management time from 31 to 60 minutes to <30 minutes after completing all interventions. P2 also reported a normalized frequency of bowel movement from 2 to 3 times per week to daily management, something that P2 had not achieved since his injury.
Both participants managed their bladder with intermittent catheterization at the beginning of the study. P1’s caregiver anecdotally reported the measured amount of urine drained via the straight catheter, the “residual amount” of urine, after P1’s attempt to self-void using a condom catheter. Although the Neurogenic Bladder Symptom Score questionnaire did not capture a change for either participant (Suppl. Tab. 3), P1 experienced decreased residual urine volume throughout the protocol (estimated 125 mL to 30 mL). Eventually, 2 months after completing all interventions, P1 discontinued the use of intermittent catheterization. Anecdotally, he has reported still being able to void his bladder without intermittent catheterization for 1.8 years since the study (Suppl. Tab. 3). Whereas he often contracted a urinary tract infection prior to the study, he has not experienced a single urinary tract infection since the completion of the study. Finally, P2 reported that he improved tolerance in cold weather and was able to work in low-temperature environments for more extended time periods following the tSCS phase of the study.
Spatiotemporal and Kinematic Outcomes
At baseline, both participants presented with impaired gait, including reduced step length, cadence, and joint excursions. After completing the stimulation treatment, P1 improved all gait parameters except for bilateral step time and cadence. P2 improved all gait parameters except for the right hip joint excursion (Suppl. Tab. 4). Almost all of these improvements were maintained throughout follow-up (Fig. 4).
Figure 4.
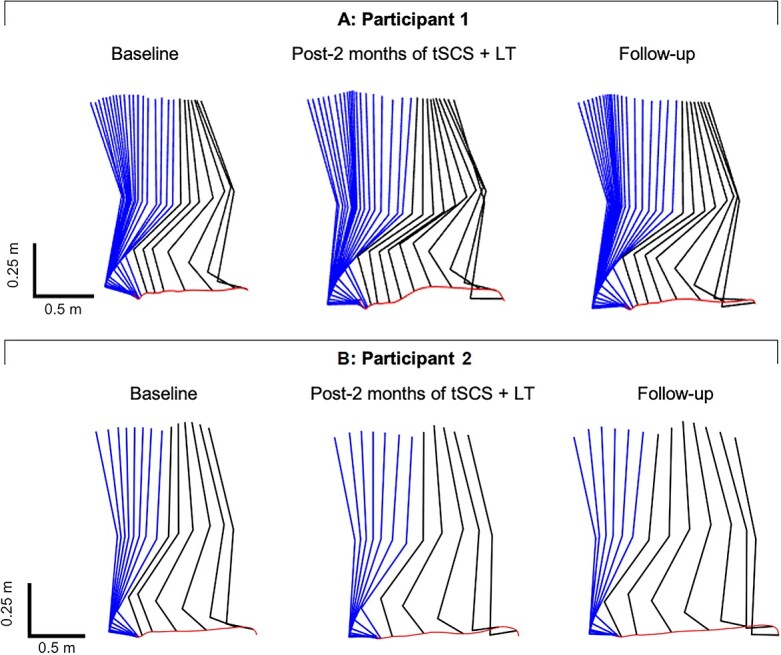
Comparisons of right lower extremity stepping patterns at baseline, after the transcutaneous spinal cord stimulation phase, and in follow-up. Average right lower extremity movements during stance (blue) and swing (black) together with successive trajectories per 0.05 second of the joint endpoint. The red line indicates toe trajectory. A: Participant 1 demonstrated improved step length after the transcutaneous spinal cord stimulation and maintained the improvement throughout follow-up. B: Participant 2 improved step length after transcutaneous spinal cord stimulation phase and further improved during follow-up. LT = locomotor training; tSCS = transcutaneous spinal cord stimulation. The foot length appears to change due to rotation not captured in this sagittal plane view.
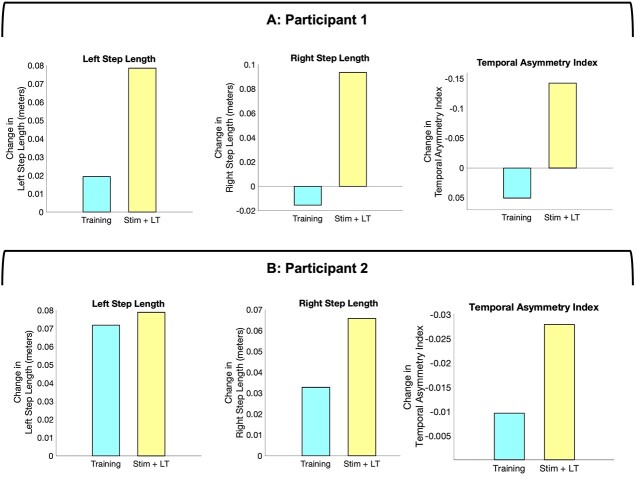
We compared kinematic changes resulting from 2 months of locomotor training with and without tSCS. Both participants presented greater improvement of right and left step length and temporal interlimb coordination during tSCS compared with locomotor training alone (Fig. 5). We also compared kinematic parameters with stimulation turned off and on during the same day, but no consistent changes were seen.
Figure 5.
Comparisons of spatiotemporal changes following locomotor training alone and following transcutaneous spinal cord stimulation combined with training (Stim + LT). Changes in spatiotemporal characteristics during each phase of the intervention. The changes were obtained by calculating the cumulative differences of mean values across phases. Blue bars show the total change during the locomotor training alone phase. Yellow bars show the total change during the transcutaneous spinal cord stimulation phase. The left and middle figures show right step length and left step length, respectively, in Participant 1 (A) and Participant 2 (B). The right figures show temporal asymmetry indices. The values of the y-axis are reversed as a negative value indicates improved interlimb coordination. Both participants improved step length and temporal interlimb coordination during the transcutaneous spinal cord stimulation. LT = locomotor training; Stim = transcutaneous spinal cord stimulation.
Temporary Improvement of Spasticity With tSCS
Lower extremity muscle spasticity improved only after tSCS with locomotor training and not after locomotor training alone (Suppl. Tab. 2). The MAS sum score was similar or slightly worse following locomotor training relative to baseline (+2 points in P1 and +3 points in P2). Less spasticity was observed after tSCS relative to baseline (−5 points in P1 and −2 points in P2). The spasticity, however, returned close to the baseline levels 2 months after the end of stimulation.
Sensory Recovery
Both participants improved the ISNCSCI sensory score and SSEP P40 latency during the study (Tab. 1). In P1, we could not differentiate the effect of tSCS and locomotor training as a result of the study design. P2, however, demonstrated greater improvement in the pinprick sensation during the tSCS phase (+13 points) than during the locomotor training–only phase (+4 points; Suppl. Fig. 2). In both participants, the pinprick sensation improved after all interventions (+20 points in P1 and +17 points in P2; Table). There was no improvement of light touch sensation in either participant. The P40 latencies of the tibial SSEPs showed a delayed response at baseline in both participants, which improved to shorter latencies following the tSCS phases of the study (Table).
Table.
Progress of Neurological Outcomesa
| Baseline | After 1 Mo of LT Alone and 1 Mo of tSCS+LT | After 2 Mo of LT Alone | After 2 Mo of LT Alone and 2 Mo of tSCS+LT | |
|---|---|---|---|---|
| Participant 1 | ||||
| NLI & AIS | C4 AIS D | C4 AIS D | C4 AIS D | |
| UEMS (R | L) | 13 | 21 | 11 | 21 | 16 | 22 | |
| LEMS (R | L) | 19 | 19 | 19 | 22 | 21 | 25 | |
| Light touch (R | L) | 33 | 32 | 32 | 33 | 31 | 34 | |
| Pinprick (R | L) | 18 | 23 | 25 | 32 | 25 | 36 | |
| Tibial SSEP P40 latency (R Stim | L Stim; ms) | 64.1 | 60.5 | 56.9 | 58.1 | 57.5 | 58.3 | |
| Participant 2 | ||||
| NLI & AIS | C6 AIS D | C6 AIS D | C6 AIS D | |
| UEMS (R | L) | 22 | 25 | 22 | 25 | 22 | 25 | |
| LEMS (R | L) | 21 | 25 | 22 | 24 | 23 | 25 | |
| Light touch (R | L) | 48 | 47 | 49 | 48 | 52 | 52 | |
| Pinprick (R | L) | 40 | 41 | 42 | 43 | 49 | 49 | |
| Tibial SSEP P40 latency (R Stim | L Stim; ms) | 55.6 | 54.5 | 54.2 | 54.3 | 54.2 | 53.2 | |
a Bold values show improved parameters after the intervention compared with the baseline measures. AIS = American Spinal Cord Injury Impairment Scale; LEMS = Lower Extremity Motor Score; LT = locomotor training; NLI = Neurologic Level of Injury; R | L = right | left; Tibial SSEP = tibial nerve stimulation-induced somatosensory evoked potential recorded at Cz’-Fz; tSCS = transcutaneous spinal cord stimulation; UEMS = Upper Extremity Motor Score.
Discussion
Our findings demonstrate that multisite tSCS with locomotor training leads to recovery of walking and autonomic function in 2 participants with incomplete cervical SCI. Walking distance improved threefold more with tSCS combined with locomotor training compared with locomotor training alone. Locomotor recovery was sustained for at least 2 months after the intervention. Additionally, both participants demonstrated notable recovery of bowel function. Lastly, P1 no longer required intermittent catheterization.
Walking Function
Prior studies using lumbosacral tSCS presented some recovery of motor function, including muscle strength and EMG activity, as well as voluntary control of joints in people with complete and incomplete SCI.26–28,49 Lumbosacral epidural stimulation with intensive training led to improved walking function for people with complete and incomplete SCI.15–17
After completing tSCS with intensive training in the present study, 6MWT distance improved threefold compared with intensive training alone. The improvement was sustained without further tSCS throughout the follow-up stage. For example, 7 months after the intervention, P2 maintained his improved stair management ability (Suppl. Video 3). After the intervention, P1 was able to complete walking exercises at home with a caregiver, and P2 reported fewer falls during and after the stimulation phase of the study.48 Our results indicate substantial benefits from the rehabilitation program because the improvements exceeded the minimum detectable changes (Suppl. Tab. 5).
Although we did not directly compare lumbosacral stimulation with combined cervical and lumbosacral stimulation, prior studies illustrate the potential additive effect of cervical stimulation on walking function. Cervical stimulation may improve walking function by inducing arm-leg synchronizations,33,50 activating propriospinal pathways,34 and directly modulating supraspinal inputs by recruiting the corticospinal tract.51
Autonomic Function
The disruption of the signal transmissions in the spinal cord after an SCI can cause bowel dysfunction and interruptions to urinary tract function.52 Restoration of bowel and bladder function is one of the highest treatment priorities following cervical SCI,3 but currently available treatment options are limited.53 The necessity of research for neurogenic bowel and bladder dysfunction is rapidly emerging.54,55
Both participants improved bowel function after the stimulation that may be due to the modulation of the spinal excitability and improvement of abdominal muscle control.56 Future studies will benefit from using a more comprehensive questionnaire57 and an assessment of abdominal muscle function. We observed no consistent improvement of the Neurogenic Bladder Symptom Score in either participant. Nevertheless, P1 was able to eliminate the need for intermittent catheterization and has had no urinary tract infections since the completion of the study, which significantly improved his quality of life.58 Similarly, a recent study found that multiple sessions of tSCS improved detrusor activity and lower urinary tract sensation in 5 individuals with SCI.32 Several reports also demonstrate that the modulation of spinal excitability through epidural stimulation improved bladder management time, detrusor-sphincter synergy, and voiding function.20,21 Based on the activated neural structures25,59 and clinical observations,32 we hypothesize that increased sensory inputs, activation of interneurons, and lumbosacral circuits responsive for bowel and bladder function may impact supraspinal inputs and sensory feedback for voluntary and autonomic control.
P2 reported impaired tolerance for low temperatures since his injury, which is common following SCI.60 After the tSCS with locomotor training phase, he reported that he could tolerate much longer time in low-temperature environments compared with pre-intervention. Taken together, these findings provide encouraging evidence of the efficacy of spinal stimulation to improve autonomic function.55
Gait Analysis
The kinematic analysis suggests that tSCS with locomotor training could improve gait via different mechanisms than intensive locomotor training alone. Conventional gait training can improve walking function somewhat, but the functional improvement via compensatory strategies reaches a plateau approximately 1.5 years after injury.61 Although high-intensity locomotor training shows some potential for further improving lower extremity coordination instead of amplifying the aberrant compensatory strategies,62 the ability to normalize gait is still limited with traditional care.63
Previous studies reported that temporal interlimb coordination might be primarily regulated through the spinal cord, brainstem, and subcortical regions.64 This regulation mechanism may allow the automaticity of the rhythmic gait pattern to be influenced by tSCS.37 Thus, tSCS paired with activity-based training may enhance the control of reciprocal and autogenic inhibitory circuitry in the spinal cord and modulation of subcortical inputs for locomotor recovery.65 In people with stroke, better interlimb coordination resulted in less energy cost for walking.66 Thus, the improved interlimb coordination and 6MWT in both participants suggest the improvement of energy efficiency in walking from tSCS. Better lower extremity coordination may also explain how P2 showed greater improvement of walking function than P1, despite the smaller increase in lower extremity motor score. Further research is needed to investigate an association of neural recovery with limb coordination.
Spasticity
In both participants, tSCS with locomotor training reduced spasticity (Suppl. Tab. 2). Spasticity in SCI is commonly thought to reflect increased excitability of Ia afferents caused by disrupted presynaptic and postsynaptic regulatory mechanisms following SCI.67 We observed a slight increase in spasticity with locomotor training alone. Previous studies also reported either a slight increase or no change in spasticity after intensive training depending on the duration.62,68 Therefore, the intensity and duration of locomotor training alone used here may lead to the increased spasticity, which can be mitigated by combining training with stimulation. Early epidural stimulation studies reported a reduction of spasticity following SCI.69 Similarly, a recent study illustrated the effect of repetitive tSCS on reducing lower extremity spasticity,70 which paralleled our findings. In addition to autogenic inhibitory circuitry discussed above, we postulate that high-frequency stimulation of the spinal cord improved spasticity by either activating silent inhibitory interneurons at the lumbosacral spinal cord or by providing additional activation of motor neurons to potentially suppress hyperexcitability to sensory inputs.71 When stimulation was discontinued for several weeks, reversal of this mechanism may lead to the return of spasticity observed during the follow-up period.
Sensory Improvement
Both participants experienced modest sensory recovery during stimulation treatment. The evoked responses of the tibial SSEPs are thought to reflect the functional status in SCI72; moreover, the shortened latencies that we observed in the present study may indicate improved transmission along the dorsal column pathways and recovery of somatosensory function. Improved conduction along proprioceptive pathways could contribute to the faster locomotor recovery rate observed here and in prior work.73 Notably, the ISNCSCI sensory tests revealed consistent improvements of pinprick sensation in both participants, similar to previous studies.49,74
Limitations
Limitations of the study include lack of establishing a stable baseline for the functional outcomes, only anecdotal evidence of improved autonomic function, a small sample of individuals receiving combined cervical and lumbar spinal cord stimulation, and the fact that we did not directly compare the benefits of lumbosacral stimulation alone. We designed this study to test the ability to combine cervical and lumbosacral spinal stimulation with locomotor training rather than testing the superiority of the multisite stimulation. Prior studies have demonstrated modest26,49 to similar27 improvements with lumbosacral stimulation, although the importance of cervicolumbar pathways for lower extremity function is well documented.34 We did not conduct blinding for spinal stimulation, so the sensation associated with stimulation and knowledge of receiving a new treatment may lead to a placebo effect. Although the stimulation frequency and pulse width were selected based on prior studies,28,29,35,36 further investigations may optimize the stimulation parameter to maximize the therapeutic effect. Future research is also needed to determine the lower extremity and autonomic benefits to people with more severe impairments, including motor-complete injury, as suggested by our recent study of cervical transcutaneous stimulation that restored upper extremity movement for 2 individuals with motor complete SCI.36
The current study demonstrates that cervical and lumbosacral tSCS combined with locomotion training can improve walking and autonomic function after chronic cervical SCI. Two participants with motor-incomplete tetraplegia experienced a sustained increase in walking ability after the rehabilitation program. Furthermore, the tSCS with intensive locomotor training induced substantial improvement of autonomic function, including bowel and bladder function.
Supplementary Material
Author Contributions
Concept/idea/research design: S. Samejima, K.M. Steele, R. Saigal, C.T. Moritz
Writing: S. Samejima, C.D. Caskey, F. Inanici, R. Saigal, C.T. Moritz
Data collection: S. Samejima, C.D. Caskey, F. Inanici, S.R. Shrivastav, L.N. Brighton, J. Pradarelli, V. Martinez, R. Saigal
Data analysis: S. Samejima, C.D. Caskey, J. Pradarelli, K.M. Steele, R. Saigal
Project management: S. Samejima, C.T. Moritz
Fund procurement: S. Samejima, C.T. Moritz
Providing participants: S. Samejima
Providing facilities/equipment: V. Martinez, C.T. Moritz
Providing institution liaisons: C.T. Moritz
Clerical/secretarial support: S.R. Shrivastav
Consultation: S.R. Shrivastav, V. Martinez, K.M. Steele, C.T. Moritz
Acknowledgments
The authors thank the research participants and their families for their contributions and acknowledge ONWARD Medical Inc for the use of the transcutaneous spinal stimulators.
Funding
This work was supported by the Center for Neurotechnology, the National Science Foundation-Engineering Research Center (EEC-1028725), the Washington State Spinal Cord Injury Consortium, the Christopher and Dana Reeve Foundation, and the University of Washington Institute for Neuroengineering established by a grant from the Washington Research Foundation.
Disclosures
J.P. was hired by ONWARD Medical Inc after the conclusion of the current study. C.T.M. began serving as a paid consult for ONWARD after the conclusion of the study, and this conflict of interest is actively managed by the University of Washington.
References
- 1. Dewan MC, Rattani A, Fieggen G, et al. Global neurosurgery: the current capacity and deficit in the provision of essential neurosurgical care. Executive summary of the global neurosurgery initiative at the program in global surgery and social change. J Neurosurg. 2019;130:1055–1064. [DOI] [PubMed] [Google Scholar]
- 2. NSCISC . Spinal Cord Injury Facts and Figures at a Glance. Birmingham AL, USA: University of Alabama at Birmingham; 2020. [Google Scholar]
- 3. Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–1383. [DOI] [PubMed] [Google Scholar]
- 4. Ditunno PL, Patrick M, Stineman M, Ditunno JF. Who wants to walk? Preferences for recovery after SCI: a longitudinal and cross-sectional study. Spinal Cord. 2008;46:500–506. [DOI] [PubMed] [Google Scholar]
- 5. Dunn RB, Walter JS, Lucero Y, et al. Follow-up assessment of standing mobility device users. Assist Technol. 1998;10:84–93. [DOI] [PubMed] [Google Scholar]
- 6. Harkema SJ, Schmidt-Read M, Lorenz DJ, Edgerton VR, Behrman AL. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training–based rehabilitation. Arch Phys Med Rehabil. 2012;93:1508–1517. [DOI] [PubMed] [Google Scholar]
- 7. Hornby TG, Reisman DS, Ward IG, et al. Clinical practice guideline to improve locomotor function following chronic stroke, incomplete spinal cord injury, and brain injury. J Neurol Phys Ther. 2020;44:49–100. [DOI] [PubMed] [Google Scholar]
- 8. Hubscher CH, Herrity AN, Williams CS, et al. Improvements in bladder, bowel and sexual outcomes following task-specific locomotor training in human spinal cord injury. PLoS One. 2018;13:e0190998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morawietz C, Moffat F. Effects of locomotor training after incomplete spinal cord injury: a systematic review. Arch Phys Med Rehabil. 2013;94:2297–2308. [DOI] [PubMed] [Google Scholar]
- 10. Courtine G, Sofroniew MV. Spinal cord repair: advances in biology and technology. Nat Med. 2019;25:898–908. [DOI] [PubMed] [Google Scholar]
- 11. Waltz JM. Spinal cord stimulation: a quarter century of development and investigation. A review of its development and effectiveness in 1,336 cases. Stereotact Funct Neurosurg. 1997;69:288–299. [DOI] [PubMed] [Google Scholar]
- 12. Minassian K, Jilge B, Rattay F, et al. Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials. Spinal Cord. 2004;42:401–416. [DOI] [PubMed] [Google Scholar]
- 13. Carhart MR, Jiping He, Herman R, D'Luzansky S, Willis WT. Epidural spinal-cord stimulation facilitates recovery of functional walking following incomplete spinal-cord injury. IEEE Trans Neural Syst Rehabil Eng. 2004;12:32–42. [DOI] [PubMed] [Google Scholar]
- 14. Herman R, He J, D'Luzansky S, Willis W, Dilli S. Spinal cord stimulation facilitates functional walking in a chronic, incomplete spinal cord injured. Spinal Cord. 2002;40:65–68. [DOI] [PubMed] [Google Scholar]
- 15. Angeli CA, Boakye M, Morton RA, et al. Recovery of over-ground walking after chronic motor complete spinal cord injury. N Engl J Med. 2018;379:1244–1250. [DOI] [PubMed] [Google Scholar]
- 16. Gill ML, Grahn PJ, Calvert JS, et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med. 2018;24:1677–1682. [DOI] [PubMed] [Google Scholar]
- 17. Wagner FB, Mignardot JB, Le Goff-Mignardot CG, et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature. 2018;563:65–71. [DOI] [PubMed] [Google Scholar]
- 18. Harkema SJ, Legg Ditterline B, Wang S, et al. Epidural spinal cord stimulation training and sustained recovery of cardiovascular function in individuals with chronic cervical spinal cord injury. JAMA Neurol. 2018;75:1569–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Darrow D, Balser D, Netoff TI, et al. Epidural spinal cord stimulation facilitates immediate restoration of dormant motor and autonomic supraspinal pathways after chronic neurologically complete spinal cord injury. J Neurotrauma. 2019;36:2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herrity AN, Williams CS, Angeli CA, Harkema SJ, Hubscher CH. Lumbosacral spinal cord epidural stimulation improves voiding function after human spinal cord injury. Sci Rep. 2018;8:8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walter M, Lee AHX, Kavanagh A, Phillips AA, Krassioukov AV. Epidural spinal cord stimulation acutely modulates lower urinary tract and bowel function following spinal cord injury: a case report. Front Physiol. 2018;9:1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taccola G, Barber S, Horner PJ, Bazo HAC, Sayenko D. Complications of epidural spinal stimulation: lessons from the past and alternatives for the future. Spinal Cord. 2020;58:1049–1059. [DOI] [PubMed] [Google Scholar]
- 23. Shipley J, North RB. A review of spinal cord stimulation cost studies. In: Krames ES, Peckham PH, Rezeai AR, eds., Neuromodulation. 2nd ed. Cambridge, MA, USA: Academic Press; 2018:701–719. [Google Scholar]
- 24. Sayenko DG, Atkinson DA, Dy CJ, et al. Spinal segment-specific transcutaneous stimulation differentially shapes activation pattern among motor pools in humans. J Appl Physiol (1985). 2015;118:1364–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hofstoetter US, Freundl B, Binder H, Minassian K. Common neural structures activated by epidural and transcutaneous lumbar spinal cord stimulation: elicitation of posterior root-muscle reflexes. PLoS One. 2018;13:e0192013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hofstoetter US, Krenn M, Danner SM, et al. Augmentation of voluntary locomotor activity by transcutaneous spinal cord stimulation in motor-incomplete spinal cord-injured individuals. Artif Organs. 2015;39:E176–E186. [DOI] [PubMed] [Google Scholar]
- 27. McHugh LV, Miller AA, Leech KA, Salorio C, Martin RH. Feasibility and utility of transcutaneous spinal cord stimulation combined with walking-based therapy for people with motor incomplete spinal cord injury. Spinal Cord Ser Cases. 2020;6:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gerasimenko YP, Lu DC, Modaber M, et al. Noninvasive reactivation of motor descending control after paralysis. J Neurotrauma. 2015;32:1968–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gad P, Gerasimenko Y, Zdunowski S, et al. Weight bearing over-ground stepping in an exoskeleton with non-invasive spinal cord neuromodulation after motor complete paraplegia. Front Neurosci. 2017;11:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Phillips AA, Squair JW, Sayenko DG, Edgerton VR, Gerasimenko Y, Krassioukov AV. An autonomic neuroprosthesis: noninvasive electrical spinal cord stimulation restores autonomic cardiovascular function in individuals with spinal cord injury. J Neurotrauma. 2018;35:446–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gad PN, Kreydin E, Zhong H, Latack K, Edgerton VR. Non-invasive neuromodulation of spinal cord restores lower urinary tract function after paralysis. Front Neurosci. 2018;12:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kreydin E, Zhong H, Latack K, Ye S, Edgerton VR, Gad P. Transcutaneous electrical spinal cord neuromodulator (TESCoN) improves symptoms of overactive bladder. Front Syst Neurosci. 2020;14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou R, Parhizi B, Assh J, et al. Effect of cervicolumbar coupling on spinal reflexes during cycling after incomplete spinal cord injury. J Neurophysiol. 2018;120:3172–3186. [DOI] [PubMed] [Google Scholar]
- 34. Barss TS, Parhizi B, Mushahwar VK. Transcutaneous spinal cord stimulation of the cervical cord modulates lumbar networks. J Neurophysiol. 2020;123:158–166. [DOI] [PubMed] [Google Scholar]
- 35. Inanici F, Samejima S, Gad P, Edgerton VR, Hofstetter CP, Moritz CT. Transcutaneous electrical spinal stimulation promotes long-term recovery of upper extremity function in chronic tetraplegia. IEEE Trans Neural Syst Rehabil Eng. 2018;26:1272–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Inanici F, Brighton LN, Samejima S, Hofstetter CP, Moritz CT. Transcutaneous spinal cord stimulation restores hand and arm function after spinal cord injury. IEEE Trans Neural Syst Rehabil Eng. 2021;29:310–319. [DOI] [PubMed] [Google Scholar]
- 37. Gerasimenko Y, Gorodnichev R, Puhov A, et al. Initiation and modulation of locomotor circuitry output with multisite transcutaneous electrical stimulation of the spinal cord in noninjured humans. J Neurophysiol. 2015;113:834–842. [DOI] [PubMed] [Google Scholar]
- 38. Linacre JM, Heinemann AW, Wright BD, Granger CV, Hamilton BB. The structure and stability of the functional independence measure. Arch Phys Med Rehabil. 1994;75:127–132. [PubMed] [Google Scholar]
- 39. Behrman AL, Lawless-Dixon AR, Davis SB, et al. Locomotor training progression and outcomes after incomplete spinal cord injury. Phys Ther. 2005;85:1356–1371. [PubMed] [Google Scholar]
- 40. Gad P, Lee S, Terrafranca N, et al. Non-invasive activation of cervical spinal networks after severe paralysis. J Neurotrauma. 2018;35:2145–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Curt A, Van Hedel HJ, Klaus D, Dietz V. Recovery from a spinal cord injury: significance of compensation, neural plasticity, and repair. J Neurotrauma. 2008;25:677–685. [DOI] [PubMed] [Google Scholar]
- 42. Kadaba MP, Ramakrishnan HK, Wootten ME. Measurement of lower extremity kinematics during level walking. J Orthop Res. 1990;8:383–392. [DOI] [PubMed] [Google Scholar]
- 43. Delp SL, Anderson FC, Arnold AS, et al. OpenSim: open-source software to create and analyze dynamic simulations of movement. IEEE Trans Biomed Eng. 2007;54:1940–1950. [DOI] [PubMed] [Google Scholar]
- 44. Thibaudier Y, Tan AQ, Peters DM, Trumbower RD. Differential deficits in spatial and temporal interlimb coordination during walking in persons with incomplete spinal cord injury. Gait Posture. 2020;75:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Krogh K, Christensen P, Sabroe S, Laurberg S. Neurogenic bowel dysfunction score. Spinal Cord. 2006;44:625–631. [DOI] [PubMed] [Google Scholar]
- 46. Welk B, Lenherr S, Elliott S, et al. The neurogenic bladder symptom score (NBSS): a secondary assessment of its validity, reliability among people with a spinal cord injury. Spinal Cord. 2018;56:259–264. [DOI] [PubMed] [Google Scholar]
- 47. Abou L, Ilha J, Romanini F, Rice LA. Do clinical balance measures have the ability to predict falls among ambulatory individuals with spinal cord injury? A systematic review and meta-analysis. Spinal Cord. 2019;57:1001–1013. [DOI] [PubMed] [Google Scholar]
- 48. Shumway-Cook A, Baldwin M, Polissar NL, Gruber W. Predicting the probability for falls in community-dwelling older adults. Phys Ther. 1997;77:812–819. [DOI] [PubMed] [Google Scholar]
- 49. Alam M, Ling YT, Wong AYL, Zhong H, Edgerton VR, Zheng YP. Reversing 21 years of chronic paralysis via non-invasive spinal cord neuromodulation: a case study. Ann Clin Transl Neurol. 2020;7:829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weersink JB, Jong BM, Halliday DM, Maurits NM. Intermuscular coherence analysis in older adults reveals that gait-related arm swing drives lower limb muscles via subcortical and cortical pathways. J Physiol. 2021;599:2283–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zewdie ET, Roy FD, Okuma Y, Yang JF, Gorassini MA. Long-latency, inhibitory spinal pathway to ankle flexors activated by homonymous group 1 afferents. J Neurophysiol. 2014;111:2544–2553. [DOI] [PubMed] [Google Scholar]
- 52. Taweel WA, Seyam R. Neurogenic bladder in spinal cord injury patients. Res Rep Urol. 2015;7:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sanders PM, Ijzerman MJ, Roach MJ, Gustafson KJ. Patient preferences for next generation neural prostheses to restore bladder function. Spinal Cord. 2011;49:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wheeler TL, Groat W, Eisner K, et al. Translating promising strategies for bowel and bladder management in spinal cord injury. Exp Neurol. 2018;306:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bourbeau D, Creasey G, French J, et al. A roadmap for advancing neurostimulation approaches for bladder and bowel function after spinal cord injury. Spinal Cord. 2020;58:1227–1232. [DOI] [PubMed] [Google Scholar]
- 56. Korsten MA, Fajardo NR, Rosman AS, Creasey GH, Spungen AM, Bauman WA. Difficulty with evacuation after spinal cord injury: colonic motility during sleep and effects of abdominal wall stimulation. J Rehabil Res Dev. 2004;41:95–100. [DOI] [PubMed] [Google Scholar]
- 57. Krogh K, Perkash I, Stiens SA, Biering-Sørensen F. International bowel function extended spinal cord injury data set. Spinal Cord. 2009;47:235–241. [DOI] [PubMed] [Google Scholar]
- 58. Theisen KM, Mann R, Roth JD, et al. Frequency of patient-reported UTIs is associated with poor quality of life after spinal cord injury: a prospective observational study. Spinal Cord. 2020;58:1274–1281. [DOI] [PubMed] [Google Scholar]
- 59. Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol. 1997;77:797–811. [DOI] [PubMed] [Google Scholar]
- 60. Handrakis JP, Rosado-Rivera D, Singh K, et al. Self-reported effects of cold temperature exposure in persons with tetraplegia. J Spinal Cord Med. 2017;40:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fawcett JW, Curt A, Steeves JD, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45:190–205. [DOI] [PubMed] [Google Scholar]
- 62. Leech KA, Kinnaird CR, Holleran CL, Kahn J, Hornby TG. Effects of locomotor exercise intensity on gait performance in individuals with incomplete spinal cord injury. Phys Ther. 2016;96:1919–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Awai L, Curt A. Intralimb coordination as a sensitive indicator of motor-control impairment after spinal cord injury. Front Hum Neurosci. 2014;8:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Malone LA, Bastian AJ, Torres-Oviedo G. How does the motor system correct for errors in time and space during locomotor adaptation? J Neurophysiol. 2012;108:672–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thompson AK, Wolpaw JR. H-reflex conditioning during locomotion in people with spinal cord injury. J Physiol. 2019;599:2453–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Awad LN, Palmer JA, Pohlig RT, Binder-Macleod SA, Reisman DS. Walking speed and step length asymmetry modify the energy cost of walking after stroke. Neurorehabil Neural Repair. 2015;29:416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Adams MM, Hicks AL. Spasticity after spinal cord injury. Spinal Cord. 2005;43:577–586. [DOI] [PubMed] [Google Scholar]
- 68. Gross R, Leboeuf F, Rémy-Néris O, Perrouin-Verbe B. Unstable gait due to spasticity of the rectus femoris: gait analysis and motor nerve block. Ann Phys Rehabil Med. 2012;55:609–622. [DOI] [PubMed] [Google Scholar]
- 69. Richardson RR, Siqueira EB, Cerullo LJ. Spinal epidural neurostimulation for treatment of acute and chronic intractable pain: initial and long term results. Neurosurgery. 1979;5:344–348. [PubMed] [Google Scholar]
- 70. Hofstoetter US, Freundl B, Danner SM, et al. Transcutaneous spinal cord stimulation induces temporary attenuation of spasticity in individuals with spinal cord injury. J Neurotrauma. 2020;37:481–493. [DOI] [PubMed] [Google Scholar]
- 71. Lee KY, Bae C, Lee D, et al. Low-intensity, kilohertz frequency spinal cord stimulation differently affects excitatory and inhibitory neurons in the rodent superficial dorsal horn. Neuroscience. 2020;428:132–139. [DOI] [PubMed] [Google Scholar]
- 72. Spiess M, Schubert M, Kliesch U, EM-SCI Study group, Halder P. Evolution of tibial SSEP after traumatic spinal cord injury: baseline for clinical trials. Clin Neurophysiol. 2008;119:1051–1061. [DOI] [PubMed] [Google Scholar]
- 73. Chisholm AE, Qaiser T, Williams AMM, Eginyan G, Lam T. Acquisition of a precision walking skill and the impact of proprioceptive deficits in people with motor-incomplete spinal cord injury. J Neurophysiol. 2019;121:1078–1084. [DOI] [PubMed] [Google Scholar]
- 74. Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain J Neurol. 2014;137:1394–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lam T, Noonan VK, Eng JJ. A systematic review of functional ambulation outcome measures in spinal cord injury. Spinal Cord. 2008;46:246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.