Abstract
Aims
Heart failure with preserved ejection fraction (HFpEF) is a heterogeneous syndrome with various causes that may influence prognosis.
Methods and results
We extracted the electronic medical records for 2180 consecutive patients hospitalized between 2016 and 2019 for decompensated heart failure. Using a text mining algorithm looking for a left ventricular ejection fraction ≥50% and plasma brain natriuretic peptide level >100 pg/mL, we identified 928 HFpEF patients. We screened for a prevailing cause of HFpEF according to European guidelines and found that 418 (45.0%) patients had secondary HFpEF due to either myocardial (n = 125, 13.5%) or loading condition abnormalities (n = 293, 31.5%), while the remaining 510 (55.0%) patients had idiopathic HFpEF. We assessed the association between the causes of HFpEF and survival collected up to 31 December 2020 using Cox proportional hazards analysis. Even though patients with idiopathic HFpEF were older, frequently female, and had frequent co‐morbidities and a higher crude mortality rate compared with secondary HFpEF patients, their prognosis was similar after adjustment for age and sex. Unsupervised clustering analysis revealed three main phenogroups with different distribution of idiopathic vs. secondary HFpEF. The phenogroup with the highest proportion of idiopathic HFpEF (69%) had (i) an excess rate of non‐cardiac co‐morbidities including chronic obstructive pulmonary disease (31%) or obesity (41%) and (ii) a better prognosis compared with the two other phenogroups enriched with secondary HFpEF.
Conclusions
Aetiological classification provides clinical and prognostic information and may be useful to better decipher the clinical heterogeneity of HFpEF.
Keywords: Heart failure with preserved ejection faction, Prognosis, Classification, Aetiologies
Condensed abstract
The identification of specific aetiologies was possible in almost half of patients with heart failure with preserved ejection fraction (HFpEF). Compared with patients with secondary HFpEF, patients with idiopathic HFpEF were older, frequently female, and had a higher proportion of co‐morbidities and high crude mortality rates, but their prognosis was similar after adjustment for age and sex. Unsupervised cluster analysis, however, indicated that idiopathic HFpEF patients were mostly grouped into separate cluster with a better prognosis. Aetiological classification provides clinical and prognostic information and may be useful to better decipher the clinical heterogeneity of HFpEF.
Introduction
A growing number of patients present with heart failure (HF) symptoms and an apparently normal left ventricular ejection fraction (LVEF ≥ 50%). 1 , 2 These patients are commonly referred to as having HF with preserved ejection fraction (HFpEF), 3 which however represents a heterogeneous syndrome with very different clinical phenotypes. 4 Typically, HFpEF is thought to result from a combination of risk factors and co‐morbidities that progressively lead to cardiac remodelling associated with high left ventricular filling pressures and consequent congestive symptoms. 5 In rarer cases, however, HFpEF is related to causes that can involve specific pathophysiological mechanisms that directly affect the myocardium (e.g. ischaemia, infiltrative disorders, or genetic cardiomyopathies) or that profoundly change the cardiac loading conditions (e.g. hypervolaemic states, severe heart valve diseases, acute hypertensive urgencies, or high‐output states). 4 Screening for aetiologies is recommended in patients with HFpEF under current international guidelines (Class Ic) 4 , 6 , 7 and is a practical way to categorize HFpEF patients. However, whether the identification of an underlying cause impacts the management and prognosis of HFpEF patients remains unclear.
In contrast to HF with reduced ejection fraction (HFrEF), therapeutic options are currently limited for HFpEF patients, 6 in part due to significant pathophysiological heterogeneity within the broad population of HFpEF patients. 5 The different causes of HFpEF are likely to involve distinct mechanisms and specific therapeutic targets. 8 , 9 Novel data‐driven methods have been developed to allow classification of complex and heterogeneous medical disorders. Their application using main baseline characteristics and co‐morbidities has suggested that HFpEF encompasses different phenogroups of patients who likely share similar pathophysiological profiles. 10 , 11 , 12 However, whether an aetiological classification could further improve this classification of HFpEF patients has not been clearly shown.
Although Borlaug previously reported high mortality rates in HFpEF patients, 13 data about the prevalence of different causes of HFpEF and their prognostic value are limited. Therefore, the goals of this retrospective study were to identify the different prevailing causes of HFpEF, evaluate the extent of residual heterogeneity among HFpEF patients using aetiological classification, and determine the relationship between different causes of HFpEF and prognosis.
Methods
Patient identification and study population
We used the inpatient clinical data warehouse at Hôpital Européen Georges‐Pompidou, Paris, France 14 to identify all consecutive patients hospitalized for decompensated congestive HF between 1 January 2016 and 31 December 2019. The identification of patients was first performed with an automated query of the clinical, biological, and free text reports using the following search criteria: (i) a diagnosis of HF based on the ICD‐10 codes for HF (I50.0, I50.1, and I50.9), (ii) a plasma B‐type natriuretic peptide (BNP) level >100 pg/mL, and (iii) an estimation of the LVEF value extracted by a text mining algorithm applied to the reports of echocardiography or cardiac magnetic resonance imaging or cardiac scintigraphy. Demographic, clinical, co‐morbidity, and cardiac diagnoses were extracted using complementary ICD‐10 codes (Supporting Information, Table S1 ). Patients with prior cardiac transplantation, an implanted left ventricular assistance device, acute endocarditis, primary pulmonary arterial hypertension, or isolated severe tricuspid regurgitation were excluded.
A list of patients corresponding to these search criteria was generated, and the individual patients' electronic medical records (EMRs) were independently reviewed by three cardiologists for further validation. The diagnosis of decompensated congestive HF was confirmed for patients with symptoms and clinical signs typical of congestive HF 4 , 6 who required intravenous diuretic therapy or an increase in diuretic oral therapy during the hospital stay. Patients who did not meet this definition were excluded. When multiple hospital stays for HF were identified, the first recorded event that met the definition of congestive HF was considered as the index hospitalization. The BNP levels and LVEF values at admission were extracted by the text mining algorithm and confirmed in the EMR data. LVEF was used for subgroup stratification as recommended by current guidelines. 6 A cut‐off value of LVEF ≤ 40% was used for HFrEF and of LVEF ≥ 50% for HFpEF. Patients with LVEF between 40% and 50% were classified as having HF with mildly reduced ejection fraction (HFmrEF). For patients with multiple estimations of LVEF, the lowest recorded value was used. Patients with LVEF ≥ 50% during the HF index hospitalization but with evidence of a prior LVEF < 50% (i.e. recovered LVEF) were excluded, leaving only HFpEF patients who had not evidence of reduced LVEF (<50%) during or prior to the index hospitalization. The estimated glomerular filtration rate was estimated by the modification of diet in renal disease equation.
This study was approved by the institutional review board of Paris (Institutional Review Board Registration #00011928, CERAPHP.5, Project 2018‐12‐10). Patients included in this study were all informed that their medical data could be used for research purposes in accordance with the General Data Protection Regulation 2016/679.
Specific aetiologies in heart failure with preserved ejection fraction patients and classification
The EMRs of all patients with LVEF ≥ 50% were screened in order to assign a prevailing cause of HF. All medical records were individually reviewed by three independent cardiologists. As there is currently no consensus for classification, we defined 12 categories according to the list of specific aetiologies underlying HFpEF as recently published in the guidelines for HFpEF diagnosis. 4 We then classified these categories into two major groups to distinguish between abnormalities of the myocardium and the abnormalities of the loading conditions.
Briefly, the abnormalities of the myocardium consisted of the following categories and are defined as follows:
- Ischaemic
- Myocardial post‐infarction: patients with a history of myocardial infarction (MI) prior the hospitalization for decompensated HF.
- Myocardial stunning: decompensated HF with troponin elevation and typical electrocardiogram modifications related to cardiac ischaemia, with regional akinetic segment(s) identified on cardiac imaging and non‐occlusive coronary lesions or stenosis on coronary artery angiography.
- Microvascular dysfunction: decompensated HF with evident myocardial ischaemia (diagnosed by elevated troponin + typical electrocardiogram modifications), with preserved ejection fraction but without evidence of coronary lesions on the coronary angiogram, and with a perfusion imaging technique identifying a decreased coronary reserve.
- Genetic
- Direct affections of the myocardium: hypertrophic cardiomyopathy and restrictive cardiomyopathy not caused by a pathological loading conditions, with or without identified mutations in sarcomeric genes.
- Sickle cell disease‐associated cardiomyopathy.
- Infiltrative
- Amyloidosis: presence of a myocardial infiltration linked to a diagnosed amyloidosis on cardiac imaging ± biopsy‐proven histological type.
- Storage disease: evidence for Fabry disease.
- Toxic
- Medications: decompensated HF related to the administration of a cardiac‐offending drug ± relief of symptoms after drug withdrawal.
- Radiation: patients with history of cardiac radiation (total mediastinal doses >3 Gy).
- Immune and inflammatory
- Related to infection: HF related to a cardiotropic virus or to HIV infection.
- Not related to infection: HF occurring in the context of an autoimmune disease such as lupus erythematosus, hypersensitivity, and eosinophilic myocarditis.
- Metabolic
- HF occurring in the immediate context of pregnancy or peripartum.
Abnormalities of the loading conditions consisted of the following categories and are defined as follows:
- Valvular defect
- Acquired degeneration of the native valve with significant and severe haemodynamic impact, including aortic stenosis, aortic regurgitation, mitral regurgitation, and stenosis.
- Patients with a previous left‐sided valvular surgery including aortic valve replacement and/or mitral valve replacement or repair.
- Volume overload
- HF in the context of a terminal renal failure with hypervolaemia and requiring dialysis.
- HF immediately following a fluid overload.
- High‐output state
- Severe anaemia: decompensated HF occurring in the context of a severe acute anaemia (haemoglobin <7 g/dL).
- Presence of a large arteriovenous fistula or multiple small arteriovenous shunts.
- Decompensated HF occurring in the context of thyrotoxicosis.
- Hypertensive crisis
- Acute hypertensive crisis: HF in the context of severe elevation of blood pressure with target organ damage ± related to an identified secondary form of hypertension.
- Structural defect
- Congenital heart disease with septal defect or single ventricle.
- Pericardial pathologies
- Constrictive pericarditis or chronic pericardial effusion with significant impact on the loading conditions.
All co‐morbidities were also recorded and analysed. Essential hypertension, hypercholesterolaemia, diabetes, obesity, lung disorders with chronic obstructive pulmonary disease (COPD), sleep apnoea, and atrial fibrillation (AF) were considered to be underlying co‐morbidities or potential factors favouring HF decompensation but not primary causes of HFpEF.
Patients with characterized atherosclerotic plaque(s) in the coronary arteries were noted as having a coronary artery disease (CAD). Ischaemia was not considered to be a prevailing cause of HF when there was no evidence of significantly obstructive CAD on the coronary angiogram during explorations for HF or in the case of a known obstructive CAD with prior appropriate revascularization and without evidence of residual ischaemia. All patients with a known or identified MI were considered to have an ischaemic HFpEF.
When none of the earlier potential causes were identified, patients were considered to have idiopathic HFpEF (i.e. primary HFpEF). All patients with an identified underlying aetiology were labelled as having secondary HFpEF. In patients with more than one potential cause of HFpEF, the single most likely cause was chosen based on the chronology and the resolution of decompensated HF with specific treatment of the aetiology. All patient's medical records were reviewed independently by three cardiologists. The few discordant cases were resolved upon re‐review and discussion between two senior cardiologists (A. F and J.‐S. H). Final diagnoses were assigned blinded to each patient's status in terms of long‐term survival.
Follow‐up
Follow‐up information about the vital status of the patients was obtained through a search of the official statistics from the National Institute of Statistics and Economic Studies using the matchID routine (https://deces.matchid.io/search). Data were collected up to 31 December 2020.
Statistical analyses
Continuous variables are presented as means ± standard deviation, whereas categorical variables are shown as total numbers and frequencies (%). Between‐group comparisons of continuous variables were performed using unpaired t‐tests for two groups or one‐way analysis of variance for three groups, as indicated. Categorical variables were compared using the χ 2 or Fisher's exact test as appropriate. We used Kaplan–Meier survival curves and the log‐rank test to compare differences in event‐free survival between groups. Overall survival was analysed using a two‐sided log‐rank test, and the hazard ratio (HR) and two‐sided 95% confidence interval (CI) values were calculated using the Cox proportional hazard model. Cox models were used to evaluate the impact of the different aetiological groups on survival with adjustment for age and sex. Aetiological groups with fewer than five patients were not considered in the survival analyses. As sex and age are strong confounders for survival, we further performed a sex‐matched and age‐matched survival analysis for the different paired groups of interest. All statistical analyses were performed using R (Version 3.6.2; R Foundation for Statistical Computing, Vienna, Austria). Two‐sided P‐values <0.05 were considered to be statistically significant.
Clustering analysis
We conducted an unsupervised clustering analysis of the subgroups of HFpEF patients, based on the following independent variables at baseline: age; sex; diabetes; obesity; hypercholesterolaemia; hypertension; AF; history of MI; CAD; COPD and amyloidosis; and values for LVEF, BNP, and modification of diet in renal disease.
We used the Gower metric to compute distances between patients and Ward's method for hierarchical agglomerative clustering. The optimal number of clusters was inferred using the silhouette method. 15 The results are presented as a heat map of all clustering parameters, with the associated dendrogram for the hierarchical clustering. We also conducted bivariate analyses (categorical variables were compared using Fisher's exact test, and continuous variables were compared using the Kruskal–Wallis one‐way analysis of variance) and a Kaplan–Meier survival analysis between the clusters identified by the hierarchical clustering, with a significance threshold of P < 0.05. The clustering analysis and attached survival analysis were performed using R and the NbClust package. 16
Results
Population characteristics
The final cohort consisted of 2180 patients hospitalized for congestive HF, of which we identified 977 (44.8%) as HFrEF patients, 275 (12.6%) as HFmrEF patients, and 928 (42.6%) as HFpEF patients (Figure 1 ). Clinical characteristics were significantly different among the three groups according to LVEF (Table 1 ). Similar to previous studies, patients with HFpEF were older, more often female, and had lower proportions of CAD and lower BNP levels compared with HFrEF or HFmrEF patients. Overall, HFpEF patients had higher proportions of risk factors and co‐morbidities, including arterial hypertension, COPD, and renal dysfunction. In contrast, the number of patients with type 2 diabetes, obesity (as defined by a body mass index ≥30 kg/m2), and hypercholesterolaemia did not differ across the three subgroups of patients. A majority of HFpEF patients (58.6%) had a history of AF, with a high proportion of them presenting with paroxysmal AF.
Figure 1.
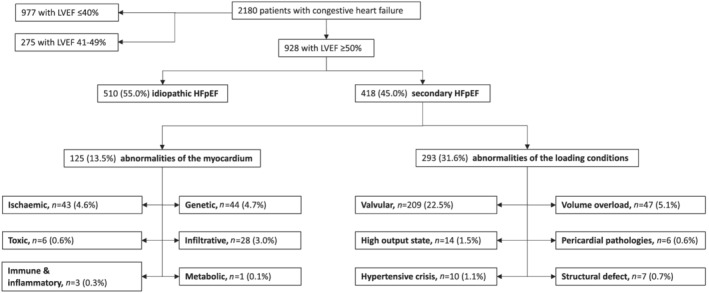
Classification by left ventricular ejection fraction (LVEF) and by aetiologies of identified heart failure patients. Secondary heart failure with preserved ejection fraction (HFpEF) patients are grouped into two distinct categories with aetiologies related to abnormalities of the myocardium vs. abnormalities of the loading conditions. Patients for whom none of these causes were identified were classified as having idiopathic HFpEF.
Table 1.
Baseline characteristics of the study population according to LVEF classification
| Characteristic |
Overall N = 2180 |
HFrEF patients N = 977 |
HFmrEF patients N = 275 |
HFpEF patients N = 928 |
P‐value |
|---|---|---|---|---|---|
| Age (years) | 75.5 ± 15.0 | 71.2 ± 15.3 | 75.9 ± 14.3 | 80.0 ± 13.6 | <0.0001 |
| Median [IQR] | 79 [67–86] | 72 [61–83] | 79 [68–86] | 83 [74–89] | |
| Female, n (%) | 947 (43.4) | 311 (31.8) | 121 (44.0) | 515 (55.5) | <0.0001 |
| Hypertension, n (%) | 1474 (67.6) | 582 (59.6) | 199 (72.4) | 693 (74.7) | <0.0001 |
| Type 2 diabetes, n (%) | 534 (24.5) | 242 (24.8) | 72 (26.2) | 220 (23.7) | 0.67 |
| Hypercholesterolaemia, n (%) | 756 (34.7) | 342 (35.0) | 97 (35.3) | 317 (34.2) | 0.90 |
| Obesity, n (%) | 364 (16.7) | 151 (15.5) | 53 (19.3) | 160 (17.3) | 0.27 |
| CAD, n (%) | 797 (36.6) | 474 (48.5) | 121 (44.0) | 202 (21.8) | <0.0001 |
| COPD, n (%) | 203 (9.3) | 59 (6.0) | 35 (12.7) | 109 (11.7) | <0.0001 |
| Sleep apnoea, n (%) | 147 (6.7) | 63 (6.4) | 20 (7.3) | 64 (6.9) | 0.86 |
| Atrial fibrillation | |||||
| Any type, n (%) | 1219 (55.9) | 507 (51.9) | 172 (62.5) | 540 (58.2) | 0.0009 |
| Paroxysmal, n (%) | 487 (22.3) | 173 (17.7) | 33 (12.0) | 281 (30.3) | <0.0001 |
| Sustained, n (%) | 732 (33.6) | 334 (34.2) | 139 (50.6) | 259 (27.9) | |
| LVEF (%) | 44.9 ± 16.0 | 29.7 ± 7.5 | 45.1 ± 2.1 | 60.8 ± 6.7 | <0.0001 |
| Median [IQR] | 45 [31–60] | 30 [25–35] | 45 [44–46] | 60 [55–65] | |
| BNP level (pg/mL) | 824 ± 845 | 1050 ± 1004 | 805 ± 787 | 591 ± 570 | <0.0001 |
| Median [IQR] | 525 [283–1013] | 692 [340–1352] | 588 [298–932] | 399 [235–758] | |
| eGFR (mL/min/1.73 m2) | 59 ± 29 | 60 ± 28 | 60 ± 30 | 57 ± 30 | 0.017 |
| Median [IQR] | 55 [38–74] | 57 [39–76] | 55 [41–75] | 54 [36–73] | |
| All‐cause death, n (%) | 855 (39.2) | 364 (37.3) | 108 (39.3) | 383 (41.3) | 0.20 |
CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IQR, inter‐quartile range; LVEF, left ventricular ejection fraction. The bold font indicates significant P‐values.
Causes of heart failure with preserved ejection fraction and classification
The EMRs of the 928 HFpEF patients were screened, and a specific cause was identified in 418 (45.0%) of them. The underlying causes in these patients with secondary HFpEF were classified into 12 aetiological categories: six related to abnormalities of the myocardium (n = 125, 13.5% of total) and six related to abnormalities of the loading conditions (n = 293, 31.5% of total) (Figure 1 and Supporting Information, Table S2 ). Ischaemic (post‐MI and myocardial stunning), genetic (mainly hypertrophic cardiomyopathy), and infiltrative (mainly cardiac amyloidosis) disorders were the most frequent aetiologies related to abnormalities of the myocardium. Severe valvular disorders (mainly linked to the aortic valve), volume overload (mainly acute renal failure precipitating HF), and high‐output HF were the most frequent causes related to abnormalities of the loading conditions. Other causes were rare (<1%) but were very distinctive causes of HF, such as toxic origins linked to anticancer drugs or mediastinal radiation therapy, immune systemic disorders, peripartum, acute hypertensive emergencies, structural left ventricular defects, or constrictive pericarditis (Supporting Information, Table S2 ). None of these causes were identified in the remaining 510 (55.0%) patients, who were thus classified as having idiopathic HFpEF (Figure 1 ).
This aetiological classification resulted in subgroups of HFpEF patients with significant differences in characteristics (Table 2 ). Overall, patients with idiopathic HFpEF were older, frequently female, and had a higher proportion of risk factors and co‐morbidities, including hypertension, COPD, and AF. In contrast, patients with secondary HFpEF related to abnormalities of the myocardium were significantly younger and more frequently male, and a higher proportion of patients had underlying CAD and slightly lower LVEF. Patients with abnormalities of the loading conditions were less different compared with patients with idiopathic HFpEF; the sex ratio was similar, but patients were slightly younger and had lower rates of AF. Supporting Information, Table S3 provides a detailed description of the patients' characteristics according to the specific cause and further illustrates the substantial heterogeneity among the groups. For instance, the distributions of age differed significantly among patients with idiopathic HFpEF, secondary HFpEF, and HFrEF (Supporting Information, Table S3 and Figure S1 ). Similar differences were noted with regard to sex ratio and associated co‐morbidities, which were, however, concordant with the identified clinical diagnoses (Supporting Information, Table S3 ).
Table 2.
Baseline characteristics of HFpEF patients according to aetiologies
| Characteristics |
Idiopathic HFpEF N = 510 |
Secondary: abnormalities of the myocardium N = 125 |
Secondary: abnormalities of the loading conditions N = 293 |
P‐value |
|---|---|---|---|---|
| Age (years) | 83.5 ± 10.2 | 70.7 ± 19.0 | 78.0 ± 13.9 | <0.0001 |
| Median [IQR] | 85 [78–90] | 74 [63–85] | 81 [71–88] | |
| Female, n (%) | 294 (57.7) | 52 (41.6) | 169 (57.7) | 0.003 |
| Hypertension, n (%) | 406 (79.6) | 85 (68.0) | 202 (68.9) | 0.001 |
| Type 2 diabetes, n (%) | 126 (24.7) | 26 (20.8) | 68 (23.2) | 0.63 |
| Hypercholesterolaemia, n (%) | 182 (35.7) | 48 (38.4) | 87 (29.7) | 0.12 |
| Obesity, n (%) | 96 (18.8) | 25 (20.0) | 39 (13.3) | 0.09 |
| CAD, n (%) | 91 (17.8) | 49 (39.2) | 62 (21.2) | <0.0001 |
| COPD, n (%) | 84 (16.5) | 8 (6.4) | 17 (5.8) | <0.0001 |
| Sleep apnoea, n (%) | 42 (8.2) | 9 (7.2) | 13 (4.4) | 0.12 |
| Atrial fibrillation | ||||
| Any type, n (%) | 325 (63.7) | 69 (55.2) | 146 (49.8) | 0.001 |
| Paroxysmal, n (%) | 153 (30.0) | 43 (34.4) | 85 (29.0) | 0.0002 |
| Sustained, n (%) | 172 (33.7) | 26 (20.8) | 61 (20.8) | |
| LVEF (%) | 61.0 ± 6.5 | 58.9 ± 7.1 | 61.1 ± 6.6 | 0.002 |
| Median [IQR] | 60 [55–65] | 58 [53–65] | 60 [56–65] | |
| BNP level (pg/mL) | 520 ± 481 | 617 ± 546 | 703 ± 693 | 0.0001 |
| Median [IQR] | 364 [217–648] | 438 [281–749] | 440 [260–912] | |
| eGFR‐MDRD (mL/min/1.73 m2) | 57 ± 28 | 66 ± 32 | 51 ± 31 | 0.0001 |
| Median [IQR] | 53 [37–73] | 60 [46–78] | 50 [28–70] | |
| All‐cause death, n (%) | 233 (45.7) | 45 (36.0) | 105 (35.9) | 0.01 |
CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HFpEF, heart failure with preserved ejection fraction; IQR, inter‐quartile range; LVEF, left ventricular ejection fraction; MDRD, modification of diet in renal disease. The bold font indicates significant P‐values.
Clinical outcomes
Out of the 2180 patients, we identified 855 (39.2%) deaths during the follow‐up period (2.17 ± 1.38 years). The all‐cause mortality did not differ significantly among the three groups of patients (HFrEF vs. HFmEF vs. HFpEF) (Table 1 and Figure 2 A ). When considering the aetiological classification among the HFpEF patients, however, survival was significantly worse among patients with idiopathic HFpEF than among those with HFrEF (unadjusted HR 1.29, 95% CI 1.09–1.53, P = 0.002) as well as those with secondary HFpEF (unadjusted HR 1.34, 95% CI 1.09–1.64, P = 0.005, Figure 2 B ), independent of the categories of secondary HFpEF (Figure 2 C ). However, after adjustment for age and sex, survival among the patients with idiopathic HFpEF did not differ from that among patients with HFrEF (adjusted HR, 0.92, 95% CI 0.84–1.01, P = 0.08) or those with secondary HFpEF (adjusted HR, 1.07, 95% CI 0.86–1.32, P = 0.54). To further account for the potential influence of age and sex on prognosis, we conducted a sex‐matched and age‐matched (±2 years) analysis to compare survival between the different subgroups of HF patients (HFrEF vs. all HFpEF patients and idiopathic HFpEF vs. secondary HFpEF patients globally and then considering secondary HFpEF patients with abnormalities of the loading conditions and HFpEF patients with abnormalities of the myocardium separately). As shown in Figure 2 D – 2 G , the differences in survival between the different subgroups were not statistically significant.
Figure 2.

Unadjusted cumulative curves for all‐cause mortality in (A) heart failure with reduced ejection fraction (HFrEF) vs. heart failure with mildly reduced ejection fraction (HFmrEF) and heart failure with preserved ejection fraction (HFpEF), (B) HFrEF vs. idiopathic HFpEF vs. secondary HFpEF, and (C) HFrEF vs. idiopathic HFpEF vs. secondary HFpEF linked to abnormalities of the myocardium vs. abnormalities of the loading conditions. Age‐matched and sex‐matched analyses of all‐cause mortality in (D) HFrEF vs. HFpEF, (E) idiopathic HFpEF vs. secondary HFpEF, (F) idiopathic HFpEF vs. secondary HFpEF abnormalities of the loading conditions, and (G) idiopathic HFpEF vs. secondary HFpEF linked to abnormalities of the myocardium. P‐values are from log‐rank tests. The mean age and proportion of female are reported for each group in the matched analyses.
Crude mortality rates among patients with secondary HFpEF significantly differed according to the aetiological subgroups (Supporting Information, Figure S2 ). Survival was substantially better among the patients with hypertensive crisis (as no death was observed in this aetiological group during the follow‐up period). In contrast, survival was substantially worse among the patients with toxic origin of HFpEF and for patients with high‐output HF. Survival among the patients with other causes of HFpEF (including ischaemic or infiltrative causes) did not differ significantly from that among patients with idiopathic HFpEF.
Overall, these results show that HFpEF patients have a prognosis similar to that of other HF patients irrespective of LVEF. The classification of HFpEF patients according to aetiologies helped identify patients with specific clinical profiles but did not provide a greater prognostic information when used as the sole classifier.
Unsupervised clustering, aetiologies of heart failure with preserved ejection fraction, and outcomes
Clustering analysis of the 928 HFpEF patients in an aetiology‐independent analysis identified three clusters as the optimal number of groupings (Figure 3 A ). Each phenogroup was associated with specific patients' characteristics as shown in heat maps in Figure 3 A . While the mean age was balanced among groups (85 vs. 84 vs. 81 years old, respectively), the distribution of HFpEF causes was significantly imbalanced between the three phenogroups (P = 4.10−11). Phenogroup 1 included 327 patients with the highest proportion of idiopathic HFpEF (n = 225, 69%), and high rates of extra‐cardiac co‐morbidities, including COPD (n = 100, 31%), obesity (n = 135, 41%), dyslipidaemia (n = 219, 67%), and diabetes (n = 101, 31%). Phenogroup 2 contained a smaller number of patients (n = 147) and had a high proportion of secondary HFpEF (n = 92, 63%), which notably was linked to myocardial abnormalities; all 147 patients presented with CAD, and the majority were male (n = 98, 67%). Phenogroup 3 (n = 454) had a balance of idiopathic (n = 230, 51%) and secondary forms of HFpEF (n = 224, 49%), which were notably linked to loading abnormalities (n = 166, 37%). This phenogroup had the lowest rates of all co‐morbidities and disorders with the exception of amyloidosis. Almost all patients with amyloidosis (n = 22, 4.8%) were in this phenogroup. AF was highly prevalent in all phenogroups and was the only characteristic that was not significantly imbalanced among groups.
Figure 3.
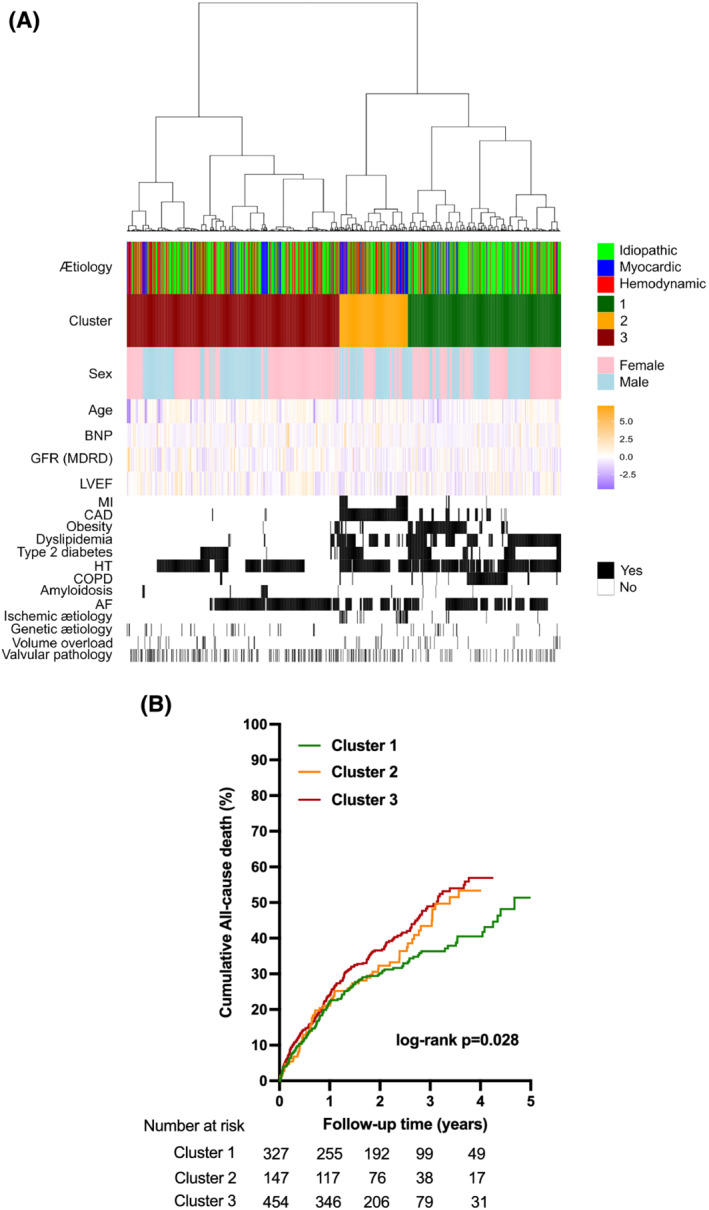
(A) Heat maps of patients' characteristics stratified according to clusters identified by an unsupervised and aetiology‐independent clustering analysis. The distribution of heart failure with preserved ejection fraction aetiologies within the three clusters is represented a posteriori [idiopathic in green, myocardial abnormalities in blue, and loading condition (i.e. haemodynamic) abnormalities in red]. (B) Cumulative curves for all‐cause mortality in the three clusters. P‐values are from log‐rank tests. AF, atrial fibrillation; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; HT, hypertension; LVEF, left ventricular ejection fraction; MDRD, modification of diet in renal disease; MI, myocardial infarction.
We also found that this phenogroup classification successfully stratified patients according to clinical outcomes (Figure 3 B ). All‐cause mortality was significantly lower in patients from Phenogroup 1 compared with the other two phenogroups (HR 0.76, 95% CI 0.61–0.94, P = 0.01). Reciprocally, patients from Phenogroup 3 (with the lowest rates of major co‐morbidities) generally had the worst outcomes.
Discussion
In this study, we examined the prevalence of causes underlying HFpEF and the association between these causes and long‐term survival in a large group of patients evaluated at a single tertiary care centre that is a national reference centre for HF and cardiomyopathies. Using these real‐world data and strict definitions for HF causes, we identified an underlying cause for HFpEF in almost 50% of these patients (referred to as patients with secondary HFpEF), whereas these aetiologies were not be observed in the other half of the patients (referred to as patients with idiopathic HFpEF). This prevalence of these causes is strikingly high and illustrates the importance of screening for aetiologies in patients with HFpEF as recommended by international guidelines. 4 , 6 , 7 , 17 The heterogeneous nature of HFpEF has been previously recognized in different cohorts 2 and in studies that used unsupervised machine learning techniques to propose classification of HFpEF patients (so‐called phenogroups) with distinct clinical characteristics, outcomes, and responses to drugs. 10 , 11 , 12 , 18 In our study, we initially used a simpler and more pragmatic classification of patients into 12 aetiological groups based on the medical assessment of prevailing cause of HFpEF. The aetiological classification was inspired by recent guidelines that recognize potential specific aetiologies underlying HFpEF or HFpEF‐like syndromes. 4 We found that the most frequent causes of HFpEF patients could be classified into a limited number of groups that were almost exclusive (i.e. with limited overlap). The ischaemic aetiology was challenging to define as a large proportion of HF patients have atherosclerotic risk factors or a history of CAD. In this study, we recorded an ischaemic origin of HFpEF for patients with evidence of an underlying ischaemic cardiac disease (i.e. a prior MI or cardiac ischaemia during the index hospitalization for HF), but not for those patients with a history of appropriately treated CAD without residual obstruction. However, our clustering analysis revealed a specific phenogroup containing all patients with CAD. This indicates that an ischaemic cause of HFpEF should be considered in all patients with evidence of coronary disease, whatever the revascularization status. Various researchers have proposed ischaemic HFpEF as a distinct entity. 19 , 20 Shah et al. proposed that abnormalities in coronary microcirculation can support the occurrence of HFpEF in some patients, 21 but there is currently no routine evaluation of microvascular dysfunction in HFpEF patients. Recent studies have suggested a high prevalence of coronary microvascular dysfunction in HFpEF patients. 19 Whether abnormal coronary microcirculation occurs in almost all HFpEF patients with evidence of macroscopic CAD deserves further investigations.
Other causes of HFpEF were rare but corresponded to very specific medical contexts, such as acute hypertensive emergencies, toxic origins linked to anticancer drugs or mediastinal radiation therapy, immune systemic disorders, peripartum, or evidence of constrictive pericarditis. Overall, our final aetiological classification illustrates the large heterogeneity in clinical profiles and physiopathological mechanisms within HFpEF patients.
Those patients without these apparent causes were classified as having idiopathic HFpEF. It could be argued that the true HFpEF population corresponds only to patients with idiopathic HFpEF. However, many studies have reported a potential under‐diagnosis of specific aetiologies underlying HFpEF, as notably exemplified by the increasing recognition of the importance of cardiac amyloidosis in HFpEF patients. 22 , 23 It is also possible that patients with a suspected specific cause underlying HF were more likely to be referred to a reference centre, which would have led to an overestimation of some causes (such as ischaemia, valvular, or hypertrophic cardiomyopathies) in our study. However, our standardized investigations of these patients should have limited the under‐detection of specific aetiologies in idiopathic HFpEF patients. We used a conservative approach, and patients that did not show clear evidence for an underlying cause were considered as having primary HFpEF. Because of the real‐world nature of these data, we cannot ensure that a systematic screening of all potential causes was performed in all of our patients, notably in those presenting with the most severe forms of HF and with a rapidly unfavourable prognosis. Therefore, the proportion of patients with primary HFpEF in our study is potentially overestimated, further supporting the need for an aetiological screening in these patients. The characteristics of our HFpEF patients were, however, concordant with expectations in a real world and unselected cohort of HFpEF patients, 18 thus reinforcing the representativity of our data.
Idiopathic HFpEF remains an obscure group of HFpEF. Compared with patients with secondary HFpEF, those with primary diseases were older, frequently female, and had a higher proportion of risk factors and co‐morbidities, including hypertension and COPD. However, none of these differences indicate a specific and easily recognizable mechanism leading to HF. Similarly, we considered this category after excluding other causes, suggesting that idiopathic HFpEF is supported by other unknown mechanisms or by undetected abnormalities. The unsupervised and aetiology‐independent clustering analysis also led to a significant clustering of most of these idiopathic HFpEF patients into a specific phenogroup with high rates of extra‐cardiac co‐morbidities but low rates of specific cardiac abnormalities. We note that most of patients presenting with obesity and diabetes were found in this cluster. Obesity and diabetes have long been listed as frequent co‐morbidities found in HFpEF patients and important risk factors for the development of cardiac remodelling leading to HFpEF, 4 , 24 and we did not consider these metabolic disorders as a primary cause of HFpEF in our aetiological classifier. However, even if we cannot conclude on a causality link, our clustering result suggests obesity‐related and diabetes‐related HFpEF as a specific entity. Further investigations will be needed to understand a specific underlying mechanism in these patients who more frequently present with low‐grade inflammation. 25 The crude all‐cause mortality rates were particularly high in patients with idiopathic HFpEF, who are thus among patients with the poorest prognosis, but we found that this result was mainly explained by the older age of idiopathic HFpEF patients. After age and sex matching, there was no significant difference in survival of idiopathic HFpEF patients as compared with HFrEF patients or to patients with secondary HFpEF.
In line with previous studies, our data however indicate that prognosis of HF patients remains poor, 26 , 27 independent of LVEF. 12 , 28 Moreover, our data show that the prognosis is relatively independent of the underlying causes of HF when used as sole classifier. However, the clustering analysis indicated that the phenogroup enriched with idiopathic HFpEF was associated with a significantly better prognosis as compared with the two other phenogroups enriched with secondary HFpEF patients. These results suggest that the aetiological classification could provide greater prognostic information when considered in conjunction with patients' other characteristics.
Limitations
This study was based on a retrospective registry of unselected patients presenting with decompensated HF who were hospitalized in a single reference medical centre with expertise in HF management. The single‐centre nature of this study could limit the generalizability of our results, but the characteristics of our patient population are similar to other HF populations in the literature. We included a large population of patients hospitalized for a decompensated HF and used real‐life available data to define the aetiological classification. There was no requirement for systematic investigations; thus, we cannot exclude the possibility that some patients were misclassified as having idiopathic HFpEF as a result of missing medical results. However, investigations in patients hospitalized for decompensated HF are well standardized, and the single nature of the study over a limited time period (2016–19) ensured a higher homogeneity in the diagnostic strategies applied to the patients than would be anticipated in multicentric studies. In addition, while efforts are ongoing to generate prospective cohorts of HFpEF patients based on systemic phenotyping (NCT04189029), access to diagnostic and aetiological data as presented in our study is currently limited.
As in other medico‐administrative databases, the diagnosis of HF was based on ICD codes, which does not provide information about cardiac ejection fraction. In this study, we successfully extracted LVEF values by applying a specific text mining algorithm, and the results were further confirmed by EMRs (with a 97.8% agreement). All‐cause mortality was the only accessible outcome in our study, but other studies have reported higher non‐cardiovascular mortality in HFpEF patients compared with patients with other forms of HF. 29 Notably, we identified some subgroups with particularly severe prognosis as being associated with other non‐cardiovascular disorders (including toxic origins and cancer, and high‐output HF), but sample sizes of these subgroups were limited.
Conclusions
The identification of specific aetiologies was possible in almost half of HFpEF patients and thus has clinical and prognostic value. Compared with patients with secondary HFpEF, patients with idiopathic HFpEF were older, frequently female, and had a higher proportion of co‐morbidities and high crude mortality rates. Survival analyses accounting for differences in age and sex showed that prognosis of HFpEF patients was independent of the underlying causes of HFpEF. Unsupervised cluster analysis, however, indicated that idiopathic HFpEF patients were mostly grouped into separate cluster with a better prognosis. The aetiological classification may be useful to better decipher the large heterogeneity of HFpEF.
Perspectives
Competencies in medical knowledge
The identification of specific underlying aetiologies is possible in almost half of HFpEF patients. The most frequent causes could be classified into a limited number of groups, thus proposing an aetiological classification of HFpEF.
Translational outlook
The application of an aetiological classification may be useful to better decipher the large heterogeneity of HFpEF. Additional research is needed to understand the therapeutic impact of such classification of the disease.
Conflict of interest
J.‐S.H. reports research grants from BioSerenity, Sanofi, Servier, and Novo Nordisk and speaker, advisory board, or consultancy fees from Amgen, AstraZeneca, Bayer, BioSerenity, Bristol Myers Squibb, Novartis, and Novo Nordisk, all unrelated to the present work. C.C. reports advisory board or consultancy fees from AstraZeneca, Lilly, Novartis Pharma, MSD France, Ipsen Pharma, Pfizer, and Publicis Health, all unrelated to the present work. The other authors declare no competing financial interests.
Funding
This work was supported by Assistance Publique ‐ Hôpitaux de Paris (AP‐HP), French Investments for the Future Programme (PIA) Project (2018‐PSPC‐07, PACIFIC‐PRESERVED, BPIfrance), and the University Research Federation against Heart Failure (FHU2019, PREVENT_Heart Failure).
Supporting information
Table S1. List of ICD‐10 Codes.
Table S2. Details about the underlying causes identified in patients with HF with preserved ejection fraction.
Table S3. Baseline characteristics of HFpEF patients according to the underlying aetiology.
Figure S1. Distribution of age in HFrEF (green), idiopathic HFpEF (red) and main etiologies found in secondary HFpEF patients (related to myocardial abnormalities in dark red; related to loading conditions abnormalities in blue).
Figure S2. Cumulative curves for all‐cause mortality in patients with secondary HFpEF according to the underlying aetiology.
Acknowledgement
We thank Pierre‐Yves Hervé for helpful discussions on statistical analyses.
Fayol, A. , Wack, M. , Livrozet, M. , Carves, J.‐B. , Domengé, O. , Vermersch, E. , Mirabel, M. , Karras, A. , Le Guen, J. , Blanchard, A. , Azizi, M. , Amar, L. , Bories, M.‐C. , Mousseaux, E. , Carette, C. , Puymirat, E. , Hagège, A. , Jannot, A.‐S. , and Hulot, J.‐S. (2022) Aetiological classification and prognosis in patients with heart failure with preserved ejection fraction. ESC Heart Failure, 9: 519–530. 10.1002/ehf2.13717.
References
- 1. van Riet EE, Hoes AW, Wagenaar KP, Limburg A, Landman MA, Rutten FH. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail 2016; 18: 242–252. [DOI] [PubMed] [Google Scholar]
- 2. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017; 14: 591–602. [DOI] [PubMed] [Google Scholar]
- 3. Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite‐Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007; 28: 2539–2550. [DOI] [PubMed] [Google Scholar]
- 4. Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske‐Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P, Filippatos G. How to diagnose heart failure with preserved ejection fraction: the HFA–PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019; 40: 3297–3317. [DOI] [PubMed] [Google Scholar]
- 5. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62: 263–271. [DOI] [PubMed] [Google Scholar]
- 6. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 7. Bozkurt B, Hershberger RE, Butler J, Grady KL, Heidenreich PA, Isler ML, Kirklin JK, Weintraub WS. 2021 ACC/AHA key data elements and definitions for heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Clinical Data Standards for Heart Failure). J Am Coll Cardiol 2021; 77: 2053–2150. [DOI] [PubMed] [Google Scholar]
- 8. Frisk M, Le C, Shen X, Røe ÅT, Hou Y, Manfra O, Silva GJJ, van Hout I, Norden ES, Aronsen JM, Laasmaa M, Espe EKS, Zouein FA, Lambert RR, Dahl CP, Sjaastad I, Lunde IG, Coffey S, Cataliotti A, Gullestad L, Tønnessen T, Jones PP, Altara R, Louch WE. Etiology‐dependent impairment of diastolic cardiomyocyte calcium homeostasis in heart failure with preserved ejection fraction. J Am Coll Cardiol 2021; 77: 405–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hulot JS, Livrozet M. HFpEF: should we consider diabetic patients separately?: the cardiomyocytes say yes. J Am Coll Cardiol 2021; 77: 420–422. [DOI] [PubMed] [Google Scholar]
- 10. Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 2015; 131: 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen JB, Schrauben SJ, Zhao L, Basso MD, Cvijic ME, Li Z, Yarde M, Wang Z, Bhattacharya PT, Chirinos DA, Prenner S, Zamani P, Seiffert DA, Car BD, Gordon DA, Margulies K, Cappola T, Chirinos JA. Clinical phenogroups in heart failure with preserved ejection fraction: detailed phenotypes, prognosis, and response to spironolactone. JACC Heart Fail 2020; 8: 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gevaert AB, Tibebu S, Mamas MA, Ravindra NG, Lee SF, Ahmad T, Ko DT, Januzzi JL Jr, van Spall HGC. Clinical phenogroups are more effective than left ventricular ejection fraction categories in stratifying heart failure outcomes. ESC Heart Fail 2021; 8: 2741–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Borlaug BA. Heart failure with preserved and reduced ejection fraction: different risk profiles for different diseases. Eur Heart J 2013; 34: 1393–1395. [DOI] [PubMed] [Google Scholar]
- 14. Jannot AS, Zapletal E, Avillach P, Mamzer MF, Burgun A, Degoulet P. The Georges Pompidou University Hospital Clinical Data Warehouse: a 8‐years follow‐up experience. Int J Med Inform 2017; 102: 21–28. [DOI] [PubMed] [Google Scholar]
- 15. Rousseeuw PJ. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. Journal of computational and applied mathematics 1987; 20: 53–65. [Google Scholar]
- 16. Charrad M, Ghazzali N, Boiteau V, Niknafs A. NbClust: an R package for determining the relevant number of clusters in a data set. J Stat Softw 2014; 61: 1–36. [Google Scholar]
- 17. Tsutsui H, Isobe M, Ito H, Ito H, Okumura K, Ono M, Kitakaze M, Kinugawa K, Kihara Y, Goto Y, Komuro I, Saiki Y, Saito Y, Sakata Y, Sato N, Sawa Y, Shiose A, Shimizu W, Shimokawa H, Seino Y, Node K, Higo T, Hirayama A, Makaya M, Masuyama T, Murohara T, Momomura SI, Yano M, Yamazaki K, Yamamoto K, Yoshikawa T, Yoshimura M, Akiyama M, Anzai T, Ishihara S, Inomata T, Imamura T, Iwasaki YK, Ohtani T, Onishi K, Kasai T, Kato M, Kawai M, Kinugasa Y, Kinugawa S, Kuratani T, Kobayashi S, Sakata Y, Tanaka A, Toda K, Noda T, Nochioka K, Hatano M, Hidaka T, Fujino T, Makita S, Yamaguchi O, Ikeda U, Kimura T, Kohsaka S, Kosuge M, Yamagishi M, Yamashina A, Japanese Circulation Society and the Japanese Heart Failure Society Joint Working Group . JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure—digest version. Circ J 2019; 83: 2084–2184. [DOI] [PubMed] [Google Scholar]
- 18. Uijl A, Savarese G, Vaartjes I, Dahlström U, Brugts JJ, Linssen GCM, Empel V, Brunner‐la Rocca HP, Asselbergs FW, Lund LH, Hoes AW, Koudstaal S. Identification of distinct phenotypic clusters in heart failure with preserved ejection fraction. Eur J Heart Fail 2021; 23: 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rush CJ, Berry C, Oldroyd KG, Rocchiccioli JP, Lindsay MM, Touyz RM, Murphy CL, Ford TJ, Sidik N, McEntegart MB, Lang NN, Jhund PS, Campbell RT, McMurray JJV, Petrie MC. Prevalence of coronary artery disease and coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. JAMA Cardiol 2021; 6: 1130–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rahimi G, Tecson KM, Elsaid O, McCullough PA. Role of ischemic heart disease in major adverse renal and cardiac events among individuals with heart failure with preserved ejection fraction (from the TOPCAT trial). Am J Cardiol 2021; 142: 91–96. [DOI] [PubMed] [Google Scholar]
- 21. Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan RS, Beussink‐Nelson L, Ljung Faxén U, Fermer ML, Broberg MA, Gan LM, Lund LH. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS‐HFpEF. Eur Heart J 2018; 39: 3439–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Devesa A, Camblor Blasco A, Pello Lázaro AM, Askari E, Lapeña G, Gómez Talavera S, Taibo Urquía M, Rodríguez Olleros C, Tuñón J, Ibáñez B, Aceña Á. Prevalence of transthyretin amyloidosis in patients with heart failure and no left ventricular hypertrophy. ESC Heart Fail 2021; 8: 2856–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hahn VS, Yanek LR, Vaishnav J, Ying W, Vaidya D, Lee YZJ, Riley SJ, Subramanya V, Brown EE, Hopkins CD, Ononogbu S, Perzel Mandell K, Halushka MK, Steenbergen C Jr, Rosenberg AZ, Tedford RJ, Judge DP, Shah SJ, Russell SD, Kass DA, Sharma K. Endomyocardial biopsy characterization of heart failure with preserved ejection fraction and prevalence of cardiac amyloidosis. JACC Heart Fail 2020; 8: 712–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Packer M, Lam CSP, Lund LH, Maurer MS, Borlaug BA. Characterization of the inflammatory‐metabolic phenotype of heart failure with a preserved ejection fraction: a hypothesis to explain influence of sex on the evolution and potential treatment of the disease. Eur J Heart Fail 2020; 22: 1551–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sabbah MS, Fayyaz AU, de Denus S, Felker GM, Borlaug BA, Dasari S, Carter RE, Redfield MM. Obese‐inflammatory phenotypes in heart failure with preserved ejection fraction. Circ Heart Fail 2020; 13: e006414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tribouilloy C, Rusinaru D, Mahjoub H, Souliere V, Levy F, Peltier M, Slama M, Massy Z. Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population‐based study. Eur Heart J 2008; 29: 339–347. [DOI] [PubMed] [Google Scholar]
- 27. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 28. Triposkiadis F, Butler J, Abboud FM, Armstrong PW, Adamopoulos S, Atherton JJ, Backs J, Bauersachs J, Burkhoff D, Bonow RO, Chopra VK, de Boer RA, de Windt L, Hamdani N, Hasenfuss G, Heymans S, Hulot JS, Konstam M, Lee RT, Linke WA, Lunde IG, Lyon AR, Maack C, Mann DL, Mebazaa A, Mentz RJ, Nihoyannopoulos P, Papp Z, Parissis J, Pedrazzini T, Rosano G, Rouleau J, Seferovic PM, Shah AM, Starling RC, Tocchetti CG, Trochu JN, Thum T, Zannad F, Brutsaert DL, Segers VF, de Keulenaer GW. The continuous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J 2019; 40: 2155–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wolsk E, Claggett B, Køber L, Pocock S, Yusuf S, Swedberg K, McMurray JJV, Granger CB, Pfeffer MA, Solomon SD. Contribution of cardiac and extra‐cardiac disease burden to risk of cardiovascular outcomes varies by ejection fraction in heart failure. Eur J Heart Fail 2018; 20: 504–510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of ICD‐10 Codes.
Table S2. Details about the underlying causes identified in patients with HF with preserved ejection fraction.
Table S3. Baseline characteristics of HFpEF patients according to the underlying aetiology.
Figure S1. Distribution of age in HFrEF (green), idiopathic HFpEF (red) and main etiologies found in secondary HFpEF patients (related to myocardial abnormalities in dark red; related to loading conditions abnormalities in blue).
Figure S2. Cumulative curves for all‐cause mortality in patients with secondary HFpEF according to the underlying aetiology.


