Abstract
Aims
Although diabetes mellitus (DM) is a common co‐morbidity in chronic heart failure (HF) patients, European data on concurrent HF and DM treatment are lacking. Therefore, we have studied the HF treatment of patients with and without DM. Additionally, with the recent breakthrough of sodium–glucose cotransporter 2 (SGLT2) inhibitors in the field of HF, we studied the potential impact of this new drug in a large cohort of HF patients.
Methods and results
A total of 7488 patients with chronic HF with a left ventricular ejection fraction <50% from 34 Dutch outpatient HF clinics between 2013 and 2016 were analysed on diabetic status and background HF therapy. Average age of the total population was 72.8 years (±11.7 years), and 64% of the patients were male. Diabetes was present in 29% of the patients (N = 2174). Diabetics had a worse renal function (mean estimated glomerular filtration rate 56 vs. 61 mL/min/1.73 m2, P < 0.001). Renin–angiotensin system inhibitors were less often prescribed in diabetics compared with non‐diabetics (79% vs. 82%, P = 0.001), while no significant differences regarding other guideline‐recommended HF drugs were found. Target doses of beta‐blockers (23% vs. 16%, P < 0.001), renin–angiotensin system inhibitors (47% vs. 43%, P = 0.009), and mineralocorticoid receptor antagonists (57% vs. 51%, P = 0.005) were more often prescribed in diabetics than non‐diabetics. Based on the latest trials on SGLT2 inhibitors, 31–64% of all HF patients would fulfil the eligibility or enrichment criteria (with vs. without N‐terminal prohormone BNP criterion).
Conclusions
In this large real‐world HF registry, a high prevalence of DM was observed and diabetics more often received guideline‐recommended target doses. Based on current evidence, the majority of patients would fulfil the enrichment criteria of SGLT2 trials in HF and the impact of this new drug class will be large.
Keywords: Heart failure, Diabetes mellitus, Guideline adherence
Introduction
Diabetes mellitus (DM) is a common co‐morbidity in patients suffering from heart failure (HF) and is associated with increased hospitalization and mortality rates in chronic HF. 1 , 2 Despite DM being a well‐established risk factor for worse outcome in HF, guideline‐directed medical therapy does not specifically target the subgroup of patients who also suffer from DM. This might be because the effects of DM therapy on cardiovascular events are not fully clear yet. 3 Recently, the American CHAMP‐HF investigators aimed to characterize treatment patterns and outcomes of patients with HF with reduced ejection fraction (HFrEF) and co‐morbid DM in a real‐world outpatient setting. Besides slight differences in prescription rates and doses, higher HF hospitalization and mortality rates among patients with DM were found. 4 Unfortunately, there is paucity of data when it comes to DM and concurrent HF treatment for the Western European setting. The Dutch CHECK‐HF registry studied in detail prescription rates and dosages of HF medication among subgroups of HF patients in an outpatient setting. 5 , 6
This CHECK‐HF subanalysis aimed to study differences in HF treatment between diabetics and non‐diabetics in a Western European country and compares these findings to the recent analysis in the USA (CHAMP‐HF). Furthermore, the percentage of patients that would be eligible for treatment with sodium–glucose cotransporter 2 (SGLT2) inhibitors was investigated based on trial criteria from the recently completed DAPA‐HF and EMPEROR‐Reduced clinical trials, which could have major impact in upcoming years. 2 , 7
Methods
The design and methods of the CHECK‐HF registry have been published in detail elsewhere. 8 Briefly, a total of 10 910 chronic HF patients from 34 participating Dutch centres between 2013 and 2016 were included in this cross‐sectional observational cohort. All included patients were diagnosed with HF and treated according to the 2012 European Society of Cardiology (ESC) HF guidelines, and almost all were seen at a dedicated outpatient HF clinic (96%). 9 Detailed information on patient characteristics, echocardiographic values, and guideline‐recommended HF drug prescription and dosages was recorded. The study was conducted according to the Declaration of Helsinki. Ethical approval was provided for anonymously analysing existing patient data by the Ethical Committee of the Maastricht University Medical Center, the Netherlands.
Patients were divided based on left ventricular (LV) ejection fraction (LVEF) or visual assessment of the left ventricle into HF with an LVEF <50% (N = 8360) or HF with an LVEF ≥50% (N = 2267) and treated according to the 2012 ESC HF guidelines. In 283 patients, recording of the LV function in the database was insufficient to classify patients into HF type or standard baseline demographic data were missing, and they were excluded from this analysis as well as patients with an LVEF ≥50%. Additionally, patients with missing information on diabetes (N = 872) were excluded from this analysis, and a total of 7488 patients were included in this analysis. All patients were divided into those with and without diabetes, based on patient records and medical history. Distinction between type 1 and 2 diabetes was made, but the CRF contained no information on antidiabetic therapy.
In a subanalysis, we investigated the treatment differences according to renal function. Additionally, we compared the treatment differences between the Western European and the American CHAMP‐HF population. 4
In order to provide a detailed insight in the reduced LVEF population according to the 2016 ESC HF guidelines, an additional subanalysis was performed. 3 Patients with a reduced LVEF (LVEF <50%) and known diabetic status were categorized into HF with mid‐range ejection fraction (HFmrEF) {LVEF 40–49% [n = 1417 (18.9%)]} and HFrEF {LVEF<40% [n = 5073 (67.7%)]}, only in those patients with an exactly specified LVEF. Patients without exact ejection fraction, though visually reduced LV function, were presented separately as semi‐quantitative patient group [n = 998 (13.3%)].
Indication for sodium–glucose cotransporter‐2 inhibitors
Based on the inclusion and exclusion criteria of the DAPA‐HF and EMPEROR‐Reduced trials, we investigated what percentage of patients would be eligible for an SGLT2 inhibitor. An overview of the used criteria is shown in Supporting Information, Table S1 . 2 , 10
Statistical analysis
Continuous data are expressed as mean value ± standard deviation or median and inter‐quartile range, depending on the distribution of the data, and compared by the one‐way analysis of variance or Mann–Whitney U test. Categorical data are expressed as counts and percentages, and compared by the Pearson χ 2 test.
In order to investigate whether the observed differences between patients with and without diabetes were independent of potential clinical predictors, univariable and multivariable logistic regression analyses were used. The results of these regression analyses are expressed as odds ratios (ORs) with 95% confidence intervals (CIs). In Model 1, we adjusted for age and sex only. In Model 2, we further adjusted for New York Heart Association (NYHA) classification and LVEF. At last, in Model 3, we further adjusted for age, gender, NYHA classification, LVEF, hypertension, chronic obstructive pulmonary disease, obstructive sleep apnoea syndrome, thyroid disease, renal insufficiency [defined as estimated glomerular filtration rate (eGFR) <60 mL/min or a history of renal insufficiency], and atrial fibrillation. Missing data occurred in the variables included in the multivariable analysis, which were imputed using multiple imputation as has been described previously. 11 All analyses were performed with SPSS Statistical Package Version 25.0 (SPSS Inc., Chicago, IL). A two‐sided P‐value of <0.05 was considered statistically significant.
Results
In total, 2174 (29%) diabetic and 5314 (71%) non‐diabetic patients were included in this analysis, and their baseline characteristics are shown in Table 1 . In brief, diabetics were significantly more often severely symptomatic in NYHA Class III–IV, more often had an ischaemic aetiology, and suffered from higher rates of co‐morbidities such as hypertension, obstructive sleep apnoea syndrome, and renal insufficiency.
Table 1.
Baseline characteristics of HF patients with an LVEF < 50%
| Total population (N = 7488) | Diabetes (N = 2174) | No diabetes (N = 5314) | P‐value | |
|---|---|---|---|---|
| Age (years) (N = 7480) | 72.8 ± 11.7 | 72.9 ± 10.3 | 72.2 ± 12.2 | 0.022 |
| Men (N = 7459) | 4756 (63.8) | 1380 (63.6) | 3376 (63.8) | 0.874 |
| BMI, kg/m2 (N = 6980) | 27.3 ± 5.2 | 29.0 ± 5.6 | 26.5 ± 4.8 | <0.001 |
| NYHA (N = 7416) | ||||
| I | 1200 (16.2) | 255 (11.9) | 945 (17.9) | <0.001 |
| II | 4181 (56.4) | 1154 (53.7) | 3027 (57.5) | |
| III | 1893 (25.5) | 681 (31.7) | 1212 (23.0) | |
| IV | 142 (1.9) | 60 (2.8) | 82 (1.6) | |
| LVEF, % (N = 5468) | 32.7 ± 10.4 | 33.3 ± 10.6 | 32.4 ± 10.4 | 0.004 |
| Cause of HF (N = 7360) | ||||
| Ischaemic | 3842 (52.2) | 1264 (59.1) | 2578 (49.4) | <0.001 |
| Non‐ischaemic | 3518 (47.8) | 874 (40.9) | 2644 (50.6) | |
| Systolic BP, mmHg (N = 7413) | 126.0 ± 20.8 | 126.4 ± 20.5 | 125.9 ± 21.0 | 0.363 |
| Diastolic BP, mmHg (N = 7419) | 71.3 ± 11.4 | 70.5 ± 11.4 | 71.6 ± 11.4 | <0.001 |
| Heart rate, b.p.m. (N = 7392) | 72.0 ± 13.9 | 73.0 ± 13.6 | 71.6 ± 14.0 | <0.001 |
| Atrial fibrillation (N = 7399) | 1902 (25.7) | 589 (27.4) | 1313 (25.0) | 0.031 |
| LBBB (N = 7488) | 1283 (17.1) | 350 (16.1) | 933 (17.6) | 0.129 |
| QRS ≥ 130 ms (N = 6337) | 2534 (40.0) | 738 (40.8) | 1796 (39.7) | 0.419 |
| NT‐proBNP, pg/mL (N = 2873) | 978.0 [311.0–2850.0] | 954.0 [323.0–2622.0] | 990.7 [304.1–2901.1] | 0.943 |
| Co‐morbidities (N = 7488) | ||||
| Hypertension | 2978 (39.8) | 1067 (49.1) | 1911 (36.0) | <0.001 |
| COPD | 1381 (18.4) | 401 (18.4) | 980 (18.4) | 0.997 |
| OSAS | 495 (6.6) | 198 (9.1) | 297 (5.6) | <0.001 |
| Thyroid disease | 557 (7.4) | 180 (8.3) | 377 (7.1) | 0.076 |
| Kidney insufficiency a | 3583 (57.2) | 1247 (64.9) | 2336 (53.8) | <0.001 |
BMI, body mass index; BP, blood pressure; COPD, chronic obstructive pulmonary disease; HF, heart failure; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal prohormone BNP; NYHA, New York Heart Association; OSAS, obstructive sleeping apnoea syndrome.
Defined as an estimated glomerular filtration rate <60 or a documented history of kidney insufficiency.
Pharmacological treatment
The pharmacological HF treatment of patients according to diabetic status is shown in Figure 1 . As shown, diabetic HF patients less often received renin–angiotensin system (RAS) inhibitors, but more often received the guideline‐recommended target dose of beta‐blockers, RAS inhibitors, and mineralocorticoid receptor antagonists (MRAs) and had triple therapy more often prescribed at ≥50% of the guideline‐recommended target dose, while no significant difference was observed in the prescription rate of triple therapy. Of all diabetic patients who had a RAS inhibitor prescribed, 64.2% and 35.8% received an angiotensin‐converting enzyme inhibitor and angiotensin receptor blocker, respectively, compared with 68.9% and 31.1% for the non‐diabetic patients, respectively.
Figure 1.
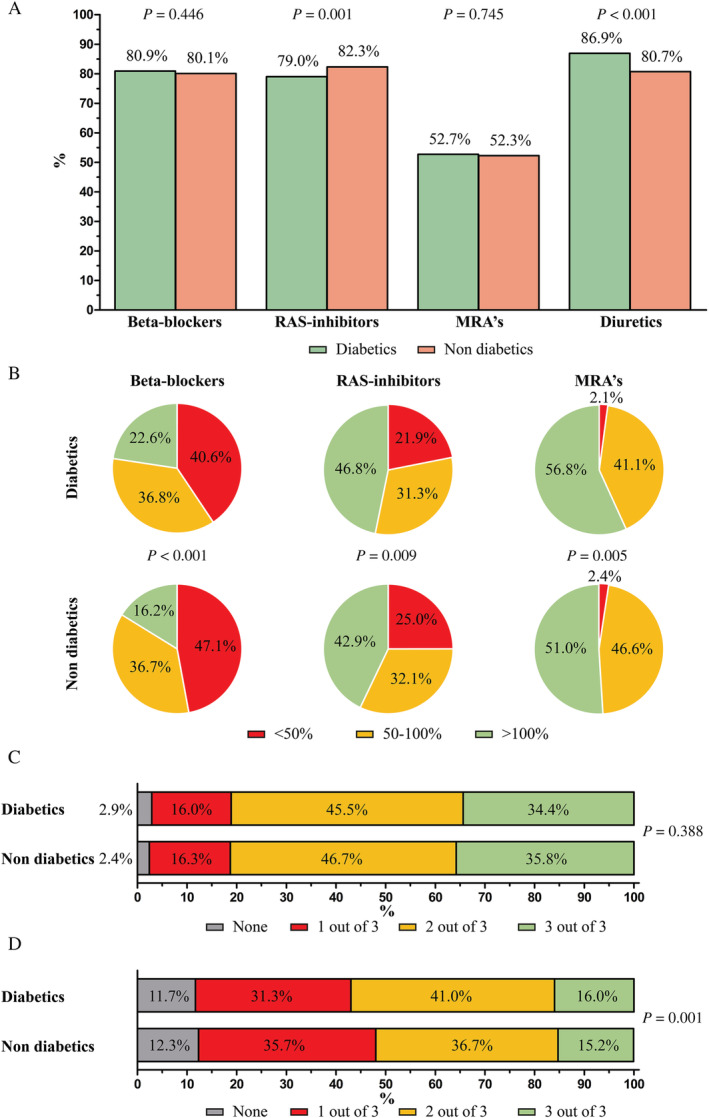
Guideline‐recommended heart failure therapy use according to diabetes in heart failure patients with a left ventricular ejection fraction <50%, shown as (A) prescription rates, (B) percentage of the recommended target dose prescribed, (C) prescription of triple therapy, and (D) prescription of triple therapy at ≥50% of guideline‐recommended target dose. MRAs, mineralocorticoid receptor antagonists; RAS, renin–angiotensin system.
After multivariable adjustments, diabetic HF patients had a lower likelihood to receive a RAS inhibitor (OR 0.853, 95% CI [0.747–0.975]), but a higher likelihood to receive diuretics compared with non‐diabetic patients (OR 1.284, 95% CI [1.103–1.495]), as shown in Table 2 .
Table 2.
Univariable and multivariable analysis: the likelihood (displayed as odds ratio [95% confidence interval]) of receiving guideline‐recommended therapy for diabetics compared with non‐diabetics
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||
| Beta‐blocker | 1.060 [0.932–1.205] | 1.065 [0.998–1.138] | 1.082 [0.950–1.231] | 1.042 [0.914–1.188] |
| RAS inhibitor | 0.804 [0.708–0.912] | 0.803 [0.752–0.857] | 0.848 [0.744–0.966] | 0.853 [0.747–0.975] |
| MRA | 1.007 [0.910–1.114] | 1.011 [0.960–1.065] | 0.965 [0.871–1.070] | 0.952 [0.857–1.058] |
| Diuretics | 1.564 [1.355–1.806] | 1.526 [1.417–1.643] | 1.393 [1.202–1.616] | 1.284 [1.103–1.495] |
COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; OSAS, obstructive sleep apnoea syndrome; RAS, renin–angiotensin system.
Model 1 included age and gender. Model 2 included age, gender, NYHA classification, and LVEF. Model 3 included age, gender, NYHA classification, LVEF, hypertension, COPD, OSAS, thyroid disease, renal insufficiency (defined as eGFR < 60 mL/min or a history of renal insufficiency), and atrial fibrillation.
Diabetes and renal function
As shown in Table 3 , diabetic HF patients had a worse renal function compared with non‐diabetic patients (mean eGFR 55.8 ± 22.6 vs. 60.8 ± 23.8 mL/min/1.73 m2, P<0.001). A detailed overview of HF therapy stratified by renal function in diabetic HF patients is shown in Supporting Information, Figure S1 . Most importantly, among all diabetics, patients with an eGFR <30 mL/min less often received RAS inhibitors and, when prescribed, less often received the recommended target dose. Furthermore, those patients with an eGFR <30 mL/min less often received triple therapy. The patients on triple therapy with an eGFR <30 mL/min less often received the recommended target dose.
Table 3.
Renal function of HF patients with an LVEF < 50%
| Total population (N = 7488) | Diabetes (N = 2174) | No diabetes (N = 5314) | P‐value | |
|---|---|---|---|---|
| eGFR, mL/min/1.73 m2 (N = 5169) | 59.3 ± 24.8 | 55.8 ± 26.6 | 60.8 ± 23.8 | <0.001 |
| eGFR (N = 5169) | ||||
| <30 mL/min/1.73 m2 | 613 (11.9) | 286 (17.6) | 327 (9.2) | <0.001 |
| 30–44 mL/min/1.73 m2 | 1065 (20.6) | 381 (23.4) | 684 (19.3) | |
| 45–59 mL/min/1.73 m2 | 1087 (21.0) | 302 (18.6) | 785 (22.2) | |
| ≥60 mL/min/1.73 m2 | 2404 (46.5) | 658 (40.4) | 1746 (49.3) | |
eGFR, estimated glomerular filtration rate; HF, heart failure; LVEF, left ventricular ejection fraction.
CHAMP‐HF (American) vs. CHECK‐HF (Western European)
Heart failure therapy in the American CHAMP‐HF registry and the CHECK‐HF registry according to diabetic status is shown in Figure 2 . In general, both diabetic and non‐diabetic patients in the CHECK‐HF registry more often received RAS inhibitors and MRAs, and more often received RAS inhibitors at the recommended target dose.
Figure 2.
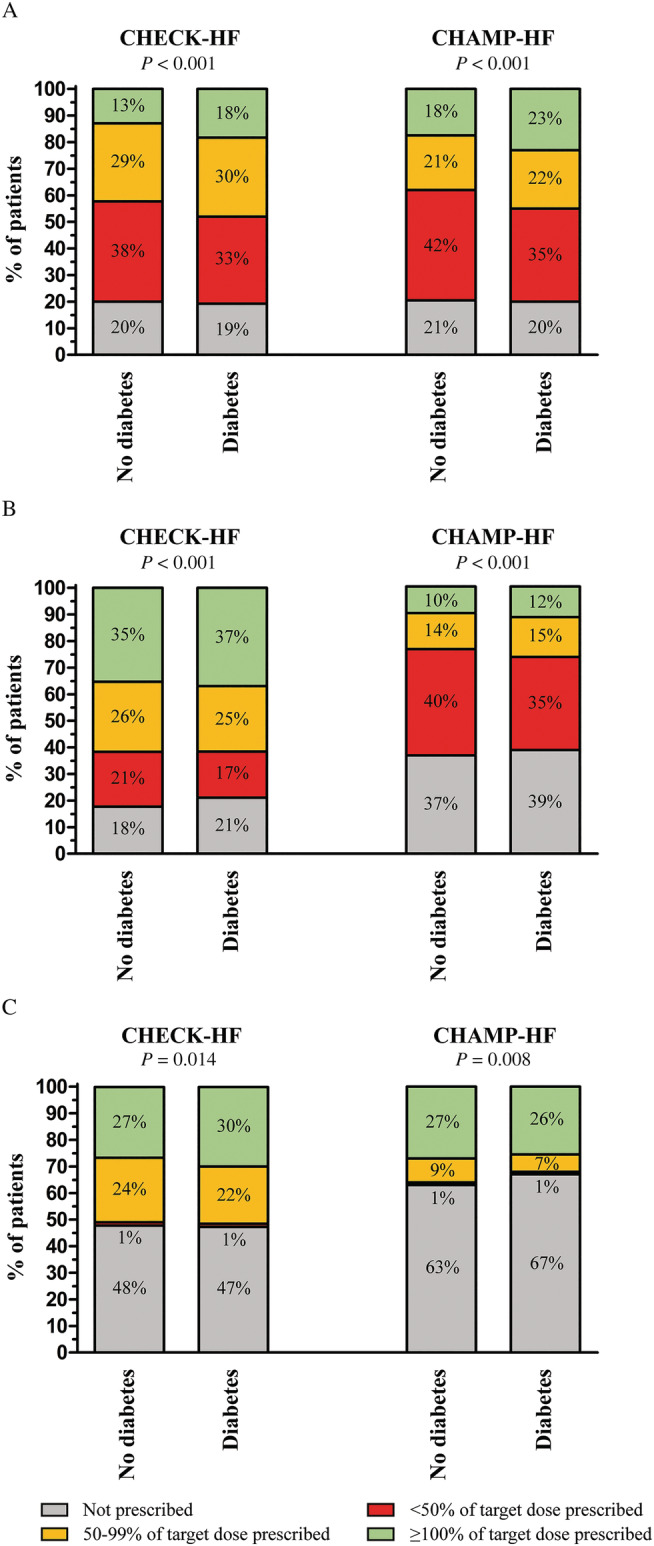
Guideline‐recommended heart failure therapy use: (A) beta‐blockers, (B) renin–angiotensin system inhibitors, and (C) mineralocorticoid receptor antagonists, in the CHAMP‐HF and CHECK‐HF registries according to diabetes in patients with a reduced left ventricular ejection fraction. Data from the CHAMP‐HF registry were used with permission.
Medical therapy in heart failure with reduced ejection fraction and heart failure with mid‐range ejection fraction patients according to the 2016 European Society of Cardiology guidelines
The baseline characteristics of HFrEF, HFmrEF, and the subgroup of semi‐quantitative LV function patients are shown in Supporting Information, Table S2 . The prescription rates of HF medication in these subgroups are shown in Supporting Information, Figure S2 . As shown, diabetic HFrEF patients less often received RAS inhibitors, and diabetic patients with HFrEF, HFmrEF, and semi‐quantitative LV function more often received diuretics.
Potential impact of sodium–glucose cotransporter‐2 inhibitors in a real‐world heart failure population
The patients with complete data on age, NYHA class, LVEF, serum N‐terminal prohormone BNP (NT‐proBNP) levels, systolic blood pressure, and eGFR (31.7% of the total population) were analysed based on inclusion and exclusion criteria of several large trials (Supporting Information, Table S1 ) to assess the percentage of patients potentially eligible for treatment with SGLT2 inhibitors. As shown in Table 4 , up to 31% of the patients with an LVEF ≤40% would fulfil trial criteria for treatment with an SGLT2 inhibitor. The proportion of eligible patients was similar for diabetics and non‐diabetics. Detailed reasons for being ineligible are shown in Supporting Information, Figure S3 , and as seen, ineligibility was largely caused by violation of the NT‐proBNP criterion. Analyses were therefore repeated without applying NT‐proBNP serum levels as enrichment criteria, and as such, up to 64% of the CHECK‐HF population with complete available data would fulfil trial criteria (Supporting Information, Figure S3 ).
Table 4.
Indication for SGLT2 inhibitors according to the eligibility criteria of the two major HF SGLT2 inhibitor trials
| Total CHECK‐HF population | Diabetics | Non‐diabetics | |
|---|---|---|---|
| DAPA‐HF trial a | |||
| Eligible | 742 (31.3) | 184 (25.4) | 558 (33.8) |
| Not eligible | 1632 (68.7) | 539 (74.6) | 1093 (66.2) |
| EMPEROR‐Reduced trial a | |||
| Eligible | 571 (24.1) | 175 (24.2) | 396 (24.0) |
| Not eligible | 1801 (75.9) | 547 (75.8) | 1254 (76.0) |
HF, heart failure; SGLT2, sodium–glucose cotransporter 2.
Eligibility criteria are shown in Supporting Information, Table S2 .
Discussion
In this large cross‐sectional Dutch registry of chronic HF patients, diabetes was prevalent in ~30% of patients. Renal function is very relevant in this patient category. The number of patients with eGFR <45 mL/min/1.73 m2 was 41% in diabetic and 29% in non‐diabetic HF patients. Regarding HF treatment, only slight differences in prescription rates and doses were found between diabetics and non‐diabetics. Especially diuretics were more often prescribed in diabetics compared with non‐diabetics. This could be relevant for the mechanisms of action of SGLT2 inhibitors (natriuresis). Diabetic HF patients more often received the guideline‐recommended target dose of beta‐blockers, RAS inhibitors, and MRAs as compared with non‐diabetic patients.
Based on current evidence, up to 64% of all HF patients with an LVEF ≤40% would fulfil the eligibility or enrichment criteria of the recent SGLT2 inhibitor trials.
Heart failure treatment in diabetic patients
The effectivity of HF drugs in diabetics and non‐diabetics has been investigated in several studies. The relative risk reduction for mortality by beta‐blockers, RAS inhibitors, and MRAs was comparable between diabetic and non‐diabetic HF patients. 12 , 13 , 14 , 15 , 16 Diabetic HF patients have a higher mortality risk, and therefore, HF drugs have a greater absolute risk reduction in this patient group. 12 , 13 Additionally, HF drugs might have favourable effects on the glycaemic control as well. It has been shown that beta‐blockers with alpha‐blockade properties (such as carvedilol) might improve insulin sensitivity and glycaemic control. 17 , 18 , 19 RAS inhibitors might reduce the incidence of DM in HF patients, although data on the effect of RAS inhibitors on glycaemic control in HF patients with pre‐existent DM are lacking. 20 , 21 MRAs do not increase the risk of developing DM. 22 It has been suggested that spironolactone increases haemoglobin A1(c) serum levels in DM patients, while eplerenone does not. 23 The overall positive effects of HF drugs on diabetic treatment and the lack of negative effects of DM on the effectivity of HF drugs might have contributed to the higher prescription rates observed in our registry. Chronic HF and DM are independently associated with a worse renal function, and the presence of both conditions further increases the risk of worse renal function, as also shown by our results. 24 , 25 Both antidiabetic and HF drugs are associated with a decline in renal function. 26 This could prevent prescription and limit up‐titration of the recommended drugs. Additionally, impaired renal function increases the risk of hyperkalaemia, especially if RAS inhibitors are combined with an MRA. 27 This could lead to lower prescription rates of the combination of RAS inhibitors and MRAs in diabetic HF patients.
Diabetes and heart failure treatment in CHECK‐HF compared with CHAMP‐HF
The American CHAMP‐HF study recently explored differences in treatment patterns and clinical outcomes between diabetic and non‐diabetic HFrEF patients in a real‐world US outpatient setting. 4 Remarkably, diabetes was more often present in the US HF population (42%) compared with Dutch HF patients (29%). This observation might be explained by the overall higher body mass index in the CHAMP‐HF compared with the CHECK‐HF registry (29.3 vs. 27.3 kg/m2, respectively). A clear association between an elevated body mass index and incidence of DM in chronic HF has been described. 28 Small differences in HF treatment between diabetics and non‐diabetics were observed in both HF registries with only a minority of HF patients receiving the guideline‐recommended target doses. However, slight differences between the CHECK‐HF and CHAMP‐HF registries were observed. Dutch HF patients more often received RAS inhibitors and MRAs. Furthermore, RAS inhibitors were more often prescribed at the recommended target dose compared with the US HF patients. When comparing baseline patient characteristics of both registries, several differences become apparent. Firstly, average renal function was worse in the CHECK‐HF registry with a higher proportion of patients having an eGFR <45. This might indicate that Dutch physicians are more likely to accept the decline in renal function introduced by RAS inhibitors and MRAs in order to optimize HF treatment. 26 Furthermore, average age was slightly higher in the CHECK‐HF population (mean age of 72.8 vs. 68 years in the CHAMP‐HF registry). Other important baseline characteristics reflecting disease severity such as distribution of NYHA class and LVEF were comparable between both registries.
Potential range of sodium–glucose cotransporter‐2 inhibitors in heart failure
Although SGLT2 inhibitors were initially developed as antidiabetic drugs, the secondary cardiovascular effects became especially clear as patients without DM surprisingly benefited from SGLT2 inhibitors as well. 29 The efficacy of SGLT2 inhibitors on top of the guideline‐recommended HF drugs in patients with an LVEF <40% has been evaluated by the DAPA‐HF and EMPEROR‐Reduced trials. 2 , 7 However, the percentage of HF patients eligible for SGLT2 inhibitor therapy is still unknown. When applying the inclusion and exclusion criteria of the DAPA‐HF and EMPEROR‐Reduced trials, we calculated that up to 31% of the Dutch HF patients with an LVEF ≤40% could be eligible for treatment with an SGLT2 inhibitor. Ineligibility was largely caused by violation of the NT‐proBNP criterion (Supporting Information, Figure S3 ). However, serum NT‐proBNP levels are unlikely to affect implementation in routine HF care, and low NT‐proBNP levels may actually reflect adequate HF therapy rather than a factor advocating against use of SGLT2 inhibitors. After performing the analyses without strict NT‐proBNP criteria, the proportion of eligible patients increased up to 64% (Supporting Information, Figure S3 ). Similar analyses were performed after the early angiotensin receptor‐neprilysin inhibitor studies in which (NT‐pro)BNP criteria were used as well, while in clinical practice, angiotensin receptor neprilysin inhibitor therapy is nowadays initiated without fulfilling these criteria. 30 , 31 , 32
Recently, the 2021 ESC guidelines on HF have been published, and the SGLT2 inhibitors dapagliflozin and empagliflozin have received a Class IA indication for the treatment of patients with HF and an LVEF ≤40% to reduce the risk of HF hospitalization and death, regardless of the presence of concomitant DM. 33 This current large HF registry contributes to recent developments in pharmacological HF therapy and may help to learn about the initiation of SGLT2 inhibitors in Western European countries. In light of the positive DAPA‐HF and EMPEROR‐Reduced trials, we have shown the potentially wide scope of SGLT2 inhibitors in our chronic HF population. 2 , 7 According to the latest ESC guidelines on HF, type 1 diabetes is not an absolute contraindication for the initiation of SGLT2 inhibitors, which may imply that a larger proportion of our HF population would be eligible according to the enrolment criteria of the DAPA‐HF trial (Supporting Information, Figure S3 ). Unfortunately, information on concomitant use of other antidiabetic drugs was unavailable in our study. When interpreting our data, it is therefore essential to keep in mind the potential hazard of concurrent diabetic treatment before starting an SGLT2 inhibitor. Although diabetic ketoacidosis was rare in clinical trial setting with just three cases in the DAPA‐HF trial and zero cases in the EMPEROR‐Reduced trial, it is among the most serious complications, especially with concomitant use of insulin. 2 , 7 The risk of developing ketoacidosis has to be taken into account, and it is recommended to eliminate factors that increase the risk of ketoacidosis. In the case of hypoglycaemia, modification of other diabetic drugs is indicated with consultation of nurses and doctors specialized in diabetes care. 33
Sodium–glucose cotransporter‐2 inhibitors are also likely to play an important role in the treatment of HF patients with an LVEF >40% after the positive results of the recently published EMPEROR‐Preserved trial in which empagliflozin has been shown to significantly reduce the risk of cardiovascular death or HF hospitalization as compared with placebo, again regardless of diabetic status. 34 Additional analyses of the trial showed that SGLT2 inhibition by empagliflozin also reduced the risk of severe hospitalizations (such as admissions requiring intravenous positive inotropic or vasopressor drugs and/or intensive care) and outpatient worsening HF events (including emergency or urgent care visits and intensification of diuretic therapy). 35 Currently, SGLT2 inhibitors are not yet incorporated in the ESC guidelines for the treatment of HF with preserved ejection fraction, but recommendations will most likely be updated as new evidence is emerging. 34 , 35
Strengths and limitations
The CHECK‐HF registry encompasses a large number of HF patients in a real‐world Western European outpatient setting and thus provides valuable insights into the characteristics and treatment patterns in this population. However, this registry has some limitations. First of all, because of the cross‐sectional design, data on clinical outcomes and prognosis are unavailable. Furthermore, no data on insulin use or oral antidiabetic use were available as this subanalysis was not planned when designing the study.
Conclusions
In this large registry of HF patients with an LVEF <50% and concurrent DM, patients with diabetes were generally well treated and more often received the guideline‐recommended target doses as compared with patients without diabetes. Based on current evidence, we have shown that a considerable proportion, up to 64%, of our HF population fulfils clinical trial criteria for treatment with an SGLT2 inhibitor, and adding this new drug class will have a major impact on contemporary HF treatment.
Conflict of interest
None declared.
Funding
This work was supported by Servier, the Netherlands, who funded the inclusion of data and software program. The steering committee (H.‐P.B.‐L.R., G.C.M.L., and J.J.B.) received no funding for this project. This analysis was initiated by the authors and was designed, conducted, interpreted, and reported independently of the sponsor. The current study had no other funding source or any with a participating role in outcome assessment or writing of the manuscript.
Supporting information
Table S1. Eligibility criteria for the different SGLT2 inhibitor HF trials.
Table S2. Baseline characteristics of HF patients according to 2016 ESC HF Guidelines.
Figure S1. Guideline‐recommended heart failure therapy use in diabetic HF patients with an LVEF <50%, shown as A prescription rates, B percentage of the recommended target dose prescribed, C prescription of triple therapy and, D prescription of triple therapy at ≥50% of guideline recommended target dose, stratified according to renal function.
Figure S2. Guideline‐recommended heart failure therapy use according to the 2016 ESC HF Guidelines as stratified by diabetic status, A in patients with a reduced ejection fraction. B in patients with midrange ejection fraction, and C in patients with a semi quantitative analysis.
Figure S3. Flowchart of eligibility for SGLT2 inhibitors according to the eligibility criteria of the A DAPA‐HF trial and B the EMPEROR‐Reduced trial.
Radhoe, S. P. , Veenis, J. F. , Linssen, G. C. M. , van der Lee, C. , Eurlings, L. W. M. , Kragten, H. , Al‐Windy, N. Y. Y. , van der Spank, A. , Koudstaal, S. , Brunner‐La Rocca, H.‐P. , and Brugts, J. J. (2022) Diabetes and treatment of chronic heart failure in a large real‐world heart failure population. ESC Heart Failure, 9: 353–362. 10.1002/ehf2.13743.
References
- 1. Bozkurt B, Aguilar D, Deswal A, Dunbar SB, Francis GS, Horwich T, Jessup M, Kosiborod M, Pritchett AM, Ramasubbu K, Rosendorff C, Yancy C, American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology , Council on Cardiovascular Surgery and Anesthesia , Council on Cardiovascular and Stroke Nursing , Council on Hypertension , Council on Quality and Outcomes Research . Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the American Heart Association. Circulation 2016; 134: e535–e578. [DOI] [PubMed] [Google Scholar]
- 2. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM, DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 4. Vaduganathan M, Fonarow GC, Greene SJ, DeVore AD, Kavati A, Sikirica S, Albert NM, Duffy CI, Hill CL, Patterson JH, Spertus JA, Thomas LE, Williams FB, Hernandez AF, Butler J. Contemporary treatment patterns and clinical outcomes of comorbid diabetes mellitus and HFrEF: the CHAMP‐HF registry. JACC Heart Fail 2020; 8: 469–480. [DOI] [PubMed] [Google Scholar]
- 5. Brunner‐La Rocca HP, Linssen GC, Smeele FJ, van Drimmelen AA, Schaafsma HJ, Westendorp PH, Rademaker PC, van de Kamp HJ, Hoes AW, Brugts JJ, CHECK‐HF Investigators . Contemporary drug treatment of chronic heart failure with reduced ejection fraction: the CHECK‐HF registry. JACC Heart Fail 2019; 7: 13–21. [DOI] [PubMed] [Google Scholar]
- 6. Veenis JF, Brunner‐La Rocca HP, Linssen GCM, Van Gent MWF, Hoes AW, Brugts JJ, Investigators C‐H. Treatment differences in chronic heart failure patients with reduced ejection fraction according to blood pressure. Circ Heart Fail 2020; 13: e006667. [DOI] [PubMed] [Google Scholar]
- 7. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F, EMPEROR‐Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 383: 1413–1424. [DOI] [PubMed] [Google Scholar]
- 8. Brugts JJ, Linssen GCM, Hoes AW, Brunner‐La Rocca HP, CHECK‐HF investigators . Real‐world heart failure management in 10,910 patients with chronic heart failure in the Netherlands: design and rationale of the Chronic Heart failure ESC guideline‐based Cardiology practice Quality project (CHECK‐HF) registry. Neth Heart J 2018; 26: 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, ESC Committee for Practice Guidelines . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 10. Packer M, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, Anker SD, Zannad F, EMPEROR‐Reduced Trial Committees and Investigators . Evaluation of the effect of sodium–glucose co‐transporter 2 inhibition with empagliflozin on morbidity and mortality of patients with chronic heart failure and a reduced ejection fraction: rationale for and design of the EMPEROR‐Reduced trial. Eur J Heart Fail 2019; 21: 1270–1278. [DOI] [PubMed] [Google Scholar]
- 11. Veenis JF, Brunner‐La Rocca HP, Linssen GC, Geerlings PR, Van Gent MW, Aksoy I, Oosterom L, Moons AH, Hoes AW, Brugts JJ, CHECK‐HF investigators . Age differences in contemporary treatment of patients with chronic heart failure and reduced ejection fraction. Eur J Prev Cardiol 2019; 26: 1399–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bell DS, Lukas MA, Holdbrook FK, Fowler MB. The effect of carvedilol on mortality risk in heart failure patients with diabetes: results of a meta‐analysis. Curr Med Res Opin 2006; 22: 287–296. [DOI] [PubMed] [Google Scholar]
- 13. Deedwania PC, Giles TD, Klibaner M, Ghali JK, Herlitz J, Hildebrandt P, Kjekshus J, Spinar J, Vitovec J, Stanbrook H, Wikstrand J, MERIT‐HF Study Group . Efficacy, safety and tolerability of metoprolol CR/XL in patients with diabetes and chronic heart failure: experiences from MERIT‐HF. Am Heart J 2005; 149: 159–167. [DOI] [PubMed] [Google Scholar]
- 14. Eschalier R, McMurray JJ, Swedberg K, van Veldhuisen DJ, Krum H, Pocock SJ, Shi H, Vincent J, Rossignol P, Zannad F, Pitt B, EMPHASIS‐HF Investigators . Safety and efficacy of eplerenone in patients at high risk for hyperkalemia and/or worsening renal function: analyses of the EMPHASIS‐HF study subgroups (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure). J Am Coll Cardiol 2013; 62: 1585–1593. [DOI] [PubMed] [Google Scholar]
- 15. MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, Solomon SD, Granger CB, Swedberg K, Yusuf S, Pfeffer MA, McMurray JJ, CHARM Investigators . Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J 2008; 29: 1377–1385. [DOI] [PubMed] [Google Scholar]
- 16. Shekelle PG, Rich MW, Morton SC, Atkinson CS, Tu W, Maglione M, Rhodes S, Barrett M, Fonarow GC, Greenberg B, Heidenreich PA, Knabel T, Konstam MA, Steimle A, Warner SL. Efficacy of angiotensin‐converting enzyme inhibitors and beta‐blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta‐analysis of major clinical trials. J Am Coll Cardiol 2003; 41: 1529–1538. [DOI] [PubMed] [Google Scholar]
- 17. Bakris GL, Fonseca V, Katholi RE, McGill JB, Messerli FH, Phillips RA, Raskin P, Wright JT Jr, Oakes R, Lukas MA, Anderson KM, Bell DS, GEMINI Investigators . Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA 2004; 292: 2227–2236. [DOI] [PubMed] [Google Scholar]
- 18. Kovacic D, Marinsek M, Gobec L, Lainscak M, Podbregar M. Effect of selective and non‐selective β‐blockers on body weight, insulin resistance and leptin concentration in chronic heart failure. Clin Res Cardiol 2008; 97: 24–31. [DOI] [PubMed] [Google Scholar]
- 19. Wai B, Kearney LG, Hare DL, Ord M, Burrell LM, Srivastava PM. Beta blocker use in subjects with type 2 diabetes mellitus and systolic heart failure does not worsen glycaemic control. Cardiovasc Diabetol 2012; 14: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vermes E, Ducharme A, Bourassa MG, Lessard M, White M, Tardif JC. Enalapril reduces the incidence of diabetes in patients with chronic heart failure: insight from the Studies Of Left Ventricular Dysfunction (SOLVD). Circulation 2003; 107: 1291–1296. [DOI] [PubMed] [Google Scholar]
- 21. Yusuf S, Ostergren JB, Gerstein HC, Pfeffer MA, Swedberg K, Granger CB, Olofsson B, Probstfield J, McMurray JV, Candesartan in Heart Failure—Assessment of Reduction in Mortality and Morbidity Program (CHARM) Investigators . Effects of candesartan on the development of a new diagnosis of diabetes mellitus in patients with heart failure. Circulation 2005; 112: 48–53. [DOI] [PubMed] [Google Scholar]
- 22. Preiss D, van Veldhuisen DJ, Sattar N, Krum H, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, Zannad F, McMurray JJ. Eplerenone and new‐onset diabetes in patients with mild heart failure: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS‐HF). Eur J Heart Fail 2012; 14: 909–915. [DOI] [PubMed] [Google Scholar]
- 23. Yamaji M, Tsutamoto T, Kawahara C, Nishiyama K, Yamamoto T, Fujii M, Horie M. Effect of eplerenone versus spironolactone on cortisol and hemoglobin A1c levels in patients with chronic heart failure. Am Heart J 2010; 160: 915–921. [DOI] [PubMed] [Google Scholar]
- 24. Ahmed A, Campbell RC. Epidemiology of chronic kidney disease in heart failure. Heart Fail Clin 2008; 4: 387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patel PA, Liang L, Khazanie P, Hammill BG, Fonarow GC, Yancy CW, Bhatt DL, Curtis LH, Hernandez AF. Antihyperglycemic medication use among Medicare beneficiaries with heart failure, diabetes mellitus, and chronic kidney disease. Circ Heart Fail 2016; 9: e002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J, ADHERE Scientific Advisory Committee and Investigators . High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail 2007; 13: 422–430. [DOI] [PubMed] [Google Scholar]
- 27. Michel A, Martín‐Pérez M, Ruigómez A, García Rodríguez LA. Risk factors for hyperkalaemia in a cohort of patients with newly diagnosed heart failure: a nested case–control study in UK general practice. Eur J Heart Fail 2015; 17: 205–213. [DOI] [PubMed] [Google Scholar]
- 28. Thomas MC. Type 2 diabetes and heart failure: challenges and solutions. Curr Cardiol Rev 2016; 12: 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause‐Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS, DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 30. Lainscak M, Coats AJ. The PARADIGM of ARNI's: assessing reasons for non‐implementation in heart failure. Int J Cardiol 2016; 212: 187–189. [DOI] [PubMed] [Google Scholar]
- 31. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Investigators and Committees . Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 32. Pellicori P, Urbinati A, Shah P, MacNamara A, Kazmi S, Dierckx R, Zhang J, Cleland JGF, Clark AL. What proportion of patients with chronic heart failure are eligible for sacubitril–valsartan? Eur J Heart Fail 2017; 19: 768–778. [DOI] [PubMed] [Google Scholar]
- 33. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 34. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, Brunner‐La Rocca HP, Choi DJ, Chopra V, Chuquiure‐Valenzuela E, Giannetti N, Gomez‐Mesa JE, Janssens S, Januzzi JL, Gonzalez‐Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Pina IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M, EMPEROR‐Preserved Trial Investigators . Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021; 385: 1451–1461. [DOI] [PubMed] [Google Scholar]
- 35. Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, Carson P, Anand I, Doehner W, Haass M, Komajda M, Miller A, Pehrson S, Teerlink JR, Schnaidt S, Zeller C, Schnee JM, Anker SD. Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: EMPEROR‐Preserved trial. Circulation 2021; 144: 1284–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Eligibility criteria for the different SGLT2 inhibitor HF trials.
Table S2. Baseline characteristics of HF patients according to 2016 ESC HF Guidelines.
Figure S1. Guideline‐recommended heart failure therapy use in diabetic HF patients with an LVEF <50%, shown as A prescription rates, B percentage of the recommended target dose prescribed, C prescription of triple therapy and, D prescription of triple therapy at ≥50% of guideline recommended target dose, stratified according to renal function.
Figure S2. Guideline‐recommended heart failure therapy use according to the 2016 ESC HF Guidelines as stratified by diabetic status, A in patients with a reduced ejection fraction. B in patients with midrange ejection fraction, and C in patients with a semi quantitative analysis.
Figure S3. Flowchart of eligibility for SGLT2 inhibitors according to the eligibility criteria of the A DAPA‐HF trial and B the EMPEROR‐Reduced trial.


