Abstract
Aims
Fontan palliation is a surgical strategy for patients with complex congenital heart disease, in whom biventricular circulation cannot be achieved. Long‐term survival is negatively affected by the absence of sub‐pulmonary ventricle and increased systemic venous pressure. Exercise capacity is a known predictor of overall survival and quality of life in congenital heart defects. We aim to track individual trends of peak oxygen uptake (V̇O2peak) after total cavopulmonary connection (TCPC), identify predictors of deterioration, and derive a disease‐specific reference V̇O2peak dataset.
Methods and results
A retrospective study of serial cardiopulmonary exercise testing (CPET) data, gathered from all patients who underwent TCPC in the Czech Republic between 1992 and 2016. Of 354 consecutive patients with TCPC, 288 (81.4%) patients underwent one or more CPETs yielding 786 unique V̇O2peak values used as a reference dataset. Longitudinal data were available in 206 (58.2%) patients, who underwent a median (inter‐quartile range) of 3.0 (2.0–5.0) CPETs over a mean (standard deviation) of 8.9 (5.5) years. The decline of exercise capacity with age was linear and not faster than in healthy peers (P = 0.47), but relative values of V̇O2peak in TCPC patients were 12.6 mL/min/kg lower. Single ventricular morphology and pulmonary artery size had no significant influence on the exercise capacity dynamics. V̇O2peak decline correlated negatively with the trend of body mass index z‐score (P = 0.006) and was faster in women than men (P = 0.008).
Conclusions
Total cavopulmonary connection patients have significantly reduced exercise capacity. The age‐related decline paralleled the healthy population and correlated negatively with the body mass index trend. The presented V̇O2peak reference dataset may help the clinicians to grade the severity of exercise capacity impairment in individual TCPC patients.
Keywords: Fontan circulation, Total cavopulmonary connection, Cardiopulmonary exercise testing, Oxygen consumption, Exercise capacity
Introduction
Fontan circulation is associated with suboptimal long‐term outcomes due to the absence of sub‐pulmonary ventricle associated with chronic systemic venous congestion. Failing Fontan circulation presents one of the major concerns in paediatric and adult congenital cardiology. Although many diagnostic and imaging modalities can describe patient's circulatory system function in detail, prediction of speed of worsening remains unclear. Cardiopulmonary exercise test (CPET) is a sensitive non‐invasive method of functional assessment in patients after surgical repair of congenital heart disease. Peak oxygen uptake (V̇O2peak) predicts unscheduled hospitalizations and is an independent predictor of all‐cause morbidity and mortality in total cavopulmonary connection (TCPC) patients. 1 Clinicians are encouraged to perform CPET as a routine part of the follow‐up, as exercise capacity is difficult to derive from resting diagnostic measures, and available studies show that V̇O2peak is severely reduced in the majority of Fontan patients. 2 , 3 Comparing measured data with healthy population, reference values might thus be misleading. Reference values for exercise capacity among adults with congenital heart disease are available including the subgroup of adult patients with mixed types of Fontan palliation. 4 Our study aims to describe long‐term (decades) dynamics of exercise performance in patients after TCPC from childhood to adulthood and to present a disease‐specific age‐related reference dataset.
Methods
Patients
Study group was recruited from all consecutive patients in the Czech Republic, who underwent TCPC procedure between 1992 and 2016 at a median [inter‐quartile range (IQR)] age of 4.7 (3.5–6.4) years at our institution. A total of 354 patient records were identified in the nationwide single‐centre institutional database. Of these, 288 patients (female 115 = 39.9%) underwent at least one cardiopulmonary exercise test. From the remaining 66 patients, 12 patients have died, 4 patients have had the TCPC taken down, and 2 patients had a heart transplant. Forty‐eight patients were not able or willing to perform a CPET or were lost in follow‐up. Body mass index (BMI) z‐score of the CPET group was mean [standard deviation (SD)] + 0.13 (1.33) z. Underlying morphology of systemic ventricle was left in 148 (51.4%) patients, right in 130 (45.1%) patients, biventricular in 7 (2.4%) patients, and undetermined in 3 (1.0%) patients. Respective structural diagnoses are listed in Table 1 . TCPC using an intra‐atrial tunnel was performed in 153 (53.1%) patients, whereas extracardiac conduit was used in 135 patients (46.9%). Fenestration in the TCPC tunnel was created in 112 patients (38.9%), but it was patent in only 19 (6.6%) patients at the time of CPET. In the remaining patients, fenestration had been closed by catheter intervention (N = 69) or closed spontaneously (N = 24). List of pharmacotherapies used in patients is shown in Table 2 . In course of follow‐up, seven patients have died (2.4%), and five patients have had a heart transplant or have been listed for a heart transplant (1.7%).
Table 1.
Main diagnosis
| Diagnosis | Count (n) | % |
|---|---|---|
| Tricuspid atresia | 67 | 23.3 |
| Single ventricle/double‐inlet left ventricle | 52 | 18.1 |
| Double‐outlet right ventricle | 51 | 17.7 |
| Hypoplastic left heart syndrome | 26 | 9.0 |
| Transposition of the great arteries | 18 | 6.2 |
| Single ventricle/double‐inlet right ventricle | 18 | 6.2 |
| Mitral atresia | 16 | 5.6 |
| Pulmonary atresia with intact interventricular septum | 14 | 4.9 |
| Congenitally corrected transposition of the great arteries | 9 | 3.1 |
| Complete atrioventricular septal defect | 6 | 2.1 |
| Hypoplastic right ventricle | 3 | 1.0 |
| Other | 8 | 2.8 |
| Total | 288 | 100.0 |
Table 2.
List of pharmacotherapies
| Pharmacotherapy | Count (n) | % |
|---|---|---|
| Angiotensin‐converting enzyme inhibitors | 90 | 31.2 |
| Digoxin | 41 | 14.2 |
| Furosemide | 22 | 7.6 |
| Beta‐blockers | 18 | 6.3 |
| Spironolactone | 9 | 3.1 |
| Propafenone | 4 | 1.4 |
| Sotalol | 4 | 1.4 |
| Sildenafil | 4 | 1.4 |
| Bosentan | 3 | 1.0 |
| Calcium channel blockers | 1 | 0.3 |
Imaging
Semi‐quantitative echocardiographic evaluation of atrioventricular valve regurgitation severity was available in 240 (83.3%) patients at the time of the last CPET. Regurgitation was mild to moderate in 226 (78.5%) patients and moderate to severe or severe in 14 (4.9%) patients. Seven (2.4%) patients underwent atrioventricular valve surgery for severe regurgitation after TCPC. Pulmonary arteries were measured by angiography during catheterization before TCPC, and the mean (SD) Nakata index and McGoon ratio were 305.8 (132.5) mm2/m2 and 2.10 (0.48), respectively. Ejection fraction of systemic ventricle at the time of the last CPET was quantified by magnetic resonance imaging (MRI) in 81 (28.1%) patients using the 1.5 T MRI machines. Mean ejection fraction of systemic ventricle was 51.2 (8.9)%.
Cardiopulmonary exercise test
In total, 1006 CPETs were performed between 1994 and 2019 at a mean (SD) age of 19.0 (6.9) years at the time of testing. Only 786 tests (78.1%) were declared as true maximal stress tests with valid gas analysis data and meeting the criteria for maximal patient effort (respiratory exchange ratio ≥1.05 and perceived exertion ≥16 at the modified Borg scale). These data were used to create disease‐specific reference values for maximal oxygen uptake in TCPC patients. Longitudinal data were available in 206 patients (58.2%), who underwent a median (IQR) of 3.0 (2.0–5.0) CPETs. The duration of follow‐up between first and last testing was mean (SD) 8.9 (5.5) years. The longest period between tests in an individual patient was 25 years. In a subgroup of 59 patients (20.4%), detailed gas analysis dataset was available at the time of their last CPET, including VE′/VCO2 slopes and oxygen uptake to work rate slopes (V̇O2/WR). Oxygen uptake efficiency slope (OUES) was calculated according to standards and normalized for patient weight. 5 Oxygen pulse was calculated as V̇O2 (mL/min) divided by heart rate (1/min) and presented in millilitres per beat. Patient data were compared with national reference values of exercise indices for healthy peers. 6
All CPETs were performed on a bicycle ergometer (Ergoline 900 and 150, Bitz, Germany) at a single lab facility. Only tests using fast gas analysers were included. Gas exchange analysers used consecutively throughout 25 year period were Oxycon Beta (Mijnhardt, the Netherlands) and Oxycon Pro (Jaeger, Germany) with both paramagnetic and electrochemical oxygen sensors. Older tests with Douglas bags and slow analysers were excluded. The incremental stepwise or combined protocol was used in all tests until patient exhaustion. Each test result was retrospectively checked for consistency in the physiological response of the data by an experienced exercise physiologist.
Statistical analysis
Data are presented as mean (SD) or median (IQR) as appropriate according to the pattern of distribution tested for normality with Shapiro's test. If applicable, z‐scores were used, and pathological limits were defined as those exceeding ±2 SD (z‐score ±2). V̇O2peak results were separated by gender, and second‐order polynomial quantile regression was used to create reference V̇O2peak percentile graphs. Individual linear trends were derived from discrete values by the least squares method. Then the influence of gender, systemic ventricle morphology, and preoperative pulmonary dimensions on the steepness of exercise capacity slope decline was analysed. Normally distributed parameters were tested by the Pearson correlation coefficient and the resulting P‐value for testing non‐correlation. Non‐parametric variables were tested by the Spearman rank‐order test. Multivariate analysis using a generalized additive model was applied to test for the effect of gender and trend of BMI on V̇O2peak slope. Statistical analysis was computed using the Python and R programming languages and SciPy, quantreg, and mgcv packages, respectively. P values <0.05 were considered significant.
Ethics
The locally appointed ethics committee has approved the research protocol (Reference Number EK‐125/21). Informed consent was not required for this retrospective anonymised study with no direct impact on individual patient management.
Results
Age‐related and sex‐related V̇O2peak values
Age‐related and sex‐related V̇O2peak values expressed as percentile nomographs are shown in Figures 1 and 2 . The highest values of V̇O2peak in men are noted in early childhood between 6 and 10 years of age, and then the trajectory shows a slow linear decline until the third decennium. There is no visible peak of exercise capacity in puberty as compared with healthy peers. The decline during childhood and early adulthood is steeper in women, relatively stabilizing in the third decennium.
Figure 1.
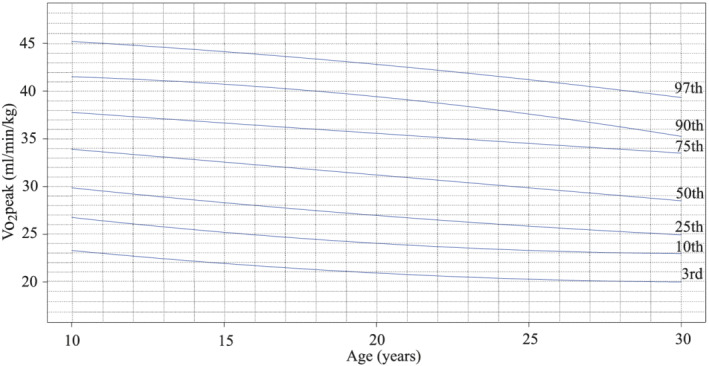
V̇O2peak quantile regression in men with percentiles, the total number of tests (N = 434).
Figure 2.
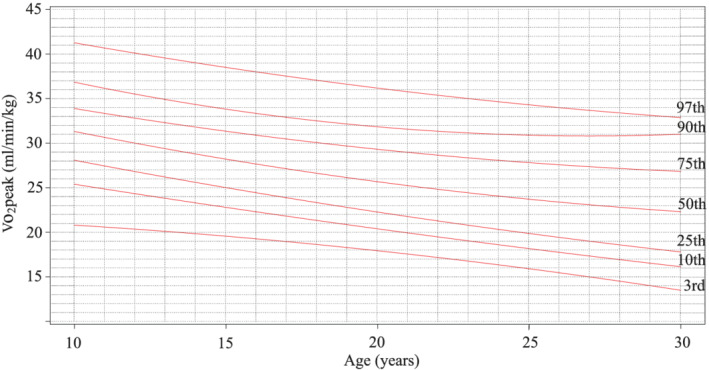
V̇O2peak quantile regression in women with percentiles, the total number of tests (N = 312).
Cross‐sectional group
Detailed gas analysis data at the time of the last stress test were available in 54 (18.8%) patients. Mean calculated OUES/kg was 32.8 (8.3) mL/min/kg, mean VE′/VCO2 slope was 32.9 (4.0), and mean V̇O2/WR slope was 9.4 (1.7) mL/min/W. OUES/kg and V̇O2/WR slopes both correlated positively with V̇O2peak at the last CPET (R = 0.77 and P < 0.001, and R = 0.35 and P = 0.04, respectively). Values of VE′/VCO2 slope correlated inversely with V̇O2peak at the last CPET (R = −0.48, P = 0.003). Values of oxygen pulse were overall significantly lower in both male and female patients than in healthy peers at multiple stages of exercise (Figures 3 and 4 ). Increase of oxygen pulse from low to maximal effort was lower in patients, 1.8 (1.1) mL in TCPC women vs. 2.3 (0.5) mL in healthy peers, and 2.9 (1.7) mL in TCPC men vs. 4.4 (0.9) mL in healthy peers (P = 0.03 and P = 0.0007, respectively). Despite lower increase during exercise and lower absolute values, the ability to augment oxygen pulse until maximal exertion is preserved in both sex groups and the steepness of the slope of oxygen pulse throughout incremental exercise is not significantly different from healthy controls (P = 0.50 for men and P = 0.08 for women, respectively). Ejection fraction of the systemic ventricle at the time of the last CPET was quantified by MRI in 81 patients (36 women). Systemic ventricle contractility did not influence the speed of V̇O2peak decrease (P = 0.25) nor z‐score of V̇O2peak at the last CPET (P = 0.92). Haemoglobin levels at the time of last CPET were available in 54 (18.8%) patients. Mean haemoglobin levels were 160.7 (17.8) g/L, and they did not influence the speed of V̇O2peak decline (P = 0.96) nor z‐score of V̇O2peak at the last CPET (P = 0.06).
Figure 3.
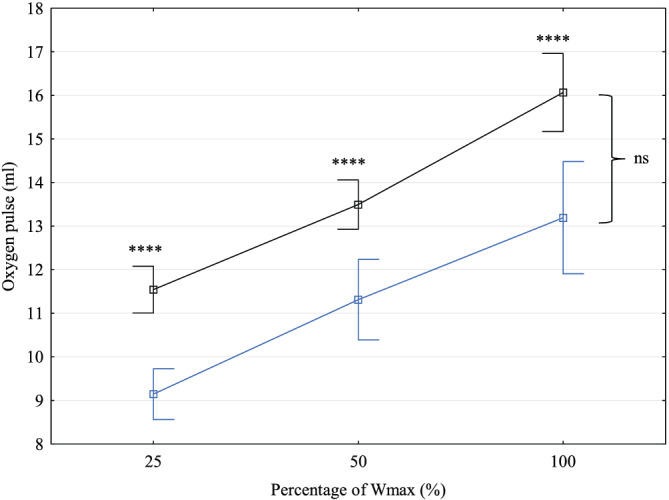
Oxygen pulse increase during exercise in men with total cavopulmonary connection (blue) compared to healthy controls (black). ns, non‐significant; Wmax, maximal power output in watts. ****P < 0.00001.
Figure 4.
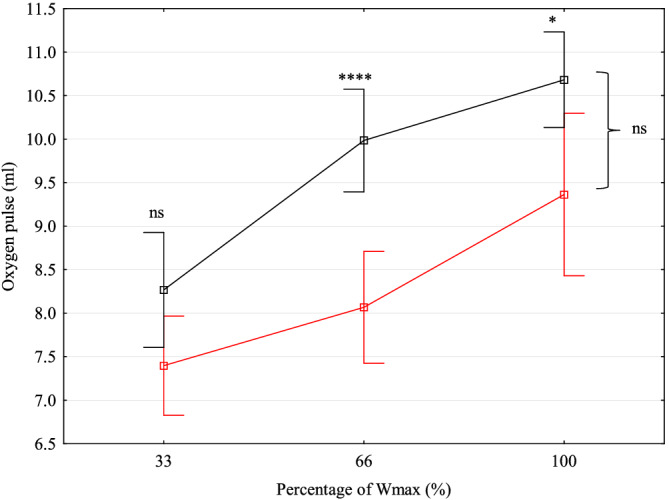
Oxygen pulse increase during exercise in women with total cavopulmonary connection (red) compared to healthy controls (black). ns, non‐significant; Wmax, maximal power output in watts. *P < 0.01. ****P < 0.00001.
Longitudinal group
Individual trajectories of V̇O2peak were studied in 206 patients who had at least two valid V̇O2peak values throughout follow‐up. Discrete values from individual CPETs were transformed to linear vectors fitted by the method of least squares. Figure 5 shows the resulting graph where individual trajectories and length of follow‐up can be appreciated. The mean trend of decline in all patients was 36.6 mL/min/kg − 0.41 * age (years), which implicates a similar speed of exercise capacity deterioration when compared with national reference values for healthy peers, where V̇O2peak is equal to 49.2 mL/min/kg − 0.41 * age (P = 0.47). The mean annual rate of deterioration in predicted V̇O2peak was 0.39 (2.20)% per year. On average, the difference in exercise capacity between TCPC patients and healthy peers of a given age was 12.6 mL/min/kg of V̇O2 (P < 0.00001).
Figure 5.
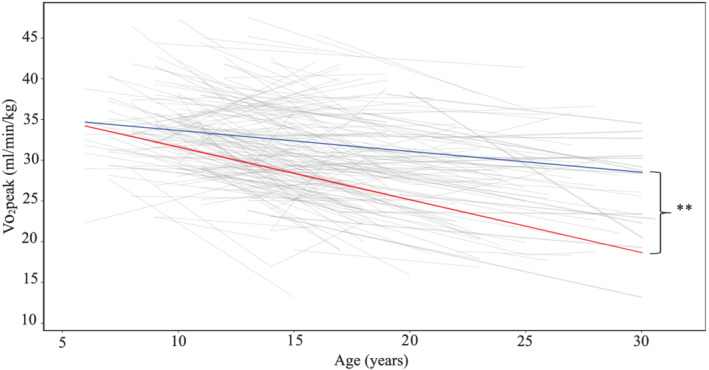
Individual linear trajectories of V̇O2peak fitted by the method of least squares from first to last cardiopulmonary exercise test and calculated means for both genders (grey lines for individual trajectories, blue line for mean decline rate in men, and red line for mean decline rate in women). **P < 0.001.
Influence of gender
Linear trajectories were divided into two subgroups according to gender. Means with SDs were calculated for men and women, respectively. Faster decline was observed in women, where V̇O2peak is equal to 38.1 mL/min/kg − 0.65 (0.81) * age, vs. in men 36.2 mL/min/kg − 0.26 (1.1) * age (P = 0.008). In multivariate analysis adjusted for BMI, gender remained a significant predictor of decline in exercise capacity over time (P = 0.016).
Influence of ventricular morphology
Two hundred six patients had at least two V̇O2peak values and known morphology of systemic ventricle; 109 patients had morphologically left ventricle, 87 patients had morphologically right ventricle, and 10 patients had undetermined ventricle. The steepness of decline in exercise capacity over time was not dependent on ventricle morphology (P = 0.23).
Influence of preoperative pulmonary dimensions
One hundred eighty patients had at least two V̇O2peak values and known preoperative value of Nakata index, whereas 169 patients had at least two V̇O2peak values and known preoperative value of McGoon ratio. The steepness of decline in exercise capacity in these subgroups over time was not influenced by the size of pulmonary arteries (P = 0.64 for Nakata index and P = 0.50 for the McGoon ratio, respectively).
Influence of body mass index
Two hundred six patients had at least two BMI and V̇O2peak values from separate CPETs. Mean BMI z‐score was +0.05 (1.29) z in men and +0.26 (1.39) z in women (P = 0.22). The mean overall increase in BMI z‐score throughout follow‐up was 0.06 (0.17) z * age. In subgroups divided by gender, the mean increase of BMI z‐score was 0.064 (0.17) z * age in men and 0.053 (0.16) z * age in women (P = 0.60). V̇O2peak trajectory correlated negatively with BMI trend (P = 0.006), but the effect tended to be less predictive than gender in multivariate analysis (P = 0.06).
Discussion
Patients in our study group showed different dynamics of exercise capacity during childhood and adolescence as compared with healthy peers. Exercise capacity in boys with TCPC was highest at the start of follow‐up around 6–10 years of age, and then it slowly declined throughout puberty, adolescence, and early adulthood. On the contrary, in healthy boys, exercise capacity peaks between the 14th and 16th years of age as the pubertal gain can be appreciated. Exercise tolerance in girls with TCPC peaked right at the start of the follow‐up between 6 and 10 years of age, and then it progressively declined until the third decennium. That trend was similar to reference data for healthy girls, but the absolute values in our patients were lower. 6 Same finding was reported by Müller et al. in 57 paediatric TCPC patients. 7 We, therefore, suggest the use of disease‐specific nomographs for evaluation of functional status in paediatric TCPC patients (Figures 1 and 2 ) as the routine use of whole population reference values could be misleading.
Our data suggest that between 15 and 30 years of age, exercise capacity of TCPC patients ranges between 60% and 70% of healthy peers. In absolute numbers, V̇O2peak values at all ages are on average 12.6 mL/min/kg lower than in healthy peers. This fits well with published cross‐sectional studies that report the aerobic capacity of Fontan patients in the range of 60–72% predicted for age and sex. 3 , 8 , 9
The observed deterioration rate was on the other hand slower than expected at only 0.39 (2.20)% of predicted V̇O2peak per year. Preceding studies report inconsistent rates of deterioration in their cohorts. In the first published serial study, Nir et al. reported no significant deterioration of V̇O2peak in course of 3.5 years in Fontan patients, but the cohort was rather small and composed of 21 patients with atriopulmonary connection and 4 patients with atrioventricular connection. 10 V̇O2peak was stable although low at only 56–57% predicted within 2.2–5.9 years after surgery, demonstrating inferior functional outcomes of the early surgical era. In the following studies, Giardini et al. demonstrated deterioration at 2.6% of predicted V̇O2peak per year. This study involved a mixed cohort of 53 Fontan patients with higher proportion of those with atriopulmonary anastomosis vs. TCPC. 11 Fernandes et al. demonstrated a decline of 1.25% of predicted V̇O2peak per year in 78 TCPC patients during a follow‐up of 3.0 years. 12 More recently, Egbe et al. reported deterioration at 1.7% of predicted V̇O2peak per year during 3.8 years follow‐up in a group of 71 patients, where the decline in per cent predicted V̇O2peak ≥ 3 percentage points per year was a predictor of 5 year risk of cardiovascular adverse events. 13 In the newly published Paediatric Heart Network Fontan study, the per cent predicted V̇O2peak declined by 0.8 (1.7)% per year in 95 patients, which comes closest to our findings. 14 Different reported deterioration rates in literature could be affected by shorter periods of follow‐up, a smaller number of patients, or mixed cohorts from different surgery eras. Moreover, authors usually do not report their reference datasets for the prediction of V̇O2peak, which hinders direct comparison. When comparing studies with exclusively TCPC cohorts, those with longer follow‐up periods and larger patient count tend to report better results at around 1.0% per year or lower. Strongest independent predictor of V̇O2peak deterioration in our cohort was female gender and increase of BMI during follow‐up. Association of BMI with a steeper decline of V̇O2peak slope was shown also in a large longitudinal anthropometric study of Lambert et al. 15
Preoperative dimensions of the pulmonary artery had no significant effect on the observed decline of exercise capacity. Despite being crucial for perioperative management, the Nakata index and McGoon ratio could not predict the dynamics of functional outcomes in paediatric patients and young adults.
Single ventricular morphology had no significant impact on V̇O2peak decline. In the literature, the influence of ventricular morphology on Fontan outcomes remains controversial. Atz et al. demonstrated that the risk of death or cardiac transplantation was not associated with ventricular morphology. 16 Interestingly in the work of Giardini et al., type of surgery and ventricle morphology were identified as predictors of exercise capacity decline. A slower decline of exercise capacity was observed in patients with an underlying left ventricular morphology and TCPC. 11 Moon et al. concluded that right ventricular morphology is negatively associated with the long‐term survival following the Fontan procedure, possibly due to a tendency towards progressive atrioventricular valve regurgitation and deterioration of the single ventricle function. 17 The relationship of OUES with values of V̇O2peak in our patient group is consistent with the previously published study of Bongers et al. 18 Absolute values of V̇O2/WR and VE′/VCO2 slopes are similar to those reported by Bossers et al. 19 Correlations of those indices with peak oxygen consumption were both weak. Our data also showed that values of oxygen pulse were overall significantly lower than in healthy peers, but the ability to augment oxygen pulse until maximal exertion was preserved in both sex groups and the steepness of slope was not different from healthy controls. As previously hypothesized by La Gerche et al., performance of the systemic ventricle may not be a major limiting factor in oxygen delivery cascade in paediatric patients and young adults with TCPC without significant atrioventricular valve regurgitation. 20 Lack of significant impact of systemic ventricle contractility on V̇O2peak values in our patient group is also approving this hypothesis. This argument is further supported by preserved augmentation of oxygen pulse throughout incremental exercise testing. Diastolic filling, ventricular contractility, and peripheral oxygen extraction all interact comprehensively during exercise in Fontan patients, but the true mechanism of the increase in oxygen pulse will remain elusive until direct invasive measurement. Although scarce, stress echocardiography data favour enhancement of peripheral oxygen extraction as a major contributing factor. 21 On the other hand, cardiac magnetic resonance data during exercise show a fair increase in cardiac index during incremental supine exercise. 22 Evaluation of oxygen pulse dynamics may be further complicated by dynamic changes in haemoglobin saturation during exercise.
Given the significance of V̇O2peak in the prognosis of TCPC patients, finding ways to maintain their exercise capacity is of utmost importance. Bosentan improved peak oxygen consumption in TCPC patients by 2.0 mL/min/kg compared with only 0.6 mL/min/kg increase while on placebo in the TEMPO trial. 23 Sildenafil did not improve peak oxygen consumption in the double‐blinded placebo‐controlled study, but increased cardiac index when assessed by stress MRI, especially in high intensities of exercise. 22 , 24 Several studies show promising results of cardiac rehabilitation in improving exercise capacity and symptoms in Fontan patients. Most recently, Turquetto et al. reported an increase in peak oxygen consumption by 6.3 mL/min/kg in Fontan patients after 4 months of supervised aerobic exercise training. 25 Although no controlled cardiac rehabilitation programme was implemented in our centre at the time of the study, all patients were being routinely educated on exercise and leisure time activities by sports medicine specialist. Symptomatic patients (New York Heart Association >I) were referred for balneotherapy and/or chest physiotherapy combined with inspiratory muscle training (Coach 2 or Threshold IMT) under the supervision of an experienced physiotherapist. Participation in school physical education and encouragement in leisure time sport activities from early childhood should be the cornerstone of acquiring healthy exercising habits in adulthood. In those who are sedentary, overweight, or symptomatic, a cardiac rehabilitation programme and/or nutritional intervention should be encouraged to maintain their exercise capacity. Chest physiotherapy and respiratory muscle training should be incorporated into perioperative care for these patients.
Limitations
This study is limited by observing mainly trends in time and not the absolute values in cross section. Assessment of ventricular function, haemoglobin levels, and detailed gas analysis data were available only in a subgroup of patients, leading to possible selection bias. Also, the severity of atrioventricular valve regurgitation was not taken into consideration as being mild to moderate in the majority of patients in our study group. The influence of pharmacotherapy was not analysed because of the heterogeneity of treatment regimen and suspected influence of other imperceptible confounders during a long period of follow‐up.
Conclusions
Total cavopulmonary connection patients have significantly reduced exercise capacity with a linear decline from adolescence to third decade of life. The rate of decline is faster in women and is not significantly affected by ventricular morphology or pre‐TCPC pulmonary artery size. Despite lower absolute values, the ability to augment oxygen pulse is, however, maintained until maximal exertion. An increase in BMI negatively affects V̇O2peak; thus, weight management should be encouraged in all patients, but substantially stressed out in women to maintain their exercise capacity. We advise using disease‐specific reference datasets for oxygen consumption in TCPC patients as their lifetime V̇O2peak trends differ from healthy peers.
Conflict of interest
None declared.
Funding
This work was supported by Ministry of Health of the Czech Republic (Ministerstvo Zdravotnictví České Republiky)—Conceptual Development of Research Organization, Motol University Hospital, Prague (00064203).
Illinger, V. , Materna, O. , Slabý, K. , Jičínská, D. , Kovanda, J. , Koubský, K. , Pokorný, J. , Procházka, M. , Antonová, P. , Hoskovec, A. , Radvanský, J. , Chaloupecký, V. , and Janoušek, J. (2022) Exercise capacity after total cavopulmonary anastomosis: a longitudinal paediatric and adult study. ESC Heart Failure, 9: 337–344. 10.1002/ehf2.13747.
References
- 1. Ohuchi H, Negishi J, Noritake K, Hayama Y, Sakaguchi H, Miyazaki A, Kagisaki K, Yamada O. Prognostic value of exercise variables in 335 patients after the Fontan operation: a 23‐year single‐center experience of cardiopulmonary exercise testing. Congenit Heart Dis 2015; 10: 105–116. [DOI] [PubMed] [Google Scholar]
- 2. McCrindle BW, Zak V, Sleeper LA, Paridon SM, Colan SD, Geva T, Mahony L, Li JS, Breitbart RE, Margossian R, Williams RV, Gersony WM, Atz AM. Laboratory measures of exercise capacity and ventricular characteristics and function are weakly associated with functional health status after Fontan procedure. Circulation 2010; 121: 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paridon SM, Mitchell PD, Colan SD, Williams RV, Blaufox A, Li JS, Margossian R, Mital S, Russell J, Rhodes J. A cross‐sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol 2008; 52: 99–107. [DOI] [PubMed] [Google Scholar]
- 4. Kempny A, Dimopoulos K, Uebing A, Moceri P, Swan L, Gatzoulis MA, Diller GP. Reference values for exercise limitations among adults with congenital heart disease. Relation to activities of daily life—single centre experience and review of published data. Eur Heart J 2012; 33: 1386–1396. [DOI] [PubMed] [Google Scholar]
- 5. Hollenberg M, Tager IB. Oxygen uptake efficiency slope: an index of exercise performance and cardiopulmonary reserve requiring only submaximal exercise. J Am Coll Cardiol 2000; 36: 194–201. [DOI] [PubMed] [Google Scholar]
- 6. Máček M, Vávra J, Radvanský J. Spotřeba kyslíku při zátěži na bicyklovém ergumetru. In Fyziologie a patofyziologie tělesné zátěže. Avicenum Prague; 1988. p 230. [Google Scholar]
- 7. Müller J, Christov F, Schreiber C, Hess J, Hager A. Exercise capacity, quality of life, and daily activity in the long‐term follow‐up of patients with univentricular heart and total cavopulmonary connection. Eur Heart 2009; 30: 2915–2920. [DOI] [PubMed] [Google Scholar]
- 8. Qu J, Shi H, Chen X, Li K, Liang H, Cui Y. Evaluation of physical fitness in children with congenital heart diseases versus healthy population. Semin Thorac Cardiovasc Surg 2020; 32: 906–915. [DOI] [PubMed] [Google Scholar]
- 9. Minter MM, Deshpande S, Ayers R, Stark M. Cardiopulmonary exercise performance in children after Fontan. Am Acad Pediatrics 2019; 144: 308–308. [Google Scholar]
- 10. Nir A, Driscoll DJ, Mottram CD, Offord KP, Puga FJ, Schaff HV, Danielson GK. Cardiorespiratory response to exercise after the Fontan operation: a serial study. J Am Coll Cardiol 1993; 22: 216–220. [DOI] [PubMed] [Google Scholar]
- 11. Giardini A, Hager A, Napoleone CP, Picchio FM. Natural history of exercise capacity after the Fontan operation: a longitudinal study. Ann Thorac Surg 2008; 85: 818–821. [DOI] [PubMed] [Google Scholar]
- 12. Fernandes SM, McElhinney DB, Khairy P, Graham DA, Landzberg MJ, Rhodes J. Serial cardiopulmonary exercise testing in patients with previous Fontan surgery. Pediatr Cardiol 2010; 31: 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Egbe AC, Driscoll DJ, Khan AR, Said SS, Akintoye E, Berganza FM, Connolly HM. Cardiopulmonary exercise test in adults with prior Fontan operation: the prognostic value of serial testing. Int J Cardiol 2017; 235: 6–10. [DOI] [PubMed] [Google Scholar]
- 14. Goldberg DJ, Zak V, McCrindle BW, Ni H, Gongwer R, Rhodes J, Garofano RP, Kaltman JR, Lambert LM, Mahony L, Margossian R, Spector ZZ, Williams RV, Atz AM, Paridon SM. Exercise capacity and predictors of performance after Fontan: results from the Pediatric Heart Network Fontan 3 study. Pediatr Cardiol 2020; 25: 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lambert LM, McCrindle BW, Pemberton VL, Hollenbeck‐Pringle D, Atz AM, Ravishankar C, Campbell MJ, Dunbar‐Masterson C, Uzark K, Rolland M, Trachtenberg FL, Menon SC. Longitudinal study of anthropometry in Fontan survivors: Pediatric Heart Network Fontan study. Am Heart J 2020; 224: 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Atz AM, Zak V, Mahony L, Uzark K, D'agincourt N, Goldberg DJ, Williams RV, Breitbart RE, Colan SD, Burns KM, Margossian R, Henderson HT, Korsin R, Marino BS, Daniels K, McCrindle BW. Longitudinal outcomes of patients with single ventricle after the Fontan procedure. J Am Coll Cardiol 2017; 69: 2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moon J, Shen L, Likosky DS, Sood V, Hobbs RD, Sassalos P, Romano JC, Ohye RG, Bove EL, Si MS. Relationship of ventricular morphology and atrioventricular valve function to long‐term outcomes following Fontan procedures. J Am Coll Cardiol 2020; 76: 419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bongers BC, Hulzebos HJ, Blank AC, Van Brussel M, Takken T. The oxygen uptake efficiency slope in children with congenital heart disease: construct and group validity. Eur J Cardiovasc Prev Rehabil 2011; 18: 384–392. [DOI] [PubMed] [Google Scholar]
- 19. Bossers SS, Helbing WA, Duppen N, Kuipers IM, Schokking M, Hazekamp MG, Bogers AJ, Ten Harkel AD, Takken T. Exercise capacity in children after total cavopulmonary connection: lateral tunnel versus extracardiac conduit technique. J Thorac Cardiovasc Surg 2014; 148: 1490–1497. [DOI] [PubMed] [Google Scholar]
- 20. La Gerche A, Gewillig M. What limits cardiac performance during exercise in normal subjects and in healthy Fontan patients? Int J Pediatr 2010; 2010: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wittekind S, Mays W, Gerdes Y, Knecht S, Hambrook J, Border W, Jefferies JL. A novel mechanism for improved exercise performance in pediatric Fontan patients after cardiac rehabilitation. Pediatr Cardiol 2018; 39: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 22. Van De Bruaene A, La Gerche A, Claessen G, De Meester P, Devroe S, Gillijns H, Bogaert J, Claus P, Heidbuchel H, Gewillig M, Budts W. Sildenafil improves exercise hemodynamics in Fontan patients. Circ Cardiovasc Imaging 2014; 7: 265–273. [DOI] [PubMed] [Google Scholar]
- 23. Hebert A, Mikkelsen UR, Thilen U, Idorn L, Jensen AS, Nagy E, Hanseus K, Sørensen KE, Søndergaard L. Bosentan improves exercise capacity in adolescents and adults after Fontan operation: the TEMPO (treatment with endothelin receptor antagonist in Fontan patients, a randomized, placebo‐controlled, double‐blind study measuring peak oxygen consumption) study. Circulation 2014; 130: 2021–2030. [DOI] [PubMed] [Google Scholar]
- 24. Goldberg DJ, French B, McBride MG, Marino BS, Mirarchi N, Hanna BD, Wernovsky G, Paridon SM, Rychik J. Impact of oral sildenafil on exercise performance in children and young adults after the Fontan operation: a randomized, double‐blind, placebo‐controlled, crossover trial. Circulation 2011; 123: 1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Turquetto AL, Dos Santos MR, Agostinho DR, Sayegh AL, de Souza FR, Amato LP, Barnabe MS, de Oliveira PA, Liberato G, Binotto MA, Negrão CE. Aerobic exercise and inspiratory muscle training increase functional capacity in patients with univentricular physiology after Fontan operation: a randomized controlled trial. Int J Cardiol 2021; 330: 50–58. [DOI] [PubMed] [Google Scholar]


