Abstract
Aims
The present study investigated the prognostic impact of either isolated left atrial (LA) impairment, or its association with right ventricular (RV) failure, in heart failure (HF) with reduced ejection fraction (HFrEF), using basic and speckle tracking echocardiography (STE).
Methods and results
One hundred and six outpatients with HFrEF were enrolled in this prospective observational study. Patients with primary lung diseases, non‐sinus rhythm, previous cardiac surgery, and poor acoustic window were excluded. After clinical examination and basic echocardiography, STE was used to measure peak atrial longitudinal strain (PALS) and a new marker of RV performance and pulmonary circulation relation: free‐wall RV longitudinal strain (fwRVLS)/systolic pulmonary artery pressure (sPAP). Patients were followed for all‐cause/cardiovascular death and HF hospitalization. Of 84 eligible patients (60.1 ± 11.5 years; 82% male patients), 48 reached the combined endpoint (cardiovascular death and/or HF hospitalization). Population was divided into three groups: Group 1 (PALS ≥ 15 and fwRVLS/sPAP ≤ −0.5), Group 2 (PALS ≤ 15 and fwRVLS/sPAP ≤ −0.5), and Group 3 (PALS ≤ 15 and fwRVLS/sPAP > −0.5). Mean follow up was 3.5 ± 0.3 years. The higher severity groups were associated with higher LA volume index (P < 0.0001), New York Heart Association class (P = 0.02), mitral regurgitation (P = 0.0004) and tricuspid regurgitation grades (P < 0.0001), lower left ventricular (LV) ejection fraction (P = 0.0003), LV global longitudinal strain (P < 0.0001), PALS (P < 0.0001), tricuspid annular plane systolic excursion (P < 0.007), sPAP (P < 0.0001), and RV strain (P < 0.0001). Reduced PALS and fwRVLS/sPAP were independent predictors of the combined endpoint with adjusted Cox models (hazard ratio = 9.54; 95% confidence interval = 2.95–30.92; P = 0.0002 for Group 3 vs. Group 1). Kaplan–Meier curves showed early and persistent divergence between the three groups for the prediction of the combined endpoint and of all‐cause death (P < 0.0001).
Conclusions
The combination of LA and right heart damage entails worse prognosis in patients with HFrEF. The evaluation of PALS and fwRVLS/sPAP could aid risk stratification of HFrEF patients to provide them early treatment.
Keywords: Heart failure, Echocardiography, Left atrial, Right ventricular, Pulmonary pressure, Prognosis
Introduction
In the previous decades, chronic heart failure (HF) was primarily regarded as a left ventricular (LV) disease, with a mortality mostly related to left HF. Conversely, in the last years, thanks to the improvements in HF treatment, 1 , 2 a longer survival of patients with established LV dysfunction led to a more frequent progression to advanced HF, with increasing rates of death for end‐stage right HF. It is known that advanced HF is characterized by a significantly worse outcome, also due to limited therapeutic resources 3 ; thus, it is paramount to recognize those patients with a higher risk to develop right HF before it occurs. The clinical history of chronic HF mirrors the gradual increase in intracardiac filling pressures, originating from LV pressure or volume overload and gradually reflecting firstly on the left atrium (LA), which is the primary barrier before the onset of HF symptoms, 4 and later on the pulmonary circulation (PC) and on the right ventricle (RV), which have a strict anatomical and functional connection that further delays the development of severe biventricular impairment. Therefore, in patients with chronic LV dysfunction, the researchers should focus on the identification of the transition point to advanced HF. Accordingly, new indices would be useful to reclassify patients with known HF with reduced ejection fraction (HFrEF) based on the other chambers' impairment. Speckle tracking echocardiography (STE) emerged as a reliable and available tool to investigate not only LV but also LA and RV function in chronic HF. 5 While the prognostic value of LA strain in patients with HF with reduced 6 and preserved EF, 7 , 8 and of RV strain in patients with advanced HF, 9 , 10 , 11 has already been shown, less is known about their utility to early predict the progressive myocardial damage in HFrEF before overt biventricular dysfunction occurs. The purpose of this echocardiographic study was to assess the prognostic value of isolated LA dysfunction or its association with RV failure in patients with HFrEF, using basic and speckle tracking echocardiographic parameters.
Methods
In this prospective observational study, consecutive patients with HFrEF according to the latest European Society of Cardiology HF guidelines definition [i.e. patients with signs (pulmonary crackles, peripheral oedema, & elevated jugular venous pressure) and/or symptoms (dyspnoea, fatigue, & ankle swelling) of HF and LV EF < 40%] 12 referred to our HF ambulatories for a cardiologic visit including echocardiography between 2015 and 2017 were enrolled. Exclusion criteria were primary lung diseases (chronic obstructive pulmonary disease, sarcoidosis, & idiopathic pulmonary fibrosis) or known pulmonary hypertension (PH), history of coronary artery disease involving the right heart, previous heart transplantation (HTX) or left ventricular assist device (LVAD) implantation, and more than mild valvular stenosis.
The patients were prospectively followed for a primary combined endpoint, consisting in the occurrence of cardiovascular death and hospitalization for HF. Secondary endpoint was all‐cause death. Follow‐up data were collected via phone calls and electronic medical records. All subjects gave their written informed consent for participation in this study. All work was in compliance with the Declaration of Helsinki and obtained the approval from our Local Ethic Committee on 15 December 2014.
Basic echocardiography
Echocardiographic examination was performed according to the American Society of Echocardiography/European Association of Cardiovascular Imaging recommendations for chamber quantification, 13 using a high‐quality ultrasound machine (Vivid E9; GE Medical System, Horthen, Norway) with patients in the left lateral recumbent position.
Left ventricular and RV dimensions, RV fractional area change (RVFAC), and sphericity index were calculated using standard views. LV ejection fraction (LVEF) and LA volume and area were assessed using the biplane modified Simpson method from the apical four‐chamber and two‐chamber views. LV dimensions and LA volume were indexed to body surface area obtaining LV mass index and LA volume index (LAVI). From the four‐chamber view, mitral and tricuspid annulus plane systolic excursion (TAPSE) were measured by M‐mode; maximum early diastolic (E) and late diastolic (A) velocities were assessed by trans‐mitral pulsed wave Doppler to calculate E/A ratio; then, peak systolic (S'), early diastolic (E'), and late diastolic (A') annular velocities were obtained by tissue Doppler imaging, E/E' ratio was calculated and used as index of the LV filling pressure. Mitral and tricuspid regurgitation (MR & TR) were quantified by two‐dimensional (2D)‐echocardiography according to American Society of Echocardiography recommendations. 13 Systolic pulmonary artery pressure (sPAP) was estimated as the sum of systolic trans‐tricuspid pressure gradient and of right atrial pressure derived from the diameter and collapsibility of the inferior vena cava.
Speckle tracking echocardiography
Speckle tracking echocardiography analysis was conducted on apical two‐chamber, three‐chamber, and four‐chamber images, obtained by 2D grey‐scale echocardiography, with a stable electrocardiographic recording. Care was taken to obtain a good visualization of all chambers and a reliable delineation of the endocardial border. Measurements from three consecutive heart cycles were recorded and averaged. The frame rate was 60–80 frames per second. Analysis was performed offline by a single experienced and independent echocardiographer, who was not directly involved in the image acquisition and blinded to basic echocardiographic parameters, using a semi‐automated 2D‐strain software (EchoPac, GE, Milwaukee, Wisconsin). The endocardial border was manually traced in apical views, delineating a region of interest (ROI) of six segments for each view. Then, necessary manual adjustments of the ROI were performed, and the longitudinal strain curves for each segment were generated by the software. LV global longitudinal strain (GLS) was calculated as the average of four‐chamber, two‐chamber, and three‐chamber longitudinal strain curves. Global peak atrial longitudinal strain (PALS) was calculated at the end of the reservoir phase as the average of all LA segments in four‐chamber and two‐chamber views, using QRS as starting point.14, 15 Global RV longitudinal strain (RVLS) was calculated as the average strain of all RV and interventricular septum segments. Free‐wall RVLS (fwRVLS) was derived by a ROI of three segments (basal, medial, & apical) including only RV free‐wall. In patients in whom some segments were excluded for impossible adequate tracking, strain was calculated by averaging values measured in the remaining segments. A new parameter, fwRVLS/sPAP ratio, was assessed as marker of RV impairment deriving from RV adaptation to high pressures generated by overloaded PC.
Statistical analysis
Data are expressed as means ± SD (continuous variables) or as counts and percentages (binary variables).
Receiver operating characteristic (ROC) curves allowed to obtain optimal cut‐off values of PALS and fwRVLS/sPAP for the prediction of the primary composite endpoint (cardiovascular mortality and/or hospitalization). Using these cut‐offs, patients were divided into three groups based on the presence of preserved PALS and normal fwRVLS/sPAP, impaired PALS and normal fwRVLS/sPAP, and impaired PALS and pathological fwRVLS/sPAP. Differences between the three groups were analysed using Student t‐tests for continuous variables and χ 2 analyses for categorical variables.
Kaplan–Meier curves and log‐rank test were used to assess the correlation of the three groups with events‐free survival. Univariate and multivariate analyses were performed with Cox proportional hazard model that was fitted for the three groups as predictors of the composite endpoint; adjustment models were built using LV GLS LAVI, TR, and RVFAC. The covariates were chosen based on their univariable association with the dependent variable as well as based on biological plausibility.
Analyses were performed using the Statistical Package for Social Sciences software, release 20.0 (SPSS, Chicago, Illinois). P values < 0.05 were considered statistically significant.
Results
A total of 84 patients were included in the study. Mean age was 60.1 ± 11.5, 82% were male patients, and 35.7% had NYHA class 3 or 4 (P = 0.02). Mean LVEF was 28 ± 5%, and mean LV GLS = −7.5 ± 2.6%. ROC curves revealed the optimal cut‐off values of PALS (15%) and fwRVLS/sPAP (−0.5) for the prediction of prognosis (Figure 1 & Supporting Information, Table S1 ), allowing us to divide the population into three groups: Group 1 with preserved LA function and normal fwRVLS/sPAP (n = 28 patients with PALS ≥ 15% and fwRVLS/sPAP ≤ −0.5), Group 2 with impaired LA function and normal fwRVLS/sPAP (n = 24 patients with PALS < 15% and fwRVLS/sPAP ≤ −0.5), and Group 3 with impaired LA and fwRVLS/sPAP (n = 32 patients with PALS < 15% and fwRVLS/sPAP > −0.5).
Figure 1.
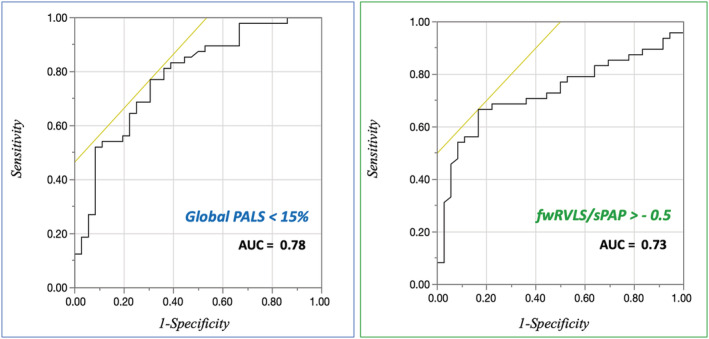
Receiver operating characteristic (ROC) curves for the accuracy of reduced global peak atrial longitudinal strain (PALS) and free‐wall right ventricular longitudinal strain (fwRVLS)/systolic pulmonary artery pressure (sPAP) ratio in the prediction of the composite endpoint. AUC, area under curve.
General, clinical, and biochemical characteristics and medications of the study cohort divided into the three groups are shown in Table 1 .
Table 1.
General and clinical characteristics of the study population
| Total sample | Group 1 (PALS ≥ 15%, fwRVLS/sPAP ≤ −0.5) (n = 28) | Group 2 (PALS < 15%, fwRVLS/sPAP ≤ −0.5) (n = 24) | Group 3 (PALS < 15% and fwRVLS/sPAP > −0.5) (n = 32) | Overall P value | |
|---|---|---|---|---|---|
| Age (years) | 60.1 ± 11.5 | 58.2 ± 12.4 | 61.9 ± 11.5 | 60.2 ± 10.9 | P = 0.5 |
| Male (%) | 82.14% (n = 69) | 26.19% (n = 22) | 23.81% (n = 20) | 32.14% (n = 27) | P = 0.83 |
| BMI | 27.12 ± 5.14 | 27.1 ± 4.9 | 27.1 ± 5.6 | 27.1 ± 4.9 | P = 0.99 |
| sBP (mmHg) | 118.3 ± 18 | 122.1 ± 18.4 | 122.2 ± 19.5 | 112.3 ± 16.6 | P = 0.06 |
| HR (b.p.m.) | 70.5 ± 10.6 | 68.9 ± 8.5 | 70.4 ± 11.4 | 71.9 ± 11.7 | P = 0.54 |
| Hypertension (%) | 44% (n = 37) | 15.5% (n = 13) | 12% (n = 10) | 16.7% (n = 14) | P = 0.94 |
| DM (%) | 15.5% (n = 13) | 5.9% (n = 5) | 4.8% (n = 4) | 4.8% (n = 4) | P = 0.83 |
| Dyslipidaemia (%) | 34.5% (n = 29) | 17.9% (n = 15) | 8.3% (n = 7) | 8.3% (n = 7) | P = 0.03 |
| CAD (%) | 41% (n = 30) | 16.4% (n = 12) | 11% (n = 8) | 13.7% (n = 10) | P = 0.3 |
| CKD (%) | 21% (n = 18) | 1.2% (n = 1) | 3.6% (n = 3) | 16.7% (n = 14) | P = 0.002 |
| NT‐proBNP (pg/mL) | 1875 ± 196.5 | 1282.8 ± 278.39 | 1075.9 ± 885.9 | 2752.9 ± 1611 | P = 0.01 |
| NYHA > 2 (%) | 35.7% (n = 30) | 4.8% (n = 4) | 9.5% (n = 8) | 21.4% (n = 18) | P = 0.02 |
| Creatinine (mg/dL) | 1.22 ± 0.44 | 0.98 ± 0.23 | 1.09 ± 0.35 | 1.5 ± 0.6 | P = 0.0007 |
| Diuretics (%) | 90.9% (n = 60) | 33.3% (n = 22) | 19.7% (n = 13) | 37.9% (n = 25) | P = 0.025 |
| Beta‐blockers (%) | 93.5% (n = 58) | 33.8% (n = 21) | 24.2% (n = 15) | 35.5% (n = 22) | P = 0.2 |
| ACE‐inhibitors (%) | 50% (n = 31) | 20.1% (n = 13) | 14.5% (n = 9) | 14.5% (n = 9) | P = 0.2 |
| ARBs (%) | 29% (n = 18) | 6.45% (n = 4) | 4.8% (n = 3) | 17.7% (n = 11) | P = 0.1 |
| MRA (%) | 88.7% (n = 55) | 32.2% (n = 20) | 21% (n = 13) | 35.5% (n = 22) | P = 0.9 |
| ARNi (%) | 10% (n = 10) | 2% (n = 2) | 3.5% (n = 3) | 8% (n = 5) | P = 0.06 |
ACE, angiotensin converting enzyme‐2; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor‐nepylisin inhibitor; BSA, body surface area; CAD, coronary artery disease; CKD, chronic kidney disease; fwRVLS, free‐wall right ventricular longitudinal strain; HR, heart rate; MRA, mineralcorticoid receptor antagonist; NT‐proBNP, N‐terminal brain natriuretic peptide; PALS, peak atrial longitudinal strain, sBP, systolic blood pressure; sPAP, systolic pulmonary artery pressure
Echocardiographic features of the study cohort stratified into the three groups are shown in Table 2 . Mitral E/A, LVEF, LAVI, RV functional indices (TAPSE, S'tricuspid, & RVFAC), sPAP, and STE parameters (LV GLS, global PALS and PACS, global RVLS, & fwRVLS) showed statistically significant variations between the groups. Group 3 showed a significantly higher percentage of patients with MR and TR. Overall, 14 patients (16%) had severe MR, of which only 6 were included in Group 3 while only 4 patients (5%) had severe TR, with no patient having massive or torrential TR.
Table 2.
Echocardiographic parameters of the study population
| Total sample | Group 1 (n = 28) | Group 2 (n = 24) | Group 3 (n = 32) | Overall P value | |
|---|---|---|---|---|---|
| LV mass (g) | 300.5 ± 93.7 | 299.4 ± 107 | 299 ± 100.9 | 302.5 ± 73.6 | P = 0.98 |
| LVEDVi (mL/m2) | 106.4 ± 33.3 | 97.7 ± 6.2 | 107.8 ± 6.8 | 113 ± 5.9 | P = 0.2 |
| LVESVi (mL/m2) | 77.5 ± 28.9 | 68.1 ± 3.6 | 76.9 ± 31 | 86.2 ± 34 | P = 0.05 |
| LVEF (%) | 28 ± 5% | 30 ± 5% | 30 ± 6% | 25 ± 6% | P = 0.0003 |
| E/A | 1.8 ± 1 | 1.02 ± 0.56 | 1.7 ± 0.98 | 2.5 ± 1.32 | P < 0.0001 |
| DT (m/s) | 163.7 ± 54.9 | 164.9 ± 10.6 | 168.7 ± 11.2 | 158.9 ± 9.7 | P = 0.79 |
| Mean E/E' | 14.6 ± 8.9 | 10.5 ± 1.7 | 15.9 ± 1.8 | 17.3 ± 1.5 | P = 0.012 |
| LAVI (mL/m2) | 55.3 ± 16.7 | 40.5 ± 12.6 | 57.3 ± 17.9 | 66.9 ± 18.9 | P < 0.0001 |
| MR grade > 2 (%) | 48.8% (n = 41) | 7.1% (n = 6) | 16.7% (n = 14) | 25% (n = 21) | P < 0.0004 |
| TR grade > 2 (%) | 19.05% (n = 16) | 0 | 5.95% (n = 5) | 13.09% (n = 11) | P < 0.0001 |
| TAPSE (mm) | 17.6 ± 3.7 | 19.5 ± 4.1 | 18 ± 3.2 | 15.7 ± 3.7 | P < 0.007 |
| RVFAC (%) | 38.6 ± 9% | 41.6 ± 8.5% | 40.3 ± 8.6% | 34.6 ± 9.8% | P < 0.077 |
| sPAP (mmHg) | 37.6 ± 10.7 | 27.7 ± 4.8 | 37.6 ± 11.7 | 46.2 ± 13.2 | P < 0.0001 |
| S't (m/s) | 0.1 ± 0.03 | 0.11 ± 0.03 | 0.09 ± 0.03 | 0.1 ± 0.03 | P < 0.043 |
| GLS (%) | −7.5 ± 2.6% | −9.1 ± 2.7 | −8.4 ± 2.7% | −5.5 ± 2.4% | P < 0.0001 |
| Global PALS (%) | 14 ± 4.6% | 21.8 ± 5.9% | 13.9 ± 4.8% | 7.3 ± 2.8% | P < 0.0001 |
| Global PACS (%) | 7.8 ± 3.1% | 13.04 ± 3.5% | 7.6 ± 3.5% | 3.5 ± 2.4% | P < 0.0001 |
| Global RVLS (%) | −13.7 ± 3.7 | −16.2 ± 3.8% | −14.8 ± 4.6% | −10.6 ± 2.7% | P < 0.0001 |
| fwRVLS (%) | −19.3 ± 4.5% | −21.39 ± 4% | −21.25 ± 5.2% | −15.7 ± 4.4% | P < 0.0001 |
E/A, trans‐mitral early diastolic E wave/late diastolic A wave ratio; E', end‐diastolic mitral annular velocity; fwRVLS, free‐wall right ventricular longitudinal strain; GLS, global longitudinal strain; LAVI, left atrial volume index; LV, left ventricular; LVEDVi, left ventricular end‐diastolic volume/BSA; LVESVi, left ventricular end‐systolic/BSA; MR, mitral regurgitation; PACS, peak atrial contraction strain; PALS, peak atrial longitudinal strain; RVFAC, right ventricular functional area change; RVLS, right ventricular longitudinal strain; sPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
Mean follow up was 3.2 ± 0.3 years, during which 48 patients reached the combined endpoint (Group 1: 7 patients, Group 2: 13 patients, Group 3: 13 patients; Figure 2 , Table S 2 ). All patients without events were followed until the end of follow up. Kaplan–Meier curves showed early divergence and persistent separation between the three groups for the prediction of the primary outcome of cardiovascular death and HF hospitalization (Figures 2 , 3 Kaplan Meier survival curves showing the risk stratification of the three groups for the composite endpoint of cardiovascular death and heart failure (HF) hospitalization. fwRVLS,free wall right ventricular longitudinal strain; PALS,peak atrial longitudinal strain; sPAP, systolic pulmonary artery pressure) and for the secondary outcome of overall death (Figure S1 ).
Figure 2.
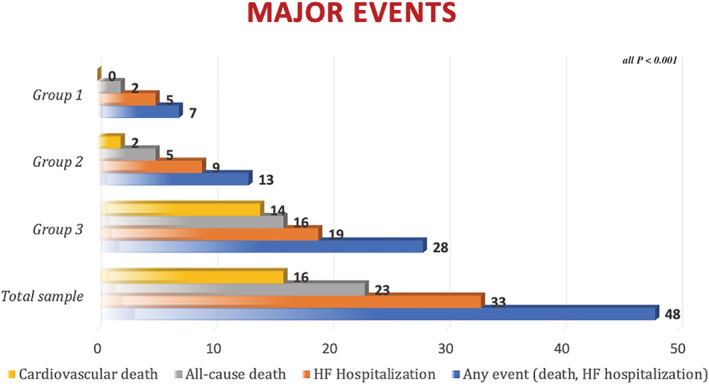
Distribution of major events in the study population (over a mean follow up of 3,5 ± 0,3 years).
Figure 3.
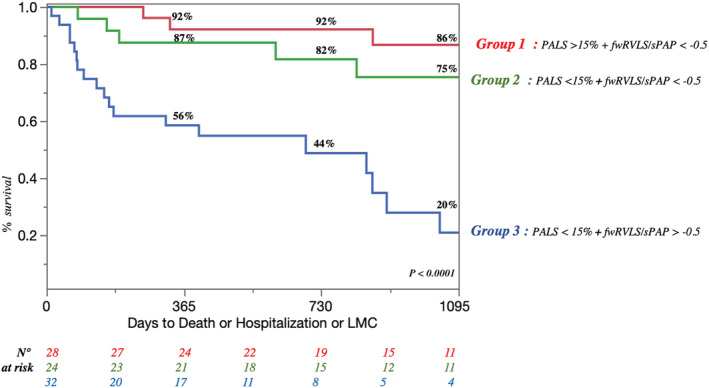
Kaplan Meier survival curves showing the risk stratification of the three groups for the composite endpoint of cardiovascular death and heart failure (HF) hospitalization. fwRVLS, free wall right ventricular longitudinal strain; PALS, peak atrial longitudinal strain; sPAP, systolic pulmonary artery pressure.
Cox proportional hazard model revealed a high predictive value of reduced PALS and fwRVLS/sPAP vs. both normal [Group 3 vs. Group 1, hazard ratio (HR) = 10.61, P < 0.0001] and vs. impaired PALS (Group 3 vs. Group 2, HR = 3.9, P < 0.0002) for the prediction of the composite endpoint, which was maintained at bivariate analysis after adjustment for GLS alone (HR = 10.24, P < 0.0001 and HR = 3.82, P = 0.0008, respectively) and at multivariate analysis adjusted for GLS, LAVI, TR grade > 2, and RVFAC (HR = 9.54, P = 0.0002 and HR = 3.78, P = 0.002, respectively) (Table 3 ). PALS was also an independent predictor of events (P < 0.0001) in a dedicated survival model.
Table 3.
Cox hazard models for Group 3 prediction of the combined endpoint compared with Groups 2 and 1
| Unadjusted HR [95% CI] | Adjusted for GLS,HR [95% CI] | Adjusted for GLS, LAVi, TR, and RVFAC,HR [95% CI] | Adjusted for renal function, valvular function, NT‐proBNP, and NYHA class,HR [95% CI] | |
|---|---|---|---|---|
| Group 3 vs. 1 | 10.61 [4.16–27.06], P < 0.0001 | 10.24 [3.49–30.02], P < 0.0001 | 9.54 [2.95–30.92], P = 0.0002 | 12.10 [2.29–63.87], P = 0.02 |
| Group 3 vs. 2 | 3.90 [1.92–7.93], P = 0.0002 | 3.82 [1.74–8.36], P = 0.0008 | 3.78 [1.66–8.61], P = 0.002 | 4.07 [1.22–13.56], P = 0.003 |
| Group 2 vs. 1 | 2.72 [1.03–7.20], P = 0.04 | 2.69 [0.99–7.25], P = 0.05 | 2.53 [0.84–7.58], P = 0.1 | 2.97 [0.61–14.4], P = 0.2 |
CI, confidence interval; EF, ejection fraction; HR, hazard ratio; LAVI, left atrial volume index; MR, mitral regurgitation, RVFAC, right ventricular fractional area change; TR, tricuspid regurgitation.
The first column represents the univariate analysis of comparisons between groups; the second column represents bivariate analysis of comparisons between groups adjusted for GLS; the third column represents multivariate analysis of comparisons between groups, adjusted for GLS, LAVI, TR, and RVFAC; and the fourth columns represents multivariate analysis of comparisons between groups, adjusted for renal function, valvular function, NT‐proBNP, and NYHA class.
Discussion
The present study constitutes an echocardiographic investigation of the best indices to early identify, among patients with HFrEF, those with atrial and/or right HF and worse prognosis. Following the pathophysiologic model of chronic HF and using advanced echocardiography, the assessment of LA dysfunction by STE and RV/PC involvement, estimated with a new measure of fwRVLS/sPAP correlating RV deformation with sPAP, revealed to provide important information for risk stratification of these patients. In fact, after calculating the optimal cut‐off values for this population sample, three risk groups with worsening outcome was depicted: (1) patients with medium‐stage chronic HF and reduced LV systolic function (low LVEF and GLS) in which LA, PC and RV still work to counteract the increased intracardiac filling pressures; (2) those with initial involvement of LA as a result of chronic HF damage; (3) patients in the last stage of the disease, in which LA function is totally compromised and RV compliance and function worsen, with dramatic prognostic consequences.
Recent change in heart failure phenotype
In the last decades, a progressive change in HF phenotype has been observed. In fact, mortality was previously mostly due to a decompensated LV failure; thus, LV functional indices such as LVEF were the most used diagnostic and prognostic markers. Contrarily, the optimization of HF therapy led to the longer survival of patients with isolate LV impairment despite underlying low LVEF. However, the presence of a ‘stiff’ LV chronically exposes them to higher LV filling pressures, which gradually leads to LA maladaptive remodelling and impairment, 4 whose estimation correlates with overt HF symptoms and significant reduction in functional capacity, 16 and provides important prognostic information.6, 17 Accordingly, a clear raise in NYHA class has been shown in Groups 2 and 3 of our cohort (Figure 4 ), in which all patients had abnormal PALS (normality range = 38–41% 18 ). Moreover, a higher prevalence of right HF has recently been reported, characterized by peripheric congestion and PH, with poor prognosis, primarily due to its challenging management. 19
Figure 4.
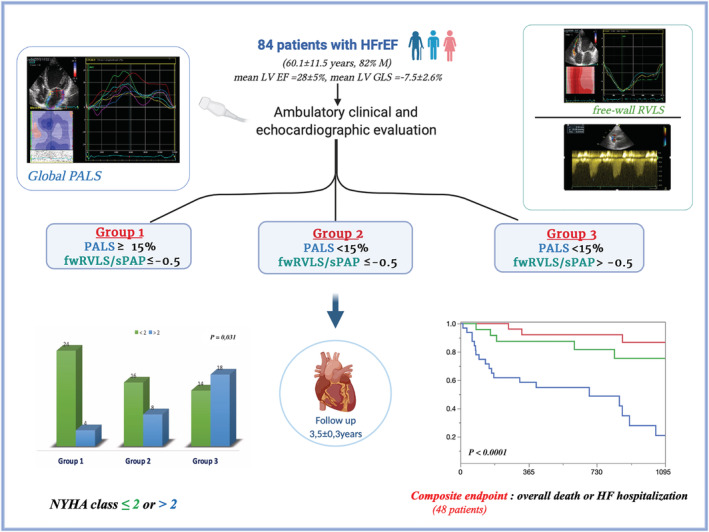
Echocardiographic prognostic indices based on pathophysiologic progressive damage of chronic heart failure. EF, ejection fraction; fwRVLS, free‐wall right ventricular longitudinal strain; GLS; global longitudinal strain; HFrEF, heart failure with reduced ejection fraction; LV, left ventricular; NYHA, New York Heart Association; PALS, peak atrial longitudinal strain; sPAP, systolic pulmonary artery pressure.
FwRVLS/sPAP: a new early marker of right heart failure
It is well‐known that PC has a barrage action between left and right heart; in fact, when PH appears, because of a greater haemodynamic load in chronic HF, it acts as a pressure afterload on the RV. Because RV function is afterload‐dependent, it is highly affected by the chronic increase in pulmonary vascular resistance. When RV remodelling and contractile function become insufficient to compensate PH, overt RV failure occurs. 20 Of note, RV remodelling would affect RV diastolic properties, 21 increasing RV stiffness and worsening PH, with poor prognosis. In HF, particularly in advanced stages, it is crucial to consider the balance between RV function and pulmonary circulation, which could strongly influence prognosis of these patients due to HF pathophysiology. Therefore, investigating the ratio between RV strain, which is a pure and less load‐dependent index of myocardial performance and PC by haemodynamical indices, would provide additive information on patients' clinical conditions and help to identify patients more prone to develop acute HF and with less capability to adapt to medical therapy, with consequent higher mortality risk. In fact, some authors have already highlighted the prognostic importance of invasive indices of RV failure/PH in patients with HF and PH. 22 , 23 However, the strict link between RV function and pulmonary vascular resistance for global RV response to haemodynamic changes makes the evaluation of RV/PC interaction paramount for non‐invasive prognostic evaluation. This relationship, previously called ‘RV‐pulmonary arterial coupling’, could be assessed using pressure–volume loops during cardiopulmonary exercise testing. 24 However, this is not routinely performed, because of technical complexity or patients' inability to exercise. Some authors have introduced TAPSE/sPAP as non‐invasive parameter to estimate RV‐pulmonary coupling, 25 also proving its correlation with prognosis in medium cohorts of HF patients, 26 , 27 with a proposed cut‐off value < 0.36. Nevertheless, it is known that TAPSE does not offer a thorough evaluation of RV contractile performance, being a mono‐dimensional angle‐dependent measure and analysing only RV basal segment movement. STE could overcome these limitations, providing a complete assessment of RV regional and global deformation. In fact, Iacoviello et al. used RV GLS/sPAP and fwRVLS/sPAP to estimate prognosis in chronic HF patients, showing an independent association with all‐cause mortality at univariate and multivariate analysis (HR: 0.66, P = 0.008 for RV GLS/sPAP < 0.36 and HR: 0.65, P = 0.002 for fwRVLS/sPAP < 0.66). 28 The results of our study confirm and complete these findings, integrating them into a comprehensive approach for risk stratification. Also, our absolute cut‐off value (0.5) is lower than that of Iacoviello et al., 28 hopefully resulting in higher specificity for the detection of severe RV/PC interaction impairment. Of note, it is also easier to remind for daily use in clinical practice.
The additive value of speckle tracking echocardiography
After more than 10 years of research showing its advantages for the improvement of non‐invasive diagnostic and prognostic paradigms of different cardiovascular disease, 5 , 29 STE has now been integrated in clinical practice, also thanks to its wide availability. As regards HF prognosis, LV strain has already shown to be superior to LVEF, 30 which is known to be limited by loading and operator dependence, underestimation in case of relevant MR, and lower accuracy, especially in the advanced stages of the disease. 31 However, GLS were considerably impaired in all the three groups of our cohort, and multivariate analysis showed the prognostic independence of PALS and fwRVLS/sPAP from GLS.
PALS is an early index of LA fibrosis caused by maladaptive remodelling in chronic HF, even in non‐dilated atria, 4 and a superior predictor of LV filling pressures than E/e' ratio in HF. 5 , 32 Its prognostic role has been well‐established in chronic HF with different grades of LVEF reduction, with influence on clinical outcome and exercise capacity 6 , 33 ; moreover, it has also shown to be a useful index of response to HF therapy. 2 , 34 Even though in previous studies investigating PALS prognostic value in HFpEF the derived cut‐off values were considerably higher than our (>30%), cut‐off values found in cohorts with HFrEF were 15%, 33 15.5%, 35 and 17%, 6 very similar to our ROC‐curves‐derived cut‐off (15%).
As previously discussed, RV strain by STE allows a reliable and angle‐independent evaluation of RV deformation also in early stages of its dysfunction. In fact, it has demonstrated its superiority over other conventional echocardiographic indices of RV function (TAPSE, S', & RVFAC) for the prediction of cardiovascular outcome in patients with HFrEF, 9 also in patients referred for HTX or LVAD, with a cut‐off value of −15%. 10 , 11 As stated in the latest European Association of Cardiovascular Imaging standardization document, fwRVLS should be preferred to RV GLS as it specifically analyses RV wall motion, avoiding the influence of interventricular septum. 36 In a previous study, we have shown that fwRVLS is comparable with invasive measures of LV filling pressures with optimum accuracy (area under curve = 0.90). 37
Need for early diagnosis of right hear failure
In patients with chronic HF, the transition from left‐sided to right‐sided HF represents a hallmark of dramatic prognosis. This is due to the lack of specific therapies for right HF and to the limited advanced therapeutic resources, such as HTX or LVAD. Our study shows a crucial role of PC as a gateway between left and right heart haemodynamics, suggesting that its interaction with RV function should not be overlooked. Moreover, RV dysfunction and high sPAP constitutes exclusion criteria for patients with HFrEF to access to HTX and LVAD implantation. Therefore, assuming that PALS < 15% and fwRVLS/sPAP > −0.5 are potential risk factors for developing RV failure, we suggest that patients with similar echocardiographic characteristics of the Group 2 of our study should be considered as belonging to an early‐stage RV failure, having at least one of the two risk factors. This would possibly lead to early diagnosis, providing adequate management and strict follow up to these patients. Therefore, we suggest the use of these advanced echocardiographic indices with our proposed cut‐off values as adjunctive tools in daily clinical practice, which would represent an easy and quick tool to enhance the risk stratification of HFrEF patients after a complete clinical evaluation and basic echocardiographic exam. This may improve the selection of treatment strategies among more or less aggressive pharmacologic therapy and optimize the allocation of advanced therapeutic resources (e.g. LVAD/HTX).
Limitations
Despite presenting promising results, which are corroborated by previous evidence, some limitations of this study should be discussed. Firstly, it was a single‐centre study with a small cohort; therefore, validation in bigger studies is needed to generalize these results. Secondly, STE technique has some limitations: it requires optimal apical views to permit easy delineation of the endocardial border; moreover, LA and RV strain were assessed using a vendor‐specific software designed for LV, since at the time of analysis a dedicated software had not yet been released. Lastly, in the multivariate analysis, there was the impossibility to account for all clinical covariates due to the limited sample size; however, our analysis focused on the most important echocardiographic features of HF patients, which we found overall quite complete.
Conclusions
In patients with chronic HF, the risk of mortality has lately moved from left‐sided to right‐sided HF, which represents the last stage of the disease. Therefore, it would be crucial to recognize patients with higher risk to develop end‐stage right HF before it occurs, in order to provide early and optimal treatment. In this study, a combination of LA damage assessed by PALS < 15% and pathological fwRVLS/sPAP, assessed by fwRVLS/sPAP, has shown to provide accurate risk stratification of patients with HFrEF independently from other HF known prognostic indices (Figure 4 ). These STE parameters could be used to classify such patients as belonging to lowest grades of RV failure.
Conflict of interest
None declared.
Funding
None.
Supporting information
Table S1. Area under curves (AUC) calculated by ROC curves of tricuspid annular plane systolic excursion (TAPSE), TAPSE/systolic pulmonary artery pressure (sPAP), TAPSE x peak systolic trans‐tricuspid gradient, peak atrial longitudinal strain (PALS), free wall right ventricular longitudinal strain (fwRVLS)/PAPs for the prediction of cardiovascular death, hospitalizations for heart failure and the composite outcome (cardiovascular death or hospitalizations for heart failure).
Table S2. distribution of the events in the three groups of our study population.
Figure S1. Kaplan Meier survival curves showing the stratification of the three groups for all‐cause death. fwRVLS, free wall right ventricular longitudinal strain; PALS, peak atrial longitudinal strain; sPAP, systolic pulmonary artery pressure.
Mandoli, G. E. , Pastore, M. C. , Benfari, G. , Setti, M. , Nistor, D. , D'Ascenzi, F. , Focardi, M. , Baccani, B. , Patti, G. , Valente, S. , Mondillo, S. , and Cameli, M. (2022) New echocardiographic indices of shift to biventricular failure to optimize risk stratification of chronic heart failure. ESC Heart Failure, 9: 476–485. 10.1002/ehf2.13722.
References
- 1. Desai AS, Solomon SD, Shah AM, Claggett BL, Fang JC, Izzo J, McCague K, Abbas CA, Rocha R, Mitchell GF, EVALUATE‐HF Investigators . Effect of sacubitril‐valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2019; 322: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deferm S, Martens P, Verbrugge FH, Bertrand PB, Dauw J, Verhaert D, Dupont M, Vandervoort PM, Mullens W. LA mechanics in decompensated heart failure: insights from strain echocardiography with invasive hemodynamics. JACC Cardiovasc Imaging 2020; 13: 1107–1115. [DOI] [PubMed] [Google Scholar]
- 3. Pastore MC, Mandoli GE, Aboumarie HS, Santoro C, Bandera F, D’Andrea A, Benfari G, Esposito R, Evola V, Sorrentino R, Cameli P, Valente S, Mondillo S, Galderisi M, Cameli M. Basic and advanced echocardiography in advanced heart failure: an overview. Heart Fail Rev. 2020; 25(6): 937–948. 10.1007/s10741-019-09865-3 [DOI] [PubMed] [Google Scholar]
- 4. Cameli M, Pastore MC, Henein MY, Mondillo S. The left atrium and the right ventricle: two supporting chambers to the failing left ventricle. Heart Fail Rev 2019; 24: 661–669. [DOI] [PubMed] [Google Scholar]
- 5. Cameli M, Mandoli GE, Loiacono F, Dini FL, Henein M, Mondillo S. Left atrial strain: a new parameter for assessment of left ventricular filling pressure. Heart Fail Rev 2016; 21: 65–76. [DOI] [PubMed] [Google Scholar]
- 6. Malagoli A, Rossi L, Bursi F, Zanni A, Sticozzi C, Piepoli MF, Villani GQ. Left atrial function predicts cardiovascular events in patients with chronic heart failure with reduced ejection fraction. J Am Soc Echocardiogr 2019; 32: 248–256. [DOI] [PubMed] [Google Scholar]
- 7. Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, Klein DA, Dixon D, Baldridge A, Rasmussen‐Torvik LJ, Maganti K, Shah SJ. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circ Cardiovasc Imaging 2016; 9: e003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Santos AB, Roca GQ, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Fang JC, Zile MR, Pitt B, Solomon SD, Shah AM. Prognostic relevance of left atrial dysfunction in heart failure with preserved ejection fraction. Circ Heart Fail 2016; 9: e002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carluccio E, Biagioli P, Alunni G, Murrone A, Zuchi C, Coiro S, Riccini C, Mengoni A, D'Antonio A, Ambrosio G. Prognostic value of right ventricular dysfunction in heart failure with reduced ejection fraction: superiority of longitudinal strain over tricuspid annular plane systolic excursion. Circ Cardiovasc Imaging 2018; 11: e006894. [DOI] [PubMed] [Google Scholar]
- 10. Cameli M, Pastore MC, Mandoli GE, Nistor D, Lisi E, Tok ÖÖ, Cavigli L, Romano A, Mondillo S. Prognosis and risk stratification of patients with advanced heart failure (from PROBE). Am J Cardiol 2019; 124: 55–62. [DOI] [PubMed] [Google Scholar]
- 11. Cameli M, Righini FM, Lisi M, Bennati E, Navarri R, Lunghetti S, Padeletti M, Cameli P, Tsioulpas C, SoniaBernazzali MM, Sani G, Henein M, Mondillo S. Comparison of right versus left ventricular strain analysis as a predictor of outcome in patients with systolic heart failure referred for heart transplantation. Am J Cardiol 2013; 112: 1778–1784. [DOI] [PubMed] [Google Scholar]
- 12. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Piepoli MF, Price S, Rosano GMC, Ruschitzka F, Skibelund AK. ESC Scientific Document Group, 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2021; 42(36): 3599–3726. 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 13. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270. [DOI] [PubMed] [Google Scholar]
- 14. Cameli M, Miglioranza MH, Magne J, Mandoli GE, Benfari G, Ancona R, Sibilio G, Reskovic Luksic V, Dejan D, Griseli L, Van De Heyning CM, Mortelmans P, Michalski B, Kupczynska K, Di Giannuario G, Devito F, Dulgheru R, Ilardi F, Salustri A, Abushahba G, Morrone D, Fabiani I, Penicka M, Katbeh A, Sammarco G, Esposito R, Santoro C, Pastore MC, Comenale Pinto S, Kalinin A, Pičkure Ž, Ažman Juvan K, Zupan Mežnar A, Coisne A, Coppin A, Opris MM, Nistor DO, Paakkanen R, Biering‐Sørensen T, Olsen FJ, Lapinskas T, Vaškelyté JJ, Galian‐Gay L, Casas G, Motoc AI, Papadopoulos CH, Loizos S, Ágoston G, Szabó I, Hristova K, Tsonev SN, Galli E, Vinereanu D, Mihaila Baldea S, Muraru D, Mondillo S, Donal E, Galderisi M, Cosyns B, Edvardsen T, Popescu BA. Multicentric atrial strain comparison between two different modalities: MASCOT HIT study. Diagnostics (Basel) 2020; 10: 946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zoghbi WA, Adams D, Bonow RO, Enriquez‐Sarano M, Foster E, Grayburn PA. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017; 30: 303–371. [DOI] [PubMed] [Google Scholar]
- 16. D'Andrea A, Caso P, Romano S, Scarafile R, Cuomo S, Salerno G, Riegler L, Limongelli G, Di Salvo G, Romano M, Liccardo B, Iengo R, Ascione L, Del Viscovo L, Calabrò P, Calabrò R. Association between left atrial myocardial function and exercise capacity in patients with either idiopathic or ischemic dilated cardiomyopathy: a two‐dimensional speckle strain study. Int J Cardiol 2009; 132: 354–363. [DOI] [PubMed] [Google Scholar]
- 17. Kebed KY, Addetia K, Lang RM. Importance of the left atrium: more than a bystander? Heart Fail Clin 2019; 15: 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pathan F, D'Elia N, Nolan MT, Marwick TH, Negishi K. Normal ranges of left atrial strain by speckle‐tracking echocardiography: a systematic review and meta‐analysis. J Am Soc Echocardiogr 2017. Jan; 30: 59–70.e8. [DOI] [PubMed] [Google Scholar]
- 19. Chizinga M, Fares WH. Chronic right heart failure: expanding prevalence and challenges in outpatient management. Heart Fail Clin 2018; 14: 413–423. [DOI] [PubMed] [Google Scholar]
- 20. Khush KKTG, Butler J, McGlothlin D, De Marco T, ESCAPE Investigators . Effect of pulmonary hypertension on clinical outcomes in advanced heart failure: analysis of the Evaluation Study of Congestive heart failure And Pulmonary artery catheterization Effectiveness (ESCAPE) database. Am Heart J 2009; 157: 1026–1034. [DOI] [PubMed] [Google Scholar]
- 21. Cameli P, Pastore MC, Mandoli GE, Vigna M, De Carli G, Bergantini L, d’Alessandro M, Ghionzoli N, Bargagli E, Cameli M Strain Echocardiography Is a Promising Tool for the Prognostic Assessment of Sarcoidosis. Life 2021; 11(10): 1065. 10.3390/life11101065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rain S, Andersen S, Najafi A, Gammelgaard Schultz J, da Silva Gonçalves Bós D, Handoko ML, Bogaard H‐J, Vonk‐Noordegraaf A, Andersen A, van der Velden J, Ottenheijm CAC, de Man FS. Right ventricular myocardial stiffness in experimental pulmonary arterial hypertension: relative contribution of fibrosis and myofibril stiffness. Circ Heart Fail 2016; 9: e002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alaa M, Abdellatif M, Tavares‐Silva M, Oliveira‐Pinto J, Lopes L, Leite S, Leite‐Moreira AF, Lourenço AP. Right ventricular end‐diastolic stiffness heralds right ventricular failure in monocrotaline‐induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 2016; 311: H1004–H1013. [DOI] [PubMed] [Google Scholar]
- 24. Singh I, Rahaghi FN, Naeije R, Oliveira RKF, Vanderpool RR, Waxman AB, Systrom DM. Dynamic right ventricular‐pulmonary arterial uncoupling during maximum incremental exercise in exercise pulmonary hypertension and pulmonary arterial hypertension. Pulm Circ 2019; 9: 2045894019862435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ghio S, Guazzi M, Scardovi AB, Klersy C, Clemenza F, Carluccio E. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur J Heart Fail 2017; 19: 873–879. [DOI] [PubMed] [Google Scholar]
- 26. Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti L, Ghio S, Temporelli PL, Arena R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol 2013; 305: H1373–H1381. [DOI] [PubMed] [Google Scholar]
- 27. Guazzi M, Naeijie R, Arena R, Corrà U, Ghio S, Forfia P, Rossi A, Cahalin LP, Bandera F, Temporelli P. Echocardiography of right ventriculoarterial coupling combined with cardiopulmonary exercise testing to predict outcome in heart failure. Chest 2015; 148: 226–234. [DOI] [PubMed] [Google Scholar]
- 28. Iacoviello M, Monitillo F, Citarelli G, Leone M, Grande D, Antoncecchi V, Rizzo C, Terlizzese P, Romito R, Caldarola P, Ciccone MM. Right ventriculo‐arterial coupling assessed by two‐dimensional strain: a new parameter of right ventricular function independently associated with prognosis in chronic heart failure patients. Int J Cardiol 2017; 241: 318–321. [DOI] [PubMed] [Google Scholar]
- 29. Pastore MC, De Carli G, Mandoli GE, D'Ascenzi F, Focardi M, Contorni F, Mondillo S, Cameli M. The prognostic role of speckle tracking echocardiography in clinical practice: evidence and reference values from the literature. Heart Fail Rev 2020; 26(6): 1371–1381. [DOI] [PubMed] [Google Scholar]
- 30. Sengeløv M, Jorgensen PG, Jensen JS, Bruun NE, Olsen FJ, Fritz‐Hansen T, Nochioka K, Biering‐Sørensen T. Global longitudinal strain is a superior predictor of all‐cause mortality in heart failure with reduced ejection fraction. JACC Cardiovasc Imaging 2015; 8: 1351–1359. [DOI] [PubMed] [Google Scholar]
- 31. Cameli M, Pastore MC, De Carli G, Henein MY, Mandoli GE, Lisi E, Cameli P, Lunghetti S, D'Ascenzi F, Nannelli C, Rizzo L, Valente S, Mondillo S. ACUTE HF score, a multiparametric prognostic tool for acute heart failure: a real‐life study. Int J Cardiol 2019; 296: 103–108. [DOI] [PubMed] [Google Scholar]
- 32. Smiseth OA, Morris DA, Cardim N, Cikes M, Delgado V, Donal E, Flachskampf FA, Galderisi M, Gerber BL, Gimelli A, Klein AL, Knuuti J, Lancellotti P, Mascherbauer J, Milicic D, Seferovic P, Solomon S, Edvardsen T, Popescu BA. Multimodality imaging in patients with heart failure and preserved ejection fraction: an expert consensus document of the European Association of Cardiovascular Imaging. European Heart Journal ‐ Cardiovascular Imaging 2021. 10.1093/ehjci/jeab154 [DOI] [PubMed] [Google Scholar]
- 33. Sugimoto T, Barletta M, Bandera F, Generati G, Alfonzetti E, Rovida M, Ruscone TG, Rossi A, Cicoira M, Guazz M. Central role of left atrial dynamics in limiting exercise cardiac output increase and oxygen uptake in heart failure: insights by cardiopulmonary imaging. Eur J Heart Fail 2020; 22(7): 1186–1198. [DOI] [PubMed] [Google Scholar]
- 34. Cameli M, Pastore MC, Pagliaro A, Di Tommaso C, Reccia R, Curci V, Mandoli GE, Mondillo S. Sacubitril/Valsartan in an elderly patient with heart failure: a case report. Cardiology 2017; 138: 3–6. [DOI] [PubMed] [Google Scholar]
- 35. Carluccio E, Biagioli P, Mengoni A, Francesca Cerasa M, Lauciello R, Zuchi C, Bardelli G, Alunni G, Coiro S, Gronda EG, Ambrosio G. Left atrial reservoir function and outcome in heart failure with reduced ejection fraction. Circ Cardiovasc Imaging 2018; 11: e007696. [DOI] [PubMed] [Google Scholar]
- 36. Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, D'Hooge J, Donal E, Fraser AG, Marwick T, Mertens L. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two‐dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2018; 19: 591–600. [DOI] [PubMed] [Google Scholar]
- 37. Cameli M, Lisi M, Righini FM, Tsioulpas C, Bernazzali S, Maccherini M, Sani G, Ballo P, Galderisi M, Mondillo S. Right ventricular longitudinal strain correlates well with right ventricular stroke work index in patients with advanced heart failure referred for heart transplantation. J Card Fail 2012; 18: 208–215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Area under curves (AUC) calculated by ROC curves of tricuspid annular plane systolic excursion (TAPSE), TAPSE/systolic pulmonary artery pressure (sPAP), TAPSE x peak systolic trans‐tricuspid gradient, peak atrial longitudinal strain (PALS), free wall right ventricular longitudinal strain (fwRVLS)/PAPs for the prediction of cardiovascular death, hospitalizations for heart failure and the composite outcome (cardiovascular death or hospitalizations for heart failure).
Table S2. distribution of the events in the three groups of our study population.
Figure S1. Kaplan Meier survival curves showing the stratification of the three groups for all‐cause death. fwRVLS, free wall right ventricular longitudinal strain; PALS, peak atrial longitudinal strain; sPAP, systolic pulmonary artery pressure.


