Abstract
Aims
The aim of this study was to evaluate the prognostic value of feature tracking (FT) derived cardiac magnetic resonance (CMR) strain parameters of the left ventricle (LV)/right ventricle (RV) in ischaemic cardiomyopathy (ICM) patients treated with an implantable cardioverter‐defibrillator (ICD). Current guidelines suggest a LV‐ejection fraction ≤35% as major criterion for ICD implantation in ICM, but this is a poor predictor for arrhythmic events. Supplementary parameters are missing.
Methods and results
Ischaemic cardiomyopathy patients (n = 242), who underwent CMR imaging prior to primary and secondary implantation of ICD, were classified depending on EF ≤ 35% (n = 188) or >35% (n = 54). FT parameters were derived from steady‐state free precession cine views using dedicated software. The primary endpoint was a composite of cardiovascular mortality (CVM) and/or appropriate ICD therapy. There were no significant differences in FT‐function or LV‐/RV‐function parameters in patients with an EF ≤ 35% correlating to the primary endpoint. In patients with EF > 35%, standard CMR functional parameters, such as LV‐EF, did not reveal significant differences. However, significant differences in most FT parameters correlating to the primary endpoint were observed in this subgroup. LV‐GLS (left ventricular‐global longitudinal strain) and RV‐GRS (right ventricular‐global radial strain) revealed the best diagnostic performance in ROC curve analysis. The combination of LV‐GLS and RV‐GRS showed a sensitivity of 85% and a specificity of 76% for the prediction of future events.
Conclusions
The impact of FT derived measurements in the risk stratification of patients with ICM depends on LV function. The combination of LV‐GLS/RV‐GRS seems to be a predictor of cardiovascular mortality and/or appropriate ICD therapy in patients with EF > 35%.
Keywords: Magnetic resonance imaging, Non‐contrast media, Feature tracking, Strain, Implantable cardioverter defibrillator, Ejection fraction, Ischaemic cardiomyopathy, Ventricular tachycardia, Sudden cardiac death
Introduction
Life‐threatening ventricular arrhythmias (VA) are a common risk in heart failure patients with a reduced left ventricular ejection fraction (LVEF) as a result of ischaemic cardiomyopathy (ICM). Current guidelines for the prevention of sudden cardiac death (SCD) recommend the implantable cardioverter defibrillator (ICD) therapy based on EF as primary preventive measure in patients with an LVEF ≤35%, and as secondary prevention (SP) in patients after an aborted SCD or unstable sustained VT, irrespective of LVEF>35%. 1 However, it is apparent that appropriate therapy is delivered in only a minority of patients with EF ≤ 35%. Additionally, specific patients with mild or moderate reduction of EF (>35%), who would also benefit from ICD implantation, cannot be properly identified as the current guidelines are not exhaustive enough to define this subgroup. 1
In this context, the analysis of myocardial strain could potentially contribute to bridge this gap. 2
Feature tracking (FT) is a novel cardiovascular magnetic resonance (CMR) postprocessing tool, which uses optical flow algorithms to follow myocardial image characteristics (‘features’) on cine SSFP sequences throughout the phases of the cardiac cycle in order to acquire strain parameters. This yields the potential of a contrast agent independent analysis of 3D cardiac mechanics, thereby demonstrating its potential in risk stratification for dilated cardiomyopathy (DCM) and ICM patients. 3 , 4 , 5 , 6
Most of the above‐mentioned studies investigated the impact of strain measurements in mixed populations including patients with various cardiomyopathies and a broad spectrum of LV dysfunction. However, we hypothesize that the impact of strain analysis in the risk stratification is dependent on the functional impairment and underlying aetiology. Thus, our study aims to evaluate the prognostic value of CMR‐FT‐derived parameters classified according to the EF in a population consisting solely of patients with ICM and an implanted ICD.
Methods
Study protocol
A total of 251 consecutive patients from a single centre, who underwent CMR imaging prior to ICD implantation between 2005 and 2016, were included in this retrospective study and their data analysed.
Nine patients had to be excluded due to insufficient imaging quality or missing CMR sequences resulting in a total of 242 (96%) patients for final analysis.
The indication for ICD implantation was according to the current ESC guidelines for prevention of SCD. 1 Fifteen patients of our population received an upgrade to a cardiac resynchronization therapy defibrillator device during follow‐up due to an indication for cardiac resynchronization therapy. In these patients the follow‐up ended at the date of the upgrade. No further inclusive or exclusion criteria were defined.
The choice and implantation of the ICD device was performed by two physicians in standard technique and the programming of the ICD was in consensus with existing current recommendations. The follow‐up of patients took place 1 month after ICD implantation and subsequently after every 4–6 months in regular intervals. The ICD was regularly monitored for cardiac events. Medical records were documented in our Hospital Information System and additional data was acquired by telephone interview with the patients' general practitioner or cardiologist.
The follow‐up took place for an average of 1342 days (interquartile range 475–2064) and all patients were recruited in a single, tertiary teaching hospital in Germany (University Medical Center, Mannheim).
The primary endpoint was defined as a composite of cardiovascular mortality, defined as SCD, heart failure, myocardial infarction with attributed death and/or appropriate ICD therapy, defined as antitachycardia pacing and/or shock.
This study was approved by the local ethics committee (Medical Faculty Mannheim) and was performed according to standards of the Health Insurance Portability and Accountability Act (HIPAA) and the Declaration of Helsinki.
Data acquisition
The CMR was performed on 1.5 Tesla MR scanners (MAGNETOM Avanto and Sonata, Siemens Healthineers, Erlangen, Germany).
Patients underwent a standard retrospective ECG‐triggered CMR protocol including multiple short axis steady‐state free precession steady‐state free precision (SSFP) and three long axis SSFP sequences (2‐/3‐/4‐chamber view) for functional assessment. All sequences were performed in the end‐expiratory breath hold state with coverage of the heart from base to apex. The sequences were defined by following parameters: slice thickness 8 mm; interslice gap 2 mm; temporal resolution 35 ms; time to echo (TE) 1.6 ms; time of repetition (TR) 3.2 ms.
Body weight adapted late gadolinium enhancement (LGE) images of the standard axis were performed 10–15 min after intravenous injection of 0.2 mmol kg−1 Gadoteric acid (Dotarem, Guerbet, Roissy CdG Cedex, France, Germany). Standard short and long axis views were obtained in end‐diastole with segmented inversion recovery gradient echo pulse sequences.
The myocardial signal was ‘nulled’ by adjusting inversion recovery time individually for every patient, typically resulting in an inversion recovery time between 240 and 300 ms.
Image analysis
Dedicated software (CVi42, Circle Cardiovascular Imaging Inc., Calgary, Canada) was used for all CMR analysis. As previously described, SSFP short‐axis views were used to obtain left ventricular mass and volumes 7 as well as right ventricular 8 volumes. Mitral (MAPSE) and tricuspid (TAPSE) annular plane systolic excursion were assessed on four‐chamber view cine images.
3D‐LV‐FT parameters [LV global radial strain (LV‐GRS), LV global circumferential strain (LV‐GCS), LV global longitudinal strain (LV‐GLS)] and 2D‐RV‐FT parameters [RV global radial strain (RV‐GRS), RV global circumferential strain (RV‐GCS), and RV global longitudinal strain (RV‐GLS)] were generated with manual endocardial and epicardial contour tracing in end‐diastole for short axis views from base to apex and two long axis views (2‐/4‐chamber) (Figure 1 ). RV upper and lower connection point for each end‐diastolic SA view were selected. Additional manually tracing of mitral‐plane was necessary for every short‐axis plane. Papillary muscles were excluded. RV‐strain analysis was performed with 2D‐FT values. Although 3D‐FT algorithms for the right ventricle are available, it is unclear if these represent data and analyses, which are robust enough for use in a clinical setting.
Figure 1.
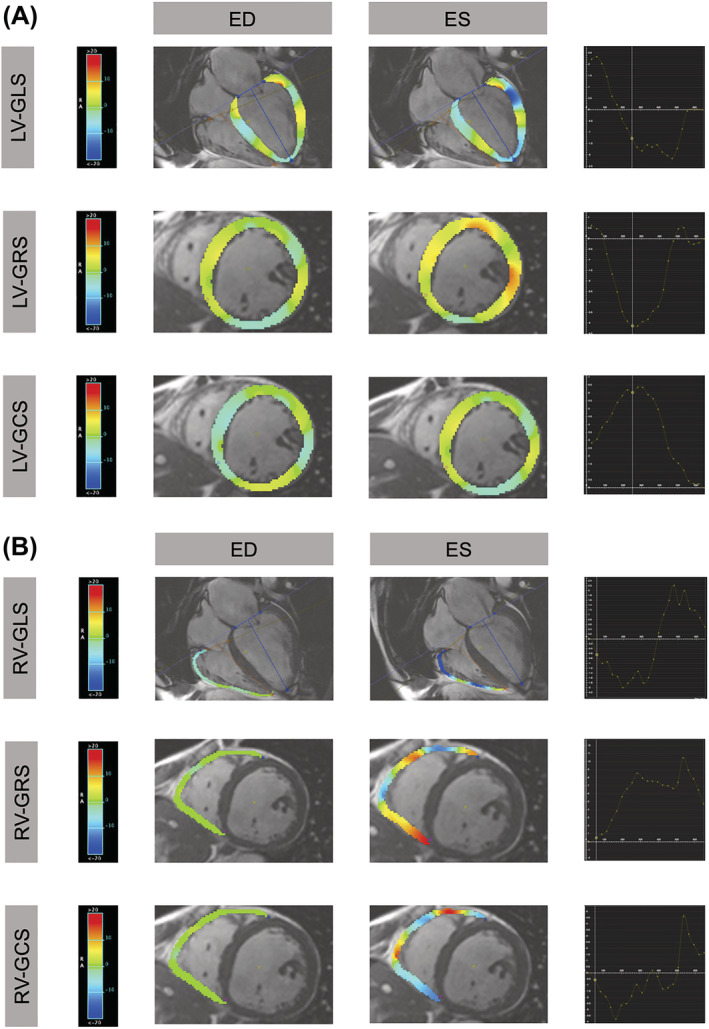
Illustration of the colour coded strain for the left (A) and right (B) ventricle of a Patient with severe reduced EF (≤35) and reduced strain parameters. ED, end‐diastolic; ES, end‐systolic; GCS, global circumferential strain; GLS, global longitudinal strain; GRS, global radial strain; LV, left ventricle; RV, right ventricle.
Late gadolinium enhancement was determined and quantitatively analysed with manual endocardial and epicardial contour tracing with the same dedicated software. A threshold of ≥3SD in comparison with mean nulled myocardial signal in contrast enhanced segmented inversion recovery gradient echo pulse sequences to define the core of myocardial infarction was defined. 9 , 10 Nulled myocardial signal was defined by manual tracing of the largest continuous region of nulled myocardium.
All the analyses were performed in consensus by a cardiologist with more than 15 years of experience and a cardiovascular radiologist with more than 10 years of experience in CMR.
Reproducibility
To determine the inter‐observer variability of the different strain parameters, 20 patients were reanalysed by an experienced cardiologist and the intraclass correlation coefficient was determined.
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (IBM SPSS Statistics, version 25; SPSS, Inc., Chicago, IL, USA).
Normal distribution was assumed due to central limit theorem. All continuous data are expressed as mean ± SD. Student's t test for independent samples was performed to analyse the means of two groups for continuous data. χ 2 test was used for comparison of ordinal data.
To predict an optimal cut‐off value for the composite of CVM and/or appropriate therapy, receiver operating characteristic (ROC) curves were generated for LV‐GRS, LV‐GLS, LV‐GCS and RV‐GRS and RV‐GCS and Area under the curves (AUC) were calculated. Youden index was performed for the optimization of FT cut‐off values.
Binary logistic regression was performed to create a model for the combination of FT parameters. ROC curve analysis and the AUC were performed for each model. ROC curves were compared with log‐rank test. The level of significance was set to <0.05.
Kaplan–Meier curves were acquired for the combination of FT parameters.
Results
Study population
Fifty‐four patients with EF > 35% and 188 patients with EF ≤ 35% were included into the study. Their baseline clinical characteristics and CMR parameters are summarized in Tables 1 and 2 .
Table 1.
Baseline characteristics of patients according to the presence of composite endpoint of cardiovascular mortality and appropriate ICD therapy
| Variables | Patients with EF > 35% | Patients with EF > 35% | P value | Patients with EF ≤ 35% | Patients with EF ≤ 35% | P value | ||
|---|---|---|---|---|---|---|---|---|
| Total n = 54 | No event n = 41 | Event n = 13 | Total n = 188 | No event n = 135 | Event n = 53 | |||
| Sex (male/female) | 44 (82%)/10 (18%) | 32 (78%)/9 (22%) | 12 (92%)/1 (8%) | 0.249 | 154 (82%)/34 (18%) | 111 (82%)/24 (18%) | 43 (81%)/10 (29%) | 0.861 |
| Age (years) | 60 ± 15 | 61 ± 15 | 59 ± 14 | 0.543 | 63 ± 15 | 64 ± 14 | 62 ± 15 | 0.470 |
| Hypertension | 46 (85%) | 35 (76%) | 11 (24%) | 0.947 | 170 (90%) | 120 (71%) | 50 (29%) | 0.253 |
| Hyperlipidaemia | 41 (76%) | 31 (76%) | 10 (24%) | 0.923 | 150 (80%) | 112 (75%) | 38 (25%) | 0.084 |
| Family history CAD (%) | 14 (26%) | 13 (93%) | 1 (7%) | 0.085 | 37 (20%) | 25 (68%) | 12 (22%) | 0.538 |
| DM | 20 (37%) | 12 (60%) | 8 (40%) | 0.036 | 77 (41%) | 52 (67%) | 25 (33%) | 0.278 |
| current smoker | 13 (24%) | 8 (62%) | 5 (38%) | 0.164 | 44 (23%) | 36 (82%) | 8 (18%) | 0.092 |
| Medication | ||||||||
| ACE inhibitor/AT1 antagonist | 51 (94%) | 39 (76%) | 12 (24%) | 0.700 | 171 (91%) | 120 (70%) | 51 (30%) | 0.141 |
| ASA | 40 (74%) | 31 (78%) | 9 (22%) | 0.647 | 128 (68%) | 93 (73%) | 35 (27%) | 0.706 |
| β–blocker (%) | 41 (98%) | 12 (77%) | 61 (23%) | 0.073 | 184 (98%) | 133 (72%) | 51 (28%) | 0.327 |
| NYHA | ||||||||
| NYHA I | 12 (22%) | 9 (75%) | 3 (25%) | 0.923 | 7 (4%) | 6 (86%) | 1 (14%) | 0.405 |
| NYHA II | 27 (50%) | 19 (70%) | 8 (30%) | 0.340 | 55 (29%) | 45 (82%) | 10 (18%) | 0.050 |
| NYHA III | 13 (24%) | 11 (85%) | 2 (15%) | 0.400 | 107 (57%) | 72 (67%) | 35 (33%) | 0.113 |
| NYHA IV | 2 (4%) | 2 (100%) | 0 (0%) | 0.417 | 19 (10%) | 12 (63%) | 7 (36%) | 0.377 |
| CAD | ||||||||
| 1‐Vessel‐Disease | 17 (31%) | 11 (65%) | 6 (35%) | 0.090 | 30 (16%) | 22 (73%) | 8 (30%) | 0.840 |
| 2‐Vessel‐Disease | 14 (26%) | 11 (76%) | 3 (24%) | 0.788 | 50 (27%) | 39 (78%) | 11 (22%) | 0.256 |
| 3‐Vessel‐Disease | 23 (43%) | 19 (83%) | 4 (17%) | 0.322 | 108 (57%) | 74 (69%) | 34 (31%) | 0.244 |
Values are mean ± SD, n (%), The P values in bold indicate the significance of differences, significance level P < 0.05.
ASA, acetylsalicyclid acid; AT1, angiotensin 1; CAD, coronary artery disease; NYHA, New York Heart Association.
Table 2.
CMR parameters of patients according to the presence of composite endpoint of cardiovascular mortality and appropriate ICD therapy
| Variables | Patients with EF > 35% | Patients with EF > 35% | P value | Patients with EF ≤ 35% | Patients with EF ≤ 35% | P value | ||
|---|---|---|---|---|---|---|---|---|
| Total n = 54 | No event n = 41 | Event n = 13 | Total n = 188 | No event n = 135 | Event n = 53 | |||
| LVEF (%) | 42.6 ± 6.8 | 42.8 ± 7.3 | 41.9 ± 5.1 | 0.676 | 24.5 ± 3.4 | 24.6 ± 7.3 | 24.2 ± 7.3 | 0.756 |
| LV‐EDV/BSA (mL/m2) | 100.0 ± 24.6 | 99.6 ± 24.7 | 101.4 ± 24.7 | 0.816 | 132.8 ± 41.6 | 130.1 ± 20.6 | 139.7 ± 43.5 | 0.159 |
| LV‐ESV/BSA (mL/m2) | 56.7 ± 18.4 | 55.7 ± 18.7 | 59.7 ± 18.0 | 0.504 | 101.6 ± 38.5 | 99.7 ± 37.6 | 106.7 ± 40.4 | 0.258 |
| LV‐SV/BSA (mL/m2) | 42.0 ± 9.6 | 42.0 ± 9.8 | 41.8 ± 9.1 | 0.945 | 31.5 ± 11.2 | 31.2 ± 11.1 | 32.9 ± 11.6 | 0.535 |
| LV‐EDM/BSA (gr/m2) | 82.5 ± 19.5 | 82.26 ± 19.26 | 83.26 ± 0.95 | 0.898 | 100.2 ± 53.7 | 101.25 ± 62.1 | 97.48 ± 20.6 | 0.667 |
| LV‐EDD (mm) | 58.9 ± 7.3 | 58.44 ± 7.59 | 60.38 ± 6.48 | 0.409 | 67.2 ± 18.2 | 67.5 ± 20.7 | 66.5 ± 9.6 | 0.750 |
| Septal wall thickness (mm) | 12.0 ± 4.5 | 11.66 ± 3.73 | 13.15 ± 6.40 | 0.301 | 10.8 ± 3.0 | 11.0 ± 3.1 | 10.3 ± 2.7 | 0.165 |
| Posterior wall thickness (mm) | 9.7 ± 6.5 | 10.00 ± 7.20 | 8.62 ± 3.23 | 0.505 | 7.9 ± 3.3 | 8.02 ± 3.6 | 7.6 ± 2.4 | 0.395 |
| RV‐EF (%) | 57.0 ± 9.7 | 58.41 ± 9.53 | 52.43 ± 9.23 | 0.052 | 44.5 ± 14.6 | 44.1 ± 14.0 | 45.7 ± 16.0 | 0.503 |
| RV‐EDV/BSA (mL/m2) | 71.8 ± 16.0 | 69.72 ± 14.71 | 78.50 ± 18.55 | 0.084 | 75.2 ± 27.5 | 73.8 ± 27.0 | 78.9 ± 28.7 | 0.258 |
| RV‐ESV/BSA (mL/m2) | 33.6 ± 13.9 | 32.19 ± 13.89 | 37.96 ± 13.55 | 0.195 | 43.5 ± 23.8 | 43.5 ± 23.8 | 45.5 ± 27.3 | 0.621 |
| RV‐SV/BSA (mL/m2) | 40.4 ± 8.2 | 40.40 ± 7.80 | 40.54 + 9.78 | 0.958 | 30.9 ± 10.4 | 30.9 ± 10.4 | 33.2 ± 12.0 | 0.188 |
| TAPSE (mm) | 14 ± 5 | 14 ± 5 | 13 ± 5 | 0.657 | 10 ± 6 | 10 ± 6 | 10 ± 5 | 0.845 |
| MAPSE‐lateral (mm) | 8 ± 4 | 8 ± 4 | 7 ± 4 | 0.125 | 11 ± 6 | 11 ± 6 | 6 ± 4 | 0.517 |
| LGE (3 SD) | 33.6 ± 11.9 | 34.9 ± 11.8 | 29.9 ± 11.6 | 0.195 | 36.5 ± 8.9 | 36.6 ± 8.9 | 33.7 ± 8.3 | 0.055 |
Values are mean ± SD.
BSA, body surface area; EDD, end‐diastolic diameter; EDM, end‐diastolic mass; EDV, end‐diastolic volume; ESV, end‐systolic volume; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; MAPSE, mitral annular plane excursion; SV, stroke volume; TAPSE, tricuspid annular plane excursion.
Intraclass correlation coefficient showed a good to very good inter‐observer reproducibility for all FT parameters. Our analysis showed a good to very good agreement for all strain parameters (LV‐GLS: 0.768; LV‐GRS: 0.853; LV‐GCS: 0.868; RV‐GLS: 0.922; RV‐GRS: 0.852; RV‐GCS:0.900).
Primary vs. secondary prevention
Although most studies showed that patients who had an ICD as a secondary prevention had more frequent appropriate therapies, patients in our study who had an ICD implantation as a secondary prevention showed only a trend towards higher rates of appropriate therapy compared to the patients who had an ICD implantation as a primary prevention (23.6% vs. 20.5%; P = 0.6, respectively) (Table 3 ).
Table 3.
Distribution of patients according to indication for an ICD implantation as well as LVEF
| ICD as a primary prevention | ICD as a secondary prevention | |||||
|---|---|---|---|---|---|---|
| LVEF ≤ 35% (n = 176) | LVEF > 35% (n = 28) a | Total group (n = 204) | LVEF ≤ 35% (n = 12) | LVEF > 35% (n = 26) | Total group (n = 38) | |
| Appropriate therapy | 36 (20.4%) | 6 (21.4%) | 42 (20.5%) | 2 (16.7%) | 7 (26.9%) | 9 (23.6%) |
| No therapy | 140 (79.6%) | 22 (78.6%) | 162 (79.5%) | 10 (84%) | 19 (73.1%) | 29 (26.4%) |
All patients who had an ICD as a primary prevention and >35% in CMR had LVEF≤35% in echocardiography. The indication for an ICD implantation as a primary prevention was based on echocardiographic LVEF measurement in those patients.
Patients with ejection fraction ≤35% (n = 188)
The all‐cause mortality was 16% (30 patients) during follow‐up. CVM occurred in 20 of 188 (11%) patients. 38 (20%) patients were treated with ATP or shock. There was no difference regarding appropriate therapy, when we compare the patients according to indication for an ICD implantation. 36 (20.5%) of 176 patients from primary prevention group and 2 (16.7%) of 12 patients from secondary prevention group had appropriate therapy (P = 0.75) (Table 3 ).
The primary endpoint was noted in 53 patients (28%) with a median follow‐up of 1286 days (interquartile range 387–1880 days).
For this subgroup as shown in Tables 2 and 4 neither standard CMR LV or RV function parameters, nor LV‐FT or RV‐FT parameters or LGE revealed significant differences between event and no‐event group. Mean LVEF was 24 ± 3%.
Table 4.
CMR feature tracking parameters of left (3D) and right (2D) ventricle of patients according to the presence of composite endpoint of cardiovascular mortality and appropriate ICD therapy
| Variables | Patients with EF > 35% | Patients with EF > 35% | P value | Patients with EF ≤ 35% | Patients with EF ≤ 35% | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| Total n = 54 | No event n = 41 | Event n = 13 | Total n = 188 | No event n = 135 | Event n = 53 | ||||
| LV‐GLS (%) | −8.3 ± 3.0 | −8.9 ± 2.5 | −6.2 ± 3.5 | 0.004 | −5.8 ± 1.8 | −5.9 ± 1.9 | −5.7 ± 1.6 | 0.628 | |
| LV‐GRS (%) | 22.7 ± 7.9 | 24.0 ± 8.1 | 18.5 ± 5.8 | 0.028 | 13.0 ± 5.5 | 13.1 ± 5.8 | 12.5 ± 4.6 | 0.525 | |
| LV‐GCS (%) | −11.3 ± 2.7 | −11.7 ± 2.9 | −9.9 ± 1.5 | 0.034 | −7.1 ± 2.1 | −7.1 ± 2.1 | −7.0 ± 2.1 | 0.846 | |
| RV‐GLS (%) | −21.4 ± 4.7 | −22.0 ± 4.7 | −19.5 ± 4.7 | 0.089 | −16.6 ± 6.2 | −16.6 ± 5.8 | −16.4 ± 7.0 | 0.821 | |
| RV‐GRS (%) | 15.2 ± 5.2 | 16.1 ± 5.0 | 12.4 ± 5.0 | 0.025 | 11.8 ± 5.5 | 11.8 ± 5.9 | 11.9 ± 4.5 | 0.866 | |
| RV‐GCS (%) | −9.8 ± 3.4 | −10.4 ± 2.8 | −8.1 ± 4.5 | 0.036 | −8.1 ± 3.4 | −8.1 ± 3.6 | −8.2 ± 2.8 | 0.737 | |
Values are mean ± SD, The P values in bold indicate the significance of differences, significance level P < 0.05.
LV‐GCS, left ventricular‐global circumferential strain; LV‐GLS, left ventricular‐global longitudinal strain; LV‐GRS, left ventricular‐global radial strain; RV‐GCS, right ventricular‐global circumferential strain; RV‐GLS, right ventricular‐global longitudinal strain; RV‐GRS, right ventricular‐global radial strain.
Patients with ejection fraction >35% (n = 54)
All‐cause mortality was 9% (5 patients) during follow‐up. CVM occurred in 2 of 54 (4%) patients. 13 (24%) patients were treated with ATP or shock. When we compare the patients according to indication for an ICD implantation there was a trend towards higher rates of appropriate therapy in secondary prevention patients but without reaching a significant level. 6 (21.4%) of 28 patients from primary prevention group and 7 (26.9%) of 26 patients from secondary prevention group had appropriate therapy (P = 0.63) (Table 3 ).
The primary endpoint was noted in 13 patients (24%) with a median follow‐up of 1534 days (interquartile range 549–2277 days). Mean LV‐EF was 43 ± 7%.
Extent of LGE showed no significant differences (Table 2 ).
Feature tracking and survival analysis for patients with ejection fraction >35%
Standard LV and RV CMR parameters did not show any significant differences between event and non‐event group. RV‐EF (58.4 ± 9.5% vs. 52.4 ± 9.2% P = 0.052) did show a trend towards lower values (within the normal range) in patients with events.
Feature tracking parameters are summarized in Table 4 . While comparing patients with and without events, we encountered significant differences in means of LV‐GRS (24.0 ± 8.1% vs.18.5 ± 5.8% P = 0.028), LV‐GLS (−8.9 ± 2.5% vs. −6.2 ± 3.5% P = 0.004), LV‐GCS (−11.7 ± 2.9% vs. −9.9 ± 1.5% P = 0.034), RV‐GRS (16.1 ± 5.0% vs. 12.4 ± 5.0% P = 0.025) and RV‐GCS (−10.4 ± 2.9% vs. −8.10 ± 4.5% P = 0.036). RV‐GLS did not differ significantly between patients with and without events.
For FT parameters with a significant difference between the event and non‐event group in the t‐test, a ROC‐analysis was performed and optimal cut‐off values were estimated by Youden index (Figure 2 ).
Figure 2.
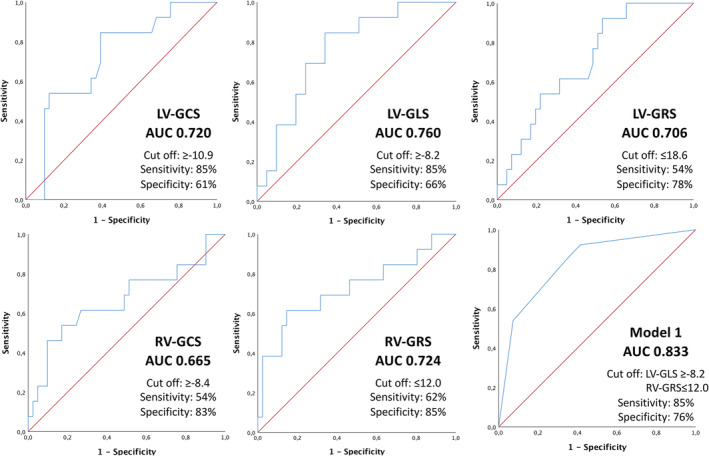
Receiver operating characteristic (ROC) curves with optimized cut‐off values for left and right‐ventricular strain and related sensitivity and specificity. LV‐GCS, left ventricular‐global circumferential strain; LV‐GLS, left ventricular‐global longitudinal strain; LV‐GRS, left ventricular‐global radial strain; RV‐GCS, right ventricular‐global circumferential strain; RV‐GLS, right ventricular‐global longitudinal strain; RV‐GRS, right ventricular‐global radial strain.
Linear binary regression models were created for different combinations of LV and RV FT parameters according to above‐calculated cut‐off values. ROC curves were generated for the different models and the AUC was calculated. The model with combination of LV‐GLS and RV‐GRS showed an AUC value (0.833) with the least amount of FT parameters (Figure 2 ).
Kaplan–Meier survival analysis demonstrated a significant higher event free survival for patients with the combination of LV‐GLS ≤ −8.2% and RV‐GRS ≥ 12.0% (Figure 3 ).
Figure 3.
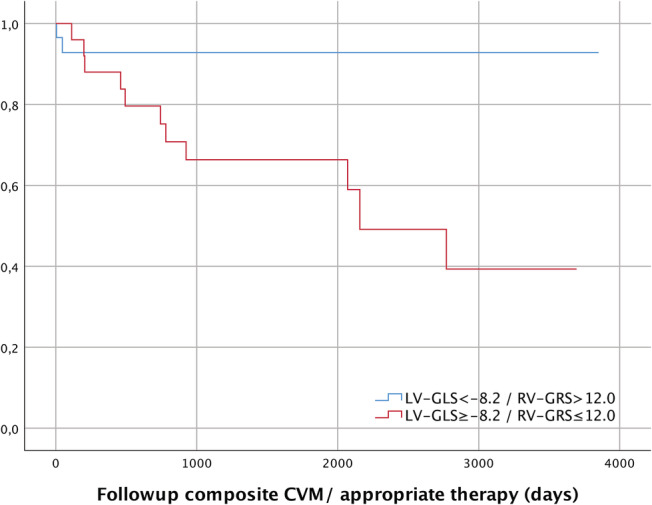
Kaplan–Meier estimates for event free survival for Model LV‐GLS/RV‐GRS and EF > 35%. EF, ejection fraction; LV‐GLS, left ventricular‐global longitudinal strain; RV‐GRS, right ventricular‐global radial strain.
Discussion
The present study provides evidence that the use of FT analysis could serve as a predictor for severe arrhythmic events and cardiovascular mortality in patients with ICM and EF > 35%. Patients with EF > 35% and a combination of LV‐GLS ≤ −8.2% and RV‐GRS ≥ 12.0% seem to have a significant lower risk of CVM and severe arrhythmic events. Additionally, FT seems to have no added value in risk stratification in patients with EF ≤ 35%, possibly due to the highly impaired myocardial function. To our knowledge, this is the first study focusing on FT‐based risk stratification in ICM patients 3 , 4 , 5 , 11 , 12 dependent of their LV dysfunction.
According to the current guidelines LVEF is recommended to discriminate whether to implant an ICD in patients with ICM as primary or secondary prevention of SCD. 1 Interestingly, in the big MADIT‐II cohort, 65% of patients, who received an ICD after myocardial infarction due to primary prevention, did not receive any appropriate ICD therapy. 13 Especially for patients with mild or moderate impaired EF (>35%) clinical data or supplement factors are missing. It should be noted that in clinical routine as well as in the mentioned MADIT‐II study, EF is determined by echocardiography and not by CMR, which is used in our study setting.
In our study, the extent of LGE did not reach significance which is in line with the results of Romano et al. 5 who could show that LGE presence or extent are not predictors of all cause death in multivariate analysis. Tulumen et al. and a recent meta‐analysis could show that especially the extent of the periinfarct zone is a predictor for risk stratification of appropriate ICD therapy. 14 , 15
In this context, cardiac deformation parameters derived by FT are coming ever more in focus. 4 , 5 The key advantages of FT parameters are that they can be derived from contrast‐free standard cine‐SSFP sequences with dedicated software, which makes it appealing to patient groups with potential repetitive intravenous contrast use over time or impaired renal function.
Several studies investigated the relationship between FT parameters and different cardiovascular diseases and could show significant correlation to different clinical endpoints, for example, MACE and cardiac death. 3 , 4 , 16 , 17 , 18 , 19 , 20 , 21 , 22 The primary endpoint of our study was a composite of CVM and/or appropriate ICD therapy.
Recent studies showed an association between LV and RV dysfunction, and furthermore, an increased risk in 1 year mortality in myocardial infarction patients with reduced RV‐EF in combination with reduced LV‐EF compared with patients with an exclusively decreased LV‐EF. 23 , 24 Thus, we decided to include the RV strain parameters in the analysis.
It should be noted that although FT parameters have good reproducibility for global values, they have a reduced reproducibility for segmental strain analyses. 25 , 26 Therefore, we focused on global strain values. For segmental strain analysis, for example, the strain‐encoded magnetic resonance method seems to be superior to the FT method. 27 , 28
Patients with ejection fraction ≤35%
Our study cohort shows no significant differences in LV, RV, or FT CMR parameters between the event and non‐event group. Results of a large multicentre study by Romano et al. found LV‐GLS as an independent predictor of all‐cause mortality in ICM and non‐ischaemic DCM. 5 Romano et al. noted a lower all‐cause mortality over 5 years in patients with LV‐GLS < ‐13% and EF ≤ 35% in comparison to patients with LV‐GLS > ‐8.7% and EF ≤ 35% in a mixed cardiomyopathy (DCM/ICM) population. There are, however, two significant differences when we compare this to our results. First, our study comprised solely of an ICM population. Second, Romano et al. chose all‐cause mortality as a primary endpoint, while we focused on cardiovascular mortality and severe arrhythmic events.
Patients with ejection fraction >35%
In our study, all three LV‐FT parameters individually showed a significant difference between the event and non‐event group of patients with especially high significance for LV‐GLS (P = 0.004). The correlation between LV‐GLS and LV‐EF is well known. 29 , 30 Nevertheless, neither LV‐EF nor other LV and RV CMR parameters showed a significant difference between event and non‐event group, which underlines the need for more sophisticated discrimination of parameters in ICD implantation decision making.
The earlier referred study of Romano et al. could show a significant difference in all‐cause mortality between highest and lowest LV‐GLS tertial for EF > 35%. 5 This finding is consistent with our results acknowledging the differences in the study design. Romano et al. observed a mixed ICM/DCM population without comparison of a solely ICM population when separated by EF ≤ 35% /EF > 35%. The results of our study with a cut‐off value of ≥ −8.2% for LV‐GLS and EF > 35% are in the range of the lowest tertial of Romano et al. (GLS > −8.7%).
The results of Eitel et al. also highlighted the influence of reduced LV‐GLS while considering MACE (defined as all‐cause mortality, reinfarction, new congestive heart failure within 1 year after infarction) in ICM. 4 They could emphasize that the EF > 35% subgroup showed significant differences in MACE rate with a LV‐GLS threshold of −16.4%. Contrary to our study, Eitel et al. investigated FT parameters in the acute phase of the myocardial infarction. In our study the assessment of FT parameters was mainly obtained in the chronic phase of myocardial infarction. This could serve as an explanation for the difference in cut‐off values of LV‐GLS in comparison to our study.
Guerra et al. demonstrated that reduced LV‐GLS derived by echocardiography is associated with an increased risk for appropriate ICD therapy. 6 This concurs with our results. Considering that Guerra et al. examined a mixed population with different cardiomyopathies and did not differentiate patient groups with different levels of EF impairment, we hypothesize that for patients with an EF ≤ 35% the underlying structural damage and the resulting impairment in cardiac strain is too pronounced to sufficiently add any value in risk stratification.
Left ventricular‐global circumferential strain also showed significant differences between event and non‐event group. Previous studies have shown that GCS has its strength in assessing reduction of regional LV function and transmurality of myocardial infarction. 31 , 32 , 33 Plausible reasons for the lower statistical significance in comparison to LV‐GLS could be, that a reduction in GCS is due to subepicardial myocardial dysfunction and the fact that the impairment of this location correlates with the transmurality of myocardial infarction. 34 Patients with mild or moderate reduction of LV‐EF (>35%) have smaller infarcted area (Table 2 ); thus; LV‐GCS has to have a lower impairment than LV‐GLS. Nucifora et al. could show that LV‐GCS can predict poorer long term prognosis in a group of novel ST‐elevation myocardial Infarction patients, which is not surprising considering that a greater reduction in LV‐GCS value indicates more severe transmural myocardial damage. 35
Global radial strain implicates fibres from endocardium to epicardium. Thus, LV‐GRS is not limited by fibre localization when compared with LV‐GCS. On the other hand, GRS shows a lower reproducibility than other FT parameters in 2D measurements 36 ; a downside which could be reduced with 3D FT‐CMR. 37 3D FT‐CMR of the LV offers superior reproducibility compared with 2D FT‐CMR with excellent intra‐observer and inter‐observer variability and lower normal values. 37
Right ventricle strain
Right ventricular‐global radial strain individually was associated with the most significant differences between the event and non‐event group of patients only to be followed by RV‐GCS.
Right ventricular‐global longitudinal strain showed no significant difference between event and non‐event patients as well as RV‐EF. This concurs with previous echocardiographic speckle tracking studies, which could demonstrate that RV‐EF and RV‐GLS share a close relationship. 38
As seen in Figure 2 LV‐GLS and RV‐GRS demonstrated the highest AUC values in ROC curves with 0.76 and 0.72. The optimized cut‐off values for each FT parameter are presented in Figure 2 . LV‐GLS has a sensitivity of 85% and a specificity of 66% at a cut‐off value of ≥ −8.2% whereas RV‐GRS has a sensitivity of 62% and a specificity of 85% at a cut‐off value of ≤12.0%. The combination of both FT parameters shows an AUC for ROC curve of 0.83, with a sensitivity of 85% and a specificity of 76%. This elucidates the potential for more precise clinical decision making and takes into consideration the function of both, LV and RV, for risk stratification. 39 , 40 This is also supported by the Kaplan–Meier estimates for survival as shown in the Figure 3 .
When LV‐GLS and LV‐GRS are combined with the defined cut‐off values, there is a significant difference in event free survival (P = 0.007).
Study limitations
Our study yields limitations. The study was performed in a single‐centre setting and the population with EF > 35% is rather small. Six strain parameters were considered in combination. This may overestimate the predictability of the examined parameters.
Additionally, all patients were treated with an ICD. This enabled the good verifiability of arrhythmic events but could possibly have led to a selection bias. Further studies especially for patients with EF > 35% are needed to verify our findings.
Training has an impact on intra‐observer and inter‐observer reproducibility in FT parameters. 41 Thus, we used only experienced observers with high level of training in deriving FT parameters. This should be taken into consideration when transcribing the results of this study in clinical practice.
Conclusions
The impact of FT derived measurements in the risk stratification of patients with ICM seems to be LV‐function dependent. CMR‐FT could serve as a surrogate marker in clinical decision making of ICD implantation in ICM patients with mild or moderate reduced LVEF>35%. The combination of LV‐GLS (≥ −8.2%) and RV‐GRS (≤12.0%) shows a high predictive value for the composite of CVM and appropriate ICD therapy with a sensitivity of 85% and a specificity of 76% for patients with EF > 35%.
For patients with severe impaired EF < 35%, FT showed no further value in risk stratification when describing CVM and ICD appropriate therapy.
Conflict of interest
There is no potential conflict of interest to disclose for any of the co‐authors.
Acknowledgements
Open Access funding enabled and organized by Projekt DEAL.
Overhoff, D. , Ansari, U. , Hohneck, A. , Tülümen, E. , Rudic, B. , Kuschyk, J. , Lossnitzer, D. , Baumann, S. , Froelich, M. F. , Waldeck, S. , Akin, I. , Borggrefe, M. , Schoenberg, S. O. , and Papavassiliu, T. (2022) Prediction of cardiac events with non‐contrast magnetic resonance feature tracking in patients with ischaemic cardiomyopathy. ESC Heart Failure, 9: 574–584. 10.1002/ehf2.13712.
References
- 1. Priori SG, Blomstrom‐Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: theTask Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Europace 2015; 17: 1601–1687. [DOI] [PubMed] [Google Scholar]
- 2. Kawakami H, Nerlekar N, Haugaa KH, Edvardsen T, Marwick TH. Prediction of ventricular arrhythmias with left ventricular mechanical dispersion: a systematic review and meta‐analysis. JACC Cardiovasc Imaging 2020; 13: 562–572. [DOI] [PubMed] [Google Scholar]
- 3. Buss SJ, Breuninger K, Lehrke S, Voss A, Galuschky C, Lossnitzer D, Andre F, Ehlermann P, Franke J, Taeger T, Frankenstein L, Steen H, Meder B, Giannitsis E, Katus HA, Korosoglou G. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging 2015; 16: 307–315. [DOI] [PubMed] [Google Scholar]
- 4. Eitel I, Stiermaier T, Lange T, Rommel KP, Koschalka A, Kowallick JT, Lotz J, Kutty S, Gutberlet M, Hasenfuß G, Thiele H, Schuster A. Cardiac magnetic resonance myocardial feature tracking for optimized prediction of cardiovascular events following myocardial infarction. JACC Cardiovasc Imaging 2018; 11: 1433–1444. [DOI] [PubMed] [Google Scholar]
- 5. Romano S, Judd RM, Kim RJ, Kim HW, Klem I, Heitner JF, Shah DJ, Jue J, White BE, Indorkar R, Shenoy C, Farzaneh‐Far A. Feature‐tracking global longitudinal strain predicts death in a multicenter population of patients with ischemic and nonischemic dilated cardiomyopathy incremental to ejection fraction and late gadolinium enhancement. JACC Cardiovasc Imaging 2018; 11: 1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guerra F, Malagoli A, Contadini D, Baiocco E, Menditto A, Bonelli P, Rossi L, Sticozzi C, Zanni A, Cai J, Maitra P, Villani GQ, Capucci A. Global longitudinal strain as a predictor of first and subsequent arrhythmic events in remotely monitored ICD patients with structural heart disease. JACC Cardiovasc Imaging 2020; 13: 1–9. [DOI] [PubMed] [Google Scholar]
- 7. Papavassiliu T, Kuhl HP, Schroder M, Suselbeck T, Bondarenko O, Bohm CK, Beek A, Hofman MM, van Rossum AC. Effect of endocardial trabeculae on left ventricular measurements and measurement reproducibility at cardiovascular MR imaging. Radiology 2005; 236: 57–64. [DOI] [PubMed] [Google Scholar]
- 8. Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady‐state free precession imaging sequences. J Magn Reson Imaging 2003; 17: 323–329. [DOI] [PubMed] [Google Scholar]
- 9. Flett AS, Hasleton J, Cook C, Hausenloy D, Quarta G, Ariti C, Muthurangu V, Moon JC. Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc Imaging 2011; 4: 150–156. [DOI] [PubMed] [Google Scholar]
- 10. Yan AT, Shayne AJ, Brown KA, Gupta SN, Chan CW, Luu TM, di Carli MF, Reynolds HG, Stevenson WG, Kwong RY. Characterization of the peri‐infarct zone by contrast‐enhanced cardiac magnetic resonance imaging is a powerful predictor of post‐myocardial infarction mortality. Circulation 2006; 114: 32–39. [DOI] [PubMed] [Google Scholar]
- 11. Yang LT, Yamashita E, Nagata Y, Kado Y, Oshima S, Otsuji Y, Takeuchi M. Prognostic value of biventricular mechanical parameters assessed using cardiac magnetic resonance feature‐tracking analysis to predict future cardiac events. J Magn Reson Imaging 2017; 45: 1034–1045. [DOI] [PubMed] [Google Scholar]
- 12. Romano S, Romer B, Evans K, Trybula M, Shenoy C, Kwong RY, Farzaneh‐Far A. Prognostic implications of blunted feature‐tracking global longitudinal strain during vasodilator cardiovascular magnetic resonance stress imaging. JACC Cardiovasc Imaging 2020; 13: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moss AJ, Greenberg H, Case RB, Zareba W, Hall WJ, Brown MW, Daubert JP, McNitt S, Andrews ML, Elkin AD, Multicenter Automatic Defibrillator Implantation Trial‐II (MADIT‐II) Research Group . Long‐term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation 2004; 110: 3760–3765. [DOI] [PubMed] [Google Scholar]
- 14. Tulumen E, Rudic B, Ringlage H, Hohneck A, Roger S, Liebe V, Kuschyk J, Overhoff D, Budjan J, Akin I, Borggrefe M. Extent of peri‐infarct scar on late gadolinium enhancement cardiac magnetic resonance imaging and outcome in patients with ischemic cardiomyopathy. Heart Rhythm 2021; 18: 954–961. [DOI] [PubMed] [Google Scholar]
- 15. Haghbayan H, Lougheed N, Deva DP, Chan KKW, Lima JAC, Yan AT. Peri‐infarct quantification by cardiac magnetic resonance to predict outcomes in ischemic cardiomyopathy: prognostic systematic review and meta‐analysis. Circ Cardiovasc Imaging 2019; 12: e009156. [DOI] [PubMed] [Google Scholar]
- 16. Schuster A, Paul M, Bettencourt N, Morton G, Chiribiri A, Ishida M, Hussain S, Jogiya R, Kutty S, Bigalke B, Perera D, Nagel E. Cardiovascular magnetic resonance myocardial feature tracking for quantitative viability assessment in ischemic cardiomyopathy. Int J Cardiol 2013; 166: 413–420. [DOI] [PubMed] [Google Scholar]
- 17. Prati G, Vitrella G, Allocca G, Muser D, Buttignoni SC, Piccoli G, Morocutti G, Delise P, Pinamonti B, Proclemer A, Sinagra G. Right ventricular strain and dyssynchrony assessment in arrhythmogenic right ventricular cardiomyopathy: cardiac magnetic resonance feature‐tracking study. Circ Cardiovasc Imaging 2015; 8: e003647 discussion e. [DOI] [PubMed] [Google Scholar]
- 18. Nucifora G, Sree Raman K, Muser D, Shah R, Perry R, Awang Ramli KA, Selvanayagam JB. Cardiac magnetic resonance evaluation of left ventricular functional, morphological, and structural features in children and adolescents vs. young adults with isolated left ventricular non‐compaction. Int J Cardiol 2017; 246: 68–73. [DOI] [PubMed] [Google Scholar]
- 19. Meyer CG, Frick M, Lotfi S, Altiok E, Koos R, Kirschfink A, Lehrke M, Autschbach R, Hoffmann R. Regional left ventricular function after transapical vs. transfemoral transcatheter aortic valve implantation analysed by cardiac magnetic resonance feature tracking. Eur Heart J Cardiovasc Imaging 2014; 15: 1168–1176. [DOI] [PubMed] [Google Scholar]
- 20. Kutty S, Rangamani S, Venkataraman J, Li L, Schuster A, Fletcher SE, Danford DA, Beerbaum P. Reduced global longitudinal and radial strain with normal left ventricular ejection fraction late after effective repair of aortic coarctation: a CMR feature tracking study. Int J Cardiovasc Imaging 2013; 29: 141–150. [DOI] [PubMed] [Google Scholar]
- 21. Padiyath A, Gribben P, Abraham JR, Li L, Rangamani S, Schuster A, Danford DA, Pedrizzetti G, Kutty S. Echocardiography and cardiac magnetic resonance‐based feature tracking in the assessment of myocardial mechanics in tetralogy of Fallot: an intermodality comparison. Echocardiography 2013; 30: 203–210. [DOI] [PubMed] [Google Scholar]
- 22. Neisius U, Myerson L, Fahmy AS, Nakamori S, El‐Rewaidy H, Joshi G, Duan C, Manning WJ, Nezafat R. Cardiovascular magnetic resonance feature tracking strain analysis for discrimination between hypertensive heart disease and hypertrophic cardiomyopathy. PLoS ONE 2019; 14: e0221061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB, National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure . Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 2006; 114: 1883–1891. [DOI] [PubMed] [Google Scholar]
- 24. Shah PK, Maddahi J, Staniloff HM, Ellrodt AG, Pichler M, Swan HJ, Berman DS. Variable spectrum and prognostic implications of left and right ventricular ejection fractions in patients with and without clinical heart failure after acute myocardial infarction. Am J Cardiol 1986; 58: 387–393. [DOI] [PubMed] [Google Scholar]
- 25. Mangion K, Burke NMM, McComb C, Carrick D, Woodward R, Berry C. Feature‐tracking myocardial strain in healthy adults—a magnetic resonance study at 3.0 tesla. Sci Rep 2019; 9: 3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Almutairi HM, Boubertakh R, Miquel ME, Petersen SE. Myocardial deformation assessment using cardiovascular magnetic resonance‐feature tracking technique. Br J Radiol 2017; 90: 20170072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Korosoglou G, Giusca S, Hofmann NP, Patel AR, Lapinskas T, Pieske B, Steen H, Katus HA, Kelle S. Strain‐encoded magnetic resonance: a method for the assessment of myocardial deformation. ESC Heart Fail 2019; 6: 584–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Giusca S, Korosoglou G, Zieschang V, Stoiber L, Schnackenburg B, Stehning C, Gebker R, Pieske B, Schuster A, Backhaus S, Pieske‐Kraigher E, Patel A, Kawaji K, Steen H, Lapinskas T, Kelle S. Reproducibility study on myocardial strain assessment using fast‐SENC cardiac magnetic resonance imaging. Sci Rep 2018; 8: 14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Onishi T, Saha SK, Delgado‐Montero A, Ludwig DR, Onishi T, Schelbert EB, Schwartzman D, Gorcsan J III. Global longitudinal strain and global circumferential strain by speckle‐tracking echocardiography and feature‐tracking cardiac magnetic resonance imaging: comparison with left ventricular ejection fraction. J Am Soc Echocardiogr 2015; 28: 587–596. [DOI] [PubMed] [Google Scholar]
- 30. Shang Q, Patel S, Steinmetz M, Schuster A, Danford DA, Beerbaum P, Sarikouch S, Kutty S. Myocardial deformation assessed by longitudinal strain: chamber specific normative data for CMR‐feature tracking from the German competence network for congenital heart defects. Eur Radiol 2018; 28: 1257–1266. [DOI] [PubMed] [Google Scholar]
- 31. Kihlberg J, Haraldsson H, Sigfridsson A, Ebbers T, Engvall JE. Clinical experience of strain imaging using DENSE for detecting infarcted cardiac segments. J Cardiovasc Magn Res: Off J Soc Cardiovasc Magn Res 2015; 17: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khan JN, Singh A, Nazir SA, Kanagala P, Gershlick AH, McCann GP. Comparison of cardiovascular magnetic resonance feature tracking and tagging for the assessment of left ventricular systolic strain in acute myocardial infarction. Eur J Radiol 2015; 84: 840–848. [DOI] [PubMed] [Google Scholar]
- 33. Miyagi H, Nagata M, Kitagawa K, Kato S, Takase S, Sigfridsson A, Ishida M, Dohi K, Ito M, Sakuma H. Quantitative assessment of myocardial strain with displacement encoding with stimulated echoes MRI in patients with coronary artery disease. Int J Cardiovasc Imaging 2013; 29: 1779–1786. [DOI] [PubMed] [Google Scholar]
- 34. Clauss JF, Wirths W, Roos M, Wohrle B, Brischwein M, Wolf B. In‐vivo cell and tissue monitoring with active implants. Conf Proc IEEE Eng Med Biol Soc 2015;2015:7087–7090. [DOI] [PubMed] [Google Scholar]
- 35. Nucifora G, Muser D, Tioni C, Shah R, Selvanayagam JB. Prognostic value of myocardial deformation imaging by cardiac magnetic resonance feature‐tracking in patients with a first ST‐segment elevation myocardial infarction. Int J Cardiol 2018; 271: 387–391. [DOI] [PubMed] [Google Scholar]
- 36. Barreiro‐Perez M, Curione D, Symons R, Claus P, Voigt JU, Bogaert J. Left ventricular global myocardial strain assessment comparing the reproducibility of four commercially available CMR‐feature tracking algorithms. Eur Radiol 2018; 28: 5137–5147. [DOI] [PubMed] [Google Scholar]
- 37. Liu B, Dardeer AM, Moody WE, Hayer MK, Baig S, Price AM, Leyva F, Edwards NC, Steeds RP. Reference ranges for three‐dimensional feature tracking cardiac magnetic resonance: comparison with two‐dimensional methodology and relevance of age and gender. Int J Cardiovasc Imaging 2018; 34: 761–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee J‐H, Park J‐H. Strain analysis of the right ventricle using two‐dimensional echocardiography. J Cardiovasc Imaging 2018; 26: 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grant AD, Smedira NG, Starling RC, Marwick TH. Independent and incremental role of quantitative right ventricular evaluation for the prediction of right ventricular failure after left ventricular assist device implantation. J Am Coll Cardiol 2012; 60: 521–528. [DOI] [PubMed] [Google Scholar]
- 40. Matthews JC, Koelling TM, Pagani FD, Aaronson KD. The right ventricular failure risk score a pre‐operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol 2008; 51: 2163–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Backhaus SJ, Metschies G, Billing M, Kowallick JT, Gertz RJ, Lapinskas T, Pieske B, Lotz J, Bigalke B, Kutty S, Hasenfuß G, Beerbaum P, Kelle S, Schuster A. Cardiovascular magnetic resonance imaging feature tracking: impact of training on observer performance and reproducibility. PLoS ONE 2019; 14: e0210127. [DOI] [PMC free article] [PubMed] [Google Scholar]


