Abstract
Noncompaction cardiomyopathy (NCCM) is a rare condition characterized by prominent trabeculae, deep intertrabecular recesses, and a left ventricular myocardium with a two‐layered structure, characterized by a spongy endocardial layer and a thinner and compacted epicardial one. NCCM can be isolated or associated with other congenital heart diseases and complex syndromes involving neuromuscular disorders and facial dysmorphisms. To date, more than 40 genes coding for sarcomeric, cytoskeletal, ion channels, and desmosomal proteins have been identified. Clinical presentation is also highly variable, ranging from no symptoms to end‐stage heart failure (HF), lethal arrhythmias, sudden cardiac death, or thromboembolic events. In particular, the prevalence of thromboembolism in NCCM patients appears to be higher than that of a similar, age‐matched population without NCCM. Thromboembolism has a multifactorial aetiology, which is linked to genetic, as well as traditional cardiovascular risk factors. In previous studies, atrial fibrillation (AF) was observed in approximately 25–30% of adult NCCM patients and embolism had a cardiac source in ~63–69% of cases; therefore, AF represents a strong predictor of adverse events, especially if associated to HF and neuromuscular disorders. Left ventricular dysfunction is another risk factor for thromboembolism, as a result of blood stagnation and local myocardial injury. Moreover, it is not completely clarified if the presence of deep intertrabecular recesses causing stagnant blood flow can constitute per se a thrombogenic substrate even in absence of ventricular dysfunction. For the clinical management of NCCM patients, an appropriate stratification of the thromboembolic risk is of utmost importance for a timely initiation of anticoagulant therapy. The aim of the present study is to review the available literature on NCCM with particular attention on thromboembolic risk stratification and prevention and the current evidence for oral anticoagulation therapy. The use of direct oral anticoagulants vs. vitamin K antagonists is also discussed with important implications for patient treatment and prognosis.
Keywords: Noncompaction cardiomyopathy, Atrial fibrillation, Anticoagulant therapy, Left ventricular dysfunction, Thrombosis, Stroke
Introduction
Noncompaction cardiomyopathy (NCCM) is a rare condition characterized by prominent left ventricular (LV) trabeculae and deep intertrabecular recesses, first described in 1969 by Feldt et al., 1 who reported a biventricular spongy myocardium in a female patient who died at the age of 3 months. NCCM is characterized by a two‐layered myocardial structure, characterized by a spongy endocardial layer and a thinner and compacted epicardial one. Apical and lateral segments of the LV are the most commonly involved, but both ventricles can be affected.
The prevalence of NCCM is unknown, but it has been estimated to be approximately 0.05–0.27% among adults referred to echocardiography lab, 2 with males more affected than females. 3 Age at the time of diagnosis is variable from early infancy to late adulthood. 3
Noncompaction cardiomyopathy is a genetic cardiomyopathy, because it has been described in association with mutations in more than 40 genes coding for sarcomeric, cytoskeletal, Z‐line, and mitochondrial proteins 4 and even in chromosomal defects. 3 A strong genotype–phenotype correlation has been reported for Hyperpolarization Activated Cyclic Nucleotide gated potassium channel 4 (HCN4), Titin (TTN), and Lamin A/C (LMNA) mutations, with a high incidence of heart failure (HF) and ventricular arrhythmias. 5 Furthermore, NCCM can be associated to genetic syndromes, including congenital heart disease, neuromuscular disorders, and facial dysmorphisms. 5
From a pathogenetic point of view, NCCM may be due to an abnormal myocardial compaction during intrauterine cardiac development. 6 However, some authors have suggested that NCCM could be the result of abnormal persistence of the trabecular layer rather than the effect of noncompaction of the ventricular wall. 6
Clinical presentation of NCCM is highly variable, ranging from no symptoms to end‐stage HF, lethal arrhythmias, sudden cardiac death, or thromboembolic events (stroke, transient ischaemic attack, mesenteric, myocardial and renal infarction, or peripheral embolism). 3 , 7 , 8
As far as the diagnosis is concerned, transthoracic echocardiography (TTE) and cardiac magnetic resonance imaging (MRI) are the most common imaging techniques (Figure 1 ), but recent studies showed the ability of low‐dose cardiac computer tomography in identifying ventricular trabeculation 9 , 10 , 11 ; ventricular angiography can be advised in selected cases with uncertain diagnosis or in patients who need an invasive cardiac study 12 (Figure 1 ).
Figure 1.
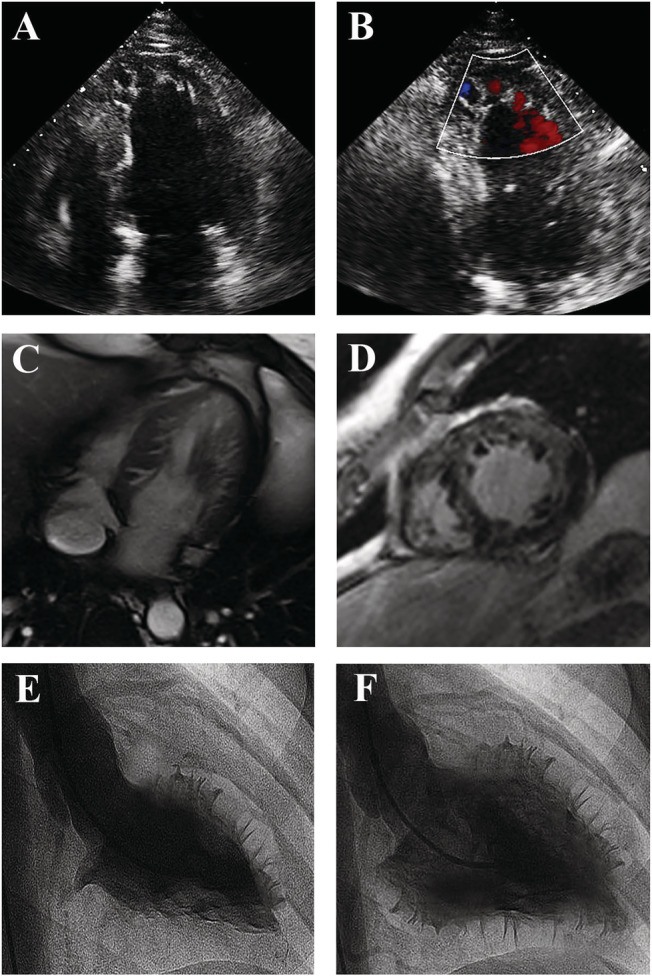
Imaging of NCCM. Echocardiographic apical four‐chamber view (Panel A) showing a dilated left ventricle with trabeculations in the apical and lateral region, and blood flow in deep recesses at colour Doppler (Panel B). Cardiac MRI four‐chamber (Panel C) and axial (Panel D) view showing NCCM at lateral and apical wall. Left ventriculogram in right long‐axis oblique view during systole (Panel E) and diastole (Panel F), showing an extensive non‐compacted layer containing numerous trabeculations.
Several diagnostic criteria have been proposed, but there is currently no gold standard. The most used criteria are the Jenni criteria 13 for TTE and the Petersen criteria 14 and Jacquier criteria 15 for cardiac MRI (Table 1 ). Endomyocardial biopsy should be considered if myocarditis is suspected or if new onset acute HF occurs in a previously haemodynamically stable NCCM patient. 20
Table 1.
Diagnostic criteria used to define noncompaction cardiomyopathy
| Echocardiographic criteria | |||
|---|---|---|---|
| Jenni et al. 13 | A two‐layer structure with a thin, compacted layer (C) and a thickened non‐compacted layer (NC) at end‐systole | A ratio of NC/C > 2 | Intertrabecular spaces are filled by blood flow on colour Doppler |
| Chin et al. 16 | Distance from the epicardial surface to the trough of the trabecular recess (X) and distance from the epicardial surface to peak of trabeculation (Y) at end‐diastole | A ratio of X/Y ≤ 0.5 | Focus on the trabeculations at the LV apex |
| Stöllberger et al. 17 | ≥3 trabeculations along the LV endocardial borders, different from the papillary muscles, false tendons, and aberrant muscle bands | Trabeculations with the same echogenicity as the myocardium and synchronous movement with ventricular contractions | Perfusion of the intertrabecular recesses from the LV cavity |
| Paterick et al. 18 | Identification of the bilayered myocardium in the short‐axis views at the middle and apical levels | A ratio of NC/C > 2, measured at end‐diastole on short‐axis parasternal views | |
| MRI criteria | |||
|---|---|---|---|
| Petersen et al. 14 | A ratio of NC/C > 2.3 measured at end‐diastole | ||
| Jacquier et al. 15 | Trabeculated LV mass > 20% of global LV mass | ||
| Grothoff et al. 19 | Percentage of LV trabeculation > 25% and trabeculated LV mass > 15 g/m2 | A ratio of NC/C ≥ 2 in segments 4–6 | A ratio of NC/C ≥ 3 in at least one of the other segments |
C, compacted; LV, left ventricular; MRI, magnetic resonance imaging; NC, non‐compacted.
As a result of the very low prevalence of the disease, there is a lack of evidence from large randomized trials about the clinical management of NCCM, especially regarding the need for anticoagulation. Therefore, it may be challenging to identify the optimal candidates for and when to start anticoagulant therapy, particularly if LV size and function are normal or in case of systolic dysfunction and sinus rhythm. Thus, we searched the Medline, Embase, and Google Scholar databases from inception to April 2021, and we found 174 articles describing the thromboembolic risk and the anticoagulation strategy adopted in NCCM patients; the full search strategies are provided in the supporting information. The aim of this review is to stratify the thromboembolic risk in NCCM patients and shed light on the potential indications for anticoagulant therapy.
Thromboembolic risk in noncompaction cardiomyopathy
The frequency of thromboembolism in NCCM patients is quite variable (0–38%) and seems to be age related: overall, adults have an increased risk of experiencing an embolic event than children (Table 2 ). 3 As an example, although Chin et al. described three children out of eight (38%) with clinically evident systemic embolism, 16 in subsequent studies, no thromboembolic events occurred during the entire follow‐up period. 30 , 31 , 32 On the contrary, in a large retrospective study of 169 NCCM adult patients, 26 15% experienced thromboembolic events: 92% of them had a stroke and 8% had a peripheral embolism. The cause of thromboembolism was cardioembolic (69%), atherosclerotic (19%), and undetermined (12%); among the 18 patients with cardioembolic stroke, only 7 (39%) had atrial fibrillation (AF) while 14 (78%) had LV systolic dysfunction. In the cardioembolic group, 50% of patients was receiving acetylsalicylic acid (ASA) 100 mg/day, 6% was receiving vitamin K antagonists (VKAs), 6% was receiving low‐molecular weight heparin, and 38% was not taking any antithrombotic or anticoagulant therapy. Another study 28 by the same authors reported similar findings: thromboembolic events occurred in 22 (15.3%) of 144 NCCM patients (21 ischaemic strokes and 1 peripheral embolism). At the time of thromboembolism, 59% of patients was receiving ASA 100 mg/day, 4.5% VKA with an international normalized ratio (INR) below the therapeutic range, 4.5% low‐molecular weight heparin, and 32% was not taking antithrombotic or anticoagulant therapy.
Table 2.
Thromboembolic events in noncompaction cardiomyopathy patients
| Study | No. of patients | Incidence of thromboembolic events | Type of thromboembolic event |
|---|---|---|---|
| Ritter et al. 21 | 17 | 29% |
|
| Oechslin et al. 22 | 34 | 24% |
|
| Aras et al. 23 | 67 | 9% |
|
| Murphy et al. 24 | 45 | 4% |
|
| Stöllberger and Finsterer 25 | 62 | 10% |
|
| Stöllberger et al. 26 | 169 | 15% |
|
| Greutmann et al. 27 | 115 | 4% |
|
| Stöllberger et al. 28 | 144 | 15% |
|
| Paediatric population | |||
|---|---|---|---|
| Ichida et al. 29 | 27 | 0 | |
| Chin et al. 16 | 8 | 38% |
|
| Pignatelli et al. 30 | 36 | 0 | |
| Wald et al. 31 | 22 | 0 | |
PE, pulmonary embolism; TIA, transient ischaemic stroke.
These findings suggest that the treatment with ASA, administered in ~60% of NCCM patients, was not able to prevent the occurrence of thromboembolic events. As a matter of fact, thromboembolism in NCCM patients has a multifactorial aetiology, including genetic and other well‐known cardiovascular risk factors. 5
A recent study by van Waning et al. 33 correlated genetics, phenotype, and outcomes; myosin heavy chain 7 (MYH7), myosin binding protein C3 (MYBPC3), and TTN mutations were the most common (71%) and were frequently associated with LV dysfunction. Furthermore, children with MYBPC3 complex mutations had a higher risk of major adverse cardiovascular events, including cardioembolic stroke, than patients with MYH7 mutations. 33
Genotype variability is associated with an equally varied phenotype presentation; one of the intriguing findings among patients with isolated LV noncompaction is the presence of associated neuromuscular disorders, reported in more than four‐fifths of subjects. 34 Patients with NCCM and neuromuscular disorders appear to have a higher thromboembolic risk and a worse prognosis, probably related to the earlier onset of severe LV dysfunction, the frequent involvement of respiratory muscles resulting from the underlying neuromuscular disorders, and the precluded decision for heart transplantation. 35 Moreover, NCCM is also associated with congenital heart diseases; cardiac abnormalities, in particular atrial (3.5%) and ventricular defects (2.5%), have been described in 12% of patients. 36 In this setting, thrombi could originate from the right ventricle or right atrium and reach the systemic circulation through a ventricular or atrial septal defect.
More challenging is the assessment of thromboembolic risk in NCCM patients without AF, LV dysfunction, and/or LV thrombus; specifically, it is still controversial whether NCCM alone is an independent risk factor for stroke. Given the complexity of the cardiomyopathy, CHADS2 score, developed for AF patients, could be used for thromboembolic risk stratification. Stöllberger et al. 26 evaluated the prognostic value of CHADS2 and CHA2DS2‐VASc scores in NCCM patients with and without previous thromboembolic events. They observed that patients with NCCM and a history of thromboembolic events have a significantly higher CHADS2/CHA2DS2‐Vasc score. Of note, CHADS2 was superior to CHA2DS2‐VASc in predicting the thromboembolic risk; this may be explained by the younger age of NCCM patients and the male preponderance. Therefore, in NCCM patients without a clear indication to anticoagulant therapy (AF, previous thromboembolism, or intracardiac thrombi), CHADS2 score may be more accurate than CHA2DS2VASc for thromboembolic risk stratification and, if the CHADS2 is ≥2, oral anticoagulation should be strongly considered.
Noncompaction cardiomyopathy and atrial fibrillation
Atrial fibrillation is most common atrial arrhythmia affecting ~25–30% of patients with NCCM. 37 In a previous study on 68 adult NCCM patients followed up for approximately 61 months, AF occurred in 29.4%. 5 A similar incidence of AF has been reported in children with NCCM. These findings suggest that this cardiomyopathy may acts as a proarrhythmic substrate for either the atria or the ventricles. 30
Noncompaction cardiomyopathy patients developing AF tend to have a higher prevalence of valvular abnormalities, a more extensive noncompaction layer, and a worst LV systolic function than NCCM patients without AF. 37 , 38 These findings could partially explain the pathophysiology of AF; in adult patients, atrial cardiopathy may be correlated to atrial dilation due to systolic dysfunction and atrioventricular valve regurgitation or to the underlying cardiomyopathy and ion channel imbalances. 39
In NCCM patients, embolism have a cardiac source in ~63–69% of cases 26 , 28 and AF represents a strong predictor for mortality, especially if associated to HF and neuromuscular disorders. 28 , 37 Therefore, an appropriate anticoagulation therapy for NCCM patients with AF or previous embolic event is mandatory. 40 In particular, there is much stronger evidence that oral anticoagulation is associated with a higher reduction in stroke rate compared with antiplatelet agents (60% vs. 20%, respectively). 41
Noncompaction cardiomyopathy and left ventricular dysfunction with and without thrombosis
Noncompaction cardiomyopathy patients with LV dysfunction (ejection fraction < 50%) and sinus rhythm should be considered as high‐risk patients for thromboembolic events. 42 Indeed, in this group, LV dysfunction plays a crucial role in the predisposition to thrombus formation due to blood stagnation and local myocardial injury within the deep intertrabecular recesses.
In a nested case–control study of patients with severe LV dysfunction without AF, the stroke incidence was 3.9% over 35 months, with a significantly higher incidence in patients affected by NCCM (P = 0.02), LV aneurysm (P < 0.01), and pulmonary hypertension (P < 0.001). 43 Therefore, in NCCM patients with LV systolic dysfunction and sinus rhythm, long‐term oral anticoagulation is generally suggested. 40
Left ventricular thrombosis in NCCM patients with LV dysfunction may be a terrible complication because it is associated with high rates of systemic embolism, morbidity, and mortality. Compared with echocardiography, cardiac MRI offers a higher resolution for the identification of myocardial trabeculations and provides a more accurate tissue characterization, allowing for the correct identification of apical thrombi and endomyocardial fibrosis. 44 , 45 Therefore, cardiac MRI should be the first choice in all NCCM patients as it can provide valuable information for a better thromboembolic risk stratification. If an LV thrombus is identified, VKA is considered the standard of care; nevertheless, there is increasing evidence that direct oral anticoagulants (DOACs) are associated with a high rate of complete resolution of intracardiac thrombosis with a low rate of thromboembolic or haemorrhagic complication. 46
Noncompaction cardiomyopathy per se
In a large retrospective study has been reported a thromboembolic event of unknown origin in NCCM patients with CHA2DS2VASc score = 0, suggesting that NCCM may have per se an intrinsic thromboembolic risk. 26 It has also been reported that NCCM patients with preserved ejection fraction and positive late gadolinium enhancement (LGE) at cardiac MRI have an increased risk of cardiovascular events, including stroke, than NCCM patients with preserved systolic function without LGE. 47
Autopsy and endomyocardial biopsy studies in patients with NCCM showed the presence of viable cells separated by large, deep, unendothelialized endomyocardial channels (Figure 2 ). 48 Deep intertrabecular recesses cause stagnant blood flow, which can result in thrombus formation even in absence of ventricular dysfunction. 49 Possible pathogenesis of thrombus formation is showed in Figure 3 .
Figure 2.
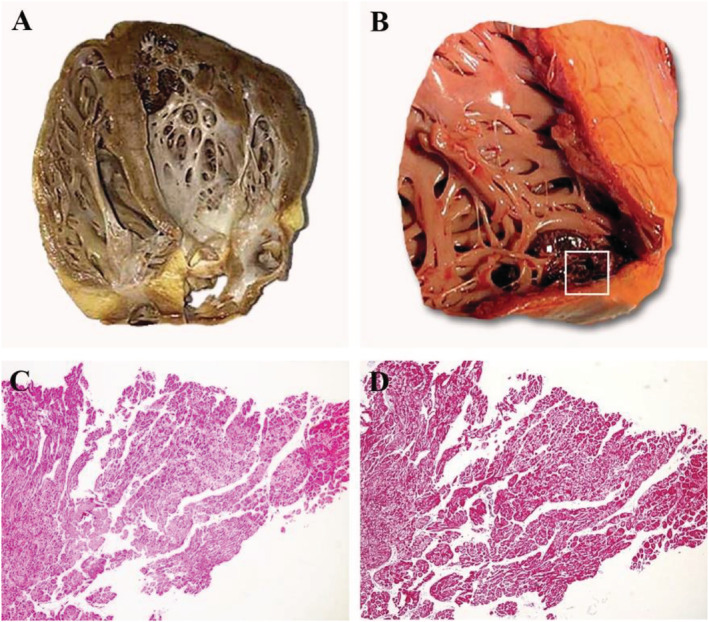
Macroscopic and microscopic aspect of NCCM. Autoptic NCCM heart showing prominent trabeculations and deep recesses in apical left ventricular wall (Panel A). Mural thrombus wedged within the intertrabecular recesses is evident in Panel B (square). Histology shows cell separated by unendothelialized large and deep spaces (haematoxylin and eosin, magn. ×4) with poor fibrous replacement (Masson‐trichrome, magn. ×4) (Panels C and D).
Figure 3.
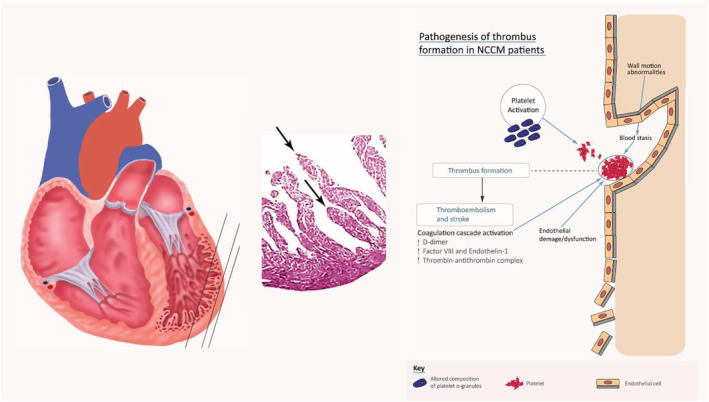
Pathogenesis of thrombus formation in NCCM patients. The Virchow's triad factors, that is, sluggish blood flow in deep intertrabecular recesses, local myocardial injury, and hypercoagulability/stasis of flow, contribute to formation of LV thrombus.
All the Virchow's triad factors—sluggish blood flow in deep intertrabecular recesses, local myocardial injury, and hypercoagulability/stasis of flow—which contribute to formation of LV thrombus, are present in NCCM, which can also be associated with a hypercoagulability state due to overexpression of factor VIII and endothelin‐1. 50
In the current literature, two large studies have highlighted a direct association between NCCM and juvenile ‘cryptogenic’ stroke: 12–14% patients with NCCM and no other concomitant comorbidities or risk factors (i.e. PFO) experienced an undetermined thromboembolic event, confirming that NCCM may carry a cardioembolic risk per se. 26 , 28
A recent case–control study in young adults (age 18–49) presenting with an imaging‐positive ischaemic stroke of undetermined aetiology showed that the percentage of non‐compacted LV volume was higher in patients with cryptogenic stroke compared with stroke‐free controls of similar age and gender. 51 Importantly, a 5% increase in non‐compacted ventricular volume at cardiac MRI has been associated with a nine‐fold increased risk of cryptogenic ischaemic stroke. 51 Furthermore, several case reports seem to confirm the close relationship between NCCM and juvenile stroke. 52 , 53
Stroke prevention in noncompaction cardiomyopathy
In the ‘2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy’, anticoagulation therapy is recommended in NCCM patients with AF and in those with previous thromboembolic events or LV thrombosis (Class I, Level of Evidence B) while may be reasonable in individuals with NCCM with LV dysfunction (Class IIb, Level of Evidence B). 40 In AF patients, the landscape of oral anticoagulant therapy has widely changed in the last decade. Randomized control trials have demonstrated the effectiveness and safety of DOACs. 54 Subsequent data from registries and real‐world experiences have established DOACs as a valid alternative to warfarin. 55 , 56 Given the remarkable safety profile of DOACs compared with VKAs, emerging evidence supports their use in high‐risk populations such as elderly, renal impairment, peripheral artery disease, and AF in the context of congenital heart disease and cardiomyopathies. 57 , 58 Similarly, several case reports have demonstrated that DOACs could be at least as effective and safe as warfarin in preventing cardioembolic events in NCCM patients. 59 , 60 Skidan et al. reported a case of a 32‐year‐old male with NCCM and isolated LV apical hypoplasia admitted to the hospital for radiofrequency catheter ablation of persistent AF. 60 Despite the high thromboembolic risk due to the concomitant presence of AF and biventricular systolic dysfunction, no thromboembolic events occurred during 2 years of follow‐up in NCCM patients receiving long‐term oral anticoagulation therapy with Rivaroxaban. Similarly, Li et al. described the history of a 54‐year‐old man with hypertension, diabetes, and end‐stage renal disease presented with 1 day palpitations and lightheadedness following a dialysis session. 59 Electrocardiogram revealed AF with normal ventricular rate while TTE revealed prominent trabeculation and normal LV systolic function, suggestive of NCCM. Although heparin was firstly administered, the patient was switched to Apixaban for outpatient treatment; during follow‐up, no thromboembolic events were reported.
Regarding the presence of LV thrombosis, long‐term VKA administration is still the most commonly adopted therapeutic strategy. In a multicentre study enrolling patients with LV thrombi, DOAC treatment was associated with a higher risk of thromboembolic events compared with warfarin. 61 Although these findings seem to corroborate the assumption that the ideal anticoagulant treatment for LV thrombus is warfarin, the authors reported several study limitations: for example, bleeding events were not considered as an endpoint and no information on DOAC dosing was provided. 61 Thus, no conclusive data are available on the use of DOACs in patients with NCCM and LV thrombus, and further studies with comparative groups are necessary.
As a matter of fact, several recent studies have shown that DOACs represent a new promising strategy for treating LV thrombus. Fledderman et al. demonstrated that in 83% of patients, DOACs safely and effectively led to complete resolution of LV thrombus. 46 Additionally, a meta‐analysis of 33 papers showed thrombus resolution in 80% of patients treated with DOACs. 62 Undoubtedly, only prospective randomized clinical trials may determine the most effective treatment strategies for LV thrombi.
In the specific setting of LV thrombosis in NCCM patients, a single case report about treatment with DOACs is currently available: in a 43‐year‐old patient, treatment with Rivaroxaban at low dose (10 mg once daily) resulted in thrombus resolution at 3 months of follow‐up. 63 Probably, DOACs may offer in the next future a new alternative therapeutic approach in NCCM patients with LV thrombosis and/or systolic dysfunction. Well‐known therapeutic advantages of DOACs, including improved safety, reduced risk of bleeding, increased adherence to therapy, and convenience during long life oral therapy, could be preciously useful in young NCCM patients, especially in children. 54 , 58 , 64 The latter category is the most challenging one because no randomized trials on DOACs have been published for paediatric patients and VKAs still represent the first‐choice anticoagulation therapy. Unfortunately, VKAs have numerous drug interactions, as well as their efficacy can be influenced by the vitamin K intake from the diet. This factor is a major limitation especially in children who rapidly change their diet altering their vitamin K consumption. Therefore, monitoring oral anticoagulant therapy in children is difficult and requires close supervision, with frequent dose adjustments. During initiation of therapy, monitoring should be daily or every few days, 65 and when INR is high, lifestyle changes are necessary and physical activity may be forbidden. Moreover, because of the increased bleeding risk associated with oral anticoagulation, children are often hovered over by their parents and may have psychological problems, such as depression and anxiety. 66 In this setting, the use of DOACs could potentially overcome several difficulties (i.e. need for INR evaluation and diet control), but further study is necessary.
Another debated issue is the need for oral anticoagulant therapy in NCCM patients with LV dysfunction alone. Although in general population the thromboembolic risk is directly proportional to the severity of LV dysfunction, current evidence does not recommend long‐term anticoagulation therapy. 42 , 67 , 68 In spite of this, NCCM patients with LV dysfunction and sinus rhythm should be considered at high risk for thromboembolic events. 26 , 28 , 37 , 38 As previously described, the presence of NCCM in patients with severe systolic dysfunction was associated with higher risk of thromboembolism. 43 Moreover, in a recent meta‐analysis, NCCM patients with LV dysfunction and LGE at cardiac MRI were compared with those without LGE; patients with LGE experienced more frequently cardiovascular adverse events, including stroke. 46 Thus, in NCCM patients with LV dysfunction (ejection fraction < 50% as defined in the 2016 Guidelines for the diagnosis and treatment of acute and chronic heart failure), long‐term anticoagulation is suggested and the use of DOACs should be preferred. 42
Regarding patients with NCCM without AF, systolic dysfunction, and/or ventricular thrombosis, current evidence suggests that NCCM is a thromboembolic substrate per se because young patients with no other cardiovascular risk factors have experienced a ‘cryptogenic’ stroke. 52 Therefore, although the prevention therapy of thromboembolic events in these patients has not been recommended yet, the use of anticoagulant therapy may be considered in patients with CHADS2 score ≥ 2.
A flow chart to summarize the current and potential indications of anticoagulation therapy in NCCM patients is shown in Figure 4 ; anticoagulation is mandatory in all NCCM patients with AF, previous thromboembolic events, or LV thrombosis, whereas it should be considered in NCCM patients with LV dysfunction and sinus rhythm. 40 In patients with normal LV size and function, better stratification of thromboembolic risk by evaluating CHADS2 score is suggested. In our opinion, the use of DOACs should be taken into consideration in all NCCM patients in absence of contraindication and following a careful evaluation of each single case, mainly because of the long‐life expectancy of affected patients, the reduced risk of bleeding, and the larger adherence and persistence to therapy.
Figure 4.
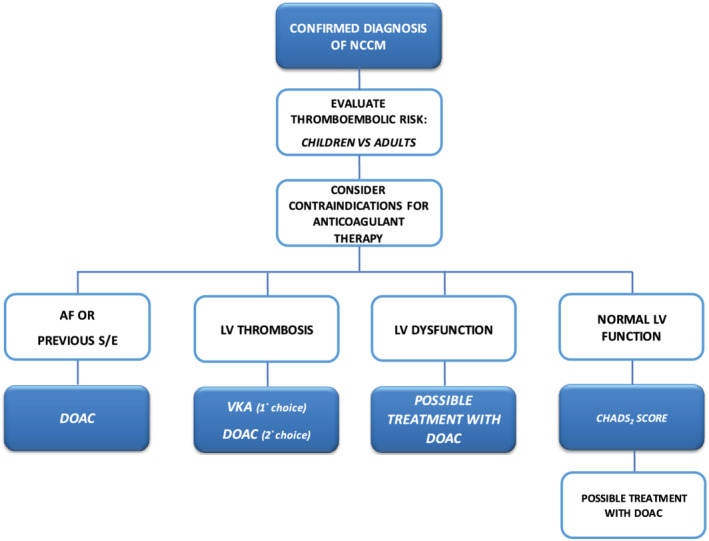
Proposed flow chart for anticoagulation therapy in NCCM patients.
Conclusions
Noncompaction cardiomyopathy is a complex, clinically and genetically heterogeneous disorder characterized by an increased thromboembolic risk. Long‐term oral anticoagulant therapy is recommended in NCCM patients with AF and previous thromboembolic events while it seems reasonable in those with LV dysfunction. Based on latest findings and our clinical experience, DOACs may be used as first choice. Although a better clinical stratification and pathophysiologic understanding are necessary, NCCM per se might represent an embolic risk factor that requires anticoagulant therapy for stroke prevention.
Conflict of interest
Nothing to disclose.
Supporting information
Figure S1. Study Strategy.
Chimenti, C. , Lavalle, C. , Magnocavallo, M. , Alfarano, M. , Mariani, M. V. , Bernardini, F. , Della Rocca, D. G. , Galardo, G. , Severino, P. , Di Lullo, L. , Miraldi, F. , Fedele, F. , and Frustaci, A. (2022) A proposed strategy for anticoagulation therapy in noncompaction cardiomyopathy. ESC Heart Failure, 9: 241–250. 10.1002/ehf2.13694.
References
- 1. Feldt RH, Rahimtoola SH, Davis GD, Swan HJC, Titus JL. Anomalous ventricular myocardial patterns in a child with complex congenital heart disease. Am J Cardiol 1969; 23: 732–734. [DOI] [PubMed] [Google Scholar]
- 2. Ronderos R, Avegliano G, Borelli E, Kuschnir P, Castro F, Sanchez G, Perea G, Corneli M, Zanier MM, Andres S, Aranda A, Conde D, Trivi M. Estimation of prevalence of the left ventricular noncompaction among adults. Am J Cardiol 2016; 118: 901–905. [DOI] [PubMed] [Google Scholar]
- 3. Towbin JA, Lorts A, Jefferies JL. Left ventricular non‐compaction cardiomyopathy. Lancet 2015; 386: 813–825. [DOI] [PubMed] [Google Scholar]
- 4. Richard P, Ader F, Roux M, Donal E, Eicher JC, Aoutil N, Huttin O, Selton‐Suty C, Coisne D, Jondeau G, Damy T, Mansencal N, Casalta AC, Michel N, Haentjens J, Faivre L, Lavoute C, Nguyen K, Tregouët DA, Habib G, Charron P. Targeted panel sequencing in adult patients with left ventricular non‐compaction reveals a large genetic heterogeneity. Clin Genet 2019; 95: 356–367. [DOI] [PubMed] [Google Scholar]
- 5. Sedaghat‐Hamedani F, Haas J, Zhu F, Geier C, Kayvanpour E, Liss M, Lai A, Frese K, Pribe‐Wolferts R, Amr A, Li DT, Samani OS, Carstensen A, Bordalo DM, Müller M, Fischer C, Shao J, Wang J, Nie M, Yuan L, Haßfeld S, Schwartz C, Zhou M, Zhou Z, Shu Y, Wang M, Huang K, Zeng Q, Cheng L, Fehlmann T, Ehlermann P, Keller A, Dieterich C, Streckfuß‐Bömeke K, Liao Y, Gotthardt M, Katus HA, Meder B. Clinical genetics and outcome of left ventricular non‐compaction cardiomyopathy. Eur Heart J 2017; 38: 3449–3460. [DOI] [PubMed] [Google Scholar]
- 6. Hussein A, Karimianpour A, Collier P, Krasuski RA. Isolated noncompaction of the left ventricle in adults. J Am Coll Cardiol 2015; 66: 578–585. [DOI] [PubMed] [Google Scholar]
- 7. Frustaci A, Galea N, Verardo R, Francone M, Alfarano M, Russo MA, Chimenti C. Kappa‐light chain amyloid overlapping hypertrophic cardiomyopathy with myocardial noncompaction. Circ Cardiovasc Imaging 2020; 13: e010379. [DOI] [PubMed] [Google Scholar]
- 8. Tarantino N, Rocca DGD, Cruz NSL, Manheimer ED, Magnocavallo M, Lavalle C, Gianni C, Mohanty S, Trivedi C, Al‐Ahmad A, Horton RP, Bassiouny M, Burkhardt JD, Gallinghouse GJ, Forleo GB, Biase LD, Natale A. Catheter ablation of life‐threatening ventricular arrhythmias in athletes. Medicina (Kaunas). 2021; 57: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fuchs TA, Erhart L, Ghadri JR, Herzog BA, Giannopoulos A, Buechel RR, Stämpfli SF, Gruner C, Pazhenkottil AP, Niemann M, Kaufmann PA, Tanner FC. Diagnostic criteria for left ventricular non‐compaction in cardiac computed tomography jefferies JL, editor. PLoS ONE 2020; 15: e0235751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ficili S, Pandozi C, Galeazzi M, Kol A, Russo M, Lavalle C, Dottori S, Santini M. Noncompacted ventricular myocardium: Characterization by intracardiac echo. J Cardiovasc Med 2011; 12: 294–296. [DOI] [PubMed] [Google Scholar]
- 11. Martín M, Barriales V, Corros C, Santamarta E. Usefulness of cardiac magnetic resonance imaging in left ventricular non‐compaction cardiomyopathy. Eur J Heart Fail 2011; 13: 577–577. [DOI] [PubMed] [Google Scholar]
- 12. Hughes ML, Carstensen B, Wilkinson JL, Weintraub RG. Angiographic diagnosis, prevalence and outcomes for left ventricular noncompaction in children with congenital cardiac disease. CTY 2007; 17: 56. [DOI] [PubMed] [Google Scholar]
- 13. Jenni R. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non‐compaction: A step towards classification as a distinct cardiomyopathy. Heart 2001; 86: 666–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, Anderson RH, Watkins H, Neubauer S. Left ventricular non‐compaction. J Am Coll Cardiol 2005; 46: 101–105. [DOI] [PubMed] [Google Scholar]
- 15. Jacquier A, Thuny F, Jop B, Giorgi R, Cohen F, Gaubert JY, Vidal V, Bartoli JM, Habib G, Moulin G. Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non‐compaction. Eur Heart J 2010; 31: 1098–1104. [DOI] [PubMed] [Google Scholar]
- 16. Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 1990; 82: 507–513. [DOI] [PubMed] [Google Scholar]
- 17. Stöllberger C, Gerecke B, Finsterer J, Engberding R. Refinement of echocardiographic criteria for left ventricular noncompaction. Int J Cardiol 2013; 165: 463–467. [DOI] [PubMed] [Google Scholar]
- 18. Paterick TE, Umland MM, Jan MF, Ammar KA, Kramer C, Khandheria BK, Seward JB, Tajik AJ. Left ventricular noncompaction: A 25‐year odyssey. J Am Soc Echocardiogr 2012; 25: 363–375. [DOI] [PubMed] [Google Scholar]
- 19. Grothoff M, Pachowsky M, Hoffmann J, Posch M, Klaassen S, Lehmkuhl L, Gutberlet M. Value of cardiovascular MR in diagnosing left ventricular non‐compaction cardiomyopathy and in discriminating between other cardiomyopathies. Eur Radiol 2012; 22: 2699–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, Levine GN, Narula J, Starling RC, Towbin J, Virmani R. The role of endomyocardial biopsy in the management of cardiovascular disease: A scientific statement from the American Heart Association, the american College of Cardiology, and the european Society of Cardiology Endorsed by the Heart Failure Society of America and the heart failure Association of the European Society of cardiology. Eur Heart J 2007; 28: 3076–3093. [DOI] [PubMed] [Google Scholar]
- 21. Ritter M, Oechslin E, Sütsch G, Attenhofer C, Schneider J, Jenni R. Isolated noncompaction of the myocardium in adults. Mayo Clin Proc 1997; 72: 26–31. [DOI] [PubMed] [Google Scholar]
- 22. Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R. Long‐term follow‐up of 34 adults with isolated left ventricular noncompaction: A distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol 2000; 36: 493–500. [DOI] [PubMed] [Google Scholar]
- 23. Aras D, Tufekcioglu O, Ergun K, Ozeke O, Yildiz A, Topaloglu S, Deveci B, Sahin O, Kisacik HL, Korkmaz S. Clinical features of isolated ventricular noncompaction in adults long‐term clinical course, echocardiographic properties, and predictors of left ventricular failure. J Card Fail 2006; 12: 726–733. [DOI] [PubMed] [Google Scholar]
- 24. Murphy RT, Thaman R, Blanes JG, Ward D, Sevdalis E, Papra E, Kiotsekolglou A, Tome MT, Pellerin D, McKenna WJ, Elliott PM. Natural history and familial characteristics of isolated left ventricular non‐compaction. Eur Heart J 2005; 26: 187–192. [DOI] [PubMed] [Google Scholar]
- 25. Stöllberger C, Finsterer J. Left ventricular Hypertrabeculation/Noncompaction and stroke or embolism. Cardiology 2005; 103: 68–72. [DOI] [PubMed] [Google Scholar]
- 26. Stöllberger C, Wegner C, Finsterer J. CHADS2‐ and CHA2DS2VASc scores and embolic risk in left ventricular Hypertrabeculation/Noncompaction. J Stroke Cerebrovasc Dis 2013; 22: 709–712. [DOI] [PubMed] [Google Scholar]
- 27. Greutmann M, Mah ML, Silversides CK, Klaassen S, Attenhofer Jost CH, Jenni R, Oechslin EN. Predictors of adverse outcome in adolescents and adults with isolated left ventricular noncompaction. Am J Cardiol 2012; 109: 276–281. [DOI] [PubMed] [Google Scholar]
- 28. Stöllberger C, Blazek G, Dobias C, Hanafin A, Wegner C, Finsterer J. Frequency of stroke and embolism in left ventricular Hypertrabeculation/Noncompaction. Am J Cardiol 2011; 108: 1021–1023. [DOI] [PubMed] [Google Scholar]
- 29. Ichida F, Hamamichi Y, Miyawaki T, Ono Y, Kamiya T, Akagi T, Hamada H, Hirose O, Isobe T, Yamada K, Kurotobi S, Mito H, Miyake T, Murakami Y, Nishi T, Shinohara M, Seguchi M, Tashiro S, Tomimatsu H. Clinical features of isolated noncompaction of the ventricular myocardium. J Am Coll Cardiol 1999; 34: 233–240. [DOI] [PubMed] [Google Scholar]
- 30. Pignatelli RH, McMahon CJ, Dreyer WJ, Denfield SW, Price J, Belmont JW, Craigen WJ, Wu J, El Said H, Bezold LI, Clunie S, Fernbach S, Bowles NE, Towbin JA. Clinical characterization of left ventricular noncompaction in children: A relatively common form of cardiomyopathy. Circulation 2003; 108: 2672–2678. [DOI] [PubMed] [Google Scholar]
- 31. Wald R, Veldtman G, Golding F, Kirsh J, McCrindle B, Benson L. Determinants of outcome in isolated ventricular noncompaction in childhood. Am J Cardiol 2004; 94: 1581–1584. [DOI] [PubMed] [Google Scholar]
- 32. Ichida F, Tsubata S, Bowles KR, Haneda N, Uese K, Miyawaki T, Dreyer WJ, Messina J, Li H, Bowles NE, Towbin JA. Novel gene mutations in patients with left ventricular noncompaction or Barth syndrome. Circulation 2001; 103: 1256–1263. [DOI] [PubMed] [Google Scholar]
- 33. van Waning JI, Caliskan K, Hoedemaekers YM, van Spaendonck‐Zwarts KY, Baas AF, Boekholdt SM, van Melle JP, Teske AJ, Asselbergs FW, Backx APCM, du Marchie Sarvaas GJ, Dalinghaus M, Breur JMPJ, Linschoten MPM, Verlooij LA, Kardys I, Dooijes D, Lekanne Deprez RH, IJpma AS, van den Berg MP, Hofstra RMW, van Slegtenhorst MA, Jongbloed JDH, Majoor‐Krakauer D. Genetics, clinical features, and long‐term outcome of noncompaction cardiomyopathy. J Am Coll Cardiol 2018; 71: 711–722. [DOI] [PubMed] [Google Scholar]
- 34. Stöllberger C, Finsterer J, Blazek G. Left ventricular hypertrabeculation/noncompaction and association with additional cardiac abnormalities and neuromuscular disorders. Am J Cardiol 2002; 90: 899–902. [DOI] [PubMed] [Google Scholar]
- 35. Stöllberger C, Blazek G, Winkler‐Dworak M, Finsterer J. Atrial fibrillation in left ventricular noncompaction with and without neuromuscular disorders is associated with a poor prognosis. Int J Cardiol 2009; 133: 41–45. [DOI] [PubMed] [Google Scholar]
- 36. Stähli BE, Gebhard C, Biaggi P, Klaassen S, Valsangiacomo Buechel E, Attenhofer Jost CH, Jenni R, Tanner FC, Greutmann M. Left ventricular non‐compaction: Prevalence in congenital heart disease. Int J Cardiol 2013; 167: 2477–2481. [DOI] [PubMed] [Google Scholar]
- 37. Stöllberger C, Blazek G, Winkler‐Dworak M, Finsterer J. Atrial fibrillation in left ventricular noncompaction with and without neuromuscular disorders is associated with a poor prognosis. Int J Cardiol 2009; 133: 41–45. [DOI] [PubMed] [Google Scholar]
- 38. Stöllberger C, Blazek G, Gessner M, Bichler K, Wegner C, Finsterer J. Neuromuscular comorbidity, heart failure, and atrial fibrillation as prognostic factors in left ventricular hypertrabeculation/noncompaction. Herz 2015; 40: 906–911. [DOI] [PubMed] [Google Scholar]
- 39. Yeung C, Enriquez A, Suarez‐Fuster L, Baranchuk A. Atrial fibrillation in patients with inherited cardiomyopathies. EP Europace 2019; 21: 22–32. [DOI] [PubMed] [Google Scholar]
- 40. Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCC, Daubert JP, de Chillou C, DePasquale EC, Desai MY, Estes NAM 3rd, Hua W, Indik JH, Ingles J, James CA, John RM, Judge DP, Keegan R, Krahn AD, Link MS, Marcus FI, McLeod CJ, Mestroni L, Priori SG, Saffitz JE, Sanatani S, Shimizu W, van Tintelen JP, Wilde AAM, Zareba W. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm 2019; 16: e301–e372. [DOI] [PubMed] [Google Scholar]
- 41. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146: 857–867. [DOI] [PubMed] [Google Scholar]
- 42. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the european Society of Cardiology (ESC)Developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 43. Mahajan N, Ganguly J, Simegn M, Bhattacharya P, Shankar L, Madhavan R, Chaturvedi S, Ramappa P, Afonso L. Predictors of stroke in patients with severe systolic dysfunction in sinus rhythm: Role of echocardiography. Int J Cardiol 2010; 145: 87–89. [DOI] [PubMed] [Google Scholar]
- 44. Zhou H, Lin X, Fang L, Zhao X, Ding H, Chen W, Xu R, Bai X, Wang Y, Fang Q. Characterization of compacted myocardial abnormalities by cardiac magnetic resonance with native T1 mapping in left ventricular non‐compaction patients – a comparison with late gadolinium enhancement –. Circ J 2016; 80: 1210–1216. [DOI] [PubMed] [Google Scholar]
- 45. Diwadkar S, Nallamshetty L, Rojas C, Athienitis A, Declue C, Cox C, Patel A, Chae SH. Echocardiography fails to detect left ventricular noncompaction in a cohort of patients with noncompaction on cardiac magnetic resonance imaging. Clin Cardiol 2017; 40: 364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fleddermann AM, Hayes CH, Magalski A, Main ML. Efficacy of direct acting Oral anticoagulants in treatment of left ventricular thrombus. Am J Cardiol 2019; 124: 367–372. [DOI] [PubMed] [Google Scholar]
- 47. Grigoratos C, Barison A, Ivanov A, Andreini D, Amzulescu MS, Mazurkiewicz L, de Luca A, Grzybowski J, Masci PG, Marczak M, Heitner JF, Schwitter J, Gerber BL, Emdin M, Aquaro GD. Meta‐analysis of the prognostic role of late gadolinium enhancement and global systolic impairment in left ventricular noncompaction. JACC Cardiovasc Imaging 2019; 12: 2141–2151. [DOI] [PubMed] [Google Scholar]
- 48. Icardo JM, Fernandez‐Terán A. Morphologic study of ventricular trabeculation in the embryonic chick heart. Acta Anat 1987; 130: 264–274. [DOI] [PubMed] [Google Scholar]
- 49. Kulhari A, Kalra N, Sila C. Noncompaction cardiomyopathy and stroke: Case report and literature review. J Stroke Cerebrovasc Dis 2015; 24: e213–e217. [DOI] [PubMed] [Google Scholar]
- 50. Fan P, Zhang Y, Lu YT, Yang KQ, Lu PP, Zhang QY, Luo F, Lin YH, Zhou XL, Tian T. Prognostic value of plasma big endothelin‐1 in left ventricular non‐compaction cardiomyopathy. Heart. 2021; 107: 836–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pöyhönen P, Kuusisto J, Järvinen V, Pirinen J, Räty H, Lehmonen L, Paakkanen R, Martinez‐Majander N, Putaala J, Sinisalo J. Left ventricular non‐compaction as a potential source for cryptogenic ischemic stroke in the young: A case‐control study bauer WR, editor. PLoS ONE 2020; 15: e0237228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sahin S, Sekban A, Ayalp S, Karsidag S. An unusual cause of cardioembolic stroke: Isolated left ventricular noncompaction. Neurologist 2008; 14: 125–127. [DOI] [PubMed] [Google Scholar]
- 53. Finsterer J, Stöllberger C. Juvenile “cryptogenic” stroke from noncompaction in a neuromuscular disease. Cardiology 2014; 127: 223–226. [DOI] [PubMed] [Google Scholar]
- 54. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta‐analysis of randomised trials. The Lancet 2014; 383: 955–962. [DOI] [PubMed] [Google Scholar]
- 55. Mariani MV, Magnocavallo M, Straito M, Piro A, Severino P, Iannucci G, Chimenti C, Mancone M, Rocca DGD, Forleo GB, Fedele F, Lavalle C. Direct oral anticoagulants versus vitamin K antagonists in patients with atrial fibrillation and cancer a meta‐analysis. J Thromb Thrombolysis 2021; 51: 419–429 Accessed October 26, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lavalle C, Di Lullo L, Bellasi A, di Lullo L, Bellasi A, Ronco C, Radicchia S, Barbera V, Galardo G, Piro A, Magnocavallo M, Straito M, Uguccioni M. Adverse drug reactions during real‐life use of direct Oral anticoagulants in Italy: An update based on data from the italian National Pharmacovigilance Network. Cardiorenal Med 2020; 10: 266–276. [DOI] [PubMed] [Google Scholar]
- 57. Yang H, Bouma BJ, Dimopoulos K, Khairy P, Ladouceur M, Niwa K, Greutmann M, Schwerzmann M, Egbe A, Scognamiglio G, Budts W, Veldtman G, Opotowsky AR, Broberg CS, Gumbiene L, Meijboom FJ, Rutz T, Post MC, Moe T, Lipczyńska M, Tsai SF, Chakrabarti S, Tobler D, Davidson W, Morissens M, van Dijk A, Buber J, Bouchardy J, Skoglund K, Christersson C, Kronvall T, Konings TC, Alonso‐Gonzalez R, Mizuno A, Webb G, Laukyte M, Sieswerda GTJ, Shafer K, Aboulhosn J, Mulder BJM. Non‐vitamin K antagonist oral anticoagulants (NOACs) for thromboembolic prevention, are they safe in congenital heart disease? Results of a worldwide study. Int J Cardiol 2020; 299: 123–130. [DOI] [PubMed] [Google Scholar]
- 58. Magnocavallo M, Bellasi A, Mariani MV, Fusaro M, Ravera M, Paoletti E, di Iorio B, Barbera V, Della Rocca DG, Palumbo R, Severino P, Lavalle C, di Lullo L. Thromboembolic and bleeding risk in atrial fibrillation patients with chronic kidney disease: Role of anticoagulation therapy. JCM 2020; 10: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li T, Mendoza L, Chan W, McFarlane IM. Non‐compaction cardiomyopathy presented with atrial fibrillation: A case report and literature review. Am J Med Case Rep 2020; 8: 281–283.32775629 [Google Scholar]
- 60. Skidan VI, Kuznetsova T, Pavlyukova EN, Nartsissova GP. Isolated left ventricular apical hypoplasia with myocardial non‐compaction: A case report cameli M, D'Amario d, aboumarie HS, akhtar MM, green P, editors. Eur Heart J ‐ Case Rep 2020; 4: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Robinson AA, Trankle CR, Eubanks G, Schumann C, Thompson P, Wallace RL, Gottiparthi S, Ruth B, Kramer CM, Salerno M, Bilchick KC, Deen C, Kontos MC, Dent J. Off‐label use of direct Oral anticoagulants compared with warfarin for left ventricular thrombi. JAMA Cardiol 2020; 5: 685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kajy M, Shokr M, Ramappa P. Use of direct Oral anticoagulants in the treatment of left ventricular thrombus: Systematic review of current literature. Am J Ther 2020; 27: e584–e590. [DOI] [PubMed] [Google Scholar]
- 63. Sun H, Zhao Q, Wang Y, Lakin R, Feng H, Fan X, Luo H, Gao D, Liu L, He Y, Yang P. Daily 10 mg rivaroxaban as a therapy for ventricular thrombus related to left ventricular non‐compaction cardiomyopathy: A case report. Medicine 2018; 97: e9670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Martinez C, Katholing A, Wallenhorst C, Freedman SB. Therapy persistence in newly diagnosed non‐valvular atrial fibrillation treated with warfarin or NOAC: A cohort study. Thromb Haemost 2016; 115: 31–39. [DOI] [PubMed] [Google Scholar]
- 65. Monagle P, Newall F. Management of thrombosis in children and neonates: Practical use of anticoagulants in children. Hematology Am Soc Hematol Educ Program 2018; 2018: 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Spijkerboer AW, Utens EMWJ, Bogers AJJC, Verhulst FC, Helbing WA. Long‐term behavioural and emotional problems in four cardiac diagnostic groups of children and adolescents after invasive treatment for congenital heart disease. Int J Cardiol 2008; 125: 66–73. [DOI] [PubMed] [Google Scholar]
- 67. Dries DL, Rosenberg YD, Waclawiw MA, Domanski MJ. Ejection fraction and risk of thromboembolic events in patients with systolic dysfunction and sinus rhythm: Evidence for gender differences in the studies of left ventricular dysfunction trials. J Am Coll Cardiol 1997; 29: 1074–1080. [DOI] [PubMed] [Google Scholar]
- 68. Hays AG, Sacco RL, Rundek T, Sciacca RR, Jin Z, Liu R, Homma S, di Tullio MR. Left ventricular systolic dysfunction and the risk of ischemic stroke in a multiethnic population. Stroke 2006; 37: 1715–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study Strategy.


