Abstract
Aims
The prevalence and the natural course of iron deficiency (ID) in acute heart failure (AHF) are still unclear. We investigated the prevalence of ID in unselected patients admitted with AHF on admission, at discharge and up to 3 months thereafter.
Methods and results
In this prospective, multicentre, observational study, 742 patients admitted with AHF were enrolled. The main study outcome was the percentage of patients with ID (ferritin <100 μg/L = absolute ID or ferritin 100–299 μg/L and transferrin saturation <20% = functional ID) at admission (T0), after clinical stabilization prior to discharge (T1), and 10 ± 6 weeks after discharge (T2). At T0, ID was present in 71.8% of the patients (44.1% absolute and 27.7% functional ID). At T1 and T2, ID was present in 56.4% (32.4% absolute and 24% functional ID) and 50.3% (36.8% absolute and 13.5% functional ID), respectively. Absolute ID persisted from T0 to T2 in 66% of the patients, while functional ID resolved in 56% of the patients. Ferritin (median [interquartile range] 124 μg/L [56–247] to 150 μg/L [73–277]), transferrin saturation (15% [10–20] to 18% [12–27]), and iron levels (9 μmol/L [6–13] to 11 μmol/L [8–16]) increased significantly (all P < 0.001) from T0 to T1. Transferrin saturation (to 21% [15–29]) and iron levels (to 13 μmol/L [9–17]) also increased significantly (both P < 0.01) from T1 to T2 without iron supplementation.
Conclusions
Iron deficiency is highly prevalent in patients with AHF, but resolves during treatment in some patients, even without iron supplementation. Absolute ID is more likely to persist over time, whereas functional ID often resolves during treatment of AHF, representing probably a reduced iron availability rather than a true deficiency.
Keywords: Acute decompensated heart failure, Iron deficiency, Worsening heart failure, Comorbidity, Functional iron deficiency, Iron availability disorder
Introduction
Iron deficiency (ID) is very common in patients with heart failure (HF), occurring in 37–50% of patients with chronic HF (CHF), 1 , 2 , 3 and in up to 52–74% of patients with acute HF (AHF). 4 , 5 , 6 , 7 ID is also present in patients admitted to the intensive care unit, irrespective of the underlying disease. 8 It is associated with worse outcome in acute diseases, including cardiac pathology such as acute coronary syndrome 9 and AHF. 4 , 6 ID is a therapeutic target in CHF. 10 Growing evidence supports the hypothesis that ID may be a new therapeutic target in acutely ill patients, 8 and, as recently investigated, in patients at discharge after hospital admission for AHF. 11 This may be of particular interest, as current therapy for AHF focuses on symptomatic treatment such as oxygen therapy, administering intravenous diuretics, inotropes, and vasodilators, but lacks interventions that improve prognosis. 12
Meta‐analyses of randomized controlled trials with intravenous iron therapy to correct ID in patients with HF with reduced left‐ventricular ejection fraction (LVEF) showed benefits in patient‐related outcomes, such as improvement in New York Heart Association (NYHA) class, 6 minute walking distance, quality of life, and hospitalization rates. 13 , 14 , 15 In the chronic setting, ID is defined as a serum ferritin level <100 μg/L (absolute ID) or a serum ferritin level 100–300 μg/L in combination with a transferrin saturation (TSAT) < 20% (functional ID). However, the assessment in AHF is less well defined. As ferritin is an acute phase protein, it is often elevated in acute conditions, 16 which may result in an underestimation of the number of iron deficient patients in the acute phase. In addition, the course of ID during admission due to AHF is less well defined as many studies in AHF only measured iron parameters once at or during admission. 4 , 5
Two small studies showed that the prevalence of ID during hospitalization for AHF and a subsequent period of one month varies considerably. 17 , 18 Therefore, it remains unclear what happens if the clinical situation stabilizes, and which time point is most suitable to assess and/or correct ID. 17 , 19 As ID in HF appears to be detrimental not only in the chronic but also acute setting, 19 it is important to investigate the time course of iron homeostasis during and after AHF in a sufficiently large, real‐world population. This may help to determine the best approach to correct ID and to improve clinical outcomes in AHF. Therefore, we investigated the prevalence of ID in a large cohort of patients with an episode of AHF on admission, at discharge and up to 3 months after discharge.
Methods
Between June 2017 and July 2020, 742 AHF patients were enrolled from 15 sites in the Netherlands. The study protocol was approved by the Medical Research Ethics Committee Zuyderland (MREC Z) and conducted in accordance with the principles of the Declaration of Helsinki (Fortaleza, Brazil, October 2013), International Conference on Harmonization Good Clinical Practice, and Medical Research Involving Human Subjects Act (WMO). Written informed consent was provided by all patients prior to any study‐related procedures. To ensure data quality, on site monitoring and source data verification has been performed by the Clinical Trial Center Maastricht.
Study design
This study was a prospective, observational, non‐interventional study assessing the prevalence of ID in a real‐life cohort of patients admitted to hospital due to AHF. ID was defined as serum ferritin <100 μg/L or serum ferritin 100–299 μg/L and transferrin saturation <20%, according to the ESC guidelines of 2016. 12 Blood samples were drawn via venipunctures that were part of the routine care procedure: within 36 h after admission (T0), after clinically stabilization and within 0–2 days prior to discharge (T1), and 10 ± 6 weeks after discharge (T2) in the outpatient clinic. Administration of iron both orally and intravenously was discouraged during the hospitalization phase because the effect of iron supplementation in the acute phase of HF was not known. Still, treatment decision was left to the physician in charge. This was also true for any clinical decision about used diagnostic tests and applied treatment.
Participants
All adult patients admitted due to an episode of AHF, irrespective of LVEF and left or right‐sided HF, were eligible to participate in this study. Patients with a history of erythropoietin stimulating agent therapy, intravenous iron therapy, and/or blood transfusion within 3 months prior to hospitalization were excluded. Patients on oral iron therapy at any doses in the past 4 weeks or iron containing multivitamins irrespective of the dose of iron were excluded as well. Furthermore, patients who received systemic chemotherapy and/or radiotherapy in the 3 months prior to hospitalization were excluded.
Measurements
Baseline characteristics of patients were recorded. They included age, sex, height, weight, vital parameters, physical examination, NYHA class, echocardiography, medication, comorbidities (hypertension, diabetes mellitus, chronic obstructive pulmonary disease/asthma, cerebrovascular accident/transient ischaemic attack, malignancy, anaemia, systemic inflammatory disorders, established atherosclerotic cardiovascular disease, valve disease, atrial fibrillation, and alcohol abuse), as well as laboratory findings such as N‐terminal pro hormone brain natriuretic peptide, C‐reactive protein (CRP), estimated glomerular filtration rate (based on the Modification of Diet in Renal Disease equation 20 ), haemoglobin (Hb), mean corpuscular volume, folic acid, and vitamin B12. Echocardiography was considered only if performed within 6 months prior to admission and end of the study to avoid reporting undue results. If the lab parameters showed an unexplained, persistent low Hb at T2, it was encouraged but left to the investigator's discretion to refer the patient to the general practitioner, or the outpatient clinic of internal medicine or gastro‐enterology for further analysis.
Study outcomes
The main study outcome was the percentage of patients with ID within the total group of patients on admission (T0), after stabilization (T1), and 10 ± 6 weeks after discharge (T2) as well as the changes from baseline to T1 and T2.
Secondary outcomes included the changes of serum ferritin levels, transferrin saturation, and serum iron levels at the three time points. In addition, cut‐off values as previously defined were applied: that is, ferritin <100 and <300 μg/L, transferrin saturation <20%, and serum iron <13 μmol/L.
Statistical analysis
Data are presented as mean (SD), median (interquartile range, IQR) or percentage as appropriate. Given the fact that most variables were not normally distributed, non‐parametric testing was used. Comparison between groups was performed using the Kruskal–Wallis H test or χ2 test as appropriate. Changes over time were calculated using the Friedman test. Adjustment for multiple testing was performed using the Bonferroni adjustment. Correlations were tested using Spearman's ρ. Given the large number of missing data, imputation analysis was applied using 5 imputations to determine the impact of the missing data on the results. The values at all three timepoints (calculated for each iron parameters separately) were imputed using the linear scale model with fully conditional specification (max 10 iterations) without applying any constraints or considering interactions. Data analysis was performed using IBM SPSS version 26.0 for Windows (Armonk, New York, USA: IBM corp.) Statistical significance was accepted at a P value of <0.05.
Results
Of the 742 patients, 9 received oral iron supplement within 4 weeks prior to admission and iron status was incomplete in another 41 patients, leaving 692 patients to be included in this analysis. Of these patients, 72 (10.4%) were only assessed at baseline as they were either included in the AFFIRM‐AHF trial (n = 12), received blood transfusion (n = 8), intravenous iron (n = 45) or both blood transfusion and intravenous iron (n = 7). Of the remaining 620 patients, iron assessment was completed at T1 in 463 (74.7%) patients, at T2 in 386 (62.3%) patients, and at both T1 and T2 in 314 (50.6%) patients. The patient flow is displayed in Figure 1 .
Figure 1.
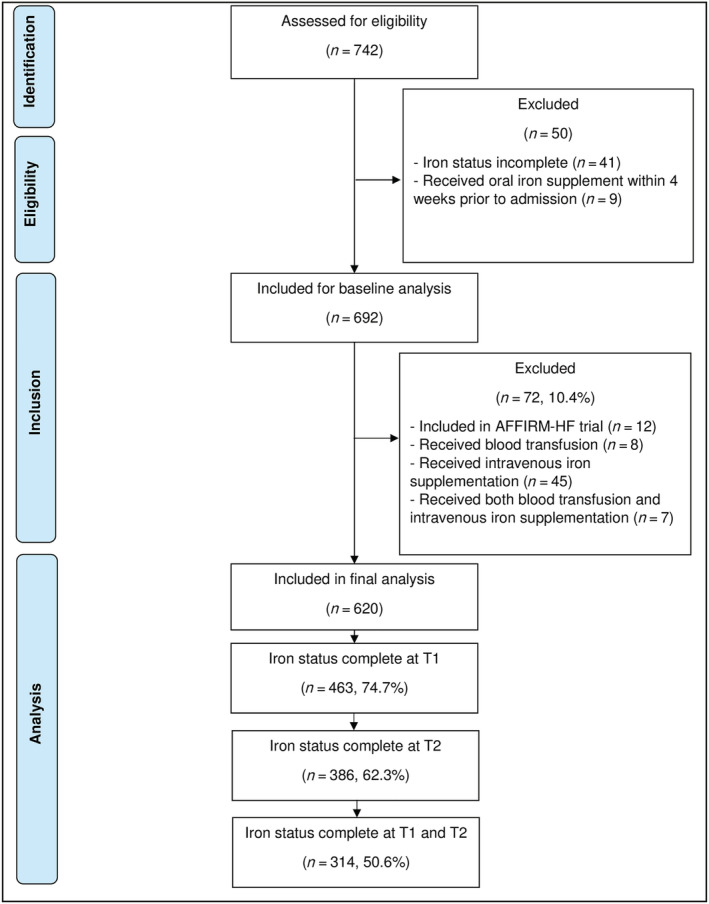
Number of patients included at different time points.
The median (IQR) age was 78 (70–84) years, 42.5% of the patients were female, and 44.1% had de novo HF. The LVEF was measured within the predefined timeframe in 465 patients (67.2%). The median (IQR) LVEF was 38% (27–50%), where 261 (56.1%) patients had an LVEF of 40% or less (HFrEF), 82 (17.6%) patients between 41% and 49% (HFmrEF), and 122 (26.2%) of 50% or more (HFpEF). Baseline characteristics are shown in Table 1 .
Table 1.
Baseline characteristics by iron status
| Variables a | All patients (N = 692) | Absolute ID at baseline (N = 305) | Functional ID at baseline (N = 192) | No ID at baseline (N = 195) | P value (χ 2 or Kruskal–Wallis H) |
|---|---|---|---|---|---|
| Age, years | 78 (70–84) | 79 (72–84) | 78 (70–84) | 76 (69–82) | 0.014 |
| Female sex, N (%) | 294 (42.5) | 158 (51.8) | 84 (43.8) | 52 (26.7) | <0.001 |
| BMI, kg/m2 | 27.8 (24.3–32.0) | 27.9 (24.1–32.7) | 27.1 (24.2–31.6) | 28.0 (24.9–31.3) | 0.842 |
| Established ASCVD, N (%) | 299 (43.4%) | 130 (42.8%) | 81 (42.6%) | 88 (45.1%) | 0.847 |
| Atrial fibrillation or flutter, N (%) | 386 (55.8%) | 180 (59%) | 108 (56.3%) | 98 (50.3%) | 0.155 |
| Valve disease, N (%) | 218 (31.5%) | 96 (31.5%) | 63 (32.8%) | 59 (30.3%) | 0.864 |
| History of HF, N (%) | 387 (55.9%) | 180 (59.0%) | 103 (53.6%) | 104 (53.3%) | 0.347 |
| LVEF, % | 38 (27–50) | 38 (27–50) | 40 (27–50) | 38 (25–50) | 0.957 |
| HFrEF, N (%) | 261 (56.1%) | 111 (56.9%) | 75 (54.7%) | 75 (56.4%) | 0.962 |
| HFmrEF, N (%) | 82 (17.6%) | 33 (16.9%) | 27 (19.7%) | 22 (16.5%) | |
| HFpEF, N (%) | 122 (26.2%) | 51 (26.2%) | 35 (25.5%) | 36 (27.1%) | |
| NYHA class | 0.235 | ||||
| I, N (%) | 7 (1.2%) | 5 (2.0%) | 2 (1.3%) | 0 (0%) | |
| II, N (%) | 85 (14.9%) | 31 (12.4%) | 24 (15.2%) | 30 (18.4%) | |
| III, N (%) | 315 (55.1%) | 134 (53.4%) | 88 (55.7%) | 93 (57.1%) | |
| IV, N (%) | 165 (28.8%) | 81 (32.3%) | 44 (27.8%) | 40 (24.5%) | |
| Systolic blood pressure, mmHg | 137 (120–157) | 138 (121–156) | 140 (120–159) | 135 (119–155) | 0.417 |
| Diabetes mellitus, N (%) | 240 (34.7%) | 121 (39.7%) | 65 (33.9%) | 54 (27.7%) | 0.022 |
| Hypertension, N (%) | 404 (58.5%) | 183 (60.2%) | 115 (59.9%) | 106 (54.4%) | 0.388 |
| CVA/TIA, N (%) | 120 (17.3%) | 62 (20.3%) | 6 (13.5%) | 32 (16.4%) | 0.139 |
| COPD/asthma, N (%) | 149 (21.5%) | 72 (23.6%) | 39 (20.3%) | 38 (19.5%) | 0.490 |
| Systemic inflammatory disease, N (%) | 36 (5.2) | 17 (5.6) | 14 (7.3) | 5 (2.6) | 0.103 |
| Malignancy, N (%) | 90 (13.0%) | 42 (13.8%) | 23 (12.0%) | 25 (12.8%) | 0.843 |
| Alcohol abuse (>6 units/day), N (%) | 29 (4.2) | 13 (4.3) | 3 (1.6) | 13 (6.7) | 0.043 |
| Oral anticoagulation, N (%) | 342 (64.9) | 151 (67.7) | 92 (65.2) | 99 (60.7) | 0.364 |
| Acetylsalicylic acid therapy, N (%) | 171 (29.0) | 69 (26.0) | 47 (29.9) | 55 (32.9) | 0.294 |
| P1Y12‐inhibitor therapy, N (%) | 92 (15.4%) | 35 (13.2%) | 28 (17.4%) | 29 (17.1%) | 0.392 |
| Serum ferritin, μg/L | 114 (58–231) | 53 (35–74) | 165 (128–224) | 342 (190–510) | NA |
| Serum iron, μmol/L | 8.9 (6.0–12.0) | 7.1 (6.0–10.0) | 8.0 (6.0–10.0) | 12.0 (8.0–15.2) | NA |
| Serum transferrin, g/L | 2.5 (2.2–2.9) | 2.8 (2.5–3.2) | 2.4 (2.1–2.7) | 2.2 (2.0–2.5) | NA |
| TSAT, % | 14 (9–19) | 10 (8–16) | 14 (10–17) | 21 (15–25) | NA |
| eGFR, mL/min/1.73 m2 | 54.7 (39.6–73.6) | 52.5 (38.8–71.2) | 55.8 (40.6–75.3) | 56.5 (40.6–76.8) | 0.349 |
| CRP, mg/L | 10 (4–24) | 8 (4–17) | 15 (6–35) | 10 (4–36) | <0.001 |
| Haemoglobin, mmol/L | |||||
| Male | 8.2 (1.2) | 7.8 (1.1) | 8.2 (1.2) | 8.5 (1.3) | <0.001 |
| Female | 7.8 (1.1) | 7.7 (1.1) | 7.9 (1.0) | 8.1 (1.3) | 0.141 |
| Anaemia, N (%) | 156 (22.5) | 88 (28.9) | 32 (16.7) | 36 (18.5) | 0.002 |
| NTproBNP, pmol/L | 520 (261–1025) | 494 (260–951) | 536 (239–1194) | 580 (276–1039) | 0.508 |
| BNP, pmol/L | 499 (234–1168) | 363 (229–856) | 548 (220–1442) | 623 (259–1606) | 0.578 |
| Length of stay, (days) | 7 (5–10) | 7 (5–11) | 8 (5–10) | 8 (5–11) | 0.639 |
| Change in body weight, (kg) b | −2.7 (−0.5 to −5.3) | −2.7 (−0.5 to −5.1) | −2.6 (−0.5 to −5.2) | −3.2 (−0.3 to −6.2) | 0.931 |
| In‐hospital mortality, N (%) | 22 (3.2%) | 8 (2.6%) | 5 (2.6%) | 9 (4.6%) | 0.409 |
All continuous variables are presented as median (interquartile range) except for haemoglobin [mean (SD)].
Change from first measured weight (usually Day 2 of hospitalization) to weight at discharge.
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; BNP, brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; CVA, cerebrovascular accident; eGFR, estimated glomerular filtration rate; HF, heart failure; HFmrEF, heart failure with mid‐range ejection fraction (40.1–49.9%); HFpEF, heart failure with preserved ejection fraction (≥50%); HFrEF, heart failure with reduced ejection fraction (≤40%); ID, iron deficiency; LVEF, left ventricular ejection fraction; NA, not applicable; NTproBNP, N‐terminal (NT)‐pro hormone Brain natriuretic peptide; NYHA, New York Heart Association; TIA, transient ischaemic attack; TSAT, transferrin saturation.
At T0, ID was present in 497 (71.8%) patients (Figure 2 ). Of the 72 patients that were either included in the AFFIRM‐AHF study, or received intravenous iron and/or blood transfusion, 68% had absolute ID, 26% functional ID, and 6% had no ID, as compared with 41%, 289%, and 31%, respectively, in the other 620 patients (P < 0.001). The following characteristics were significantly related to ID at T0 (Table 1 ): female gender, age, anaemia, diabetes mellitus, CRP levels, and alcohol abuse but not characteristics such as LVEF, NYHA class, renal function or N‐terminal (NT)‐pro hormone brain natriuretic peptide levels. Obviously, measures of iron status were related to ID. At T0, ferritin <100 μg/L was present in 305 (44.1%) patients and between 100 and 299 μg/L in 270 (39.0%) patients. Transferrin saturation <20% was present in 529 (76.7%) patients and iron serum levels of <13 μmol/L in 575 (83.1%) patients.
Figure 2.
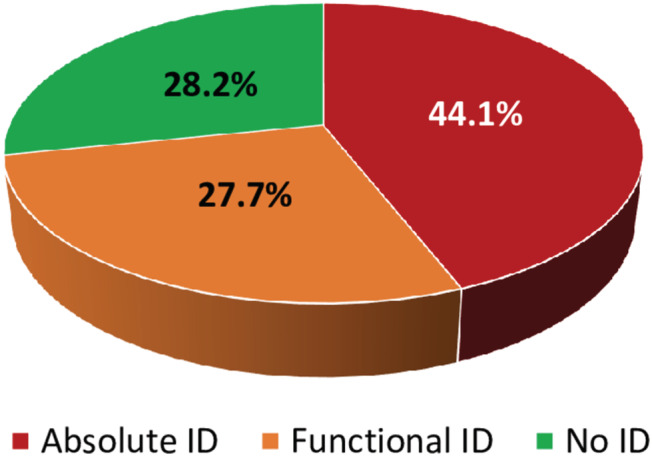
Iron deficiency (ID) at hospital admission (T0).
At T1, 261 (56.4%) patients had ID [150 absolute ID (32.4%), 111 functional ID (24.0%)] as compared with 319 (68.9%) patients at T0 [192 absolute ID (41.5%), 127 functional ID (27.4%)] (P < 0.001) if only those were considered with both measurements not receiving an iron related treatment. In hospital mortality was 3.2% (n = 22). At T2, 194 (50.3%) patients had ID [142 absolute ID (36.8%), 52 functional ID (13.5%)] as compared with 271 (70.2%) patients at T0 [170 absolute ID (44.0%), 101 functional ID (26.2%)] (P < 0.001) if only those were considered with both measurements not receiving an iron related treatment. Figure 3 shows the prevalence of ID in patients with measurements at all three time points, showing a gradual decrease of patients with ID with most changes in patients that had a functional ID. Of the 135 patients with absolute ID at T0, 97 (71.9%) also had absolute ID at T1, whereas 18 (13.3%) changed to functional ID and 20 (14.8%) no longer had ID. Only 8 patients developed a new absolute ID [4 of 99 (3.8%) with no ID at T0 and 4 of 80 (5.0%) with functional ID at T0]. Functional ID resolved in 38 of 80 (47.5%) patients and remained unchanged in another 38 (47.5%). Of the 99 patients with no ID at T0, 13 (13.1%) developed functional ID and 82 (82.8%) remained without ID. Changes from T1 to T2 were comparable with the changes from T0 to T1 (Figure 3 ). When comparing T0 with T2, absolute ID persisted in 89 of 135 (65.9%) patients, whereas only 15.2% of those with no ID developed a new ID at T2 (Table 2 ).
Figure 3.
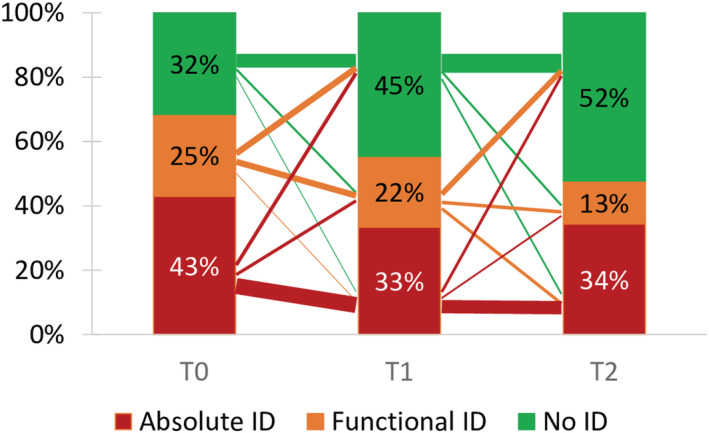
Iron deficiency (ID) at the different time points and variability between groups. Width of lines between groups indicates number of patients (1 pt = 10 patients).
Table 2.
Comparison of iron status at T0 with T2
| Iron status at T2 | ||||
|---|---|---|---|---|
| No ID | Functional ID | Absolute ID | ||
| Iron status at T0 | No ID | 84 (84.8%) | 7 (7.1%) | 8 (8.1%) |
| Functional ID | 45 (56.3%) | 24 (30.0%) | 11 (13.8%) | |
| Absolute ID | 35 (25.9%) | 11 (8.1%) | 89 (65.9%) | |
Percentage indicates % of patients with the according iron status at T0.
ID, iron deficiency.
Serum ferritin levels, transferrin saturation, and serum iron levels showed a rise from T0 to T2 (Figure 4 ). Values at T0 in this plot are slightly higher than shown in Table 1 as only those patients are included who had values measured at all time points. Ferritin levels correlated significantly more between time points than transferrin saturation and serum iron levels: Spearman's ρ between T0 and T1, T0 and T2, and T1 and T2 for serum ferritin levels was 0.83, 0.67, and 0.71, respectively, whereas this was 0.50, 0.50, and 0.59 for transferrin saturation, and 0.44, 0.44, and 0.54, respectively, for serum iron levels.
Figure 4.
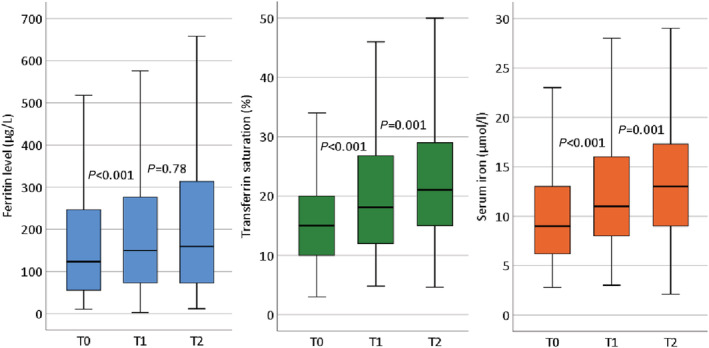
Levels of ferritin (left), transferrin saturation (middle) and serum iron (right) at the three time points in those patients having iron status measured at all time points. The boundaries of the box are Tukey's hinges; extremes are not shown.
Correlation between serum CRP, N‐terminal pro hormone brain natriuretic peptide, and estimated glomerular filtration rate and measures of iron status are depicted in supplement Table S1 , showing that mainly CRP was correlated with iron status. Interestingly, this correlation was somewhat stronger with transferrin saturation and serum iron than with ferritin.
At T1 and T2, ID was more common in women, patients with a history of anaemia and/or diabetes mellitus, as already seen at T0 (data not shown). Patients with previously known HF more often had absolute ID at T1 compared with those with a new onset of HF (37.5% vs. 26.1%) (functional ID 21.5% vs. 27.1%; no ID 41.0% vs. 46.9%; P = 0.03) with similar differences at T2. At T2, patients with atrial fibrillation had more absolute ID (42.7% vs. 28.9%) compared with those without (functional ID 13.2% vs. 13.9%, no ID 44.1% vs. 57.2%; P = 0.004).
By applying imputation, ferritin levels, transferrin saturation, and iron levels at T1 and T2 were not substantially different from levels if all available results or only those in patients with all three measurements available were used (supporting information, Table S2 ). Only ferritin levels at T1 and T2 were slightly higher with imputed data. The percentages of all imputed data (ranges of five imputations) for absolute ID at T1 and T2 were 29.1% (range 28.5–29.6%) and 29.8% (28.3–30.8%), for functional ID at T1 and T2 24.5% (23.3–25.3%) and 15.6% (14.7–16.4%), and for no ID at T1 and T2 46.4% (45.3–47.1%) and 54.6% (52.8–56.8%), respectively.
Discussion
In this large, prospective, multicentre, study in a real‐life cohort of patients with AHF, ID was highly prevalent. Moreover, we show a significant reduction in the prevalence of ID, even during the short period of hospitalization. Absolute ID is more likely to persist over time, whereas functional ID is more likely to resolve during the treatment of AHF, even without iron supplementation.
Two rather small studies showed comparable results about the prevalence of ID in patient with AHF. Van Aelst et al. found a prevalence of 83% at baseline in their cohort, while Sportouch et al. showed a prevalence of 75%. 17 , 18 We found a comparable prevalence of ID, but we demonstrated this in a larger patient population. Similarly, the changes in prevalence of ID over time are comparable. Van Aelst et al. showed that 23.4% of patients with ID at baseline had no ID at Day 30, whereas in the study of Sportouch et al. 15% of the patients with ID at baseline showed no ID after one month. 17 The AFFIRM‐AHF trial, which investigated iron supplementation with ferric carboxymaltose (FCM) in patients with ID who were stabilized after AHF, showed a similar effect in the placebo arm of the trial. TSAT increased in the placebo group (mean absolute change +5.4%) after 6 weeks, although less than in the treatment arm. Furthermore, the serum ferritin levels in the placebo arm increased from baseline to 6–12 weeks follow‐up. 11
AFFIRM‐AHF showed no statistically significant effect for the primary endpoint of FCM (P = 0.059). Still, analysing the subgroups of serum ferritin levels below and above 100 μg/L, it may be hypothesized that there is a benefit of FCM in patients with a serum ferritin <100 μg/L but not in those with a serum ferritin >100 μg/L. 11 An explanation for this difference might be our finding that functional ID more often resolved during the treatment and stabilization of AHF, whereas absolute ID more often remained unchanged. In line with this, absolute, but not functional ID at discharge, was associated with an increased risk of early re‐admission. 6 Obviously, it needs to be prospectively tested if this assumption is true.
Several studies on (acute or chronic) HF report varying percentages of ID, ranging from 37% to 74%. 1 , 6 , 21 This may reflect the heterogeneity of the patient populations in these studies, but is probably also the result of the different definitions of ID. 22 , 23 , 24 , 25 , 26 In patients without chronic conditions, absolute ID is identified by serum ferritin levels of <30 μg/L with acceptable sensitivity and specificity. 27 In conditions with multiple comorbidities accompanied with chronic inflammation, ID may be present if serum ferritin levels are <100 μg/L or even higher, in combination with a TSAT <20%. 28 Like others, we have followed the definition as advised by the European Society of Cardiology: absolute ID when serum ferritin level is <100 μg/L and functional ID when the serum ferritin level is 100–300 μg/L and the transferrin saturation is <20%.
These cut‐off values have been adopted from patients with chronic kidney disease with the assumption that the situation in patients with CHF may be similar. 25 In a study that validated this definition to the golden standard of iron content in bone marrow (in patients undergoing median sternotomy with LVEF <45%), a cut‐off value for TSAT of <19.8% was found, similar to the used definition <20%. However, that study also questioned the diagnostic value of ferritin. 29 In addition, another study in CHF patients with or without reduced LVEF showed that ID and anaemia were often concomitant, but serum iron levels and transferrin saturation were more strongly associated with anaemia than serum ferritin levels. 30 In our study however, we found a larger variation in TSAT than in ferritin. This might be explained by a variation in transferrin and iron due to reduced consumption, absorption, and distribution in the acute phase of HF.
So far, studies and trials have considered patients with absolute ID and functional ID as one homogeneous group, but this is debatable with the mentioned findings. In addition, the definition of functional ID, in the presence of normal body iron stores can be somewhat confusing. In fact, functional ID may be better understood as iron availability disorder with a serum ferritin of 100–300 μg/L AND TSAT <20%, distinct from absolute ID. It needs to be prospectively tested whether intravenous iron supplementation is only effective in absolute ID but not in functional ID (i.e. reduced iron availability only) as suggested by the AFFIRM‐AHF as discussed earlier. 11 It could be hypothesized that patients with absolute ID have greater benefit of FCM, probably not only by accomplishing reduction of rehospitalizations as shown in several meta‐analyses of FCM trials, 13 , 14 , 15 but eventually also a reduction in mortality.
Compared with most clinical intervention trials in a HF population, the patients included in this study were more often female, older, and NYHA Classes III and IV were more prevalent. Furthermore, LVEF was higher than in the HFrEF trials. 31 , 32 Importantly, ID was prevalent independently of LVEF. The differences between the study populations can be explained by the fact that our study was observational and inclusion and exclusion criteria allowed inclusion of almost all AHF patients, whereas clinical trials usually exclude the more frail patients because of safety issues. The age and sex in our study sample are comparable with those in other observational studies reporting real‐world data of AHF patients. 33 , 34 We therefore are convinced that our study population can be considered as a real‐life cohort.
Our study has several strengths and limitations. The first strength is that, to our knowledge, this is the so far largest, prospective, observational study on the prevalence of ID in AHF, investigating the course of ID during and early after hospitalization without supplementing iron. Second, our study is a real‐life cohort of patients admitted with AHF, which enhances the generalizability of our results.
The most important limitation of our study is the high amount of missing data, which was caused by several reasons: only blood samples and LVEF measurements as part of the clinical daily routine were available to reduce the burden for participating patients and the costs of this investigator‐initiated study. Additional patients had LVEF measured outside the predefined timeframe, but they were not included in the analysis to avoid reporting undue results. Also, no data on physical performance or quality of life are available, as those are not part of standard care in the Netherlands. Part of the patients were discharged to a nursing home or follow‐up by the general practitioner, which complicated follow‐up visits. In addition, limited follow‐up at the outpatient clinic was possible during the COVID‐19 pandemic. To lower the amount of missing values, we widened the follow‐up window in a protocol amendment, which was initially set to 6 (±2) weeks after discharge. In addition, we performed an analysis with imputed values, which did not show meaningful differences as compared with the main results. We are, therefore, convinced that the impact of the missing data on the results is rather small, and the analyses performed with complete cases only are representative for the whole population. Furthermore, for better understanding of the underlying pathophysiology, data about detailed clinical characteristics during hospitalization, for example, use of inotropes, hemodynamic profile, liver functions, and the long‐term follow‐up would have been helpful. However, this was an investigator‐initiated study with limited funding to collect these data. Due to the limited set of acquired data, we were able to include a representative sample of patients admitted with AHF during the time period.
Another limitation is the fact that some patients received iron therapy although this was discouraged by the protocol. Therefore, the prevalence of ID at T1 and T2 without iron supplementation is probably slightly underestimated. Another limitation is the fact that we only used those iron parameters that are available in daily clinical practice; ferritin, serum iron, and TSAT. More extensive data on iron status including, for example, reticulocyte haemoglobin content (Ret‐He), soluble transferrin receptor (sTfR), or hepcidin would have added information on the iron availability during the course of admission and stabilization of AHF.
Further research should focus on the methods and timing of diagnosing ID in patients with AHF. Moreover, it needs to be investigated if the effect of iron supplementation in AHF patients is only present with absolute ID. It should be kept in mind however, that a prolonged period of functional ID might also be harmful. Finally, the effect of iron supplementation during hospital admission should be investigated, particularly in patients with absolute ID.
Conclusions
Iron deficiency is highly prevalent in patients with AHF. Prevalence of ID, as currently defined, changes over time, even during the short period of clinical stabilization during hospitalization. It may be investigated which point of time is best to determine ID for initiation of treatment. Absolute ID is more likely to be persistent than functional ID, the latter often resolves after stabilization of HF, even without iron supplementation. Absolute and functional ID may therefore be considered as two distinct groups. Further research is required to investigate if this may have therapeutic implications.
Conflict of interest
H‐PB‐LR, CvD, and ME report receiving advisory board fees and lecture fees from Vifor Pharma. HK and CK received lecture fees from Vifor Pharma. The other authors have no conflicts of interest to declare.
Funding
This work was supported by Vifor Pharma, Ltd., Glattbrugg, Switzerland, and the Dutch Network for Cardiovascular Research (WCN). However, as this was an investigator‐initiated study, Vifor Pharma had no role in the design of the trial, the enrolment of the patients, the collection, analysis or interpretation of the data, and the writing and publication of the manuscript.
Supporting information
Table S1. Spearman ρ correlation between measures of iron status and serum CRP, NT‐proBNP and eGFR at different time points.
Table S2. Summary of ferritin levels, transferrin saturation, and iron levels using all available results including number of measurements (left), using only those with complete values at all time points (middle) and using imputation (right). Imp. median indicates the median value of all 5 imputations, Range imp. median indicated the range of median values of the 5 imputations and Range of IQR indicates the lowest 25 percentile and the highest 75 percentile of the 5 imputations. Grey areas indicate not applicable as no values were imputed.
Acknowledgements
The authors would like to thank all study participants, all participating clinicians, and all research professionals at each site. This study was initiated by the late Dr Dunselman and HK. Dr Dunselman had the intention to coordinate this study after his retirement in 2017, but unfortunately, he suddenly passed away, only a few weeks after his retirement. With these results, we want to honour him for all his scientific and clinical achievements in cardiology.
van Dalen, D. H. , Kragten, J. A. , Emans, M. E. , van Ofwegen‐Hanekamp, C. E. E. , Klaarwater, C. C. R. , Spanjers, M. H. A. , Hendrick, R. , van Deursen, C. T. B. M. , and Brunner‐La Rocca, H.‐P. (2022) Acute heart failure and iron deficiency: a prospective, multicentre, observational study. ESC Heart Failure, 9: 398–407. 10.1002/ehf2.13737.
This work was performed in the following institutions: BovenIJ Hospital, Amsterdam, the Netherlands; OLVG (West), Amsterdam, the Netherlands; Gelre Hospital, Apeldoorn, the Netherlands; IJsselland Hospital, Capelle a/d Ijssel, the Netherlands; Slingeland Hospital, Doetinchem, the Netherlands; St Anna Hospital, Geldrop, the Netherlands; Admiraal de Ruyter Hospital, Goes, the Netherlands; Beatrix Hospital, Gorinchem, the Netherlands; Saxenburgh Medical Center, Hardenberg, the Netherlands; Zuyderland Medical Center, Heerlen, the Netherlands; Jeroen Bosch Hospital, 's‐Hertogenbosch, the Netherlands; Maastricht University Medical Center, Maastricht, the Netherlands; Ikazia Hospital, Rotterdam, the Netherlands; Diakonessenhuis, Utrecht, the Netherlands; Elisabeth‐Tweesteden Hospital, Tilburg, the Netherlands; Viecuri Medical Center, Venlo, the Netherlands; Langeland Hospital, Zoetermeer, the Netherlands.
References
- 1. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin‐Nadzieja L, Banasiak W, Polonski L, Filippatos G, McMurray JJ, Anker SD, Ponikowski P. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J 2010; 31: 1872–1880. [DOI] [PubMed] [Google Scholar]
- 2. von Haehling S, Gremmler U, Krumm M, Mibach F, Schön N, Taggeselle J, Dahm JB, Angermann CE. Prevalence and clinical impact of iron deficiency and anaemia among outpatients with chronic heart failure: the PrEP Registry. Clin Res Cardiol 2017; 106: 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klip IT, Comin‐Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, Lok DJ, Rosentryt P, Torrens A, Polonski L, van Veldhuisen DJ, van der Meer P, Jankowska EA. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013; 165: 575–582.e3. [DOI] [PubMed] [Google Scholar]
- 4. Beale A, Carballo D, Stirnemann J, Garin N, Agoritsas T, Serratrice J, Kaye D, Meyer P, Carballo S. Iron deficiency in acute decompensated heart failure. J Clin Med 2019; 8: 1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen‐Solal A, Damy T, Terbah M, Kerebel S, Baguet JP, Hanon O, Zannad F, Laperche T, Leclercq C, Concas V, Duvillié L, Darné B, Anker S, Mebazaa A. High prevalence of iron deficiency in patients with acute decompensated heart failure. Eur J Heart Fail 2014; 16: 984–991. [DOI] [PubMed] [Google Scholar]
- 6. Núñez J, Comín‐Colet J, Miñana G, Núñez E, Santas E, Mollar A, Valero E, García‐Blas S, Cardells I, Bodí V, Chorro FJ, Sanchis J. Iron deficiency and risk of early readmission following a hospitalization for acute heart failure. Eur J Heart Fail 2016; 18: 798–802. [DOI] [PubMed] [Google Scholar]
- 7. Wexler D, Silverberg D, Sheps D, Blum M, Keren G, Iaina A, Schwartz D. Prevalence of anemia in patients admitted to hospital with a primary diagnosis of congestive heart failure. Int J Cardiol 2004; 96: 79–87. [DOI] [PubMed] [Google Scholar]
- 8. Litton E, Lim J. Iron metabolism: an emerging therapeutic target in critical illness. Crit Care 2019; 23: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zeller T, Waldeyer C, Ojeda F, Schnabel RB, Schäfer S, Altay A, Lackner KJ, Anker SD, Westermann D, Blankenberg S, Karakas M. Adverse outcome prediction of iron deficiency in patients with acute coronary syndrome. Biomolecules 2018; 8: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J 2013; 34: 816–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ponikowski P, Kirwan BA, Anker SD, McDonagh T, Dorobantu M, Drozdz J, Fabien V, Filippatos G, Göhring UM, Keren A, Khintibidze I, Kragten H, Martinez FA, Metra M, Milicic D, Nicolau JC, Ohlsson M, Parkhomenko A, Pascual‐Figal DA, Ruschitzka F, Sim D, Skouri H, van der Meer P, Lewis BS, Comin‐Colet J, von Haehling S, Cohen‐Solal A, Danchin N, Doehner W, Dargie HJ, Motro M, Butler J, Friede T, Jensen KH, Pocock S, Jankowska EA, AFFIRM‐AHF investigators . Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double‐blind, randomised, controlled trial. Lancet 2020; 396: 1895–1904. [DOI] [PubMed] [Google Scholar]
- 12. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 2016: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 13. Jankowska EA, Tkaczyszyn M, Suchocki T, Drozd M, von Haehling S, Doehner W, Banasiak W, Filippatos G, Anker SD, Ponikowski P. Effects of intravenous iron therapy in iron‐deficient patients with systolic heart failure: a meta‐analysis of randomized controlled trials. Eur J Heart Fail 2016; 18: 786–795. [DOI] [PubMed] [Google Scholar]
- 14. Graham FJ, Pellicori P, Ford I, Petrie MC, Kalra PR, Cleland JGF. Intravenous iron for heart failure with evidence of iron deficiency: a meta‐analysis of randomised trials. Clin Res Cardiol 2021. Epub ahead of print; 110: 1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khan MS, Usman MS, von Haehling S, Doehner W, Stewart Coats AJ. Ferric carboxymaltose for the treatment of iron‐deficient heart failure patients: a systematic review and meta‐analysis. ESC Heart Fail 2020; 7: 3392–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fitzsimons S, Doughty RN. Iron deficiency in patients with heart failure. Eur Heart J Cardiovasc Pharmacother 2015; 1: 58–64. [DOI] [PubMed] [Google Scholar]
- 17. Sportouch L, Cautela J, Resseguier N, Pinto J, Ammar C, Gaubert M, Barraud J, Peyrol M, Laine M, Bonello L, Yvorra S, Paganelli F, Thuny F. Dynamic iron status after acute heart failure. Arch Cardiovasc Dis 2019; 112: 410–419. [DOI] [PubMed] [Google Scholar]
- 18. Van Aelst LNL, Abraham M, Sadoune M, Lefebvre T, Manivet P, Logeart D, Launay JM, Karim Z, Puy H, Cohen‐Solal A. Iron status and inflammatory biomarkers in patients with acutely decompensated heart failure: early in‐hospital phase and 30‐day follow‐up. Eur J Heart Fail 2017; 19: 1075–1076. [DOI] [PubMed] [Google Scholar]
- 19. Marchi G, Busti F, Vianello A, Girelli D. Anemia and iron deficiency in heart failure: extending evidences from chronic to acute setting. Intern Emerg Med 2021; 16: 167–170. [DOI] [PubMed] [Google Scholar]
- 20. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 21. Nanas JN, Matsouka C, Karageorgopoulos D, Leonti A, Tsolakis E, Drakos SG, Tsagalou EP, Maroulidis GD, Alexopoulos GP, Kanakakis JE, Anastasiou‐Nana MI. Etiology of anemia in patients with advanced heart failure. J Am Coll Cardiol 2006; 48: 2485–2489. [DOI] [PubMed] [Google Scholar]
- 22. Gaber R, Kotb NA, Ghazy M, Nagy HM, Salama M, Elhendy A. Tissue Doppler and strain rate imaging detect improvement of myocardial function in iron deficient patients with congestive heart failure after iron replacement therapy. Echocardiography 2012; 29: 13–18. [DOI] [PubMed] [Google Scholar]
- 23. Toblli JE, Lombraña A, Duarte P, Di Gennaro F. Intravenous iron reduces NT‐pro‐brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol 2007; 50: 1657–1665. [DOI] [PubMed] [Google Scholar]
- 24. Okonko DO, Grzeslo A, Witkowski T, Mandal AK, Slater RM, Roughton M, Foldes G, Thum T, Majda J, Banasiak W, Missouris CG, Poole‐Wilson PA, Anker SD, Ponikowski P. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC‐HF: a randomized, controlled, observer‐blinded trial. J Am Coll Cardiol 2008; 51: 103–112. [DOI] [PubMed] [Google Scholar]
- 25. von Haehling S, Anker MS, Jankowska EA, Ponikowski P, Anker SD. Anemia in chronic heart failure: can we treat? What to treat? Heart Fail Rev 2012; 17: 203–210. [DOI] [PubMed] [Google Scholar]
- 26. Núñez J, Miñana G, Cardells I, Palau P, Llàcer P, Fácila L, Almenar L, López‐Lereu MP, Monmeneu JV, Amiguet M, González J, Serrano A, Montagud V, López‐Vilella R, Valero E, García‐Blas S, Bodí V, de la Espriella‐Juan R, Lupón J, Navarro J, Górriz JL, Sanchis J, Chorro FJ, Comín‐Colet J, Bayés‐Genís A. Noninvasive imaging estimation of myocardial iron repletion following administration of intravenous iron: the Myocardial‐IRON trial. J Am Heart Assoc 2020; 9: e014254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Camaschella C. New insights into iron deficiency and iron deficiency anemia. Blood Rev 2017; 31: 225–233. [DOI] [PubMed] [Google Scholar]
- 28. Camaschella C. Iron deficiency. Blood 2019; 133: 30–39. [DOI] [PubMed] [Google Scholar]
- 29. Grote Beverborg N, Klip IT, Meijers WC, Voors AA, Vegter EL, van der Wal HH, Swinkels DW, van Pelt J, Mulder AB, Bulstra SK, Vellenga E, Mariani MA, de Boer RA, van Veldhuisen DJ, van der Meer P. Definition of iron deficiency based on the gold standard of bone marrow iron staining in heart failure patients. Circ Heart Fail 2018; 11: e004519. [DOI] [PubMed] [Google Scholar]
- 30. Cleland JG, Zhang J, Pellicori P, Dicken B, Dierckx R, Shoaib A, Wong K, Rigby A, Goode K, Clark AL. Prevalence and outcomes of anemia and hematinic deficiencies in patients with chronic heart failure. JAMA Cardiol 2016; 1: 539–547. [DOI] [PubMed] [Google Scholar]
- 31. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM, DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 32. Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, Adams KF, Anand I, Arias‐Mendoza A, Biering‐Sørensen T, Böhm M, Bonderman D, Cleland JGF, Corbalan R, Crespo‐Leiro MG, Dahlström U, Echeverria LE, Fang JC, Filippatos G, Fonseca C, Goncalvesova E, Goudev AR, Howlett JG, Lanfear DE, Li J, Lund M, Macdonald P, Mareev V, Momomura SI, O'Meara E, Parkhomenko A, Ponikowski P, Ramires FJA, Serpytis P, Sliwa K, Spinar J, Suter TM, Tomcsanyi J, Vandekerckhove H, Vinereanu D, Voors AA, Yilmaz MB, Zannad F, Sharpsten L, Legg JC, Varin C, Honarpour N, Abbasi SA, Malik FI, Kurtz CE, Investigators GALACTIC‐HF. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med 2021; 384: 105–116. [DOI] [PubMed] [Google Scholar]
- 33. Marques‐Alves P, Marinho AV, Almeida JP, Gonçalves T, Costa M, Ferreira M, Baptista R, Costa S, Franco F, Fonseca I, Gonçalves L. Real‐world analysis of acute decompensated heart failure outcomes in Portugal. ESC Heart Fail 2020; 7: 551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tromp J, Bamadhaj S, Cleland JGF, Angermann CE, Dahlstrom U, Ouwerkerk W, Tay WT, Dickstein K, Ertl G, Hassanein M, Perrone SV, Ghadanfar M, Schweizer A, Obergfell A, Lam CSP, Filippatos G, Collins SP. Post‐discharge prognosis of patients admitted to hospital for heart failure by world region, and national level of income and income disparity (REPORT‐HF): a cohort study. Lancet Glob Health 2020; 8: e411–e422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Spearman ρ correlation between measures of iron status and serum CRP, NT‐proBNP and eGFR at different time points.
Table S2. Summary of ferritin levels, transferrin saturation, and iron levels using all available results including number of measurements (left), using only those with complete values at all time points (middle) and using imputation (right). Imp. median indicates the median value of all 5 imputations, Range imp. median indicated the range of median values of the 5 imputations and Range of IQR indicates the lowest 25 percentile and the highest 75 percentile of the 5 imputations. Grey areas indicate not applicable as no values were imputed.


