Notes
Editorial note
This review was split in 2012 and the review question was to be addressed according to three new protocols: (See: http://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD010267.pub2; http://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD010291.pub2; http://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD010325.pub2). These titles were withdrawn at the protocol stage in 2020 as the authors did not make any progress on the reviews. This original review will no longer be updated and may be superseded by new titles hosted by Cochrane Gut in the future.
Abstract
Background
There is evidence from experimental animals studies, prospective and retrospective observational studies that nonsteroidal anti‐inflammatory drugs (NSAIDS) may reduce the development of sporadic colorectal adenomas (CRAs) and cancer (CRC) and may induce the regression of adenomas in familial adenomatous polyposis (FAP).
Objectives
To conduct a systematic review to determine the effect of NSAIDS for the prevention or regression of CRAs and CRC.
Search methods
Randomized controlled trials (RCTs) up to September 2003 were identified.
Selection criteria
NSAIDS and aspirin (ASA) were the interventions. The primary outcomes were the number of subjects with at least one CRA, the change in polyp burden, and CRC. The secondary outcome was adverse events.
Data collection and analysis
Two reviewers independently extracted data and assessed trial quality. Dichotomous outcomes were reported as relative risks (RR) with 95% confidence intervals (CI). The data were combined with the random effects model if clinically and statistically reasonable.
Main results
Nine trials with 150 familial adenomatous polyposis (FAP) and 24,143 population subjects met the inclusion criteria. The interventions included sulindac, celecoxib, or aspirin (ASA). From the combined results of three trials, significantly fewer subjects in the low dose ASA group developed recurrent sporadic CRAs [RR 0.77 (95% CI 0.61, 0.96), (NNT 12.5 (95% CI 7.7, 25)] after one to three years. In another three trials, phenotypic FAP subjects that received sulindac or celecoxib had a greater proportional reduction (range: 11.9% to 44%) in the number of CRAs compared to those in the control group (range: 4.5% to 10%). There was no significant difference for the outcomes of CRC or adverse events in any of the trials.
Authors' conclusions
There was evidence from three pooled RCTs that ASA significantly reduces the recurrence of sporadic adenomatous polyps after one to three years. There is evidence from short‐term studies to support regression, but not elimination or prevention of CRAs in FAP.
Plain language summary
Colorectal (bowel) cancer is common worldwide but is especially prevalent in industrialised countries. Genes, diet and lifestyle all seem to be important in the development of bowel cancer.
Experimental animals studies and observational studies have suggested that nonsteroidal anti‐inflammatory drugs and aspirin may reduce the development of colorectal cancer and recurrence of adenomas in patients with familial adenomatous polyposis (FAP).
The review found some evidence to support the effectiveness of aspirin for reducing the risk of recurrent sporadic colorectal adenomas. Further, there is evidence from short‐term studies to support regression, but not elimination or prevention of colorectal adenomas in FAP with NSAIDs. The review suggests more research on the long‐term role of NSAIDs.
Background
Colorectal cancer (CRC) is a major cause of morbidity and mortality in industrialized countries. It is the third most common cause of cancer‐related deaths in North America (CCS 2002, ACS 2003). In most cases, CRC develops from a precursor lesion that becomes cancerous through a multi‐step adenoma‐carcinoma sequence (Hill 1978). Routine CRC screening has been shown to be effective in reducing cancer related mortality by the detection and removal of precancerous adenomatous polyps or by the early detection of cancer (Winawer 1993, Mandel 1993, Kronborg 1996, Hardcastle 1996, Kewenter 1988, Newcomb 1992; Trends 2001). Despite the benefits, there is an inadequate awareness and utilization of CRC screening (MMWR 2001, Vernon 1997). Therefore alternate means of preventing CRC are desirable and have been an expanding focus in cancer research.
Chemoprevention is a concept coined by Michael B. Sporn in 1976, that describes the use of a pharmacologic or natural agents for the purpose of "prevention of the initiation, promotion and progression of carcinogenesis" (Theisen 2001). There is a substantial amount of experimental and observational data that support the hypothesis that nonsteroidal anti‐inflammatory drugs (NSAIDs), including aspirin (ASA) (Sturmer 1998), have chemopreventive properties for colorectal adenomas (CRAs) and cancer. One mechanism proposed for the preventive effects of NSAIDs is through the inhibition of cyclooxygenase‐2 (Dannenberg 1999). Cyclooxygenase is an enzyme that catalyzes the reaction that produces prostaglandins from arachidonic acid. There are two isomers of cyclooxygenase. Cyclooxygenase‐1 is expressed in most tissues and produces prostaglandins that mediate normal physiologic functions. Cyclooxygenase‐2 is undetectable in most normal tissues but is elevated in CRAs and cancers. There is experimental evidence in animal models that demonstrates the potential contribution of cyclooxygenase‐2 to colorectal tumour growth and development, through its influence on apoptosis, cell migration, attachment, invasion and angiogenesis (Dannenberg 1999, Gupta 2000). Potential cyclooyxengase independent mechanisms have also been examined (Gupta 2000). Therefore it is biologically plausible that both nonselective and selective cyclooxygenase‐2 inhibitors could function as chemopreventive agents, but the selective NSAIDs cause significantly fewer serious gastrointestinal adverse effects (Fitzgerald 2001). There is evidence from retrospective and prospective observational studies that NSAIDs, the majority assessing ASA, may reduce the development of sporadic CRAs and CRC (Turner 1993, Paganini‐Hill 1994, Arber 2000, Vainio 1997). There is also observational evidence that nonaspirin NSAIDs may be effective for reducing the number and size of CRAs in the hereditary CRC syndrome, familial adenomatous polyposis (FAP) (Hawk 1999).
We have conducted a systematic review of the available evidence from randomized controlled trials that assess the influence of NSAIDs, including ASA, on the outcome of CRAs or CRC.
Objectives
The primary objectives of this systematic review were to assess the effect of NSAIDs on the incidence or recurrence of CRAs and on the incidence of CRC. A secondary objective was to identify adverse events.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs) that compare NSAIDs to a control.
Types of participants
Participants from the general population as well as individuals that have the diagnosis of FAP were included in this review. Subjects were included regardless of their personal history of adenomatous polyps or intact colon status.
Types of interventions
Studies included in this review have an intervention of a NSAID. This included nonselective and selective cyclooxygenase‐2 inhibitors and aspirin.
Types of outcome measures
The outcome of CRAs or CRC had to be confirmed pathologically. At least one of the primary outcomes have been reported in order for a study to be included.
Primary outcomes: Number of subjects with 1. at least one adenoma 2. more than one adenoma 3. at least one adenoma that is 1 cm or greater 4. at least one pathologic diagnosis of a tubulovillous or villous adenoma 5. the new diagnosis of CRC or 6. % change in polyp burden ("polyp burden" to be defined for each trial) 7. Change in polyp burden: Better/Worse/Same
Secondary outcome: Number of subjects that report: 1. an adverse event 2. a serious adverse event (Common Toxicity Criteria Grade 3 or 4) (CTEP 1998)
Search methods for identification of studies
Randomized controlled trials were identified from Medline (1966‐September 2003), Premedline (September 2003), Embase (1980‐2003 Week 43), and the Cochrane Controlled Controlled Trials Register (Cochrane Library 2003 issue 3). MeSH headings were included: randomized controlled trial, controlled clinical trial, random allocation, clinical trial, human, adenomatous polyps, adenomas, polyps, colorectal, colonic, rectal, prevention, chemoprevention, nonsteroidal anti‐inflammatory, cyclooxygenase inhibitor, aspirin, neoplasms, and cancer.
No language restriction was specified. The references from published studies and journal references were handsearched.
Data collection and analysis
There were two reviewers (TKA, RSM). The identified studies were screened by the reviewers (TKA, RSM) in order to identify articles eligible for inclusion into the review (see Criteria for considering studies for this review). The list of included studies were reviewed by a content expert to ensure that all relevant studies were included (Dr. J. Baron).
The quality of the included trials were evaluated independently by the two reviewers. Selection, performance, detection and attrition bias were detected from the following questions: 1) Concealment of allocation? 2) Blinding of intervention? 3) Intention‐to‐treat analysis? 4) Blinding of outcome measurement? 5) Complete follow‐up?
Questions 1‐4 were answered as Yes, No, or Unclear. Answers to Question 5 were described as a percentage of complete follow‐up. Complete follow‐up of at least 85% was considered to be adequate in order to minimize the risk of attrition bias. Clarification from the primary author was sought if the published data provided inadequate information for the review. From the quality assessment of the trials the potential risk of bias was summarized into three categories according to the Cochrane handbook (Clarke 2003):
Risk of Bias Interpretation Relationships to individual criteria
A. Low risk of bias Plausible bias unlikely to All of the criteria met: seriously alter the results ‐ "Yes" to Questions 1‐3 ‐ complete follow‐up >=85% B. Moderate risk of bias Plausible bias that raises One or more criteria partly met: some doubt about the results ‐ "No" or "Unclear" to one of Questions 2 or 3 or ‐ complete follow‐up 75‐84% C. High risk of bias Plausible bias that seriously One or more criteria not met: weakens confidence in the ‐ "No" or "Unclear" to results Question 1 or ‐ Two of the following: "No" or "Unclear" to Questions 2 or 3 or complete follow‐up <85%
The data from the included studies were abstracted independently by the two reviewers. Discrepancies were resolved by consensus.
Statistical analyses: For the outcomes that were discrete and numerical, the relative risk (RR) and risk difference (RD) was reported with 95% confidence intervals (CI). If the RD is statistically significant, the number needed to treat (NNT) or the number needed to harm (NNH) was calculated. Statistical heterogeneity was assessed using the chi‐square Q‐test. The studies were assessed clinically and methodologically to assess if it was reasonable to consider combining the data. If so, the more conservative random effects model was used to account for small clinical and methodologic variations between very similar high quality trials. Data from studies that were found to be at a high risk of bias from the quality assessment or were found to be statistically significantly heterogeneous were not combined. Instead, futher investigation would be undertaken to identify factors that could potentially explain the heterogeneity. An example of a potential factor is if the mean age of the subjects varied significantly between studies. A sensitivity analysis would then be conducted if data from trials were believed to be clinically appropriate to combine but involved studies that were at a high risk of bias from the quality assessment or were statistically heterogeneous. In this situation, the data from all of the studies would be combined and compared to the results obtained from the pooling of the subset of studies that were at low or moderate of bias. The primary outcomes for the secondary prevention of FAP that were reported as continuous or ordinal will be reported descriptively in the additional tables section.
Results
Description of studies
Details of the studies are listed in the Table of Included Studies. Study Participants: Nine studies with a total of 24,143 subjects met the inclusion criteria. In general, the aim of primary prevention trials is disease prevention in otherwise healthy individuals. Therefore the primary prevention trials included in this review attempted to reduce the development of CRAs or CRC in individuals without a known history of either. Whereas in the secondary prevention trials, the objective was to reduce the recurrence or induce the regression of CRAs in individuals that have a history of CRAs or CRC. For the five trials that included population based or average risk subjects; one primary prevention trial recruited 22,071 male physicians for 5 years (Gann 1993), three secondary prevention trials recruited 2,028 subjects had sporadic colorectal adenomas that were removed endoscopically with an intervention for 1 to 3 years (Baron 2003; Sandler 2003; Benamouzig 2003), and one small secondary prevention trial included 44 subjects that had polyps diagnosed on routine CRC screening with flexible sigmoidoscopies that were left in situ for 4 months (Ladenheim 1995). For the four trials that included subjects with FAP, there was one primary prevention trial that included 41 subjects for 4 years that were genotypically FAP (Giardiello 2002), but were phenotypically normal and three secondary prevention trials that recruited a total of 109 subjects that had phenotypic FAP for an intervention between 4 to 9 months (Steinbach 2000; Giardiello 1993,Labayle 1991). Types of interventions: The trials that included general population subjects generally included low dose ASA as the intervention (Gann 1993; Baron 2003; Sandler 2003) except one trial that included sulindac (Labayle 1991). The trials that included subjects with FAP included sulindac or celecoxib as an intervention.
Risk of bias in included studies
The quality assessment for each study is listed in the Table of Included Studies. Overall the nine included studies were of high methodologic quality. Eight of the included studies were determined to be at low risk for bias as they met all criteria of the quality assessment with at least 90% complete follow‐up (Baron 2003; Gann 1993; Giardiello 1993; Giardiello 2002; Labayle 1991; Ladenheim 1995; Steinbach 2000; Benamouzig 2003). Only one trial met four out of the five quality assessment criteria, because it attained an 81% complete follow‐up and as a result was considered to be at moderate risk for bias (Sandler 2003).
Effects of interventions
From the electronic search, thirteen potentially relevant references were identified and four references were identified through handsearching. One was excluded because this trial assessed only the effect of nonsteroidal‐inflammatory drugs (NSAIDs) on upper gastrointestinal polyps in familial adenomatous polyposis (FAP) subjects (Seow‐Choen 1996). As a result the manuscripts for sixteen references were retrieved and of these, seven were excluded. One trial was excluded because randomization was conducted for patients with advanced duodenal polyposis and the patients with rectal polyps were nonrandomized and were analyzed as a subgroup (Nugent 1993), four trials were ongoing (Women's Health Study; CAPP1; WHI 1998; CAPP2), and two manuscripts related to trials that were already identified (APACC 2001; MD Health 1988). The trial characteristics are listed in the TABLE OF INCLUDED STUDIES. It was often not possible to combine the data because the included trials differed significantly by the type of participants (ie: general population versus FAP patients), the study design (ie: primary prevention trials where the outcome were identified by subject self‐report versus secondary prevention where the primary outcomes were identified by endoscopy). Thus overall, a systematic review was conducted and the data were combined with meta‐analytic techniques when appropriate. The raw and summarized outcomes for the individual studies are listed in the ADDITIONAL TABLES and FIGURES sections.
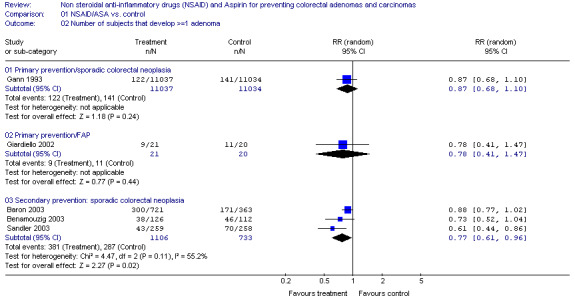
Population Based Subjects Population based subjects were subjects considered to be from the general population, or "average" risk subjects for CRAs or cancer. There were four trials comparing ASA to placebo. In all four trials, there was a trend in favour of ASA however the difference reached statistical significance in only one trial (Sandler 2003). One trial was a population based primary prevention study in which male physicians received 325mg of ASA on alternate days and the outcome was the incidence of self‐reported CRAs or CRC (Gann 1993). No statistically significant reduction for the incidence of sporadic CRAs was noted after five years [RR 0.87 (95% CI 0.68,1.10)]. Results were pooled from three secondary prevention trials as the outcomes were not statistically significantly heterogeneous (p=0.11) and the study designs were similar. However a random effects model was used because the lengths of ASA intervention varied from 1 to 3 years and unlike the other two trials, one included subjects with a history of CRC (Sandler 2003).
When the results were combined there was a statistically significant reduction in the recurrence of sporadic CRAs with an intervention of low‐dose ASA for one to three years [RR 0.77 (95% CI 0.61, 0.96), RD ‐0.08 (95% CI ‐0.13,‐0.04), NNT 12.5 (95% CI 7.7, 25)] (Baron 2003; Benamouzig 2003; Sandler 2003) (Figure 1). In one of these secondary prevention trials, 517 subjects with previous CRC were randomized to ASA 325 mg daily or placebo (Sandler 2003). This trial was terminated early because there was a statistically significant reduction in the recurrence of sporadic CRAs in the intervention group after a median of 12.8 months after randomization [RR 0.61 (95% CI 0.44,0.86), RD ‐0.11 (95% CI ‐0.18,‐0.03), NNT 9 (95% CI 6, 33)] (Sandler 2003). The other two trials were similar in design, except subjects with CRC were excluded and individually there was no statistically significant reduction in the recurrence of CRAs (Baron 2003; Benamouzig 2003). However, in a subgroup receiving 81mg of ASA daily there was a significant reduction in recurrent CRAs [RR 0.81 (95% CI 0.69,0.96)] that was not observed in a subgroup receiving 325mg daily [RR 0.96 (95% CI 0.81‐1.13)] by Baron and colleagues however the opposite nonstatistically significant trend was found by Benzamouzig and colleagues [25% rates of recurrence with 300mg of lysine acetylsalicylate and 35% recurrence with 160 mg (p=0.23)]. There was a fifth small secondary prevention trial where no significant regression of identified small sporadic CRAs was observed after four months of sulindac compared to a placebo group [RR 1.67 (95% CI 0.45,6.14)] (Ladenheim 1995).
1.
Polyps in Familial Adenomatous Polyposis There were four RCTs that included subjects with FAP. There was one primary prevention trial that recruited subjects with a disease‐causing mutation of the adenomatous polyposis coli gene but no phenotypic expression of FAP, and after four years of intervention with sulindac had no statistically significant difference in the incidence of CRAs compared to a control [RR 0.78 (95% CI 0.41,1.147)] (Giardiello 2002). Three trials were secondary prevention RCTs with phenotypic FAP subjects who received sulindac or celecoxib for four to nine months (Giardiello 1993; Labayle 1991; Steinbach 2000). Investigators attempted to standardize outcome assessment within their own trial, but because of the numerous polyps, standardization between trials was not possible and therefore the results have been reported descriptively. There was a greater proportional reduction (range: 11.9% to 44%) in the number of colorectal adenomas in the intervention groups compared to those in the control group (range: 4.5% to 10%) (Table 1).
1. Secondary prevention: Familial adenomatous polyposis.
| Study | % change #CR polyp | %change CRpolyp size | Change polyp burden |
| Labayle 1991 | Treatment: Better 9 Control: Better 2 Worse 5 Same 2 | ||
| Giardello 1993 | Treatment (n=11): ‐ 44% Control (n=11): ‐ 10% (get raw data) | Treatment: ‐ 35% Control: 0% | |
| Steinbach 2000 | Treatment 1 (n=32): ‐11.9% +/‐ 30.3 Treament 2 (n=30): ‐28.0% +/‐ 24.0 Control (n=15): ‐4.5% +/‐16.4 | Treatment 1: ‐14.6% +/‐31.7 Treatment 2: ‐30.7% +/‐25.7 Treatment 3: ‐4.9% +/‐17.3 | |
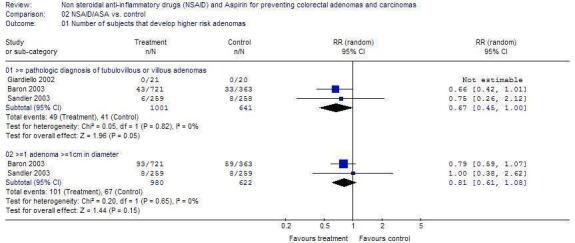
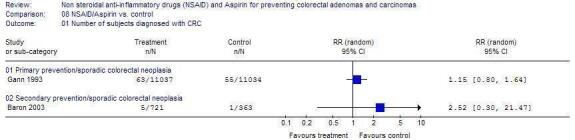
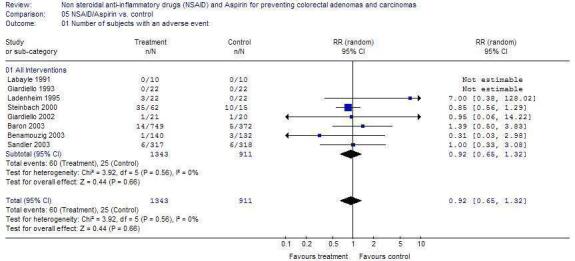
Other Outcomes There was no statistically significant difference in the outcomes of higher risk CRAs (Figure 2), CRC (Figure 3) or adverse events (Figure 4) in any of the trials.
2.
3.
4.
Discussion
DISCUSSION From nine high quality published RCTs, there is some evidence for the effectiveness of NSAIDs and ASA in the prevention of CRAs for the average risk and individuals with FAP. From the combined results of three trials, low dose ASA was found to statistically significantly reduce the rates of recurrent sporadic adenomas after one to three years (Baron 2003; Benamouzig 2003; Sandler 2003). There is no apparent biologic explanation for the significant benefit received by the subgroup from the lower dose of 81mg of aspirin that was not found in the subgroup that received 325mg daily (Baron 2003). Perhaps this may have been a chance finding.
Additionally, there are limitations to these trials. First, in the majority of the trials the end‐point was a surrogate biomarker and not the clinically more relevant end‐point of CRC. However CRAs are the most suitable intermediate biomarker as they are precursors of CRC, are clinically identifiable and resectable (Winawer 1993). Clinical support for this include results from a retrospective review of Mayo Clinic records for 226 patients with colonic polyps at least 10mm in diameter that elected for periodic radiographic follow‐up instead of excision between January 1965 and December 1970 (Stryker 1987). The cumulative risk for cancer at the polyp site at 5, 10, and 20 years was 2.5%, 8% and 24% respectively. The coloretal outcomes were published from St. Mark's Hospital for 1 618 patients that had at least one rectosigmoid polypectomy with a rigid‐sigmoidoscopy who did not undergo surveilance (Atkin 1992). The incidence of subsequent rectal cancer was found to be similar to the general population rates if the polyp had been adequately removed, and the risk of subsequent colon cancer was increased for tubulovillous, villous, multiple or large (>=1cm) polyps. Furthermore, primary prevention trials with the incidence of CRC as an endpoint would require prohibitively large sample sizes and a long follow‐up period. Despite there being 22,072 men in The Physicians' Health Study a significant decrease in the outcome of CRC was not observed after five years of follow‐up (Gann 1993). This suggests further caution when interpreting the results. Perhaps the duration of the intervention was too brief and was started too late in order to reduce the incidence CRC. The polyp‐cancer sequence is a multi‐step process that often has a natural history of 10 to 15 years and a relatively short duration intervention may not interrupt it (Winawer 1993). Also, the Physicians' Health Study relied on self‐report of CRC and CRAs and may have failed to detect a significant difference because of the low sensitivity of measuring the outcome. Finally, with regard to the FAP trials, assessment of the number of adenomas in the rectum is notoriously inaccurate especially with respect to identifying microadenomas.
Based on these results, further studies are required to clarify the effectiveness of these agents. Many questions remain unanswered, including the identification of the most effective type, dosage or duration of an NSAID intervention. It is also unknown whether these medications are equally effective as primary and secondary chemopreventive agents. There are numerous ongoing studies that may provide some answers. Of these, there are two RCTs that will test the effect of low dose aspirin for recurrent sporadic CRAs. The final four year results of trial by Benamouzig and colleagues will provide more information about optimal dosages of ASA (Benamouzig 2003) and the other is the ukCAP trial which plans to recruit 1300 subjects with a 2x2 factorial intervention that includes a 300mg ASA intervention for 4 years (ukcccr 2002). Also, one trial includes an aspirin and/or resistant starch intervention for the prevention of CRAs for hereditary nonpolyposis CRC gene carriers (CAPP2), and the Women's Health Study which is a eight to ten year primary prevention trial that includes a 100mg aspirin arm for the prevention of cardiovascular disease and site‐specific cancer in women (Women's Health Study).
Although the data from these trials suggest that NSAIDs and ASA can be taken without increased adverse events [RR 0.92 (95% 0.65, 1.32)], the results from these trials may not be generalizable because individuals at higher risk for adverse events were excluded. Also, the four largest studies (Sandler 2003; Gann 1993; Baron 2003; Benamouzig 2003) had a one to three month run‐in phase with low dose ASA, therefore individuals that had reduced compliance due to medication intolerance or adverse events would also have been excluded from the trials. Based on results from other trials, it has been estimated that low dose aspirin may cause 2 to 4 major gastrointestinal bleeding episodes and 0 to 2 excess hemorrhagic strokes per 1000 middle‐aged persons after five years (USPTF 2002). Despite the improved gastrointestinal toxicity profile, recent evidence has suggested that selective cyclooxygenase‐2 inhibitors may have a prothrombotic potential and may increase the risk of cardiovascular events(Mukherjee 2001). Thus, the risks and overall benefits of NSAIDs for individual patients must be weighed.
Finally, an important question that remains unanswered is whether chemopreventive strategies with NSAIDs have a role for individuals at average risk for the development of CRAs or CRC or whether strategies to increase compliance with screening would be more effective. Recently, two analyses concluded that aspirin 325mg daily was not a cost‐effective substitute or addition to routine CRC screening due to aspirin‐related complication rates (Suleiman 2001; Ladabaum 2001).
For patients with FAP, short‐term regression, but not complete elimination of polyps was noted in three RCTs. There is some supporting evidence from nonrandomized studies that NSAIDS may be effective for long‐term regression of polyps in FAP. In a case series, 12 FAP patients with ileorectal anastomoses who received a mean dosage of 158mg daily of sulindac had a reduced polyp burden after a mean of 63.4+/‐31.3 months (Cruz‐Correa 2002). It is important to note that rectal cancer, an increase in polyp number and refractory rectal mucosal erosions also occurred in some individuals within this same cohort. As well, there have been other published reports of rectal cancer developing in FAP patients despite sulindac treatment (Niv 1994; Thorson 1994) as well as evidence of sulindac‐resistent adenomas (Keller 2001). There is limited conflicting evidence from randomized controlled trials about the potential role of NSAIDS for the regression of duodenal polyps (Seow‐Choen 1996; Nugent 1993).
Authors' conclusions
Implications for practice.
There is some evidence from the pooled data of three RCTs to support the effectiveness of one to three years of ASA for reducing the risk of recurrent sporadic CRAs. Similarly, there is evidence from short‐term studies to support regression, but not elimination or prevention of CRAs in FAP with NSAIDs.
Implications for research.
More evidence from ongoing RCTs is necessary to establish the long‐term role of NSAIDs for the prevention of CRAs in addition to currently recommended CRC screening strategies for the average risk population or routine surveillance for post‐polypectomy or FAP patients.
What's new
| Date | Event | Description |
|---|---|---|
| 6 July 2021 | Amended | A new editorial note was added. |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 2, 2004
| Date | Event | Description |
|---|---|---|
| 5 August 2008 | Amended | Converted to new review format. |
| 17 November 2003 | New citation required and conclusions have changed | Substantive amendment |
Notes
Acknowledgements
We are grateful to: ‐ Dr. J. Baron for expert assistance regarding completeness of our list of included studies ‐ trialists Dr. R. Benamouzig, Dr. J. Baron, L.A. Mott, Dr. IM Lee for responding to requests for information ‐ Dr. H.K. Andersen & the Cochrane Colorectal Cancer Group
Data and analyses
Comparison 1. NSAID/ASA vs. control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.2 Number of subjects that develop >=1 adenoma | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.2.1 Primary prevention/sporadic colorectal neoplasia | 1 | 22071 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.68, 1.10] |
| 1.2.2 Primary prevention/FAP | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.41, 1.47] |
| 1.2.3 Secondary prevention: sporadic colorectal neoplasia | 3 | 1839 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.96] |
1.2. Analysis.

Comparison 1: NSAID/ASA vs. control, Outcome 2: Number of subjects that develop >=1 adenoma
Comparison 2. NSAID/ASA vs. control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Number of subjects that develop higher risk adenomas | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1.1 >= pathologic diagnosis of tubulovillous or villous adenomas | 3 | 1642 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.45, 1.00] |
| 2.1.2 >=1 adenoma >=1cm in diameter | 2 | 1602 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.61, 1.08] |
2.1. Analysis.

Comparison 2: NSAID/ASA vs. control, Outcome 1: Number of subjects that develop higher risk adenomas
Comparison 3. Secondary prevention: Sporadic colorectal neoplasia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Number of subjects where adenomas regressed or disappeared | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.1 Sulindac vs. control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.3 Number of subjects with >=1 adenoma | 3 | 1839 | Risk Difference (M‐H, Random, 95% CI) | ‐0.08 [‐0.13, ‐0.04] |
| 3.3.1 Secondary prevention: sporadic colorectal neoplasia | 3 | 1839 | Risk Difference (M‐H, Random, 95% CI) | ‐0.08 [‐0.13, ‐0.04] |
3.1. Analysis.

Comparison 3: Secondary prevention: Sporadic colorectal neoplasia, Outcome 1: Number of subjects where adenomas regressed or disappeared
3.3. Analysis.

Comparison 3: Secondary prevention: Sporadic colorectal neoplasia, Outcome 3: Number of subjects with >=1 adenoma
Comparison 5. NSAID/Aspirin vs. control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Number of subjects with an adverse event | 8 | 2254 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.65, 1.32] |
| 5.1.1 All Interventions | 8 | 2254 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.65, 1.32] |
| 5.2 Number of subjects with a serious adverse event | 3 | 1797 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.58, 2.45] |
| 5.2.1 All Interventions | 3 | 1797 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.58, 2.45] |
5.1. Analysis.

Comparison 5: NSAID/Aspirin vs. control, Outcome 1: Number of subjects with an adverse event
5.2. Analysis.

Comparison 5: NSAID/Aspirin vs. control, Outcome 2: Number of subjects with a serious adverse event
Comparison 8. NSAID/Aspirin vs. control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Number of subjects diagnosed with CRC | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1.1 Primary prevention/sporadic colorectal neoplasia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1.2 Secondary prevention/sporadic colorectal neoplasia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
8.1. Analysis.

Comparison 8: NSAID/Aspirin vs. control, Outcome 1: Number of subjects diagnosed with CRC
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baron 2003.
| Study characteristics | ||
| Methods | Multi‐centred, randomized, double blind, placebo controlled trial Recruitment: July 1994 to March 1998 1. Allocation concealment: Yes 2. Blinding of intervention: Yes 3. Intention to treat: Yes 4. % complete f/u: 97% 5. Blinding of outcome: measurement(s): Yes | |
| Participants | Sample size: 1,121 Incl. Criteria: age 21‐80 years, >=1 histologically documented adenoma removed <=3 months prior to study enrollment, endoscopically clean colon Excl. Criteria: FAP, HNPCC, CRC, any condition potentially worsened or treated by aspirin, other NSAIDs or folic acid Country: USA, Canada Setting: tertiary care outpatient % female: 36% Mean age: 53.2+/‐ 9.5 years Baseline characteristics similar for each group: Yes | |
| Interventions | 2 x 3 factorial design Treatment 1. Aspirin 325 mg daily 2. Aspirin 80 mg daily 3. Folic acid 1mg daily Control: Placebo daily Duration: 3 years Outcome Monitoring: Qualifying colonoscopy, repeated at 1 and 3 years |
|
| Outcomes | Primary outcomes:
1. Number of subjects with >=1 adenoma Secondary outcome: 1. Number of subjects that reported a serious adverse event |
|
| Notes | 1. 1409 eligible subjects underwent a 3 month run‐in period 2. Compliance: >90% reported >= 50% compliance | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Benamouzig 2003.
| Study characteristics | ||
| Methods | Multi‐centred, randomized double blind placebo‐controlled trial Recruitment: Dec. 1996 to Feb, 2000 1. Allocation concealment: Yes 2. Blinding of intervention: Yes 3. Intention to treat: Yes 4. % complete f/u: 87% 5. Blinding of outcome: measurement(s): Yes | |
| Participants | Sample size: 272 Incl. Criteria: age 18‐75 years, >=3 adenomas or >=1 adenoma >=6mm in diameter all histologically confirmed and removed <=3 months prior to study enrollment, endoscopically clean colon Excl. Criteria: FAP, HNPCC, CRC, any condition potentially worsened or treated by aspirin, other NSAIDs or folic acid Country: France Setting: outpatient setting % female: 30% Mean age: 58+/‐ 2 years Baseline characteristics similar for each group: Yes | |
| Interventions | Treatment:
1. Lysine acetylsalicylate 160mg
2. Lysine acetylsalicylate 300mg Control: placebo Duration: 4 years (note: interim 1 year results published 2003) Outcome Monitoring: Qualifying colonoscopy, repeated at 1 and 4 years |
|
| Outcomes | Primary outcomes:
1. Number of subjects with >=1 adenoma
2. size of new adenomas
3. polyp burden (sum of the diameters of the adenomas) Secondary outcome: 1. Number of subjects that reported a serious adverse event |
|
| Notes | 1. 291 potentially eligible subjects underwent 4‐week run‐in period (must have >=80% compliance of 300mg lysine ASA to be eligible) ‐ 19 not randomized (5 had side‐effects) 2. Compliance 87+/‐12% of sachets for the intervention and 88+/‐10% in the placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Gann 1993.
| Study characteristics | ||
| Methods | Randomized, double blind, placebo‐controlled trial Conducted: 1982 to 1988 1. Allocation concealment: Yes 2. Blinding of intervention: Yes 3. Intention to treat: Yes 4. % complete f/u: 99.7% 5. Blinding of outcome measurement(s): Yes | |
| Participants | Sample size: 22,071 Incl. Criteria: US male physicians age 41‐84 years Excl. Criteria: prior history of vascular disease, cancer, liver or renal disease, gout, PUD, current use of NSAID (>=1/week) or vitamin A Country: USA Setting: population cohort % female: 0% Mean age: 53.2+/‐ 9.5 years Baseline characteristics similar for each group: Yes | |
| Interventions | 2 X 2 factorial design Treatment 1: Aspirin 325 mg every other day Treatment 2: Beta carotene 50mg every other day Control 1 & 2: Placebo every other day Duration: 5 years Outcome Monitoring: self‐report, pathology reports, medical records, death certificates |
|
| Outcomes | Primary outcomes: 1. Number of subjects with >=1 adenoma 2. Number of subjects with the new diagnosis of CRC | |
| Notes | 1. 33,223 eligible physicians underwent an 18 week run‐in period 2. The randomized aspirin component was terminated early (5 years) due to statistically significant reduction of MIs. 3. Compliance: 85.7% for both aspirin and placebo groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Giardiello 1993.
| Study characteristics | ||
| Methods | Single‐centre, randomized, doubled blind, placebo‐controlled trial: Published: 1993 1. Allocation concealment: yes 2. Blinding of intervention: yes 3. Intention to treat: yes 4. % complete f/u: 91% 5. Blinding of outcome: measurement(s): yes | |
| Participants | Sample size: 22 Incl. Criteria: FAP, intact colon or subtotal colectomy with IRA Excl. Criteria: use of an NSAID or aspirin >1 week within 3 months of the study, absence of the use of effective birth control in women of child bearing age, abnormal serum laboratory tests (WBC, plt liver, kidney), history of PUD or GI bleed, cancer, active bacterial infection Country: USA Setting: Outpatient tertiary care centre % female: 59% Mean age: Tx: 21.9+/‐7.6 & Cx:26.3+/‐10.3 yrs % intact colon: 82% Baseline characteristics similar for each group: yes | |
| Interventions | Stratified by intact colon and subtotal colectomy with IRA Treatment: Sulindac 150 mg twice daily Control: Placebo twice daily Duration: 9 months Outcome Monitoring: Flexible sigmoidoscopy at baseline and at 3, 6, 9 and 12 months |
|
| Outcomes | Primary outcomes:
1. Percent change from baseline in polyp number
2. Percent change from baseline in polyp size Secondary outcome: 1. Number of subjects that reported a serious adverse event |
|
| Notes | 1. Apriori sample size calculation of 40, but recruitment stopped at 22 subjects because statistical guidelines were met 2. Outcomes assessment: total number of polyps were counted in the circumference of the colorectum from 20cm (tattooed) to the anal verge, videotaped, diameter of up to 5 polyps just beyond 20cm were measured with graduated scale 3. Study withdrawals: 2 patients in the sulindac group were withdrawn (one due to persistent diarrhea diagnosed as colitis secondary to clostridium difficile, another moved from the area) 4. Compliance: 85% with scheduled drug doses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Giardiello 2002.
| Study characteristics | ||
| Methods | Single‐centre, randomized, doubled blind, placebo‐controlled trial Conducted: Sept 1993 to July 2001 1. Allocation concealment: yes 2. Blinding of intervention: yes 3. Intention to treat: yes 4. % complete f/u: 93% 5. Blinding of outcome: yes measurement(s): | |
| Participants | Sample size: 41 Incl. Criteria: age >=8 years, has a disease‐causing mutation of the APC gene, clean colon on endoscopy, intact colon Excl. Criteria: use of an NSAID or aspirin >1 week within 3 months of the study, absence of the use of effective birth control in women of child bearing age, abnormal serum laboratory tests (WBC, plt liver, kidney), history of PUD or GI bleed, cancer, active bacterial infection, body weight <20kg Country: USA Setting: Outpatient tertiary care centre % female: 66% Age range: 8‐25 years % Intact colon: 100% Baseline characteristics similar for each group: yes | |
| Interventions | Treatment: Sulindac 75mg (body weight 20‐44 kg) or 150mg (body weight >44kg) twice daily
Control: Placebo twice daily
Duration: 4 years Outcome Monitoring: Flexible sigmoidoscopy at baseline and every 4 months |
|
| Outcomes | Primary outcomes:
1. Number of subjects with at least 1 adenoma
2. Number of subjects with at least one pathologic diagnosis of tubulovillous or villous adenoma Secondary outcome: 1. Number of subjects that reported a serious adverse event Other outcomes: 1. Mean number of adenomas (SD) 2. Mean size of adenomas (SD) |
|
| Notes | 1. Compliance (mean +/‐SD) : Treatment 86.9+/‐ 7.5% and Placebo 81.7+/10.4% 2. Outcomes assessment: total number of polyps were counted in the circumference of the colorectum from 20cm to the anal verge, videotaped, diameter of up to 5 polyps just beyond 20cm were measured with graduated scale 3. Study withdrawals: 5 subjects from the treatment group (3 for increasing polyps, 1 for persistent neutropenia, 1 unable to attend visits), 6 withdrew from the placebo (4 for increasing polyps, 2 unable to attend visits) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Labayle 1991.
| Study characteristics | ||
| Methods | Multi‐centre, randomized, double blind, placebo‐controlled cross‐over trial Published: 1991 Type of trial: 1. Allocation concealment: Yes 2. Blinding of intervention: Yes 3. Intention to treat: Yes 4. % complete f/u: 90% 5. Blinding of outcome measurement(s): Yes | |
| Participants | Sample size: 10 Incl. Criteria: FAP, age >18 years, previous colectomy with IRA, rectal adenomas Excl. Criteria: PUD, cirrhosis, abnormal serum creatinine Country: France Setting: Outpatient % female: 20% Mean age: 37 (24‐52) years % intact colon: 0% Baseline characteristics similar for each group: yes | |
| Interventions | Cross‐over design: 1 month wash‐out
period prior to cross‐over Treatment: Sulindac 100mg three times daily Control: Placebo three times daily Duration: 4 months Outcome Monitoring: Flexible rectoscopy at baseline and after each 4 month treatment period |
|
| Outcomes | Primary outcome:
1. Change in polyp burden (better/worse/same) Secondary outcome: 1. Number of subjects that reported an adverse event |
|
| Notes | 1. One patient did not complete study and was excluded due to lack of compliance | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Ladenheim 1995.
| Study characteristics | ||
| Methods | Multi‐centre, randomized, double blind, placebo‐controlled trial Published: 1995 1. Allocation concealment: Yes 2. Blinding of intervention: Yes 3. Intention to treat: Yes 4. % complete f/u: 100% 5. Blinding of outcome measurement(s): Yes | |
| Participants | Sample size: 44 Incl. Criteria: age >50 years old scheduled for routine screening flex sig, adenomas <=1cm in diameter Excl. Criteria: adenomas >1cm in diameter, history of GI bleeding, PUD, cancer, long‐term use of NSAIDs (except aspirin), severe lung or cardiac disease Country: USA Setting: Outpatient endoscopy % female: 13.6% Mean age: 64.4+/‐ 7.5 years Baseline characteristics similar for each group: yes except Mean number of polyps was greater in the sulindac group (2.0+/‐1.5) vs. placebo (1.3+/‐0.9) | |
| Interventions | Treatment: Sulindac 150 mg twice daily
Control: Placebo twice daily
Duration: 4 months colonoscopy at 4 months |
|
| Outcomes | Primary outcome:
1. Change in polyp burden (# patients for whom polyps either disappeared or regressed by at least 25% in diameter) Secondary outcome: 1. Number of subjects that reported an adverse event |
|
| Notes | 1. From 162 eligible patients, 44 were enrolled 2. Compliance: 89% 3. Assessment of outcomes: polyps were photographed, adenoma diameter estimated by comparison to open biopsy forcep, adenoma location estimated by distance from anal verge 4. 4 patients (sulindac group) discontinued intervention (3 side effects, 1 urosepsis) 5. 12/44 subjects (8 sulindac, 4 placebo) were taking 80‐325 mg aspirin regularly, discontinued for study duration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Sandler 2003.
| Study characteristics | ||
| Methods | Multi‐centred, randomized, double blind, placebo controlled trial Conducted: May 1993 to January 2000 1. Allocation concealment: Yes 2. Blinding of intervention: Yes 3. Intention to treat: Yes 4. % complete f/u: 81% 5. Blinding of outcome: measurement(s): Yes | |
| Participants | Sample size: 635 Incl. Criteria: age 30‐80 years, histologically documented colon or rectal cancer with a low risk of recurrent disease, within 4 months prior to study enrollment, endoscopically clean colon Excl. Criteria: FAP, cardiovascular disease, other nonmelanoma invasive cancer within 5 years, immunsuppressive therapy within 6 months, IBD, contraindication to apsirin, current use of NSAID Country: USA Setting: tertiary care outpatient % female: 48% Median age range: 60‐69 years Baseline characteristics similar for each group: Yes | |
| Interventions | Treatment
1. Aspirin 325 mg daily
Control:
Placebo daily
Duration: planned for 3 years terminated early (after 1 year) after planned interim analysis Outcome Monitoring: Qualifying colonoscopy, repeated at 1 and 3 years |
|
| Outcomes | Primary outcomes: 1. Number of subjects with >=1 adenoma 2. Number of subjects >=1adenoma >=1cm 3. Number of subjects with tubulovillous or villous adenomas Secondary outcome: 1. Number of subjects that reported a serious adverse event | |
| Notes | 1. 719 eligible subjects underwent a 3 month run‐in period 2. Compliance: >90% reported >= 50% compliance 3. endoscopy was performed as part of the usual follow‐up for patients with CRC and was performed by each patient's own gastroenterologist or surgeon | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Steinbach 2000.
| Study characteristics | ||
| Methods | Multi‐centre, randomized, double blind, placebo‐controlled trial Conducted: Dec 1996 to Dec 1998 1. Allocation concealment: Yes 2. Blinding of intervention: Yes 3. Intention to treat: Yes 4. % complete f/u: 96% 5. Blinding of outcome measurement(s): Yes | |
| Participants | Sample size: 77 Incl. Criteria: age 18‐65 years, FAP, have not had their entire colorectum removed, >= 5 adenomas >= 2mm that can be visualized endoscopically Excl. Criteria: colectomy 12 months prior or planned 8 months after enrollment, use of NSAIDs or aspirin at least 3 times a week within 6 months or at least 1 time a week within 3 months, abnormal serum lab tests (CBC, liver, kidney) Country: USA, UK Setting: Outpatient tertiary care centres % female: 43% Mean age: P40+/‐11, T139+/‐10,T233+/‐11 years % Intact colon: 33% Baseline characteristics similar for each group: yes except for age | |
| Interventions | Randomized: 2:2:1 Treatment 1: Celecoxib 100mg twice daily Treatment 2: Celecoxib 400mg twice daily Control: Placebo twice daily Duration: 6 months Outcome Monitoring: Endoscopy at baseline and at 6 months |
|
| Outcomes | Primary outcomes:
1. Percent change from baseline in polyp burden (sum of the polyp diameters)
2. Percent change from baseline in polyp number Secondary outcome: 1. Number of subjects that reported an adverse event |
|
| Notes | 1. Testing for APC gene mutations were performed at baseline 2. 103 eligible patients: 29 had insufficient number of polyps, 2 required colectomy 3. Outcomes assessment: at baseline tattoo was placed in the rectum, colon or both near high density of polyps, video & photos were taken near tattoo, appendix & ileocecal valve for baseline & follow‐up, diameters measured with a standardized endoscopic ruler or biopsy forcep 4. Two patients were withdrawn because of noncompliance and two patients were added to Treatment 1 group. 5. Compliance: 90% of subjects that completed the study took >= 80% of study drug 6. 3 subjects did not complete the study: T1: 1 subject committed suicide T2: 1 subject had an allergic reaction, 1 subject had dyspepsia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
>= greater than or equal to; > greater than; mm3 cubic millimeter; CBC complete blood count; plt platelet; mg/dl milligram per deciliter; mg milligrams; kg kilograms; APC adenomatous polyposis coli; NSAID nonsteroidal anti‐inflammatory drug; PUD peptic ulcer disease; GI gastrointestinal; MI myocardial infarction Flex sig flexible sigmoidoscopy; FAP familial adenomatous polyposis; HNPCC hereditary nonpolyposis colorectal cancer; IRA ileorectal anastomosis; IBD inflammatory bowel disease; T treatment; P placebo;
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Nugent 1993 | This double‐blind randomized controlled trial of sulindac versus placebo for FAP patients with advanced duodenal polyposis was excluded because patients with rectal polyposis were not randomized and were analysed as a subgroup |
FAP familial adenomatous polyposis
Characteristics of ongoing studies [ordered by study ID]
APACC 2001.
| Study name | APACC |
| Methods | |
| Participants | Incl. criteria: Adults age 18‐75 years, >=1 histologically confirmed colorectal adenoma, clean colon on endoscopy Excl. criteria: CRC, FAP, IBD |
| Interventions | 1) aspirin 160mg daily 2) aspirin 300mg daily 3) placebo daily Duration: 4 years |
| Outcomes | Recurrence of colorectal adenomas Colonoscopy at 1 and 4 years |
| Starting date | November 1996 |
| Contact information | robert.benamouzig@avc.ap‐hop‐paris.fr |
| Notes | 1 year interim results published in Gastroneterology August 2003 |
CAPP1.
| Study name | Concerted Action Polyposis Prevention 1 |
| Methods | |
| Participants | FAP gene carriers in 14 European countries |
| Interventions | 2 x 2 factorial design 1) aspirin 100mg 2) corn starch 30g (13.2 resistant starch) Duration: 2 years |
| Outcomes | Incidence or progression of colorectal adenomas |
| Starting date | Ongoing |
| Contact information | http://www.ncl.ac.uk/capp/index.html |
| Notes |
CAPP2.
| Study name | Concerted Action Polyposis Prevention 2 |
| Methods | |
| Participants | Hereditary nonpolyposis colorectal cancer (HNPCC) gene carriers in 25 centres around the world. |
| Interventions | 2 x 2 factorial design aspirin 600mg, corn starch 30g (13.2 resistant starch) Duration: 2 years |
| Outcomes | Incidence of colorectal adenomas (extracolonic malignancy, proliferation, apoptosis, genotype as secondary endpoints) |
| Starting date | March 1999 |
| Contact information | http://www.ncl.ac.uk/capp/index.html |
| Notes |
MDA‐ID‐00109.
| Study name | Phase II Randomized study of celecoxib with or without eflornithine for the prevention of colorectal cancer in participants with familial adenomatous polyposis of the colorectum |
| Methods | |
| Participants | Adults age 18‐65 years of age with a history of FAP and no anticipated colectomy within 8 months after randomization |
| Interventions | 1) celecoxib twice daily and placebo once daily 2) oral celecoxib twice daily and oral DFMO daily Duration: 6 months |
| Outcomes | 1) % change in number of polyps 2) % change in polyp size |
| Starting date | |
| Contact information | http://www.cancer.gov/search/clinical trials |
| Notes |
NCI‐P00‐0150.
| Study name | PhaseIIB randomized study of eflornithine (DFMO) and sulindac in the prevention of colorectal carcinoma in patients with previously resected colorectal adenoma |
| Methods | |
| Participants | Adults age 40‐80 years of age with a history of at least 1 prior resected colorectal adenoma <=5years |
| Interventions | 1) oral sulindac and oral DFMO daily 2) placebo daily Duration: 3 years |
| Outcomes | Recurrence of colorectal adenomas |
| Starting date | |
| Contact information | http://www.cancer.gov/search/clinical trials |
| Notes |
ukCAP.
| Study name | Multi‐centre, double blind, randomized controlled trial of aspirin and/or folic acid as prevention of recurrent colorectal adenomas |
| Methods | |
| Participants | Adults <=75 years of age with a history of histologically confirmed colorectal adenomas, clean colon by endoscopy within 6 months of enrollment, intact colon |
| Interventions | 2 X 2 factorial design 1) aspirin 300 mg daily 2) folic acid 0.5mg daily 3) placebo 1 or 2 daily Duration: 3 years |
| Outcomes | Recurrence of colorectal adenomas |
| Starting date | May 1997 |
| Contact information | http://www.controlled‐trials.com contact: MRC Clinical Trials Unit tel: +44 (0) 20 76704723 |
| Notes | March 2003: current accrual 937, projected accrual 1,300 |
WHI 1998.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Women's Health Study.
| Study name | The Women's Health Study |
| Methods | |
| Participants | Female health professionals >= 45 years of age |
| Interventions | 2 X 2 X2 factorial design 1) aspirin 100mg every other day 2) vitamin E 600 IU every other day 3) B‐carotene 50 mg every other day 4) placebo 1, 2, or 3 Duration: |
| Outcomes | Site‐specific cancer or cardiovascular disease |
| Starting date | April 1993 |
| Contact information | I_Min Lee<ilee@rics.bwh.harvard.edu> |
| Notes |
<= less than or equal to; FAP Familial adenomatous polyposis; DFMO eflornithine; IBD Inflammatory bowel disease
Contributions of authors
Dr. Tracey Asano Literature search and identification of trials for inclusion Evaluation of methodologic quality of included trials Abstraction of data Verifying and entering data into RevMan Writing the text of the draft review Writing the final manuscript.
Dr. Robin McLeod
Reviewed the results of the literature search to identify trials for inclusion Evaluation of methodologic quality of included trials Abstraction of data Review and substantial editing to the draft review Writing the final manuscript.
Sources of support
Internal sources
Mount Sinai Hospital, Canada
University of Toronto, Department of Surgery, Canada
External sources
Dr. TK Asano ‐ Research Fellow of the National Cancer Institute of Canada with funds provided by the Canadian Cancer Society, Canada
Declarations of interest
none known
Edited (no change to conclusions)
References
References to studies included in this review
Baron 2003 {published and unpublished data}
- Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, McKeowyn-Eyssen G, Summers RW, Rothstein R, Burke CA, Snover DC, Church TR, Allen JI, Beach M, Beck, GJ, Bond JH, Byers T Greenberg ER, Mandel JS, Marcon N, Mott LA, Pearson L, Saibil F vanStok RU. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med 2003;348:891-9. [DOI] [PubMed] [Google Scholar]
Benamouzig 2003 {published data only}
- Benamouzig R, Deyra J, Martin A, Girard B, Jullian E, Piednoir B, Couturier D, Coste T, Little J, Chaussade S. Daily soluble aspirin and prevention of colorectal adenoma recurrence: One-yr results of the APACC Trial. Gastroeneterology 2003;125:328-336. [DOI] [PubMed] [Google Scholar]
Gann 1993 {published data only}
- Gann PH, Manson JE, Glynn RJ, Buring JE, Hennekens CH. Low-dose aspirin and incidence of colorectal tumors in a randomized trial. J Natl Cancer Inst 1993;85:1220-24. [DOI] [PubMed] [Google Scholar]
- The Steering Committee of the Physicians' Health Group. Preliminary report: findings from the aspirin component of the ongoing physicians' health study. N Engl J Med 1988;318:262-64. [DOI] [PubMed] [Google Scholar]
Giardiello 1993 {published data only}
- Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus JA. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med 1993;328:1313-6. [DOI] [PubMed] [Google Scholar]
Giardiello 2002 {published data only}
- Giardiello FM, Yang VW, Hylind LM, Krush AJ, Petersen GM, Trimbath JD, Piantadosi S, Garrett E, Geiman GE, Hubbard W, Offerhaus JA, Hamilton SR. Primary chemoprevention of familial adenomatous polyposis with sulindac. N Engl J Med 2002;346:1054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Labayle 1991 {published data only}
- Labayle D, Fischer D, Vielh P, Drouhin F, Pariente A, Bories C, Duhamel O, Trousset M, Attali P. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology 1991;101:635-39. [DOI] [PubMed] [Google Scholar]
Ladenheim 1995 {published data only}
- Ladenheim J, Garcia G, Titzer D, Herzenberg H, Lavori P, Edson R, Omary MB. Effect of sulindac on sporadic colonic polyps. Gastroenterology 1995;108:1083-7. [DOI] [PubMed] [Google Scholar]
Sandler 2003 {published data only}
- Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, petrelli N, Pipas JM, Karp DD, Loprinzi CL, Steinbach G, Schilsky R. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med 2003;348:883-90. [DOI] [PubMed] [Google Scholar]
Steinbach 2000 {published data only}
- Steinbach G, Lynch PM, Phillips RKS, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, Su LK, Levin B. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med 2000;342:1946-52. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Nugent 1993 {published data only}
- Nugent KP, Famer KCR, Spigelman AD, Williams CB, Phillips RKS. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial polyposis. Br J Surg 1993;80:1618-19. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
APACC 2001 {published data only}
- Benamouzig R, Yoon H, Little J, Martin A, Douturier D, Deyra J, Coste T, Chaussade S. APACC, a French prospective study on aspirin efficacy in reducing colorectal adenoma recurrence: design and baseline findings. Eur J Cancer Prev 2001;10:327-35. [DOI] [PubMed] [Google Scholar]
CAPP1 {published data only}
- Burns J, Chapman PD, Bertario L, Bishop DT, Bulow S, Cummings J, Mathers J, Phillips R, Vasen H. The protocol for a European double-blind trial of aspirin and resistant starch in Familial Adenomatous Polyposis: The CAPP Study. E J Cancer 1995;31A:1385-86. [DOI] [PubMed] [Google Scholar]
CAPP2 {published data only}
- Burns J, Chapman PD, Mathers J, Bishop DT, Lynch H. CAPP2: An international controlled trial of aspirin and resistant starch in carriers of HNPCC. J Med Genet 1997;34[Supplement 1]:S53. [Google Scholar]
MDA‐ID‐00109 {published data only}
- Phase II Randomized study of celecoxib with or without eflornithine for the prevention of colorectal cancer in participants with familial adenomatous polyposis of the colorectum (Summary last modified 04/2002). BioMed Central, [Protocol available online. Accessed Nov 2002 at http://www.cancer.gov/search/clinical trials].
NCI‐P00‐0150 {published data only}
- PhaseIIB randomized study of eflornithine (DFMO) and sulindac in the prevention of colorectal carcinoma in patients with previously resected colorectal adenoma (Summary last modified 07/2002). BioMed Central, [Protocol available online. Accessed Nov 2002 at http://www.cancer.gov/search/clinical trials].
ukCAP {published data only}
- Randomized study of aspirin and/or folic acid as prevention of recurrent colorectal adenomas in patients who have had colorectal adenomas in patients who have had colorectal adenomas removed (Summary last modified 04/2002). BioMed Central, [Protocol available online. (Accessed March 2003 at http://www.controlled-trials.com)].
WHI 1998 {published data only}
- Anonymous. Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group.. Controlled Clinical Trials 1998;19(1):61-109. [DOI] [PubMed] [Google Scholar]
Women's Health Study {published data only}
- Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH. B-Carotene supplementation and incidence of cancer and cardiovascular disease: the Women's Health Study. J Natl Cancer Inst 1999;91:2102-6. [DOI] [PubMed] [Google Scholar]
Additional references
ACS 2003 [Computer program]
- Cancer Facts & Figures. [available on-line: accessed on Feb. 2003, http://www.cancer.org]. American Cancer Society. American Cancer Society, Inc, 2003.
Arber 2000
- Arber N. Do NSAIDs prevent colorectal cancer? Canadian Journal of Gastroenterology 2000;14:299-307. [DOI] [PubMed] [Google Scholar]
Atkin 1992
- Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med 1992;326:658-62. [DOI] [PubMed] [Google Scholar]
CCS 2002 [Computer program]
- Canadian Cancer Statistics. [available on-line: Accessed Feb. 2003, http://www.cancer.ca]. Canadian Cancer Society. Canadian Cancer Society, 2002.
Clarke 2003 [Computer program]
- Cochrane Reviewers' Handbook 4.1.6 [updated January 2003]. http://www.cochrane.dk/cochrane/handbook/handbook.htm (accessed 12 May 2003). Clarke M, Oxman AD, editors. John Wiley and Sons, 2003.
Cruz‐Correa 2002
- Cruz-Correa M, Hylinda LM, Romans KE, Booker SV, Giardiello FM. Long-term treatment with sulindac in familial adenomatous polyposis: A prospective cohort study. Gastroenterology 2002;122:641-45. [DOI] [PubMed] [Google Scholar]
CTEP 1998 [Computer program]
- Common toxicity criteria. [available on-line: accessed August 13. 2002, at http//ctep.cancer.gov/reporting.ctc.html]. National Cancer Institute. National Cancer Institute, 1998.
Dannenberg 1999
- Dannenberg AJ, Zakim D. Chemoprevention of colorectal cancer through inhibition of cyclooxygenase-2. Seminars in Oncology 1999;26:499-504. [PubMed] [Google Scholar]
Fitzgerald 2001
- Fitzgerald GA, Patrono C. The Coxibs, selective inhibitors of cyclooxygenase-2. New England Journal of Medicine 2001;345:433-42. [DOI] [PubMed] [Google Scholar]
Gupta 2000
- Gupta RA, Dubois RN. Translational studies on Cox-2 inhibitors in the prevention and treatment of colon cancer. Annals of the New York Academy of Sciences 2000;910:196-206. [DOI] [PubMed] [Google Scholar]
Hardcastle 1996
- Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996;348(9040):1472-7. [DOI] [PubMed] [Google Scholar]
Hawk 1999
- Hawk E, Lubet R, Limburg P. Chemoprevention in hereditary colorectal cancer syndromes. Cancer 1999;86:1731-43. [DOI] [PubMed] [Google Scholar]
Hill 1978
- Hill MJ, Morson BC, Bussey HJR. Aetiology of adenoma - carcinoma sequence in large bowel. The Lancet 1978;1:245-247. [DOI] [PubMed] [Google Scholar]
Keller 2001
- Keller JJ, Offerhaus GJA, Drillenburg P, Caspers E, Musler A, Ristimaki A, et al. Molecular analysis of sulindac-resistant adenomas in familial adenomatous polyposis. Clinical Cancer Research 2001;7:4000-7. [PubMed] [Google Scholar]
Kewenter 1988
- Kewenter J, Bjork S, Haglind E, Smith L, Svanvik J, Ahren C. Screening and rescreening for colorectal cancer. A controlled trial of fecal occult blood testing in 27,700 subjects. Cancer 1988;62:645-51. [DOI] [PubMed] [Google Scholar]
Kronborg 1996
- Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996;348:1467-71. [DOI] [PubMed] [Google Scholar]
Ladabaum 2001
- Ladabaum U, Chopra CL, Huanga G, Scheiman JM, Chernew ME, A. M. F. Aspirin as an adjunct to screening for prevention of sporadic colorectal cancer: A cost-effectiveness analysis. Ann Intern Med 2001;135:769-81. [DOI] [PubMed] [Google Scholar]
Mandel 1993
- Mandel JS, Bond JH, Church TR. Reducing mortality from colorectal cancer by screening for fecal occult blood. New England Journal of Medicine 1993;328(19):1365-71. [DOI] [PubMed] [Google Scholar]
MD Health 1988
- The Steering Committee of the Physicians' Health Group. Preliminary report: findings from the aspirin component of the ongoing physicians' health study. N Engl J Med 1988;318:262-4. [DOI] [PubMed] [Google Scholar]
MMWR 2001
- anonymous. Morbidity and Mortality Weekly Report [Trends in screening for colorectal cancer - United States, 1997 and 1999]. 2001;50:162-6. [PubMed]
Mukherjee 2001
- Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 2001;286:954-59. [DOI] [PubMed] [Google Scholar]
Newcomb 1992
- Newcomb PA, Norfleet RG, Storer BE, Surawicz TS, Marcus PM. Screening sigmoidoscopy and colorectal cancer mortality. Journal of the National Cancer Institute 1992;84:1572-5. [DOI] [PubMed] [Google Scholar]
Niv 1994
- Niv Y, Fraser GM. Adenocarcinoma in the rectal segment in familial polyposis coli is not prevented by sulindac therapy. Gastroenterology 1994;107:854-7. [DOI] [PubMed] [Google Scholar]
Paganini‐Hill 1994
- Paganini-Hill A. Aspiring and the prevention of colorectal cancer: A review of the evidence. Seminars in Surgical Oncology 1994;10:158-164. [DOI] [PubMed] [Google Scholar]
Seow‐Choen 1996
- Seow-Choen F, Pappalardo G, Maiani G, et al. Prospective randomized study of sulindac versus calcium and calciferol for upper gastrointestinal polyps in familial adenomatous polyposis. Br J Surgery 1996;83:1763-6. [DOI] [PubMed] [Google Scholar]
Stryker 1987
- Stryker SJ, Wolff BG, Culp CE, Libbe SD, Ilstrup DM, MacCarty RL. Natural history of untreated colonic polyps. Gastroenterology 1987;93:1009-13. [DOI] [PubMed] [Google Scholar]
Sturmer 1998
- Sturmer T, Glynn RJ, Lee IM, Manson JE, Buring JE, Hennekens CH. Aspirin use and colorectal cancer: Post-trial follow-up datafrom the Physicians' Health Study. Ann Intern Med 1998;128(9):713-20. [DOI] [PubMed] [Google Scholar]
Suleiman 2001
- Suleiman S, Rex DK, Sonnenberg A. Chemoprevention of colorectal cancer by aspirin: A cost-effectiveness analysis. Gastroenterology 2002;122:78-84. [DOI] [PubMed] [Google Scholar]
Theisen 2001
- Theisen C. Chemoprevention: What's in a name? Journal of the National Cancer Institute 2001;93:743. [DOI] [PubMed] [Google Scholar]
Thorson 1994
- Thorson AG, Lynch HT, Smyrk TC. Rectal cancer in FAP patient after sulindac. Lancet 1994;343:180. [DOI] [PubMed] [Google Scholar]
Trends 2001
- Trends in screening for colorectal cancer - United States, 1997 and 1999. Trends in screening for colorectal cancer - United States, 1997 and 1999. Morbidity and Mortality Weekly Report 2001;50:162-66. [PubMed] [Google Scholar]
Turner 1993
- Turner D, Berkel HJ. Nonsteroidal anti-inflammatory drugs for the prevention of colon cancer. Canadian Medical Association Journal 1993;149:595-602. [PMC free article] [PubMed] [Google Scholar]
ukcccr 2002 [Computer program]
- In: http://www.ctu.mrc.ac. A multi-centre double blind randomised controlled trial of Aspirin and / or Folate supplementation for the prevention of recurrent colorectal adenomas. 2002.
USPTF 2002
- anonymous. US Preventive Task Force. Aspirin for the primary prevention of cardiovascular events: Recommendations and rationale. Ann Intern Med 2002;136:161-172. [DOI] [PubMed] [Google Scholar]
Vainio 1997
- Vainio H, Morgan G, Kleihues P. An international evaluation of the cancer-preventive potential of nonsteroidal anti-inflammatory drugs. Cancer Epidemiology, Biomarkers and Prevention 1997;6:749-53. [PubMed] [Google Scholar]
Vernon 1997
- Vernon SW. Participation in colorectal cancer screening: A review. Journal of the National Cancer Institute 1997;89(19):1406-22. [DOI] [PubMed] [Google Scholar]
Winawer 1993
- Winawer SJ, Zauber AG, Ho MN. Prevention of colorectal cancer by colonoscopic polypectomy. The New England Journal of Medicine 1993;329(27):1977-81. [DOI] [PubMed] [Google Scholar]


