Abstract
Background
Campylobacter spp. are zoonotic pathogens, ubiquitous and are found naturally as commensals in livestock from where they can be transmitted to humans directly or through animal products. The genomic diversity and antimicrobial resistance profile of Campylobacter was investigated with a focus on C. jejuni and C. coli in humans and livestock (poultry and cattle) from Nigeria.
Methods
586 human stool samples and 472 faecal samples from livestock were cultured for thermophilic Campylobacter species on modified charcoal cefoperazone deoxycholate agar (mCCDA). Culture in combination with whole genome sequencing identified and confirmed the presence of Campylobacter in humans and animals from the study area. Further analysis of the sequences was performed to determine multilocus sequence types and genetic determinants of antimicrobial resistance to fluoroquinolone, betalactam, tetracycline and macrolide classes of antimicrobials.
Results
From the human stool samples tested, 50 (9%) were positive of which 33 (66%) were C. jejuni, 14 (28%) were C. coli while 3 (6%) were C. hyointestinalis. In livestock, 132 (28%) were positive. Thirty one (7%) were C. jejuni while 101 (21%) were C. coli. Whole genome sequencing and MLST of the isolates revealed a total of 32 sequence types (STs) identified from 47 human isolates while 48 STs were identified in 124 isolates from livestock indicating a population which was overall, genetically diverse with a few more dominant strains. The antimicrobial resistance profiles of the isolates indicated a higher prevalence of resistance in Campylobacter isolated from livestock than in humans. Generally, resistance was greatest for betalactams (42%) closely followed by fluoroquinolones (41%), tetracyclines (15%) and lastly macrolides (2%). Multidrug resistance to three or more antimicrobials was observed in 24 (13%) isolates from humans (n = 1, 4%) and chicken (n = 23, 96%).
Conclusions
This study has further contributed information about the epidemiology, genetic diversity and antimicrobial resistance profile of thermophilic Campylobacter in Nigeria.
Keywords: Campylobacter, Multilocus sequence typing, Clonal complex, Sequence type, Antimicrobial resistance
Background
Campylobacter is considered to be the biggest cause of human bacterial gastroenteritis globally. Diarrhoea, one of the most common outcomes of infection, leads to the mortality of about 1.4 million children yearly, the majority occurring in the developing world where poor hygiene as well as contaminated water and foods are more prevalent [1].
Campylobacter has been isolated from apparently healthy people as well as children having diarrhoea in developing countries and could be due to early exposure with a subsequent development of protective immunity [2, 3].
In humans, Campylobacter jejuni is the main cause of disease though about 10% is by C. coli [4]. The reference Campylobacter jejuni NCTC11168 genome sequence has a circular chromosome of approximately 1.6 Mb with a G + C content of 30.6%. Predicted coding sequences (CDS) number slightly more than 1,600 with an average gene length of 948 bp and more than 90% of the genome coding for proteins. The genome also contains hypervariable sequences in genes which function to modify surface structures and is thought to aid survival strategy of the pathogen [5].
Studies on the emergence, spread, persistence and evolution of Campylobacter species in environmental niches have been carried out using molecular tools to examine genome diversity [6, 7] and how this relates to human and animal health. These molecular and computational tools are used to infer the population structure of the organism and to understand evolutionary relationships within geographical locations and the various hosts [8]. These tools have also enabled researchers to track pathogen movements, to understand their origins and how environmental drivers of disease spread play a role [9, 10]. Due to infrastructural deficits though, these tools, are hardly used to study pathogens in Africa creating a knowledge gap.
Campylobacter induced enteritis is usually self-limiting in many individuals and treatment is generally not recommended. However, in cases of extra-intestinal infections, in immunocompromised patients, the elderly and very young, antimicrobial agents such as erythromycin and ciprofloxacin are the drugs of choice [11]. Studies of human infections caused by resistant and sensitive Campylobacter strains has shown that ill-health is more severe and prolonged in infection with resistant strains [12, 13]. The growing rate at which resistance to these drugs is developing though, has made treatment harder, more so in poorer countries such as Nigeria. Resistance to ciprofloxacin, tetracycline and erythromycin have been observed to be the most common forms of resistance in Campylobacter spp. by various authors mostly employing disc diffusion methods [14].
The level of impact antimicrobial resistant Campylobacter of livestock origin has had on human health is still not fully understood, however, the close association between humans and animals in Nigeria will likely make livestock ownership a major risk factor for pathogen and antimicrobial resistance transfer.
Study of Campylobacter isolated from animals and humans in Nigeria using whole genome sequence analysis is currently unavailable. That makes this study a first of its kind and aims to characterize, determine genetic diversity and antimicrobial resistance in Campylobacter from Nigeria.
Methods
Statement of ethical approval and consent to participate
The study of human participants was approved by the Health Research Ethics Committee, Plateau State Specialist Hospital, Plateau State, Nigeria – NHREC /05/01/20106 (Appendix I). An informed consent was also written and signed by all the human subjects in this study or from parents/guardians of minors.
Isolation of Campylobacter from humans and animals
A total of 568 human stool samples were collected from 248 symptomatic participants described as having diarrhoea, abdominal pain with or without other symptoms such as stomach upsets, fever, vomiting, blood in the stool, who had submitted stool samples to a medical facility as part of their diagnostic requirements. The other 320 samples were collected in schools from asymptomatic participants showing no self-reported clinical symptoms as described above. All the samples were collected in sterile specimen bottles, placed in a cool box with ice, transported and processed within 3 h of collection at the laboratory. Samples were collected from patients aged between 2 months and 89 years.
A simple questionnaire was completed by each of the human participants. The information gathered from each participant through the questionnaire was linked to each isolate, in a spreadsheet and used for subsequent analyses.
Chicken and other poultry faecal samples were collected from live poultry markets, slaughter/processing points and veterinary clinics at post mortem. Three hundred and twelve poultry faecal samples were collected at slaughter (n = 176), at post mortem from veterinary clinics (n = 71), poultry farm (n = 19) and from indigenous Fulani ecotype chicken at rural live bird markets (n = 46). Individual samples were collected immediately after excretion from live birds and directly from the caecum at slaughter or post mortem in sterile bijou bottles, placed in a cooler box and transported to the laboratory for culturing within 3 h post collection.
Cattle faecal samples totalled 160 of which 149 were collected at the abattoir and the remaining eleven at a cattle farm. Samples collected at the abattoir were from animals bought by butchers from herdsmen to be slaughtered and carcasses dressed for subsequent sale to local meat sellers. Most of these animals were not raised on ranches or farms but kept by pastoralist herdsmen who extensively graze them through the practice of transhumance. Samples from slaughtered cattle at the abattoir were taken from the lower intestinal contents. On the farm, freshly voided faeces were collected. All samples were collected in sterile bijou bottles, placed in a cooler box, taken to the laboratory and cultured within 3 h of collection.
The geographical (GPS) locations for the samples collected and number of positive cases in humans and animals from these locations are attached in Appendix II.
A loopful of each sample was directly streaked onto mCCDA (Blood free Campylobacter specific media, modified charcoal cefoperazone deoxycholate agar (Oxoid, UK) containing a selective supplement (SR155E, Oxoid, UK)), immediately on receipt at the laboratory. Plates were incubated in anaerobic jars under micro aerobic conditions of 5% oxygen, 10% carbon dioxide and 85% nitrogen using gas generating packs (Campy-Gen, Oxoid, UK) at 40 °C for 2–4 days. Presumptive colonies were sub-cultured and single colony re-plates made onto blood agar plates.
Campylobacter colony growth was identified as greyish, flat and moist, with a tendency to spread and having a metallic sheen. Single colony re-plates, from presumptive positive colonies from each plate, were stored in cryovials containing nutrient broth with 20% (v/v) glycerol at -80 °C.
Whole Genome sequencing and assembly
From each of the isolates, genomic DNA was extracted using a Wizard® Genomic DNA Purification Kit (Promega, USA) according to the manufacturers’ protocol. The DNA extracts went through a genome prep similar to Sanger Institutes Illumina Truseq DNA sample preparation protocol (https://support.illumina.com/downloads/truseq_dna_sample_preparation_guide). Briefly, this involved preparing libraries for unidirectional sequencing from 5 µg of genomic DNA from each isolate. Adaptor sequences were added to the ends of the DNA fragments which match the surface bound amplification primers on the flow cells. Sequencing was done on an Illumina HiSeq 2000 analyser with 100 bp paired end runs. Each isolate produced about 400 Mb of 100 bp raw reads which were de novo assembled using the Velvet assembler [15].
Multilocus sequence typing
Assembled sequences were uploaded into BIGSdb (Bacterial Isolate Genome Sequence Database) at http://pubmlst.org/software/database/bigsdb/ [16]. This software was developed by the University of Oxford and is able to store and analyse sequence information for bacterial species and was used to identify and define loci along the genome from each isolate genome. These identified loci were then organised using an MLST scheme within PubMLST (http://pubmlst.org/campylobacter). Allele number designation to the seven housekeeping loci (aspA, glnA, gltA, glyA, pgm, tkt, uncA), allowed assignment of sequence types (STs) to each isolate [17]. Sequences of new alleles for each locus were submitted to the curators for assignment of new allele numbers. Isolates were then separated into STs and based on clonal relationships, grouped into clonal complexes (CC).
Genome diversity
Genome diversity was investigated using eBURST, Simpson’s Diversity Index, Nei’s Genetic Distance and Rarefaction analysis. Genetic diversity could not be measured or compared for the isolates from cattle as all were the same ST and CC so were excluded from the analysis above except for eBURST.
7-locus allelic profiles of all the isolates in this study were uploaded and analysed in eBURST to determine relatedness and explore diversity within the isolates. The whole input population was analysed as a single group and visualized as the number and sizes of the linked STs in eBURST (http://eburst.mlst.net). The minimum number of identical loci for a group definition was six while the minimum single locus variant (SLV) count for each group definition was three. Bootstrapping with resampling (1000 times) was conducted to provide statistical confidence in ST assignment. The eBURST algorithm subdivides MLST datasets into related groups of STs or CCs and then determines the best arrangement of isolates in the group to the predicted founder [18].
To quantify the genetic diversity within the sets of isolates, Simpson’s Diversity Index was used. This considers the relative frequency of STs in a specific source [19]. A diversity index of 0 indicates that all the isolates are genetically identical while a value of 1 indicates all the isolates are different. To generate confidence intervals, the isolates were bootstrap resampled with replacement 10,000 times using Pop Tools (add-in for Microsoft Excel) allowing the Simpson’s Diversity Index and 95% confidence interval (CI) to be calculated.
To investigate the genetic distance between Campylobacter isolates in different pairs of sources, Nei’s genetic distance was used [20]. Nei’s genetic distance compares genetic relatedness between two populations and was calculated using 7-locus MLST data with randomisation and replacement 10,000 times in Microsoft Excel to calculate confidence intervals and P-values. A value of 1 indicates an absence of any similar genotype between the pair while a value of 0 shows that both populations are identical.
To evaluate whether the maximum number of genotypes from a particular animal source had been sampled, rarefaction analysis was employed. Rarefaction is a resampling technique that assesses the proportion of the genotypes from a given source that have been sampled. The results are displayed as a curve which reaches a plateau if all the genotypes have been sampled or the curve keeps increasing where the genotypes in the population remain under sampled [21]. The slope of the curve is also an indication of the strength of diversity within the source with a steep curve indicating a greater diversity than a gradual curve.
In silico identification of genetic determinants of antimicrobial resistance
Assembled sequences which had been uploaded into BIGSdb, as detailed above, were screened using the autotagger functionality within BIGSdb. Loci were defined by nucleotide sequences with automatic recognition of the query type and the appropriate BLAST algorithm called. This was used to identify loci responsible for fluoroquinolone resistance gyrA (PubMLST-Camp 0950), macrolide resistance 23S rRNA (PubMLST-23S rRNA), tetracycline resistance (PubMLST-Camp1698) and beta-lactam resistance (PubMLST-CAMP0265). Resistance was inferred by the presence of one or more alleles at these loci.
Statistical analysis
Statistical analysis was performed to determine odds ratio (OR), 95% confidence interval and P-values. The OR measures association between the presence or absence of two properties with confidence intervals indicating the spread of variation associated with the value. Comparisons using OR selected one character as the reference. Statistical significance was assumed if the confidence interval for a comparison did not overlap the OR of 1 and P ≤ 0.05.
Results
Distribution of Campylobacter in humans
From the 50 human isolates recovered, 30 were C. jejuni (60%), 17 C. coli (34%) and three C. hyointestinalis (6%). Only four of the isolates were from patients at health centres while 46 were from asymptomatic carriers in various schools.
The significance of the human characteristics age, gender and clinical manifestation, in relation to Campylobacter carriage, was calculated (Table 1). Campylobacter was more likely to be isolated from stool samples of asymptomatic individuals in relation to symptomatic ones. Based on age, individuals aged ≥ 15 were significantly less likely to be infected than individuals < 5, with an odds ratio < 1 and P-value < 0.05. Age group 6–14 also had an odds ratio < 1, although this effect only approached significance (P = 0.1). Therefore, isolation of Campylobacter tends to decrease with age. Calculations based on gender suggest it is not statistically significant for Campylobacter infections.
Table 1.
Campylobacter jejuni and Campylobacter coli from human samples by age, health status and gender
| Category | No. Samples | Positive | Negative | OR (95% CI) | P-value | |
|---|---|---|---|---|---|---|
| All | 568 | 47 | 521 | |||
| Age | 0 - 5 | 293 | 39 | 253 | 1 | |
| 6-14 | 96 | 7 | 89 | 0.5 (0.2, 1.15) | 0.1 | |
| ≥ 15 | 179 | 1 | 178 | 0.04 (0.005, 0.26) | 0.001 | |
| Health Status | Symptomatic | 248 | 4 | 244 | 1 | |
| Asymptomatic | 320 | 43 | 273 | 9.6 (3.40,27.16) | < 0.0001 | |
| Gender | Male | 268 | 21 | 247 | 1 | |
| Female | 300 | 26 | 270 | 1.13(0.62,2.06) | 0.7 |
Odds ratio and p-values have also been calculated
Distribution of Campylobacter in livestock
A total of 312 poultry samples were analysed. Faecal samples were collected and cultured from chicken (layers, broilers, Nigeria indigenous Fulani ecotype) and other poultry (duck, geese, quails). 124 (40%) samples were confirmed positive for Campylobacter, and these were identified at different frequencies from all the poultry types. Species specific PCR revealed only C. jejuni (25%) and C. coli (75%) (Table 2).
Table 2.
Campylobacter spp. isolation from poultry and cattle
| Category | No. Samples | No. Positive Samples | |||
|---|---|---|---|---|---|
| C. jejuni | C. coli | Total (%) | |||
| Poultry | Layers | 155 | 6 | 63 | 69 (45) |
| Broilers | 96 | 7 | 22 | 29 (30) | |
| Indigenous Breeds | 47 | 15 | 6 | 21 (45) | |
| Other | 14 | 3 | 2 | 5 (36) | |
| Total | 312 | 31 | 93 | 124 (40) | |
| Cattle | Abattoir | 149 | 0 | 8 | 8 (5) |
| Farm | 11 | 0 | |||
| Total | 160 | 0 | |||
Cattle faecal samples were collected from the abattoir (93%) and from a farm (7%). Of the 160 cattle faecal samples analysed, 8 (5%) were positive for Campylobacter. Speciation of the isolates through multiplex PCR revealed all of them to be C. coli (Table 2). All the positive samples were sourced from the abattoir.
Genomic diversity of Campylobacter strains in humans and livestock
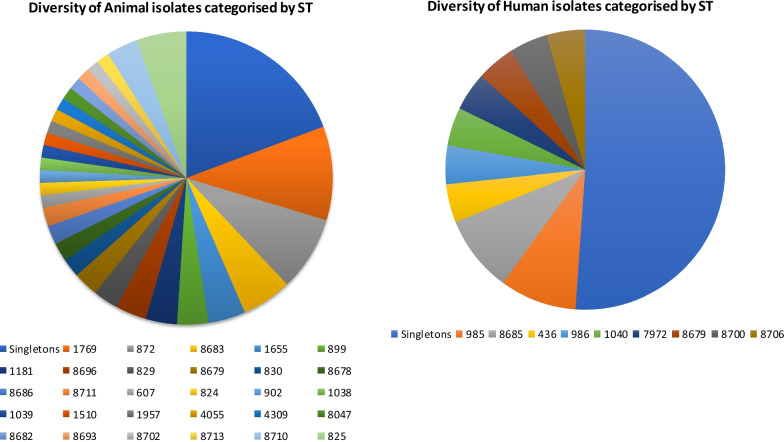
A total of 80 STs were identified from 132 Campylobacter isolates from animals and 47 isolates from human sources (Fig. 1).
Fig. 1.
Sequence Type diversity of isolates in samples from animals and humans
Thirty seven STs were identified from 108 (60%) isolates and had been previously described in PubMLST. The remaining 43 STs from 71 (40%) of the isolates were previously undescribed and these were uploaded into the PubMLST database, curated and assigned new ST numbers (Table 3).
Table 3.
Novel STs, MLST allelic profiles and host source
| Source | aspA | glnA | gltA | glyA | pgm | tkt | uncA | ST (MLST) | clonal_complex (MLST) |
|---|---|---|---|---|---|---|---|---|---|
| Human stool | 273 | 39 | 516 | 82 | 248 | 43 | 17 | 8679 | ST-828 complex |
| Human stool | 8 | 604 | 5 | 53 | 11 | 3 | 1 | 8684 | ST-607 complex |
| Human stool | 33 | 39 | 30 | 82 | 189 | 678 | 17 | 8685 | ST-828 complex |
| Human stool | 20 | 164 | 24 | 604 | 27 | 19 | 555 | 8689 | UA |
| Human stool | 63 | 164 | 24 | 188 | 878 | 19 | 557 | 8690 | UA |
| Human stool | 28 | 34 | 27 | 33 | 223 | 428 | 213 | 8691 | UA |
| Human stool | 63 | 164 | 24 | 722 | 780 | 19 | 18 | 8694 | UA |
| Human stool | 63 | 164 | 557 | 21 | 780 | 266 | 18 | 8695 | UA |
| Human stool | 461 | 34 | 559 | 723 | 879 | 682 | 33 | 8698 | UA |
| Human stool | 131 | 39 | 150 | 79 | 188 | 534 | 17 | 8700 | UA |
| Human stool | 1 | 2 | 42 | 4 | 90 | 9 | 8 | 8703 | ST-42 complex |
| Human stool | 103 | 195 | 103 | 140 | 188 | 368 | 79 | 8704 | ST-1150 complex |
| Human stool | 33 | 38 | 30 | 79 | 880 | 64 | 17 | 8705 | UA |
| Human stool | 28 | 648 | 27 | 33 | 697 | 61 | 303 | 8706 | UA |
| Human stool | 33 | 39 | 150 | 174 | 104 | 35 | 17 | 8707 | ST-828 complex |
| Human stool | 1 | 2 | 42 | 4 | 90 | 683 | 8 | 8708 | ST-362 complex |
| Chicken | 33 | 603 | 65 | 82 | 113 | 47 | 17 | 8677 | UA |
| Chicken | 131 | 38 | 150 | 79 | 805 | 534 | 17 | 8678 | UA |
| Chicken | 273 | 39 | 516 | 82 | 248 | 43 | 17 | 8679 | ST-828 complex |
| Chicken | 22 | 61 | 4 | 64 | 74 | 3 | 23 | 8680 | ST-1034 complex |
| Chicken | 22 | 364 | 4 | 64 | 74 | 3 | 23 | 8681 | ST-1034 complex |
| Chicken | 33 | 39 | 66 | 82 | 104 | 47 | 41 | 8682 | ST-828 complex |
| Chicken | 33 | 39 | 30 | 82 | 678 | 43 | 17 | 8683 | ST-828 complex |
| Chicken | 8 | 604 | 5 | 53 | 11 | 3 | 1 | 8684 | ST-607 complex |
| Chicken | 33 | 39 | 30 | 115 | 104 | 47 | 17 | 8686 | ST-828 complex |
| Chicken | 103 | 110 | 103 | 350 | 188 | 368 | 79 | 8688 | ST-1150 complex |
| Chicken | 131 | 39 | 30 | 79 | 805 | 534 | 17 | 8692 | UA |
| Chicken | 435 | 39 | 30 | 79 | 805 | 534 | 17 | 8693 | UA |
| Chicken | 273 | 39 | 30 | 82 | 248 | 35 | 17 | 8696 | ST-828 complex |
| Chicken | 24 | 30 | 515 | 2 | 89 | 59 | 6 | 8697 | ST-460 complex |
| Chicken | 436 | 39 | 30 | 79 | 805 | 534 | 17 | 8699 | UA |
| Chicken | 7 | 27 | 1 | 10 | 11 | 3 | 6 | 8701 | ST-574 complex |
| Chicken | 273 | 39 | 30 | 82 | 188 | 35 | 17 | 8702 | ST-828 complex |
| Chicken | 2 | 4 | 1 | 25 | 11 | 358 | 5 | 8709 | ST-48 complex |
| Chicken | 33 | 38 | 150 | 82 | 877 | 43 | 41 | 8711 | UA |
| Chicken | 103 | 195 | 103 | 140 | 188 | 368 | 17 | 8713 | ST-1150 complex |
| Chicken | 8 | 2 | 5 | 451 | 11 | 5 | 1 | 8716 | ST-607 complex |
UA unassigned
The five most common STs overall were ST-1769 (n = 15), ST-872 (n = 12), ST-8683 (n = 8), ST-825 (n = 8) and ST-1655 (n = 7). Most of the STs (n = 144) clustered into eighteen different clonal complexes, however, 35 (20%) of the isolates could not be assigned to a CC. This included nineteen isolates from human and sixteen from chicken sources. The most common clonal complex and by far the largest group, was ST-828 which had 99 isolates (55% of the total isolates). This was followed by the ST-607 complex which comprised of 9 isolates (5%). By diversity of STs within the two species, 25 STs were identified from 103 C. coli isolates and 30 STs in 76 C. jejuni isolates.
Interestingly, nine STs were common to both humans and chicken. These were ST-8679, ST-353, ST-523, ST-607, ST-824, ST-899, ST-1181, ST-1655 and ST-8684. These clustered into five CCs with CC-828 having the highest number of STs (n = 4).
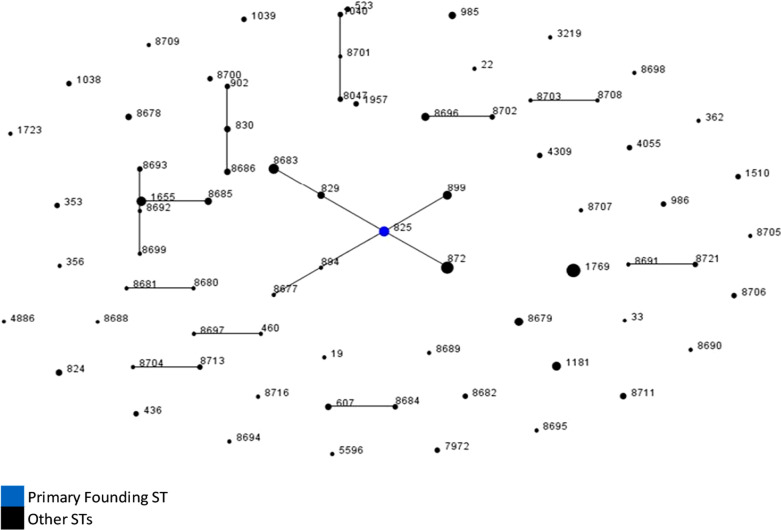
Population relationship
The phylogenetic model eBURST showed relatedness between strains from Nigeria at the 7-locus MLST level and revealed twelve groups of linked STs and 40 singletons. Most of the STs though, were unlinked and were not SLVs of other STs in the population (Fig. 2).
Fig. 2.
A population snapshot of human, chicken and cattle strains using eBURST. ** STs are indicated by dots which are linked by a line if they are SLVs while the primary founder by a blue dot. Relative abundance of the STs is reflected by the area of the dot
Simpson’s diversity index was determined using 7-locus MLST data for chicken and human isolates. Diversity was based on the variation in the frequency of individual STs in the total population within each source (Fig. 3).
Fig. 3.
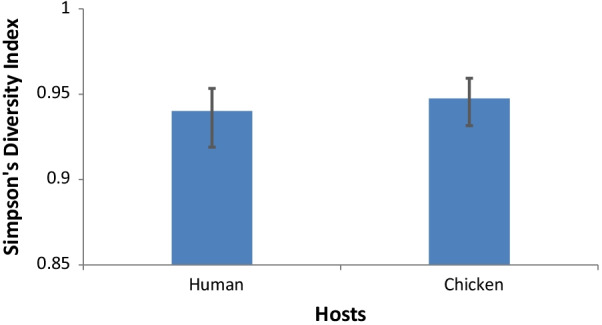
Simpson’s Diversity Index of Campylobacter STs in human and chicken
The diversity index for both human and chicken isolates was only marginally different at 0.940 and 0.947. These values which are close to 1 would suggest a high genetic diversity within the sets of isolates.
Nei genetic distance between isolates from humans and chickens was calculated at the level of 7-locus MLST where a value of 0 indicates identical genotypes between the pair, and a value of 1 shows an absence of similarity (Fig. 4).
Fig. 4.
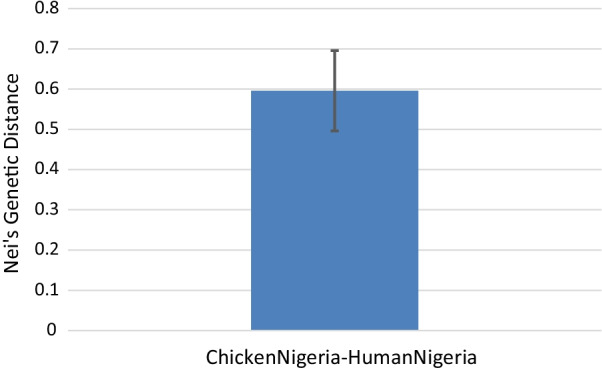
Pairwise Nei’s genetic distance between human and chicken isolates calculated at the 7-locus MLST level
The computed Nei’s genetic distance between chicken and human strains was 0.596 (CI = 0.65, 0.54) and indicates some genetic overlap between human and chicken Campylobacter strains.
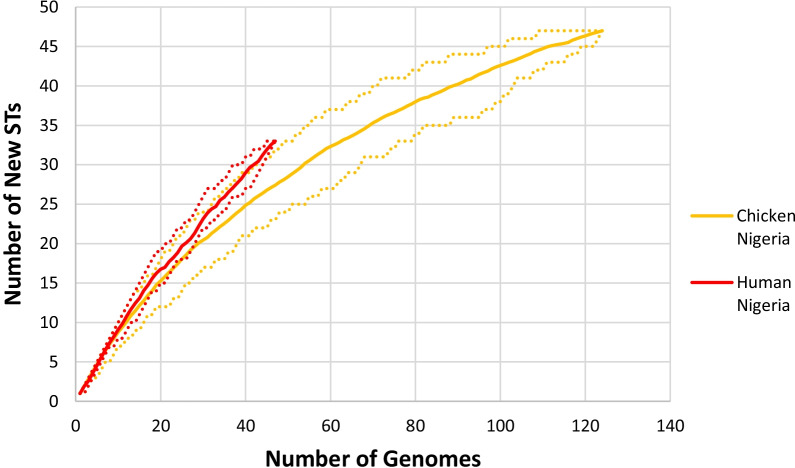
Diversity within hosts and range of sampling was also assessed by rarefaction analysis using the ST of isolates from humans and chicken (Fig. 5).
Fig. 5.
Rarefaction analysis of human and chicken strains. *Dashed lines indicate 95% bootstrapped CI
Rarefaction curves of ST accumulation in humans and chicken revealed similar steepness in the curves implying that all the sources had very diverse strain sets. None of the rarefaction curves had plateaued indicating that the detection of STs from individual hosts had not yet been saturated. The rarefaction curve in humans though revealed a somewhat higher number of new STs as indicated by the slightly steeper curve.
Resistance to antibiotics
Identification of antibiotic resistance determinants
Resistance determinants for beta-lactam, fluoroquinolone, tetracycline and macrolide classes of antimicrobials in Campylobacter are varied and those identified in this study have been listed in Table 4. OXA beta-lactamases, which confer resistance to beta-lactam antimicrobials, were the most frequently occurring resistance determinants and were identified in 68% (122/179) of the Campylobacter isolates in this study. Within the group, OXA-193 (49%, n = 87) was the most prevalent OXA beta-lactamase detected while nine other OXA beta-lactamases (n = 35) were detected but at much lower frequencies. Four novel OXA beta-lactams were also detected and have been highlighted in Table 4. Fluoroquinolone resistance was the next most prevalent resistance and was primarily mediated by a mutation at T86I (66%, n = 119). This mutation was the single most frequently occurring resistance determinant in the study. Also observed in the same group was a double mutation, P104S/T86I which occurred in two isolates. For resistance to tetracycline, two determinants were identified. The first, tetO, was more common and occurred in 23% (n = 42) of the isolates, followed by the chimeric tet(O/32/O) gene which occurred in 1% (n = 2) of the study isolates. Resistance to macrolides was the least occurring, observed in only 2.8% (n = 5) of the isolates and was determined by a change in the 23S rRNA gene, specifically A2075G.
Table 4.
Antibiotic resistance determinants identified from the study isolates
| Betalactams | Fluoroquinolones | Tetracyclines | Macrolides | ||||
|---|---|---|---|---|---|---|---|
| Oxacillinase | Frequency (%) | gyrA | Frequency (%) | Tet gene | Frequency (%) | 23S rRNA | Frequency (%) |
| Oxa-193 | 87 (49) | T86I | 119 (66) | tet(O) | 42 (23) | A2075G | 5 (2.8) |
| Oxa-449 | 6 (3) | P104S, T86I | 2 (1) | tet(O/32/O) | 2 (1) | ||
| Oxa-460 | 3 (2) | ||||||
| Oxa-184 | 2 (1) | ||||||
| Oxa-61 | 2 (1) | ||||||
| Oxa-461 | 1 (0.6) | ||||||
| Oxa-783* | 9 (5) | ||||||
| Oxa-784* | 9 (5) | ||||||
| Oxa-785* | 2 (1) | ||||||
| Oxa-786* | 1 (0.6) | ||||||
| Total (%) | 122 (68) | 121 (67.6) | 44 (25) | 5 (2.8) | |||
*Previously unidentified OXA beta-lactams
Resistance to all the antibiotics was found in only one isolate, 23 were resistant for up to three antibiotics, 60 to two, 168 to at least one while 24 did not have any resistance characters and were assumed to be susceptible to the four antibiotics. Multi-resistance was observed in only one human isolate while the rest were from chicken.
Discussion
Human health in developing countries is inseparably associated with the livestock they own with these animals serving as a source of nutrition, labour and extra income. An outcome of this is increased direct contact between humans and livestock or their faeces making it likely for human exposure to zoonotic disease causing pathogens such as Campylobacter.
This study showed significant correlation between age group and Campylobacter isolation. Children are more likely to be infected with Campylobacter because of exposure to faecal contaminated playgrounds [22, 23] and picking items from the ground to put in their mouths. This agrees with age related studies in Nigeria which identified Campylobacter to be more common in children [24–26] than adults. A significant association was also determined between Campylobacter infection and asymptomatic individuals (OR = 10.5, CI = 3.73, 29.57) who were again mostly children. The likely reasons for the high levels of asymptomatic cases could be increased immunity due to constant exposure to the pathogen through contact. Primary episodes of campylobacteriosis in naïve individuals has also been shown to prevent a subsequent more serious bloody diarrhoea from occurring, and over time the disease totally [27]. However, a study in Malawi comparing the epidemiology of Campylobacter infections within the context of a resource poor setting, reported a lower detection rate for Campylobacter in non- diarrhoeic children (14%, 71/507) and a higher rate (21%, 415/1941) in children with diarrhoea [28].
The strain diversity both within and between the sources of Campylobacter strains in Nigeria revealed a genetically diverse population. A total of 179 isolates were typed with 80 different STs identified. This diversity and relatively high percentage of new STs reflects a heterogenous Campylobacter population in Nigeria which also points to the limited number of studies on indigenous Campylobacter in Nigeria [26] with only one previous molecular based study uploading MLST sequences into the PubMLST database [29].
The two most frequent human STs were ST-985 and ST-8685 together totalling 17% of the Campylobacter population. ST-8685 belongs to CC-828, was unique to this study and could be an ST which has been geographically restricted to Nigeria or has evolved over time to sustain itself within the local environment. On the other hand, ST-985 (CC-403) was not novel to this study and was reported in humans from the Netherlands, Luxembourg, Bangladesh and the UK (pubmlst.org/campylobacter/).
MLST typing of the 124 chicken isolates revealed 47 STs assigned to 16 CCs with 21 STs previously undescribed while 16 STs were unassigned to any CC. Poultry are considered a natural reservoir of Campylobacter spp. with studies indicating diverse ST populations in broiler flocks at slaughter [30, 31]. These strains may be circulating within chicken populations or may originate from other sources such as the farm environment, wild animals or other livestock.
A dominance of C. coli species (75%) was observed from Campylobacter isolates in chicken and which were mostly CC-828 (85%, n = 81). Similar findings where CC-828 was the dominant CC among C. coli isolates, have been shown [32]. However, the prevalence of C. coli in chicken is higher in this study than reported elsewhere [33, 34].
The two dominant STs in chicken from this study were ST-1769 (n = 12) and ST-872 (n = 10) both of which are C. coli, CC-828 strains. This type of population structure where a few genotypes are predominant within the group seems to occur regularly in chicken flocks [35, 36] and could be due to geography or host specific factors [37]. Isolation of ST-1769 has been previously documented from chicken meat in a study from Croatia [38] and in humans and poultry from other countries like Botswana, USA, Germany and Portugal (https://pubmlst.org/campylobacter/). The over-abundance of ST-1769 strain in chickens in Nigeria could suggest a natural selection and maintenance of phenotypic variations that confer an advantage to survive within its host [39, 40].
A total of 22 C. jejuni STs were identified in chicken. None of the STs had a frequency of more than two isolates revealing a high diversity of the C. jejuni population in chicken. Only a little evidence exists of a phylogenetic relationship between the numerous clonal complexes in the C. jejuni population structure [41, 42] making the species highly varied with many STs identified in chicken [43].
In cattle, the 5% incidence of Campylobacter isolation in this study is most probably an under estimation of the colonisation in the general cattle population in the state as an earlier study had detected an 18.5% prevalence rate [44]. Also, all the 8 isolates from this source turned out to be from the same ST, ST-825 and CC-828. Previous studies of cattle from Plateau State yielded a greater number of STs and CC’s [29].
Nine Campylobacter STs from this study’s’ isolates were found in both human and chicken sources. The ability of Campylobacter to colonise animal reservoirs and subsequently infect humans has been shown in STs such as ST-21 which have been described as generalist because they show high genetic flexibility and express diverse fitness factors enabling adaptation in changing host environments [45]. These genetic characteristics has enabled certain livestock specific Campylobacter strains to infect and cause disease in humans. Genetic relatedness of Campylobacter within the same country between humans and chicken has previously been reported in the UK with the assertion that chicken is the main source for human campylobacteriosis [46, 47].
The extent of diversity from the two main sources, was also seen in the analysis using eBURST. Only one founding genotype, ST-825, was identified within the study population. Most of the other STs were individually unlinked and not single locus variants of the other STs. This level of diversity is possible in instances where high rates of recombination decrease the degree of clonality [48]. Humans also harboured more diversity than chickens as seen by the slope of the curve in the rarefaction analysis and is similar to what was discovered by Gormley et al. (2008) in their analysis of Campylobacter in Scotland [49]. Quantifying genetic diversity within both hosts (chickens and humans) indicated a lack of significant difference in the variety of infective strains. This could be attributed to exposure to a large diversity of infecting strains from the environment through foraging for food in the case of chickens or drinking contaminated water and outdoor activities in humans [50–52]. Constant interaction between humans and chicken within the same environment in Nigeria implies that either chickens contribute in part to human infection or both humans and chickens have at least one similar infection source. Nei’s genetic distance supports the assumption that chickens are an important source of infection for humans in Nigeria, however, other sources are likely to also be important. This is also a view shared by other researchers [50].
Antimicrobial resistance in Campylobacter isolated from livestock, especially chicken, is of significant public health concern as poultry (indigenous Fulani ecotypes, broilers and layers) are kept by many households at a subsistence or commercial level [53]. Resistance to similar antimicrobials used for human therapy makes it an even more important problem as human contact with faecal droppings from extensively reared chicken, cattle and other livestock increase the risk of infection from these resistant strains and a possible transfer of resistance to humans. Resistance to betalactams, fluoroquinolones and tetracyclines were observed in this study both in livestock and humans with resistance in macrolides found solely in livestock.
Resistance to beta-lactams (42%) and fluoroquinolones (41%) were overrepresented within the study dataset. Beta-lactam resistance is mediated majorly by OXA-61 beta-lactamases and other beta-lactamases [54]. Beta-lactams such as ampicillin are hydrolysed by beta-lactamase enzymes which are widely distributed in Campylobacter [55]. This was shown in a UK survey of poultry associated Campylobacter where 52% were ampicillin resistant of which 92% possessed blaOXA-61 [54]. The high rate of this resistance in Nigeria may be due to widespread use of betalactams such as ampicillin in humans and livestock which has exerted selective pressure on Campylobacter to acquire betalactamases [56, 57].
Resistance to fluoroquinolones occurs due to a mutation in the quinolone resistance determining region on gyrA [58] and apart from two isolates which had double mutations (T86I and P104S), mutation of gyrA was limited to T86I which confirms this as the most common gyrA mutation. Other studies in Botswana and Portugal also detected T86I mutation in gyrA as the most common type of mutation [59, 60]. It is established that treatment in poultry with fluoroquinolones leads to resistant Campylobacter phenotypes [61] and of interest, fluoroquinolone resistant Campylobacter do not display a fitness cost and can compete favourably with susceptible Campylobacter in the absence of the antibiotic [62].
Tetracycline was the most imported class of antimicrobial to Nigeria in 2014- 2015 [63]. Resistance to this antimicrobial in this study though, was the third most common with an overall detection rate of 23% (44/192) and was lower than what was reported in Spain (72%) [64] and Canada (50%) [65]. Tetracycline resistance is mediated primarily by tetO, a ribosomal protection protein transferable as a plasmid encoded gene [66] or is chromosomally located [67]. Other tetracycline resistance genes exist and are able to confer resistance [68]. TetO gene was the most common identified in the study isolates occurring in all the tetracycline resistant isolates except two which had a mosaic derivative tetO/32/O gene. Mosaic tetracycline resistance genes have been identified previously occurring in human and animal faecal samples [69] and in other bacteria [70, 71].
Mutations in the 23S rRNA gene which confers resistance to macrolides was observed in only five isolates (2%) and was the least common resistance. Low detection of macrolide resistance has been reported in Campylobacter from humans and chicken in Botswana [59] and Poland [72] though higher numbers were detected in an Italian study where the A2075G 23S rRNA mutations were observed in 49 (25%) of Campylobacter isolates [73]. Interestingly, it was observed that all the isolates that were resistant to macrolides were also multidrug resistant to tetracyclines and fluoroquinolones.
Multidrug resistance, defined as resistance to three or more antimicrobials [74], was detected in 24 (13%) of the study isolates. Most of the multidrug resistance was to beta-lactam, fluoroquinolone and tetracycline class of antibiotics and may reflect the indiscriminate use of these classes of antibiotics in humans and livestock. A similar finding was observed in China where a study reflecting on the overuse of different antimicrobials in the poultry industry, identified up to 90% prevalence rates of multidrug resistance in Campylobacter species [75].
Conclusion
A thorough understanding of Campylobacter, its epidemiology, infection in humans, reservoir status of animal hosts and risk factors for human infection remains limited in the developing world and especially Nigeria. This is because little research has been carried out in this setting to ascertain the pathogenesis and socio-economic burden of the infection.
The use of molecular typing techniques such as MLST and whole genome sequencing to detect genetic traits and characterize isolates in this study proved to be extremely useful in determining the abundant diversity in circulating Campylobacter spp. The different genotypes within human and livestock sources in Nigeria was demonstrated and can prove to be very useful in future studies to further understand the pathogen.
Resistance to antimicrobials has been shown both in humans and animals although in humans, treatment for Campylobacter enteritis remains controversial except for the immunocompromised and during pregnancy. Severe disease in the undernourished in developing countries though is common and for these group of individuals, options for susceptible oral therapies is diminishing.
In Nigeria, Campylobacter infections in humans and animals is poorly understood and more research needs to be done to understand the pathogen. This knowledge would provide links to human infective sources where targeted interventions can be applied to reduce human disease.
Acknowledgements
Staff of Biotechnology Centre, NVRI, technicians and staff of hospitals, clinics and schools where human stool samples were collected and livestock farmers for their cooperation and input.
Abbreviations
- bp
Base Pair
- CC
Clonal Complex
- DNA
Deoxyribonucleic acid
- mCCDA
Modified Charcoal Cefoperazone Deoxycholate Agar
- MLST
Multilocus Sequence Type
- PCR
Polymerase Chain Reaction
- rRNA
Ribosomal Ribonucleic acid
- SLV
Single Locus Variant
- Spp
Species
- ST
Sequence Type
Appendix
Appendix I. Ethical clearance for human sampling
Appendix II. Geographical relationships between positive cases and sampling locations
| Humans | Animals | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total sampled | No. +ve | C. jejuni | C. coli | Total sampled | No. +ve | C. jejuni | C.coli | ||
| Jos North | |||||||||
| 9° 54' 50.364'' N | School | ||||||||
| 8° 53' 22.524'' E | Health Centers | 73 | 0 | 0 | 0 | ||||
| Markets | 149 | 8 | 0 | 8 | |||||
| Farms | |||||||||
| Jos South | |||||||||
| 9° 54' 50.364'' N | School | 125 | 18 | 9 | 9 | ||||
| 8° 53' 22.524'' E | Health Centers | 194 | 4 | 2 | 2 | ||||
| Markets | 293 | 99 | 12 | 87 | |||||
| Farms | 42 | 12 | 4 | 8 | |||||
| Pankshin | |||||||||
| 9° 19' 42.348'' N | School | 42 | 7 | 3 | 4 | ||||
| 9° 25' 17.724'' E | Health Centers | ||||||||
| Markets | 27 | 13 | 11 | 2 | |||||
| Farms | |||||||||
| Barkin Ladi | |||||||||
| 9° 32' 27.204'' N | School | 69 | 11 | 11 | 0 | ||||
| 8° 53' 37.788'' E | Health Centers | ||||||||
| Markets | |||||||||
| Farms | |||||||||
| Bokkos | |||||||||
| 9° 13' 42.727'' N | School | 34 | 5 | 4 | 1 | ||||
| 8° 53' 41.288'' E | Health Centers | ||||||||
| Markets | 20 | 8 | 4 | 4 | |||||
| Farms | |||||||||
Authors' contributions
BJA was involved in conceptualization, formal analysis, investigation writing original draft and writing review and editing. SN and KJF were involved in conceptualization, resources and supervision. LB was involved in data curation and formal analysis. RM and MM were involved in project administration, methodology and validation. All authors read and approved the final manuscript.
Funding
This work was supported through a studentship by an Elphinstone Scholarship from the University of Aberdeen, Scotland.
Availability of data and materials
The datasets used and/or analysed during the current study are stored at https://pubmlst.org/organisms/campylobacter-jejunicoli, and can be made available from the corresponding author on reasonable request.
Declarations
Ethical approval and consent to participate
The study of human participants was approved by the Health Research Ethics Committee, Plateau State Specialist Hospital, Plateau State, Nigeria. Reference number: PSSH/ADM/ETH.CO/2015/004 (Appendix I).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Benshak J. Audu, Email: benshakng@gmail.com
Strachan Norval, Email: n.strachan@abdn.ac.uk.
Lopes Bruno, Email: brunoldlopez@gmail.com.
Ramjee Meenakshi, Email: m.ramjee@abdn.ac.uk.
Macrae Marion, Email: m.macrae@abdn.ac.uk.
Ken J. Forbes, Email: k.forbes@abdn.ac.uk
References
- 1.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 2.Baqar S, Bourgeois AL, Schultheiss PJ, Walker RI, Rollins DM, Haberberger RL, et al. Safety and immunogenicity of a prototype oral whole-cell killed Campylobacter vaccine administered with a mucosal adjuvant in non-human primates. Vaccine. 1995;13(1):22–28. doi: 10.1016/0264-410x(95)80006-y. [DOI] [PubMed] [Google Scholar]
- 3.Rao MR, Naficy AB, Savarino SJ, Abu-Elyazeed R, Wierzba TF, Peruski LF, et al. Pathogenicity and convalescent excretion of Campylobacter in rural Egyptian children. Am J Epidemiol. 2001;154(2):166–173. doi: 10.1093/aje/154.2.166. [DOI] [PubMed] [Google Scholar]
- 4.Gillespie IA, O'Brien SJ, Frost JA, Adak GK, Horby P, Swan AV, et al. A case-case comparison of Campylobacter coli and Campylobacter jejuni infection: a tool for generating hypotheses. Emerg Infect Dis. 2002;8(9):937–942. doi: 10.3201/eid0809.10.3201/eid0809.010187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parkhill J, Wren BW, Mungall K, Ketley JM, Churcher C, Basham D, et al. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403(6770):665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 6.Frasao BDS, Marin VA, Conte-Junior CA. Molecular detection, typing, and quantification of Campylobacter spp in foods of animal origin. Comprehen Rev Food Sci Food Safety. 2017;16(4):721–734. doi: 10.1111/1541-4337.12274. [DOI] [PubMed] [Google Scholar]
- 7.El-Adawy H, Hotzel H, Tomaso H, Neubauer H, Taboada EN, Ehricht R, et al. Detection of genetic diversity in Campylobacter jejuni isolated from a commercial turkey flock using flaA typing, MLST analysis and microarray assay. PLoS ONE. 2013;8(2):e51582. doi: 10.1371/journal.pone.0051582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohan V, Stevenson M, Marshall J, Fearnhead P, Holland BR, Hotter G, et al. Campylobacter jejuni colonization and population structure in urban populations of ducks and starlings in New Zealand. MicrobiologyOpen. 2013;2(4):659–673. doi: 10.1002/mbo3.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boccia S, Pasquarella C, Colotto M, Barchitta M, Quattrocchi A, Agodi A. Molecular epidemiology tools in the management of healthcare-associated infections: Towards the definition of recommendations. Epidemiol Prev. 2015;39(4 Suppl 1):21–26. [PubMed] [Google Scholar]
- 10.Magana M, Chatzipanagiotou S, Burriel AR, Ioannidis A. Inquiring into the gaps of Campylobacter surveillance methods. Veter Sci. 2017;4(3):36. doi: 10.3390/vetsci4030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engberg J, Aarestrup FM, Taylor DE, Gerner-Smidt P, Nachamkin I. Quinolone and macrolide resistance in Campylobacter jejuni and C coli: resistance mechanisms and trends in human isolates. Emerg Infect Dis. 2001;7(1):24–34. doi: 10.3201/eid0701.010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helms M, Simonsen J, Olsen KE, Mølbak K. Adverse health events associated with antimicrobial drug resistance in Campylobacter species: a registry-based cohort study. J Infect Dis. 2005;191(7):1050–1055. doi: 10.1086/428453. [DOI] [PubMed] [Google Scholar]
- 13.Engberg J, Neimann J, Nielsen EM, Aerestrup FM, Fussing V. Quinolone-resistant Campylobacter infections: risk factors and clinical consequences. Emerg Infect Dis. 2004;10(6):1056–1063. doi: 10.3201/eid1006.030669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abubakar SM, Maiha A, Adamu YA, Talabi AO, Abubakar MB. Antimicrobial Resistance Profiles of Thermophilic Campylobacter Species in Rural Poultry in the North Western Nigeria. Multidiscip Adv Veter Sci. 2018;21:268–275. [Google Scholar]
- 15.Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18(5):821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jolley KA, Maiden MC. BIGSdb: scalable analysis of bacterial genome variation at the population level. BMC Bioinform. 2010;11(1):595. doi: 10.1186/1471-2105-11-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maiden MC, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, et al. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci U S A. 1998;95(6):3140–3145. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J Bacteriol. 2004;186(5):1518–1530. doi: 10.1128/JB.186.5.1518-1530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simpson EH. Measurement of diversity. Nature 1949.
- 20.Nei M. Genetic distance between populations. Am Nat. 1972;106(949):283–292. [Google Scholar]
- 21.Heck KL, van Belle G, Simberloff D. Explicit calculation of the rarefaction diversity measurement and the determination of sufficient sample size. Ecology. 1975;56(6):1459–1461. [Google Scholar]
- 22.French NP, Midwinter A, Holland B, Collins-Emerson J, Pattison R, Colles F, et al. Molecular epidemiology of Campylobacter jejuni isolates from wild-bird fecal material in children's playgrounds. Appl Environ Microbiol. 2009;75(3):779–783. doi: 10.1128/AEM.01979-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moriarty E, Karki N, Mackenzie M, Sinton L, Wood D, Gilpin B. Faecal indicators and pathogens in selected New Zealand waterfowl. N Z J Mar Freshwat Res. 2011;45(4):679–688. [Google Scholar]
- 24.Aboderin AO, Smith SI, Oyelese AO, Onipede AO, Zailani SB, Coker AO. Role of Campylobacter jejuni/coli in diarrhoea in Ile-Ife. Nigeria East Afr Med J. 2002;79(8):423–426. [PubMed] [Google Scholar]
- 25.Cardinale E, Rose V, GrosClaude JDP, Tall F, Rivoal K, Mead G, et al. Genetic characterization and antibiotic resistance of Campylobacter spp isolated from poultry and humans in Senegal. J Appl Microbiol. 2006;100(1):209–217. doi: 10.1111/j.1365-2672.2005.02763.x. [DOI] [PubMed] [Google Scholar]
- 26.Coker AO, Isokpehi RD, Thomas BN, Amisu KO, Larry OC. Human campylobacteriosis in developing countries. Emerg Infect Dis. 2002;8(3):237–243. doi: 10.3201/eid0803.010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calva J, Lopez-Vidal A, Ruiz-Palacios G, Ramos A, Bojalil R. Cohort study of intestinal infection with Campylobacter in Mexican children. The Lancet. 1988;331(8584):503–506. doi: 10.1016/s0140-6736(88)91297-4. [DOI] [PubMed] [Google Scholar]
- 28.Mason J, Iturriza-Gomara M, O’Brien SJ, Ngwira BM, Dove W, Maiden MC, et al. Campylobacter infection in children in Malawi is common and is frequently associated with enteric virus co-infections. PLoS ONE. 2013;8(3):e59663. doi: 10.1371/journal.pone.0059663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ngulukun S, Oboegbulem S, Klein G. Multilocus sequence typing of Campylobacter jejuni and Campylobacter coli isolates from poultry, cattle and humans in Nigeria. J Appl Microbiol. 2016;121(2):561–568. doi: 10.1111/jam.13185. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs-Reitsma W, Bolder N, Mulder R. Cecal carriage of Campylobacter and Salmonella in Dutch broiler flocks at slaughter: a one-year study. Poult Sci. 1994;73(8):1260–1266. doi: 10.3382/ps.0731260. [DOI] [PubMed] [Google Scholar]
- 31.Colles FM, McCarthy ND, Sheppard SK, Layton R, Maiden MC. Comparison of Campylobacter populations isolated from a free-range broiler flock before and after slaughter. Int J Food Microbiol. 2010;137(2):259–264. doi: 10.1016/j.ijfoodmicro.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vinueza-Burgos C, Wautier M, Martiny D, Cisneros M, Van Damme I, De Zutter L. Prevalence, antimicrobial resistance and genetic diversity of Campylobacter coli and Campylobacter jejuni in Ecuadorian broilers at slaughter age. Poult Sci. 2017;9:45. doi: 10.3382/ps/pew487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newell DG, Fearnley C. Sources of Campylobacter colonization in broiler chickens. Appl Environ Microbiol. 2003;69(8):4343–4351. doi: 10.1128/AEM.69.8.4343-4351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saleha A. Epidemiological study on the colonization of chickens with Campylobacter in broiler farms in Malaysia: possible risk and management factors. Int J Poult Sci. 2004;3(2):129–134. [Google Scholar]
- 35.Griekspoor P, Engvall EO, Olsen B, Waldenström J. Multilocus sequence typing of Campylobacter jejuni from broilers. Vet Microbiol. 2010;140(1–2):180–185. doi: 10.1016/j.vetmic.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 36.Llarena A, Huneau A, Hakkinen M, Hänninen M. Predominant Campylobacter jejuni sequence types persist in Finnish chicken production. PLoS ONE. 2015;10(2):e0116585. doi: 10.1371/journal.pone.0116585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.French N, Yu S, Biggs P, Holland B, Fearnhead P, Binney B, et al. Evolution of Campylobacter species in New Zealand. Campylobacter Ecol Evol. 2014;9:221–240. [Google Scholar]
- 38.Mikulić M, Humski A, Njari B, Ostović M, Duvnjak S, Cvetnić Ž. Prevalence of thermotolerant Campylobacter spp. in chicken meat in croatia and multilocus sequence typing of a small subset of Campylobacter jejuni and Campylobacter coli isolates. Food Technol Biotechnol. 2016;54(4):475. doi: 10.17113/ftb.54.04.16.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carja O, Plotkin JB. The evolutionary advantage of heritable phenotypic heterogeneity. Sci Rep. 2017;7(1):5090. doi: 10.1038/s41598-017-05214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Woude MW, Baumler AJ. Phase and antigenic variation in bacteria. Clin Microbiol Rev. 2004;17(3):581–611. doi: 10.1128/CMR.17.3.581-611.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colles F, Maiden M. Campylobacter sequence typing databases: applications and future prospects. Microbiology. 2012;158(11):2695–2709. doi: 10.1099/mic.0.062000-0. [DOI] [PubMed] [Google Scholar]
- 42.Maiden MC, Dingle KE. Population biology of Campylobacter jejuni and related organisms. Campylobacter, Third Edition: American Society of Microbiology; 2008. pp. 27–40. [Google Scholar]
- 43.Wieczorek K, Denis E, Lachtara B, Osek J. Distribution of Campylobacter jejuni multilocus sequence types isolated from chickens in Poland. Poult Sci. 2017;96(3):703–709. doi: 10.3382/ps/pew343. [DOI] [PubMed] [Google Scholar]
- 44.Ngulukun S, Oboegbulem S, Fagbamila I, Bertu W, Odugbo M. Prevalence and molecular characterization of thermophilic Campylobacter species isolated from cattle in Plateau State Nigeria. Niger Veter J. 2011;32:4. [Google Scholar]
- 45.Gripp E, Hlahla D, Didelot X, Kops F, Maurischat S, Tedin K, et al. Closely related Campylobacter jejuni strains from different sources reveal a generalist rather than a specialist lifestyle. BMC Genomics. 2011;12(1):584. doi: 10.1186/1471-2164-12-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheppard SK, Dallas JF, Strachan NJ, MacRae M, McCarthy ND, Wilson DJ, et al. Campylobacter genotyping to determine the source of human infection. Clin Infect Dis. 2009;48(8):1072–1078. doi: 10.1086/597402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strachan NJ, Gormley FJ, Rotariu O, Ogden ID, Miller G, Dunn GM, et al. Attribution of Campylobacter infections in northeast Scotland to specific sources by use of multilocus sequence typing. J Infect Dis. 2009;199(8):1205–1208. doi: 10.1086/597417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Litrup E, Torpdahl M, Nielsen E. Multilocus sequence typing performed on Campylobacter coli isolates from humans, broilers, pigs and cattle originating in Denmark. J Appl Microbiol. 2007;103(1):210–218. doi: 10.1111/j.1365-2672.2006.03214.x. [DOI] [PubMed] [Google Scholar]
- 49.Gormley FJ, MacRae M, Forbes KJ, Ogden ID, Dallas JF, Strachan NJC. Has retail chicken played a role in the decline of human Campylobacteriosis? Appl Environ Microbiol. 2008;74(2):383–390. doi: 10.1128/AEM.01455-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopes BS, Rotariu O, Strachan NJC, Forbes KJ. I-CaMPS-3 impact of interventions - campylobacter MLST project in Scotland; 2016. http://www.foodstandards.gov.scot/sites/default/files/A15572266%20i-CaMPS3%20Report%20-%20Campylobacter%20MLST%20Project%20in%20Scotland.pdf. Accessed 18 May 2018.
- 51.Kapperud G, Espeland G, Wahl E, Walde A, Herikstad H, Gustavsen S, et al. Factors associated with increased and decreased risk of Campylobacter infection: a prospective case-control study in Norway. Am J Epidemiol. 2003;158(3):234–242. doi: 10.1093/aje/kwg139. [DOI] [PubMed] [Google Scholar]
- 52.Niederer L, Kuhnert P, Egger R, Buttner S, Hachler H, Korczak BM. Genotypes and antibiotic resistances of Campylobacter jejuni and Campylobacter coli isolates from domestic and travel-associated human cases. Appl Environ Microbiol. 2012;78(1):288–291. doi: 10.1128/AEM.06194-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guèye E. The role of family poultry in poverty alleviation, food security and the promotion of gender equality in rural Africa. Outlook Agric. 2000;29(2):129–136. [Google Scholar]
- 54.Griggs DJ, Peake L, Johnson MM, Ghori S, Mott A, Piddock LJ. Beta-lactamase-mediated beta-lactam resistance in Campylobacter species: prevalence of Cj0299 (bla OXA-61) and evidence for a novel beta-Lactamase in C. jejuni. Antimicrob Agents Chemother. 2009;53(8):3357–3364. doi: 10.1128/AAC.01655-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alfredson DA, Korolik V. Isolation and expression of a novel molecular class D beta-lactamase, OXA-61, from Campylobacter jejuni. Antimicrob Agents Chemother. 2005;49(6):2515–2518. doi: 10.1128/AAC.49.6.2515-2518.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iroha I, Adikwu M, Esimone C, Aibinu I, Amadi E. Extended spectrum beta lactamase (ESBL) in E. coli isolated from a tertiary hospital in Enugu State. Nigeria. Pak J Med Sci. 2009;25(2):279–282. [Google Scholar]
- 57.Ayeni FA, Olujobi OF, Alabi OS. A preliminary investigation of prevalence of extended spectrum beta lactamases among enterobacteriaceae isolated from poultry farms in Ibadan. Nigeria Niger J Pharm Res. 2016;11(1):46–51. [Google Scholar]
- 58.Padungton P, Kaneene JB. Campylobacter spp. in human, chickens, pigs and their antimicrobial resistance. J Veter Med Sci. 2003;65(2):161–170. doi: 10.1292/jvms.65.161. [DOI] [PubMed] [Google Scholar]
- 59.De Vries SP, Vurayai M, Holmes M, Gupta S, Bateman M, Goldfarb D, et al. Phylogenetic analyses and antimicrobial resistance profiles of Campylobacter spp from diarrhoeal patients and chickens in Botswana. PLoS ONE. 2018;13(3):e0194481. doi: 10.1371/journal.pone.0194481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duarte A, Santos A, Manageiro V, Martins A, Fraqueza MJ, Canica M, et al. Human, food and animal Campylobacter spp isolated in Portugal: high genetic diversity and antibiotic resistance rates. Int J Antimicrob Agents. 2014;44(4):306–313. doi: 10.1016/j.ijantimicag.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 61.Luangtongkum T, Jeon B, Han J, Plummer P, Logue CM, Zhang Q. Antibiotic resistance in Campylobacter: emergence, transmission and persistence. 2009. [DOI] [PMC free article] [PubMed]
- 62.Luo N, Pereira S, Sahin O, Lin J, Huang S, Michel L, et al. Enhanced in vivo fitness of fluoroquinolone-resistant Campylobacter jejuni in the absence of antibiotic selection pressure. Proc Natl Acad Sci U S A. 2005;102(3):541–546. doi: 10.1073/pnas.0408966102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.World Organization for Animal Health (OIE). Terrestrial Animal Health Code. Paris: World Organization for Animal Health. 2016.
- 64.Prats G, Mirelis B, Llovet T, Munoz C, Miro E, Navarro F. Antibiotic resistance trends in enteropathogenic bacteria isolated in 1985–1987 and 1995–1998 in Barcelona. Antimicrob Agents Chemother. 2000;44(5):1140–1145. doi: 10.1128/aac.44.5.1140-1145.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gibreel A, Tracz DM, Nonaka L, Ngo TM, Connell SR, Taylor DE. Incidence of antibiotic resistance in Campylobacter jejuni isolated in Alberta, Canada, from 1999 to 2002, with special reference to tet(O)-mediated tetracycline resistance. Antimicrob Agents Chemother. 2004;48(9):3442–3450. doi: 10.1128/AAC.48.9.3442-3450.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wardak S, Szych J, Zasada AA, Gierczynski R. Antibiotic resistance of Campylobacter jejuni and Campylobacter coli clinical isolates from Poland. Antimicrob Agents Chemother. 2007;51(3):1123–1125. doi: 10.1128/AAC.01187-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001;65(2):232–260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roberts MC. Update on acquired tetracycline resistance genes. FEMS Microbiol Lett. 2005;245(2):195–203. doi: 10.1016/j.femsle.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 69.Patterson AJ, Rincon MT, Flint HJ, Scott KP. Mosaic tetracycline resistance genes are widespread in human and animal fecal samples. Antimicrob Agents Chemother. 2007;51(3):1115–1118. doi: 10.1128/AAC.00725-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Melville CM, Scott KP, Mercer DK, Flint HJ. Novel tetracycline resistance gene, tet(32), in the Clostridium-related human colonic anaerobe K10 and its transmission in vitro to the rumen anaerobe Butyrivibrio fibrisolvens. Antimicrob Agents Chemother. 2001;45(11):3246–3249. doi: 10.1128/AAC.45.11.3246-3249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stanton TB, McDowall JS, Rasmussen MA. Diverse tetracycline resistance genotypes of Megasphaera elsdenii strains selectively cultured from swine feces. Appl Environ Microbiol. 2004;70(6):3754–3757. doi: 10.1128/AEM.70.6.3754-3757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wieczorek K, Kania I, Osek J. Prevalence and antimicrobial resistance of Campylobacter spp isolated from poultry carcasses in Poland. J Food Prot. 2013;76(8):1451–1455. doi: 10.4315/0362-028X.JFP-13-035. [DOI] [PubMed] [Google Scholar]
- 73.Pergola S, Franciosini M, Comitini F, Ciani M, De Luca S, Bellucci S, et al. Genetic diversity and antimicrobial resistance profiles of Campylobacter coli and Campylobacter jejuni isolated from broiler chicken in farms and at time of slaughter in central Italy. J Appl Microbiol. 2017;122(5):1348–1356. doi: 10.1111/jam.13419. [DOI] [PubMed] [Google Scholar]
- 74.Piddock L, Griggs D, Johnson M, Ricci V, Elviss N, Williams L, et al. Persistence of Campylobacter species, strain types, antibiotic resistance and mechanisms of tetracycline resistance in poultry flocks treated with chlortetracycline. J Antimicrob Chemother. 2008;62(2):303–315. doi: 10.1093/jac/dkn190. [DOI] [PubMed] [Google Scholar]
- 75.Chen X, Naren G, Wu C, Wang Y, Dai L, Xia L, et al. Prevalence and antimicrobial resistance of Campylobacter isolates in broilers from China. Vet Microbiol. 2010;144(1–2):133–139. doi: 10.1016/j.vetmic.2009.12.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are stored at https://pubmlst.org/organisms/campylobacter-jejunicoli, and can be made available from the corresponding author on reasonable request.