Abstract
As COVID-19 continues to spread globally, monitoring the disease at different scales is critical to support public health decision making. Surveillance for SARS-CoV-2 RNA in wastewater can supplement surveillance based on diagnostic testing. In this paper, we report the results of wastewater-based COVID-19 surveillance on Emory University campus that included routine sampling of sewage from a hospital building, an isolation/quarantine building, and 21 student residence halls between July 13th, 2020 and March 14th, 2021. We examined the sensitivity of wastewater surveillance for detecting COVID-19 cases at building level and the relation between Ct values from RT-qPCR results of wastewater samples and the number of COVID-19 patients residing in the building. Our results show that weekly wastewater surveillance using Moore swab samples was not sensitive enough (6 of 63 times) to reliably detect one or two sporadic cases in a residence building. The Ct values of the wastewater samples over time from the same sampling location reflected the temporal trend in the number of COVID-19 patients in the isolation/quarantine building and hospital (Pearson's r < −0.8), but there is too much uncertainty to directly estimate the number of COVID-19 cases using Ct values. After students returned for the spring 2021 semester, SARS-CoV-2 RNA was detected in the wastewater samples from most of the student residence hall monitoring sites one to two weeks before COVID-19 cases surged on campus. This finding suggests that wastewater-based surveillance can be used to provide early warning of COVID-19 outbreaks at institutions.
Keywords: COVID-19, SARS-CoV-2, Wastewater surveillance, Campus, Residence hall
Graphical abstract
1. Introduction
The ongoing COVID-19 pandemic has posed great risks for public health and created unprecedented challenges for safely operating businesses, travelling, and reopening schools, etc. COVID-19 surveillance tracks the numbers of cases, hospitalization, and deaths at different geographic scales to monitor the temporal and spatial trends of COVID-19. Currently, COVID-19 surveillance relies mainly on diagnostic testing, such as nucleic acid amplification tests and rapid antigen tests, for individuals (Centers for Disease Control and Prevention, 2021). However, such a surveillance strategy has several limitations. First, it underestimates the disease incidence. Cases with mild or no symptoms are less likely to get a diagnostic test. In addition, the capacity of diagnostic testing can be inadequate, especially during a surge of infections or in areas with limited resources, resulting in even more undetected cases. Second, trends in observed cases, hospitalizations or deaths are delayed with respect to transmission. Delays exist between exposure to SARS-CoV-2 and symptom onset, as well as between symptom onset and confirmation by a diagnostic test. The median incubation period of COVID-19 was estimated to be 5.1 days (95% CI, 4.5 to 5.8 days) (Lauer et al., 2020). A study in South Korea reported that the estimated median period between the date of symptom onset and the date of COVID-19 confirmation was 4 days (Kim et al., 2020). In addition, the costs of population-wide repeated diagnostic testing impose a heavy financial burden on communities. The cost of a typical COVID-19 PCR test for saliva samples is $1.29 to $4.30 per test, and the cost of an antigen test is $5 per test (Greenwood, 2021; Park, 2020).
Detection of SARS-CoV-2 RNA in wastewater offers a sensitive, non-invasive, rapid, and low-cost surveillance approach to supplement clinical surveillance. Infected subjects may shed SARS-CoV-2 in their feces for a prolonged period, regardless of whether they are symptomatic or asymptomatic (C. Chen et al., 2020; Park et al., 2020; X. Wang et al., 2020; Wu et al., 2020), and fecal shedding of SARS-CoV-2 may start before the appearance of any symptoms (Wu et al., 2022). A study in New Haven, Connecticut observed an increased concentration of SARS-CoV-2 RNA in sewage sludge 6–8 days ahead of a rise in reported cases (Peccia et al., 2020). Wastewater samples can usually be collected and processed within 1–2 days. In addition, testing wastewater requires only a small number of samples to provide population-level data, which enables wastewater surveillance to be sustained with low cost.
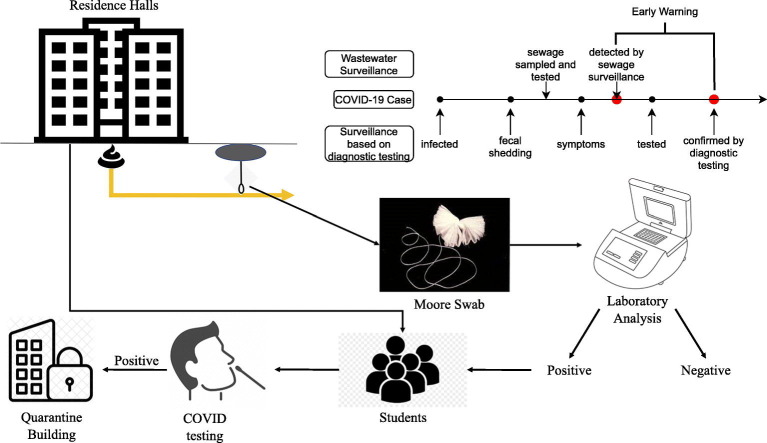
When the prevalence of COVID-19 decreases and restrictions like shelter-in-place have been lifted, wastewater surveillance could play a critical role to monitor the disease spread and inform public health interventions. For universities to safely reopen, it is critical to maintain COVID-19 prevalence on campus at a low level. Early warning of outbreaks could help universities respond rapidly and prevent further disease transmission. Emory University transitioned to full remote learning on March 23rd, 2020 and reopened on August 19th, 2020 for online and in-person classes. COVID-19 screening tests using a rapid point of care antigen test (Quidel) were mandatory for all faculty, staff, and students returning to campus. The number of cases was monitored on a daily basis and reported on a publicly-available dashboard (https://www.emory.edu/forward/covid-19/dashboard/index.html). Throughout the semester, weekly routine diagnostic testing using antigen test (Quidel) was conducted for students who lived in residence halls on campus regardless of whether or not they had symptoms. In December 2020, it changed to the Qaunterix (Simo) platform with RT-PCR confirmation for positive antigen tests twice a week. Whenever a COVID-19 case was confirmed, isolation and contact tracing were implemented. Wastewater-based surveillance at residence halls was also conducted, as a part of an effort to monitor and control COVID-19 transmission on campus. When a wastewater sample was positive by RT-PCR for SARS-CoV-2 RNA, students living in the corresponding building were instructed to screen themselves for symptoms using an online COVID-19 symptom checker. Diagnostic testing compliance was reviewed for all the residents of the building. Those students who had not been tested 48 h prior to the time the wastewater sample collected, were instructed to get tested immediately. This study reports the results of wastewater-based surveillance, along with numbers of confirmed COVID-19 cases, in all residence hall buildings on three campuses of Emory University, one isolation/quarantine building, and one Emory University Hospital building with wards for confirmed COVID-19 patients.
2. Material and methods
2.1. Study areas and sample collection
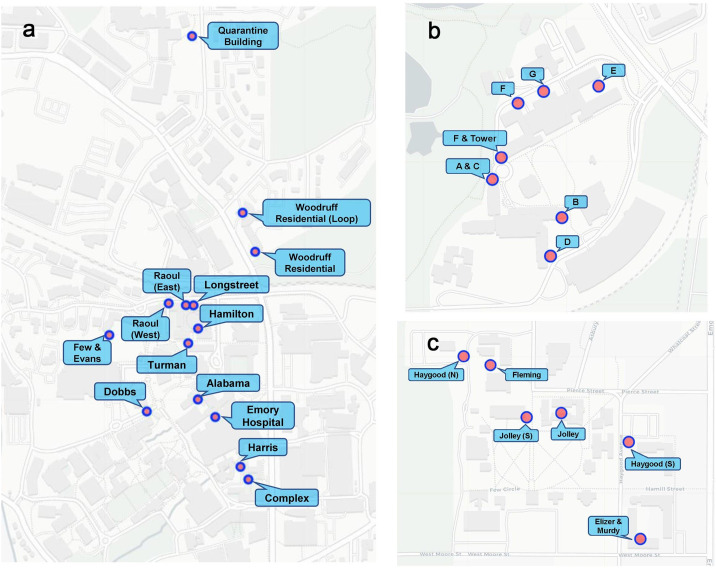
Emory University has three campuses: the Atlanta Campus and Clairmont Campus in Atlanta, Georgia and the Oxford Campus in Oxford, Georgia. During the Fall 2019 semester, Emory University had 15,398 students enrolled, and over 13,200 faculty and staff members. 6.4%. of students were in Oxford Campus. There are ten residence halls (Alabama, Complex, Dobbs, Few and Evens, Hamilton, Harris, Longstreet, Raoul, Turman, and Woodruff) at the Emory Atlanta Campus, three residence halls (Clairmont Residential Center, Clairmont Tower Apartments, and Clairmont Undergraduate Residential Center) at the Clairmont Campus, and four residence halls (Elizer and Murdy, Fleming, Haygood, and Jolley Residential Center) at the Oxford Campus. The capacity of residence halls ranges from 91 to 600. About 1600 undergraduate students lived on campus during the Fall 2020 semester. Weekly Moore swab samples (Liu et al., 2021) were collected at 25 manholes located adjacent to the residence halls between August 21st, 2020 to March 14th, 2021 (Fig. 1 ). Of these 25 manholes, twelve were at the Atlanta Campus, seven were at the Clairmont Campus, and six were at the Oxford Campus. The majority of the manhole sites collected all the wastewater directly from the targeted building. In some cases, the targeted building was served by two manhole sites, and we collected samples from both manholes.
Fig. 1.
Sampling sites distribution on Emory Campuses. a. Atlanta Campus, including the Emory University Hospital building sampling site and isolation/quarantine building sampling site. b. Clairmont Campus. c. Oxford Campus.
The students living on campus who either were exposed to SARS-CoV-2 virus or tested positive but did not require hospitalization were transferred to a hotel near the Emory Atlanta Campus (Fig. 1a) for isolation and quarantine. There were 325 rooms available for use at the hotel, divided between the South wing (used exclusively for isolation and quarantine) and the North wing. Isolated/quarantined students remained in isolation for at least 10 days and were discharged after a negative test and showing no active symptoms. Between September 8th, 2020 to March 14th, 2021, weekly Moore swabs were taken at one of the manholes near the South wing of the isolation/quarantine building.
Emory University Hospital is located at the center of the Emory Atlanta Campus (Fig. 1a) and started admitting COVID-19 patients in mid-February 2020. Between July 13th, 2020 and March 14th, 2021, weekly grab samples and Moore swab samples were collected at a manhole that was directly downstream from one of the Emory University Hospital buildings with COVID-19 inpatients. The sampled hospital building had an average of 540 daily inpatients (SD: 26.7), including both COVID-19 and non-COVID-19 patients, during our study period.
Moore swabs, comprised of cotton gauze tied with fishing line, were placed in selected manholes in the flowing wastewater from the target building and retrieved after 24–72 h. The collection period for swabs on Main Atlanta Campus alternated between 24 and 48 h (often set on Monday and collected on Wednesday), on Clairmont Campus was 48 h (set on Tuesday and collected on Thursday), and on Oxford campus was 72 h (set on Friday and collected on Monday). For grab samples, 1 L of wastewater was collected from the outflow of the hospital building. All samples were transported on ice to the laboratory for analysis. Further details of sample collection are described in (Liu et al., 2021).
2.2. Sample processing and lab testing
Moore swab samples were rinsed with an elution buffer, consisting of 0.01% sodium polyphosphate, 0.01% Tween 80, and 0.001% antifoam Y-30 emulsion, resulting in sample volumes of 250 mL, which were then processed primarily by a skimmed milk method (Liu et al., 2012; Liu et al., 2021). Initially, a small number of swab samples were concentrated by PEC precipitation which had similar recovery efficiency (results not shown). Because this method was more expensive and complicated than the skimmed milk method, we did not continue using it. For grab samples, 500 mL sample volumes were concentrated using membrane filtration with 0.45-μm-pore-size, 47-mm diameter nitrocellulose filters (Millipore Sigma, Burlington MA). RNA was extracted using a RNeasy Mini Kit (QIAGEN, Hilden, Germany) with Bovine Respiratory Syncytial Virus (BRSV) (INFORCE 3, Zoetis, Parsippany, NJ) as an extraction control. SARS-CoV-2 RNA was detected via real-time quantitative reverse-transcription polymerase chain reaction (RT-qPCR) using the N1 primer developed by the Centers of Disease Control and Prevention (CDC) (Lu et al., 2020) and the TaqPathTM qPCR Master Mixture (ThermoFisher Scientific, Waltham, MA). A diluted synthetic SARS-CoV-2 RNA (ATCC® VR-3276SD, Manassas, VA) served as a positive control and was included in each RT-qPCR run. Consistent Ct values from this positive control were observed [mean = 24.10; standard deviation (SD) = 0.51] in a total of 49 RT-qPCR runs throughout the study period. All samples were tested in duplicate. A wastewater sample was considered to be positive when both replicate PCR tests had a quantifiable Ct value (Ct values below 41 and the PCR curve showed amplification) with difference of Ct values between replicates less than 2. The turnaround time from sample collection to final lab results was between 2 and 3 days. Details of sample processing and lab analyses can be found in (Liu et al., 2021).
2.3. Confirmed COVID-19 cases
Emory University offered free COVID-19 tests for all the students, faculty and staff since early in the pandemic. Mandatory diagnostic screenings were done for all students returning to campus for the Fall 2020 and Spring 2021 semesters. Weekly diagnostic screening tests were required for all students living on campus. The majority of these tests used saliva samples. A confirmed case was defined as a confirmation of SARS-CoV-2 infection by either a nucleic acid test or rapid antigen test. As CDC guidance changed, presumptive positive antigen tests were reflexed to RT-PCR testing for confirmation. Contact tracing was leveraged to rapidly identify and isolate close contacts to mitigate any outbreaks of COVID-19 on campus. For students living on campus who tested positive, information collected included confirmed infection date, the last date they stayed on campus, and the residence halls where they lived.
The numbers of confirmed COVID-19 cases and isolated close contacts in the Emory isolation/quarantine building and the number of confirmed COVID-19 patients in the building of Emory University Hospital, where we collected wastewater samples were also acquired for the study period.
2.4. Data analysis
For hospital samples and isolation/quarantine building samples, quantitative analyses of Ct values and COVID-19 patient numbers were conducted. Ct values of negative PCR results were substituted (with 40 for hospital samples and 44 for isolation/quarantine building samples) based on the lowest Ct values found. To compare trends, we plotted LOESS (Locally Estimated Scatterplot Smoothing) lines with 95% confidence intervals, and we examined the correlation between two time series of confirmed COVID-19 case numbers in the building and Ct values in the wastewater samples from the same building.
For the residence halls, we explored the sensitivity and specificity of using the wastewater sample results to identify resident COVID-19 cases using weekly building-level wastewater surveillance results. Also, by comparing the temporal trend of wastewater surveillance results with case numbers living in corresponding buildings, we examined the application of wastewater surveillance as an early warning system for COVID-19 outbreaks on campus. Wastewater surveillance results and reported cases identified by diagnostic tests were aggregated by week. Since a single COVID-19 case could spread the disease rapidly, any detection of SARS-CoV-2 RNA in wastewater should trigger interventions to prevent outbreaks. Therefore, wastewater testing results were presented as binary outcomes, positive or negative. When multiple samples were collected at the same site in a week, the weekly outcome for that site was marked positive if any of those samples was positive. Confirmed cases with missing last date on campus or with testing date 14 days later than the last day on campus were excluded from analysis. All the analyses were conducted in R (version 4.0.1) (R Core Team, 2013).
The Emory Institutional Review Board (IRB) determined that this analysis was exempt from the requirement for IRB review, and approval and informed consent were not required.
3. Results
3.1. Overview of wastewater surveillance results
Wastewater surveillance results between July 13th, 2020 to March 14th, 2021 are summarized in Table 1 . At the hospital building, the Moore swab method (82.4%) detected more positive samples (p = 0.005) compared to matched grab sample method (51.5%). At the isolation/quarantine building, where the number of COVID-19 patients was smaller compared to the hospital building, 65.2% of the Moore swab samples were positive. Only 4.9% of the wastewater samples from the residence halls were positive in the Fall 2020 semester, and during this semester the number of COVID-19 cases among resident students was low. However, when the students returned to campus for the Spring 2021 semester, an increase in COVID-19 cases was observed on campus and about 40% of the wastewater samples were positive.
Table 1.
Summary of wastewater surveillance results for the hospital building, isolation/quarantine building, and residence halls in three Emory campuses. †Average number of cases in the building during the study period. ‡Results from wastewater samples from the same site within a calendar week were consolidated into one sample. The Fall 2020 semester covered the period from August 20th, 2020 to December 31st, 2020; The Spring 2021 semester covered the period from January 1st, 2021 to March 28th, 2021.
| Location | Number of cases | Number of sites | ‡Number of samples | Positive samples (%) |
|---|---|---|---|---|
| Hospital building (grab sample) | †65 | 1 | 66 | 34 (51.5) |
| Hospital building (Moore swab sample) | †65 | 1 | 34 | 28 (82.4) |
| Isolation/quarantine building | †9 | 1 | 23 | 15 (65.2) |
| Atlanta campus residence halls (Fall 2020 semester) | 26 | 10 | 92 | 10 (10.9) |
| Clairmont campus residence halls (Fall 2020 semester and winter break) | 14 | 7 | 111 | 3 (2.7) |
| Oxford campus residence halls (Fall 2020 semester) | 6 | 6 | 63 | 0 (0) |
| Atlanta campus residence halls (Spring 2021 semester) | 140 | 10 | 41 | 26 (63.4) |
| Clairmont campus residence halls (Spring 2021 semester) | 19 | 7 | 63 | 14 (22.2) |
| Oxford campus residence halls (Spring 2021 semester) | 19 | 6 | 22 | 13 (59.1) |
3.2. Wastewater surveillance at the hospital building
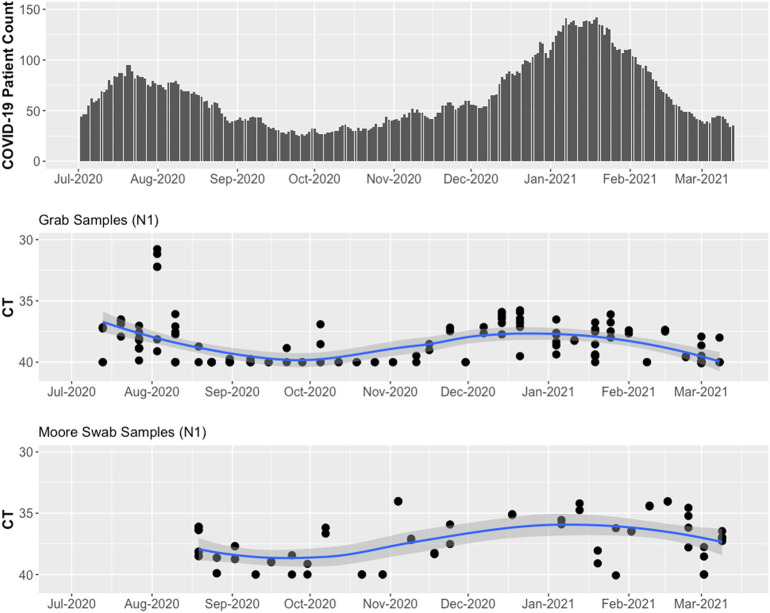
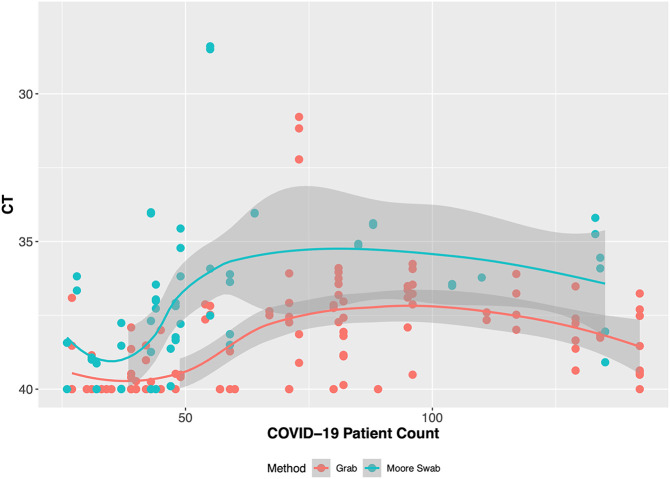
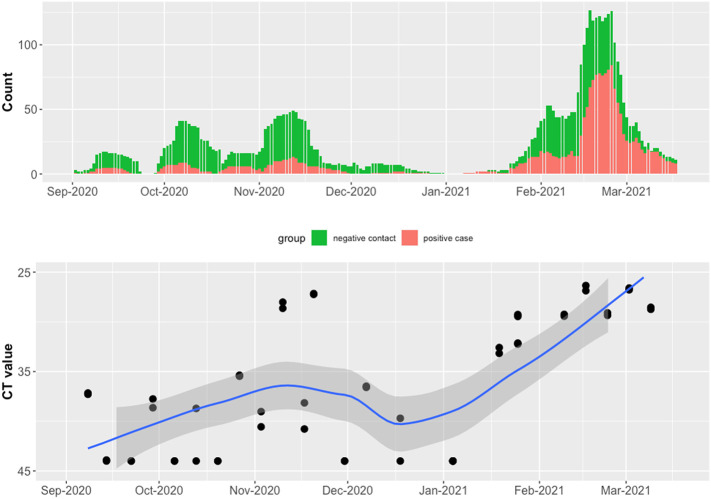
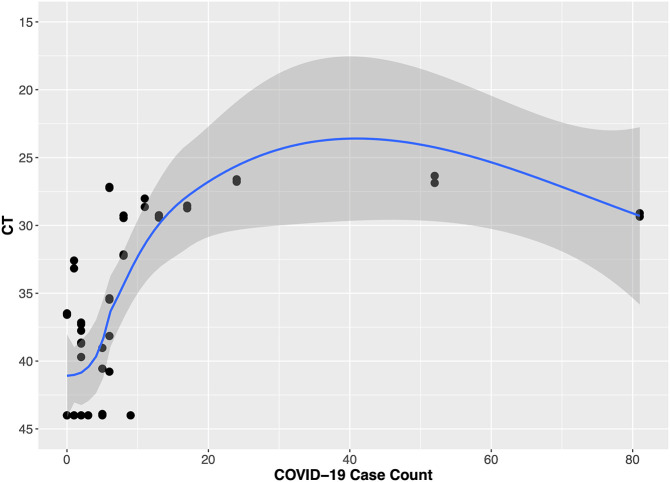
The hospital building where we conducted wastewater surveillance had a steady number of inpatients (range 471–606) during the study period. The number of confirmed COVID-19 patients was low (<40) during October and November 2020 and high (>100) in January 2021 (Fig. 2 ). When the number of COVID-19 patients in the building was low (<50), the Moore swabs (9 of 12 samples were positive) were more likely to detect SARS-CoV-2 RNA compared to the grab samples (8 of 15 samples were positive). As the number of COVID-19 patients increased, both methods detected SARS-CoV-2 RNA in the wastewater from the hospital building. Fig. 2 shows that the Ct values tended to decrease as the number of cases increased. The smoothed LOESS curves show that the temporal trend in average Ct values for grab samples and Moore swabs was correlated with the trend in COVID-19 patient counts (Pearson's r = −0.822 for grab samples and Pearson's r = −0.835 for Moore swab samples). In Fig. 3 , the Ct values are shown relative to the COVID-19 patient count. When the number of COVID-19 cases was low, the Ct value of grab samples and Moore swab samples decreased with the increasing number of COVID patients. For higher numbers of cases, the Ct values appeared to plateau between 36 and 38.
Fig. 2.
Wastewater surveillance results for grab samples and Moore swab samples compared to the number of COVID-19 patients in the hospital building between July 13th, 2020 to March 14th, 2021.
Fig. 3.
Wastewater surveillance Ct value results for grab samples and Moore swab samples compared to the number of COVID-19 patients in the hospital building.
3.3. Wastewater surveillance at the isolation/quarantine building
When the number of confirmed COVID-19 patients in the isolation/quarantine building was relatively low (≤10) before January 25th, 2021 (first day of spring semester), the Moore swabs did not consistently detect SARS-CoV-2 RNA (11 of the 17 samples were positive) in the wastewater. When the spring semester started and the number of cases was high (>10), all 6 Moore swab samples were positive. The smoothed LOESS line shows a decrease in Ct values when the number of COVID-19 patients in the isolation/quarantine building increased (Fig. 4 ). The Pearson's correlation between the time series of COVID-19 patient numbers and Ct values was −0.888. When the number of cases in the building was low (<25), the Ct values increased with the number of COVID-19 patients. Once the number of cases exceeded a certain level (around 25), the Ct values did not decrease further (Fig. 5 ).
Fig. 4.
Wastewater surveillance results from Moore swabs placed near the isolation/quarantine building and the number of students isolated/quarantined. The number of students in the isolation/quarantine building included those who tested positive for COVID-19 and close contacts who tested negative.
Fig. 5.
Wastewater surveillance Ct value results for Moore swab samples with the number of students in the isolation/quarantine building.
3.4. Wastewater surveillance at residence halls
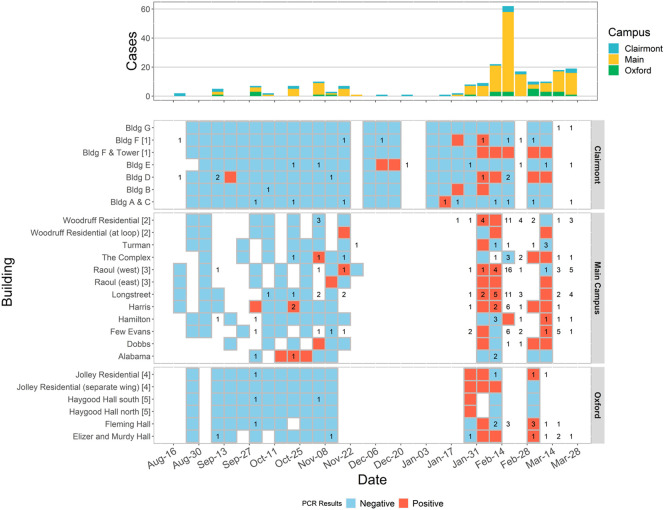
Fig. 6 shows the wastewater surveillance results at the student residence halls for the Fall 2020 semester and Spring 2021 semester along with the epidemic curve of confirmed COVID-19 cases in the residence halls. In the Fall 2020 semester, there were no major outbreaks on campus, and the numbers of cases in residence halls were low (≤2 cases per week). In the Fall 2020 semester, we were able to detect SARS-CoV-2 RNA in the wastewater only a few times (6 of 63) when a case was confirmed in a building in the same week or a week later (Supplementary Table S6).
Fig. 6.
Wastewater surveillance results of residence hall and case data at Emory University Campus. Wastewater surveillance results are displayed with blue (negative) and red (positive) tiles by sampling site. Data were aggregated weekly. Numbers of confirmed cases are marked in the tiles corresponding to their residence halls. Some residence halls shared data of confirmed cases and have the same manhole index number following the residence hall name. Weeks without wastewater surveillance results are left blank.
In the Spring 2021 semester, there was a surge of COVID-19 cases on campus in mid-February, three weeks after the semester began (Fig. 6). The majority (54.2%) of wastewater surveillance samples from January 31st, 2020 to February 14th, 2021 (1–2 weeks in advance of the surge in diagnosed cases) were positive for SARS-CoV-2 RNA. The wastewater samples from Feb 28th to March 14th, 2021 also had a high proportion of positives (50.0%) followed by another wave of COVID-19 cases on campus between March 14th to March 28th.
We observed that the proportion of positive wastewater surveillance samples in a given week reflected the trajectory of the case numbers. However, our wastewater surveillance method was not sensitive enough to reliably capture sporadic single cases that emerged in individual residence halls.
4. Discussion
4.1. Sensitivity of wastewater surveillance at the building level
This study examined the feasibility of detecting SARS-CoV-2 RNA in wastewater from three types of buildings when COVID-19 cases were present in the building. Compared to grab samples, Moore swab samples were more sensitive for detecting the presence of SARS-CoV-2 in wastewater. Probability of SARS-CoV-2 RNA detection in wastewater depended on COVID-19 prevalence, the type of building and type of residents. The sensitivity of detection for SARS-CoV-2 RNA from a single wastewater sample was low when the number of COVID-19 cases in the building was small. As the number of COVID-19 cases in the building increased, we were able to detect SARS-CoV-2 RNA in the majority of the wastewater samples.
The hospital building was a large building with a large number of patients (500–600) and a high prevalence of COVID-19 cases. In addition, COVID-19 patients admitted to the hospital usually had severe symptoms. In such a setting, both the grab sample method and Moore swab method performed equally well with a high number of COVID-19 patients in the building. When the number of COVID-19 patients in the hospital was relatively low (<50), Moore swab samples, as composite samples of wastewater over 24–48 h, were more likely to detect SARS-CoV-2 RNA than grab samples, for which virus detection depends on the presence of virus in the wastewater flow at the time of sample collection.
The isolation/quarantine building at the Emory Conference Center Hotel contained fewer confirmed case-patients. Wastewater samples were collected from one of the three manholes serving the sewage system of the isolation/quarantine building. Therefore, the catchment population of the wastewater samples from the isolation/quarantine building was smaller compared to those from the hospital building. And the isolated/quarantined students included confirmed COVID-19 cases and close contacts identified through tracing, usually had mild or no symptoms (less fecal shedding). Still, Moore swab samples were sufficiently sensitive to detect the virus in the wastewater from this building. We observed lower Ct values with more variance in samples from the isolation/quarantine building compared to samples from the hospital building. The reason could be that normal activities in the hospital building use a large amount of water, leading to diluted wastewater samples, whereas the isolation/quarantine building had less water flow with less diluted human fecal waste.
Wastewater surveillance of the residence halls was used to monitor COVID-19 cases on campus along with weekly diagnostic tests. The catchment population of each residential hall sampling site was relatively small (91 to 600), and COVID-19 prevalence within the residence halls was generally low. Unlike the hospital and isolation/quarantine buildings where the number of confirmed COVID-19 cases is known and is a relatively high proportion of the building residents, wastewater surveillance of residence halls is attempting to identify a low numbers of student COVID-19 cases possibly before they have symptoms or are aware that they are infected. During the Fall 2020 semester, the incidence of COVID-19 on campus was low, and wastewater surveillance of the residence halls detected SARS-CoV-2 RNA in only a few samples when there were confirmed COVID-19 cases in the sampled buildings. This was in agreement with what we found from wastewater surveillance at the hospital building and the isolation/quarantine building: our wastewater surveillance approach was not sensitive enough to capture a single sporadic case at the building level. This changed during the Spring 2021 semester when surges in COVID-19 cases were observed on the Emory Campus, and the majority of the residence hall wastewater samples were RT-PCR positive.
There are several recent reports of building-level wastewater surveillance at institutions (Betancourt et al., 2021; Bivins et al., 2022; Gibas et al., 2021; Harris-Lovett et al., 2021; Karthikeyan et al., 2021). Although the building size, disease prevalence, sampling method, and lab assay varied, all the studies successfully detected the SARS-CoV-2 RNA in the wastewater. Some of the studies used the wastewater surveillance results to trigger clinical testing for residents in the building and asserted that the wastewater surveillance at the building level could detect a single asymptomatic COVID-19 case (Gibas et al., 2021). However, such results do not guarantee that the sensitivity of wastewater surveillance is always adequate to reliably detect a single case. Similar to the study by Karthikeyan et al. (2021), the current study examined the relationship between building-level case numbers identified by diagnostic testing and the wastewater surveillance RT-PCR results. This enabled us to evaluate the sensitivity and specificity of wastewater surveillance at the building level. However, we found a lower sensitivity of wastewater surveillance in our study (45.5%) compared to the study by Karthikeyan et al. (2021) (84.7%). This could be due to the different collection methods (Moore swab vs. composite sample by an autosampler), sewerage structure, and building and resident population size, etc.
Several studies have reported that the probability of fecal shedding of SARS-CoV-2 in infected subjects is between 30 and 75% (Y. Chen et al., 2020; W. Wang et al., 2020). A meta-analysis reported that the overall detection rate of SARS-CoV-2 RNA in feces of COVID-19 patients was 43.7% (95% CI 32.6%–55.0%) (Wong et al., 2020). It is likely the amount of virus shed in feces is higher when the symptoms are more severe (Lin et al., 2021; Zheng et al., 2020). In situations where the building is large and the sampling site only covers part of the building, it may be challenging to detect the virus from wastewater when there are a small number of cases in the building. On the Emory campuses, students may move around between residence halls, which means that they could have used toilets in other buildings. To increase the likelihood of finding cases who are shedding SARS-CoV-2 and thus improve the sensitivity of wastewater surveillance for COVID-19 case detection, it may be necessary to collect wastewater samples from more sites and use a greater sampling frequency.
4.2. Estimation of case numbers at the building level
One of the applications of wastewater surveillance for COVID-19 that has been suggested is for estimating the prevalence of COVID-19 in the catchment population (Daughton, 2020). This has been attempted for wastewater surveillance systems that collect samples at wastewater treatment plants with large catchment populations (Gonzalez et al., 2020; Peccia et al., 2020; Saguti et al., 2021). However, there is no universal model to estimate the prevalence of COVID-19 directly from the results of wastewater analyses because the catchment population, dilution factor, and topology of the sewage network vary by sampling site. At the building level, the trend of Ct values from either the grab samples or Moore swab samples of wastewater mirrored the trend of COVID-19 prevalence. Generally, the Ct values, which had considerable variation, decreased as the case number increased in the building but flatten when the case number became large. The Moore swabs were left in the manhole near the hospital for 24 h (sometimes 48 h) and may have become saturated with virus. The correlation between Ct values from wastewater samples and the number of COVID-19 patients in the hospital building and isolation/quarantine building was strong, which is consistent with the results reported by Karthikeyan et al. (2021). Because there is very limited information about the proportion of COVID-19 cases that shed SARS-CoV-2 in feces, the magnitude and duration of SARS-CoV-2 fecal shedding, and the fecal dilution of the wastewater, it is challenging to try to directly estimate the number of COVID-19 cases using wastewater surveillance results at the building level.
4.3. Early warning at the institution level
Although wastewater testing may not be sensitive enough to reliably identify sporadic cases in individual buildings, when wastewater data from multiple buildings were combined, the overall wastewater surveillance system was sufficiently sensitive to provide early warning of COVID-19 outbreaks. During the Spring 2021 semester, large proportions of wastewater samples across campus (Clairmont: 6 of 14; Main: 14 of 23; Oxford: 6 of 10) were positive for SARS-CoV-2 RNA 1–2 weeks before the surges of cases detected by diagnostic testing. At the start of an outbreak, when the numbers of infected subjects are increasing, people infected with SARS-CoV-2 could shed SARS-CoV-2 virus in their feces before their infection is identified by diagnostic testing (Jones et al., 2020). This early detection/warning of COVID-19 cases has also been reported by other campus wastewater surveillance studies (Betancourt et al., 2021; Gibas et al., 2021; Karthikeyan et al., 2021). For students living on campus and doing weekly diagnostic tests, transmission could be prevented by rapid isolation/quarantine and contact tracing. Any students who were infected but did not comply with weekly testing or who lived off campus, could “escape” detection and cause further transmission on campus. COVID-19 cases missed by diagnostic testing may use toilets in the residence halls or other buildings. Our findings suggest that it is more practical to examine the results of campus wastewater surveillance at the institution level rather than by individual buildings. Essentially, a positive signal from wastewater collected at any campus sampling site indicates SARS-CoV-2 circulation in the campus population, including: students who live on campus; students living off campus but taking in-person classes; faculty and staff working on campus. Such transmission could be detected by weekly diagnostic testing at the expense of a large number of tests. Wastewater surveillance is a much more economical approach and could potentially cover a greater proportion of the population on campus as a screening approach to guide the use of diagnostic testing. Moving forward, wastewater sampling at buildings with classes, libraries, student centers, or gyms could further expand the coverage of the surveillance. Therefore, wastewater surveillance has the potential to supplement diagnostic testing and provide early warning of outbreaks at the institutional level.
4.4. Designing wastewater surveillance
In order to effectively use wastewater surveillance, the system design should be based on the objectives of the surveillance, sanitation infrastructures, disease dynamics, and sensitivity of the lab assay (Harris-Lovett et al., 2021; Y. Wang et al., 2020). When monitoring large-scale trends in disease prevalence, such as in cities, when the disease prevalence is high, samples of wastewater that are collected at downstream points (e.g., inlets of wastewater treatment plants) with large catchment populations are appropriate. Composite and frequent samples are usually recommended, and quantification of viral RNA concentration in the wastewater is required for examining temporal trends (Peccia et al., 2020).
When monitoring infection in smaller communities and institutions (e.g., schools, nursing homes, jails) and when the disease prevalence is low, samples can be collected at upstream points (e.g., manholes, toilets) with small catchment populations, thus providing early warning of potential COVID-19 outbreaks to enable mitigations measures. Such a surveillance strategy may need a large number of sample collection sites to cover a large area, but results indicating the presence or absence of virus could be sufficient to inform response (Gibas et al., 2021).
As the COVID-19 vaccine coverage increases, the COVID-19 prevalence is expected to decrease to a low level. At some point, routine diagnostic testing for monitoring COVID-19 incidence/prevalence in large populations may become too expensive. Wastewater surveillance at a building level and institution level could complement surveillance based on diagnostic testing or symptom-based surveillance. Such a surveillance approach could be considerably less expensive to sustain. In addition, wastewater surveillance can be deployed to monitor for multiple pathogens simultaneously. Wastewater surveillance has been used globally for polio and typhoid (Andrews et al., 2020; Asghar et al., 2014; Hovi et al., 2012; Y. Wang et al., 2020) and could serve as a valuable tool at a national level to monitor current and emerging disease threats (Kirby et al., 2021).
4.5. Limitations
The current study had several limitations. First, it was not possible to collect weekly samples for all the residence halls, especially in the Fall 2020 semester, due to a shortage of some critical lab supplies caused by the pandemic. Second, some COVID-19 cases may shed virus in their feces for weeks to a month after they had negative conversion of respiratory samples and no symptoms. After students recovered from COVID-19 and were released from quarantine back to their residence hall, they could have continued shedding virus in their feces which would create “background noise” in the wastewater RT-PCR signal. Third, the majority of the university campus population was young healthy adults, who could have different fecal shedding of SARS-CoV-2, transmission patterns, and behavior compared to the general population.
Furthermore, the sensitivity of our wastewater surveillance method could still be improved. In our wastewater surveillance of the residence halls, we only had one sample per site per week for most of the study period, which could only capture fecal shedding for 1–2 days in a week. Also, in the early phase of this study we explored and compared several different methods for sample collection, virus concentration, and RNA extraction which may have affected our analyses of temporal trends in the first two months of the study. Finally, we did not have enough data to assess measurement error, which is necessary to understand the quantification of SARS-CoV-2 viral RNA in wastewater.
5. Conclusion
From July 2020 through March 2021, we examined the feasibility of routine wastewater-based surveillance for COVID-19 in three settings on the Emory University campus: a hospital building, an isolation/quarantine building, and multiple student residence halls in three Emory campuses. We used two wastewater sampling methods: grab samples of the wastewater from the hospital building and Moore swab samples of the wastewater from the hospital, the isolation/quarantine building, and 21 student residence halls. We observed that the wastewater surveillance results clearly reflected the trajectory of COVID-19 case numbers in the isolation/quarantine building and in the hospital building. However, this wastewater surveillance method was not sensitive enough to reliably capture sporadic single cases that emerged in individual residence halls. Routine wastewater sampling and analyses at an institution level with multiple sampling sites was a useful surveillance tool and provided an early warning of a surge in COVID-19 infections. Carefully-designed wastewater-based surveillance can be a low-cost strategy to supplement surveillance based on diagnostic testing.
CRediT authorship contribution statement
Yuke Wang: Conceptualization, Methodology, Software, Validation, Formal analysis, Writing – original draft, Writing – review & editing, Visualization, Supervision. Pengbo Liu: Conceptualization, Methodology, Investigation, Writing – review & editing, Supervision, Funding acquisition. Haisu Zhang: Software, Formal analysis, Writing – review & editing, Visualization. Makoto Ibaraki: Investigation, Writing – review & editing. Jamie VanTassell: Investigation, Writing – review & editing, Project administration. Kelly Geith: Investigation, Writing – review & editing. Matthew Cavallo: Investigation, Writing – review & editing. Rebecca Kann: Investigation, Writing – review & editing. Lindsay Saber: Investigation, Writing – review & editing. Colleen S. Kraft: Investigation, Writing – review & editing, Resources, Supervision. Morgan Lane: Investigation, Writing – review & editing. Samuel Shartar: Investigation, Writing – review & editing, Resources, Supervision. Christine Moe: Conceptualization, Methodology, Writing – review & editing, Resources, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was funded by the Rollins School of Public Health Dean's Rapid COVID-19 Pilot Awards and Emory University. We appreciate the support and critical discussions with Dr. Peter Teunis from Emory University. And we thank Sean Mann from Emory University for extracting Emory COVID-19 case information.
Editor: Warish Ahmed
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2022.153291.
Appendix A. Supplementary data
Supplementary material
References
- Andrews J.R., et al. Environmental surveillance as a tool for identifying high-risk settings for typhoid transmission. Clin. Infect. Dis. 2020;71(Supplement_2):S71–S78. doi: 10.1093/cid/ciaa513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar H., et al. Environmental surveillance for polioviruses in the global polio eradication initiative. J. Infect. Dis. 2014;210(Suppl. 1):S294–S303. doi: 10.1093/infdis/jiu384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt W.Q., et al. COVID-19 containment on a college campus via wastewater-based epidemiology, targeted clinical testing and an intervention. Sci. Total Environ. 2021;779 doi: 10.1016/j.scitotenv.2021.146408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivins A., et al. Building-level wastewater surveillance using tampon swabs and RT-LAMP for rapid SARS-CoV-2 RNA detection [10.1039/D1EW00496D] Environ. Sci. Water Res. Technol. 2022 doi: 10.1039/D1EW00496D. [DOI] [Google Scholar]
- Centers for Disease Control and Prevention COVID-19 Testing Overview. 2021. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/testing.html
- Chen C., et al. SARS-CoV-2–Positive sputum and feces after conversion of pharyngeal samples in patients with COVID-19. Ann. Intern. Med. 2020;172(12):832–834. doi: 10.7326/m20-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J. Med. Virol. 2020;92(7):833–840. doi: 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- Daughton C.G. Wastewater surveillance for population-wide Covid-19: the present and future. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibas C., et al. Implementing building-level SARS-CoV-2 wastewater surveillance on a university campus. Sci. Total Environ. 2021;782 doi: 10.1016/j.scitotenv.2021.146749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R., et al. COVID-19 surveillance in southeastern Virginia using wastewater-based epidemiology. Water Res. 2020;186 doi: 10.1016/j.watres.2020.116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood M. SalivaDirect: What You Need to Know About the New COVID-19 Test. Yale School of Medicine. 2021. https://medicine.yale.edu/news-article/27120/
- Harris-Lovett S., et al. Wastewater surveillance for SARS-CoV-2 on college campuses: initial efforts, lessons learned, and research needs. Int. J. Environ. Res. Public Health. 2021;18(9):4455. doi: 10.3390/ijerph18094455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovi T., et al. Role of environmental poliovirus surveillance in global polio eradication and beyond. Epidemiol. Infect. 2012;140(1):1–13. doi: 10.1017/s095026881000316x. [DOI] [PubMed] [Google Scholar]
- Jones D.L., et al. Shedding of SARS-CoV-2 in feces and urine and its potential role in person-to-person transmission and the environment-based spread of COVID-19. Sci. Total Environ. 2020;749 doi: 10.1016/j.scitotenv.2020.141364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikeyan S., et al. Rapid, large-scale wastewater surveillance and automated reporting system enable early detection of nearly 85% of COVID-19 cases on a university campus. mSystems. 2021;6(4) doi: 10.1128/mSystems.00793-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.-J., et al. The delay in confirming COVID-19 cases linked to a religious group in Korea. J. Prev. Med. Public Health. 2020;53(3):164–167. doi: 10.3961/jpmph.20.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby A.E., et al. Using wastewater surveillance data to support the COVID-19 response — United States, 2020–2021. MMWR Morb. Mortal. Wkly Rep. 2021;70(36):1242–1244. doi: 10.15585/mmwr.mm7036a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer S.A., et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020;172(9):577–582. doi: 10.7326/m20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., et al. Association between detectable SARS-COV-2 RNA in anal swabs and disease severity in patients with coronavirus disease 2019. J. Med. Virol. 2021;93(2):794–802. doi: 10.1002/jmv.26307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., et al. Hollow-fiber ultrafiltration for simultaneous recovery of viruses, bacteria and parasites from reclaimed water. J. Microbiol. Methods. 2012;88(1):155–161. doi: 10.1016/j.mimet.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Liu P., et al. A sensitive, simple, and low-cost method for COVID-19 wastewater surveillance at an institutional level. Sci. Total Environ. 2021:151047. doi: 10.1016/j.scitotenv.2021.151047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., et al. US CDC real-time reverse transcription PCR panel for detection of severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis. 2020;26(8):1654. doi: 10.3201/eid2608.201246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park A. For $5 and in 15 minutes you can learn if you have COVID-19. Time. 2020. https://time.com/5884012/15-minute-coronavirus-test/
- Park S.-K., et al. Detection of SARS-CoV-2 in fecal samples from patients with asymptomatic and mild COVID-19 in Korea. Clin. Gastroenterol. Hepatol. 2020 doi: 10.1016/j.cgh.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J., et al. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020;38(10):1164–1167. doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . 2013. R: a language and environment for statistical computing. [Google Scholar]
- Saguti F., et al. Surveillance of wastewater revealed peaks of SARS-CoV-2 preceding those of hospitalized patients with COVID-19. Water Res. 2021;189 doi: 10.1016/j.watres.2020.116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020 doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., et al. Fecal viral shedding in COVID-19 patients: clinical significance, viral load dynamics and survival analysis. Virus Res. 2020;289 doi: 10.1016/j.virusres.2020.198147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., et al. Designing a typhoid environmental surveillance study: a simulation model for optimum sampling site allocation. Epidemics. 2020;31 doi: 10.1016/j.epidem.2020.100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M.C., et al. Detection of SARS-CoV-2 RNA in fecal specimens of patients with confirmed COVID-19: a meta-analysis. J. Infect. 2020;81(2):e31–e38. doi: 10.1016/j.jinf.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., et al. SARS-CoV-2 RNA concentrations in wastewater foreshadow dynamics and clinical presentation of new COVID-19 cases. Sci. Total Environ. 2022;805 doi: 10.1016/j.scitotenv.2021.150121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020;5(5):434–435. doi: 10.1016/s2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material