Abstract
Objectives
Understanding the role of certain salivary components, such as TNF-⍺, IL-6, IL-10, lactoferrin, lysozyme, IgG, IgA, and IgM, in airway defense during the ongoing SARS-CoV-2 pandemic is essential. The salivary immune barrier of patients with COVID-19 may play a role in their prognosis. The present study aims to evaluate the impact of SARS-CoV-2 on saliva composition.
Methods
A longitudinal study was carried out with male and female firefighters aged 24–48 years. The study sample (n = 34) was divided into 3 groups: asymptomatic volunteers with a negative polymerase chain reaction (PCR) test for SARS-CoV-2 (group 1, Control, n = 21); patients with symptoms of COVID-19 of less than 7 days’ duration and a diagnosis of SARS-CoV-2 infection by PCR (group 2, COVID-19, n = 13); and recovered patients from group 2 who were free of COVID-19 symptoms for at least 2 months (group 3, post-COVID-19 recovery, n = 13). All groups underwent real-time PCR to detect the presence of SARS-CoV-2, as well as analysis of the salivary concentrations of TNF-⍺, IL-6, IL-10, lactoferrin, lysozyme, IgG, IgA, and IgM by the ELISA method.
Results
Lactoferrin concentrations were significantly decreased in the infected group (COVID-19) when compared to those not infected by SARS-CoV-2 (control) (p = 0.032). IgA concentrations were decreased in the COVID-19 and post-COVID-19 groups compared to the control group (p = 0.005 and p = 0.016, respectively). Comparison of the COVID-19 and post-COVID-19 groups also revealed an increase in IgM concentrations during acute SARS-CoV-2 infection (p = 0.010).
Conclusion
SARS-CoV-2 alters the composition of the salivary immune barrier.
Keywords: Saliva, COVID-19, Antimicrobial peptides, Interleukins, Immunoglobulins
Abbreviation: ACE2, Angiotensin-converting enzyme II; COVID-19, Coronavirus disease 2019; CoVs, Coronaviruses; IFN, Interferon; IL, Interleukins; Lf, Lactoferrin; TNF-⍺, Tumor necrosis factor-alpha; WHO, World Health Organization
1. Introduction
In December 2019, a cluster of pneumonia cases of unknown etiology emerged in Wuhan, China [1]. As the disease continued to spread rapidly, an epidemiological and etiological investigation detected a novel type of coronavirus [2]. This infection was named coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO) [3]. In a short time, the virus spread globally and was declared a public health emergency of international concern by WHO [4].
Coronaviruses (CoVs) are enveloped, single-stranded RNA viruses belonging to the Coronaviridae family [5]. Person-to-person spread occurs through close contact, either with patients or with asymptomatic carriers [6]. Close contact or short-range transmission of infectious saliva droplets is the primary mode of SARS-CoV-2 spread, while long-range transmission of saliva via aerosols is highly dependent on the environment, occurring mostly in aerosol-generating indoor settings [7]. Once in the body, the coronavirus enters host cells by binding via its spike glycoprotein to the angiotensin-converting enzyme II (ACE2) receptor [5,8]. After fusion, viral RNA is released into the cytoplasm and forms a complex for replication and transcription, which will produce proteins for the assembly of new virions [6].
COVID-19 has an incubation period of approximately 4–6 days [9]. It affects mostly males (55.6%), with a mean age of 46.62 years [10], although the age range is quite broad [11,12]. The clinical manifestations of COVID-19 are protean, varying widely in terms of both symptoms and severity. Signs and symptoms include fever, conjunctival injection, headache, cough, hemoptysis, fatigue, nausea, vomiting, diarrhea, arthralgia, dyspnea, anorexia, myalgia, abdominal pain, and weakness [4,13]. Fever, cough, and dyspnea are the most common manifestations [14].
The innate immune response is the first to be triggered in the presence of the virus, inducing the expression of inflammatory factors, dendritic cell maturation, and interferon (IFN) synthesis; this speeds virus phagocytosis by macrophages, thus slowing its spread [15]. However, previous studies of SARS-CoV-2 have shown that its N protein is capable of interfering with the RNA recognition step of the innate immune response, thus suppressing IFN synthesis [16].
The adaptive immune response begins with the activation of CD4 and CD8 T lymphocytes, which aim to stimulate the production of antibodies by B cells and pro-inflammatory cytokines by T cells, respectively. Another mechanism of CoV pathogenesis is the inhibition of T-cell functions, culminating in their apoptosis [5]. During the disease course, there is an increase in levels of cytokines such as interleukins (IL) (IL-1, IL-2, IL-4, IL-7, IL-10, IL12, IL-13, IL-17), granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN gamma, and tumor necrosis factor; this is one of the major mechanisms implicated in clinical deterioration [6,17].
Saliva is a fluid secreted by salivary glands in the oral cavity. It is composed of water, electrolytes, and proteins, among other constituents. Among the component proteins of saliva, lactoferrin, lysozyme, cytokines, and immunoglobulins are key, as they act as a first line of immune defense against the entry of harmful agents [18].
Lactoferrin (Lf) is a naturally occurring, multifunctional globular glycoprotein present in saliva, tears, sperm, and colostrum, with the highest available concentrations in the latter. In vitro, Lf has shown antiviral efficacy against a wide range of viruses, including SARS-CoV-2. In addition, it has unique immunomodulatory and anti-inflammatory effects that may be especially relevant to the pathophysiology of severe cases of COVID-19 [19].
Lysozyme, in turn, is an enzyme capable of hydrolyzing bacterial cell wall bonds. It can be found in tears, mucus, breast milk, and saliva. By its mechanism of action, it contributes to the immune response against infections [20].
Cytokines are glycoproteins capable of modulating the host inflammatory response. Interleukin 6 (IL-6) and tumor necrosis factor-alpha (TNF-⍺) are two of the main pro-inflammatory cytokines. IL-6 is released by macrophages and T cells, playing an important role in fever induction and in the response to acute inflammation, stimulating the production of other cytokines and B cell growth. TNF-⍺ is produced by macrophages, lymphocytes, and monocytes, and its release recruits neutrophils and monocytes to the site of inflammation [21].
Conversely, interleukin 10 (IL-10) is a major immunosuppressant and antipyretic agent. It is a macrophage deactivating factor and has neutrophil-inhibiting effects. In fact, some infectious agents induce IL-10 synthesis or encode their own IL-10 homologs, resulting in suppression of the host immune response [22]. There are five different classes of immunoglobulins (IgM, IgG, IgE, IgA, and IgD). IgA protects against infections of the mucous membranes that line the mouth, airways, and gastrointestinal tract. It is also found in saliva, tears, and breast milk. IgM is the first immunoglobulin to appear in the immune response, clearing pathogens in their earliest stages. IgG is the main type of antibody (accounting for three-quarters of total blood immunoglobulin), especially in secondary responses, and its levels can be elevated in several conditions, including autoimmune diseases and infections. It is capable of entering tissues to fight infection [23].
Understanding the role of certain salivary components in airway defense is essential in a pandemic such as COVID-19. Furthermore, saliva itself offers an ecological niche for colonization by oral microorganisms, which can prevent the overgrowth of other, more aggressive pathogens. It is also useful as a noninvasive diagnostic tool for virus detection and surveillance of immune status. On the other hand, it is also the main substrate for viral transmission, through droplets and aerosols [24].
In COVID-19, respiratory failure is the dominant condition in more severe cases, whether due to diffuse alveolar damage caused by inflammation or to direct infection of the lung tissues. Since secondary pulmonary infections can be partially facilitated or hindered by changes in the innate and adaptive barriers of the upper airways, such as saliva, research into the effects of SARS-CoV-2 on the oral mucosa is essential to understand whether this virus modifies this immune barrier and, if so, how and to which extent this influences its penetration into the host [25].
2. Materials and methods
2.1. Population
This longitudinal study was carried out on a sample of firefighters from the city of São Paulo, of both sexes, aged between 24 and 48 years. The project was approved by the Research Ethics Committee and registered on Plataforma Brasil (CAAE: 39223320.9.1.1001.5505).
The inclusion criteria were age between 18 and 65 years and having passed the annual fire department physical evaluation. The exclusion criteria were: unwillingness to participate in the study; chronic or acute sinusitis; history of chronic diseases of the oral cavity, pharynx, or larynx; systemic or autoimmune diseases; and chronic intake of corticosteroids and/or anti-inflammatory drugs up to one month before specimen collection.
The sample comprised 34 volunteers. For research purposes, it was divided into 3 groups: asymptomatic volunteers with a negative PCR test for SARS-CoV-2 (group 1, Control, n = 21); patients with symptoms of COVID-19 of less than 7 days’ duration, no indications for hospital admission, and a diagnosis of SARS-CoV-2 infection by PCR (group 2, COVID-19, n = 13); and participants from group 2 once they had been free of COVID-19 symptoms for at least 2 months (group 3, recovery, n = 13). In all groups, concentrations of TNF-⍺, IL-6, IL-10, lactoferrin, lysozyme, IgG, IgA, and IgM in saliva were analyzed.
2.2. Laboratory testing of collected specimens
Participants’ saliva was stored at −80 °C at an Otorhinolaryngology Research Laboratory where TNF-⍺, IL-6, IL-10, lactoferrin, lysozyme, IgG, IgA, and IgM measurements were also performed.
Cytokines (IL-6, IL-10, and TNF-α) were quantitated using commercial ELISA assays (Thermo Fisher Scientific, Invitrogen, Vienna, Austria; catalog numbers 88-7066, 88-7106, and 88-7346 respectively), following the manufacturer's instructions, and results expressed as pg/mL. The antimicrobial peptides (lactoferrin and lysozyme) were also measured by ELISA (EZ Assays, Florida, USA). Results were expressed as μg/mL for lactoferrin and ng/mL for lysozyme (catalog numbers Ck-bio-12222 and Ck-bio-12360, respectively).
Concentrations of anti-SARS-CoV-2 IgA, IgM, and IgG were also determined by ELISA in the saliva specimens. Reaction plates were coated with optimal concentrations of SARS-CoV-2 N, M, and S antigens. After blocking of non-specific binding sites, saliva specimens were diluted in PBS-Tween 0.1% buffer containing 1M NaCl, at a 1:2000 ratio for IgA and IgM and 1:5000 for IgG, added to the previously coated and blocked plate, and left to incubate for at least 2 h at 37 °C. After incubation and washing of each well of the plate, human anti-IgA (1:2000), anti-IgM (1:2000), and anti-IgG (1:10,000) conjugate were added and the plate kept for at least 1 h at 37 °C. The reaction was assessed by adding TMB solution to each well of the plate, followed by a stop buffer (4N H2SO4) to interrupt the reaction. The reaction was then read at 450 nm in a microplate reader (Labsystem Mulitskan MS).
The measured concentrations of antimicrobial peptides (lactoferrin and lysozyme), cytokines (IL-6, IL-10, TNF-⍺), and immunoglobulins (IgA, IgM, and IgG) in saliva were then compared between groups to assess the impact of SARS-CoV-2.
2.3. Statistical analysis
Data were analyzed in Jamovi version 1.8.4. Quantitative variables were described as mean (standard deviation) or median (interquartile range) as appropriate. The Shapiro–Wilk test was used to test the assumption of normality for quantitative variables. Between-group homogeneity of age and sex distribution was evaluated using Student's t-test for independent samples and Fisher's exact test, respectively. The nonparametric Wilcoxon test was used to compare the expression of the markers of interest between the COVID-19 and post-COVID-19 groups. Comparisons among the COVID-19, post-COVID-19, and Control groups were performed using Student's t-test or the nonparametric Mann–Whitney test. P-values <0.05 were considered statistically significant.
3. Results
First, groups were examined for homogeneity regarding age and sex. Table 1 below presents descriptive statistics for these two variables across groups, with the respective p-values.
Table 1.
Comparison of groups by age and sex.
| Variable | Category | Group |
p∗ | |
|---|---|---|---|---|
| Control (n = 21) | COVID (n = 13) | |||
| Age (years) | Mean ± SD Range (min–max) | 34.4 ± 6.0 (24–44) | 39.8 ± 6.4 (28–48) | 0.019∗ |
| Sex | Male | 19 (90.5%) | 12 (92.3%) | |
| Female | 2 (9.5%) | 1 (7.7%) | 1 | |
∗p < 0.05.
The results indicate that there is a significant difference in participant age between the groups (p = 0.019). On average, participants in the COVID-19 group were 5.3 years older than those in the control group. No significant difference was found regarding sex distribution (p = 1).
Analysis of TNF-⍺, IL-6, IL-10, lactoferrin, lysozyme, IgG, IgA, and IgM was done considering comparisons of the study groups (COVID-19 and post-COVID-19) with the control group, as well as comparison of the two-time points of evaluation of the study groups (COVID-19 versus post-COVID-19) (Table 2 ; Fig. 1 ; Fig. 2 ).
Table 2.
Comparative analysis of cytokines in saliva of study groups (COVID-19 and post-COVID-19) and control group.
| Variable | Group | n | Mean ± sd | Median (IQR) | p∗ (COVID-19 vs. post-COVID-19) | p∗∗ (COVID-19 vs.Ctrl) | p∗ (post-COVID-19 vs. Ctrl) |
|---|---|---|---|---|---|---|---|
| TNF-α | COVID-19 | 13 | 75.4 ± 51.2 | 56.3 (10.1) | |||
| Post-COVID-19 | 13 | 57.7 ± 21.2 | 53.6 (20.9) | ||||
| Control |
21 |
64.1 ± 32.7 |
52.5 (9.7) |
0.946 |
0.405 |
0.701 |
|
| IL-6 | COVID-19 | 13 | 8.25 ± 2.14 | 7.54 (1.27) | |||
| Post-COVID-19 | 13 | 7.83 ± 1.51 | 7.38 (1.07) | ||||
| Control |
21 |
9.41 ± 6.59 |
8 (0.78) |
0.839 |
0.523 |
0.190 |
|
| IL-10 | COVID-19 | 13 | 10.01 ± 2.05 | 9.51 (2.74) | |||
| Post-COVID-19 | 13 | 9.08 ± 1.17 | 8.55 (1.29) | ||||
| Control |
21 |
10.83 ± 2.02 |
11.11 (2.75) |
0.244 |
0.263 |
0.011∗ |
|
| Lactoferrin | COVID-19 | 13 | 5.82 ± 6.99 | 3.39 (3.08) | |||
| Post-COVID-19 | 13 | 7.97 ± 5.93 | 5.34 (9.4) | ||||
| Control |
21 |
8.43 ± 6.94 |
6.12 (3.2) |
0.191 |
0.032∗ |
0.861 |
|
| Lysozyme | COVID-19 | 13 | 61.2 ± 31.4 | 45.7 (28.6) | |||
| Post-COVID-19 | 13 | 56.6 ± 37.3 | 43.1 (25.1) | ||||
| Control |
21 |
46.2 ± 22.0 |
37.6 (26.4) |
0.588 |
0.111 |
0.478 |
|
| IgG | COVID-19 | 13 | 0.073 ± 0.015 | 0.077 (0.021) | |||
| Post-COVID-19 | 13 | 0.085 ± 0.063 | 0.067 (0.006) | ||||
| Control |
21 |
0.094 ± 0.037 |
0.098 (0.053) |
0.376 |
0.064 |
0.178 |
|
| IgA | COVID-19 | 13 | 0.173 ± 0.048 | 0.148 (0.075) | |||
| Post-COVID-19 | 13 | 0.193 ± 0.066 | 0.195 (0.059) | ||||
| Control |
21 |
0.290 ± 0.202 |
0.225 (0.068) |
0.376 |
0.005∗ |
0.016∗ |
|
| IgM | COVID-19 | 13 | 0.557 ± 0.995 | 0.233 (0.216) | |||
| Post-COVID-19 | 13 | 0.146 ± 0.067 | 0.153 (0.106) | ||||
| Control | 21 | 0.230 ± 0.205 | 0.177 (0.108) | 0.010∗ | 0.292 | 0.381 |
sd: standard deviation.
∗Nonparametric Wilcoxon test; p < 0.05.
∗∗Student's t-test for independent samples or nonparametric Mann–Whitney test; p < 0.05.
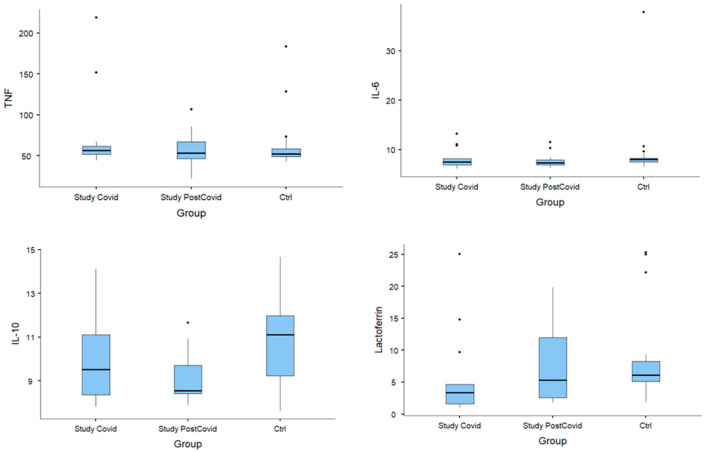
Fig. 1.
Median; 25%–75%; minimum–maximum considering COVID-19 group, post-COVID-19 group, and control group. TNF, IL-6, IL-10 and Lactoferrin.
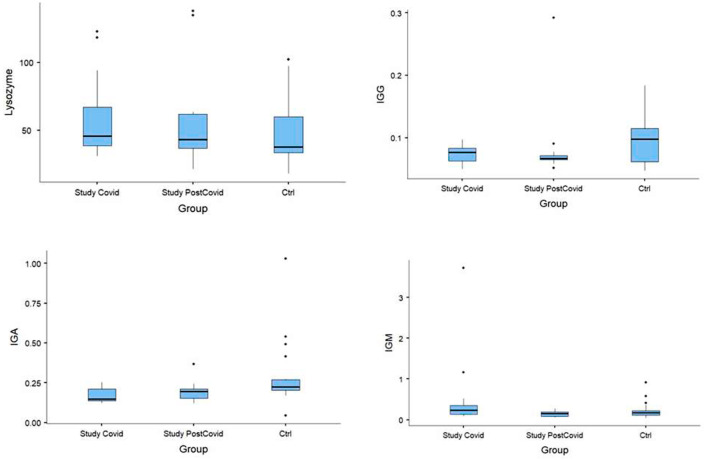
Fig. 2.
Median; 25%–75%; minimum–maximum considering COVID-19 group, post-COVID-19 group, and control group. Lysozyme, IgG, IgA and IgM.
Compared to the control group, the COVID-19 group had statistically significant reductions in lactoferrin (p = 0.032) and IgA (p = 0.005). IgA also remained below control levels in the post-COVID-19 group (p = 0.016). IgM concentrations were increased in the acute phase of infection (i.e., the COVID-19 group), with p = 0.01. The post-COVID-19 group showed a decrease in IL-10 concentrations compared to the control (p = 0.011). There was no statistically significant difference in between-group comparisons of TNF-⍺, IL-6, lysozyme, or IgG.
4. Discussion
Although there is a statistical difference in age between the control group and the infected group, this difference was not clinically important, as the mean age ranged from 34 to 39 years between groups. Regarding sex, studies have reported an alternating predominance among those infected with SARS-CoV-2 [13,26]. In our study, as our sample was composed of firefighters and firefighting is a predominantly male occupation, a higher prevalence of male patients was expected. No significant difference in sex distribution was found between the control and COVID-19 groups.
The pro-inflammatory cytokines IL-6 and TNF were not significantly altered in patients with COVID-19, suggesting that there was no intense inflammatory response in the saliva. However, a decrease in concentrations of IL-10 was observed in the post-COVID-19 group when compared to the control. This cytokine promotes immune regulation, inactivating macrophages and limiting the inflammatory response. A prior study, however, found that one of the features of the immune response in COVID-19 is marked elevation of serum concentrations of IL-10, especially in patients with a severe and critical illness. Although the exact mechanism of this response remains unknown, meta-analyses have found that high levels of IL-10 could be used as predictors of disease severity. In short, this cytokine is believed to play a prognostic role in the pathophysiology of SARS-CoV-2 infection [22]. In blood samples from 102 hospitalized patients with COVID-19, categorized into moderate, severe, and critical conditions, high serum concentrations of TNF-⍺, IFN-gamma, IL-2, IL-4, IL-6, and IL-10 were found in comparison to a control group. The same study also found that measurement of IL-6 and IL-10 could be used as a rapid means of identifying patients at higher risk of progressing to severe disease [18].
Research has reported elevation of IL-1, IL-2, IL-4, IL-7, IL-10, IL12, IL-13, IL-17, GM-CSF, IFN-gamma, TNF during the disease course of COVID-19, and suggested that this is one of the major mechanisms implicated in clinical deterioration [6,17]. Many studies have measured cytokine levels in the peripheral blood of critically ill patients during the acute phase of illness, unlike this study, in which salivary cytokine levels were evaluated in patients with mild COVID-19. In contrast, Merza et al. compared cytokine levels in the blood of patients with moderate COVID-19, severe COVID-19, and recovered from COVID-19, and found IL-10 concentrations to be significantly higher in the group of recovered patients compared to those with active disease; this may corroborate its role in regulating inflammation [27].
In the present study, there was no significant difference in IL-6 concentrations between the control and COVID-19 groups. However, in other studies which evaluated serum levels of inflammatory markers, IL-6 values were found to be increased in patients with COVID-19. Furthermore, this increase occurred progressively with the severity of the condition. In addition, some studies reported that IL-10 levels increased earlier than those of IL-6 in the blood of critically ill patients [22]. As a pro-inflammatory cytokine, IL-6 is commonly increased in sepsis, acute respiratory distress syndrome, and in major burns [28]. Similarly, in another study with oral samples, the increase of IL-6, IL-5, IL-2, and TNF-⍺ in the COVID-19 group was correlated to a distinctive microbiota: smaller species richness and predominance of Prevotella salivae and Veilonella infantium, which shows the importance of these changes [23].
Other results were found in analyzes of patients infected with influenza A (H1N1) during the 2009 pandemic. In these individuals, significantly elevated concentrations of IL-2, IL-12, IFN-gamma, IL-6, TNF-⍺, IL-5, IL-10, IL-17, and IL-23 were observed in comparison with healthy controls. Furthermore, even more, marked elevations of IL-6 and IL-10 were observed in patients with severe disease during the pandemic [29]. Our study suggests that levels of inflammatory markers in the saliva may not correspond to the systemic inflammatory process of COVID-19, at least in mild to moderate cases.
The decrease in lactoferrin in acutely infected patients suggests a loss of antiviral protection against a wide range of viruses, including SARS-CoV-2. Furthermore, as lactoferrin has unique immunomodulatory and anti-inflammatory effects that may play an important role in the pathophysiology of severe cases of COVID-19 [19], these patients may be susceptible to secondary airway infections.
The role of lactoferrin in the treatment of SARS-CoV-2 infection is still being studied. One of the ways this protein may prevent COVID-19 is by interfering with receptors used by the virus to bind to the host cells, such as heparan sulfate proteoglycans. In addition, lactoferrin is involved in the sequestration of iron and inflammatory molecules, which are elevated in a viral infection-induced cytokine storm. Furthermore, lactoferrin improves NK cell activity and stimulates neutrophil aggregation, both of which are important in the immune response against infectious diseases [30].
Regarding IgA, we found reduced concentrations of this immunoglobulin among patients who contracted COVID-19, and this reduction persisted even after recovery. As IgA protects against infection of the mucous membranes that line the oral cavity, airways, and digestive tract [21], reduced levels may compromise this defense mechanism and facilitate infection by SARS-CoV-2 and by other pathogens, both during and after infection.
Our findings are interesting insofar as they raise the question of whether IgA production was impaired by SARS-CoV-2 infection (which would have repercussions for the development of secondary infections) or whether patients with a preexisting low level of IgA were thus predisposed to SARS-CoV-2 infection. The latter hypothesis is most tempting because IgA levels remained low after complete recovery from COVID-19 symptoms. Further studies are needed to elucidate this finding.
Analysis of IgM concentrations showed an expected increase in the acute phase of the infection, followed by a decline in the convalescent phase. Despite the small sample size and the predominance of young males with no co-morbidities as participants, we believe that the changes observed in salivary lactoferrin and IgA suggest impairment of the immunoprotective mechanisms of the mucosal barrier in patients infected with SARS-CoV-2. Further studies are necessary to elucidate these causal links and their prognostic role for host responses.
5. Conclusion
The findings of this study demonstrate that the salivary immune defenses are altered in patients with COVID-19, with a particular decrease in lactoferrin and IgA concentration and an increase in IgM concentration.
Ethical approval
The project was approved by the Research Ethics Committee and registered on Plataforma Brasil (CAAE: 39223320.9.1.1001.5505).
CRediT authorship contribution statement
Júlia Gaspar de Oliveira Santos: Conceptualization, Funding acquisition, Project administration, Resources, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Débora Petrungaro Migueis: Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Jônatas Bussador do Amaral: Conceptualization, Funding acquisition, Project administration, Resources, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. André Luis Lacerda Bachi: Conceptualization, Funding acquisition, Project administration, Resources, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Alexandre Coelho Boggi: Conceptualization, Funding acquisition, Project administration, Resources, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Andrew Thamboo: Conceptualization, Funding acquisition, Project administration, Resources, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Richard Louis Voegels: Conceptualization, Funding acquisition, Project administration, Resources, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Rogério Pezato: Conceptualization, Funding acquisition, Project administration, Resources, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Conflict of interest
No potential conflicts of interest are disclosed.
Acknowledgments
We acknowledge the São Paulo Fire Department and its medical department for their support with the recruitment of volunteers for this study.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel Coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Coronavirus disease (COVID-19) outbreak. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed 30 Set 2021.
- 4.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi Y., Lagniton P.N.P., Ye S., Li E., Xu R.-H. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16(10):1753–1766. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu R., Cui B., Duan X., Zhang P., Zhou X., Yuan Q. Saliva: potential diagnostic value and transmission of 2019-nCoV. Int J Oral Sci. 2020;12(1):11. doi: 10.1038/s41368-020-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11(1):1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park M., Cook A.R., Lim J.T., Sun Y., Dickens B.L. A systematic review of COVID-19 epidemiology based on current evidence. J Clin Med. 2020;9(4):967. doi: 10.3390/jcm9040967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao Y., Liu X., Xiong L., Cai K. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: a systematic review and meta-analysis. J Med Virol. 2020;92(9):1449–1459. doi: 10.1002/jmv.25822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China, of novel Coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parri N., Lenge M., Buonsenso D., Coronavirus Infection in Pediatric Emergency Departments (CONFIDENCE) Research Group Children with Covid-19 in pediatric emergency departments in Italy. N Engl J Med. 2020;383(2):187–190. doi: 10.1056/NEJMc2007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A., et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Oto-Rhino-Laryngol. 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krajewska J., Krajewski W., Zub K., Zatoński T. COVID-19 in otolaryngologist practice: a review of current knowledge. Eur Arch Oto-Rhino-Laryngol. 2020;277(7):1885–1897. doi: 10.1007/s00405-020-05968-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ben Addi A., Lefort A., Hua X., Libert F., Communi D., Ledent C., et al. Modulation of murine dendritic cell function by adenine nucleotides and adenosine: involvement of the A(2B) receptor. Eur J Immunol. 2008;38(6):1610–1620. doi: 10.1002/eji.200737781. [DOI] [PubMed] [Google Scholar]
- 16.Lu X., Pan J., Tao J., Guo D. SARS-CoV nucleocapsid protein antagonizes IFN-β response by targeting initial step of IFN-β induction pathway, and its C-terminal region is critical for the antagonism. Virus Gene. 2011;42(1):37–45. doi: 10.1007/s11262-010-0544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C., Zhang X.R., Ju Z.Y., He W.F. Advances in the research of mechanism and related immunotherapy on the cytokine storm induced by coronavirus disease 2019. Zhonghua Shaoshang Zazhi. 2020;36(6):471–475. doi: 10.3760/cma.j.cn501120-20200224-00088. [DOI] [PubMed] [Google Scholar]
- 18.Humphrey S.P., Williamson R.T. A review of saliva: normal composition, flow, and function. J Prosthet Dent. 2001;85(2):162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 19.Chang R., Ng T.B., Sun W.Z. Lactoferrin as a potential preventative and adjunct treatment for COVID-19. Int J Antimicrob Agents. 2020;56(3):106118. doi: 10.1016/j.ijantimicag.2020.106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ragland S.A., Criss A.K. From bacterial killing to immune modulation: recent insights into the functions of lysozyme. PLoS Pathog. 2017;13(9):e1006512-e. doi: 10.1371/journal.ppat.1006512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreira V.L., Borba H.H.L., Bonetti A.F., Leonart L.P., Pontarolo R. Autoantibodies and cytokines [Working Title] IntechOpen; 2018. Cytokines and interferons: types and functions. [Google Scholar]
- 22.Lu L., Zhang H., Dauphars D.J., He Y.W. A potential role of interleukin 10 in COVID-19 pathogenesis. Trends Immunol. 2021;42(1):3–5. doi: 10.1016/j.it.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iebba V., Zanotta N., Campisciano G., Zerbato V., Di Bella S., Cason C., et al. Profiling of oral microbiota and cytokines in COVID-19 patients. Front Microbiol. 2021;12:671813. doi: 10.3389/fmicb.2021.671813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baghizadeh Fini M. Oral saliva and COVID-19. Oral Oncol. 2020;108(104821):104821. doi: 10.1016/j.oraloncology.2020.104821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elezkurtaj S., Greuel S., Ihlow J., Michaelis E.G., Bischoff P., Kunze C.A., et al. Causes of death and comorbidities in hospitalized patients with COVID-19. Sci Rep. 2021;11(1):4263. doi: 10.1038/s41598-021-82862-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merza M.Y., Hwaiz R.A., Hamad B.K., Mohammad K.A., Hama H.A., Karim A.Y. Analysis of cytokines in SARS-CoV-2 or COVID-19 patients in Erbil city, Kurdistan Region of Iraq. PLos One. 2021;16(4):e0250330. doi: 10.1371/journal.pone.0250330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Q.Q., Cheng A., Wang Y., Li H., Hu L., Zhao X., et al. Cytokines and their relationship with the severity and prognosis of coronavirus disease 2019 (COVID-19): a retrospective cohort study. BMJ Open. 2020;10(11):e041471. doi: 10.1136/bmjopen-2020-041471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Influenza virus induced cytokine responses: an evaluation of host-pathogen association. Immunome Res. 2014;1(s2) doi: 10.4172/1745-7580.s2.001. [Internet]. Available from: [DOI] [Google Scholar]
- 30.Kell D.B., Heyden E.L., Pretorius E. The biology of lactoferrin, an iron-binding protein that can help defend against viruses and bacteria. Front Immunol. 2020;11:1221. doi: 10.3389/fimmu.2020.01221. [DOI] [PMC free article] [PubMed] [Google Scholar]