Abstract
Aims
Chronic heart failure (CHF) has an increasing burden of comorbidities, which affect clinical outcomes. Few studies have focused on the clustering and hierarchical management of patients with CHF based on comorbidity. This study aimed to explore the cluster model of CHF patients based on comorbidities and to verify their relationship with clinical outcomes.
Methods and results
Electronic health records of patients hospitalized with CHF from January 2014 to April 2019 were collected, and 12 common comorbidities were included in the latent class analysis. The Fruchterman–Reingold layout was used to draw the comorbidity network, and analysis of variance was used to compare the weighted degrees among them. The incidence of clinical outcomes among different clusters was presented on Kaplan–Meier curves and compared using the log‐rank test, and the hazard ratio was calculated using the Cox proportional risk model. Sensitivity analysis was performed according to the left ventricular ejection fraction. Four different clinical clusters from 4063 total patients were identified: metabolic, ischaemic, high comorbidity burden, and elderly‐atrial fibrillation. Compared with the metabolic cluster, patients in the high comorbidity burden cluster had the highest adjusted risk of combined outcome and all‐cause mortality {1.67 [95% confidence interval (CI), 1.40–1.99] and 2.87 [95% CI, 2.17–3.81], respectively}, followed by the elderly‐atrial fibrillation and ischaemic clusters. The adjusted readmission risk of patients with ischaemic, high comorbidity burden, and elderly‐atrial fibrillation clusters were 1.35 (95% CI, 1.08–1.68), 1.39 (95% CI, 1.13–1.72), and 1.42 (95% CI, 1.14–1.77), respectively. The comorbidity network analysis found that patients in the high comorbidity burden cluster had more and higher comorbidity correlations than those in other clusters. Sensitivity analysis revealed that patients in the high comorbidity burden cluster had the highest risk of combined outcome and all‐cause mortality (P < 0.05).
Conclusions
The difference in adverse outcomes among clusters confirmed the heterogeneity of CHF and the importance of hierarchical management. This study can provide a basis for personalized treatment and management of patients with CHF, and provide a new perspective for clinical decision making.
Keywords: Chronic heart failure, Comorbidity, LCA, Mortality, Readmission
Introduction
Comorbidities are very common in patients with heart failure (HF), especially in elderly patients and those in advanced stages of disease, and the burden of comorbidities is increasing. 1 In Asia, 64% of patients with HF have two or more comorbidities. 2 The existence of multiple comorbidities increases the heterogeneity of HF patients, and makes the diagnosis, treatment, and management of patients face great challenges, affecting the precision medicine of patients and increasing the risk of adverse outcomes. 3 , 4
Most previous clinical studies have focused on the relationship between single comorbidities such as atrial fibrillation (AF), 5 chronic kidney disease, 6 depression, 7 and the clinical outcome of HF, while few studies have focused on the cluster model of multiple comorbidities in HF. Understanding the clustering of comorbidities in patients with HF and its impact on major adverse outcome events can lay the foundation for clinically personalized treatment programmes. 8 HF is currently classified by left ventricular ejection fraction (LVEF), which does not reflect the true nature of HF as a complex heterogeneous syndrome that includes cardiovascular and non‐cardiovascular factors related to its pathophysiology and prognosis, 9 , 10 , 11 especially when there may be a two‐way causality between HF and comorbidities, and there may be an interaction between LVEF and comorbidities.
This study used the latent class analysis (LCA) method to determine the clustering pattern of patients with CHF based on comorbidities, analyse the composition and network relationship of comorbidities in different clusters and the impact on clinical outcomes to reduce treatment complexity for patients with multiple comorbidities, and provide a new perspective for patients' personalized decision making.
Methods
Data sources and study population
This was a prospective cohort study. Patients who were hospitalized and diagnosed with CHF at the First Hospital of Shanxi Medical University and Shanxi Cardiovascular Hospital from January 2014 to April 2019 were selected. The investigation conforms with the principles outlined in the Declaration of Helsinki, and written informed consent for participation was obtained from all patients.
Eligible patients met the following criteria: (i) age ≥18 years, (ii) diagnosed with HF according to the guidelines, 12 (iii) New York Heart Association (NYHA) function Class II–IV, and (iv) use of HF drugs and other treatment measures.
The exclusion criteria were patients with acute cardiovascular events in the past 2 months, concurrent mental illness, inability to understand or complete the questionnaire due to speech and intellectual disabilities, and refusal to participate in this project.
Comorbidity variables
The inpatient information was collected according to the case report form of chronic heart failure (CHF‐CRF) developed by our group based on the content of the case records and the guidelines for HF. 3 , 12 Comorbidity variables used in the LCA 13 included AF, old myocardial infarction (OMI), valvular heart disease (VHD), hyperlipidaemia (HLP), stroke, lung disease, type II diabetes, hypertension, renal disease, obesity, anaemia, and cancer. The definitions of comorbidities are provided in Supporting information, Table S1. Age, sex, treatment, and other variables were not used in the LCA analysis, but these variables were adjusted in the final analysis stage.
Data pre‐processing
To make full use of clinical information, the factorial analysis for mixed data (FAMD) method was used to fill in the missing data, and there were no missing values for comorbid variables.
Outcomes
The patients were followed up at 1, 3, 6, and 12 months after discharge, and then once a year. The primary outcome was the combined outcome (readmission or death), and the secondary outcomes were HF‐specific readmission and all‐cause mortality, including death from HF, cardiovascular disease, and other causes. The patient's HF‐specific readmission records of patients were indexed based on each patient's unique hospitalization code. The death information was composed of two parts: one was that the follow‐up personnel conducted regular follow‐up of the patient, and the other was to inquire in the information system of the cause of death registration report of Shanxi Province based on the patient's identification number.
Statistical analyses
Continuous variables of baseline data are presented as median and inter‐quartile range, and categorical variables are presented as numbers (percentages). The analysis of variance (ANOVA) or Kruskal–Wallis H test was used for continuous variable analysis, and the χ 2 test was used for categorical variable analysis.
The poLCA package was used for LCA by using comorbid variables; clusters of two to seven were identified using maximum likelihood estimation, and 100 iterations were performed. Finally, the Akaike information criterion, Bayesian information criterion, and χ 2 statistics were used to determine that four was the optimal number of latent clusters (Table S2). The Spearman correlation coefficient and the Fruchterman–Reingold layout of the elastic model were used to draw each cluster's comorbidity network and completed by Gephi 0.9.2.
The incidence of adverse outcomes among different clusters was presented on the Kaplan–Meier curve and compared using the log‐rank test, and the hazard ratio and 95% confidence interval (CI) were calculated using the Cox proportional risk model. Sensitivity analysis was performed according to the LVEF. The test level was set at 0.05, and the least significant difference or Bonferroni correction was used for post‐hoc test. The statistical analysis was performed using the R software version 3.6.1 and Python version 3.7.3, and our research flow chart is shown in Figure S1.
Results
Baseline patient characteristics
The baseline characteristics of the patients are shown in Table 1 . Overall, the median age of the 4063 patients with CHF was 69 years (inter‐quartile range 60–77 years), and 65.8% were male. Hypertension (66.4%) and OMI (51.6%) were the most common comorbidities, followed by VHD, diabetes, and anaemia. Beta‐blockers (74.0%) were the most commonly used drug, followed by mineralocorticoid receptor antagonist and loop diuretics (specific drugs are shown in Table S3).
Table 1.
Baseline patient characteristics (n = 4063)
| Characteristics | n (%) |
|---|---|
| Age, years, median (IQR) | 69 (60–77) |
| Male, n (%) | 2673 (65.8) |
| Medical insurance | |
| City medical insurance | 2438 (60.0) |
| Rural medical insurance | 1087 (26.8) |
| Self‐paying | 538 (13.2) |
| NYHA class, n (%) | |
| III/IV | 2447 (60.2) |
| LVEF, n (%) | |
| HFrEF | 906 (22.3) |
| HFmrEF | 863 (21.2) |
| HFpEF | 2294 (56.5) |
| PCI or CABG, n (%) | 1288 (31.7) |
| Valvular surgery | 6 (0.1) |
| IHD | |
| Angina pectoris | 2951 (72.6) |
| OMI | 2095 (51.6) |
| Comorbidities, n (%) | |
| AF | 1266 (31.2) |
| OMI | 2095 (51.6) |
| VHD | 1436 (35.3) |
| HLP | 1251 (30.8) |
| Stroke | 1034 (25.4) |
| Lung disease | 1055 (26.0) |
| Diabetes | 1330 (32.7) |
| Hypertension | 2696 (66.4) |
| Renal disease | 1246 (30.7) |
| Obesity | 741 (18.2) |
| Anaemia | 1329 (32.7) |
| Medication, n (%) | |
| Beta‐blocker | 3008 (74.0) |
| Ivabradine | 16 (0.4) |
| ACEI/ARB | 1873 (46.1) |
| ARNI | 18 (0.4) |
| MRA | 2445 (60.2) |
| Loop diuretics | 2033 (50.0) |
ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HLP, hyperlipidaemia; IHD, ischaemic heart disease; IQR, inter‐quartile range; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; OMI, old myocardial infarction; PCI, percutaneous coronary intervention; VHD, valvular heart disease.
Cluster analysis
Based on the LCA, four comorbid clinical clusters were identified. Of the 4063 patients, 1977(48.7%), 666 (16.4%), 725 (17.8%), and 695 (17.1%) patients were assigned to Clusters 1–4, respectively. Table 2 shows the characteristics of patients in each cluster, and the comorbidity variables used in the LCA showed high discrimination between clusters (P < 0.01).
Table 2.
Patient characteristics per cluster (n = 4063)
| Cluster1(Ref) | Cluster 2 | Cluster 3 | Cluster 4 | P value | |
|---|---|---|---|---|---|
| Patients, n (%) | 1977 (48.7) | 666 (16.4) | 725 (17.8) | 695 (17.1) | — |
| Age, years | 64 (57–72) | 66 (59–74) | 73 (65–79) | 75 (65–80) | <0.001 |
| Male, n (%) | 1300 (65.8) | 514 (77.2) | 442 (61.0) | 417 (60.0) | <0.001 |
| Non‐manual workers | 695 (35.2) | 251 (37.7) | 172 (23.7) | 192 (27.6) | <0.001 |
| Medical insurance | |||||
| City medical insurance | 1111(56.2) | 359 (53.9) # | 491 (67.7) | 477 (68.6) | <0.001 |
| Rural medical insurance | 583 (29.5) | 205 (30.8) # | 157 (21.7) | 142 (20.4) | |
| Self‐paying | 283 (14.3) | 102 (15.3) # | 77 (10.6) | 76 (10.9) | |
| NYHA class, n (%) | |||||
| III/IV | 930 (47.0) | 410 (61.6) | 571 (78.8) | 536 (77.1) | <0.001 |
| Heart rate, bpm | 68 (61–77) | 72 (63–83) | 76 (65–89) | 77 (66–92) | <0.001 |
| ALT, U/L | 20 (14–30) | 19 (14–32) # | 23 (18–37) | 18(12–32) # | <0.001 |
| AST, U/L | 23 (17–32) | 24 (18–33) # | 23 (17–37) # | 25 (18–38) | <0.001 |
| Albumin, g/L | 43 (40–46) | 42 (39–45) | 41 (38–44) | 41 (38–44) | <0.001 |
| TBIL, ummol/L | 13.5 (10.3–17.6) | 15.2 (11.4–20.7) | 14.9 (10.8–20.4) | 19.7 (14.5–26.7) | <0.001 |
| eGFR, mL/min/1.73 m2 | 88.1 (75.0–97.0) | 84.7 (72.3–94.3) | 59.5 (42.7–81.1) | 72.4 (52.2–84.8) | <0.001 |
| NT‐pro BNP, pg/mL | 999.8 (643.7–1464.4) | 1566.2 (1008.2–2246.2) | 2004.6 (1319.8–2786.1) | 2004.0 (1485.7–3198.0) | <0.001 |
| Echocardiography | |||||
| LA, mm | 37 (35–40) | 39 (36–43) | 41 (38–45) | 44 (40–48) | <0.001 |
| LVDD, mm | 50 (47–55) | 57 (51–63) | 55 (49–62) | 54 (48–62) | <0.001 |
| LVSD, mm | 33 (30–37) | 39 (34–45) | 39 (33–45) | 40 (35–47) | <0.001 |
| LVEF, n (%) | |||||
| HFrEF | 212 (10.7) | 257 (38.6) | 234 (32.3) | 203 (29.2) | <0.001 |
| HFmrEF | 316 (16.0) | 203 (30.5) | 210 (29.0) | 134 (19.3) | |
| HFpEF | 1449 (73.3) | 206 (30.9) | 281 (38.8) | 358 (51.5) | |
| PCI or CABG, n (%) | 717 (36.3) | 236 (35.4)# | 214 (29.5) | 121 (17.4) | <0.001 |
| ICD, n (%) | 1 (0.1) | 3 (0.5) | 4 (0.6) | 2 (0.3) | 0.071 |
| Valvular surgery, n (%) | 0 (0.0) | 0 (0.0) # | 0 (0.0)# | 6 (0.9) | <0.001 |
| Comorbidities, n (%) | |||||
| AF | 320 (16.2) | 70 (10.5) | 249 (34.3) | 627 (90.2) | <0.001 |
| OMI | 766 (38.7) | 570 (85.6) | 547 (75.4) | 212 (30.5) | <0.001 |
| VHD | 91 (4.6) | 371 (55.7) | 364 (50.2) | 610 (87.8) | <0.001 |
| HLP | 962 (48.7) | 77 (11.6) | 134 (18.5) | 78 (11.2) | <0.001 |
| Stroke | 539 (27.3) | 62 (9.3) | 262 (36.1) | 171 (24.6) # | <0.001 |
| Lung disease | 119 (6.0) | 217 (32.6) | 406 (56.0) | 313 (45.0) | <0.001 |
| Diabetes | 647 (32.7) | 113 (17.0) | 488 (67.3) | 82 (11.8) | <0.001 |
| Hypertension | 1491 (75.4) | 150 (22.5) | 622 (85.8) | 433 (42.3) | <0.001 |
| Renal disease | 311 (15.7) | 104 (15.6) # | 556 (76.7) | 275 (39.6) | <0.001 |
| Obesity | 516 (26.1) | 11 (1.7) | 129 (17.8) | 85 (12.2) | <0.001 |
| Anaemia | 526 (26.6) | 141 (21.2) | 406 (56.0) | 256 (36.8) | <0.001 |
| Cancer | 20 (1.0) | 8 (1.2) # | 21 (2.9) | 15 (2.2) | 0.002 |
| Medication, n (%) | |||||
| Beta‐blocker | 1555 (78.7) | 507 (76.1) # | 495 (68.3) | 451 (64.9) | <0.001 |
| Ivabradine | 3 (0.2) | 5 (0.8) | 3 (0.4) | 5 (0.7) | 0.072 |
| ACEI/ARB | 904 (45.7) | 318 (47.7) | 342 (47.2) | 309 (44.5) | 0.588 |
| ARNI | 7 (0.4) | 3 (0.5) | 2 (0.3) | 6 (0.9) | 0.308 |
| MRA | 911 (46.1) | 474 (71.2) | 546 (75.3) | 514 (74.0) | <0.001 |
| Loop diuretics | 590 (29.8) | 384 (57.7) | 544 (75.0) | 515 (74.1) | <0.001 |
The P value of multiple test did not reach statistical significance (P > 0.05).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate; ICD, implantable cardiac defibrillator; LA, left atrial diameter; LVDD, left ventricular end diastolic diameter; LVSD, left ventricular end systolic diameter; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; TBIL, total bilirubin.
Cluster 1 was classified as a metabolic cluster (Figure 1A ). Patients in this cluster were the youngest, the prevalence of HLP (48.7%) and obesity (26.1%) was higher than those in other clusters, and the severity of HF was mild. Of the patients in Cluster 2, OMI (85.6%) was the most common, followed by VHD; this group can be classified as the ischaemic cluster (Figure 1B ). Cluster 3 was classified as a high comorbidity burden cluster (Figure 1C ). The patients in this cluster had proportionately higher comorbidities than did those in the other clusters. The proportions of patients with diseases such as hypertension (85.8%), renal disease (76.7%), OMI (75.4%), and diabetes (67.3%) were higher than those in other clusters. The patients in Cluster 4 demonstrated the highest median age; as this group was also characterized by the highest prevalence of AF (90.4%), it could be referred to as the elderly‐AF cluster (Figure 1D, Table 2 ). The probability of each comorbidity variable in each cluster is provided in the Table S4.
Figure 1.
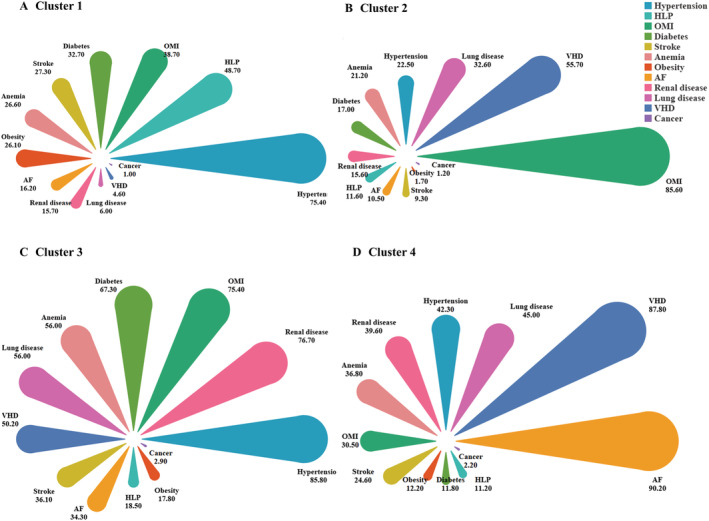
(A–D) Patient comorbidity profiles within clusters. The colour of the petals represents the comorbidity variable, and the area represents the constituent ratio of each comorbidity in the cluster, arranged in descending order. AF, atrial fibrillation; HLP, hyperlipidaemia; OMI, old myocardial infarction; VHD, valvular heart disease.
Drug use profile
The medication usage of patients with CHF in the different clusters is shown in Table 2 . Compared with other clusters, Cluster 1 received fewer mineralocorticoid receptor antagonists and loop diuretics. The beta‐blocker drug administration rate of patients in each cluster was higher, but there was no difference in the usage rate of other drugs among clusters.
Comorbidity networks
The average weighted degrees of Clusters 1–4 were 2.503, 1.778, 5.794, and 3.813, respectively. The ANOVA results showed that there were significant differences between the comorbidity networks (F = 11.063, P < 0.001). The two major correlations of Cluster 1 patients were hypertension‐HLP and hypertension‐OMI (Figure 2A ). Patients in Cluster 2 were more likely to have OMI and VHD simultaneously (Figure 2B ), and the comorbidities of Cluster 3 patients had more and higher correlations than those in other clusters (Figure 2C ). The correlation intensity of AF, VHD, and hypertension in Cluster 4 patients was higher than that in other clusters (Figure 2D ).
Figure 2.
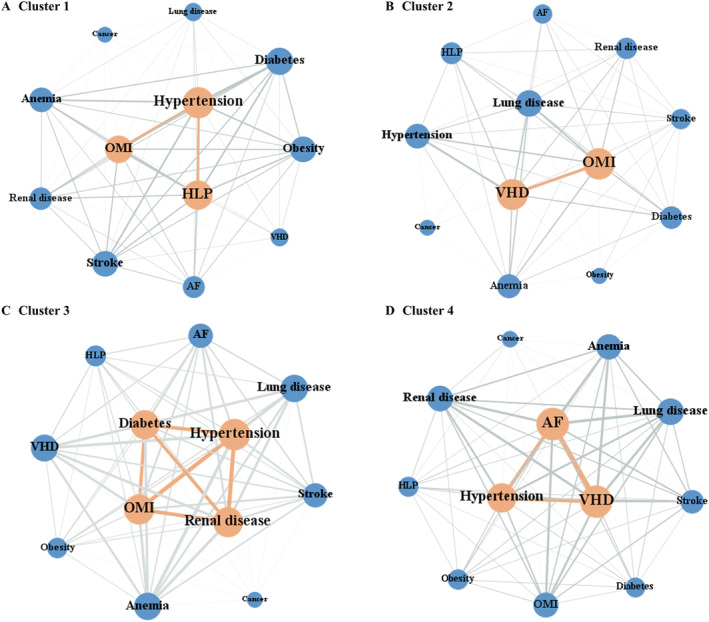
(A–D) Patient comorbidity networks within clusters. In the network diagram, each node represents the comorbidity variable, the node size represents the weighted degree, and the connecting line between nodes represents the correlation. The thicker the line, the greater the correlations of nodes. AF, atrial fibrillation; HLP, hyperlipidaemia; OMI, old myocardial infarction; VHD, valvular heart disease.
Clinical outcomes
For the entire cohort, we evaluated the relationship between different clusters and combined outcomes, all‐cause mortality, and readmission rates. Log‐rank test results showed that there were differences in outcomes among clusters (Figure 3 , P < 0.001). After controlling for age and other influencing factors, the Cox proportional hazards regression model showed that the risk of adverse outcomes in patients in Clusters 2–4 patients were higher than that of patients in Cluster 1 (P < 0.05). Compared with Cluster 1, patients in Cluster 3 had the highest adjusted risk of combined outcome and all‐cause mortality, which were 1.67 (95% CI, 1.40–1.99) and 2.87 (95% CI, 2.17–3.81), respectively, followed by Clusters 4 and 2. The adjusted readmission risks of patients in Clusters 2–4 were 1.35 (95% CI, 1.08–1.68), 1.39 (95% CI, 1.13–1.72), and 1.42 (95% CI, 1.14–1.77), respectively (Table 3 , Figure 4 ).
Figure 3.
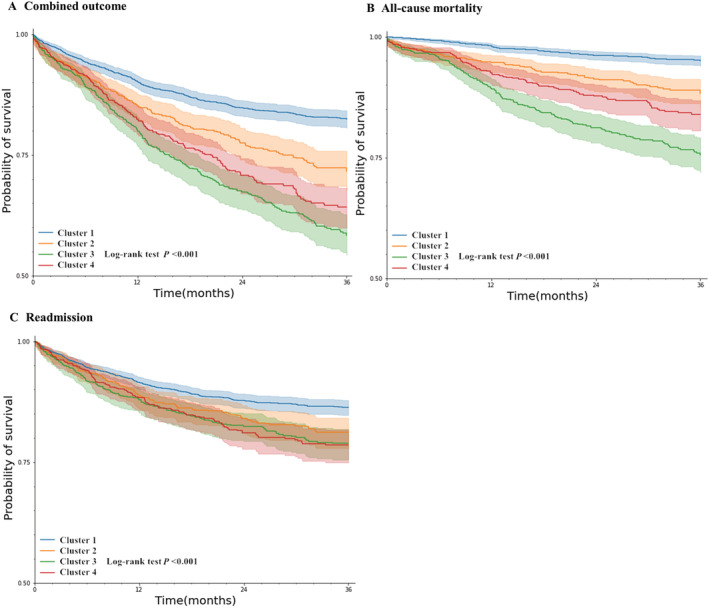
(A–C) Kaplan–Meier curve and log‐rank test showed that there were differences in outcomes among clusters.
Table 3.
Association between different clusters and clinical outcomes in all patients (n = 4063)
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Overall | |
|---|---|---|---|---|---|
| Combined outcome, n (%) | 332 (16.8) | 170 (25.5) | 267 (36.8) | 207 (29.8) | 976 (24.0) |
| HR (95% CI) | 1.00 (Ref) | 1.68 (1.40–2.02) | 2.61 (2.22–3.07) | 2.18 (1.83–2.60) | |
| Adjusted HR (95% CI) a | 1.00 (Ref) | 1.33 (1.09–1.61) | 1.67 (1.40–1.99) | 1.43 (1.19–1.72) | |
| All‐cause mortality, n (%) | 91 (4.6) | 69 (10.4) | 157 (21.7) | 92 (13.2) | 409 (10.1) |
| HR (95% CI) | 1.00 (Ref) | 2.46 (1.80–3.36) | 5.51 (4.25–7.13) | 3.47 (2.60–4.64) | |
| Adjusted HR (95% CI) b | 1.00 (Ref) | 1.75 (1.26–2.42) | 2.87 (2.17–3.81) | 1.81 (1.33–2.45) | |
| Readmission, n (%) | 260 (13.2) | 115 (17.3) | 140 (19.3) | 129 (18.6) | 644 (15.9) |
| HR (95% CI) | 1.00 (Ref) | 1.39 (1.12–1.73) | 1.59 (1.29–1.95) | 1.59 (1.29–1.97) | |
| Adjusted HR (95% CI) c | 1.00 (Ref) | 1.35 (1.08–1.68) | 1.39 (1.13–1.72) | 1.42 (1.14–1.77) |
CI, confidence interval; HR, hazard ratio; Ref, reference.
Adjusted for age, NYHA, LVEF, and beta‐blocker.
Adjusted for age, NYHA, PCI or CABG, LVEF, and beta‐blocker.
Adjusted for age, PCI or CABG, and medical insurance.
Figure 4.
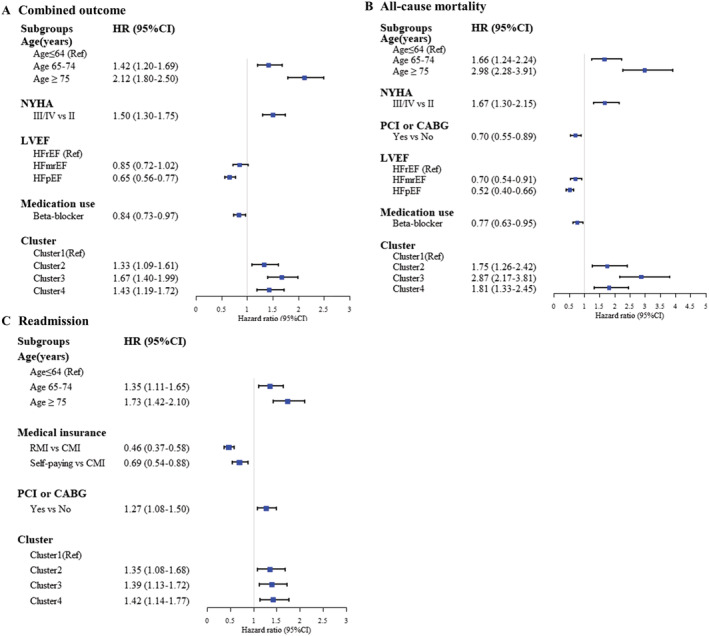
(A–C) The hazard ratio showed the association between clusters and outcomes after adjustment for baseline covariates. CABG, coronary artery bypass grafting; CI, confidence interval; HR, hazard ratio; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
Sensitivity analysis
Using the LCA method, HFpEF patients, and HFrEF patients were divided into four clusters, respectively. The Cox proportional hazards regression model revealed that in the HFpEF and HFrEF subgroups, patients in Cluster 3 had the highest risk of combined outcome and all‐cause mortality (P < 0.05, Tables 4 and S5).
Table 4.
Association between different clusters and clinical outcomes in HFpEF patients (n = 2294)
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Overall | |
|---|---|---|---|---|---|
| Combined outcome, n (%) | 101 (10.9) | 57 (18.4) | 123 (32.5) | 177 (26.1) | 458 (20.0) |
| HR (95% CI) | 1.00 (Ref) | 1.83 (1.32–2.53) | 3.79 (2.91–4.93) | 2.70 (2.12–3.45) | |
| All‐cause mortality, n (%) | 20 (2.2) | 17 (5.5) | 58 (15.3) | 70 (10.3) | 165 (7.2) |
| HR (95% CI) | 1.00 (Ref) | 2.72 (1.43–2.37) | 8.76 (5.27–14.58) | 5.22 (3.18–6.52) | |
| Readmission, n (%) | 83 (8.9) | 42 (13.6) | 69 (18.3) | 131(19.4) | 325 (14.2) |
| HR (95% CI) | 1.00 (Ref) | 1.60 (1.11–2.32) | 2.34 (1.70–3.22) | 2.34 (1.78–3.08) |
CI, confidence interval; HR, hazard ratio; Ref, reference.
Discussion
In this study, we used the LCA method to cluster CHF patients into four clinical clusters: metabolic, ischaemic, high comorbidity burden, and elderly‐AF. The risk of adverse outcomes of the metabolic cluster was the lowest, while the risk of combined outcome and all‐cause mortality in the high comorbidity burden cluster was the highest. The different comorbidity patterns and comorbidity burden reflect the different clinical phenotypes of patients with HF, highlighting the importance of comorbidity research.
Studies on heart failure cluster and geographic and ethnic differences
In previous studies on the subgroup of patients with HF, Lee et al. 14 used the latent mixture model to identify the common characteristics of comorbidities of adult patients with HF. Uijl et al. 15 chose to cluster patients with HFpEF into five categories using easily available clinical information, and identified an elderly‐AF cluster similar to that in our study, but the study cohort only included patients with HFpEF. Gulea et al. 16 performed the LCA of patients with HF and identified five subgroups, which proved the feasibility of using LCA to classify HF. However, their study data came only from patients across the USA, and there were no data on the severity of HF. Tromp et al. 17 conducted a prospective study on the multimorbidity pattern of Asian patients with HF and observed significant geographic differences in the distribution of multimorbidity groups across Asia. Both Tromp et al. and Gulea et al. identified ischaemia and metabolism subgroups; however, Tromp et al. identified an lower average age of patients (61.6 vs. 73 years), and a higher proportion of male patients (73% vs. 48.6%). The incidence of diabetes and obesity in the metabolic group, and of non‐cardiovascular comorbidities in the ischaemic group, was lower.
It is generally believed that 50% of inpatients with HF have HFpEF/HFmrEF 18 , 19 and 16% of HF outpatients have HFpEF. 20 The study on the prevalence of HF and left ventricular dysfunction in China by Hao et al. 21 found that 60% of patients had HFpEF, echoing our data distribution. Previous studies have revealed that there are regional and ethnic differences in patients with HF. Therefore, it is necessary to explore the comorbidity patterns of patients with CHF in different areas.
This study extended the previous studies by using comorbidity information to cluster CHF patients into different clinical phenotypes and analysing the comorbidity network of clustered patients. We found that the clinical outcome of the metabolic cluster was the best, which may be due to the younger age of patients in this cluster, the lighter severity of HF, and most of the patients being NYHA Class II. The high comorbidity burden cluster had the highest risk of adverse outcome events, which was consistent with the conclusions of Schmidt et al. 22 The analysis of comorbidity network revealed that the correlation of hypertension, diabetes and renal disease, and OMI was greater in high comorbidity burden cluster than that in other clusters, suggesting that a special combination of cardiovascular and non‐cardiovascular comorbidities may increase the risk of adverse events. Studies have found that renal dysfunction often occurs in HF patients with all phenotypes, with higher mortality and morbidity. 23 There was a vicious circle between diabetes, HF, and kidney disease, and this interaction worsens the patient's prognosis. 24 , 25 The reason for the poorer prognosis of patients in the elderly‐AF cluster than that in the metabolic cluster was that most of these patients were elderly, and the proportion of patients with AF and VHD was the highest. Studies have found that the clinical outcomes are particularly poor when AF is comorbid with HF. In patients with HF, the development of AF doubled the mortality, while in AF patients, it tripled the mortality. 26 , 27 Although the ischaemic cluster had fewer comorbidities, 77.1% of the patients were NYHA Class III/IV. At the same time, the prevalence of cardiovascular complications OMI and VHD was higher; therefore, patients in this cluster had poorer outcomes.
The results of the sensitivity analysis echoed those of the whole population cohort, indicating that LCA has a good clustering effect on CHF patients. Previous studies have indicated that most comorbidities have similar effects on patients in the HFpEF and HFrEF subgroups. 28 , 29 After adjusting for EF, the risk of adverse outcomes in different clusters still differed, which was consistent with the previous results. 16
Management of chronic heart failure patients
It is extremely challenging to manage CHF patients with multiple comorbidities. The existence of comorbidities may interfere with the process of diagnosing HF, aggravate HF symptoms, and further impair the quality of life. Preventing HF‐related comorbidities is crucial, and measures should be taken to reduce obesity, diabetes, and hypertension, which are powerful risk factors for major comorbidities related to HF. 30 The clinical characteristics of patients in the same cluster are similar, which is conducive to taking similar preventive measures and treatment plans. The hypothesis that treatment of comorbidities may improve patients' prognosis needs to be verified in prospective cohort studies. Polypharmacy often appears in the management of CHF patients with multimorbidity; however, the drugs used to treat HF and those used to treat comorbidities may interact, resulting in reduced efficacy, poor safety, and adverse effects. 31 The impact of polypharmacy related to multimorbidity on the effectiveness of HF management merits further study.
Strengths and limitations
This study used the LCA method to cluster CHF patients, which reduced the heterogeneity of patients. By analysing the comorbidity network of patients in different clusters, we explored the comorbidity relationship in different clusters, and the same network research was not found in the previous analysis of the CHF subgroup. We found that multiple comorbidities are very common in patients with HF and are associated with adverse outcomes. This study can provide a theoretical basis for the study of the relationship between the cluster pattern of comorbidities and clinical outcomes for patients with HF, and provide the basis for clinicians to perform hierarchical management and more precise medical treatment for complex patients with multiple comorbidities.
There were some limitations in this study. First, this study only included CHF patients from two hospitals, which may result in some selection bias. At present, the data collection has been extended to several hospitals, which will further verify the conclusions of this study. Second, this study did not consider changes in the clustering of comorbidities over time and could not explain the progress of HF. The time trajectory changes in the comorbidity patterns of CHF patients should be explored further in future studies. Even if the proportions were different, there were overlapping clinical features among the clusters. This is because the clustering method based on the LCA model classifies patients according to the maximum likelihood probability.
Conclusions
Patients with CHF who also have multiple comorbidities had an increased risk of adverse outcomes, and the differences among clusters confirmed the heterogeneity of CHF. This study can provide a basis for personalized treatment and hierarchical management of CHF patients, as well as provide a new perspective for clinical decision‐making.
Conflict of interest
None declared.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 81872714 and 82173631); Shanxi Provincial Key Laboratory of Major Diseases Risk Assessment (grant number 201805D111006); Youth Science and Technology Research Foundation of Shanxi Province (grant number 201801D221423).
Supporting information
Figure S1 Study flow chart.
Table S1 Definition of comorbidity in patients with chronic heart failure.
Table S2 Fit statistics for 2–7 latent class models.
Table S3 Medication classes.
Table S4 Probabilities for each comorbidity variable per cluster.
Table S5 Association between different clusters and clinical outcomes in HFrEF patients (n = 906).
Zheng, C. , Han, L. , Tian, J. , Li, J. , He, H. , Han, G. , Wang, K. , Yang, H. , Yan, J. , Meng, B. , Han, Q. , and Zhang, Y. (2022) Hierarchical management of chronic heart failure: a perspective based on the latent structure of comorbidities. ESC Heart Failure, 9: 595–605. 10.1002/ehf2.13708.
Contributor Information
Qinghua Han, Email: syhqh@sohu.com.
Yanbo Zhang, Email: sxmuzyb@126.com.
References
- 1. Palazzuoli A, Ruocco G, Gronda E. Noncardiac comorbidity clustering in heart failure: An overlooked aspect with potential therapeutic door. Heart Fail Rev 2020: 1–12. [DOI] [PubMed] [Google Scholar]
- 2. Lam CS, Teng TK, Tay WT, Anand I, Zhang S, Shimizu W, Narasimhan C, Park SW, Yu CM, Ngarmukos T, Omar R, Reyes EB, Siswanto BB, Hung CL, Ling LH, Yap J, MacDonald M, Richards AM. Regional and ethnic differences among patients with heart failure in Asia: The asian sudden cardiac death in heart failure registry. Eur Heart J 2016; 37: 3141–3153. [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. Authors/Task Force Members; Document Reviewers2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 4. Braunstein JB, Anderson GF, Gerstenblith G, Weller W, Niefeld M, Herbert R, Wu AW. Noncardiac comorbidity increases preventable hospitalizations and mortality among medicare beneficiaries with chronic heart failure. J Am Coll Cardiol 2003; 42: 1226–1233. [DOI] [PubMed] [Google Scholar]
- 5. Reinhardt SW, Chouairi F, Miller PE, Clark KAA, Kay B, Fuery M, Guha A, Freeman JV, Ahmad T, Desai NR, Friedman DJ. National trends in the burden of atrial fibrillation during hospital admissions for heart failure. J Am Heart Assoc 2021; 10: e019412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luttik ML, Jaarsma T, van Geel PP, Brons M, Hillege HL, Hoes AW, de Jong R, Linssen G, Lok DJ, Berge M, van Veldhuisen DJ. Long‐term follow‐up in optimally treated and stable heart failure patients: Primary care vs. Heart failure clinic. Results of the COACH‐2 study. Eur J Heart Fail 2014; 16: 1241–1248. [DOI] [PubMed] [Google Scholar]
- 7. Sbolli M, Fiuzat M, Cani D, O'Connor CM. Depression and heart failure: The lonely comorbidity. Eur J Heart Fail 2020; 22: 2007–2017. [DOI] [PubMed] [Google Scholar]
- 8. Bayes‐Genis A, Voors AA, Zannad F, Januzzi JL, Mark Richards A, Díez J. Transitioning from usual care to biomarker‐based personalized and precision medicine in heart failure: Call for action. Eur Heart J 2018; 39: 2793–2799. [DOI] [PubMed] [Google Scholar]
- 9. Lawson CA, Solis‐Trapala I, Dahlstrom U, Mamas M, Jaarsma T, Kadam UT, Stromberg A. Comorbidity health pathways in heart failure patients: A sequences‐of‐regressions analysis using cross‐sectional data from 10,575 patients in the swedish heart failure registry. PLoS Med 2018; 15: e1002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chamberlain AM, Boyd CM, Manemann SM, Dunlay SM, Gerber Y, Killian JM, Weston SA, Roger VL. Risk factors for heart failure in the community: Differences by age and ejection fraction. Am J Med 2020; 133: e237–e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gevaert AB, Tibebu S, Mamas MA, Ravindra NG, Lee SF, Ahmad T, Ko DT, Januzzi JL Jr, Van Spall HGC. Clinical phenogroups are more effective than left ventricular ejection fraction categories in stratifying heart failure outcomes. ESC Heart Fail 2021; 8: 2741–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yancy CW, Jessup M, Bozkurt B, Butler J, de Casey Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride P, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the american College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation 2017; 136: e137–e161. [DOI] [PubMed] [Google Scholar]
- 13. Linzer DA, Lewis JB. poLCA: An R package for polytomous variable latent class analysis. J Stat Softw 2011; 42: 1–29. [Google Scholar]
- 14. Lee CS, Chien CV, Bidwell JT, Gelow JM, Denfeld QE, Masterson Creber R, Buck HG, Mudd JO. Comorbidity profiles and inpatient outcomes during hospitalization for heart failure: An analysis of the U.S. Nationwide inpatient sample. BMC Cardiovasc Disord 2014; 14: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uijl A, Savarese G, Vaartjes I, Dahlström U, Brugts JJ, Linssen GCM, van Empel V, Brunner‐La Rocca HP, Asselbergs FW, Lund LH, Hoes AW, Koudstaal S. Identification of distinct phenotypic clusters in heart failure with preserved ejection fraction. Eur J Heart Fail 2021; 23: 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gulea C, Zakeri R, Quint JK. Model‐based comorbidity clusters in patients with heart failure: Association with clinical outcomes and healthcare utilization. BMC Med 2021; 19: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tromp J, Tay WT, Ouwerkerk W, Teng TK, Yap J, MacDonald MR, Leineweber K, McMurray JJV, Zile MR, Anand IS, Richards AMR, Lam CSP. ASIAN‐HF authorsMultimorbidity in patients with heart failure from 11 Asian regions: A prospective cohort study using the ASIAN‐HF registry. PLoS Med 2018; 15: e1002541 Erratum in: PLoS Med 2018;15:e1002583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, Deckers JW, Witteman JC, Stricker BH. Quantifying the heart failure epidemic: Prevalence, incidence rate, lifetime risk and prognosis of heart failure the Rotterdam study. Eur Heart J 2004; 25: 1614–1619. [DOI] [PubMed] [Google Scholar]
- 19. Koh AS, Tay WT, Teng THK, Vedin O, Benson L, Dahlstrom U, Savarese G, Lam CSP, Lund LH. A comprehensive population‐based characterization of heart failure with mid‐range ejection fraction. Eur J Heart Fail 2017; 19: 1624–1634. [DOI] [PubMed] [Google Scholar]
- 20. Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo‐Leiro MG, Harjola VP, Parissis J, Laroche C, Piepoli MF, Fonseca C, Mebazaa A, Lund L, Ambrosio GA, Coats AJ, Ferrari R, Ruschitzka F, Maggioni AP, Filippatos G. Epidemiology and one‐year outcomes in patients with chronic heart failure and preserved, mid‐range and reduced ejection fraction: An analysis of the ESC heart failure long‐term registry. Eur J Heart Fail 2017; 19: 1574–1585. [DOI] [PubMed] [Google Scholar]
- 21. Hao G, Wang X, Chen Z, Zhang L, Zhang Y, Wei B, Zheng C, Kang Y, Jiang L, Zhu Z, Zhang J, Wang Z, Gao R. China Hypertension Survey Investigators Prevalence of heart failure and left ventricular dysfunction in China: the China Hypertension Survey, 2012‐2015. Eur J Heart Fail 2019; 21: 1329–1337 Erratum in: Eur J Heart Fail 2020; 22:759. [DOI] [PubMed] [Google Scholar]
- 22. Schmidt M, Ulrichsen SP, Pedersen L, Bøtker HE, Sørensen HT. Thirty‐year trends in heart failure hospitalization and mortality rates and the prognostic impact of co‐morbidity: A danish nationwide cohort study. Eur J Heart Fail 2016; 18: 490–499. [DOI] [PubMed] [Google Scholar]
- 23. Damman K, Testani JM. The kidney in heart failure: An update. Eur Heart J 2015; 36: 1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Braunwald E. Diabetes, heart failure, and renal dysfunction: The vicious circles. Prog Cardiovasc Dis 2019; 62: 298–302. [DOI] [PubMed] [Google Scholar]
- 25. van Deursen VM, Urso R, Laroche C, Damman K, Dahlström U, Tavazzi L, Maggioni AP, Voors AA. Co‐morbidities in patients with heart failure: An analysis of the european heart failure pilot survey. Eur J Heart Fail 2014; 16: 103–111. [DOI] [PubMed] [Google Scholar]
- 26. Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The Framingham heart study. Circulation 2003; 107: 2920–2925. [DOI] [PubMed] [Google Scholar]
- 27. Ling LH, Kistler PM, Kalman JM, Schilling RJ, Hunter RJ. Comorbidity of atrial fibrillation and heart failure. Nat Rev Cardiol 2016; 13: 131–147. [DOI] [PubMed] [Google Scholar]
- 28. Triposkiadis F, Giamouzis G, Parissis J, Starling RC, Boudoulas H, Skoularigis J, Butler J, Filippatos G. Reframing the association and significance of co‐morbidities in heart failure. Eur J Heart Fail 2016; 18: 744–758. [DOI] [PubMed] [Google Scholar]
- 29. Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH, Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 2012; 59: 998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haug N, Sorger J, Gisinger T, Gyimesi M, Kautzky‐Willer A, Thurner S, Klimek P. Decompression of multimorbidity along the disease trajectories of diabetes mellitus patients. Front Physiol 2021; 11: 612604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reddel HK, Bateman ED, Becker A, Boulet LP, Cruz AA, Drazen JM, Haahtela T, Hurd SS, Inoue H, de Jongste JC, Lemanske RF Jr, Levy ML, O'Byrne PM, Paggiaro P, Pedersen SE, Pizzichini E, Soto‐Quiroz M, Szefler SJ, Wong GW, FitzGerald JM. A summary of the new GINA strategy: A roadmap to asthma control. Eur Respir J 2015; 46: 622–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Study flow chart.
Table S1 Definition of comorbidity in patients with chronic heart failure.
Table S2 Fit statistics for 2–7 latent class models.
Table S3 Medication classes.
Table S4 Probabilities for each comorbidity variable per cluster.
Table S5 Association between different clusters and clinical outcomes in HFrEF patients (n = 906).


