Abstract
Patients with Gorham–Stout disease (GSD) present progressive destruction and resorption of bone.
Typical bone-related symptoms include swelling, pain and functional impairment in the region involved.
The three aspects of GSD etiopathology are osteoclasts, angiogenesis/lymphangiogenesis and osteoblast function.
Multi-targeted pharmacological approach includes innovative options and represent milestones of treatment, sometimes associated with radiotherapy.
Surgery is mainly used to treat complications: pathologic/impending fractures, spinal instability or deformities and chylothorax.
In this narrative review, we highlight current standards in diagnosis, clinical management and therapeutic strategies.
Keywords: phantom bone disease, osteolysis, differential diagnosis, radiotherapy, bone tumors, treatment, diagnosis, symptoms, surgery
Search strategy
A systematic search of the literature was done to identify studies reporting on patients treated for Gorham–Stout disease (GSD). English and non-English language literature were searched in Pubmed using the terms ‘GSD’, ‘Gorham-Stout’, ‘progressive osteolysis’, ‘phantom bone disease’, ‘disappearing bone’, ‘lymphangiogenesis’, ‘syndrome’, in different combinations and in ISI Web of Knowledge database. The search was done on literature published in the past 70 years (from 1955 to date), resulting in about 270 articles (mainly case reports) describing over 350 cases (Supplementary Table 1 can be found online, see section on supplementary materials given at the end of this article). The focus of each reference varied including: series of patients with GSD irrespective of locations, case reports and articles investigating specific aspects from preclinic research, imaging, treatment and outcomes.
Historical overview and epidemiology
First described by Jackson in 1838 (1), in a patient with progressive osteolysis of the humerus, GSD takes its name from LW Gorham and AP Stout who first correlated the massive bone lysis with hemangiomatosis in their report in 1955 (2). GSD, also called phantom bone disease, is a rare syndrome (around 350 cases reported in the literature are summarized in Supplementary Table 1) (2, 3, 4, 5) characterized by massive osteolysis affecting one or more bones and by the substitution with bone lymphatics. This syndrome is considered as the type IV of osteolysis in the classification of Hardegger (6) (Table 1).
Table 1.
Classification of ‘idiopathic osteolysis’ according to Hardegger.
| Type I | Multicentric osteolysis (+ carpotarsal) | Dominant trasmission | Infancy | Pain and edema in hands and feet |
| Type 2 | Multicentric osteolysis (+ carpotarsal) | Recessive transmission | Infancy | Generalized osteoporosis |
| Type 3 | Multicentric osteolysis (+carpo-metacarpal) | Non-hereditary | Infancy | Nephropaty |
| Type 4 (GSD) | Single center osteolysis | Non-hereditary | Aspecific (usually under 40) | Bone lymphatics |
| Type 5 (WC) | Monocentric osteolysis (+ carpotarsal) | Recessive autosomal | Infancy | Contractures, generalized osteoporosis and short stature |
GSD, Gorham–Stout disease; WC, Winchester syndrome.
GSD does not seem to have any kind of preference for race, sex (although some authors evidenced a male prevalence (7, 8)) or geographic area; the only noteworthy epidemiologic feature is the age. GSD can be diagnosed at any age, but it exhibits a clear preference for a young patient; it affects patients younger than 40 years of age with an average age at diagnosis of 25 years of age (5, 9, 10, 11, 12, 13, 14). This syndrome can affect every skeletal segment, but it is mostly found in the upper part of the body with a slight predilection for the maxillo-facial bones (5). Other frequently involved bones are vertebrae, ribs and the pelvic girdle. In the majority of the reported cases, the disease affects only one bone segment even if multiple bones involvement has been described (13).
GSD is a sporadic disease with no definite pattern of genetic inheritance described since now. No cases with a familiar history have been reported. The only mutation reported today is a heterozygous splicing mutation NM_032638.4 (GATA2): c.379C >A described by Oguz et al in 2019 in a patient clinically diagnosed with GSD with a severe case of cardiac tamponade and multiple vertebral lytic lesions (15).
Molecular characteristics
Etiopathogenetic mechanisms in GSD are still unclear and uncertain, nevertheless, several hypotheses have been made since the Gorham and Stout article of 1955 (2). There are three fundamental features in GSD etiopathology, which are the role of osteoclasts, angiogenesis/lymphangiogenesis and osteoblast function. About the role of osteoclasts, these cells have been found by some investigators in osteolytic area’s biopsies (9, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26) whereas others have not (2, 27, 28, 29, 30). The reason for this discrepancy is unclear. It may be due to evaluations conducted at different phases of the disease (31). Those who have not found osteoclasts argue that osteolysis may be secondary to lymphangiogenesis (the second main feature in GSD). The improvement in number and activity of the osteoclast has been confirmed by Devlin et al. in 1996 (32) and by Hirayama et al. in 2001 (22). Both of these studies confirm the fundamental role of humoral factors to promote osteoclastogenesis and the survival of osteoclast besides their activity (22, 32). Irnterleukin -6 (IL-6), macrophage colony-stimulating factor (M-CSF) and Receptor activator of NF-κB ligand (RANK-L) seem to play an important role in this pathway. Osteoclastogenesis can also be induced in a RANK-L independent way using a combination of TNFa and IL-1 in presence of M-CSF (33). Colucci et al. reported that the onset of GSD is secondary to the production of high level of osteoclastogenic and angiogenic molecules from mesenchymal cells belonging to the monocyte lineage and that there is an increased sensitivity of the monocytic cell to osteoclastogenic factors that may lead those cells to differentiate in osteoclasts (34). The increased production of soluble mediators (such as IL-6 and vascular endothelial growth factor A (VEGF-A)) and their altered loops seem to be necessary but not sufficient for the pathogenesis of GSD (34). Furthermore, an augmented number of macrophage-like cells have been found in GSD lesions (28, 35), which are thought to be the osteoclast’s progenitors capable of producing VEGF-A, VEGF-C and VEGF-D (36) that can stimulate osteoclast differentiation and lymphangiogenesis (37, 38, 39, 40). As mentioned above, mononuclear cells in GSD are stimulated to differentiate into osteoclasts, and these osteoclasts are more active on resorbing bone; therefore, they are more motile as motility is fundamental for their activity (41). All these features in differentiation and activity of osteoclasts seem to be correlated with PTEN pathway as it had already been reported in a patient with Hamartoma Tumor Syndrome and Gorham–Stout Phenomenon a germline heterozygous mutation defensin β113 belongs to a family of innate host defense peptides with pleiotropic activities (42).
Some studies have been conducted recently on the role of VEGF-C in GSD patients. This growth factor, which promotes lymphangiogenesis in embryos and in adult tissue, has been found in higher levels than normal in transgenic mice expressing VEGF-C under the control of osterix promoter causing a phenotype of osteoporosis and bone lymphatics. It is interesting that increased levels of VEGF-C were found only locally, in the site of the bone loss (43). Another etiopathogenetic hypothesis is that the main part is played by lymphangiogenesis. The mechanism under this theory could be the uncontrolled growth of fluid-filled lymphatic vessel that causes osteolysis by mechanically compressing the bone. The secretion of growth factor by lymphatic endothelial cells may influence the activity of osteoclasts and osteoblasts (13). The fundamental role of lymphangiogenesis in GSD is also evidenced by the presence of lymphatic vascular endothelial hyaluronan marker on endothelial GS patient’s cells (44) and confirmed from the elevated levels of circulating platelet-derived growth factor-BB (PDGF-BB), a lymphangiogenic cytokine, in affected patients (45). Some studies have also pointed out that lymphatic endothelial cells (LECs) can stimulate osteoclast formation through the expression of M-CSF without any activity on osteoblasts. LEC-produced M-CSF by the way is not able to stimulate osteoclastogenesis on its own but needs more factors to ‘help’, such as for example, RANK-L (46). Another hypothesis on the proliferation of lymphatic vessels is that there might be a decrease in the level of an antilymphangiogenic factor such as soluble vascular endothelial growth factor receptors 2 (sVEGFR2) (47), transforming growth factor-β (TGF-β) (48), interferon-γ (IFN-γ) (49), etc. that could promote the uncontrolled growth of lymphatic vessels in the bones of patients with GSD (13).
Besides IL-6, many other serum biomarkers have been highlighted as potentially useful in the diagnosis of GSD, for example sclerostin, which is a biomarker of defective bone regeneration belonging to the β-catenin/Wnt pathway of signaling; collagen type 1 carboxy terminal telopeptide (ICTP), which is also a marker of bone resorption, and the aforementioned VEGF family. All of these potential markers are usually found in increased levels due to the bone resorption activity of the disease (especially ICTP), as well as VEGF-A which is a marker of angiomatous bone proliferation.
The last crucial feature of GSD is the role of osteoblasts. What has been found up to now about osteoclast’s role is that they are lacking in activity in GSD not counterbalancing bone resorption. Among the many factors that can influence osteoblast’s activity, the increased levels of sclerostin have been found to regulate mineralization and ALP activity. Sclerostin indeed upregulates RANK-L and downregulates osteoprotegerin (OPG) (50, 51) in osteoblast’s lineage cells (41).
Orthopedic manifestations
All possible symptoms and clinical manifestations are summarized in Table 2. Orthopedic clinical features in GSD depend on the bone that has been affected. The ribs, spine, pelvis, skull, clavicle and jaw are the most commonly affected bones, although every bone may be potentially involved. Typical symptoms include swelling, pain and functional impairment in the region involved. Pain can arise spontaneously or be caused by pathologic fractures that can be either atraumatic or following minor trauma. If the disease affects long bones, it can result in bone deformity and limb shortening. If it affects the lower limbs, walking and weight-bearing may become difficult and often the use of crutches is required. Upper limb involvement is less debilitating as the patient can walk normally, but all the movements are impaired and painful. Hand involvement is rarely reported (24, 52, 53) compared to the other cases of spontaneous osteolysis classified by Hardegger. Some patients may experience a dull pain or generalized weakness that increases over time. Life-threatening complications can arise if the lesion involves vertebrae; neurological complications may be present due to the spinal cord involvement, which can lead to paraplegia or to major neurological deficits (54, 55). Vertebral involvement can be either unifocal or multifocal. All spinal segments can be affected with different symptoms based on the segment involved.
Table 2.
Clinical manifestations and symptoms of Gorham–Stout disease.
| Orthopedic | - Pain - Swelling - Pathologic fractures - Absence of bone/soft tissue consistency - Bone deformities, kyphosis |
| Neurologic | - Paraplegia - Cerebrospinal fluid leakage (rhinorrhea, otorrhea, headache, migraine, nausea, vomiting) - Hearing problems |
| Temporo-mandibular joint | - Tinnitus - Egophony - Loose teeth - Facial deformity |
| Thorax | - Pleural or pericardial effusion - Mediastinal mass - Chylothorax - Dyspnea - Respiratory failure |
| Cutaneous | - Lymphangiogenic malformations |
Other symptoms and visceral involvement
Cerebro-spinal fluid (CSF) leakage is another severe complication that may arise if the lesion is located in the cranial bones, especially the skull base, or in the spine, and can be caused by the formation of a fistula. CSF leakage can cause symptoms like headache, vomiting, nausea and cranial hypotension and may require surgical repair (56, 57). Temporal bones and skull base seem to be the most involved cranial bones, apart from the maxilla and mandibula. CSF leak may decrease its pressure causing cerebral hypotension and, in some cases, have led to Chiari I malformation. This malformation can occur in GSD also following occiput deformities or following intracranial hypertension originated from the lymphatic flow in the CSF space following lymphatic anomalies (58, 59, 60, 61, 62, 63). Other complications in temporal or petrous bones may be hearing problems such as hearing loss (64, 65), tinnitus and egophony (66) or there may be sight problem (67). Temporo-mandibular joint is frequently involved causing face deformities and impairment in mouth opening. In 2019, Chrcanovic et al. conducted a study reviewing all the GSD involving face bone (in particular jaws) and he found 89 cases in 86 studies (68).
Chest involvement may lead to pleural effusion and chylothorax (64) if the lymphangiogenic effusion involves the pleural cavity or the thoracic duct. Chylothorax is an abnormal accumulation of chyle in the space between the lungs and chest cavity. Chylothorax has been reported to be the consequence of a duro-pleural fistula in a patient, and obviously, the symptoms were related to the duration and the acuity of the fistula formation (69). Cytological and biochemical examinations are mandatory and have always revealed the presence of exudate with a moderate predominance of lymphocytes (70). Chylothorax is a quite common severe complication of this disease (25%) (13) and it can cause respiratory failure and consequently, death. Patients affected by chylothorax have been seen to have, generally, a worse outcome since this effusion may be relapsing and it may require frequent thoracentesis. Fatal outcome has been reported around 43.6% in patients with chylothorax, which is significantly more than in patients without it.
Lymphatic malformation affecting the mediastinum may lead to pericardial effusion or to the formation of a mediastinal mass. These findings are often associated with other lymphatic diffuse anomalies such as chylothorax (since the establishment mechanism is the same), lymphatic flow into the pericardial area or involvement of thoracic duct. However, these are rare findings in GSD (less than 10 cases reported in the literature), but they can be life-threatening (56, 71, 72, 73, 74, 75, 76, 77, 78).
Cutaneous malformations are other clinical features observed in GSD. Several authors have mentioned these lesions in their patients, but no one specifically focuses attention on this aspect. Bruch Geharz et al in 2007 reported that in only five cases (16, 65, 79, 80, 81) out of the many described at that time were expressly reported cutaneous malformations, hypothesizing a possible role in anticipating diagnostic process. Since then, to the best of our knowledge, eight more cases of skin involvement have been reported in the literature with different manifestation going from verrucous lesions (82) to lymphangiogenic malformation with endothelial proliferation in dermis causing dark plaque, sometimes with cutaneous fistula formation (23).
Another organ often involved in lymphatic anomalies related to GSD is the spleen. In fact, many cases of asymptomatic splenic cyst have been reported in the literature. We did not consider this as a true complication of GSD since it is usually asymptomatic, but it can be taken into account in the diagnostic process (78).
GSD in childhood and adolescence
Gorham–Stout disease, as aforementioned, mainly affects children and young adults, with an average age at the diagnosis of 25 years old. More than half of the cases reported in the Supplementary Table 1 have been diagnosed under the age of 18. In particular, as far as we know from our systematic review, 80 cases have been diagnosed under the age of 10 and 79 more cases have been diagnosed between 11 and 18 years old. Clinical manifestations do not differ from the classical clinical picture. In children, the involvement of growing bones may appear serious, even if young children have more possibility to have a good outcome due to their plasticity (75, 83). Complication rates are comparable to the older population. In particular, we observed 40 cases of major complications in the first group (0–10 years old) and 38 major complications in the second one (10–18 years old). The most frequent are pleural effusion and chylothorax with 25 cases in the younger group and 29 cases in the older one. Unfortunately, 20 patients had fatal outcome; all of them had developed chylothorax during the course of the disease except for 3 patients. Of those 3 patients, one died of cervical spine compression, one died aged 65 years of severe depression and food refusal (84) and one died of septic shock (85).
Imaging
Radiological findings are essential for diagnosis, even if there are no specific imaging that definitively diagnose GSD, which is partly a diagnosis of exclusion.
Radiographs
Plain X-ray features depend on the stage of the lesion: initially X-rays show subcortical and intramedullary radiolucent foci (Fig. 1), but in a later stage of the disease, the classic pattern of osteolysis without osteosclerosis or periosteal reaction becomes evident and may appear as pathological fractures. Additionally, tubular bones undergo concentric shrinkage with a so-called ‘sucked candy’ appearance. These aspects usually lead to second-level exams such as CT scan or MRI (Fig. 2).
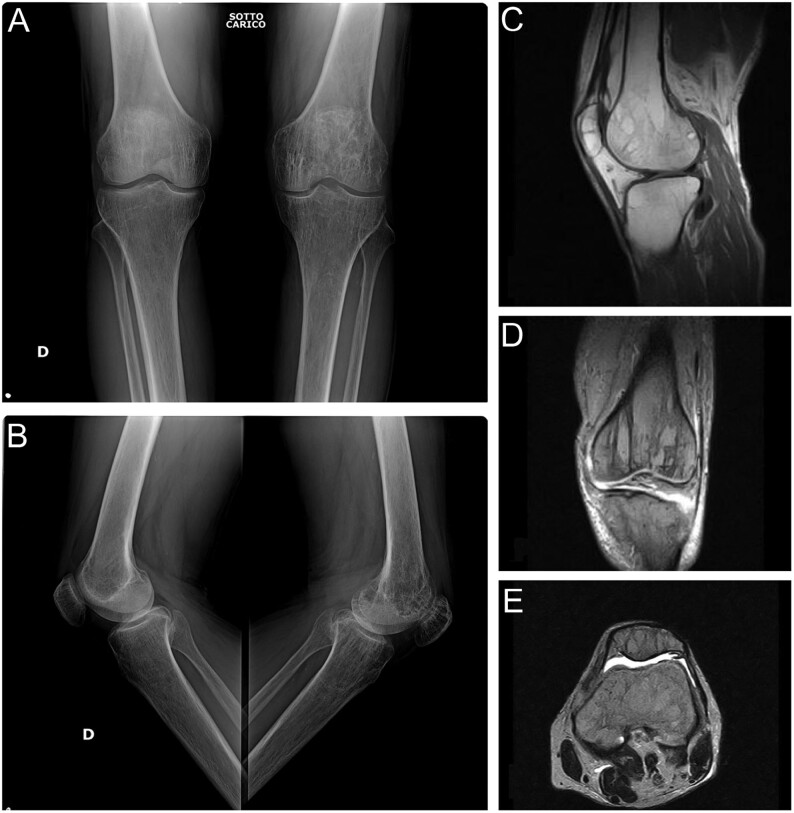
Figure 1.
First stage of GSD characterized by ‘patchy’ intramedullary and subcortical lucencies resembling osteoporosis. Typical imaging appearance with (A) frontal and (B) lateral radiograph of both knee and (C) sagittal, (D) coronal and (E) axial MR images.
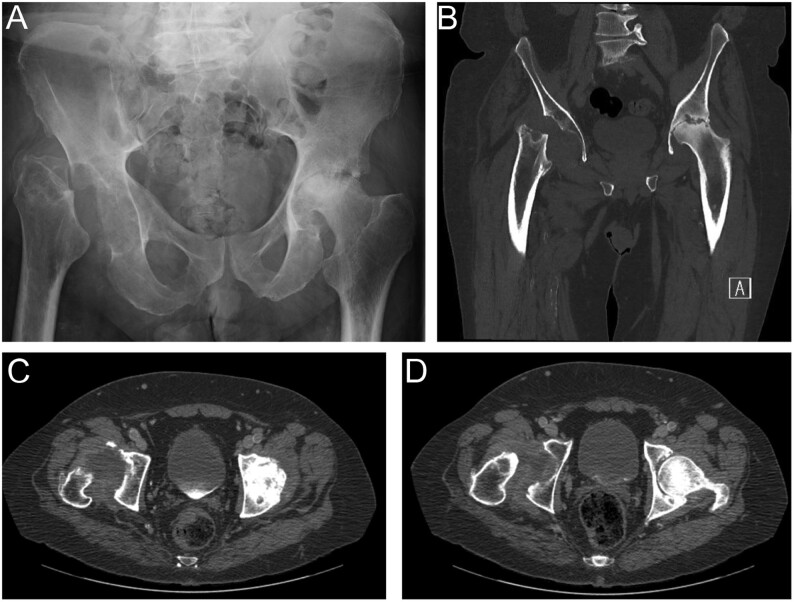
Figure 2.
59 years old male with progressive osteolysis of right hip and osteonecrosis of proximal left hip. (A) X-ray, (B) coronal and (C and D) axial CT scan show the final stage of GSD with complete resorption of bone replaced by fibrous tissue.
Computed tomography
The findings may be variable, but bone loss and its dissolution are usually observed (Fig. 3). CT scans can also be useful in showing the extent of soft tissue involvement. Vessel-shaped defects at the edge of osteolysis are sometimes observed.
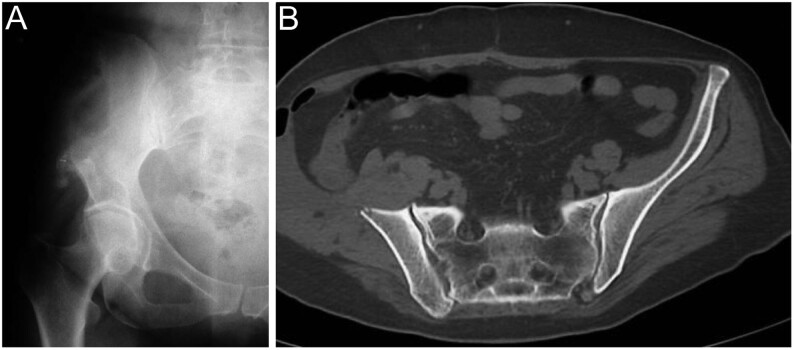
Figure 3.
Gorham–Stout disease. (A) Plain radiographs and (B) CT scan of the pelvis show the classic pattern of osteolysis without osteosclerosis or periosteal reaction.
Magnetic resonance images
The lesion appears hypointense in T1-weighted and hyperintense at T2-weighted MRI (86, 87). The osteolytic pattern is also confirmed on second-level examination and, especially if contrast is used, a soft mass substituting the reabsorbed bone with a reticular pattern may be observed. MRI can clearly show the vascular and/or lymphatic vessels within the bone, with contrast enhancement at the region of active osteolysis.
Bone scintigraphy
Bone scintigraphy mostly shows increased uptake in the areas with increased lymphatic and vascular proliferation and a decreased uptake at the osteolytic region of vanished bone. In 2009, Kobayashi et al evaluated for the first time the extension and the activity of the GSD using 99 mTc(V)-DMSA scintigraphy (88). Some years later, in 2015, Alves et al confirmed the usefulness of total body 99 mTc(V)-DMSA SPECT-CT in evaluating the activity, extension and multifocality of the disease (89).
PET/CT
Different types of agents have been used in PET/CT, and specifically, 18F-NaF has shown higher specificity and sensibility in identifying osteolytic foci when compared to 18F-FDG. In fact, in GSD, there is no higher metabolic activity and 18F-NaF is a bone-specific marker reflecting blood flow by being deposited on the surface of the hydroxyapatite matrix-forming fluorapatite (90).
Differential diagnosis
A diagnosis of GSD may be quite difficult and requires the exclusion of all the other potential causes of osteolysis such as cancer, infection, inflammation and hereditary diseases. The main disorders that have been reported in the differential diagnosis are lymphangiomatosis, multiple myeloma, lytic metastasis from an unknown primary tumor, Hajdu–Cheney syndrome, Paget’s disease, rheumatoid arthritis, fibrous dysplasia, Langerhans cell histiocytosis, Winchester syndrome (type V of Hardegger classification), carpal tarsal osteolysis (type I–III of Hardegger classification), idiopathic multicentric osteolysis, multicentric osteolysis with nephropathy and eosinophilic granulomatosis. Blood tests are almost completely negative and are neither helpful for diagnosis nor for follow-up of the disease as there are no specific markers except for alkaline phosphatase (which may be slightly elevated) (14). Heffez et al proposed eight criteria that can be used for the diagnosis of GSD: (i) positive biopsy (angiomatous tissue with abnormal lymphatic channels and numerous osteoclasts – Fig. 4); (ii) absence of cellular atypia; (iii) minimal or no osteoblastic response and absence of dystrophic calcifications; (iv) evidence of local progressive bone resorption; (v) non-expansive, non-ulcerative lesion; (vi) absence of visceral involvement; (vii) osteolytic radiographic pattern and (viii) negative hereditary, metabolic, neoplastic, immunologic and infectious etiology (31).
Figure 4.
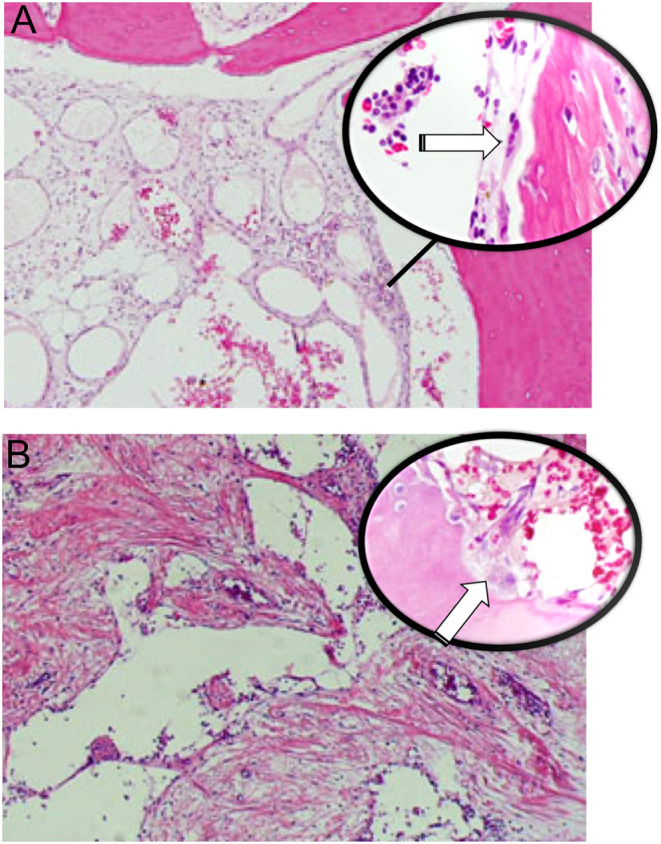
Gorham–Stout disease. (A) Enlarged abnormal lymphatic channels vary in size with numerous osteoclasts (white arrow in higher magnification), (B) dilated vessels with thin walls, loss of cortex and bone trabeculae surrounded by fibrous tissue. Active osteoclasts (white arrow) are present.
Radiotherapy and medical treatments
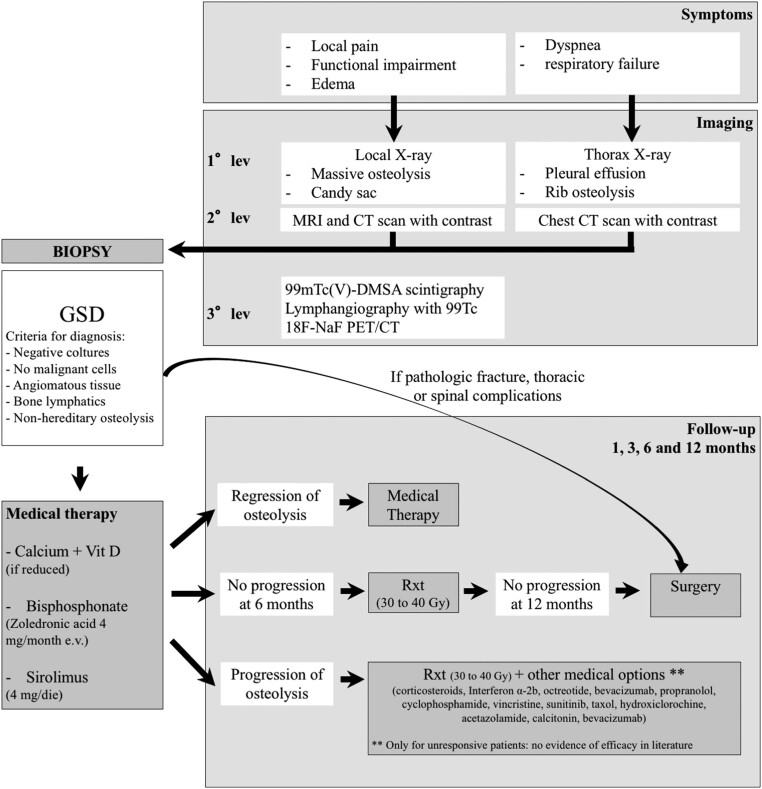
Many treatments have been proposed since the first description of this disease. The results of the accurate systematic review of the literature were summarized in a diagnostic and treatment algorithm (Fig. 5).
Figure 5.
Diagnostic and treatment algorithm for Gorham–Stout disease.
Radiotherapy and medical treatments play a very important role in the management of the disease, and many pharmacological approaches have been tried during the past years. Moderate doses of radiotherapy have been often administered to treat this disease. Commonly, the total dose of radiation ranges between 30.6 and 45 Gy in 1.8/2 Gy fractions (8, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103). Radiotherapy can be used in association with surgery, pre- or post-operatively, to reduce the size of the lesion, stopping the angiogenesis and consequently the progression of osteolysis. Results are quite convincing: in 1993, Dunbar et al reviewed the literature confirming the successful effect of radiation therapy in achieving good clinical results if administered with aforementioned posology (30–40 Gy in 2 Gy fractions) (91). In 2011, Heyd et al published another review (up to 2009) with ten additional cases, confirming local control of the disease in 77.2% of the cases in the literature and in eight out of ten patients in their series (92). To the best of our knowledge, since the last literature review, nearly 22 further patients have been treated with radiation therapy, and in most of the cases, a stop of the angiogenesis has been achieved (8, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103). Concerns about the use of radiotherapy are later adverse effects including secondary malignancy and all the other complications related to its use (104), although only one case of radiation-induced sarcoma has been reported (105).
Another option to treat GSD is the pharmacological approach which can either be monotherapeutic or multi-targeted. Monotherapeutic approach has initially been tried with usually poor results. Therefore, the use of different drugs is preferred with more encouraging results. Many pharmacological approaches have been tried during the past years. Some of them have been used since the first case and are still used nowadays like bisphosphonates, vitamin D, calcium, corticosteroids and interferon α-2b. Other drugs such as octreotide, bevacizumab, propranolol, cyclophosphamide, vincristine, sunitinib, taxol, hydroxychloroquine, acetazolamide, calcitonin and bevacizumab have been used in patients unresponsive to the conventional ‘first line treatments’, but they did not lead to great results. Recently (2016), sirolimus (also known by the name of rapamicine) has been used with encouraging results and has never been abandoned since then. Sirolimus is an antibiotic belonging to the class of macrolides, which works as an immunosuppressant by inhibiting both cell proliferation and angiogenesis. Its action is to block IL-2 signaling pathway by inhibiting mTOR (mammalian target of rapamicine), a serine/threonine protein kinase that stimulates cell growth and angiogenesis. In 2016, Triana et al. reported a large study on the use of sirolimus on various vascular anomalies: Authors observed an overall successful response in 33 out of 41 patients (80.4%) and specifically 6/7 of the patients affected by GSD responded well (106). Since its first use, sirolimus has been used many other times with good results; in some cases, a decrease of more than a half of the size of the lesion has been observed (107, 108, 109, 110, 111).
Since the first report by Lagberg in 1997, another widely used pharmaceuticals are bisphosphonates. These drugs have anti-osteolytic activity inhibiting osteoclast-mediated bone resorption but does not promote the stop of angiogenesis or bone formation. This supports their association with RT or with interferon-alpha (Ifn-α) to stop angiogenesis, or with calcium or cholecalciferol to promote bone formation. Many different types of bisphosphonates have been used in the treatment of GSD. Zoledronic acid has been widely used by many authors and has been administered intravenously with a dose of 4 mg/month. Clodronate, pamidronate and etidronate have been used as a monotherapy (7, 8, 21, 97, 109, 112, 113, 114, 115, 116), but more often, they have been administered in combination with other therapies such as RT (3, 23, 92, 97, 99, 100), Ifn-α (65, 93, 117, 118, 119, 120, 121, 122, 123), calcium and/or Vit D (19, 124, 125, 126, 127). A triple approach using a combination of biphosphonates with RT and Ifn-α has also been reported (95, 98).
Interferon α-2b (Ifn-a2b) is another pharmacological approach used in GSD for its anti-angiogenetic effect aimed at reducing angiogenesis-cased osteolysis. This drug has been commonly used for different types of leukemia and other tumors such as Kaposi sarcoma or as an adjuvant in melanoma. The first results date back to 1997 (117) when interferon α-2b had been used for the first time to treat GSD in a patient who had previously undergone radiotherapy and simultaneously clodronate therapy. However, it was not clear whether the regression in the symptoms was due to the action of clodronate or Ifn-a2b. The first satisfying results attributable to the Ifn-a2b therapy are reported in a study in 2005 (128) on a 2 year old patient with multicentric osteolysis previously treated with local injection of ok-432 (lyophilized incubation mixture of group A Streptococcus pyogenes of human origin) and simultaneously treated with steroids pulse therapy, which was reported to be only partially effective. After the administration of Ifn-a2b (1.5 MU daily then reduced to 1.5 MU weekly), she had complete remission of the symptoms and almost total disappearance of the lesions. Ifn-a2b has been used many other times since then, usually combined with other treatments such as RT (94, 98, 129) or bisphosphonates (65, 93, 117, 118, 119, 120, 121, 122, 123). Rarely, has it been tried as a monotherapeutic approach with Ifn-a2b but with variable results. In one case (65), the therapy was stopped and switched with sirolimus due to adverse reactions (fever, mild depression, fatigue); whilst, in another case, it was administered in monotherapy with good results (reduction in the size of the lesion and symptoms relief) (130).
Beta-blocking agent propranolol has been used only in few cases of GSD, with the rationale of downregulating the Raf mitogen-activated protein kinase signaling pathway lowering the expression of VEGF-A (81, 102, 111, 131, 132). However, more evidence has been found on the role of propranolol in lymphangiomatosis and in hemangiomatosis especially in young patients showing good effectiveness in reducingthe size of the tumor (19, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136).
Surgical treatment
In GSD, surgery is mainly used to treat complications. The orthopedic surgeon is involved in case of pathological fracture, impending fracture or when the patient complains about impaired function, reduced mobility or hard pain. Surgical treatments have been used to reduce the size of the osseous lesion, to fill the affected area (137) or to completely remove the bone segment (91, 138) with consequent reconstruction with prostheses or bone grafts (3, 26, 93, 124, 139, 140, 141) (Fig. 6). Bone grafts are usually resorbed and vanish in phases of activity of the GSD (142). The longest surviving rate in bone grafts has been achieved using cortical homologous bone graft, which has lasted more than 20 years, according to a case–control study conducted by Turra et al. (143). Spinal lesions can also be managed surgically if the lesion leads to spinal instability, deformity or pain. Posterior spinal stabilization with screws and rods associated with decompression, or anterior stabilization with plate and screws to induce vertebral fusion, can be used in the most severe cases of cervical and thoracic deformities (101, 115, 117, 144, 145, 146, 147, 148, 149). The use of vertebroplasty has to be considered when the spine is involved, but the osteolytic lesion does not lead to severe deformity or to major instability (98, 137). In spinal surgery, bone grafts are often used (114, 144, 150).
Figure 6.
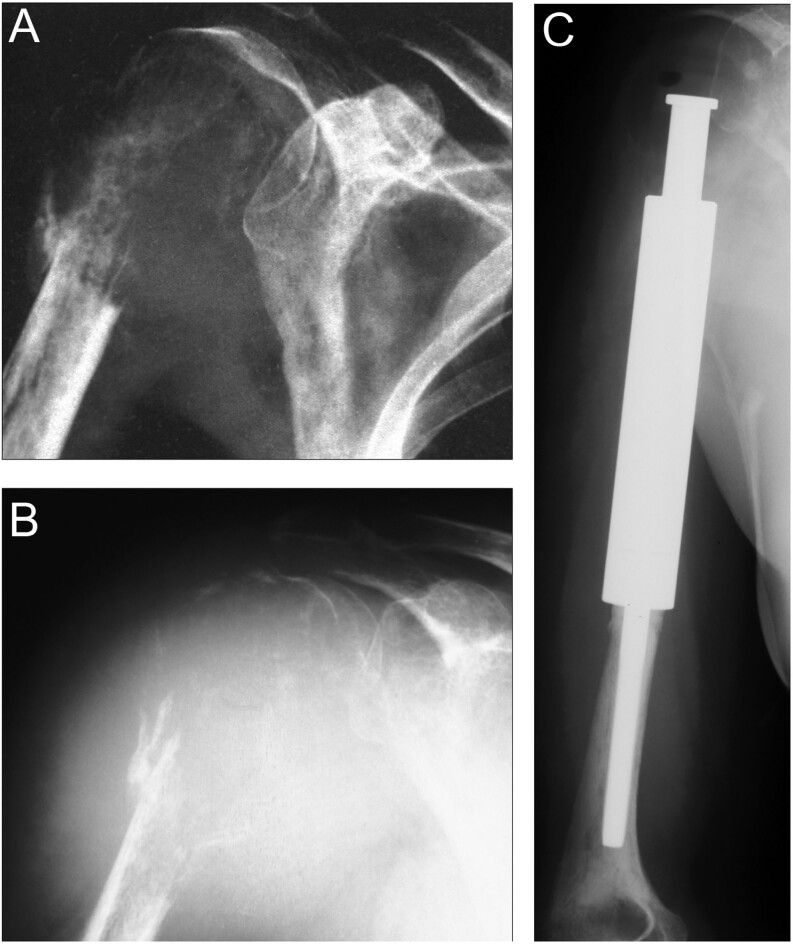
(A) Pathologic fracture of the proximal humerus with extensive osteolysis. A biopsy confirmed the diagnosis of Gorham–Stout disease. (B) Repeated plain x-ray AP view after 3 months reveals progressive resorption of bone despite medical treatment. (C) Resection and reconstruction with conventional modular mega prosthesis have been performed.
Surgery also plays a key role in thoracic complications, such as chylothorax or even minor pleural fluid leakage, which have been treated with pleural drainage (119, 149, 151, 152), pleurectomy, pleurodesis (64, 153, 154) and/or thoracic duct ligation or embolization (14, 27, 65, 155). In most of the cases, the best outcome has been obtained by combining these surgical techniques rather than using them in isolation (154, 156).
Conclusions
Gorham–Stout disease is an extremely rare bone disease characterized by progressive osteolysis with lymphatic and vascular proliferation. Diagnosis is challenging; once the GSD is suspected, the patient should be referred to a specialized center in musculoskeletal oncology or rare bone disease. Treatments and care are usually directed toward the specific symptoms and should require a multidisciplinary team approach with a long-term follow-up. Multimodal medical therapy with/without radiotherapy might be helpful in arresting bone lesion progression of GSD. Surgery has a role in stabilizing the disease or in the presence of complications (such as pathologic fracture, chylothorax or spinal complications). Chylothorax during the course of the disease represents the worst prognostic factor for survival.
Supplementary Material
ICMJE Conflict of Interest Statement
P R reports receiving royalties and consultancy fees for Stryker and Exactech are not related to the research reported here. The other authors have nothing to disclose.
Funding Statement
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
References
- 1.Jackson J.A boneless arm. Boston Medical and Surgical Journal 183818398–399. [Google Scholar]
- 2.Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. Journal of Bone and Joint Surgery: American Volume 200437-A985–1004. [PubMed] [Google Scholar]
- 3.Ellati R, Attili A, Haddad H, Al-Hussaini M, Shehadeh A. Novel approach of treating Gorham-Stout disease in the humerus – case report and review of literature. European Review for Medical and Pharmacological Sciences 201620426–432. [PubMed] [Google Scholar]
- 4.Ganau M, Prasad V, Ligarotti GKI, Syrmos NC, Ellamushi H. An overview of the neurosurgical implications, pathophysiology, diagnosis and recent treatment strategies for grade IV idiopathic osteolysis, also known as Gorham-Stout or phantom bone disease. Hellenic Journal of Nuclear Medicine 201821198–201. ( 10.1967/s002449910905) [DOI] [PubMed] [Google Scholar]
- 5.Nikolaou VS, Chytas D, Korres D, Efstathopoulos N. Vanishing bone disease (Gorham-Stout syndrome): a review of a rare entity. World Journal of Orthopedics 20145694–698. ( 10.5312/wjo.v5.i5.694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardegger F, Simpson LA, Segmueller G. The syndrome of idiopathic osteolysis. Classification, review, and case report. Journal of Bone and Joint Surgery: British Volume 19856788–93. ( 10.1302/0301-620X.67B1.3968152) [DOI] [PubMed] [Google Scholar]
- 7.de Keyser CE, Saltzherr MS, Bos EM, Zillikens MC. A large skull defect due to Gorham-Stout disease: case report and literature review on pathogenesis, diagnosis, and treatment. Frontiers in Endocrinology 202011 37. ( 10.3389/fendo.2020.00037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu P, Yuan XG, Hu XY, Shen FR, Wang JA. Gorham-Stout syndrome in mainland China: a case series of 67 patients and review of the literature. Journal of Zhejiang University: Science B 201314729–735. ( 10.1631/jzus.B1200308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. Journal of Bone and Joint Surgery: British Volume 199981501–506. ( 10.1302/0301-620x.81b3.9468) [DOI] [PubMed] [Google Scholar]
- 10.Boyer P, Bourgeois P, Boyer O, Catonné Y, Saillant G. Massive Gorham-Stout syndrome of the pelvis. Clinical Rheumatology 200524551–555. ( 10.1007/s10067-005-1088-7) [DOI] [PubMed] [Google Scholar]
- 11.Lee S, Finn L, Sze RW, Perkins JA, Sie KC. Gorham Stout syndrome (disappearing bone disease): two additional case reports and a review of the literature. Archives of Otolaryngology: Head and Neck Surgery 20031291340–1343. ( 10.1001/archotol.129.12.1340) [DOI] [PubMed] [Google Scholar]
- 12.Mendez AA, Keret D, Robertson W, MacEwen GD. Massive osteolysis of the femur (Gorham’s disease): a case report and review of the literature. Journal of Pediatric Orthopedics 19899604–608. ( 10.1097/01241398-198909010-00019) [DOI] [PubMed] [Google Scholar]
- 13.Dellinger MT, Garg N, Olsen BR. Viewpoints on vessels and vanishing bones in Gorham-Stout disease. Bone 20146347–52. ( 10.1016/j.bone.2014.02.011) [DOI] [PubMed] [Google Scholar]
- 14.Patel DV.Gorham’s disease or massive osteolysis. Clinical Medicine and Research 2005365–74. ( 10.3121/cmr.3.2.65) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oguz MM, Oguz B, Dogan V, Aydin B, Eyuboglu TS, Yesil S, Ceylaner S, Senel S. Cardiac tamponade in Gorham-Stout syndrome associated with GATA2 mutation. Indian Journal of Pediatrics 202087239–240. ( 10.1007/s12098-019-03174-1) [DOI] [PubMed] [Google Scholar]
- 16.Jones GB, Midgley RL, Smith GS. Massive osteolysis: disappearing bones. Journal of Bone and Joint Surgery: British Volume 195840-B494–501. ( 10.1302/0301-620X.40B3.494) [DOI] [PubMed] [Google Scholar]
- 17.Pazzaglia UE, Andrini L, Bonato M, Leutner M. Pathology of disappearing bone disease: a case report with immunohistochemical study. International Orthopaedics 199721303–307. ( 10.1007/s002640050173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruder E, Perez-Atayde AR, Jundt G, Alomari AI, Rischewski J, Fishman SJ, Mulliken JB, Kozakewich HP. Vascular lesions of bone in children, adolescents, and young adults. A clinicopathologic reappraisal and application of the ISSVA classification. Virchows Archiv 2009454161–179. ( 10.1007/s00428-008-0709-3) [DOI] [PubMed] [Google Scholar]
- 19.Avelar RL, Martins VB, Antunes AA, de Oliveira Neto PJ, Andrade ES. Use of zoledronic acid in the treatment of Gorham’s disease. International Journal of Pediatric Otorhinolaryngology 201074319–322. ( 10.1016/j.ijporl.2009.12.007) [DOI] [PubMed] [Google Scholar]
- 20.Choma ND, Biscotti CV, Bauer TW, Mehta AC, Licata AA. Gorham’s syndrome: a case report and review of the literature. American Journal of Medicine 1987831151–1156. ( 10.1016/0002-9343(8790959-4) [DOI] [PubMed] [Google Scholar]
- 21.Hammer F, Kenn W, Wesselmann U, Hofbauer LC, Delling G, Allolio B, Arlt W. Gorham-Stout disease – stabilization during bisphosphonate treatment. Journal of Bone and Mineral Research 200520350–353. ( 10.1359/JBMR.041113) [DOI] [PubMed] [Google Scholar]
- 22.Hirayama T, Sabokbar A, Itonaga I, Watt-Smith S, Athanasou NA. Cellular and humoral mechanisms of osteoclast formation and bone resorption in Gorham-Stout disease. Journal of Pathology 2001195624–630. ( 10.1002/path.989) [DOI] [PubMed] [Google Scholar]
- 23.Lehmann G, Pfeil A, Böttcher J, Kaiser WA, Füller J, Hein G, Wolf G. Benefit of a 17-year long-term bisphosphonate therapy in a patient with Gorham-Stout syndrome. Archives of Orthopaedic and Trauma Surgery 2009129967–972. ( 10.1007/s00402-008-0742-3) [DOI] [PubMed] [Google Scholar]
- 24.Silva S.Gorham-Stout disease affecting both hands: stabilisation during biphosphonate treatment. Hand 2011685–89. ( 10.1007/s11552-010-9292-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spieth ME, Greenspan A, Forrester DM, Ansari AN, Kimura RL, Gleason-Jordan I. Gorham’s disease of the radius: radiographic, scintigraphic, and MRI findings with pathologic correlation. A case report and review of the literature. Skeletal Radiology 199726659–663. ( 10.1007/s002560050306) [DOI] [PubMed] [Google Scholar]
- 26.Poirier H.Massive osteolysis of the humerus treated by resection and prosthetic replacement. Journal of Bone and Joint Surgery: British Volume 196850158–160. [PubMed] [Google Scholar]
- 27.Fujiu K, Kanno R, Suzuki H, Nakamura N, Gotoh M. Chylothorax associated with massive osteolysis (Gorham’s syndrome). Annals of Thoracic Surgery 2002731956–1957. ( 10.1016/s0003-4975(0203413-6) [DOI] [PubMed] [Google Scholar]
- 28.Heyden G, Kindblom LG, Nielsen JM. Disappearing bone disease. A clinical and histological study. Journal of Bone and Joint Surgery: American Volume 19775957–61. ( 10.2106/00004623-197759010-00009) [DOI] [PubMed] [Google Scholar]
- 29.Ross JL, Schinella R, Shenkman L. Massive osteolysis. An unusual cause of bone destruction. American Journal of Medicine 197865367–372. ( 10.1016/0002-9343(7890834-3) [DOI] [PubMed] [Google Scholar]
- 30.Tauro B.Multicentric Gorham’s disease. Journal of Bone and Joint Surgery: British Volume 199274928–929. ( 10.1302/0301-620X.74B6.1447260) [DOI] [PubMed] [Google Scholar]
- 31.Heffez L, Doku HC, Carter BL, Feeney JE. Perspectives onmassive osteolysis. Report of a case and review of the literature. Oral Surgery, Oral Medicine, and Oral Pathology 198355331–343. ( 10.1016/0030-4220(8390185-8) [DOI] [PubMed] [Google Scholar]
- 32.Devlin RD, Bone HG, Roodman GD. Interleukin-6: a potential mediator of the massive osteolysis in patients with Gorham-Stout disease. Journal of Clinical Endocrinology and Metabolism 1996811893–1897. ( 10.1210/jcem.81.5.8626854) [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima Net al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. Journal of Experimental Medicine 2000191275–286. ( 10.1084/jem.191.2.275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colucci S, Taraboletti G, Primo L, Viale A, Roca C, Valdembri D, Geuna M, Pagano M, Grano M, Pogrel AMet al. Gorham-Stout syndrome: a monocyte-mediated cytokine propelled disease. Journal of Bone and Mineral Research 200621207–218. ( 10.1359/JBMR.051019) [DOI] [PubMed] [Google Scholar]
- 35.Dickson GR, Hamilton A, Hayes D, Carr KE, Davis R, Mollan RA. An investigation of vanishing bone disease. Bone 199011205–210. ( 10.1016/8756-3282(9090215-k) [DOI] [PubMed] [Google Scholar]
- 36.Granata F, Frattini A, Loffredo S, Staiano RI, Petraroli A, Ribatti D, Oslund R, Gelb MH, Lambeau G, Marone Get al. Production of vascular endothelial growth factors from human lung macrophages induced by group IIA and group X secreted phospholipases A2. Journal of Immunology 20101845232–5241. ( 10.4049/jimmunol.0902501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Motokawa M, Tsuka N, Kaku M, Kawata T, Fujita T, Ohtani J, Matsuda Y, Terao A, Tanne K. Effects of vascular endothelial growth factor-C and -D on osteoclast differentiation and function in human peripheral blood mononuclear cells. Archives of Oral Biology 20135835–41. ( 10.1016/j.archoralbio.2012.06.010) [DOI] [PubMed] [Google Scholar]
- 38.Niida S, Kaku M, Amano H, Yoshida H, Kataoka H, Nishikawa S, Tanne K, Maeda N, Nishikawa S, Kodama H. Vascular endothelial growth factor can substitute for macrophage colony-stimulating factor in the support of osteoclastic bone resorption. Journal of Experimental Medicine 1999190293–298. ( 10.1084/jem.190.2.293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niida S, Kondo T, Hiratsuka S, Hayashi S, Amizuka N, Noda T, Ikeda K, Shibuya M. VEGF receptor 1 signaling is essential for osteoclast development and bone marrow formation in colony-stimulating factor 1-deficient mice. PNAS 200510214016–14021. ( 10.1073/pnas.0503544102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Q, Guo R, Lu Y, Zhao L, Zhou Q, Schwarz EM, Huang J, Chen D, Jin ZG, Boyce BFet al. VEGF-C, a lymphatic growth factor, is a RANKL target gene in osteoclasts that enhances osteoclastic bone resorption through an autocrine mechanism. Journal of Biological Chemistry 200828313491–13499. ( 10.1074/jbc.M708055200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossi M, Buonuomo PS, Battafarano G, Conforti A, Mariani E, Algeri M, Pelle S, D’Agostini M, Macchiaiolo M, De Vito Ret al. Dissecting the mechanisms of bone loss in Gorham-Stout disease. Bone 2020130115068. ( 10.1016/j.bone.2019.115068) [DOI] [PubMed] [Google Scholar]
- 42.Hopman SM, Van Rijn RR, Eng C, Bras J, Alders M, van der Horst CM, Hennekam RC, Merks JH. PTEN hamartoma tumor syndrome and Gorham-Stout phenomenon. American Journal of Medical Genetics: Part A 2012158A1719–1723. ( 10.1002/ajmg.a.35406) [DOI] [PubMed] [Google Scholar]
- 43.Hominick D, Silva A, Khurana N, Liu Y, Dechow PC, Feng JQ, Pytowski B, Rutkowski JM, Alitalo K, Dellinger MT. VEGF-C promotes the development of lymphatics in bone and bone loss. eLife 20187 e34323. ( 10.7554/eLife.34323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hagendoorn J, Padera TP, Yock TI, Nielsen GP, di Tomaso E, Duda DG, Delaney TF, Gaissert HA, Pearce J, Rosenberg AEet al. Platelet-derived growth factor receptor-beta in Gorham’s disease. Nature Clinical Practice: Oncology 20063693–697. ( 10.1038/ncponc0660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radhakrishnan K, Rockson SG. Gorham’s disease: an osseous disease of lymphangiogenesis? Annals of the New York Academy of Sciences 20081131203–205. ( 10.1196/annals.1413.022) [DOI] [PubMed] [Google Scholar]
- 46.Wang W, Wang H, Zhou X, Li X, Sun W, Dellinger M, Boyce BF, Xing L. Lymphatic endothelial cells produce M-CSF, causing massive bone loss in mice. Journal of Bone and Mineral Research 201732939–950. ( 10.1002/jbmr.3077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albuquerque RJ, Hayashi T, Cho WG, Kleinman ME, Dridi S, Takeda A, Baffi JZ, Yamada K, Kaneko H, Green MGet al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nature Medicine 2009151023–1030. ( 10.1038/nm.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oka M, Iwata C, Suzuki HI, Kiyono K, Morishita Y, Watabe T, Komuro A, Kano MR, Miyazono K. Inhibition of endogenous TGF-beta signaling enhances lymphangiogenesis. Blood 20081114571–4579. ( 10.1182/blood-2007-10-120337) [DOI] [PubMed] [Google Scholar]
- 49.Shao X, Liu C. Influence of IFN-alpha and IFN-gamma on lymphangiogenesis. Journal of Interferon and Cytokine Research 200626568–574. ( 10.1089/jir.2006.26.568) [DOI] [PubMed] [Google Scholar]
- 50.Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS ONE 20116 e25900. ( 10.1371/journal.pone.0025900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiong L, Jung JU, Wu H, Xia WF, Pan JX, Shen C, Mei L, Xiong WC. Lrp4 in osteoblasts suppresses bone formation and promotes osteoclastogenesis and bone resorption. PNAS 20151123487–3492. ( 10.1073/pnas.1419714112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klein C, Haraux E, Gouron R. Gorham-Stout disease: hand involvement in an 8-year-old child. Jurnalul Pediatrului 2017191277–277.e1. [DOI] [PubMed] [Google Scholar]
- 53.Lehnhardt M, Steinau HU, Homann HH, Steinstraesser L, Druecke D. Das Gorham-Stout-Syndrom der Hand: Fallbericht eines progressiven Krankheitsverlaufes über 24 Jahre. (Gorham-Stout disease: report of a case affecting the right hand with a follow-up of 24 years). Handchirurgie, Mikrochirurgie, Plastische Chirurgie 200436249–254. ( 10.1055/s-2004-821048) [DOI] [PubMed] [Google Scholar]
- 54.Halliday DR, Dahlin DC, Pugh DG, Young HH. Massive osteolysis and angiomatosis. Radiology 196482637–644. ( 10.1148/82.4.637) [DOI] [PubMed] [Google Scholar]
- 55.Esmailiejah AA, Kamalian N, Abbasian M. Temporary paraplegia resulting from Gorham’s disease involving the third lumbar vertebra and proximal femur: a five-year follow-up and review of the literature. Archives of Iranian Medicine 201316686–690. ( 10.31611/AIM.0016) [DOI] [PubMed] [Google Scholar]
- 56.Tie ML, Poland GA, Rosenow EC. Chylothorax in Gorham’s syndrome. A common complication of a rare disease. Chest 1994105208–213. ( 10.1378/chest.105.1.208) [DOI] [PubMed] [Google Scholar]
- 57.Hernández-Marqués C, Serrano González A, Cordobés Ortega F, Alvarez-Coca J, Sirvent Cerda S, Carceller Lechón F, Azorín Cuadrillero D. Gorham-Stout disease and cerebrospinal fluid otorrhea. Pediatric Neurosurgery 201147299–302. ( 10.1159/000336877) [DOI] [PubMed] [Google Scholar]
- 58.Coulter IC, Khan SA, Flanagan AM, Marks SM. Chiari I malformation associated with Gorham’s disease of the skull base. Clinical Neurology and Neurosurgery 201411683–86. ( 10.1016/j.clineuro.2013.11.007) [DOI] [PubMed] [Google Scholar]
- 59.Nagashima H, Mizukawa K, Taniguchi M, Yamamoto Y, Kohmura E. Cerebrospinal fluid leakage and Chiari I malformation with Gorham’s disease of the skull base: a case report. Neurologia i Neurochirurgia Polska 201751427–431. ( 10.1016/j.pjnns.2017.06.007) [DOI] [PubMed] [Google Scholar]
- 60.Yoshimoto S, Takai K, Takahashi K, Yasui T, Taniguchi M. Intracranial hypotension and hypertension: reversible Chiari malformation due to dynamic cerebrospinal fluid abnormalities in Gorham-Stout disease. Case report. Journal of Neurosurgery: Pediatrics 201822508–512. ( 10.3171/2018.5.PEDS1859) [DOI] [PubMed] [Google Scholar]
- 61.Stephens S, Squires L, Campbell R, Davies J, Chaseling R. Multifocal Gorham-Stout disease associated with Chiari I malformation and recurrent aseptic meningitis: case report and review of literature. Journal of Clinical Neuroscience 202072486–492. ( 10.1016/j.jocn.2019.12.033) [DOI] [PubMed] [Google Scholar]
- 62.Aouad P, Young NM, Saratsis AM, Reynolds MA, Ryan ME. Gorham Stout disease of the temporal bone with cerebrospinal fluid leak. Child’s Nervous System 2021. ( 10.1007/s00381-021-05245-1) [DOI] [PubMed] [Google Scholar]
- 63.Morimoto N, Ogiwara H, Miyazaki O, Kitamuara M, Nishina S, Nakazawa A, Maekawa T, Morota N. Gorham-Stout syndrome affecting the temporal bone with cerebrospinal fluid leakage. International Journal of Pediatric Otorhinolaryngology 2013771596–1600. ( 10.1016/j.ijporl.2013.06.004) [DOI] [PubMed] [Google Scholar]
- 64.Girn HR, Towns G, Chumas P, Holland P, Chakrabarty A. Gorham’s disease of skull base and cervical spine–confusing picture in a two year old. Acta Neurochirurgica 2006148909–13; discussion 913. ( 10.1007/s00701-005-0806-x) [DOI] [PubMed] [Google Scholar]
- 65.Nozawa A, Ozeki M, Kuze B, Asano T, Matsuoka K, Fukao T. Gorham-Stout disease of the skull base with hearing loss: dramatic recovery and antiangiogenic therapy. Pediatric Blood and Cancer 201663931–934. ( 10.1002/pbc.25886) [DOI] [PubMed] [Google Scholar]
- 66.Mowry S, Canalis R. Gorham-Stout disease of the temporal bone. Laryngoscope 2010120598–600. ( 10.1002/lary.20788) [DOI] [PubMed] [Google Scholar]
- 67.Domínguez Fuentes B, García-Gil D, Jimeno A, García-Lechuz JM. Síndrome de Gorham-Stout en un paciente con infección por el VIH. (Gorham-Stout syndrome in a patient with HIV infection). Medicina Clinica 200813136–37. ( 10.1157/13123040) [DOI] [PubMed] [Google Scholar]
- 68.Chrcanovic BR, Gomez RS. Gorham-Stout disease with involvement of the jaws: a systematic review. International Journal of Oral and Maxillofacial Surgery 2019481015–1021. ( 10.1016/j.ijom.2019.03.002) [DOI] [PubMed] [Google Scholar]
- 69.Agrawal R, Mohammed I, Reilly PG. Duropleural fistula as a consequence of Gorham-Stout syndrome: a combination of 2 rare conditions. Journal of Thoracic and Cardiovascular Surgery 20061311205–1206. ( 10.1016/j.jtcvs.2006.01.023) [DOI] [PubMed] [Google Scholar]
- 70.Momanu A, Caba L, Gorduza NC, Arhire OE, Popa AD, Ianole V, Gorduza EV. Gorham-Stout disease with multiple bone involvement-challenging diagnosis of a rare disease and literature review. Medicina 202157 681. ( 10.3390/medicina57070681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mawk JR, Obukhov SK, Nichols WD, Wynne TD, Odell JM, Urman SM. Successful conservative management of Gorham disease of the skull base and cervical spine. Child’s Nervous System 199713622–625. ( 10.1007/s003810050155) [DOI] [PubMed] [Google Scholar]
- 72.Duffy BM, Manon R, Patel RR, Welsh JS. A case of Gorham’s disease with chylothorax treated curatively with radiation therapy. Clinical Medicine and Research 2005383–86. ( 10.3121/cmr.3.2.83) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang Y, Hou G, Cheng W. 99mTc-SC lymphoscintigraphy and SPECT/CT findings in a case report of Gorham-Stout disease presenting with chylothorax and bone pain. Medicine 201998 e15023. ( 10.1097/MD.0000000000015023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ricci KW, Hammill AM, Mobberley-Schuman P, Nelson SC, Blatt J, Bender JLG, McCuaig CC, Synakiewicz A, Frieden IJ, Adams DM. Efficacy of systemic sirolimus in the treatment of generalized lymphatic anomaly and Gorham-Stout disease. Pediatric Blood and Cancer 201966 e27614. ( 10.1002/pbc.27614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramaroli DA, Cavarzere P, Cheli M, Provenzi M, Barillari M, Rodella G, Gaudino R, Antoniazzi F. A child with early-onset Gorham-Stout disease complicated by chylothorax: near-complete regression of bone lesions with interferon and bisphosphonate treatment. Hormone Research in Paediatrics 201991406–410. ( 10.1159/000495364) [DOI] [PubMed] [Google Scholar]
- 76.Davalos EA, Gandhi NM, Barank D, Varma RK. Gorham-Stout disease presenting with dyspnea and bone pain in a 9-year-old girl. Radiology Case Reports 201510 1110. ( 10.2484/rcr.v10i2.1110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Demir MK, Yapıcıer Ö, Toktaş ZO, Yılmaz B, Akakın A, Konya D. Gorham’s disease-vanishing bone of the cervical spine. Spine Journal 201616e19–e21. ( 10.1016/j.spinee.2015.08.045) [DOI] [PubMed] [Google Scholar]
- 78.González Luna A, Nuñez Pizarro JL, Rodríguez Echegaray CI. Presentación atípica del síndrome de Gorham-Stout: caso clínico. (Atypical presentation of Gorham-Stout syndrome: case report). Archivos Argentinos de Pediatria 2015113e153–e156 (Spanish). ( 10.5546/aap.2015.e153) [DOI] [PubMed] [Google Scholar]
- 79.Bruch-Gerharz D, Gerharz CD, Stege H, Krutmann J, Pohl M, Koester R, Ruzicka T. Cutaneous lymphatic malformations in disappearing bone (Gorham-Stout) disease: a novel clue to the pathogenesis of a rare syndrome. Journal of the American Academy of Dermatology 200756 (Supplement) S21–S25. ( 10.1016/j.jaad.2006.01.063) [DOI] [PubMed] [Google Scholar]
- 80.Frost JF, Caplan RM. Cutaneous hemangiomas and disappearing bones with a review of cutaneovisceral hemangiomatosis. Archives of Dermatology 196592501–508. [PubMed] [Google Scholar]
- 81.Fornasier VL.Hemangiomatosis with massive osteolysis. Journal of Bone and Joint Surgery: British Volume 197052B444–451. [PubMed] [Google Scholar]
- 82.Ogawa Y, Shimada S, Kawamura T. Case of Gorham-Stout disease with multiple verrucous skin lesions. Journal of Dermatology 202047e388–e390. ( 10.1111/1346-8138.15557) [DOI] [PubMed] [Google Scholar]
- 83.Liang Y, Tian R, Wang J, Shan Y, Gao H, Xie C, Li J, Xu M, Gu S. Gorham-Stout disease successfully treated with sirolimus (rapamycin): a case report and review of the literature. BMC Musculoskeletal Disorders 202021 577. ( 10.1186/s12891-020-03540-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bode-Lesniewska B, von Hochstetter A, Exner GU, Hodler J. Gorham-Stout disease of the shoulder girdle and cervico-thoracic spine: fatal course in a 65-year-old woman. Skeletal Radiology 200231724–729. ( 10.1007/s00256-002-0568-y) [DOI] [PubMed] [Google Scholar]
- 85.Kery L, Wouters HW. Massive osteolysis. Report of two cases. Journal of Bone and Joint Surgery: British Volume 197052452–459. [PubMed] [Google Scholar]
- 86.Lo CP, Chen CY, Chin SC, Juan CJ, Hsueh CJ, Chen A. Disappearing calvarium in Gorham disease: MR imaging characteristics with pathologic correlation. American Journal of Neuroradiology 200425415–418. [PMC free article] [PubMed] [Google Scholar]
- 87.Yoo SY, Hong SH, Chung HW, Choi JA, Kim CJ, Kang HS. MRI of Gorham’s disease: findings in two cases. Skeletal Radiology 200231301–306. ( 10.1007/s00256-002-0487-y) [DOI] [PubMed] [Google Scholar]
- 88.Kobayashi H, Shigeno C, Sakahara H, Hosono M, Hosono M, Yao ZS, Endo K, Konishi J. Intraosseous hemangiomatosis: technetium-99m(V)dimercaptosuccinic acid and technetium-99m-hydroxymethylene diphosphonate imaging. Journal of Nuclear Medicine 1994351482–1484. [PubMed] [Google Scholar]
- 89.Alves VM, Vieira TS, Amorim NS, Oliveira A, Rodrigues A, Pereira JG. 99mTc(V)-DMSA SPECT-CT findings in a case of Gorham-Stout disease. Nuclear Medicine Review: Central and Eastern Europe 20151897–101. ( 10.5603/NMR.2015.0023) [DOI] [PubMed] [Google Scholar]
- 90.Papadakis GZ, Millo C, Bagci U, Blau J, Collins MT. 18F-NaF and 18F-FDG PET/CT in Gorham-Stout disease. Clinical Nuclear Medicine 201641884–885. ( 10.1097/RLU.0000000000001369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dunbar SF, Rosenberg A, Mankin H, Rosenthal D, Suit HD. Gorham’s massive osteolysis: the role of radiation therapy and a review of the literature. International Journal of Radiation Oncology, Biology, Physics 199326491–497. ( 10.1016/0360-3016(9390968-2) [DOI] [PubMed] [Google Scholar]
- 92.Heyd R, Micke O, Surholt C, Berger B, Martini C, Füller J, Schimpke T, Seegenschmiedt MH. & German Cooperative Group on Radiotherapy for Benign Diseases. Radiation therapy for Gorham-Stout syndrome: results of a national patterns-of-care study and literature review. International Journal of Radiation Oncology, Biology, Physics 201181e179–e185. ( 10.1016/j.ijrobp.2011.01.006) [DOI] [PubMed] [Google Scholar]
- 93.Ruggieri P, Montalti M, Angelini A, Alberghini M, Mercuri M. Gorham-Stout disease: the experience of the Rizzoli Institute and review of the literature. Skeletal Radiology 2011401391–1397. ( 10.1007/s00256-010-1051-9) [DOI] [PubMed] [Google Scholar]
- 94.Brodszki N, Länsberg JK, Dictor M, Gyllstedt E, Ewers SB, Larsson MK, Eklund EA. A novel treatment approach for paediatric Gorham-Stout syndrome with chylothorax. Acta Paediatrica 20111001448–1453. ( 10.1111/j.1651-2227.2011.02361.x) [DOI] [PubMed] [Google Scholar]
- 95.Leite I, Hernández-Martín A, Colmenero I, López-Gutiérrez JC, Torrelo A. Invasive lymphatic malformation (gorham-stout) of the pelvis with prominent skin involvement. Pediatric Dermatology 201330374–378. ( 10.1111/j.1525-1470.2012.01814.x) [DOI] [PubMed] [Google Scholar]
- 96.Ozbayrak M, Yilmaz MH, Kantarci F, Ozer H, Harmanci K, Babacan M, Dervisoglu S. A case of an idiopathic massive osteolysis with skip lesions. Korean Journal of Radiology 201314946–950. ( 10.3348/kjr.2013.14.6.946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu Y, Zhong DR, Zhou PR, Lv F, Ma DD, Xia WB, Jiang Y, Wang O, Xing XP, Li M. Gorham-Stout disease: radiological, histological, and clinical features of 12 cases and review of literature. Clinical Rheumatology 201635813–823. ( 10.1007/s10067-014-2780-2) [DOI] [PubMed] [Google Scholar]
- 98.Carbó E, Riquelme Ó, García A, González JL. Vertebroplasty in a 10-year-old boy with Gorham-Stout syndrome. European Spine Journal 201524 (Supplement 4) S590–S593. ( 10.1007/s00586-015-3764-x) [DOI] [PubMed] [Google Scholar]
- 99.Yerganyan VV, Body JJ, De Saint Aubain N, Gebhart M. Gorham-Stout disease of the proximal fibula treated with radiotherapy and zoledronic acid. Journal of Bone Oncology 2015442–46. ( 10.1016/j.jbo.2015.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tolis K, Triantafyllopoulos IK, Tournis S, Papaioannou NA. Gorham-Stout disease of the pelvis: seven years follow up with complete radiological evaluation. Journal of Musculoskeletal and Neuronal Interactions 20161679–82. [PMC free article] [PubMed] [Google Scholar]
- 101.Tateda S, Aizawa T, Hashimoto K, Kanno H, Ohtsu S, Itoi E, Ozawa H. Successful management of Gorham-Stout disease in the cervical spine by combined conservative and surgical treatments: a case report. Tohoku Journal of Experimental Medicine 2017241249–254. ( 10.1620/tjem.241.249) [DOI] [PubMed] [Google Scholar]
- 102.Koto K, Inui K, Itoi M, Itoh K. Gorham-Stout disease in the rib and thoracic spine with spinal injury treated with radiotherapy, zoledronic acid, vitamin D, and propranolol: a case report and literature review. Molecular and Clinical Oncology 201911551–556. ( 10.3892/mco.2019.1934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tena-Sanabria ME, Jesús-Mejenes LY, Fuentes-Herrera G, Álvarez-Martínez FA, Victorio-García NP, Núñez-Enríquez JC. A report of two children with Gorham-Stout disease. BMC Pediatrics 201919 206. ( 10.1186/s12887-019-1561-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mavrogenis AF, Angelini A, Pala E, Calabro T, Bianchi G, Casadei R, Ruggieri P. Radiation-induced sarcomas. Journal of Long-Term Effects of Medical Implants 201121233–240. ( 10.1615/jlongtermeffmedimplants.v21.i3.70) [DOI] [PubMed] [Google Scholar]
- 105.Rodriguez-Vazquez JR, Chandra SR, Albertson ME, Hansen NJ, Johnson CM. Radiation-induced sarcoma on 18F-FDG PET/CT after treatment of Gorham-Stout disease of the maxilla. Clinical Nuclear Medicine 201944e607–e608. ( 10.1097/RLU.0000000000002761) [DOI] [PubMed] [Google Scholar]
- 106.Triana P, Dore M, Cerezo VN, Cervantes M, Sánchez AV, Ferrero MM, González MD, Lopez-Gutierrez JC. Sirolimus in the treatment of vascular anomalies. European Journal of Pediatric Surgery 20172786–90. ( 10.1055/s-0036-1593383) [DOI] [PubMed] [Google Scholar]
- 107.García V, Alonso-Claudio G, Gómez-Hernández MT, Chamorro AJ. Sirolimus on Gorham-Stout disease. Case report. Colombia Medica 201647213–216. ( 10.25100/cm.v47i4.2406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ozeki M, Nozawa A, Yasue S, Endo S, Asada R, Hashimoto H, Fukao T. The impact of sirolimus therapy on lesion size, clinical symptoms, and quality of life of patients with lymphatic anomalies. Orphanet Journal of Rare Diseases 201914 141. ( 10.1186/s13023-019-1118-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mo AZ, Trenor CC, Hedequist DJ. Sirolimus therapy as perioperative treatment of Gorham-Stout disease in the thoracic spine: a case report. JBJS Case Connector 20188 e70. ( 10.2106/JBJS.CC.17.00287) [DOI] [PubMed] [Google Scholar]
- 110.Schneider KN, Gosheger G, Andreou D. Gorham-Stout syndrome. Deutsches Ärzteblatt International 2019116 507. ( 10.3238/arztebl.2019.0507a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cho S, Kang SR, Lee BH, Choi S. Chylous manifestations and management of Gorham-Stout syndrome. Korean Journal of Thoracic and Cardiovascular Surgery 20195244–46. ( 10.5090/kjtcs.2019.52.1.44) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zheng MW, Yang M, Qiu JX, Nan XP, Huang LY, Zhang WD, Gong L, Huang ZZ. Gorham-Stout syndrome presenting in a 5-year-old girl with a successful bisphosphonate therapeutic effect. Experimental and Therapeutic Medicine 20124449–451. ( 10.3892/etm.2012.622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gem M, Özkul E, Arslan H. Gorham-Stout’s disease in the metatarsus: a case report. Acta Orthopaedica et Traumatologica Turcica 201448467–471. ( 10.3944/AOTT.2014.13.0132) [DOI] [PubMed] [Google Scholar]
- 114.Maillot C, Cloche T, Le Huec JC. Thoracic osteotomy for Gorham-Stout disease of the spine: a case report and literature review. European Spine Journal 2018272285–2290. ( 10.1007/s00586-014-3613-3) [DOI] [PubMed] [Google Scholar]
- 115.Matsumoto T, Naito M, Hirose J, Nakada I, Morikawa T, Tanaka S. Gorham-Stout syndrome of the shoulder girdle successfully controlled by antiresorptive agents: a report of 2 cases. JBJS Case Connector 20199 e0285. ( 10.2106/JBJS.CC.18.00285) [DOI] [PubMed] [Google Scholar]
- 116.Schneider KN, Masthoff M, Gosheger G, Klingebiel S, Schorn D, Röder J, Vogler T, Wildgruber M, Andreou D. Gorham-Stout disease: good results of bisphosphonate treatment in 6 of 7 patients. Acta Orthopaedica 202091209–214. ( 10.1080/17453674.2019.1709716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hagberg H, Lamberg K, Aström G. Alpha-2b interferon and oral clodronate for Gorham’s disease. Lancet 19973501822–1823. ( 10.1016/S0140-6736(0563639-2) [DOI] [PubMed] [Google Scholar]
- 118.Pfleger A, Schwinger W, Maier A, Tauss J, Popper HH, Zach MS. Gorham-Stout syndrome in a male adolescent-case report and review of the literature. Journal of Pediatric Hematology/Oncology 200628231–233. ( 10.1097/01.mph.0000203721.83566.e6) [DOI] [PubMed] [Google Scholar]
- 119.Deveci M, Inan N, Corapçıoğlu F, Ekingen G. Gorham-Stout syndrome with chylothorax in a six-year-old boy. Indian Journal of Pediatrics 201178737–739. ( 10.1007/s12098-010-0328-2) [DOI] [PubMed] [Google Scholar]
- 120.Kuriyama DK, McElligott SC, Glaser DW, Thompson KS. Treatment of Gorham-Stout disease with zoledronic acid and interferon-α: a case report and literature review. Journal of Pediatric Hematology/Oncology 201032579–584. ( 10.1097/MPH.0b013e3181edb464) [DOI] [PubMed] [Google Scholar]
- 121.Shimizu T, Sato K, Yoshida T, Takahashi A, Yanagawa T, Wada N, Sohmiya M, Shirakura K, Watanabe H. A case report of Gorham-Stout syndrome remission. Journal of Orthopaedic Science 201217199–204. ( 10.1007/s00776-011-0080-0) [DOI] [PubMed] [Google Scholar]
- 122.Yavaşoğlu İ, Çakiroğlu U. Diffuse lymphangiomatosis: Gorham-Stout syndrome. Internal Medicine 20145375–76. ( 10.2169/internalmedicine.53.0987) [DOI] [PubMed] [Google Scholar]
- 123.Cramer SL, Wei S, Merrow AC, Pressey JG. Gorham-Stout disease successfully treated with sirolimus and zoledronic acid therapy. Journal of Pediatric Hematology/Oncology 201638e129–e132. ( 10.1097/MPH.0000000000000514) [DOI] [PubMed] [Google Scholar]
- 124.Garbers E, Reuther F, Delling G. Report of a rare case of Gorham-Stout disease of both shoulders: bisphosphonate treatment and shoulder replacement. Case Reports in Rheumatology 20112011565142. ( 10.1155/2011/565142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brance ML, Castiglioni A, Cóccaro N, Palatnik M. Two cases of Gorham-Stout disease with good response to zoledronic acid treatment. Clinical Cases in Mineral and Bone Metabolism 201714250–253. ( 10.11138/ccmbm/2017.14.2.250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Illeez OG, Ozkan K, Ozkan FU, Bostan AB, Akpinar F, Bilgic B, Aktas I. Zoledronic acid : treatment option for Gorham-Stout disease. Der Orthopade 2018471032–1035. ( 10.1007/s00132-018-3655-z) [DOI] [PubMed] [Google Scholar]
- 127.Zheng C, Tang F, Min L, Zhou Y, Luo Y, Tu C, Zhang S. Gorham-Stout disease of the malleolus: a rare case report. BMC Musculoskeletal Disorders 201921 3. ( 10.1186/s12891-019-3027-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Takahashi A, Ogawa C, Kanazawa T, Watanabe H, Suzuki M, Suzuki N, Tsuchida Y, Morikawa A, Kuwano H. Remission induced by interferon alfa in a patient with massive osteolysis and extension of lymph-hemangiomatosis: a severe case of Gorham-Stout syndrome. Journal of Pediatric Surgery 200540E47–E50. ( 10.1016/j.jpedsurg.2004.11.015) [DOI] [PubMed] [Google Scholar]
- 129.Kose M, Pekcan S, Dogru D, Akyuz C, Ozcelik U, Ozsurekci Y, Gulhan B, Demircin M, Kiper N. Gorham-Stout syndrome with chylothorax: successful remission by interferon alpha-2b. Pediatric Pulmonology 200944613–615. ( 10.1002/ppul.20849) [DOI] [PubMed] [Google Scholar]
- 130.Dupond JL, Bermont L, Runge M, de Billy M. Plasma VEGF determination in disseminated lymphangiomatosis-Gorham-Stout syndrome: a marker of activity? A case report with a 5-year follow-up. Bone 201046873–876. ( 10.1016/j.bone.2009.11.015) [DOI] [PubMed] [Google Scholar]
- 131.Nir V, Guralnik L, Livnat G, Bar-Yoseph R, Hakim F, Ilivitzki A, Bentur L. Propranolol as a treatment option in Gorham-Stout syndrome: a case report. Pediatric Pulmonology 201449417–419. ( 10.1002/ppul.22869) [DOI] [PubMed] [Google Scholar]
- 132.Baud J, Lomri A, Graber D, Bikfalvi A. The therapeutic response in Gorham’s syndrome to the beta-blocking agent propranolol is correlated to VEGF-A, but not to VEGF-C or FLT1 expression. BMC Research Notes 20158 333. ( 10.1186/s13104-015-1259-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ozeki M, Fukao T, Kondo N. Propranolol for intractable diffuse lymphangiomatosis. New England Journal of Medicine 20113641380–1382. ( 10.1056/NEJMc1013217) [DOI] [PubMed] [Google Scholar]
- 134.Ozeki M, Kanda K, Kawamoto N, Ohnishi H, Fujino A, Hirayama M, Kato Z, Azuma E, Fukao T, Kondo N. Propranolol as an alternative treatment option for pediatric lymphatic malformation. Tohoku Journal of Experimental Medicine 201322961–66. ( 10.1620/tjem.229.61) [DOI] [PubMed] [Google Scholar]
- 135.Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. New England Journal of Medicine 20083582649–2651. ( 10.1056/NEJMc0708819) [DOI] [PubMed] [Google Scholar]
- 136.Peridis S, Pilgrim G, Athanasopoulos I, Parpounas K. A meta-analysis on the effectiveness of propranolol for the treatment of infantile airway haemangiomas. International Journal of Pediatric Otorhinolaryngology 201175455–460. ( 10.1016/j.ijporl.2011.01.028) [DOI] [PubMed] [Google Scholar]
- 137.Liu SZ, Zhou X, Song A, Wang YP, Liu Y. A rare case of Gorham-Stout syndrome of femur treated with cement augmentation. Chinese Medical Journal 20181311628–1629. ( 10.4103/0366-6999.235121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Feigl D, Seidel L, Marmor A. Gorham’s disease of the clavicle with bilateral pleural effusions. Chest 198179242–244. ( 10.1378/chest.79.2.242) [DOI] [PubMed] [Google Scholar]
- 139.Busilacchi A, Ramazzotti D, Ulisse S, Gigante A. Gorham-Stout disease as a complication of posterior shoulder capsulorrhaphy. Journal of Shoulder and Elbow Surgery 201221e1–e7. ( 10.1016/j.jse.2012.05.024) [DOI] [PubMed] [Google Scholar]
- 140.Brunner U, Rückl K, Konrads C, Rudert M, Plumhoff P. Gorham-Stout syndrome of the shoulder. SICOT-J 20162 25. ( 10.1051/sicotj/2016015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vaishya R, Vaish A, Singh LK, Baweja P. Management of a pathological fracture in a rare case of Gorham Stout disease of the hip with a mega prosthesis. Journal of Orthopaedics 202018177–180. ( 10.1016/j.jor.2019.08.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Woodward HR, Chan DP, Lee J. Massive osteolysis of the cervical spine: a case report of bone graft failure. Spine 19816545–549. ( 10.1097/00007632-198111000-00003) [DOI] [PubMed] [Google Scholar]
- 143.Turra S, Gigante C, Scapinelli R. A 20-year follow-up study of a case of surgically treated massive osteolysis. Clinical Orthopaedics and Related Research 1990250297–302. ( 10.1097/00003086-199001000-00039) [DOI] [PubMed] [Google Scholar]
- 144.Chong Ng L, Sell P. Gorham disease of the cervical spine-a case report and review of the literature. Spine 200328E355–E358. ( 10.1097/01.BRS.0000084557.38858.85) [DOI] [PubMed] [Google Scholar]
- 145.Schell A, Rhee JM, Allen A, Andras L, Zhou F. Surgical management of Gorham disease involving the upper cervical spine with occipito-cervical-thoracic fusion: a case report. Spine Journal 201616e467–e472. ( 10.1016/j.spinee.2016.02.020) [DOI] [PubMed] [Google Scholar]
- 146.Jaccard A, Macedo C, Castro G, Guiroy A. Thoracic spine dislocation in Gorham-Stout syndrome: case report and literature review. Surgical Neurology International 20189 223. ( 10.4103/sni.sni_311_18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Du CZ, Li S, Xu L, Zhou QS, Zhu ZZ, Sun X, Qiu Y. Spinal Gorham-Stout syndrome: radiological changes and spinal deformities. Quantitative Imaging in Medicine and Surgery 20199565–578. ( 10.21037/qims.2019.03.17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim JH, Yoon DH, Kim KN, Shin DA, Yi S, Kang J, Ha Y. Surgical management of Gorham-Stout disease in cervical compression fracture with cervicothoracic fusion: case report and review of literature. World Neurosurgery 2019129277–281. ( 10.1016/j.wneu.2019.05.235) [DOI] [PubMed] [Google Scholar]
- 149.Chang KJ, Yang MH, Li B, Huang H. Surgical management of Gorham-Stout syndrome involving the cervical spine with bilateral pleural effusion: a case report and literature review. Experimental and Therapeutic Medicine 2020193851–3855. ( 10.3892/etm.2020.8627) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kai B, Ryan A, Munk PL, Dunlop P. Gorham disease of bone: three cases and review of radiological features. Clinical Radiology 2006611058–1064. ( 10.1016/j.crad.2006.04.014) [DOI] [PubMed] [Google Scholar]
- 151.Kotaru AC, Rajput AK. Chylothorax from Gorham-Stout disease. Journal of Bronchology and Interventional Pulmonology 201825340–342. ( 10.1097/LBR.0000000000000506) [DOI] [PubMed] [Google Scholar]
- 152.Pedicelli G, Mattia P, Zorzoli AA, Sorrone A, De Martino F, Sciotto V. Gorham syndrome. JAMA 19842521449–1451. [PubMed] [Google Scholar]
- 153.Hejgaard N, Olsen PR. Massive Gorham osteolysis of the right hemipelvis complicated by chylothorax: report of a case in a 9-year-old boy successfully treated by pleurodesis. Journal of Pediatric Orthopedics 1987796–99. ( 10.1097/01241398-198701000-00020) [DOI] [PubMed] [Google Scholar]
- 154.Cho SJ, Kim SW. Chemical pleurodesis using Viscum album extract in Gorham disease complicated with chylothorax. Acute and Critical Care 201833105–109. ( 10.4266/acc.2016.00164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ferreiro L, Suárez-Antelo J, Rábade C, Abdulkader I, Bermúdez-Naveira A, Fernández-García A, Valdés L. Chylothorax as a debut form of Gorham-Stout disease. Pulmonology 201925195–197. ( 10.1016/j.pulmoe.2019.01.008) [DOI] [PubMed] [Google Scholar]
- 156.Wang P, Liao W, Cao G, Jiang Y. A rare case of Gorham-Stout syndrome involving the thoracic spine with progressive bilateral chylothorax: a case report. BMC Musculoskeletal Disorders 201920 154. ( 10.1186/s12891-019-2542-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a