Abstract
Introduction
The severity of SARS-CoV-2 induced coronavirus disease 19 (COVID-19) depends on the presence of risk factors and the hosts' gene variability. There are preliminary results that gene polymorphisms of the renin-angiotensin system can influence the susceptibility to and mortality from COVID-19. Angiotensin II type 1 receptor (AT1R) might be a gene candidate that exerts such influence.
The aim of this study was to elaborate on the association between A1166C at1r polymorphic variants and the susceptibility to and severity of COVID-19 in the Ukrainian population.
Methods
The study population consisted of the Ukrainian population (Poltava region) with COVID-19, divided into three clinical groups in accordance with oxygen requirement: patients without oxygen therapy (n = 110), with non-invasive (n = 136) and invasive (n = 36) oxygen therapy. The A1166C polymorphism of the at1r was determined by polymerase chain reaction with subsequent restrictase analysis.
In an attempt to better explain the role of the A1166C at1r polymorphism we compared its association with COVID-19, essential hypertension (n = 79), renoparenchimal hypertension (n = 30) and dyscirculatory encephalopathy (n = 112). The data for this comparison were obtained by meta-analysis.
Results
We observed significant differences in the frequency of AA, AC and CC genotypes in the groups of COVID-19 patients with non-invasive and invasive oxygen therapy in comparison with control subjects as well as in the frequency of combined AC + CC genotype between the groups of COVID-19 patients with any types of oxygen therapy and patients without oxygen therapy. The frequency of the 1166C allele was higher in COVID-19 patients with invasive oxygen therapy (OR = 2.06; CI (1.20–3.53); p = 0.013). We obtained important results indicating that there were no differences between the frequency of at1r polymorphisms in patients with cardiovascular disease and severe COVID-19 with invasive oxygen therapy as well as those who died due to COVID-19.
Conclusion
Our study indicated the presence of an association between the A1166C at1r polymorphisms and the severity of COVID-19 in the Ukrainian population. It seems that in carriers of 1166C at1r, the severity of COVID-19 and oxygen dependency is higher as compared to the A allele carriers, possibly, due to cardiovascular disorders.
Keywords: Gene, Polymorphism, Angiotensin II type 1 receptor, SARS-CoV-2, COVID-19
1. Introduction
SARS-CoV-2 is a novel coronavirus strain rapidly spread from China as a global devastating pandemic (Zhu et al., 2020; Wang et al., 2020). SARS-CoV-2 induced coronavirus disease 19 (COVID-19) and its severity depended on the presence of risk factors such as age, cardiovascular disease, metabolic disorders (diabetes, obesity) etc. (Lauc and Sinclair, 2020; Fang et al., 2020). On the other hand, viral infections accompanied human evolution through the host/virus interaction and at least the part of inter-individual differences was attributed to the host genetics profile (Albright et al., 2008; Fauci and Morens, 2012). In parallel, the spectrum of COVID-19 phenotypes raised the question as to what extent the variable response to SARS-CoV-2 is influenced by the variability of the hosts' genetic background (Di Maria et al., 2020).
SARS-CoV-2 spike protein has a strong binding affinity with the human ACE2 extracellular domain, and the virus recognized the ACE2 peptidase domain as a receptor (Lan et al., 2020). Recently, there are preliminary results that ace2 and ace polymorphisms can influence the susceptibility to and mortality from COVID-19 (Asselta et al., 2020; Delanghe et al., 2020).
ACE2 converts angiotensin I to biologically active angiotensin II which is the main effector of the renin-angiotensin system. In COVID-19, elevated concentration of angiotensin II is associated with proinflammatory, prothrombotic, profibrotic processes as well as oxidative stress (Alexandre et al., 2020). Angiotensin II realized most effects on cardiovascular and excretory systems through angiotensin II type 1 receptor (AT1R) (Li et al., 2017). Currently, the role of AT1Rs in the susceptibility and disease severity in COVID-19 patients remains the supposed fact (McLachlan, 2020). Little is known about polymorphisms of this receptor in patients with COVID-19 although AT1R polymorphisms had importance for COVID-19 risk factors such as hypertension, diabetes, cardiovascular diseases (Choi et al., 2021; Baudin, 2005; Miller et al., 2000; Feng et al., 2014) as well as oxidative stress and systemic inflammation (Reja et al., 2006). A number of the at1r polymorphisms have been identified. Bonnardeaux A. et al. screened the entire coding region (exon 5) and the 3′-untranslated region, and identified five frequent polymorphisms but only the frequency of at1r A1166C polymorphism was significantly increased (Bonnardeaux et al., 1994). Since that time the single nucleotide polymorphism A1166C (rs5186) of at1r has been one of the most studied and clinically important in Caucasian population (Katsuya and Morishita, 2013; Yang et al., 2017; Duncan et al., 2001). The aim of this study was to elaborate on the association between the A1166C at1r polymorphic variants and the susceptibility to and severity of COVID-19 in the Ukrainian population. In an attempt to better explain the role of the A1166C at1r polymorphism we compared its association between COVID-19, essential hypertension, renoparenchimal hypertension and dyscirculatory encephalopathy.
2. Methods
2.1. Study population
An observational analytic study with a case-control design was conducted at outpatient clinics and inpatient wards at the clinical settings of Poltava State Medical University, Ukraine. Written informed consent was obtained from all recruited patients. The study was approved by the local Ethics Committee of Poltava State Medical University, Ukraine.
The study population consisted of the Ukrainian population with COVID-19 who had resided in the Poltava Region (central part of Ukraine). The inclusion criteria for study group were subjects with clinical signs and symptoms of COVID-19, positive results of SARS-CoV-2 PCR tests in nasopharyngeal swabs (Taleghani and Taghipour, 2021).
All COVID-19 patients were divided on three clinical groups in accordance with oxygen requirement: group 1 – patients without oxygen therapy (n = 110), group 2 – patients with non-invasive oxygen therapy (n = 136), group 3 – patients with lung ventilation (invasive oxygen therapy) (n = 36). The control group comprised 82 healthy persons.
2.2. Genetic analysis
Venous blood was drawn and placed into tubes containing EDTA (BD Vacutainer® К2Е, Becton Dickinson, USA). Genomic DNA was isolated by DNeasy Blood&Tissue Kit using automatics station QIAcube Connect (QIAGEN, Germany).
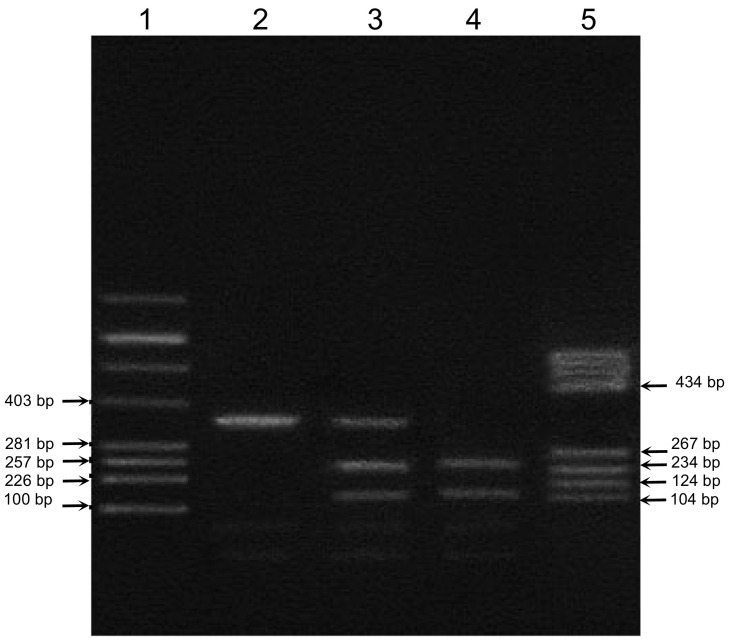
A1166C polymorphism of the at1r was determined by polymerase chain reaction (PCR) with subsequent restrictase analysis. Each DNA sample was analyzed in duplicate by PCR. For PCR, the following primers were used: F: 5’-CCTGCACCATGTTTTGAGGTTGAGTGAC-3′ and R: 5’-AAAATAACAGGACAAACGCAGGCTAGGGAG-3′. After digesting with Dde I (Promega, USA) enzyme for 2 h at 37 °C, the PCR digest was detected on 2.5% agarose gel electrophoresis stained with ethidium bromide. DNA molecular weight marker pBR322/BsuRI (Promega, USA) was used. Wild type allele A generated one fragment corresponding to size 352 bp. Two fragments of 114 bp and 238 bp were observed as mutated allele C. The heterozygote genotype (AC) generated three fragments of 114, 238 and 352 bp (Sergeeva et al., 2001). The relevant image of representative gel-electrophoresis is shown at Fig. 1 .
Fig. 1.
The electrophoresis result of polymerase chain reaction (PCR) Dde I products of A1166C AT1R polymorphism. Lane 1 – molecular marker pBR322/Alu, lane 2 – AA genotype (352 bp), lane 3 – AC genotype (352 bp, 238 bp, 114 bp), lane 4 – CC genotype (238 bp, 114 bp), and lane 5 – molecular marker pBR322/Bsu RI.
2.3. Methodology of meta-analysis
We searched PubMed, Embase, Scopus, Cochrane Library as well as Google Scholar for a relevant article. Searches of the electronic databases were conducted using terms “angiotensin II receptor type 1”, “polymorphism”, “A1166C”, “cardiovascular and vascular disease”, “Ukrainian population”, “Poltava Region”. The selected articles were analyzed to contain primary data inclusion and exclusion criteria, number of patients, proportions of A1166C alleles and genotypes. The extracted data included the study design, patients' data, polymorphism frequencies, statistic analysis. Two reviewers (O.I., O.S.) independently determined study eligibility and extracted the data. Since we included fewer than 10 studies, we did not use a funnel plot to assess publication bias.
2.4. Statistic analysis
The at1r polymorphism was analyzed for deviation of Hardy-Weinberg equilibrium by Pearson's χ2. The expected and observed heterozygosity was calculated by POPGENE (Version 1.32). Fisher's exact test or χ2 test with Yates's correction was used to compare the genotypic and allelic frequencies between controls and patients. The association between A1166C at1r polymorphism and disease susceptibility / severity was assessed by calculating the odds ratio with 95% confidential interval (CI) with GraphPad PRISM® (Version 5.03) software. P-values of less than 0.05 were considered to be significant.
3. Results
3.1. Characteristics of the studied population
Demographic characteristics of the entire studied population are shown in Table 1 .
Table 1.
Characteristics of the studied population.
| Variables | Control subjects, n = 82 | COVID-19 patients |
|||
|---|---|---|---|---|---|
| Without oxygen therapy, n = 110 | Non-invasive oxygen therapy, n = 136 | Invasive oxygen therapy |
|||
| Total, n = 36 | Of them died, n = 12 | ||||
| Sex, n (%) | |||||
| - Male | 33 (40.2) | 52 (47.3) | 56 (41.2) | 15 (41.7) | 3 (25.0) |
| - Female | 49 (59.8) | 58 (52.7) | 80 (58.8) | 21 (58.3) | 9 (75.0) |
| Age, years mean (SD) | 42.10 (1.41) | 51.11 (1.66) | 63.34 (1.32) | 65.08 (2.27) | 66,14 (5,08) |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | ||
| p1 < 0.001 | p1 < 0.001 | p1 = 0.006 | |||
| BMI, kg/m2 mean (SD) | 28.85 (0.31) | 29.08 (0.28) | 30.02 (0.42) | 31.03 (0.23) | 31,11 (0,32) |
| p = 0.030 | p ≤0.001 | p < 0.001 | |||
| p1 < 0.001 | p1 < 0.001 | ||||
| p2 = 0.040 | p2 = 0.040 | ||||
Note: p – comparison with control subjects. p1 – comparison with COVID-19 patients without oxygen therapy. p2 – comparison with COVID-19 patients with non-invasive oxygen therapy.
There were no significant differences in terms of sex between all study groups. The COVID-19 patients had significantly higher age in comparison with control subjects, and COVID-19 patients who needed oxygen therapy had higher age than those without oxygen therapy. They also tend to have significantly higher body mass index (BMI).
3.2. Frequency of at1r polymorphism A1166C in the studied population
The observed at1r genotype distribution in the study groups was significantly different from that in the Hardy-Weinberg equilibrium (patients without oxygen therapy: χ2 = 7.25; p = 0.03; Heterozygosity observed – 33%, expected – 44%; patients with invasive oxygen therapy died from COVID-19: χ2 = 6.12; p = 0.05; observed heterozygosity– 83%, expected heterozygosity – 49%).
There were significant differences in the frequency of the at1r polymorphism, i.e. AA, AC and CC genotypes, in the group of COVID-19 patients with non-invasive oxygen therapy (p = 0.008) and in the group with invasive oxygen therapy (p = 0.047) in comparison with control subjects. There were no significant differences in the frequency of at1r polymorphism between the group of COVID-19 patients without oxygen therapy and control subjects (Table 2 ).
Table 2.
Allele frequency and genotype distribution of the A1166C polymorphism of AT1R, n (%).
| Control subjects, n = 82 | COVID-19 patients |
||||
|---|---|---|---|---|---|
| Without oxygen therapy, n = 110 | Non-invasive oxygen therapy, n = 136 | Invasive oxygen therapy |
|||
| Total, n = 36 | Of them died, n = 12 | ||||
| Genotype Distribution, n (%) | |||||
| АА | 42 (51.2) | 56 (50.9) | 46 (33.8) | 10 (27.8) | 2 (16.7) |
| АС | 28 (34.1) | 36 (32.7) | 76 (55.9) | 16 (44.4) | 10 (83.3) |
| СС | 12 (14.7) | 18 (16.4) | 14 (10.3) | 10 (27.8) | 0 |
| P = 0.008 | P = 0.047 | ||||
| AC + CC | 40 (48.8) | 54 (49.1) | 90 (66.2) | 26 (72.2) | 10 (83.3) |
| p = 0.012 | p = 0.019 | p = 0.026 | |||
| OR = 2.05 | OR = 2.73 | OR = 5.25 | |||
| CI (1.17–3.60) | CI (1.17–6.38) | CI (1.08–25.46) | |||
| p1 = 0.007 | p1 = 0.016 | p1 = 0.025 | |||
| OR = 2.03 | OR = 2.70 | OR = 5.19 | |||
| CI (1.21–3.40) | CI (1.19–6.12) | CI (1.09–24.76) | |||
| Allele Frequency, n (%) | |||||
| А | 112 (68.3) | 148 (67.3) | 168 (61.8) | 36 (50.0) | 14 (58.3) |
| С | 52 (31.7) | 72 (32.7) | 104 (38.2) | 36 (50.0) | 10 (41.7) |
| p = 0.011 | |||||
| OR = 2.15 | |||||
| CI (1.22–3.80) | |||||
| p1 = 0.013 | |||||
| OR = 2.06 | |||||
| CI (1.20–3.53) | |||||
Note: p – comparison with control subjects; p1 – comparison with COVID-19 patients without oxygen therapy.
There were also significant differences in the frequency of combined AC + CC genotype between the groups of COVID-19 patients with any types of oxygen therapy and control subjects or patients without oxygen therapy.
The frequencies of combined AC + CC genotype increased in groups of patients in accordance with the volume of oxygen therapy and outcome: non-invasive oxygen therapy – 66.2% AC + CC, OR = 2.03, CI (1.21–3.40), p = 0.007; invasive oxygen therapy – 72.2% AC + CC, OR = 2.70, CI (1.19–6.12), p = 0.016; patients with invasive oxygen therapy which died – 83.3% AC + CC, OR = 5.19, CI (1.09–24.76), p = 0.025, in comparison with patients without oxygen therapy.
The frequency of C allele distribution was obviously higher in the group of COVID-19 patients with invasive oxygen therapy than that in control subjects (OR = 2.15, CI (1.22–3.80), p = 0.011) and in the group of patients without oxygen therapy (OR = 2.06, CI (1.20–3.53), p = 0.013).
Meta-analysis of at1r polymorphism in patients with essential hypertension, renoparenchimal hypertension and dyscirculatory encephalopathy in the Ukrainian population (Poltava Region). Our searches of the allele frequency and the genotype distribution of the A1166C polymorphism associated with cardiovascular diseases in the Ukrainian population (Poltava Region) identified 10 non-duplicated citations of which 6 were deemed eligible for inclusion based on their titles and abstracts. Three studies were subsequently excluded because they were critical appraisal articles.
Finally, 3 studies involving 79 patients with essential hypertension (Kaĭdashev et al., 2005), 30 patients with renoparenchimal hypertension (Kaidashev et al., 2006), and 112 patients with dyscirculatory encephalopathy (Krivchun et al., 2010) were included in the analysis.
Allele frequency and genotype distribution of A1166C polymorphism of at1r in patients with essential hypertension, renoparenchimal hypertension and dyscirculatory encephalopathy are shown in Table 3 .
Table 3.
Allele frequency and genotype distribution of the A1166C polymorphism of AT1R by clinical diagnosis, n (%).
| Essential hypertension, n = 79 (Kaĭdashev et al., 2005) | Renoparenchimal hypertension, n = 30 (Kaidashev et al., 2006) | Dyscirculatory encephalopathy, n = 112 (Krivchun et al., 2010) | |
|---|---|---|---|
| Genotype Distribution, n (%) | |||
| АА | 18 (22.8) | 9 (30.0) | 24 (21.4) |
| АС | 41 (51.9) | 19 (63.3) | 62 (55.4) |
| СС | 20 (25.3) | 2 (6.7) | 26 (23.2) |
| p < 0.001 | p = 0.021 | p < 0.001 | |
| p1 = 0.001 | p2 = 0.010 | p1 = 0.001 | |
| p2 = 0.010 | p2 = 0.008 | ||
| AC + CC | 61 (77.2) | 21 (70.0) | 88 (78.6) |
| p < 0.001 | p = 0.046 | p < 0.001 | |
| OR = 3.56 | OR = 2.45 | OR = 3.85 | |
| CI (1.80–7.03) | CI (1.00–5.98) | CI (2.06–7.20) | |
| p1 < 0.001 | p1 = 0.042 | p1 < 0.001 | |
| OR = 3.51 | OR = 2.42 | OR = 3.80 | |
| CI (1.84–6.70) | CI (1.02–5.75) | CI (2.12–6.83) | |
| p2 = 0.031 | |||
| OR = 1.87 | |||
| CI (1.06–3.33) | |||
| Allele Frequency, n (%) | |||
| A | 77 (48.7) | 37 (61.7) | 110 (49.1) |
| C | 81 (51.3) | 23 (38.3) | 114 (50.9) |
| p = 0.001 | p < 0.001 | ||
| OR = 2.27 | OR = 2.23 | ||
| CI (1.44–3.57) | CI (1.47–3.40) | ||
| p1 < 0.001 | p1 < 0.001 | ||
| OR = 0.46 | OR = 0.47 | ||
| CI (0.30–0.70) | CI (0.32–0.69) | ||
| p2 = 0.011 | p2 = 0.006 | ||
| OR = 0.59 | OR = 0.60 | ||
| CI (0.40–0.87) | CI (0.42–0.85) |
Note: p – comparison with control subjects; p1 – comparison with COVID-19 patients without oxygen therapy; p2 – comparison with COVID-19 patients with non-invasive oxygen therapy.
There were significant differences in the frequencies of at1r polymorphism between patients with essential hypertension and control subjects, COVID-19 patients without oxygen therapy or with non-invasive oxygen therapy. The patients with dyscirculatory encephalopathy had the same pattern of genotype frequencies. These differences depended on the high prevalence of the combined AC + CC genotype as well as the 1166C allele.
The patients with renoparenchimal hypertension had significant differences in the frequencies of at1r polymorphisms with control subjects (p = 0.021) and COVID-19 patients with non-invasive oxygen therapy (p = 0.010). Also, there was a difference in the distribution of combined AC + CC genotype in comparison with control subjects (OR = 2.45; CI (1.00–5.98), p = 0.046) and COVID-19 patients without oxygen therapy (OR = 2.42; CI (1.02–5.75), p = 0.042).
However, no differences were observed between the frequencies of at1r polymorphisms in patients with cardiovascular diseases and COVID-19 with invasive oxygen therapy as well as those who died due to COVID-19.
4. Discussion
Despite SARS-CoV-2 spike protein had a strong binding affinity with ACE2 extracellular domain the elevated concentration of angiotensin II might be observed during COVID-19 (Alexandre et al., 2020). In contrast, a new pilot study showed no significant differences in circulating angiotensin II, angiotensin-(1–7), but reduced ACE activity compare with COVID-19-negative controls (Files et al., 2021).
Another important player in the renin-angiotensin system is AT1R, which exerted a number of angiotensin II effects such as vasoconstriction, angiogenesis, matrix synthesis, and aldosterone synthesis. AT1R is localized mostly in the adult cardiovascular tissue, it is present in the brain, kidneys, and adrenal glands (Chaudhary and Chaudhary, 2017). Moreover, this receptor participated in angiotensin II-induced oxidant stress, and NF-κB activation with the enhanced expression of IL-6, VCAM-1, and MCP-1 (MacKenzie, 2011). AT1R was predicted to be involved in the host gene variability determined COVID-19 susceptibility and severity (Kaidashev et al., 2021). SNPs in human at1r have been implicated in the pathophysiology of various diseases, such as cardiovascular diseases. rs5186 (A1166C) is the vastly steadied SNP which lies in 3’UTR and involves the binding site of miRNA-155 (Haas et al., 2012). Computer alignment analysis and reporter silencing assays demonstrated that miRNA-155 is complimentary with a specific sequence of 3’UTR of at1r harboring the A1166C polymorphism (Martin et al., 2007). In the presence of the A allele, but not C allele, miRNA-155 interacts with 3’UTR of AT1R mRNA and modulates AT1R expression. This miRNA translationally repressed the expression of AT1R and the inhibition of miRNA-155 expression by anti-miPNA-155 markedly increased AT1R protein levels (Martin et al., 2006). miRNA-155 expression was significantly decreased in subjects and patients with CC genotype in comparison to AA and CC genotypes. In the same time, patients with CC genotype had higher AT1R expression compared to AA and AC genotypes (Ceolotto et al., 2011a; Stanković et al., 2016; Blanco et al., 2012).
Transversion from A to C allele disrupts binding of miRNA-155 leading to overexpression of AT1R and development of disease (Ceolotto et al., 2011b).
Thus, A1166C is a possible SNP of at1r, which might determine susceptibility to and severity of COVID-19.
In the first part of our study selected patients with COVID-19 were divided into three clinical groups according to the need for oxygen: without oxygen therapy, with non-invasive, and with invasive oxygen therapy. Additionally, the sub-group of patients who died was selected from the group with invasive oxygen therapy.
All COVID-19 patients had significantly higher age in comparison with control subjects, and COVID-19 patients who needed oxygen had higher age than those without oxygen dependency. Similarly, oxygen-dependent COVID-19 patients had elevated BMI. The patients who died from COVID-19 had the highest BMI. These data went in parallel with studies, which showed that aging and overweight were important risk factors in COVID-19 (Sattar et al., 2020).
We observed significant differences in the frequency of AA, AC and CC genotypes in the groups of COVID-19 patients with non-invasive and invasive oxygen therapy in comparison with control subjects as well as in the frequency of combined AC + CC genotype between the groups of COVID-19 patients with any types of oxygen therapy and patients without oxygen therapy. The frequency of the 1166C allele was higher in COVID-19 patients with invasive oxygen therapy.
Thus, the presence of the 1166C allele and AC + CC genotype might be associated with the severity of COVID-19.
As the final part of our study, we compared the association of A1166C at1r variants with COVID-19 severity and cardiovascular disorders in the Ukrainian population (Poltava Region). For this purpose, the meta-analysis of at1r polymorphism in patients with essential hypertension, renoparenchimal hypertension and dyscirculatory encephalopathy was provided.
The patients with essential hypertension and dyscirculatory encephalopathy had high prevalence of combined AC + CC genotype as well as 1166C allele. The similar results were obtained for these diseases in other populations (Yang et al., 2017; Parchwani et al., 2018; Salminen et al., 2014; Szolnoki, 2007).
The patients with essential hypertension and with dyscirculatory encephalopathy had no differences in the prevalence of AC + CC genotype and 1166C allele in comparison with COVID-19 patients with invasive oxygen therapy.
The patients with renoparenchimal hypertension showed the prevalence of AC + CC genotype in comparison with control subjects and COVID-19 patients without oxygen therapy. These data are supported by other researches in part of renal disorders (Mao and Huang, 2013; Lee et al., 2009; Chang et al., 2018). Moreover, A1166C at1r polymorphism plays an important role in the pathogenesis of pulmonary hypertension and in the susceptibility to high-altitude pulmonary edema (Hotta et al., 2004). This phenomenon may be important for the severe lung damage in COVID-19.
We obtained important results indicating that there were no differences between the frequency of the at1r polymorphisms in patients with cardiovascular diseases and severe COVID-19 with invasive oxygen therapy as well as those who died due to COVID-19.
Taking together, our data support the assumption that allele 1166C (rs5186) of the at1r might be associated with the severity of COVID-19.
We suppose that allele 1166C might be associated with the severity of COVID-19 due to the impaired regulation of AT1R by miRNA-155 with further AT1R over expression. AT1R mediates major angiotensin II effects (such as vasoconstriction, increased BP, cardiac contractility, renal tubular sodium absorption and cell proliferation) and mediates detrimental effects (such as oxidative stress, endothelial dysfunction, cardiovascular diseases and inflammation) (Mehta and Griendling, 2007). These effects of angiotensin II are potentially dangerous during COVID-19 (Miesbach, 2020).
Limitations of our study are the possible influence of age and BMI as well as the preexistence of or predisposition to cardiovascular diseases.
Prospects for a future research might be described as further investigation of COVID-19 susceptibility and severity associated with A1166C at1r polymorphism using an increased number of patients and analyzing comorbid cardiovascular and renal pathologies as well as experimental results are needed to prove hypothesis that A1166C affects COVID-19. Additional studies will be required to confirm the potential role of this polymorphism in COVID-19 in other geographic populations.
5. Conclusions
Our study indicated the presence of an association between the A1166C at1r polymorphism and the severity of COVID-19 in the Ukrainian population. It seems that in carriers of 1166C at1r, the severity of COVID-19 and oxygen dependency is higher as compared to the A allele carriers, possibly, due to cardiovascular disorders.
Ethical approval
Ethics approval and consent to participate the study was approved by the Committee on Bioethics and Ethical Issues of Poltava State Medical University. The patient signed a written informed consent form.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The study was a part of research No. 0121U107440 “Genetic variants and their potential link with COVID-19 in the Ukrainian population” funded by the Ministry of Public Health of Ukraine.
References
- Albright F.S., Orlando P., Pavia A.T., Jackson G.G., Cannon Albright L.A. Evidence for a heritable predisposition to death due to influenza. J. Infect. Dis. 2008 Jan 1;197(1):18–24. doi: 10.1086/524064. (PMID: 18171280) [DOI] [PubMed] [Google Scholar]
- Alexandre J., Cracowski J.L., Richard V., Bouhanick B. Drugs, COVID-19’ working group of the French Society of Pharmacology, Therapeutics. Renin-angiotensin-aldosterone system and COVID-19 infection. Ann. Endocrinol. (Paris). 2020 Jun;81(2–3):63–67. doi: 10.1016/j.ando.2020.04.005. Epub 2020 Apr 21. PMID: 32370986; PMCID: PMC7172808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselta R., Paraboschi E.M., Mantovani A., Duga S. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. Aging (Albany NY) 2020 Jun 5;12(11):10087–10098. doi: 10.18632/aging.103415. Epub 2020 Jun 5. PMID: 32501810; PMCID: PMC7346072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudin B. Polymorphism in angiotensin II receptor genes and hypertension. Exp. Physiol. 2005 May;90(3):277–282. doi: 10.1113/expphysiol.2004.028456. Epub 2005 Jan 7. PMID: 15640279. [DOI] [PubMed] [Google Scholar]
- Blanco R.R., Austin H., Vest R.N., 3rd, Valadri R., Li W., Lassegue B., Song Q., London B., Dudley S.C., Bloom H.L., Searles C.D., Zafari A.M. Angiotensin receptor type 1 single nucleotide polymorphism 1166A/C is associated with malignant arrhythmias and altered circulating miR-155 levels in patients with chronic heart failure. J. Card. Fail. 2012 Sep;18(9):717–723. doi: 10.1016/j.cardfail.2012.06.531. Epub 2012 Aug 9. PMID: 22939041; PMCID: PMC3640363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnardeaux A., Davies E., Jeunemaitre X., Féry I., Charru A., Clauser E., Tiret L., Cambien F., Corvol P., Soubrier F. Angiotensin II type 1 receptor gene polymorphisms in human essential hypertension. Hypertension. 1994 Jul;24(1):63–69. doi: 10.1161/01.hyp.24.1.63. (PMID: 8021009) [DOI] [PubMed] [Google Scholar]
- Ceolotto G., Papparella I., Bortoluzzi A., Strapazzon G., Ragazzo F., Bratti P., Fabricio A.S., Squarcina E., Gion M., Palatini P., Semplicini A. Interplay between miR-155, AT1R A1166C polymorphism, and AT1R expression in young untreated hypertensives. Am. J. Hypertens. 2011 Feb;24(2):241–246. doi: 10.1038/ajh.2010.211. Epub 2010 Oct 21. PMID: 20966899. [DOI] [PubMed] [Google Scholar]
- Ceolotto G., Papparella I., Bortoluzzi A., Strapazzon G., Ragazzo F., Bratti P., Fabricio A.S., Squarcina E., Gion M., Palatini P., Semplicini A. Interplay between miR-155, AT1R A1166C polymorphism, and AT1R expression in young untreated hypertensives. Am. J. Hypertens. 2011 Fe;24(2):241–246. doi: 10.1038/ajh.2010.211. Epub 2010 Oct 21. PMID: 20966899. [DOI] [PubMed] [Google Scholar]
- Chang H.F., Hsiao P.J., Hsu Y.J., Lin F.H., Lin C., Su W., Chen H.C., Su S.L. Association between angiotensin II receptor type 1 A1166C polymorphism and chronic kidney disease. Oncotarget. 2018 Feb 12;9(18):14444–14455. doi: 10.18632/oncotarget.24469. PMID: 29581855; PMCID: PMC5865681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary M., Chaudhary S. Unravelling the lesser known facets of angiotensin II type 1 receptor. Curr. Hypertens. Rep. 2017 Jan;19(1):1. doi: 10.1007/s11906-017-0699-0. (PMID: 28083801) [DOI] [PubMed] [Google Scholar]
- Choi H.G., Wee J.H., Kim S.Y., Kim J.H., Il Kim H., Park J.Y., Park S., Il Hwang Y., Jang S.H., Jung K.S. Association between asthma and clinical mortality/morbidity in COVID-19 patients using clinical epidemiologic data from Korean Disease Control and Prevention. Allergy. 2021 Mar;76(3):921–924. doi: 10.1111/all.14675. Epub 2020 Dec 10. PMID: 33249591; PMCID: PMC7753771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delanghe J.R., Speeckaert M.M., De Buyzere M.L. ACE Ins/Del genetic polymorphism and epidemiological findings in COVID-19. Clin. Chem. Lab. Med. 2020 Jun 25;58(7):1129–1130. doi: 10.1515/cclm-2020-0605. (PMID: 32386189) [DOI] [PubMed] [Google Scholar]
- Di Maria E., Latini A., Borgiani P., Novelli G. Genetic variants of the human host influencing the coronavirus-associated phenotypes (SARS, MERS and COVID-19): rapid systematic review and field synopsis. Hum Genom. 2020 Sep 11;14(1):30. doi: 10.1186/s40246-020-00280-6. PMID: 32917282; PMCID: PMC7484929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J.A., Scholey J.W., Miller J.A. Angiotensin II type 1 receptor gene polymorphisms in humans: physiology and pathophysiology of the genotypes. Curr. Opin. Nephrol. Hypertens. 2001 Jan;10(1):111–116. doi: 10.1097/00041552-200101000-00017. (PMID: 11195043) [DOI] [PubMed] [Google Scholar]
- Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020 Apr;8(4) doi: 10.1016/S2213-2600(20)30116-8. Epub 2020 Mar 11. Erratum in: Lancet Respir Med. 2020 Jun;8(6):e54. PMID: 32171062; PMCID: PMC7118626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A.S., Morens D.M. The perpetual challenge of infectious diseases. N. Engl. J. Med. 2012 Feb 2;366(5):454–461. doi: 10.1056/NEJMra1108296. Erratum in: N. EnglN Engl J Med. 2012 Mar 1;366(9):868. PMID: 22296079. [DOI] [PubMed] [Google Scholar]
- Feng X., Zheng B.S., Shi J.J., Qian J., He W., Zhou H.F. A systematic review and meta-analysis of the association between angiotensin II type 1 receptor A1166C gene polymorphism and myocardial infarction susceptibility. J. Renin-Angiotensin-Aldosterone Syst. 2014 Sep;15(3):307–315. doi: 10.1177/1470320312466927. Epub 2012 Nov 23. PMID: 23178513. [DOI] [PubMed] [Google Scholar]
- Files D.C., Gibbs K.W., Schaich C.L., Collins S.P., Gwathmey T.M., Casey J.D., Self W.H., Chappell M.C. A pilot study to assess the circulating renin-angiotensin system in COVID-19 acute respiratory failure. Am. J. Phys. Lung Cell. Mol. Phys. 2021 Jul 1;321(1):L213–L218. doi: 10.1152/ajplung.00129.2021. Epub 2021 May 19. PMID: 34009036; PMCID: PMC8270515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas U., Sczakiel G., Laufer S.D. MicroRNA-mediated regulation of gene expression is affected by disease-associated SNPs within the 3’-UTR via altered RNA structure. RNA Biol. 2012 Jun;9(6):924–937. doi: 10.4161/rna.20497. Epub 2012 Jun 1. PMID: 22664914; PMCID: PMC3495750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta J., Hanaoka M., Droma Y., Katsuyama Y., Ota M., Kobayashi T. Polymorphisms of renin-angiotensin system genes with high-altitude pulmonary edema in Japanese subjects. Chest. 2004 Sep;126(3):825–830. doi: 10.1378/chest.126.3.825. (PMID: 15364762) [DOI] [PubMed] [Google Scholar]
- Kaĭdashev I.P., Rasin M.S., Savchenko L.G., Shlykova O.A., Iakimishina L.I. Polymorphism of the angiotensin II type 1 receptor in patients with essential hypertension in Ukrainian population. Tsitol. Genet. 2005 Sep-Oct;39(5):51–55. 16398146 Russian. [PubMed] [Google Scholar]
- Kaidashev I.P., Rasin M.S., Nerukh I.A., Borzikh O.A., Shlykova O.A. The polymorphism of a angiotensin II receptor gene type. Determined severity renoparenchimal hypertension. Circul. Haemost. 2006;2:54–57. (Ukrainian) [Google Scholar]
- Kaidashev I., Shlykova O., Izmailova O., Torubara O., Ya Yushchenko, Tyshkovska T., Kyslyi V., Belyaeva A., Maryniak D. Host gene variability and SARS-CoV-2 infection: a review article. Heliyon. 2021 Aug;7(8) doi: 10.1016/j.heliyon.2021.e07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuya T., Morishita R. Gene polymorphism of angiotensin II type 1 and type 2 receptors. Curr. Pharm. Des. 2013;19(17):2996–3001. doi: 10.2174/1381612811319170004. (PMID: 23176211) [DOI] [PubMed] [Google Scholar]
- Krivchun A.M., Litvinenko N.V., Kaidashev I.P., Shlikova O.A., Smirnova I.P., Gorbas I.M. Influence of angiotensin II receptor gene polymorphism of the first type on the clinical course of hypertensive dyscirculatory encephalopathy in residents of Poltava region. Probl. Ecol. Med. 2010;14(3–4):25–29. (Ukrainian) [Google Scholar]
- Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020 May;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. Epub 2020 Mar 30. PMID: 32225176. [DOI] [PubMed] [Google Scholar]
- Lauc G., Sinclair D. Biomarkers of biological age as predictors of COVID-19 disease severity. Aging (Albany NY) 2020 Apr 8;12(8):6490–6491. doi: 10.18632/aging.103052. Epub 2020 Apr 8. PMID: 32268300; PMCID: PMC7202497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.T., Chiu H.C., Huang C.T., Su H.M., Wang C.L., Lin T.H., Voon W.C., Chen H.C., Lai W.T., Sheu S.H. The A1166C polymorphism of angiotensin II type 1 receptor as a predictor of renal function decline over 4 years follow-up in an apparently healthy Chinese population. Clin. Nephrol. 2009 Dec;72(6):457–467. doi: 10.5414/cnp72457. (PMID: 19954723) [DOI] [PubMed] [Google Scholar]
- Li X.C., Zhang J., Zhuo J.L. The vasoprotective axes of the renin-angiotensin system: Physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol. Res. 2017 Nov;125(Pt A):21–38. doi: 10.1016/j.phrs.2017.06.005. Epub 2017 Jun 12. PMID: 28619367; PMCID: PMC5607101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie A. Endothelium-derived vasoactive agents, AT1 receptors and inflammation. Pharmacol. Ther. 2011 Aug;131(2):187–203. doi: 10.1016/j.pharmthera.2010.11.001. Epub 2010 Nov 27. PMID: 21115037. [DOI] [PubMed] [Google Scholar]
- Mao S., Huang S. Lack of association of angiotensin II type 1 receptor A1166C gene polymorphism with the risk of end-stage renal disease. Ren. Fail. 2013 Oct;35(9):1295–1301. doi: 10.3109/0886022X.2013.820663. Epub 2013 Aug 1. PMID: 23902432. [DOI] [PubMed] [Google Scholar]
- Martin M.M., Lee E.J., Buckenberger J.A., Schmittgen T.D., Elton T.S. MicroRNA-155 regulates human angiotensin II type 1 receptor expression in fibroblasts. J. Biol. Chem. 2006 Jul 7;281(27):18277–18284. doi: 10.1074/jbc.M601496200. Epub 2006 May 4. Retraction in: J Biol Chem. 2013 Feb 8;288(6):4226. PMID: 16675453. [DOI] [PubMed] [Google Scholar]
- Martin M.M., Buckenberger J.A., Jiang J., Malana G.E., Nuovo G.J., Chotani M., Feldman D.S., Schmittgen T.D., Elton T.S. The human angiotensin II type 1 receptor +1166 A/C polymorphism attenuates microRNA-155 binding. J. Biol. Chem. 2007 Aug 17;282(33):24262–24269. doi: 10.1074/jbc.M701050200. Epub 2007 Jun 22. Retraction in: J Biol Chem. 2013 Feb 8;288(6):4227. PMID: 17588946; PMCID: PMC2413065. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- McLachlan C.S. The angiotensin-converting enzyme 2 (ACE2) receptor in the prevention and treatment of COVID-19 are distinctly different paradigms. Clin Hypertens. 2020 Jul 15;26:14. doi: 10.1186/s40885-020-00147-x. PMID: 32685191; PMCID: PMC7360378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P.K., Griendling K.K. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am. J. Phys. Cell Phys. 2007 Jan;292(1):C82–C97. doi: 10.1152/ajpcell.00287.2006. Epub 2006 Jul 26. PMID: 16870827. [DOI] [PubMed] [Google Scholar]
- Miesbach W. Pathological role of angiotensin II in severe COVID-19. TH Open. 2020 Jun 26;4(2):e138–e144. doi: 10.1055/s-0040-1713678. PMID: 32607467; PMCID: PMC7319800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.A., Thai K., Scholey J.W. Angiotensin II type 1 receptor gene polymorphism and the response to hyperglycemia in early type 1 diabetes. Diabetes. 2000 Sep;49(9):1585–1589. doi: 10.2337/diabetes.49.9.1585. (PMID: 10969844) [DOI] [PubMed] [Google Scholar]
- Parchwani D.N., Patel D.D., Rawtani J., Yadav D. Analysis of Association of Angiotensin II Type 1 Receptor Gene A1166C Gene Polymorphism with Essential Hypertension. Indian J. Clin. Biochem. 2018 Jan;33(1):53–60. doi: 10.1007/s12291-017-0644-7. Epub 2017 Mar 13. PMID: 29371770; PMCID: PMC5766463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reja V., Goodchild A.K., Phillips J.K., Pilowsky P.M. Upregulation of angiotensin AT1 receptor and intracellular kinase gene expression in hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2006 Aug;33(8):690–695. doi: 10.1111/j.1440-1681.2006.04420.x. (PMID: 16895541) [DOI] [PubMed] [Google Scholar]
- Salminen L.E., Schofield P.R., Pierce K.D., Conturo T.E., Tate D.F., Lane E.M., Heaps J.M., Bolzenius J.D., Baker L.M., Akbudak E., Paul R.H. Impact of the AGTR1 A1166C polymorphism on subcortical hyperintensities and cognition in healthy older adults. Age (Dordr.) 2014;36(4):9664. doi: 10.1007/s11357-014-9664-x. Epub 2014 Jul 1. PMID: 24981111; PMCID: PMC4150909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar N., Ho F.K., Gill J.M., Ghouri N., Gray S.R., Celis-Morales C.A., Katikireddi S.V., Berry C., Pell J.P., McMurray J.J., Welsh P. BMI and future risk for COVID-19 infection and death across sex, age and ethnicity: Preliminary findings from UK biobank. Diabetes Metab Syndr. 2020 Sep-Oct;14(5):1149–1151. doi: 10.1016/j.dsx.2020.06.060. Epub 2020 Jun 30. PMID: 32668401; PMCID: PMC7326434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeeva T.V., Chistyakov D.A., Kobalova Zh.D., Moiseev V.S. Relationship between renin-angiotensin gene system and endothelial NO-synthase gene polymorphism and angiocomplications of type 2 diabetes mellitus. Probl. Endocrinol. 2001;47(4):18–23. [Google Scholar]
- Stanković A., Kolaković A., Živković M., Djurić T., Bundalo M., Končar I., Davidović L., Alavantić D. Angiotensin receptor type 1 polymorphism A1166C is associated with altered AT1R and miR-155 expression in carotid plaque tissue and development of hypoechoic carotid plaques. Atherosclerosis. 2016 May;248:132–139. doi: 10.1016/j.atherosclerosis.2016.02.032. Epub 2016 Mar 11. PMID: 27016615. [DOI] [PubMed] [Google Scholar]
- Szolnoki Z. Genetic variant-associated endothelial dysfunction behind small-vessel cerebral circulatory disorders: a new pathomechanism behind common cerebral phenotypes. Mini-Rev. Med. Chem. 2007 May;7(5):527–530. doi: 10.2174/138955707780619572. (PMID: 17504188) [DOI] [PubMed] [Google Scholar]
- Taleghani N., Taghipour F. Diagnosis of COVID-19 for controlling the pandemic: a review of the state-of-the-art. Biosens. Bioelectron. 2021 Feb 15;174 doi: 10.1016/j.bios.2020.112830. Epub 2020 Nov 27. PMID: 33339696; PMCID: PMC7694563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020 Feb 15;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. Epub 2020 Jan 24. Erratum in: Lancet. 2020 Jan 29;: PMID: 31986257; PMCID: PMC7135038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Tian T., Lu J., He H., Xing K., Tian G. A1166C polymorphism of the angiotensin II type 1 receptor gene contributes to hypertension susceptibility: evidence from a meta-analysis. Acta Cardiol. 2017 Apr;72(2):205–215. doi: 10.1080/00015385.2017.1291211. Epub 2017 Mar 9. PMID: 28597796. [DOI] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W., China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020 Feb 20;382(8):727–733. doi: 10.1056/NEJMoa2001017. Epub 2020 Jan 24. PMID: 31978945; PMCID: PMC7092803. [DOI] [PMC free article] [PubMed] [Google Scholar]