Abstract
Objective
The aim of the study was to determine the prevalence of coeliac disease (CD) and to recognise Human leukocyte antigen (HLA)-associated hereditary susceptibility to Sudanese CD patients with type 1 diabetes mellitus (DM1).
Design
Antitissue transglutaminase IgA (anti-TG IgA) was measured in the serum of 373 children affected with DM1 aged 1–19-year old and in 100 serum samples from non-diabetic control children. Histological examination was performed in 19 children seropositive for anti-TG IgA (17 DMI and 2 controls). Additionally, PCR-based analysis of Major histocompatibility complex, class II, DQ beta 1 (HLA-DQB1) genotyping was implemented in three study population groups as follows: group 1 (n=25) (+ve DM1 and +ve CD), group 2 (n=63) (-ve DM1 and +ve CD) and control group 3 (n=2) (+ve CD).
Results
Twenty-six Sudanese children with DM1 out of 373 (6.97%) were seropositive for anti-TG IgA. Duodenal biopsy revealed Marsh 2 and 3 in 13 out of 17 (76.47%) seropositive anti-TG IgA patients with DM1. Significant association (p<0.05) was detected between the level of anti-TG IgA autoantibodies (IU/mL) and Marsh stage. HLA DQ2 and DQ8 were found in 88% (22/25) and 8% (2/25) of examined patients with CD with DM1, respectively.
Conclusions
Anti-TG IgA titre of greater than 10 times upper limit of normal (≥10× ULN) can be useful for detecting CD in children with type 1 diabetes without duodenal biopsy. HLA testing in children with DM1 appears to provide little added benefit given the high prevalence (96%) of HLA DQ2/DQ8 in children with DM1.
Keywords: celiac disease, paediatric gastroenterology, nutrition
Summary box.
What is already known about this subject?
In Sudan, coeliac disease was first recognised in 1978 and the prevalence of the disease was 22.5% in selected high-risk children. Previous retrospective study reported seroprevalence of 27.3% among Sudanese children with clinical features suggestive the disease. Moreover, association between diabetes mellitus type 1 (DM1) and coeliac disease is well documented, as the seroprevalence of CD among Sudanese diabetic children was 8.3%.
What are the new findings?
We approve that detection of antitissue transglutaminase IgA (anti-TG IgA) at a level of ≥10× ULN in Sudanese diabetic children with symptoms of CD is highly associated with CD diagnosis without need to apply duodenal biopsy. Furthermore, HLA DQ2 and DQ8 typing is inadequate for CD screening in Sudanese children with DM1.
How might it impact on clinical practice in the foreseeable future?
Anti-TG IgA at a level of ≥10× ULN is precise test that can be performed to CD diagnosis in Sudanese diabetic children instead of intestinal biopsy, which is invasive and costly procedure although testing for endomysial antibodies was not applied for confirmation according to European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines 2020. HLA genotyping to anticipate susceptibility to CD is not constrained in Sudanese diabetic children.
Introduction
Coeliac disease (CD) is an immune-mediated enteropathy initiated by ingestion of gluten, in genetically susceptible individuals.1 The prevalence of the disease ranging from 0.6% to 0.8% in the Middle East is almost similar to that of Europe.2 A strong association between diabetes mellitus type 1 (DM1) and CD is well documented since 19693 as the prevalence of CD in DM1 patients can be up to 20 times higher than that of healthy population.4 The prevalence of CD in children with DM1 is determined to be 0.6%–16.4%.5 CD can develop in parallel with DM1, but DM1 comes before CD in around 40% of the cases.6 Factors accountable for CD progress in patients with DM1 remain obscure; however, common HLA antigens were accepted as a probably shared genetic background.7 The diagnostic gold standard for CD has long been considered to be intestinal biopsy; however, it is impractical to perform this invasive and costly procedure on all at-risk individuals. Instead, a recent technical review of The American Gastroenterological Association recommended that these individuals should first be screened for CD with IgA antitissue Transglutaminase (tTG) serology.8 Furthermore, negative serological test results do not rule out CD in patients with DM1.9
In Sudan, CD was first described in 1978.10 Mohammed et al11 reported that the prevalence of CD in selected high-risk Sudanese children was 22.5% (18/80) using anti-gliadin autoantibodies (AGA-IgA and IgG), endomysial antibody (EMA) and confirmatory biopsies.
The aim of the present study was to determine the prevalence of CD and to identify HLA-related genetic predisposition of Sudanese CD patients with DM1.
Methods
Patients
A case–control study was performed on 373 children with newly recognised DM1 including 183 girls and 190 boys, aged 1–19 years and 100 non-diabetic control children. All enrolled children attended diabetic clinics of Wad Medani (Gezira State), Gaffar Ibn Oaf and El Bulook Pediatric Hospitals (Khartoum State) during the period 2007–2009. The study protocol was approved by the Ethics Committee of the Sudan University of Science and Technology. All enrolled subjects or their parents gave verbal consent for participation in the study. The collected data included the name, age, sex, family history and duration of DM1.
Celiac serology
tTG IgA (cut-off 4 IU/mL) were analysed in serum using a commercially available Celikey ELISA kit (Pharmacia Diagnostics, Freiburg, Germany).
Histopathology
Biopsy specimens were obtained from the duodenum of 19 (17 DM1 and 2 control) children with seropositive anti-TG IgA autoantibodies, whose parents assigned informed consent for the protocol. The histological specimens were fixed in formalin 10% and stained with H&E. All specimens were examined by expert gastrointestinal pathologist according to modified Marsh criteria.12 13 Marsh 2 and 3 lesions were evaluated to be diagnostic for CD.
HLA genotyping markers
Genomic DNA was extracted from whole peripheral blood using phenol/chloroform method.14 Extracted DNA was purified using Master Pure Complete DNA and RNA Purification Kit (Cat No. MC85200, Lucigen Corporation, USA).
HLA typing for CD specific-HLA-DQB1 alleles was performed on three groups of children including 25 children in group 1 (+ve DM1 +ve CD), 63 children in group 3 (-ve DM1 +ve CD) and 2 children in group 4 (control). PCR HLA typing with sequence-specific primers was conducted according to the manufacturer’s instructions (Olerup low resolution DQ SSP kit, Oslo, Norway). Finally, the PCR products were subjected to electrophoresis in 2% agarose gel, stained with gel green dye and visualised under ultraviolet light. The typing was interpreted with the lot-specific interpretation and specificity tables from Olerup (Olerup SSP DQ low resolution).
Statistical analysis
Standard descriptive statistics were expressed as median and range and evaluated by Mann-Whitney and Pearson χ2 correlation test. Proportions were compared with the Fisher’s exact test. A p value ≤0.05 was considered significant.
Results
Serological tests for caeliac disease
Twenty-six out of 373 patients with DM1 (6.97%) were seropositive for anti-TG IgA autoantibodies including 17 (9.3%) girls and 9 (4.7%) boys, whereas 2 out of 100 non-diabetic controls (2%) were seropositive (two girls). No significant association (p>0.05) was observed between age, sex, family history and duration of DM1 and seropositivity to anti-TG IgA among Sudanese children with DM1.
Histopathological analysis and Marsh classification of duodenum biopsies
Histopathological examination was conducted in 19 seropositive children (17 diabetics and 2 controls). Abnormal findings were detected in 13/17 (76.47%) patients with DM1, of which 4/17 (23.53%) were classified as Marsh 2, 1/17 (5.88%) as Marsh 3a, 4/17 (23.53%) as Marsh 3b and 4/17 (23.53%) as Marsh 3c. In non-diabetic controls, one out of the two was seropositive patients classified as Marsh 3c and the other was classified as Marsh 2. The relationship between the levels of anti-TG IgA autoantibodies and Marsh stage among diabetic children was statistically significant (p<0.05) (figure 1).
Figure 1.
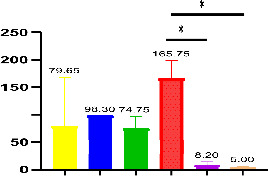
The prevalence of anti-TG IgA in patients with Marsh 2 (n=4), 3 a (n=1), 3b (n=4), 3c (n=4), 0 (n=2) and 1 (n=2) lesions. The numbers above the graph indicate mean and SE of mean. n: number of subjects. *Significant difference.
HLA genotyping for DQ2 and DQ8 antigens
Twenty-five out of 26 diabetic seropositive CD children were genotyped. HLA DQ2 gene was detected in 22 (88%) children, while 2 children (8%) carried HLA DQ8 antigen and 1 child (4%) was negative for both antigens. In 63 non-diabetic seropositive CD group, HLA DQ2 gene was determined in 54 (85.71%) children, while 2 (3.17%) carried HLA DQ8 antigen and 7 children (11.11%) were negative for both genes (table 1) while in the control group with seropositive CD, HLA DQ2 was detected in the 2 (100%) seropositive children. The ratio of HLA DQ2 + to DQ2- antigens was 6:1 in CD +ve DM1 -ve children (group 3), while it was 7:1 in DM1 +ve CD+ve children (group 1) (table 2). Furthermore, no significant correlations (p>0.05) between the seropositive DM1, Marsh grades and the HLA genotypes were detected in this study (data not shown).
Table 1.
HLA genotypes frequencies in the study population with CD as determined by the traditional Human leukocyte antigen (HLA) testing
| HLA genotype | CD +ve DM1-ve (%) | CD +ve DM1+ve (%) | Control CD+ve (%) | P value |
| DQ2−, DQ8− | 4 (6.35%) | 0 | 0 | |
| DQ2−, DQ8−, DQ7+ | 3 (4.76%) | 1 (4%) | 0 | |
| DQ2−, DQ8+ | 2 (3.17%) | 2 (8%) | 0 | |
| DQ2+, DQ8+ | 0 | 2 (8%) | 0 | 0.230 |
| DQ2+, DX | 20 (31.75%) | 8 (32%) | 1 (50%) | |
| DQ2+, DQ7+ | 17 (26.98%) | 1 (4%) | 0 | |
| DQ2+, DQ2+ | 17 (26.98%) | 11 (44%) | 1 (50%) | |
| Total | 63 (100%) | 25 (100%) | 2 (100%) |
If P value < 0.05, it considered statistically significant.
CD, coeliac disease.
Table 2.
HLA genotypes frequencies in the study population with CD as determined by the traditional Human leukocyte antigen (HLA) testing
| Group | HLA genotype | Number (%) |
| DQ2− | 9/63 (14%) | |
| 2 (CD+ve DM1-ve) | DQ2+ | 54/63 (85%) |
| DQ2+, DQX | 20/54 (37%) | |
| DQ2+, DQ7+ | 17/54 (31.5%) | |
| DQ2 +DQ2 | 17/54 (31.5%) | |
| DQ2− | 3/25 (12%) | |
| 1 (DM1+ve CD+ve) | DQ2+ | 22/25 (88%) |
| DQ2+, DQX | 8/22 (36%) | |
| DQ2+, DQ7+ | 1/22 (4.55%) | |
| DQ2 +DQ2+ | 11/22 (50%) | |
| DQ2− | 0/2 (0%) | |
| 4 (Control CD+ve) | DQ2+ | 2/2 (100%) |
| DQ2+, DQX | 1/2 (50%) | |
| DQ2+, DQ2+ | 1/2 (50%) |
Discussion
The present study documented that anti-TG IgA is accurate test that can be stratified for detecting CD in Sudanese children with type 1 diabetes. Furthermore, HLA testing in children with DM1 appears to provide little additional benefit given the high prevalence (96%) of HLA DQ2/DQ8 in children with DM1.
In the present study, 6.79% of Sudanese children with DM1 were anti-TG IgA seropositive for CD. This is similar to that encountered in Sudan by Hussien et al.15 Similar previous studies revealed that the presence of anti-TG IgA antibodies ranged between 7.6% and 9.8% in patients with DM1.4 16 The seroprevalence of CD in Sudanese DMI children was lower than that reported in Libya (21.3%), Algeria (16.4%) and Saudi Arabia (24.5%).17–19 The difference in the seroprevalence rates of CD may be attributed to the differences in exposure to gluten in the diet, genetic, environmental factors and human microbiome.20
In the current study, 76.47% (13/17) of DM1 children with seropositive anti-TG IgA autoantibodies had changes in the intestinal mucosa evaluated as Marsh 2–3. In addition, anti-TG IgA at a level of ≥10× ULN were detected in 5 out of 17 (29.4%) children. Duodenal biopsy confirmed the CD diagnosis in all five subjects, which classified as at least Marsh 2. This finding indicates that anti-TG IgA titre of greater than 10 times upper limit of normal (>10× ULN) may be beneficial in CD diagnosis in DM1 without duodenal biopsy. This is in agreement with the available literature, which demonstrated the same results.21–25
Our study indicates that 24/25 (96%) of screened DM1 children with positive CD serology carried HLA-DQ2 or DQ8 antigens, which was in line with the previous studies.5 26 Therefore, testing for HLA-DQ2/DQ8 as the first-line screening for CD in diabetic children is insufficient. This finding in accordance with Mitchell27 and Binder28 who reported that HLA genotyping is not indicated as a firstline for CD screening in DM1. Moreover, this may improperly prevent the patients with less common CD permissive HLA alleles from future screening.26
In addition, only 1 of 25 patients with DM1 did not have DQ2/DQ8. The loss of HLA-DQ2/DQ8 eliminates the CD progress, with nearly 100% of chance.29 30 In this study, 22 (88%) and 2 (8%) Sudanese children carrying alleles encoding DQ2 and DQ8 molecules, respectively. The frequency of HLA-DQ2 herein reported (88%) is higher than that reported (63.10%) in Poland,20 in Italy (68%)31 and in Czech Republic (80%).32
In Sudan, HLA genotyping is more expensive than anti-tTG IgA testing and it is not available at all hospitals and the alternate of sending blood samples to expert laboratories would be time-consuming and high-cost. Therefore, introducing a strategy of diagnosis based on high serological positivity could help local healthcare in Sudan.
The limitation of this study is that the second blood samples were not obtained and tested for EMA-IgA in children with tTG IgA ≥10× ULN to confirm no-biopsy approach for CD diagnosis according to European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines 2020.33 This was due financial, technical and social obstacles that faced the researcher.
Conclusion
This study indicates that patients with DM1 constitute a high possibility group of CD progress. In addition, clinical manifestation and immunological markers of CD in serum such as anti-TG IgA, which is confirmed herein as an accurate test, should be used to judge CD diagnosis in patients with DM1. Detection of anti-TG IgA at a level of ≥10× ULN in diabetic Sudanese children with symptoms of CD in our study was highly suggestive of CD diagnosis and could constitute a base for initiation of gluten-free diets without duodenal biopsy. However, biopsy may be suggested in both symptomatic and asymptomatic patients who have constant low titre positive anti-TG IgA antibodies. Finally, it could be concluded that anti-TG IgA titre of greater than 10 times upper limit of normal (≥10× ULN) may be beneficial in CD diagnosis in DM1 without duodenal biopsy. Moreover, HLA genotyping does not seen suitable for CD examination in DM1 Sudanese Children patients.
Footnotes
Contributors: II and MH carried out the methodology and drafted the manuscript. OS provided the samples and data collection. AM conducted the statistical analysis. NA, KK, AREH and PS contributed to the conception and design of the study and revised the manuscript. II acting as guarator. All authors have read and approved the final version of this manuscript.
Funding: This study was supported by the Central Laboratory, Ministry of Higher Education and Scientific Research, Khartoum, Sudan (Grant Number 2007/152).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article. Other Data that used to support the findings of this study are available from the corresponding author upon request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
Ethics approval
This study involves human participants and was approved by the Ethics Committee of the Ministry of Health, Khartoum, Sudan (Ethics approval ID: KMHSUD12908). Participants gave informed consent to participate in the study before taking part.
References
- 1.Catassi C, Rätsch IM, Gandolfi L, et al. Why is coeliac disease endemic in the people of the Sahara? Lancet 1999;354:647–8. 10.1016/S0140-6736(99)02609-4 [DOI] [PubMed] [Google Scholar]
- 2.Catassi C, Fabiani E, Fasano A. A journey around the celiac world. Ann Nestle 2004;62:83–94. [Google Scholar]
- 3.Walker-Smith JA, Grigor W. Coeliac disease in a diabetic child. Lancet 1969;1:1021. 10.1016/S0140-6736(69)91817-0 [DOI] [PubMed] [Google Scholar]
- 4.Barera G, Bonfanti R, Viscardi M, et al. Occurrence of celiac disease after onset of type 1 diabetes: a 6-year prospective longitudinal study. Pediatrics 2002;109:833–8. 10.1542/peds.109.5.833 [DOI] [PubMed] [Google Scholar]
- 5.Poulain C, Johanet C, Delcroix C, et al. Prevalence and clinical features of celiac disease in 950 children with type 1 diabetes in France. Diabetes Metab 2007;33:453–8. 10.1016/j.diabet.2007.06.004 [DOI] [PubMed] [Google Scholar]
- 6.Karavanaki K, Kakleas K, Paschali E, et al. Screening for associated autoimmunity in children and adolescents with type 1 diabetes mellitus (T1DM). Horm Res 2009;71:201–6. 10.1159/000201108 [DOI] [PubMed] [Google Scholar]
- 7.Ghawil M, Miotti V, Tonutti E, et al. Hla-Dq types of celiac disease in Libyan children with type 1 diabetes mellitus. Eur J Gastroenterol Hepatol 2012;24:59–63. 10.1097/MEG.0b013e32834d09d4 [DOI] [PubMed] [Google Scholar]
- 8.Rostom A, Murray JA, Kagnoff MF. American gastroenterological association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology 2006;131:1981–2002. 10.1053/j.gastro.2006.10.004 [DOI] [PubMed] [Google Scholar]
- 9.Fallahi G-H, Ahmadian JH, Rabbani A, et al. Screening for celiac disease in diabetic children from Iran. Indian Pediatr 2010;47:268–70. 10.1007/s13312-010-0048-8 [DOI] [PubMed] [Google Scholar]
- 10.Suliman GI. Coeliac disease in Sudanese children. Gut 1978;19:121–5. 10.1136/gut.19.2.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohammed IM, Karrar ZEA, El-Safi SH. Coeliac disease in Sudanese children with clinical features suggestive of the disease. East Mediterr Health J 2006;12:582–9. [PubMed] [Google Scholar]
- 12.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue'). Gastroenterology 1992;102:330–54. [PubMed] [Google Scholar]
- 13.Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol 1999;11:1185–94. 10.1097/00042737-199910000-00019 [DOI] [PubMed] [Google Scholar]
- 14.Sambrook J. Molecular cloning laboratory manual. 6. NY: Seconded Cold Spring Harbor Laboratory Press, 1989: 13–15. [Google Scholar]
- 15.Hussien M, Sara M, Rana G. Seroprevalence of celiac disease in Sudanese children: a retrospective study. Khartoum Med J 2013;6:889–93. [Google Scholar]
- 16.Sahin Y, Cakir MD, Isakoca M. Prevalence of celiac disease in children with type 1 diabetes mellitus in the South of turkey. Iran J Pediatr 2020;30:e97306. [Google Scholar]
- 17.Ashabani A, Abushofa U, Abusrewill S. The prevalence of celiac disease in Libyan children with type 1 diabetes mellitus diabetes. Metab Res Rev 2003;19:69–75. [DOI] [PubMed] [Google Scholar]
- 18.Boudraa G, Hachelaf W, Benbouabdellah M, et al. Prevalence of coeliac disease in diabetic children and their first- degree relatives in West Algeria: screening with serological markers. Acta Paediatr Suppl 1996;412:58–60. 10.1111/j.1651-2227.1996.tb14254.x [DOI] [PubMed] [Google Scholar]
- 19.Al-Hussaini A, Sulaiman N, Al-Zahrani M, et al. High prevalence of celiac disease among Saudi children with type 1 diabetes: a prospective cross-sectional study. BMC Gastroenterol 2012;12:180. 10.1186/1471-230X-12-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scher JU. The microbiome in celiac disease: beyond diet-genetic interactions. Cleve Clin J Med 2016;83:228–30. 10.3949/ccjm.83a.15123 [DOI] [PubMed] [Google Scholar]
- 21.Lewandowska K, Ciepiela O, Szypowska A, et al. Celiac antibodies in children with type 1 diabetes - A diagnostic validation study. Autoimmunity 2018;51:81–8. 10.1080/08916934.2018.1427226 [DOI] [PubMed] [Google Scholar]
- 22.Barker CC, Mitton C, Jevon G, et al. Can tissue transglutaminase antibody titers replace small-bowel biopsy to diagnose celiac disease in select pediatric populations? Pediatrics 2005;115:1341–6. 10.1542/peds.2004-1392 [DOI] [PubMed] [Google Scholar]
- 23.Werkstetter KJ, Korponay-Szabó IR, Popp A, et al. Accuracy in Diagnosis of Celiac Disease Without Biopsies in Clinical Practice. Gastroenterology 2017;153:924–35. 10.1053/j.gastro.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 24.Paul SP, Sandhu BK, Spray CH, et al. Evidence supporting serology-based pathway for diagnosing celiac disease in asymptomatic children from high-risk groups. J Pediatr Gastroenterol Nutr 2018;66:641–4. 10.1097/MPG.0000000000001757 [DOI] [PubMed] [Google Scholar]
- 25.Ylönen V, Lindfors K, Repo M, et al. Non-biopsy serology-based diagnosis of celiac disease in adults is accurate with different commercial kits and pre-test probabilities. Nutrients 2020;12:2736. 10.3390/nu12092736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joshi KK, Haynes A, Davis EA, et al. Role of HLA-DQ typing and anti-tissue transglutaminase antibody titers in diagnosing celiac disease without duodenal biopsy in type 1 diabetes: a study of the population-based pediatric type 1 diabetes cohort of Western Australia. Pediatr Diabetes 2019;20:567–73. 10.1111/pedi.12857 [DOI] [PubMed] [Google Scholar]
- 27.Mitchell RT, Sun A, Mayo A, et al. Coeliac screening in a Scottish cohort of children with type 1 diabetes mellitus: is DQ typing the way forward? Arch Dis Child 2016;101:230–3. 10.1136/archdischild-2015-309754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Binder E, Rohrer T, Denzer C, et al. Screening for coeliac disease in 1624 mainly asymptomatic children with type 1 diabetes: is genotyping for coeliac-specific human leucocyte antigen the right approach? Arch Dis Child 2019;104:354–9. 10.1136/archdischild-2018-315549 [DOI] [PubMed] [Google Scholar]
- 29.Wolters VM, Wijmenga C. Genetic background of celiac disease and its clinical implications. Am J Gastroenterol 2008;103:190–5. 10.1111/j.1572-0241.2007.01471.x [DOI] [PubMed] [Google Scholar]
- 30.Husby S, Koletzko S, Korponay-Szabó IR, et al. European Society for pediatric gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012;54:136–60. 10.1097/MPG.0b013e31821a23d0 [DOI] [PubMed] [Google Scholar]
- 31.Contreas G, Valletta E, Ulmi D, et al. Screening of coeliac disease in North Italian children with type 1 diabetes: limited usefulness of HLA-DQ typing. Acta Paediatr 2004;93:628–32. 10.1111/j.1651-2227.2004.tb02987.x [DOI] [PubMed] [Google Scholar]
- 32.Šumník Z, Koloušková S, Cinek O, et al. HLA - DQA1*05-DQB 1*0201 positivity predisposes to coeliac disease in Czech diabetic children. Acta Paediatr 2007;89:1426–30. 10.1111/j.1651-2227.2000.tb02770.x [DOI] [PubMed] [Google Scholar]
- 33.Husby S, Koletzko S, Korponay-Szabó I, et al. European Society paediatric gastroenterology, hepatology and nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr 2020;70:141–56. 10.1097/MPG.0000000000002497 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article. Other Data that used to support the findings of this study are available from the corresponding author upon request.


