Abstract
Diabetes is a group of metabolic diseases characterised by chronic hyperglycaemia caused by multiple causes, which is caused by insulin secretion and/or utilisation defects. It is characterised by increased fasting and postprandial blood glucose levels due to insulin deficiency or insulin resistance. It is reported that the harm of diabetes mainly comes from its complications, and the cardiovascular disease caused by diabetes is the primary cause of its harm. China has the largest number of diabetic patients in the world, and the prevention and control of diabetes are facing great challenges. In recent years, many kinds of literature have been published abroad, which have proved that coumarin and its derivatives are effective in the treatment of diabetic complications such as nephropathy and cardiovascular disease. In this paper, the types of antidiabetic drugs and the anti-diabetic mechanism of coumarins were reviewed.
Keywords: Diabetes, coumarin, target spots, biological activity
Introduction
Diabetes mellitus (DM) is a chronic metabolic disease characterised by the imbalance of glucose homeostasis, which leads to the increase of glucose levels in the blood. In recent decades, the incidence of diabetes has risen sharply all over the world, and the study of small molecules with potent antidiabetic activity is one of the most interesting research fields1–3.
At present, the hypoglycaemic agents in clinical use include incretin4–10 and insulin sensitizers11–14. In addition, there are other types of substances used to lower blood glucose, such as AMP-activated protein kinase AMPK15, α-glucosidase inhibitors16, amylases, and insulin analogues17. However, among the many marketed drugs, there are more or less some side effects while lowering blood glucose (Figure 1). Therefore, there is an urgent need to discover new drugs to compensate for or replace the shortcomings of current drugs. In recent years, more and more attention has been paid to the study of natural products. Coumarin compounds stand out in the process of drug research and development because of their advantages of multiple targets and less toxic side effects.
Figure 1.
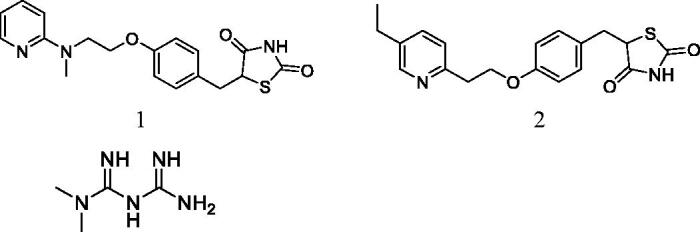
Various insulin sensitisers represent drugs (Compound 1 Rosiglitazone, Compound 2 Pioglitazone, Compound 3 Metformin).
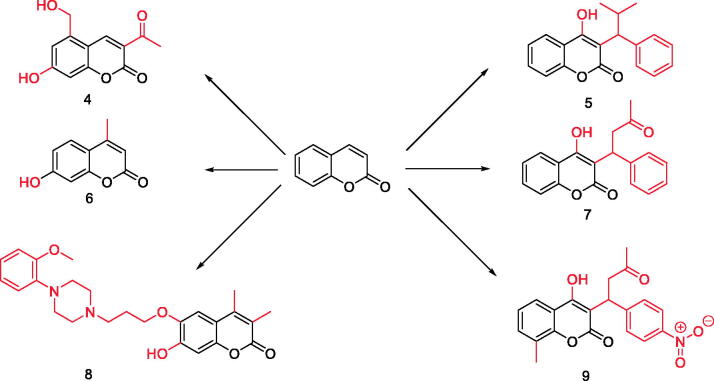
The development or discovery of new highly effective drugs with few toxic side effects is the main goal of modern medicinal chemists. In recent years, coumarin compounds have received increasing attention. Coumarin and its derivatives have extensive biological activities, anticoagulant18–21, antibacterial22–24, anti-inflammatory25–28, antioxidant29–31, antitumor32–35, antiviral36–39, and enzyme inhibition effect40–45 (Figure 2). Coumarins are the general name of cis-o-hydroxy cinnamic acid lactones, which all have the basic skeleton of the benzo α-pyranone mother nucleus. Among the various toxic activities found on it, it has a less harmful effect on normal cells, especially in anti-diabetes, and has become one of the hotspots of drug research in the future (Figure 3). Recent studies have found that coumarins have significant effects in inhibiting α-glucosidase, AGE-RAGE signalling pathway, activating PPARγ and anti-oxidation, etc. (Table 1, Figure 4). In the following part of this review, I will present the hypoglycaemic effects of coumarins on different targets discovered to date.
Figure 2.
Biological activity of coumarin and its derivatives.
Figure 3.
Various biological activities of coumarin and its derivatives (Compound 4–9).
Table 1.
Coumarins and their different structures.
| Target spot | Function | Coumarin species |
|---|---|---|
| α-glucosidase | Hydrolyzing glycosidic bonds in various sugar-containing compounds, it can degrade polysaccharides such as starch, maltose and sucrose into monosaccharides. |
|
| AGE-RAGE | AGE is one of the key factors to induce diabetes and its complications, AGEs can accelerate the ageing of the human body and can cause various chronic degenerative diseases. |
|
| Oxidative stress | In vitro antioxidant evaluation of antioxidant components; ROS is produced by hyperglycaemia, which causes damage to macromolecules and produces signal molecules. |
|
| Up-regulated expression of P2X3 after treatment | Non-selective ligand-gated cation channel, P2X3 receptors are involved in many neuropathic pain processes including DNP. |
|
| Activate PPAR-γ | Key regulatory factors of glucose metabolism, it can inhibit the AGE-RAGE system by activating PPAR-γ activity, thereby regulating oxidative stress. |
|
| Insulin receptor | It is a tyrosine kinase transmembrane receptor that is effectively involved in the regulation of glucose homeostasis through insulin-bound phosphorylation. |
|
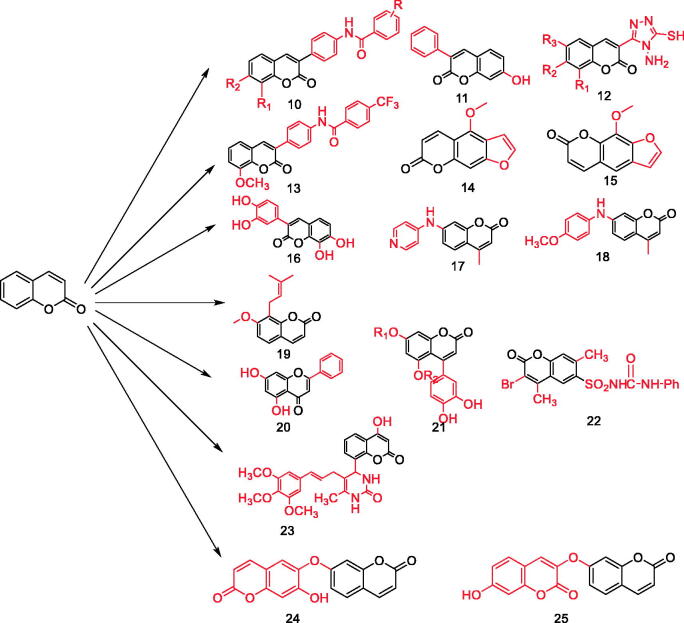
Figure 4.
Various coumarins with hypoglycaemic function (Compound 10–25).
Source
Over the years, great attention has been paid to coumarin and its derivatives, which are versatile molecules exhibiting a wide variety of biological properties including antimicrobial, antiviral, anticancer, antioxidant, anti-inflammatory, anti-tuberculosis, anti-influenza, anti-Alzheimer, and anti-hyperlipidemia activities46. Coumarins are the general name of cis-o-hydroxy cinnamic acid lactones, which all have the basic skeleton of the benzo α-pyranone mother nucleus47. Coumarins are widely distributed in roots, stems, leaves, flowers, fruits and seeds of higher plants, especially in Umbelliferae, Rutaceae, Daphne and Oleaceae, and a few of them are found in microorganisms and animals. Some coumarin compounds can also be used for artificial synthesis.
Active targets
The pathogenesis of diabetes is complex, and coumarin acts on different diabetes-related targets in multiple ways, thereby exerting a role in treating or improving diabetes-related symptoms.
α-Glucosidase
α-glucosidase is widely distributed in the brush border of the small intestinal mucosa, which has an important influence on the structure of glycosyl groups. They can degrade polysaccharides such as starch, maltose and sucrose into monosaccharides48. It hydrolyses the glycosidic bonds in various sugar compounds by endo or exo cleavage, causing the increase of blood glucose. After hydrolysis, the sugary compounds mainly exist in the following three forms: monosaccharide, oligosaccharide and carbohydrate complex. The existing traditional α-glucosidase inhibitors, such as acarbose and voglibose, have more or less gastrointestinal adverse reactions such as nausea and vomiting. Hu et al.49,50 studied 3–(4′-benzoyl amino-phenyl) coumarin derivatives (Figure 5, Table 2), and found that their inhibitory activities on α-glucosidase were different from those of positive control drugs, but their inhibitory activities were all lower than 65 μmol/L, and compound 27 had stronger inhibitory activities through screening.
Figure 5.
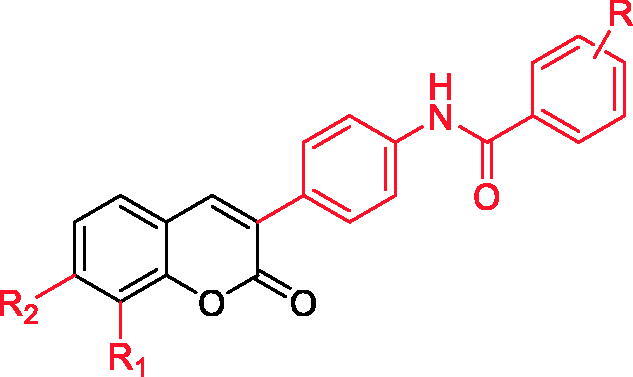
3-(4'-benzoyl amino-phenyl) coumarin derivatives.
Table 2.
3-(4'-benzoylamino-phenyl) coumarin derivatives (Compound 26–35).
| Compound | R 1 | R 2 | R |
|---|---|---|---|
| 26 | H | OH | 3-CH3 |
| 27 | H | OH | 3-Cl |
| 28 | H | OH | 2-F |
| 29 | H | OH | 4-Cl |
| 30 | H | OH | 2-CH3,3-CH3 |
| 31 | 0CH3 | H | 4-F |
| 32 | OCH3 | H | 4-CF3 |
| 33 | OCH3 | H | 3-Cl |
| 34 | OCH3 | H | 2-Cl,3-Cl |
| 35 | OH | H | 4-CH2Cl |
Wang et al. screened more than 40 kinds of 3-aryl coumarins (Figure 6). By comparing IC50 values, it was found that 3-aryl coumarins containing hydroxyl at position 7 showed strong α-glucosidase inhibitory activity, and 5,7-dihydroxy was more active than 7-hydroxy. Substituting 4′-OH on the benzene ring was another active site for inhibiting α-glucosidase. The scoring function of the best binding mode between acarbose and α-glucosidase was −129.508, and the lower scoring function showed that the ligand and protein had a high matching degree and the complex had high stability. The scores of the following compounds are all lower than −100. These seven compounds are almost completely embedded in the grid, and their conformation fits well with the binding pocket, thus achieving the best structure matching.
Figure 6.
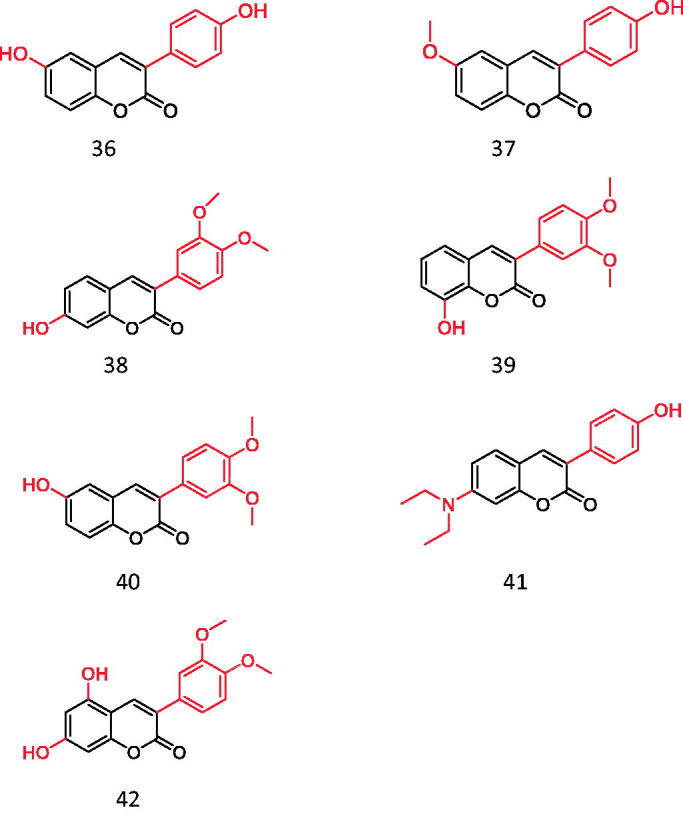
3-arylcoumarin (Compound 36–42).
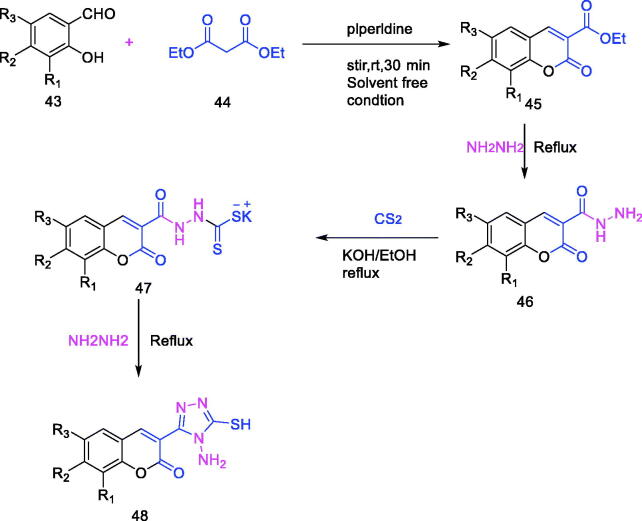
Among heterocyclic compounds, coumarin and triazole have attracted more attention because of their natural existence and great biological activity. Five-membered heterocycles, especially 1,2,4-triazoles, play a key role in pharmaceutical chemistry due to their multiple uses. Vagish Channa Basappa et al.1 synthesised a series of coumarin-triazole hybrids (Figure 7, Table 3), and screened their inhibitory effects on α-amylase. The results showed that compounds 48–3 and 48–5 exhibited good inhibition effects on enzymes, and they were expected to become lead compounds for diabetes drugs.
Figure 7.
Synthesis of 1, 2, 4-Triazole coumarin hybrids.
Table 3.
1, 2, 4-Triazole coumarin hybrids.
| Compound | R 1 | R 2 | R 3 |
|---|---|---|---|
| 48-1 | H | H | Br |
| 48-2 | H | H | Cl |
| 48-3 | OCH3 | H | H |
| 48-4 | H | OCH3 | H |
| 48-5 | OC2H5 | H | H |
Nima Sepehri et al. synthesised coumarin-1,2,3-triazole-acetamide hybrid derivatives (Figure 8, Table 4) and evaluated their α-glucosidase inhibitory activity. It was found that all the coumarin-1,2,3-triazole-acetamide hybridised derivatives were superior to acarbose in inhibiting α-glucosidase, especially compound h and d were superior to other similar derivatives in inhibiting α-glucosidase51. Compound h interacts with the important residues Arg312, Asn241, Glu304, Ser308 and Pro309 of α-glucosidase active site. Compound d interacts with the important residues His201, Ile235 and Tyr151 in the active site of α-amylase.
Figure 8.
Synthesis of Coumarin-1,2,3-triazole-acetamide hybrid derivatives.
Table 4.
Coumarin-1,2,3-triazole-acetamide hybrid derivatives.
| Compound | N | R |
|---|---|---|
| 53-1 | 0 | H |
| 53-2 | 0 | 4-CH3 |
| 53-3 | 0 | 4-OCH3 |
| 53-4 | 0 | 4-F |
| 53-5 | 0 | 4-2,4-diF |
| 53-6 | 0 | 2-Cl |
| 53-7 | 0 | 2,3-diCl |
| 53-8 | 0 | 3-Br |
| 53-9 | 0 | 4-Br |
| 53-10 | 0 | 2-CH3 3-Cl |
| 53-11 | 0 | 2-CH3 3-NO2 |
| 53-12 | 1 | H |
| 53-13 | 1 | 4-F |
Cinnamic acid is a natural compound extracted from cinnamon oil. In recent years, the research of this kind of compound and its derivatives in the treatment of type 2 diabetes has become more and more extensive Based on this, Xu et al.41 combined substituted cinnamic acid with 4-hydroxycoumarin (Figure 9, Table 5) and 7-hydroxycoumarin (Figure 10, Table 6), and evaluated the inhibitory effects of these compounds on α-glucosidase. It was found that the inhibitory activity of these compounds on α-glucosidase was much higher than that of cinnamic acid and coumarin, and the inhibitory effect of substituted cinnamic acid combined with 4-hydroxycoumarin on α-glucosidase was better than that of 7-hydroxycoumarin. In addition, the introduction of electron-donating groups such as methyl can enhance its inhibitory activity. Molecular docking studies also confirmed that the synthesised derivatives can be effectively inserted into the active bag of α-glucosidase. Therefore, these compounds may be a promising α-glucosidase inhibitor.
Figure 9.
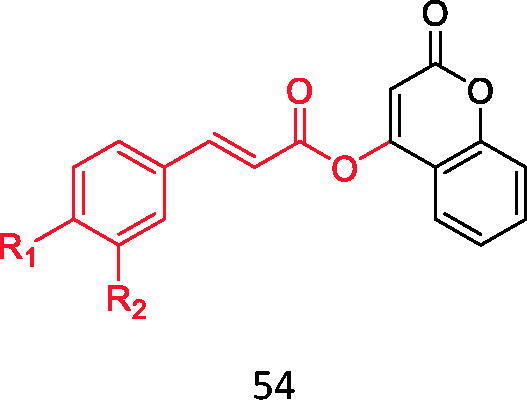
Compound 54.
Table 5.
Binding of substituted cinnamic acid to 4-hydroxycoumarin.
| Compound | R 1 | R 2 |
|---|---|---|
| 54-1 | H | H |
| 54-2 | CH3 | H |
| 54-3 | OCH3 | H |
| 54-4 | F | H |
| 54-5 | Cl | H |
| 54-6 | Br | H |
| 54-7 | CF3 | H |
| 54-8 | OH | H |
| 54-9 | OH | OCH3 |
Figure 10.
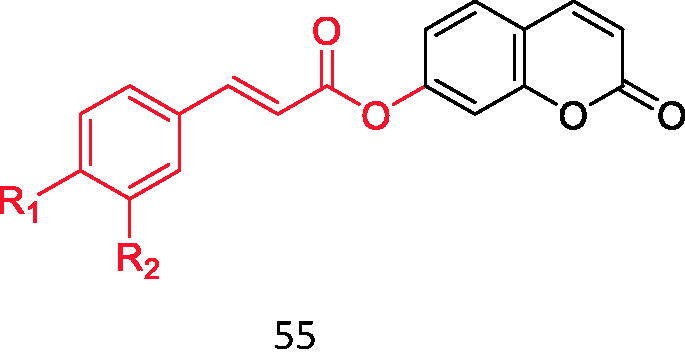
Compound 55.
Table 6.
Binding of substituted cinnamic acid to 7-hydroxycoumarin.
| Compound | R 1 | R 2 |
|---|---|---|
| 55-1 | H | H |
| 55-2 | CH3 | H |
| 55-3 | OCH3 | H |
| 55-4 | F | H |
| 55-5 | Cl | H |
| 55-6 | Br | H |
| 55-7 | CF3 | H |
| 55-8 | OH | H |
| 55-9 | OH | OCH3 |
Inhibition of advanced glycation end products (AGEs)
Advanced glycation end products are products of excessive sugar and protein combination, which is one of the hottest fields in the global medical field. Its formation is one of the key factors to induce diabetes and its complications52. Therefore, inhibiting the production of this substance has become one of the ideas for the treatment of diabetes and its complications. AGEs are the final products derived from the Maillard reaction, nonenzymatic glycation of free amino groups by sugars and aldehydes. Endogenous AGEs are mainly irreversible end products formed by non-enzymatic glycosylation of carbonyl and free amino groups in a hyperglycaemia environment, and isomerised by a series of reactions such as spontaneous rearrangement53,54. Hu50 studied 3–(4′-benzoyl amino-phenyl) coumarin derivatives and found that the inhibitory activity of these compounds on AGEs was generally higher than that of aminoguanidine hydrochloride, the positive control drug, especially the compounds shown in the figure below had the highest inhibitory activity, which was 58 times as much as that of its positive drug (Figure 11).
Figure 11.
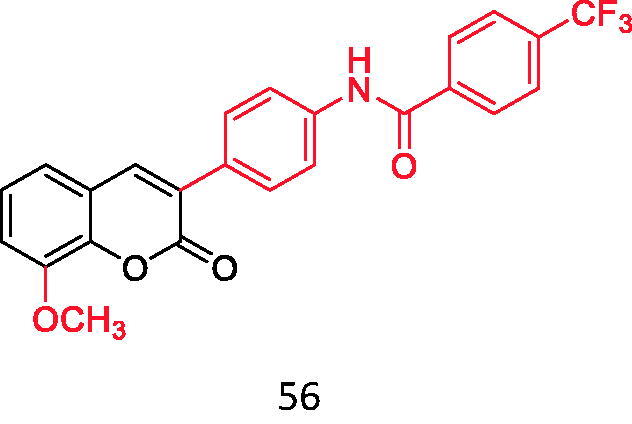
3-(4'-benzoyl amino-phenyl) coumarin derivatives.
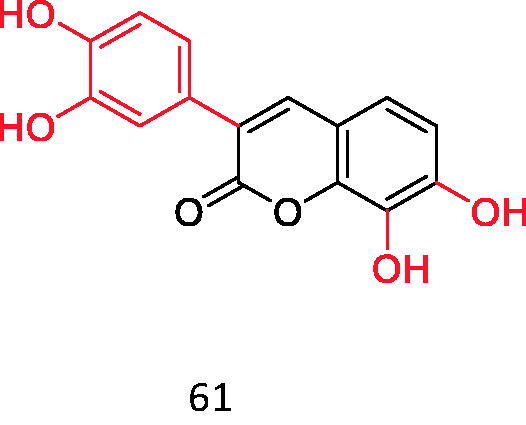
Through Wang's research on 3-aryl coumarin, it was found that most of the 3-aryl coumarins showed stronger AGEs inhibitory activity, and some of them were even stronger than the positive control drug aminoguanidine hydrochloride. Contrary to α-glucosidase inhibitory activity, 7-OH showed stronger AGEs inhibitory activity than 5,7-dihydroxy. Therefore, 3-aryl coumarins have great development potential in the treatment of diabetes (Table 7).
Table 7.
Compound 57, resveratrol and genistein have a comparative effect on a-glucosidase inhibitory activity and AGEs formation inhibitory activity.
| Compound | Structure | α-glucosidase inhibition IC50 Value (μm) | AGEs formation inhibitory activity IC50 Value (μm) |
|---|---|---|---|
| Compound 57 |
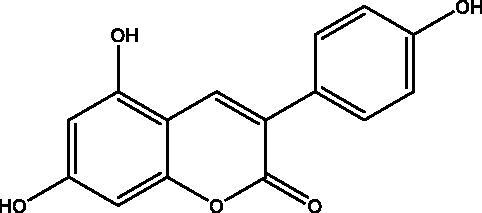
|
19.08 ± 0.26 | 3.12 ± 0,33 |
| Resveratrol |
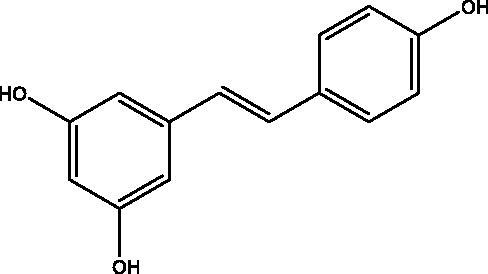
|
>1000 | 3.64 ± 0.92 |
| Genistein |
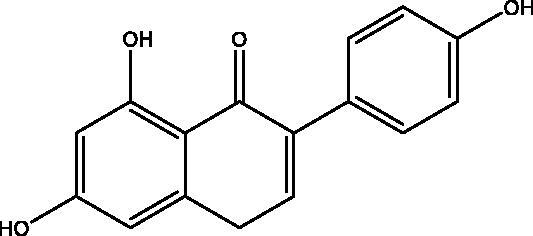
|
724.11 ± 39.74 | 11.04 ± 1.15 |
Patients with diabetes have an increased risk of osteoporotic fractures55–57. Therefore, diabetes-induced bone fragility has recently been recognised as a diabetic complication. Since the risk of fracture is not associated with a decrease in bone density, deterioration in bone quality may be a major cause of bone fragility58. In diabetic patients, persistent hyperglycaemia significantly increased AGE secretion and RAGE induction of MC3T3-E1 cells, and the interaction between AGE and RAGE destroyed osteoblast differentiation and bone formation, even during osteogenic differentiation. When the diabetic cells were treated with coumarin (≥10 μM), the production of AGE and RAGE decreased significantly. Thiazolidinediones, an antidiabetic drug59, can inhibit bone resorption of osteoclasts regardless of its lipid-forming effect, so these drugs have obvious shortcomings. Coumarins can improve bone turnover and bone remodelling in diabetic patients. Its derivatives bergamot lactone and methoxysarin can prevent diabetic osteoporosis by inhibiting osteoclast gene expression and bone absorption in diabetic bone tissue60–62; Imperatorin and bergamot lactone can enhance ALP activity, type I collagen synthesis, bone morphogenetic protein −2 expression and bone nodule formation in primary cultured osteoblasts and tibia tissue (Figure 12).
Figure 12.
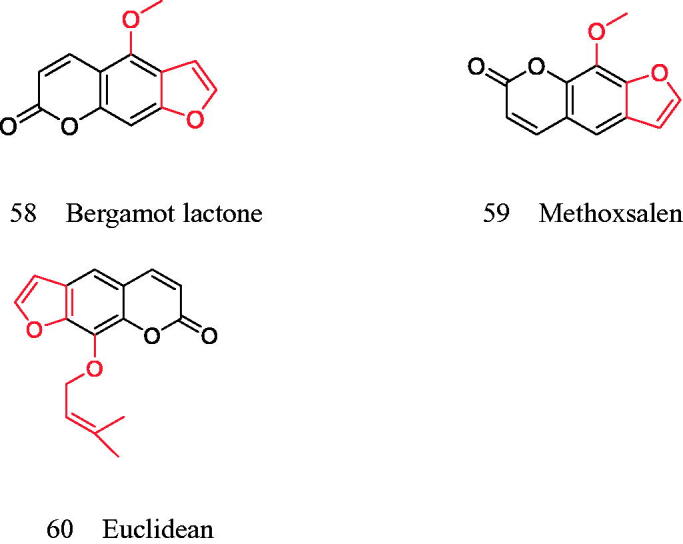
Coumarins for improving bone turnover and remodelling in diabetes.
To sum up, AGE-RAGE signalling pathway plays a role in diabetic complications (including diabetic osteopathy)63–66, while coumarin inhibits this signalling pathway, which makes coumarin improve the molecules of bone turnover and bone remodelling.
Anti-oxidative stress
Free radicals are usually produced by a series of enzymatic or non-enzymatic oxidation reactions in organisms. Many free radicals are produced in the human body due to various metabolism. In the process of metabolising organic matter such as sugars, lipids and proteins, oxygen molecules (O2) generate water (H2O) mainly by four-step single-electron reduction under the action of the mitochondrial respiratory chain. In the complete reduction process of oxygen, it is necessary to first form the superoxide anion O2−, etc., and then combine with the hydrogen ion (H+) and finally generate water. However, it is not perfect, and some superoxide anions open small differences during the reduction reaction and are not completely reduced, but receive single or double electrons halfway through the respiratory chain and are partially reduced to generate superoxide and hydrogen peroxide (H2O2), That is, free radicals are produced. When overeating or hyperglycaemia occurs, a large number of energy substrates exceed the utilisation efficiency of mitochondria and many superoxides are produced. Redox homeostasis is crucial for maintaining physiological functions67. Oxidative stress refers to the imbalance between oxidative and antioxidant activities68. The formation of oxidative stress leads to the damage of islet β cells and insulin resistance and finally leads to the occurrence and development of diabetes and its complications. Therefore, antioxidant therapy is of great significance to delay the occurrence of diabetes and its complications.
DPPH is 1,1-diphenyl-2-trinitrophenylhydrazine, which is a stable free radical with a dark purple prismatic crystal. There are two main applications of DPPH: First, as a reaction monitoring substance in chemical reactions containing free radicals, DPPH is typically used to evaluate the antioxidant activity of antioxidant components in vitro; It can also be used as a standard material for the position and intensity of electron paramagnetic resonance signals. Wang studied the DPPH activity of 44 kinds of 3-aryl coumarins and found that the compounds with o-diphenol hydroxyl structure have stronger DPPH radical scavenging ability than its positive control vitamin C, especially the compounds shown below have the highest activity, and their radical scavenging activity is twice that of vitamin C (Figure 13).
Figure 13.
Coumarins with DPPH radical scavenging ability (o-catechol structure).
Glucose can stimulate the intracellular production of ROS69. Physiologically, ROS promote insulin secretion, while excessive ROS can cause severe oxidative stress, downregulating the expression of the insulin protein, destroying pancreatic β cells and triggering insulin resistance70,71. It has been found that cardiovascular complications of diabetes are closely related to vascular calcification, and a large part of cellular calcification is due to the increase of total reactive oxygen species (ROS) content in cells. Muthipeedika et al.72 have studied the oxygen radical scavenging activity of a series of 4-methyl-7-aminocoumarin and its derivatives (Figure 14). The results show that among the 21 compounds studied, the existence of an electron-donating group or atom at the 7-position is an important feature of its enhanced antioxidant activity.
Figure 14.
4-methyl-7-aminocoumarin and its derivatives.
Hydroxycoumarin is an effective metal chelating agent, free radical scavenger and powerful chain-breaking antioxidant. Many coumarin derivatives have the unique ability to scavenge active oxygen, such as hydroxyl radical and superoxide to prevent free radical damage. The Mysore University of India, Georgia Institute of Technology and other institutions have attached pyrazole rings to hydrazine, hydrazine formamide and hydrazine methylthioamide coumarin to study their antioxidant activities. Its free radical scavenging ability was evaluated by DPPH and ROS scavenging experiments. They found that compounds 66 and 67 containing CONH2 and CSNH2 on the pyrazole ring had better antioxidant activity than ascorbic acid. The activity of compounds 68 and 69 in carbamates is lower than 66 and 67, but it is better than ascorbic acid73 (Figure 15).
Figure 15.
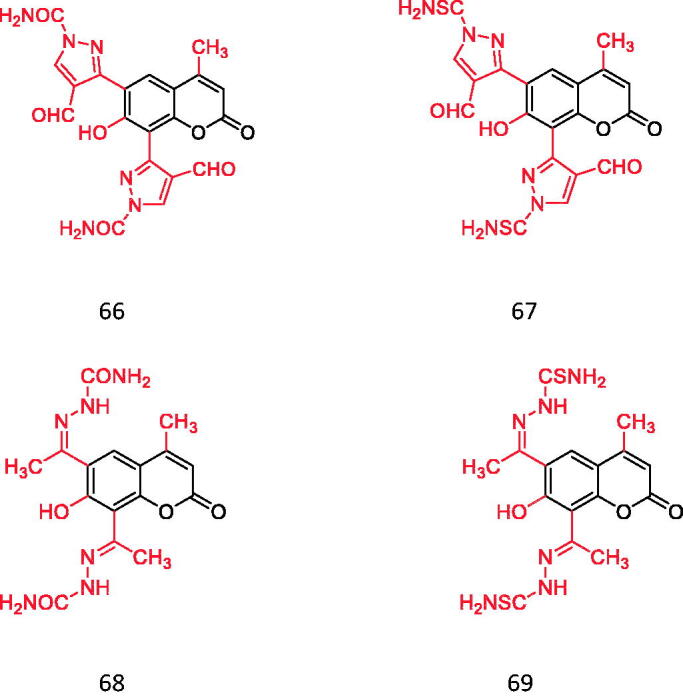
Pyrazole Cyclocoumarin (contains CONH2 and CSNH2).
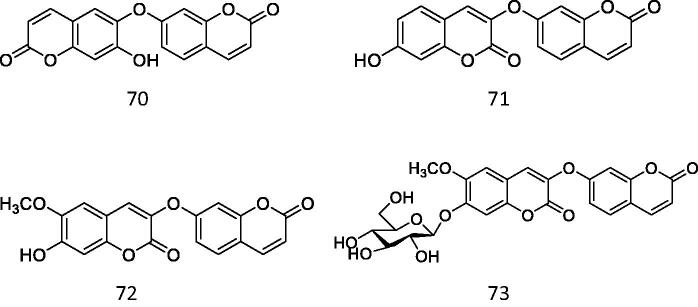
Diabetic nephropathy (DN) is a severe microvascular complication of diabetes mellitus74. Oxidative stress and fibrosis largely contribute to the progression of DN. According to the results of molecular docking by Huankai Yao et al., compound 73 can bind to Keap1 and significantly activate Nrf2. Cell-based assays have revealed compound 73 activated Nrf2 and attenuated oxidative stress and fibrosis induced by high glucose in mesangial cells75 (Figure 16).
Figure 16.
Coumarins that can activate Nrf2.
Under high glucose, mesangial cells initiate self-limited proliferation at the early stage of DN, which is followed by glomerular hypertrophy and expansion. The viability of mesangial cells cultured in a normal or high glucose medium was evaluated by CCK-8 analysis. There was no significant inhibitory effect of compound 73 on mesangial cells under normal conditions. However, high glucose-stimulated the proliferation of mesangial cells and led to an increase in their vitality. For compounds 70–72, at 10 μM they began to inhibit the proliferation stimulated by high glucose significantly though they didn’t show toxicity to normal mesangial cells even at 100 μM. However, at 5 and 10 microns, compound 73 significantly reduced the increased viability. Western blot analysis and following densitometric analysis also revealed the Nrf2 was activated by compound 73 in mesangial cells under high glucose. Compound 73 as an Nrf2 activator attenuated oxidative stress and fibrosis induced by high glucose in mesangial cells through disrupting the interaction between Keap1 and Nrf2. This investigation can provide evidence for further investigations on compound 73 in vivo and the discovery of new drugs targeting DN.
Up-regulated expression of P2X3 after treatment
Diabetic neuralgia (DNP) is one of the most common complications of diabetes76. DNP is related to the enhancement of peripheral sensory nerve excitability, involving various ion channels, receptor expression and up-regulation of function. P2X3 receptor is a member of P2X family of purine receptors and participates in many neuropathological pain processes including DNP. P2X3 receptor is a non-selective ligand-gated cation channel, which is mainly distributed in some sympathetic neurons, sensory neurons and nucleus tractus solitarius, mainly in small and medium ganglion cells. P2X3 receptor increases pain when its expression is up-regulated or its activity is enhanced and decreases pain when its expression is down or desensitised, indicating that P2X3 receptor is an important receptor for progressive pain77.
Wu et al. studied that osthol inhibited the expression of P2X3 receptor in stellate sympathetic ganglion (SG) of diabetic rats (Figure 17). They studied by immunohistochemistry and western blot and found that the integrated optical density (IOD) in the diabetic group was significantly higher than that in the control group. Compared with a diabetic group, the expression of P2X3 in the osthol group decreased significantly, which indicated that osthol treatment could resist the up-regulation of P2X3 expression in SG of diabetic rats78.
Figure 17.
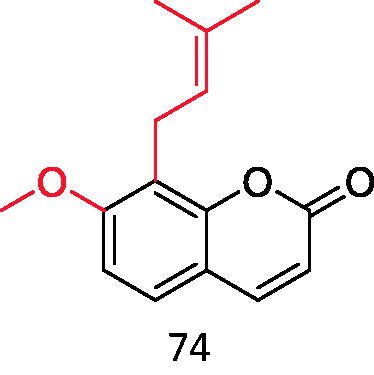
Osthole.
Activate PPAR-γ
Among the three subtypes of PPARs, peroxisome proliferator-activated receptor γ (PPAR-γ) is the most reported, which is the key regulator of glucose metabolism79–82. Chrysin and luteolin are two flavonoids with PPAR-γ stimulatory activity that protect against vascular complications associated with insulin resistance (IR) (Figure 18). Meanwhile, chrysin and luteolin significantly inhibited NO and ROS elevation in IR aortas. In conclusion, chrysin and luteolin alleviate IR-related vascular complications mainly through the PPAR-γ-dependent pathway. Coumarins are expected to improve cardiovascular problems of diabetic patients in PPAR-γ agonistic activity because of their high structural similarity with flavonoids83.
Figure 18.
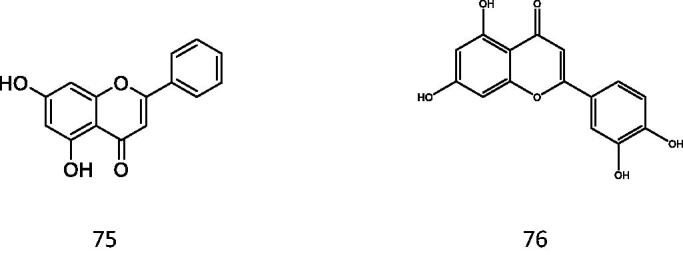
Chrysin and luteolin.
Positive effects of preparations of the bark of the Central American plant Hintonia latiflora (family Rubiaceae) on blood glucose reduction and therefore the maintenance of physiologically normal blood glucose values have been reported84–86. Jose et al. conducted mouse experiments on 4-aryl coumarin glycosides extracted from Hintonia latiflora, and found that the blood glucose concentration of diabetic mice decreased obviously and the insulin level showed an upward trend after injection of this compound. This experiment shows that 4-aryl coumarin glycosides have obvious hypoglycaemic activity (Figure 19). Its structural formula is as follows:
Figure 19.
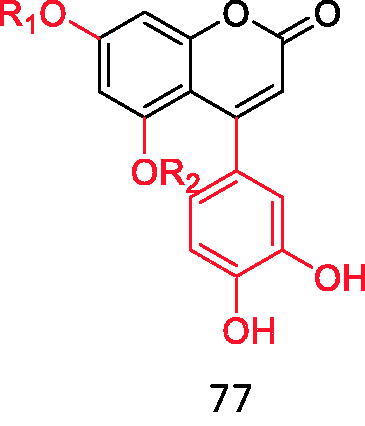
4-aryl coumarin.
The 7th position can be hydroxyl or methoxy, and R2 can be glucopyranoside, galactopyranoside, etc.
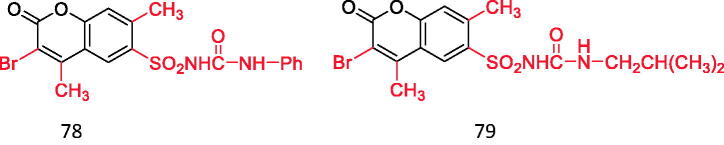
Tu et al.87 found that the introduction of sulfonylurea structure into coumarin mother nucleus showed good hypoglycaemic activity, besides, it also showed good antibacterial activity, which is expected to become a new hypoglycaemic drug different from sulfonylureas. Among the 12 selected compounds, the hypoglycaemic activity of the following two compounds is equivalent to that of positive control drugs (compounds 78 and 79) (Figure 20).
Figure 20.
Coumarin binds to sulfonylurea structures.
Insulin receptor
Type II diabetes mellitus is one type of DM found in more than 90% of cases of DM and could be attributed to obesity, overweight and lack of physical activity, marked by pancreatic insulin release, when the body has not been trained to utilise insulin developed for glucose transfer, and the emergence of insulin resistance contributes to an increase of blood glucose or hyperglycaemia.88 Therefore, the insulin receptor is a potential target for screening the anti-diabetic ligand activity of insulin receptor activator, and it is a tyrosine kinase transmembrane receptor, which effectively participates in the regulation of glucose homeostasis through phosphorylation of insulin binding89–92. A total of 54 coumarin chalcone hybrids were synthesised by the famous Biginelli synthesis, Pechmann condensation, acetylation and Claisen-Schmidt reaction. Compared with diabetic rats treated with metformin (100 mg/kg b.d), further treatment with 80 and 81 at 30 mg/kg b.d. showed that MDA in pancreas and liver tissue of diabetic rats decreased significantly and moderately, while SOD and GSH rates increased89 (Figure 21).
Figure 21.
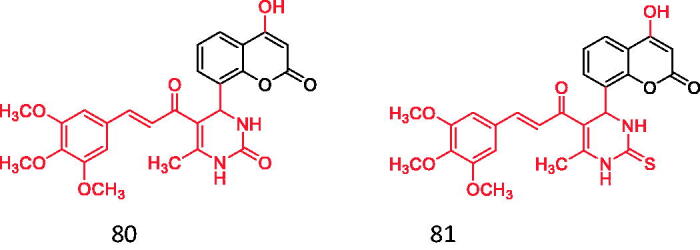
Coumarin chalcone hybrids.
Summary and prospect
The research on the therapeutic effect of coumarins in diabetes is deepening. This review summarises the hypoglycaemic activity of coumarins and their derivatives, which is conducive to the design of coumarins and their derivatives with more significant efficacy by medicinal chemists.
Among the various coumarin compounds mentioned above, 3-arylcoumarin derivatives hold promise as candidate molecules for antidiabetic drugs and further studies. Among them, compounds with hydroxyl structures at position 7 had α-glucosidase inhibitory activity; compounds with hydroxyl groups in 5,7-dihydroxyl and 4'-OH structures also had significant α-glucosidase inhibitory activity; and the above 3-arylcoumarins had AGEs inhibitory activity. The structure containing catechol hydroxyl group can effectively clear the DPPH radical; 4-methyl-7-aminocoumarins and dicoumarins also have oxygen radical scavenging activity; osthole slows down the occurrence and development of DNP, a diabetic complication, by resisting the expression of P2X3 in diabetic rats; coumarin chalcone hybrids have a significant effect on insulin resistance.
In conclusion, by summarising the hypoglycaemic activity of different structures of coumarins as well as the synthesis methods of some of them, it provides a reference for further study of the hypoglycaemic effect of coumarin compounds and opens up new horizons.
Ethics statement
We further confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
Funding Statement
This work was supported by Science and Shandong Provincial Natural Science Foundation [ZR2018LH021], the Innovation Project of Shandong Academy of Medical Sciences and Academic promotion programme of Shandong First Medical University [No. 2019LJ003], Science and Shandong Provincial Natural Science Foundation [ZR2020KH020].
Disclosure statement
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- 1.Basappa VC, Kameshwar VH, Kumara K, et al. Design and synthesis of coumarin-triazole hybrids: biocompatible anti-diabetic agents, in silico molecular docking and ADME screening. Heliyon 2020;6:e05290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohkuma T, Chalmers J, Cooper M, et al. The comparative effects of intensive glucose lowering in diabetes patients aged below or above 65 years: results from the ADVANCE trial. Diabetes Obes Metab 2021;23:1292–300. [DOI] [PubMed] [Google Scholar]
- 3.Li M, Yuan T, Li S, Sun Q.. Nomogram analysis of the influencing factors of diabetic foot in patients with diabetes mellitus. Hormones 2021;20:333–8. [DOI] [PubMed] [Google Scholar]
- 4.Santos-Pardo I, Lagerqvist B, Ritsinger V, et al. Risk of stent failure in patients with diabetes treated with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors: a nationwide observational study. Int J Cardiol 2021;330:23–9. [DOI] [PubMed] [Google Scholar]
- 5.Gaur A, Lewis EL, Hergarden AC, et al. Effects of naturally occurring genetic variations in incretin receptors on glucose homeostasis. Metabolism 2021;116:154530. [Google Scholar]
- 6.Hussain MA, Laimon-Thomson E, Mustafa SM, et al. Detour ahead: incretin hormone signaling alters its intracellular path as β-cell failure progresses during diabetes. Front Endocrinol 2021;12:665345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bianchini G, Nigro C, Sirico A, et al. A new synthetic dual agonist of GPR120/GPR40 induces GLP-1 secretion and improves glucose homeostasis in mice. Biomed Pharmacother 2021;139:111613. [DOI] [PubMed] [Google Scholar]
- 8.Nakajima Y, Ito S, Asakura M, et al. A dipeptidyl peptidase-IV inhibitor improves diastolic dysfunction in Dahl salt-sensitive rats. J Mol Cell Cardiol 2019;129:257–65. [DOI] [PubMed] [Google Scholar]
- 9.Cheng C, Jabri S, Taoka BM, Sinz CJ.. Small molecule glucagon receptor antagonists: an updated patent review (2015–2019). Expert Opin Ther Pat 2020;30:509–26. [DOI] [PubMed] [Google Scholar]
- 10.Martins FL, Bailey MA, Girardi AC.. Endogenous activation of glucagon-like peptide-1 receptor contributes to blood pressure control: role of proximal tubule Na+/H + exchanger isoform 3, renal angiotensin II, and insulin sensitivity. Hypertension 2020;76:839–48. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed S, Islam N, Shahinozzaman M, et al. Virtual screening, molecular dynamics, density functional theory and quantitative structure activity relationship studies to design peroxisome proliferator-activated receptor-γ agonists as anti-diabetic drugs. J Biomol Struct Dyn 2021;39:728–42. [DOI] [PubMed] [Google Scholar]
- 12.Kaneto H, Kimura T, Obata A, et al. Multifaceted mechanisms of action of metformin which have been unraveled one after another in the long history. Int J Mol Sci 2021;22:2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortez I, Hernandez CM, Dineley KT.. Enhancement of select cognitive domains with rosiglitazone implicates dorsal hippocampus circuitry sensitive to PPARγ agonism in an Alzheimer's mouse model. Brain Behavior 2021;11:e01973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo J, Chen J, Ren W, et al. Citrus flavone tangeretin is a potential insulin sensitizer targeting hepatocytes through suppressing MEK-ERK1/2 pathway. Biochem Biophys Res Commun 2020;529:277–82. [DOI] [PubMed] [Google Scholar]
- 15.Matumba MG, Ayeleso AO, Nyakudya T, et al. Long-term impact of neonatal intake of oleanolic acid on the expression of AMP-activated protein kinase, adiponectin and inflammatory cytokines in rats fed with a high fructose diet. Nutrients 2019;11:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaheen A, Ashiq U, Jamal RA, et al. Design and synthesis of fluoroquinolone derivatives as potent α‐glucosidase inhibitors: in vitro inhibitory screening with in silico docking studies. ChemistrySelect 2021;6:2483–91. [Google Scholar]
- 17.Jarosinski MA, Dhayalan B, Rege N, et al. ‘Smart’insulin-delivery technologies and intrinsic glucose-responsive insulin analogues. Diabetologia 2021;64:1016–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viana CC, da Silva Praxedes MF, de Sousa WJFN, et al. Influence of sex-based differences on oral anticoagulation control in patients taking coumarin derivatives: a systematic review protocol. JBI Evid Synth 2021;19:477–83. [DOI] [PubMed] [Google Scholar]
- 19.Herrera-Escandón Á, Castaño-Cifuentes O, Plata-Mosquera CA.. Use of idarucizumab to revert the anticoagulant effect of dabigatran in heart transplant surgery: an institutional experience. Case Reports Cardiol 2020;2020:6927423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Govindappa M. In silico anti-HIV and anticoagulant activity of [60] fullerene conjugated coumarin and P-coumaric acid isolated from endophytic fungi. Alternaria Species-1. Enliven: Bioinfo 2018;4:001. [Google Scholar]
- 21.Hu Y, Hu C, Pan G, et al. Novel chalcone-conjugated, multi-flexible end-group coumarin thiazole hybrids as potential antibacterial repressors against methicillin-resistant Staphylococcus aureus. Eur J Med Chem 2021;222:113628. [DOI] [PubMed] [Google Scholar]
- 22.Qin H-L, Zhang Z-W, Ravindar L, Rakesh K.. Antibacterial activities with the structure-activity relationship of coumarin derivatives. Eur J Med Chem 2020;207:112832. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Xia D-G, Chu Z-W, et al. Novel coumarin-thiazolyl ester derivatives as potential DNA gyrase inhibitors: design, synthesis, and antibacterial activity. Bioorg Chem 2020;100:103907. [DOI] [PubMed] [Google Scholar]
- 24.Alfayomy AM, Abdel-Aziz SA, Marzouk AA, et al. Design and synthesis of pyrimidine-5-carbonitrile hybrids as COX-2 inhibitors: anti-inflammatory activity, ulcerogenic liability, histopathological and docking studies. Bioorg Chem 2021;108:104555. [DOI] [PubMed] [Google Scholar]
- 25.Nayeli M-B, Maribel H-R, Enrique J-F, et al. Anti-inflammatory activity of coumarins isolated from Tagetes lucida Cav. Nat Prod Res 2020;34:3244–8. [DOI] [PubMed] [Google Scholar]
- 26.Liang H, Shi Y, Zeng K, et al. Coumarin derivatives from the leaves and twigs of Murraya exotica L. and their anti-inflammatory activities. Phytochemistry 2020;177:112416. [DOI] [PubMed] [Google Scholar]
- 27.Wang T, Peng T, Wen X, et al. Design, synthesis and evaluation of 3-substituted coumarin derivatives as anti-inflammatory agents. Chem Pharm Bull 2020;68:443–01085. [DOI] [PubMed] [Google Scholar]
- 28.Parvin K, Hasanuzzaman M, Mohsin S, et al. Coumarin improves tomato plant tolerance to salinity by enhancing antioxidant defence, glyoxalase system and ion homeostasis. Plant Biol 2021;23:181–92. [DOI] [PubMed] [Google Scholar]
- 29.Sanches K, Dias RVR, da Silva PH, et al. Grb2 dimer interacts with Coumarin through SH2 domains: a combined experimental and molecular modeling study. Heliyon 2019;5:e02869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W-B, Qiao X-P, Wang Z-X, et al. Synthesis and antioxidant activity of conjugates of hydroxytyrosol and coumarin. Bioorg Chem 2020;105:104427. [DOI] [PubMed] [Google Scholar]
- 31.Ozalp L, Danış Ö, Yuce‐Dursun B, et al. Investigation of HMG‐CoA reductase inhibitory and antioxidant effects of various hydroxycoumarin derivatives. Archiv Der Pharmazie 2020;353:1900378. [DOI] [PubMed] [Google Scholar]
- 32.Konkoľová E, Hudáčová M, Hamuľaková S, et al. Tacrine-coumarin derivatives as topoisomerase inhibitors with antitumor effects on A549 human lung carcinoma cancer cell lines. Molecules 2021;26:1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Tan Y, Li G, et al. Coumarin sulfonamides and amides derivatives: design, synthesis, and antitumor activity in vitro. Molecules 2021;26:786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohammed FZ, Rizzk YW, Abdelhamid MS, El-Deen IM.. In vivo biological evaluation of ethyl 4-(7-hydroxy-4-methyl-2-oxoquinolin-1-ylamino)-coumarin-3-carboxylate as an antitumor agent. Anti-Cancer Agents Med Chem 2020;20:2246–66. [DOI] [PubMed] [Google Scholar]
- 35.Shahzadi I, Ali Z, Baek SH, et al. Assessment of the antitumor potential of umbelliprenin, a naturally occurring sesquiterpene coumarin. Biomedicines 2020;8:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shan L-P, Zhou Y, Yan M-C, et al. A novel antiviral coumarin derivative as a potential agent against WSSV infection in shrimp seedling culture. Virus Research 2021;297:198387. [DOI] [PubMed] [Google Scholar]
- 37.Chidambaram S, El-Sheikh MA, Alfarhan AH, et al. Synthesis of novel coumarin analogues: investigation of molecular docking interaction of SARS-CoV-2 proteins with natural and synthetic coumarin analogues and their pharmacokinetics studies. Saudi J Biol Sci 2021;28:1100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Özdemir M, Köksoy B, Ceyhan D, et al. Design and in silico study of the novel coumarin derivatives against SARS-CoV-2 main enzymes. J Biomol Struct Dyn 2020;1–16. 10.1080/07391102.2020.1863263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu G-L, Liu L, Shan L-P.. Evaluation on the antiviral effect of a hydroxycoumarin against infectious hematopoietic necrosis virus infection in vitro and in vivo. Fish Shellfish Immunol 2020;102:389–99. [DOI] [PubMed] [Google Scholar]
- 40.Francisco CS, Javarini CL, de S Barcelos I, et al. Synthesis of coumarin derivatives as versatile scaffolds for GSK-3β enzyme inhibition. Curr Top Med Chem 2020;20:153–60. [DOI] [PubMed] [Google Scholar]
- 41.Xu X-T, Deng X-Y, Chen J, et al. Synthesis and biological evaluation of coumarin derivatives as α-glucosidase inhibitors. Eur J Med Chem 2020;189:112013. [DOI] [PubMed] [Google Scholar]
- 42.Kurt BZ, Sonmez F, Ozturk D, et al. Synthesis of coumarin-sulfonamide derivatives and determination of their cytotoxicity, carbonic anhydrase inhibitory and molecular docking studies. Eur J Med Chem 2019;183:111702. [DOI] [PubMed] [Google Scholar]
- 43.Supuran CT. Coumarin carbonic anhydrase inhibitors from natural sources. J Enzyme Inhib Med Chem 2020;35:1462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meleddu R, Deplano S, Maccioni E, et al. Selective inhibition of carbonic anhydrase IX and XII by coumarin and psoralen derivatives. J Enzyme Inhib Med Chem 2021;36:685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petreni A, Osman SM, Alasmary FA, et al. Binding site comparison for coumarin inhibitors and amine/amino acid activators of human carbonic anhydrases. Eur J Med Chem 2021;226:113875. [DOI] [PubMed] [Google Scholar]
- 46.Miao Y, Yang J, Yun Y, et al. Synthesis and anti-rheumatoid arthritis activities of 3-(4-aminophenyl)-coumarin derivatives. J Enzyme Inhib Med Chem 2021;36:450–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salehian F, Nadri H, Jalili-Baleh L, et al. A review: biologically active 3,4-heterocycle-fused coumarins. Eur J Med Chem 2021;212:113034. [DOI] [PubMed] [Google Scholar]
- 48.Jo YH, Lee S, Yeon SW, et al. Anti-diabetic potential of Masclura tricuspidata leaves: Prenylated isoflavonoids with α-glucosidase inhibitory and anti-glycation activity. Bioorg Chem 2021;114:105098. [DOI] [PubMed] [Google Scholar]
- 49.Hu Y, Wang B, Yang J, et al. Synthesis and biological evaluation of 3-arylcoumarin derivatives as potential anti-diabetic agents. J Enzyme Inhib Med Chem 2019;34:15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu Y-H, Yang J, Zhang Y, et al. Synthesis and biological evaluation of 3-(4-aminophenyl)-coumarin derivatives as potential anti-Alzheimer's disease agents. J Enzyme Inhib Med Chem 2019;34:1083–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sepehri N, Mohammadi‐Khanaposhtani M, Asemanipoor N, et al. Synthesis, characterization, molecular docking, and biological activities of coumarin–1, 2, 3‐triazole‐acetamide hybrid derivatives. Archiv Der Pharmazie 2020;353:2000109. [DOI] [PubMed] [Google Scholar]
- 52.Damrath JG, Creecy A, Wallace JM, Moe SM.. The impact of advanced glycation end products on bone properties in chronic kidney disease. Curr Opin Nephrol Hypertens 2021;30:411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Babel RA, Dandekar MP.. A review on cellular and molecular mechanisms linked to the development of diabetes complications. Curr Diabetes Rev 2021;17:457–73. [DOI] [PubMed] [Google Scholar]
- 54.Ali MY, Jannat S, Rahman MM.. Ginsenoside derivatives inhibit advanced glycation end-product formation and glucose-fructose mediated protein glycation in vitro via a specific structure-activity relationship. Bioorg Chem 2021;111:104844. [DOI] [PubMed] [Google Scholar]
- 55.Ni Z, Zhuge Z, Li W, et al. Inhibitory effects of hydroxysafflor yellow A on the formation of advanced glycation endproducts in vitro. Biol Pharm Bull 2012;35:2050–3. [DOI] [PubMed] [Google Scholar]
- 56.Xu X, Zhang M, Fei Z, et al. Calcification of lower extremity arteries is related to the presence of osteoporosis in postmenopausal women with type 2 diabetes mellitus: a cross-sectional observational study. Osteoporosis Int 2021;32:1185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang T-W, Chen J-Y, Wu Y-L, et al. Alterations of bone markers in obese patients with type 2 diabetes after bariatric surgery: a meta-analysis and systemic review of randomized controlled trials and cohorts. Medicine 2021;100:e26061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li C, Wang S, Du M, et al. Clinical characteristics and controllable risk factors of osteoporosis in elderly men with diabetes mellitus. Orthopaedic Surg 2021;13:1001–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaur P, Behera BS, Singh S, Munshi A.. The pharmacological profile of SGLT2 inhibitors: focus on mechanistic aspects and pharmacogenomics. Eur J Pharmacol 2021;904:174169. [DOI] [PubMed] [Google Scholar]
- 60.Chen K-Y, Wu S-M, Tseng C-H, et al. Combination therapies with thiazolidinediones are associated with a lower risk of acute exacerbations in new-onset COPD patients with advanced diabetic mellitus: a cohort-based case–control study. BMC Pulmonary Med 2021;21:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee E-J, Kang M-K, Kim Y-H, et al. Coumarin ameliorates impaired bone turnover by inhibiting the formation of advanced glycation end products in diabetic osteoblasts and osteoclasts. Biomolecules 2020;10:1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei J, Zhang X, Li Y, et al. Novel application of bergapten and quercetin with anti-bacterial, osteogenesis-potentiating, and anti-inflammation tri-effects. Acta Biochimica et Biophysica Sinica 2021;53:683–96. [DOI] [PubMed] [Google Scholar]
- 63.Chen J, Chen Y, Shu A, et al. Radix rehmanniae and corni fructus against diabetic nephropathy via AGE-RAGE signaling pathway. J Diabetes Res 2020;2020:8358102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Subedi L, Lee JH, Gaire BP, Kim SY.. Sulforaphane inhibits MGO-AGE-mediated neuroinflammation by suppressing NF-κB, MAPK, and AGE–RAGE signaling pathways in microglial cells. Antioxidants 2020;9:792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Asadipooya K, Uy EM.. Advanced glycation end products (AGEs), receptor for AGEs, diabetes, and bone: review of the literature. J Endocrine Soc 2019;3:1799–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feng Z, Hou X, Zhu C, et al. Epigallocatechin gallate ameliorates morphological changes of pancreatic islets in diabetic mice and downregulates blood sugar level by inhibiting the accumulation of AGE-RAGE. J Cell Biochem 2019;120:8510–20. [DOI] [PubMed] [Google Scholar]
- 67.Valko M, Jomova K, Rhodes CJ, et al. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol 2016;90:1–37. [DOI] [PubMed] [Google Scholar]
- 68.Wang J, Zhang W, Li M, Li X.. The new coumarin compound Bis 3 ameliorates cognitive disorder and suppresses brain-intestine-liver systematic oxidative stress in high-fat diet mice. Biomed Pharmacother 2021;137:111293. [DOI] [PubMed] [Google Scholar]
- 69.Gerber PA, Rutter GA.. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid Redox Signal 2017;26:501–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruegsegger GN, Creo AL, Cortes TM, et al. Altered mitochondrial function in insulin-deficient and insulin-resistant states. J Clin Investig 2018;128:3671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Onyango AN. Cellular stresses and stress responses in the pathogenesis of insulin resistance. Oxid Med Cell Longev 2018;2018:4321714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joy MN, Bodke YD, Telkar S.. 4-Methyl-7-amino/amido coumarin derivatives as potential antimicrobials and antioxidants. Chem Nat Comp 2020;56:614–20. [Google Scholar]
- 73.Nagamallu R, Srinivasan B, Ningappa MB, Kariyappa AK.. Synthesis of novel coumarin appended bis(formylpyrazole) derivatives: studies on their antimicrobial and antioxidant activities. Bioorganic Med Chem Lett 2016;26:690–4. [DOI] [PubMed] [Google Scholar]
- 74.Lin Y, Shao Z, Zhao M, et al. PTPN14 deficiency alleviates podocyte injury through suppressing inflammation and fibrosis by targeting TRIP6 in diabetic nephropathy. Biochem Biophys Res Commun 2021;550:62–9. [DOI] [PubMed] [Google Scholar]
- 75.Yao H, Zhang N, Zhang W, et al. Discovery of a coumarin derivative as Nrf2 activator mitigating oxidative stress and fibrosis in mesangial cells under high glucose. Bioorganic Med Chem Lett 2020;30:127490. [DOI] [PubMed] [Google Scholar]
- 76.Kolahdouz M, Jafari F, Falanji F, et al. Clavulanic acid attenuating effect on the diabetic neuropathic pain in rats. Neurochem Res 2021;46:1759–70. [DOI] [PubMed] [Google Scholar]
- 77.Shou S, Wei J, Xiaofen H, et al. Inhibitory effect of low frequency electroacupuncture on the P2 X3 receptor in dorsal root ganglion of rats suffering from type II diabetic neuropathic pain. Acta Laboratorium Animalis Scientia Sinica 2017;25:54–9. [Google Scholar]
- 78.Wu B, Sheng X, Xu Z, et al. Osthole relieves diabetics cardiac autonomic neuropathy associated with P2X3 receptor in ratstellate ganglia. Brain Res Bull 2020;157:90–9. [DOI] [PubMed] [Google Scholar]
- 79.Zheng S, Huang H, Li Y, et al. Yin-xing-tong-mai decoction attenuates atherosclerosis via activating PPARγ-LXRα-ABCA1/ABCG1 pathway. Pharmacol Res 2021;169:105639. [DOI] [PubMed] [Google Scholar]
- 80.Wang J, Guo X, Chen C, et al. Gender differences in food allergy depend on the PPAR γ/NF-κB in the intestines of mice. Life Sci 2021;278:119606. [DOI] [PubMed] [Google Scholar]
- 81.Mahmoud AM, Abd El-Ghafar OA, Alzoghaibi MA, Hassanein EH.. Agomelatine prevents gentamicin nephrotoxicity by attenuating oxidative stress and TLR-4 signaling, and upregulating PPARγ and SIRT1. Life Sci 2021;278:119600. [DOI] [PubMed] [Google Scholar]
- 82.Moon J-H, Hong J-M, Park S-Y.. The antidiabetic drug troglitazone protects against PrP (106‑126)‑induced neurotoxicity via the PPARγ‑autophagy pathway in neuronal cells. Mol Med Rep 2021;23:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.El-Bassossy HM, Abo-Warda SM, Fahmy A.. Chrysin and luteolin alleviate vascular complications associated with insulin resistance mainly through PPAR-γ activation. Am J Chin Med 2014;42:1153–67. [DOI] [PubMed] [Google Scholar]
- 84.Korecova M, Hladikova M.. Treatment of mild and moderate type-2 diabetes: open prospective trial with Hintonia latiflora extract. Eur J Med Res 2014;19:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guerrero-Analco J, Medina-Campos O, Brindis F, et al. Antidiabetic properties of selected Mexican copalchis of the Rubiaceae family. Phytochemistry 2007;68:2087–95. [DOI] [PubMed] [Google Scholar]
- 86.Guerrero-Analco JA, Hersch-Martinez P, Pedraza-Chaverri J, et al. Antihyperglycemic effect of constituents from Hintonia standleyana in streptozotocin-induced diabetic rats. Planta Medica 2005;71:1099–105. [DOI] [PubMed] [Google Scholar]
- 87.QI G, TU S-z.. Synthesis of 3-bromine-4, 7-dimethyl-6-sulfonylurea coumarin derivatives and study of their hypoglycemic activities. West China J Pharm Sci 2009;06:568–70. [Google Scholar]
- 88.Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009;32:S62–S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Konidala SK, Kotra V, Danduga RCSR, Kola PK.. Coumarin-chalcone hybrids targeting insulin receptor: design, synthesis, anti-diabetic activity, and molecular docking. Bioorg Chem 2020;104:104207. [DOI] [PubMed] [Google Scholar]
- 90.Kuwata H, Yabe D, Murotani K, et al. Effects of glucagon‐like peptide‐1 receptor agonists on secretions of insulin and glucagon and gastric emptying in Japanese individuals with type 2 diabetes: a prospective, observational study. J Diabetes Investig 2021;12:2162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sharma R, Tiwari S.. Renal gluconeogenesis in insulin resistance: a culprit for hyperglycemia in diabetes. World J Diabetes 2021;12:556–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang YX, Li P, Wang P, Zhu BT.. Insulin-induced conformational changes in the full-length insulin receptor: structural insights gained from molecular modeling analyses. Acta Biochim Biophys Sinica 2021;53:848–69. [DOI] [PubMed] [Google Scholar]