Summary
Sleep is a cross-species phenomenon whose evolutionary and biological function remain poorly understood. Clinical and animal studies suggest that sleep disturbance is significantly associated with disruptions in protein homeostasis – or proteostasis – in the brain, but the mechanism of this link has not been explored. In the cell, the protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) pathway modulates proteostasis by transiently inhibiting protein synthesis in response to proteostatic stress. In this study we examined the role of the PERK pathway in sleep regulation and provide the first evidence that PERK signaling is required to regulate normal sleep in both vertebrates and invertebrates. We show that pharmacological inhibition of PERK reduces sleep in both Drosophila and zebrafish, indicating an evolutionarily conserved requirement for PERK in sleep. Genetic knockdown of PERK activity also reduces sleep in Drosophila, while PERK overexpression induces sleep. Finally, we demonstrate that changes in PERK signaling directly impact wake-promoting neuropeptide expression, revealing a mechanism through which proteostatic pathways can affect sleep and wake behavior. Taken together, these results demonstrate that protein synthesis pathways like PERK may represent a general mechanism of sleep and wake regulation and provide greater insight into the relationship between sleep and proteostasis.
Keywords: Sleep, Drosophila, zebrafish, protein synthesis, protein translation, PERK
eTOC Blurb
Ly et al. report that a signaling pathway that inhibits protein synthesis in response to cellular stress is also required for normal sleep regulation in fruit flies and zebrafish. This finding suggests that proteostasis pathways could link sleep, cellular health and disease.
Introduction
Chronic sleep and wake disorders impact over 10% of Americans [1], with devastating long-term health consequences. Recently, emerging evidence from multiple studies is linking neurodegenerative disease risk [2–6] and even disease pathogenesis [7–9] to sleep dysfunction, although the underlying etiology remain poorly understood. A key feature of neurodegenerative disease is severe protein dyshomeostasis that is exemplified by the accumulation of misfolded protein aggregates in the brain [10]. A possible function of sleep could be to regulate proteostasis in the brain, which could explain its link to neurological health. Consistent with this theory are experiments in mice that demonstrate that during sleep, proteins and toxins are actively cleared from the brain [11] and work in C. elegans which show that external insults that cause cellular stress such as cold, toxin exposure, and heat all induce sleep [12,13]. At present, little is known about how mechanisms that regulate proteostasis are involved in sleep. Addressing this question is important as it may identify novel therapeutic targets to treat sleep disorders and possibly to enhance the restorative effects of sleep.
Cellular proteostasis involves the proper coordination of protein synthesis, folding, and trafficking inside the cell [14]. When the proteostatic balance is disrupted and misfolded proteins accumulate within the cell, intracellular signaling pathways collectively known as the unfolded protein response (UPR) become activated [15]. The UPR originates in the subcellular organelle known as the endoplasmic reticulum (ER), where secretory and membrane proteins are produced, folded, packaged, and post-translationally modified for transport out into the cell [15]. UPR activation is mediated by three signaling transducers: PKR-like ER kinase (PERK), inositol-requiring protein-1 (IRE1), and activating transcription factor-6 (ATF6) [15]. Together, these transduction molecules activate signaling cascades that inhibit protein synthesis, upregulate protein chaperone activity, and degrade transcript and protein products in the cell [15]. Thus, UPR activation restores cellular proteostasis in response to ER stress, and subsequently prevents cell death.
The UPR system is conserved across invertebrate and vertebrate species, including rats [16,17], mice [18,19], Drosophila [20], and white-crowned sparrows [21]. UPR activation by prolonged wakefulness/sleep deprivation is conserved across invertebrate and vertebrate species, including rats [16,17], mice [18,19], Drosophila [20] and white-crowned sparrows [21] as evidenced by increased expression of transcript or protein levels of the UPR chaperone molecule binding immunoglobulin protein (BiP). In Drosophila, genetic overexpression of BiP increases recovery sleep after sleep deprivation while overexpression of dominant negative BiP leads to a reduction in recovery sleep amount [20] demonstrating that UPR signaling is involved in the homeostatic regulation of sleep. However, the downstream mechanisms through which the UPR affects sleep remains unknown and it has not yet been determined whether the UPR is required not just for sleep in response to deprivation-induced stress, but also for normal sleep levels in the absence of stress.
To address this gap in knowledge, we investigated the role of the PERK pathway, a signal transduction pathway of the UPR, in regulating sleep and wake behavior. PERK transiently suppresses protein synthesis in response to ER stress [22]. Using biochemical, pharmacological, and genetic approaches, we demonstrate that the PERK pathway is necessary for normal sleep levels in both invertebrate (Drosophila melanogaster) and vertebrate (Danio rerio) model organisms, suggesting this pathway plays an ancestral role in sleep regulation. Furthermore, transgenic knockdown of PERK signaling in neurons suppressed sleep in Drosophila, while neuronal overexpression of PERK significantly increased sleep amount. Finally, we demonstrate that PERK activity in a small subset of wake-promoting neurons is sufficient to alter Drosophila sleep and wake behavior, and change the expression of the wake-promoting neuropeptide pigment dispersing factor (PDF). These results illustrate that a signaling pathway responsible for modulating protein synthesis in the cell is necessary to promote vertebrate and invertebrate sleep, and provide the first evidence of a mechanistic link between protein synthesis and sleep regulation in the brain.
Results
PERK pathway activation is associated with wakefulness
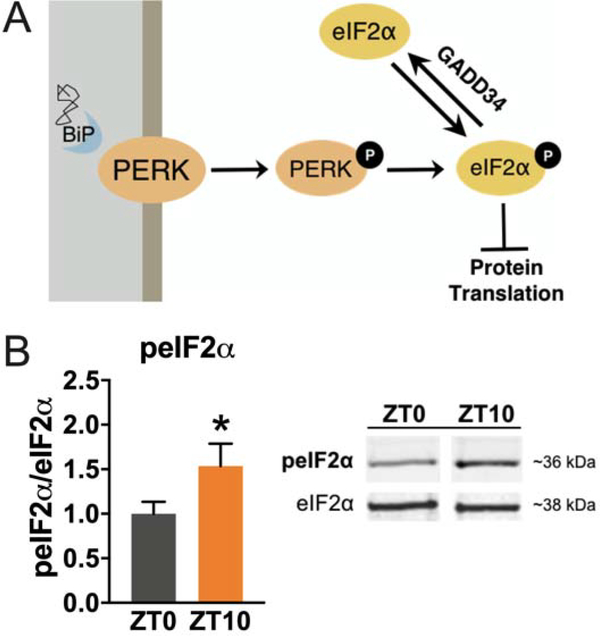
Sleep deprivation leads to ER stress [19,20] and PERK pathway activation across several species including mice and Drosophila [23,24]. We wanted to determine whether PERK pathway activation is specific to sleep deprivation or whether PERK pathway activation may occur over the course of normal wakefulness. We examined the alpha subunit of eukaryotic initiation factor 2 (eIF2α), in order to analyze PERK pathway activity. In the critical rate-limiting step of protein synthesis, GTP-bound eIF2 recruits the initiator Met-tRNAi to the 40S ribosomal subunit to form the 43S preinitiation complex necessary for the translation initiation [25]. Activated PERK phosphorylates the alpha subunit of eukaryotic initiation factor 2 (eIF2α) which forms a stable complex with the guanine nucleotide exchange factor eIF2B to halt synthesis of new proteins in the cell [26,22]. Thus, phosphorylated eIF2α (peIF2α) levels are a proxy for PERK pathway activity (Figure 1A). We examined peIF2α levels at the beginning and the end of the day to identify whether normal wakefulness across the day correlates with any changes in PERK pathway activity. We observed that peIF2α is elevated by 50% (P<0.05, student’s t-test) at the end of the day (ZT10) compared to the beginning of the day (ZT0) in Drosophila (Figure 1B). We also confirmed that peIF2α levels are elevated by sleep deprivation as previously reported [23,24] (Figure S1). Thus, phosphorylation of eIF2α appears to be a molecular correlate of wakefulness in the fly.
Figure 1. Activation of the PERK pathway is associated with wakefulness in Drosophila.
(A) Simplified schematic of PERK activation and inhibition of protein synthesis. Activated PERK phosphorylates the alpha subunit of eIF2 to inhibit protein synthesis. The phosphatase GADD34 returns eIF2α to its unphosphorylated state. (B) Quantification from western blot analysis of peIF2α expression in flies collected at the beginning of the lights-on period (ZT0) compared to the end of the day (ZT10). peIF2α protein level is 50% higher near the end of the day compared to the start of the day (N=13 animals, *P<0.05, student’s t-test). Representative peIF2α and eIF2α signal shown on the right. Bar graph shows mean ± SEM. See also Figure S1
Pharmacological inhibition of PERK reduces sleep
Until now, the relationship between PERK signaling and behavioral state has largely been described under conditions of cellular stress during sleep deprivation [19,23]. Given that we observe elevated eIF2α phosphorylation during the end of the day, we sought to test the hypothesis that PERK signaling is required for normal levels of sleep in the absence of stress. Consistent with a role of the PERK pathway in regulating sleep, a previous study in rats demonstrated that pharmacological inhibition of protein synthesis with the drug Salubrinal, which enhances peIF2α levels, induces sleep [27]. Although these findings suggest that levels of protein synthesis may directly affect behavioral state [27], a lack of genetic confirmation in this study makes it difficult to determine whether PERK signaling specifically promoted sleep in this instance. We sought to confirm this using both the fruit fly and zebrafish sleep models.
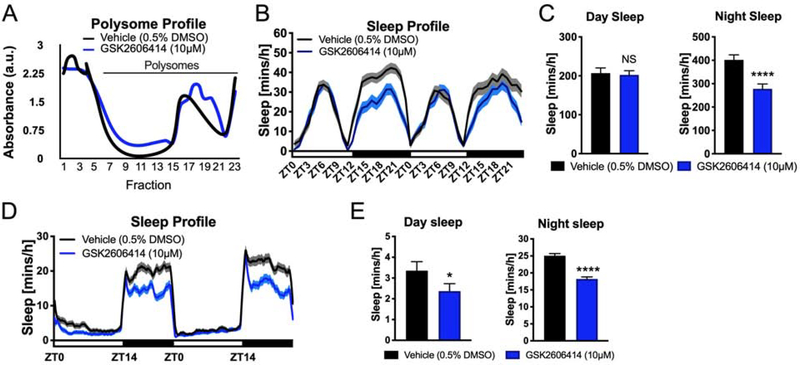
We first examined the effect of pharmacological inhibition of PERK on sleep in adult Drosophila melanogaster. Wecompared sleep in wildtype flies that were administered GSK2606414, a small molecular inhibitor of PERK [28], to isogenic controls treated with vehicle. We administered GSK2606414 at a concentration previously shown to exhibit therapeutic effects in a Drosophila model of neurodegeneration [29], in which protein folding was impaired. We conducted ribosomal profiling of brain tissue from flies treated with vehicle or GSK0606414 and found that administration of GSK2606414 resulted in a higher proportion of actively translating polysomes in the brain (Figure 2A). We then recorded the sleep behavior of flies fed vehicle or GSK2606414 and observed that GSK2606414 reduced nighttime sleep by 31% (P<0.0001, student’s t-test, Figure 2B and 2C). Analysis of sleep architecture revealed that GSK2606414 did not significantly affect sleep architecture (P=0.068, student’s t-test, Figure S2A and S2B). To validate the effect of PERK pathway inhibition in a vertebrate model of sleep, we also examined the effect of GSK2606414 treatment in zebrafish. Like Drosophila, zebrafish exhibit a diurnal sleep/wake rhythm that can be recorded and analyzed with video tracking software [30]. Here, single-housed zebrafish larvae are monitored in single wells of a 96-well plate, and drugs can be administered directly into the water while sleep is measured [31]. GSK2606414 treatment reduced zebrafish sleep by 27% at night (P< 0.0001, student’s t-test), similar to Drosophila, and also reduced sleep during the day (−29%, P< 0.05 student’s t-test) (Figure 2D and 2E).
Figure 2. Pharmacological inhibition of PERK decreases sleep in Drosophila and zebrafish.
(A) GSK0606414 alters the ribosomal profile in the Drosophila brain. The proportion of actively translating polysomes is higher following GSK2606414 administration (Fraction 7–21) (a.u. = arbitrary units). (B) Sleep profile of flies treated with GSK2606414 or vehicle (0.5% DMSO). The PERK inhibitor GSK2606414 suppresses nighttime sleep in Drosophila(N=42 animals). (C) Quantification of daytime and nighttime sleep. GSK2606414 significantly reduces sleep at night (****P<0.0001, student’s t-test). (D) Sleep profile of zebrafish during two days and nights. GSK2606414 decreases sleep at night (Vehicle: N = 102; GSK2606414: N= 114). (F) Quantification of daytime and nighttime sleep after vehicle and GSK2606414 administration. Total sleep is reduced during the day and night in zebrafish following GSK2606414 administration (* P<0.05, ****P<0.0001, student’s t-test). Line and bar graphs show mean ± SEM. White and black boxes under line graphs indicate day and night, respectively. See also Figure S2
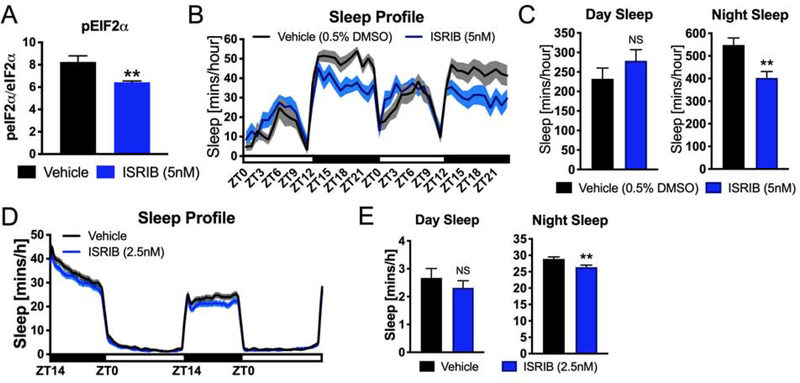
As an alternative approach to test the effect of inhibiting the PERK pathway on sleep, we treated animals with the eIF2B activator ISRIB. Similar to GSK2606414, ISRIB represses PERK pathway activation by activating eIF2B, resulting in increased protein synthesis. We found that administration of ISRIB suppressed PERK pathway activation in Drosophila, as measured by a reduction in peIF2α (Figure 3A).Similar to GSK2606414, ISRIB treatment significantly decreased sleep at night in both Drosophila (−27%, P< 0.01, student’s t-test) (Figure 3B and 3C) and zebrafish (−9%, P< 0.01, student’s t-test) (Figure 3D and 3E). Sleep architecture is not significantly changed in Drosophila following ISRIB administration (Figure S2C and S2D). Thus, two different pharmacological interventions that reduce PERK pathway activity by different mechanisms reduce sleep in both Drosophila and zebrafish. These results indicate that PERK signaling is required for normal sleep levels in both an invertebrate and a vertebrate species.
Figure 3. Pharmacological activation of eIF2B decreases sleep in Drosophila and zebrafish.
(A) ISRIB administration significantly reduces the expression of peIF2α in the brain. (N=9 animals, **P<0.01, student’s t-test). (B) Sleep profile of flies treated with ISRIB or vehicle (0.5% DMSO) (N=28 animals, shaded area represents SEM). (C) Quantification of daytime and nighttime sleep. ISRIB reduces the total amount of sleep at night (N=28, **P<0.01, student’s t-test).(D) Sleep profile of larval zebrafish over the course of two days and nights. ISRIB decreases sleep at night (Vehicle: N =232 animals; ISRIB: N = 240 animals; shaded area represents SEM). (E) Quantification of daytime and nighttime sleep. Total sleep is reduced at night in zebrafish larvae following ISRIB administration (**P<0.01, student’s t-test). Line and bar graphs show mean ± SEM. White and black boxes under line graphs indicate day and night, respectively. See also Figure S2
Pan-neuronal knockdown of the PERK pathway reduces sleep
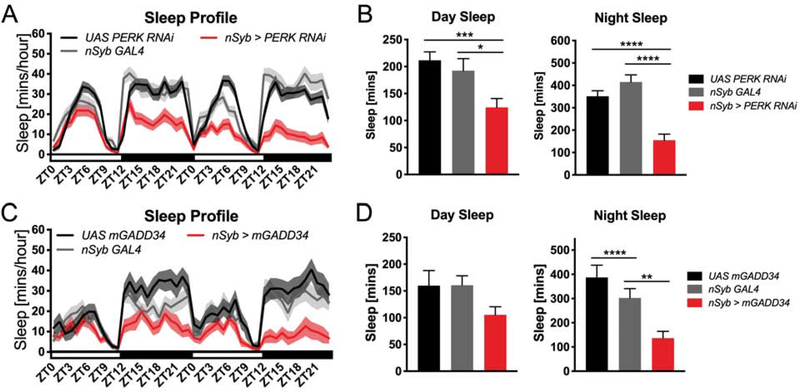
Due to caveats of pharmacology, we also tested the role of PERK signaling on sleep using genetic tools. Since pharmacological administration of a PERK inhibitor acts on PERK in all cell types without any spatial specificity, we next utilized a genetic approach to assess the role of PERK in the brain on sleep behavior. To do so, we compared sleep in transgenic neuronal Synaptobrevin (nSyb)GAL4>UAS PERK RNAi Drosophila, which express the PERK RNAi construct in all neurons, to control animals containing either the GAL4 or UAS transgene alone. We found that pan-neuronal knockdown of PERK significantly reduced sleep during both the day (P<0.01, one-way ANOVA) and night (P<0.0001, one-way ANOVA) (Figure 4A and 4B). This phenotype was due to reductions in both the number and length of sleep bouts per day (Figure S4A and S4B). To further characterize the effect of reduced eIF2α phosphorylation via a different mechanism, we pan-neuronally overexpressed a murine form of growth arrest and DNA damage-inducible protein (GADD34, also known as Protein Phosphatase 1 Regulatory Subunit 15A). Overexpression of GADD34 should result in dephosphorylation of peIF2α, similar to knockdown of PERK, resulting in increased initiation of protein synthesis (Figure 1A). We found that overexpression of mGADD34 reduced sleep at night compared to parental controls at night (P<0.0001, one-way ANOVA) (Figure 4C and 4D), similar to PERK knockdown though we did not observe a change in the amount of daytime sleep levels. Analysis of sleep architecture demonstrates that mGADD34 overexpression reduces the number of sleep bouts per day while sleep bout length is not significantly changed (Figure S4C and S4D). We also tested the effect of PERK knockdown in constant conditions and found that the reduction of sleep persists in constant dark following pan-neuronal knockdown of PERK (Figure S5) indicating that sleep changes were not due circadian rhythmicity disruption. These findings suggest that reducing phosphorylation of eIF2α results in reduced sleep in Drosophila and provide additional evidence that the PERK pathway is required for normal sleep levels.
Figure 4. Genetic inhibition of peIF2α reduces sleep.
(A) Sleep profile of flies expressing PERK RNAi in nSyb-expressing neurons compared to uncrossed parental controls. Expression of PERK RNAi in neurons reduces sleep levels (N=27 animals) (B) Quantification of daily sleep amounts. Total sleep is significantly reduced following PERK RNAi expression in neurons (N=27 animals, P<.01, one-way ANOVA; *P<0.05, ***P<0.001, ****P<0.0001). (C) Sleep profile of flies expressing mGADD34 in nSyb-expressing neurons compared to uncrossed parental controls. Expression of mGADD34 in neurons reduces sleep levels (N=18 animals). (D) Quantification of daytime and nighttime sleep amounts. Daytime sleep amount is not significantly affected by genotype (P= 0.0953, one-way ANOVA). Total nighttime sleep is significantly reduced by mGADD34 expression in neurons (N=18 animals, P<.001, one-way ANOVA; **P<0.01,****P<0.0001, student’s t-test). Total daytime sleep is not significantly changed by mGADD34 expression in neurons (P=0.09, one-way ANOVA). Line and bar graphs show mean ± SEM. White and black boxes under line graphs indicate day and night, respectively. See also Figures S3 and S4
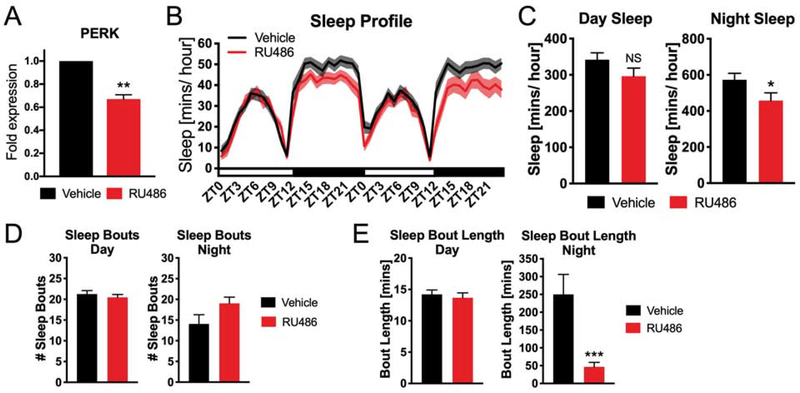
A caveat with the PERK RNAi and mGADD34 overexpression experiments is that they employ constitutive manipulations, and behavioral effects observed in adult animals may result from effects during development. To avoid this issue, we harnessed the spatial and temporal specificity of the GAL4/UAS GeneSwitch system to restrict the transgenic pan-neuronal expression of PERK RNAi to adulthood. Unlike traditional GAL4 drivers, GeneSwitch GAL4 drivers are active only in the presence of the small molecule RU486, which can be administered in the food during a selected window, thus allowing us to knock down PERK expression specifically during adulthood. We confirmed this knockdown by RT-qPCR; 24 hours of RU486 administration led to a ~30% reduction in PERK transcript expression in the brain compared to vehicle control (Figure 5A). This treatment resulted in reduced sleep at night (Figure 5B), although the magnitude of the phenotype was smaller than that observed for constitutive knockdown of PERK (Figure 4B) and sleep bouts were not significantly changed by RU486 induction of PERK RNAi (Figure 5D). However, we observe a significant reduction in average sleep bout length as a result of RU486-induced knockdown of PERK (Figure 5E). Thus, acute knockdown of PERK in neurons reduces sleep and suggests that PERK is required to maintain sleep in Drosophila.
Fig. 5. Neuronal knockdown of PERK inhibits sleep in Drosophila.
(A) RU486 administration reduces the expression of PERK mRNA in the brain of flies expressing PERK RNAi in neurons. Expression is normalized to Act5c and GAPDH housekeeping controls (P<0.01, student’s t-test). (B) Sleep profile of neuron-specific GeneSwitch>PERK RNAi flies administered either RU486 or vehicle (N=28 animals). (C) Quantification of sleep during the day and night. RU486 administration reduces the total amount of sleep at night (N=28 animals, *P<0.05, student’s t-test). (D) RU486 administration does not significantly affect the number of sleep bouts during the day and night in flies expressing PERK RNAi in neurons (N=28 animals). (E) RU486 administration does not significantly alter the average length of sleep bouts during the day but reduces the average sleep bout length at night in flies expressing PERK RNAi in neurons (N=28 animals, **P<0.01, student’s t-test). Line and bar graphs show mean ± SEM. White and black boxes under line graphs indicate day and night, respectively.
Overexpression of PERK induces sleep
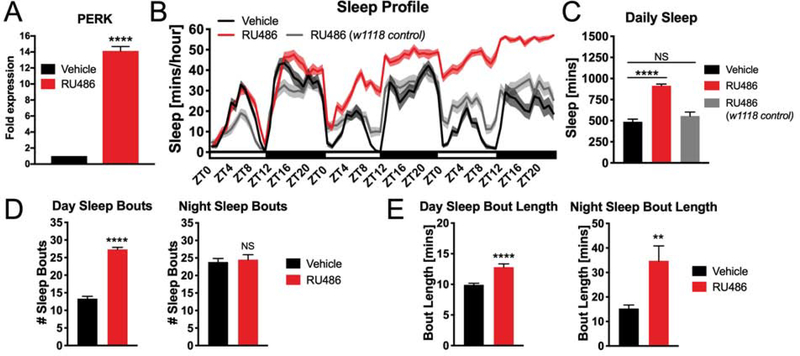
While the previous experiments demonstrate that PERK signaling is required for normal sleep levels, they also raise the question of whether induction of PERK signaling is sufficient to alter sleep behavior. To address this question, we pan-neuronally overexpressed Drosophila PERK (dPERK) specifically during adulthood using an nSyb GeneSwitch driver. Using RT-qPCR, we found that 48 hours of RU486 administration resulted in a ~14 fold increase in PERK mRNA levels (Figure 6A) and in a significant increase in sleep during both the day and night (Figure 6B and 6C). This phenotype was due to an increase in both the number and length of sleep bouts (Figure 6D and 6E). Flies on RU486 did not exhibit any basal locomotor deficits compared to vehicle controls as measured by a climbing assay (Figure S5A). Flies with RU486-induced PERK expression also displayed similar arousal threshold to control flies, indicating that PERK overexpression does not suppress the ability of flies to be aroused from sleep (Figure S5B).Further, as a control to ensure this phenotype was due to RU486-induced PERK expression, we placed a subset of the animals back on regular food for three days, and then assayed their sleep again. We found that sleep amounts were no longer significantly different from vehicle control (Figure S5C and S5D), indicating that the behavioral effects were indeed due to RU486-induced PERK expression. The behavioral phenotype was not due to RU486 itself, as RU486 treatment of flies carrying one copy of the nSyb GeneSwitch transgene but not the UAS dPERK transgene did not induce a sleep phenotype (Figure 6B and 6C). Taken together, these results indicate that overexpression of PERK is sufficient to induce sleep.
Figure 6. Neuronal overexpression of PERK induces sleep in Drosophila.
(A) RU486 administration leads to a ~14-fold enhancement in PERK transcriptional expression in the brain in nSyb GeneSwitch>dPERK flies. Expression is normalized to Act5c and GAPDH housekeeping controls (****P<0.0001, student’s t-test). (B) Sleep profile of nSybGeneSwitch>dPERK flies on vehicle compared to RU486. The gray trace shows sleep amount in progeny of nSyb GeneSwitch and w1118 wildtype controls fed RU486. Successive days on RU486 food incrementally increases sleep time in flies in nSyb GeneSwitch>dPERK transgenic animals (N=28 animals). (C) Quantification of daily sleep amount over the course of the three-day experiment on vehicle/RU486 administration. RU486 significantly increases daily sleep amount in nSyb GeneSwitch>dPERK flies but not in the w1118/nSyb GeneSwitch progeny controls (N=28 animals, ****P<0.0001, student’s t-test) (D) RU486 administration increases the number of daytime sleep bouts in nSyb GeneSwitch>dPERK flies (N=28 animals, ****P<0.0001, student’s t-test) and (E) RU486 increases the average sleep bout length during both the day and night in nSyb GeneSwitch>dPERK flies (N=28, **P<0.01, ****P<0.0001, student’s t-test). Line and bar graphs show mean ± SEM. White and black boxes under line graphs indicate day and night, respectively. See also Figure S5
Manipulation of PERK signaling in the wake-promoting PDF circuit alters sleep and wake
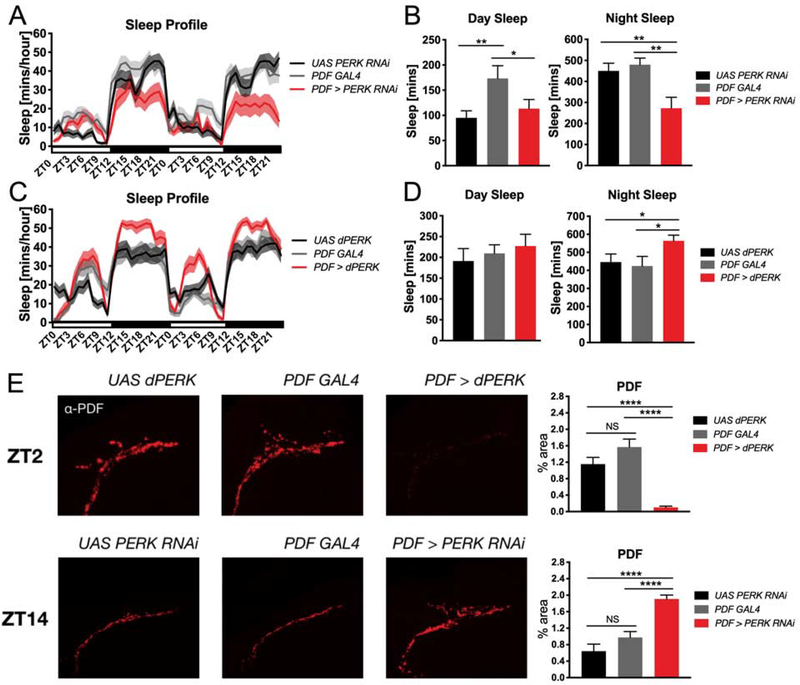
Finally, we sought to explore the potential mechanisms through which modulation of PERK activity might affect sleep. Because the effects of PERK pathway inhibition on sleep occurred mostly during the night, we posited that PERK signaling may be required to put a brake on the synthesis of wake-active molecules, in which case inhibiting PERK would allow for continued synthesis of wake-promoting molecules into the night where significant sleep reductions were observed. Thus, we chose to assess the requirement of PERK in sleep in neurons expressing the neuropeptide pigment dispersing factor (PDF), which promotes wakefulness in Drosophila [32]. Both the spatial and time-of-day expression of PDF is well-characterized; PDF is expressed in 8 neurons in each hemisphere of the brain that project to the medulla dorsal protocerebrum of the brain [33] (Figure S6). Levels of PDF are elevated during the day and its expression and release is significantly downregulated at the beginning of the night [34–36]. We used PDF GAL4 to drive PERK RNAi expression in PDF neurons and found that it significantly reduced sleep relative to parental controls (Figure 7A and 7B). Conversely, overexpression of PERK in PDF neurons significantly increased sleep compared to parental controls (Figure 7C and 7D). Next, we examined the effect of PERK knockdown and overexpression on the release of PDF from the subset of PDF-expressing clock cells known as the small lateral ventral neurons (sLNvs), in which significant time-of-day plasticity has been demonstrated [34,35]. We found that overexpression of PERK significantly reduces PDF expression at release terminals during the day (ZT 2), when PDF expression and release is normally high. Consistent with this observation, knockdown of PERK significantly increased PDF expression at night (ZT 14), when PDF levels are normally low (Figure 7E and 7F). To confirm that the low levels of PDF at the projection terminals following PERK overexpression were not the result of cell death, we also visualized the cell bodies of the sLNvs in the PDF>dPERK transgenic line. While the PDF signal is weak as expected in the cell bodies of the sLNvs with PERK overexpression, staining with PER – which is expressed in all of the LNvs [37] – shows that the cells remain undamaged and intact in the transgenic flies (Figure S7). This demonstrates that changes in PERK signaling in a small subset of wake-promoting neurons is sufficient to alter sleep and neuropeptide expression.
Figure 7. Genetic knockdown and overexpression of PERK in PDF neurons reduces and increases sleep and alters PDF expression at projection terminals.
(A) Sleep profile of flies expressing PERK RNAi in PDF neurons (PDF>PERK RNAi) compared to parental controls (N=18 animals). (B) Quantification of daytime and nighttime sleep. During the day, flies expressing PERK RNAi in PDF neurons (PDF>PERK RNAi) sleep less than PDF GAL4 parental controls but not UAS PERK RNAi controls, which also slept less than the PDF GAL4 parental line (P<0.05, one-way ANOVA; *P<0.05, **P<0.01). At night, flies expressing PERK RNAi in PDF neurons sleep less than both parental controls (P<0.01, one-way ANOVA; **P<0.01). (C) Sleep profile of flies overexpressing PERK in PDF neurons compared to parental controls (N=18 animals) (D) Quantification of daytime and nighttime sleep. Daytime sleep amount is not significantly affected by PERK overexpression in PDF neurons (P= 0.6642, one-way ANOVA). At night, flies overexpressing PERK in PDF neurons (PDF>dPERK) sleep more than parental controls (P<0.05, one-way ANOVA; *P<0.05). (E) Representative confocal images of sLNvs projections in transgenic and parental control flies and quantification of PDF expression. At ZT2 (upper panels), PERK overexpression in PDF neurons significantly suppresses PDF expression at projection terminals compared to parental controls(UAS dPERK, N=7; PDF GAL4, N=7; PDF>dPERK, N=9;, P<0.0001, one-way ANOVA; ****P<0.0001). At ZT14 (lower panels), PERK knockdown in PDF neurons significantly increases PDF expression at projection terminals compared to parental controls (UAS PERK RNAi, N=10; PDF GAL4, N=11; PDF> PERK RNAi, N=12; P<0.0001, one-way ANOVA; ****P<0.0001). (α-PDF, 1:1000; AlexaFluor 594 1:500). Line and bar graphs show mean ± SEM. White and black boxes under line graphs indicate day and night, respectively. See also Figures S6 and S7
Discussion
In this study, we identify an evolutionarily conserved role for the PERK pathway — a critical regulator of protein synthesis — in regulating sleep. In line with our previous studies, we demonstrate that wakefulness is associated with enhanced PERK activity, lending strength to the notion that ER stress is one of the molecular costs of wakefulness. We observe that pharmacological inhibition of the PERK pathway decreases sleep in both Drosophila and zebrafish, while genetic downregulation and overexpression of PERK pathway signaling reduces and increases sleep in Drosophila, respectively. This data suggests that PERK signaling promotes sleep and is consistent with previous work demonstrating that pharmacological enhancement of peIF2α by the drug salubrinal increases slow-wave sleep in rats (27).
This is the first body of work to identify a mechanism underlying a bidirectional relationship between sleep and proteostatic signaling in the brain. Across species, brain plasticity has long been shown to be modulated by sleep [38–41]. In particular, the downscaling of synapses and synaptic proteins is an observed feature of sleep across both vertebrate and invertebrate systems [38,39]. From our findings, we would posit that PERK is a general and cross-species mechanism which underlies proteostatic changes that wakefulness might inherently perturb and may play a critical role in modulating synapses during sleep. Consistent with this notion, we observed that inhibiting PERK signaling most consistently suppressed sleep during night. We interpret this result as evidence that PERK acts as a homeostatic signal in response to extended wakefulness during the day. Recently, analysis of metabolic rate in waking and sleeping flies determined that wakefulness is associated with elevated metabolic rate in Drosophila [42]. Thus, wake-induced ER stress may be triggered by higher energetic demands and greater cellular protein load that accumulate during the day in Drosophila. Altogether, our findings suggest that one of the conserved functions of sleep may be to mitigate cellular stress caused by wakefulness.
In addition to PERK, protein synthesis is regulated by many different molecular factors in the cell that may have unique implications for sleep regulation. For example, mammalian target of rapamycin complex 1 (mTORC1) activates protein translation and inhibition of mTORC1 and protein translation in hippocampal neurons is responsible for memory impairments following sleep deprivation [43]. Furthermore, local pharmacological inhibition of peptidyl transferase – which is required for peptide bond formation – with the drug anisomycin exerts region-specific changes on sleep in rats [44]. Thus, regulation of sleep by the interplay between PERK and other translational regulators will be an important line of investigation in the future. It will also be critical to parse the role of PERK from other molecules that modulate translational regulation in the cell independent of an ER-stress response. Furthermore, investigation of the temporal attributes of PERK signaling in future experiments will provide crucial information about the dynamics of translational regulation in the cell. For example, previous work in macaque monkeys has demonstrated that deep sleep is correlated with enhanced protein biosynthesis [45]. While our studies are able to determine that the PERK pathway promotes sleep, we do not yet know if PERK signaling attenuates during deeper stages of sleep in the brain. Future global proteomic analysis across sleep and wake may help address and characterize the nature of PERK-mediated translational changes in the brain under different behavioral states. Of note, many targets bypass translational inhibition during ER stress such as heat shock proteins (HSPs), which have been shown to be protective against lethality induced by sleep deprivation in Drosophila [46]. Thus, both upregulation and downregulation of proteins likely mediate the relationship between PERK and sleep and should be characterized in the future. These analyses may also identify specific molecular targets of PERK signaling that distinguish its role in sleep regulation from other translational regulatory factors. In the future, experiments should also consider the developmental role that PERK may play in circuit development and regulation as it pertains to sleep. In this study, we observed that acute knockdown of PERK in neurons produced a more modest effect on sleep than constitutive neuronal knockdown. This suggests there may be developmental factors of PERK regulation that will be important to parse apart from brain regulation in the adult animal.
In this work we uncover evidence that modulation of PERK signaling within only eight neurons in the brain is sufficient to alter sleep behavior in Drosophila. This finding does not exclude the possibility that PERK regulation of sleep occurs within multiple brain circuits and cell types. In fact, given its broad role in regulating global protein synthesis in the cell, we would posit that this is likely to be the case. In the future, analysis of sleep effects of PERK signaling in various cell types and brain regions will be important to determine whether cellular specificity of PERK regulation of sleep exists. However, these results do suggest that on a global level in the brain, downregulation of protein synthesis may be a key molecular correlate of sleep.
Our findings have broad implications for the role of sleep in disease and age-related decline. Recent studies found that pharmacological modulation of PERK signaling has therapeutic benefits in rodent models of frontotemporal dementia and prion disease, where overactive PERK signaling is comorbid with the traditional hallmarks of disease [47–49]. Given the link between poor sleep and neurodegenerative disease risk [4–6, 50], the results presented here support for the notion that the UPR may be relevant to neurodegenerative pathophysiology. As such, further investigation into how PERK and the UPR regulate sleep behavior could provide greater insight into the etiology and pathology of neurodegenerative diseases. Loss of proteostasis is also a hallmark of aging, which has been demonstrated across species such as C. elegans [51, 52], Drosophila [24], and rodents [53]. Work from our lab has identified dysregulated ER stress signaling in the brains of both old mice and Drosophila [54,24], and improvement of proteostasis using a chemical chaperone ameliorates age-related sleep loss in Drosophila [24]. As this study is the first to show a cross-species requirement for ER stress signaling in young animals, it has important implications for the relationship between sleep and proteostasis across the lifespan. If ER stress molecules are recruited to regulate sleep in young and healthy organisms, this could imply that altered ER stress signaling might underlie not only deterioration of sleep quality that accompanies old age, but also related physiological changes that impact age-related health outcomes. Overall, this work reveals a molecular mechanism of sleep regulation that points to the maintenance of cellular proteostasis as an evolutionarily conserved function of sleep. Continued investigation of molecular factors such as PERK that regulate the balance between sleep and wake will be a necessary component to building our understanding of both the vast capabilities and biological limits of brain function. Our findings suggest that a closer examination of the relationship between sleep and proteostatic signaling across species is warranted as we continue to probe the biological mysteries of sleep.
STAR Methods
LEAD CONTACT AND MATERIALS AVAILABILITY
This study did not generate new unique reagents. Further information and requests for resources and reagents should be direct to and be fulfilled by the Lead Contact, Nirinjini Naidoo (naidoo@pennmedicine.upenn.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Wildtype Drosophila melanogaster in the following study are in the White Canton-S 10 genetic (wCS10) background strain, which was originally a gift from Ronald Davis (Scripps Research Institute, Jupiter, FL). UAS PERK RNAi (#42499), and neuron-specific GeneSwitch (#40265) fly lines were purchased from the Bloomington Stock Center at Indiana University and outcrossed 7 generations into the wCS10 background in our lab before being used for behavioral experiments. PDF GAL4 was originally a gift from John Zimmerman and outcrossed 7 generations into the wCS10 background. All other fly lines were maintained in the w1118 background. UAS PERK RNAi with Dicer2, UAS dPERK, and UAS mGADD34 were a gift from Hyung Don Ryoo (New York University). The nSyb GAL4 driver (#48590) was purchased from Bloomington Stock Center and the nSyb GeneSwitch GAL4 was a gift from Amita Sehgal (University of Pennsylvania). Flies were maintained in an environmental room at 25°C in a 12:12 hour light:dark cycle on standard dextrose media (UPenn Cell Center). Female flies were used for all experiments to control for the effects of gender on all observed outcomes.
METHOD DETAILS
Drosophila and zebrafish sleep assays
Female files were collected separately under CO2anesthesia after eclosion and allowed to grow to adulthood (5–7 days of age) before recording. Individual flies were placed in 65mm × 5mm tubes containing dextrose media. For sleep experiments requiring pharmacological interventions or RU486 administration, locomotor tubes contained dextrose food that was prepared with either vehicle, drug, or RU486. Rest and activity were recorded by video as described previously (54). Sleep is defined as 5 or more minutes of continuous inactivity (55). Drosophila arousal threshold was measured using the Drosophila Activity Monitoring System (DAMS) and measured and analyzed as previously described (56). Zebrafish sleep assays were conducted as previously described (30). Briefly, individual zebrafish larva (5 days post-fertilization) are placed in separate wells within a 96-well plate. Recordings are conducted in a temperature-controlled chamber using an infrared camera which captures movement during both the day and night.
Drug administration
For Drosophila sleep assays, GSK2606414 (Tocris Bioscience) and ISRIB (Tocris Bioscience) were prepared in 50% dimethyl sulfoxide (DMSO) and incorporated into the dextrose media at a final concentration of 10 μM GSK2606414 and 5 nM ISRIB. The final concentration of DMSO vehicle is 0.5%. Mifepristone (RU486, Sigma-Aldrich) was prepared in 80% ethanol (EtOH) and incorporated into dextrose media at a concentration of 100 μM and final vehicle concentration of 0.8% EtOH. Wildtype flies, transgenic crosses, and parental control lines were placed in locomotor tubes on drug/RU486 or vehicle between 5–7 days of age at least 24 hours before the start of sleep recording. For zebrafish sleep assays, GSK2606414 (Tocris Bioscience) was prepared at a final concentration of 10 μM and ISRIB (Tocris Bioscience) was prepared at a final concentration 2.5 nM.
Fly head preparation and Western blotting
Flies were sacrificed over dry ice and protein was extracted using a standard cell lysis buffer (10 mM Tris-HCl, 1mM EDTA, 10% Glycerol, 1% Triton-X, 150 mM NaCl) containing SIG-MAFAST™ Protease Inhibitor Cocktail (Sigma-Aldrich) and Halt™ Protease Inhibitor Cocktail (ThermoFisher Scientific). Protein homogenates from fly heads were loaded on sodium dodecyl sulfate (SDS) polyacrylamide gels (10% Tris-HCl) and then transferred to nitrocellulose membranes (Bio-Rad) and blocked in Odyssey® TBS Blocking Buffer (LI-COR). Membranes were incubated with rabbit anti-phospho-eIF2α (Ser51) polyclonal antibody (1:1000, Cell Signaling) and mouse anti-eIF2α (L57A5) (1:1000, Cell Signaling), 1:1000. The membranes were subsequently incubated with goat anti-rabbit IRDye®800RD (1:10,000, LI-COR) and donkey antimouse IRDye®680RD secondary antibodies (1:10,000, LI-COR). Protein expression was detected and analyzed using the Odyssey® Infrared Scanner (LI-COR).
Immunohistochemistry and quantification of immunofluorescence
Drosophila dissection and immunostaining of whole brains was conducted as previously described (57). Brains were dissected in 1x PBS containing 0.3% Tween-20 and subsequently fixed in 4% paraformaldehyde for 20 minutes and blocked in 5% normal goat serum for 30 minutes. Antibody to PDF (PDF C7) was obtained from the Developmental Studies Hybridoma Bank (1:1000, University of Iowa). Anti-mouse AlexaFluor 594 (1:500, ThermoFisher Scientific) was used to visualize protein expression. PDF-positive neuronal projections in the dorsal protocerebrum were visualized at 126x magnification on the Leica TCS SP5 confocal microscope and images were collected in 3 μM z-stacks. Individual z-stack images were analyzed using ImageJ. Quantification of fluorescently labeled synaptic projections was calculated using % area.
Reverse transcriptase PCR and product quantification
RNA was extracted from snap-frozen fly heads using the RNeasy Mini Kit (Qiagen). RNA concentrations were measured on the NanoDrop 2000 (ThermoFisher Scientific) and quantitative real-time PCR was conducted using the TaqMan® RNA-to-Ct™ 1-Step Kit (ThermoFisher Scientific) with the following primers: PERK (Dm02137033_g1), Act5C (Dm02361909_s1) and GAPDH (Hs02758991_g1) (ThermoFisher Scientific). Each biological sample consists of a pool of approximately 50 fly heads and values were calculated from the mean of 3 technical replicates per biological sample. Fold change in PERK expression was normalized to Act5c and GAPDH housekeeping controls.
QUANTIFICATION AND STATISTICAL ANALYSIS
Student’s t-test was conducted to compare drug and vehicle groups in the Drosophila sleep assays and IR-quantified protein concentrations. For assays comparing sleep across more than two conditions, comparisons were performed using one-way analysis of variance (ANOVA) followed by pairwise t-tests. For the analysis of Drosophila arousal threshold, Mann-Whitney U Test was applied to determine statistical significance of the difference between groups. Holm-Sidak correction for multiple comparisons was applied to all t-tests. For zebrafish behavior, D’Agostino & Pearson omnibus normality test was performed to assess normality, and non-parametric Mann-Whitney test was performed to assess statistical significance between treatment groups. Statistical analysis was conducted using GraphPad Prism software (La Jolla, CA, USA).
DATA AND CODE AVAILABILITY
The custom matlab and C+ code used to analyze Drosophila sleep data has been described pre viously [55]. This study did not generate large datasets. Further information and requests for experimental data should be directed to and be fulfilled by the Lead Contact, Nirinjini Naidoo (naidoo@pennmedicine.upenn.edu).
Supplementary Material
Highlights.
Pharmacologically inhibiting PERK reduces sleep in fruit flies and zebrafish
Genetically reducing PERK reduces sleep while increasing PERK increases sleep
PERK signaling regulates wake-promoting neuropeptide expression in fruit flies
Acknowledgments
The authors thank Sarah Hou and Sophie Leon for assistance in data collection. This study was funded by NIGMS (R01GM123783) to N.N.
Footnotes
Declaration of interests:
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Colten HR, Altevogt BM (2006). Institute of Medicine Committee on Sleep Medicine and Research. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 2.Schenck CH, Boeve BF, & Mahowald MW (2013). Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med, 14(8), 744–748. doi: 10.1016/j.sleep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Zhou J, Zhang J, Lam SP, Chan JW, Mok V, Chan A,… Wing YK. (2017). Excessive Daytime Sleepiness Predicts Neurodegeneration in Idiopathic REM Sleep Behavior Disorder. Sleep, 40(5). doi: 10.1093/sleep/zsx041. [DOI] [PubMed] [Google Scholar]
- 4.Ju YE, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP,… Holtzman DM. (2013). Sleep quality and preclinical Alzheimer disease. JAMA Neurol, 70(5), 587–593. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim AS, Kowgier M, Yu L, Buchman AS, & Bennett DA (2013). Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep, 36(7), 1027–1032. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hahn EA, Wang HX, Andel R, & Fratiglioni L (2014). A change in sleep pattern may predict Alzheimer disease. Am J Geriatr Psychiatry, 22(11), 1262–1271. doi: 10.1016/j.jagp.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR,… Holtzman DM. (2009). Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science, 326(5955), 1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothman SM, Herdener N, Frankola KA, Mughal MR, & Mattson MP (2013). Chronic mild sleep restriction accentuates contextual memory impairments, and accumulations of cortical Abeta and pTau in a mouse model of Alzheimer’s disease. Brain Res, 1529, 200–208. doi: 10.1016/j.brainres.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Meco A, Joshi YB, & Pratico D (2014). Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer’s disease with plaques and tangles. Neurobiol Aging, 35(8), 1813–1820. doi: 10.1016/j.neurobiolaging.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 10.Kurtishi A, Rosen B, Patil KS,, Alves GW, &Møller SG (2018). Cellular Proteostasis in Neurodegeneration. Mol Neurobiol. doi: 10.1007/s12035-018-1334-z. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 11.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M,… Nedergaard M (2013). Sleep drives metabolite clearance from the adult brain. Science, 342(6156), 373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson MD, Lee KH, Churgin MA, Hill AJ, Van Buskirk C, Fang-Yen C, & Raizen DM (2014). FMRFamide-like FLP-13 neuropeptides promote quiescence following heat stress in Caenorhabditis elegans. Curr Biol, 24(20):2406–10. doi: 10.1016/j.cub.2014.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill AJ, Mansfield R, Lopez JM, Raizen DM, & Van Buskirk C (2014). Cellular stress induces a protective sleep-like state in C. elegans. Curr Biol, 24(20):2399–405. doi: 10.1016/j.cub.2014.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vendruscolo M, Knowles TP, & Dobson CM (2011). Protein solubility and protein homeostasis: a generic view of protein misfolding disorders. Cold Spring Harb Perspect Biol, 3(12). doi: 10.1101/cshperspect.a010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ron D, & Walter P (2007). Signal integration in the endoplasmic reticulum unfolded pro tein response. Nat Rev Mol Cell Biol, 8(7), 519–529. doi: 10.1038/nrm2199 [DOI] [PubMed] [Google Scholar]
- 16.Cirelli C, & Tononi G (2000). Gene expression in the brain across the sleep-waking cycle. Brain Res, 885(2), 303–321. [DOI] [PubMed] [Google Scholar]
- 17.Cirelli C, Gutierrez CM, & Tononi G (2004). Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron, 41(1), 35–43. [DOI] [PubMed] [Google Scholar]
- 18.Terao A, Steininger TL, Hyder K, Apte-Deshpande A, Ding J, Rishipathak D,… Kilduff TS (2003). Differential increase in the expression of heat shock protein family members during sleep deprivation and during sleep. Neuroscience, 116(1), 187–200. [DOI] [PubMed] [Google Scholar]
- 19.Naidoo N, Giang W, Galante RJ, & Pack AI (2005). Sleep deprivation induces the unfolded protein response in mouse cerebral cortex. J Neurochem, 92(5), 1150–1157. doi: 10.1111/j.1471-4159.2004.02952.x. [DOI] [PubMed] [Google Scholar]
- 20.Naidoo N, Casiano V, Cater J, Zimmerman J, & Pack AI (2007). A role for the molecular chaperone protein BiP/GRP78 in Drosophila sleep homeostasis. Sleep, 30(5), 557–565. [DOI] [PubMed] [Google Scholar]
- 21.Jones S, Pfister-Genskow M, Cirelli C, & Benca RM. (2008). Changes in brain gene expression during migration in the white-crowned sparrow. Brain Res Bull, 76(5), 536–544. doi: 10.1016/j.brainresbull.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harding HP, Zhang Y, Bertolotti A, Zeng H, & Ron D (2000). Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell, 5(5), 897–904. [DOI] [PubMed] [Google Scholar]
- 23.Naidoo N, Ferber M, Master M, Zhu Y, & Pack AI (2008). Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. J Neurosci, 28(26):6539–48. doi: 10.1523/JNEUROSCI.5685-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown MK, Chan MT, Zimmerman JE, Pack AI, Jackson NE, & Naidoo N (2014). Aging induced endoplasmic reticulum stress alters sleep and sleep homeostasis. Neurobiol Aging, 35(6):1431–41. doi: 10.1016/j.neurobiolaging.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pestova TV, de Breyne S, Pisarev AV, Abaeva IS, & Hellen CUT (2008). eIF2-dependent and eIF2-independent modes of initiation on the CSFV IRES: a common role of domain II. EMBO J, 27(7), 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clemens M (1996). Protein kinases that phosphorylate eIF2 and eIF2B, their role in eukaryotic cell translational control. (139–172) Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 27.Methippara MM, Bashir T, Kumar S, Alam N, Szymusiak R, & McGinty D (2009). Salubrinal, an inhibitor of protein synthesis, promotes deep slow wave sleep. American journal of physiology. Regulatory, integrative and comparative physiology, 296(1), R178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Axten JM, Medina JR, Feng Y, Shu A, Romeril SP, Grant SW,… Gampe RT (2012). Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}−2,3-dihydro-1H-indol-5-yl)-7H-p yrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). J Med Chem, 55(16), 7193–7207. doi: 10.1021/jm300713s. [DOI] [PubMed] [Google Scholar]
- 29.Kim HJ, Raphael AR, LaDow ES, McGurk L, Weber RA, Trojanowski JQ,… Bonini NM (2014). Therapeutic modulation of eIF2alpha phosphorylation rescues TDP-43 toxicity in amyotrophic lateral sclerosis disease models. Nat Genet, 46(2), 152–160. doi: 10.1038/ng.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prober DA, Rihel J, Onah AA, Sung RJ, &Schier AF (2006). Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J Neurosci, 26(51), 13400–13410. doi: 10.1523/jneurosci.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee DA, Andreev A, Truong TV, Chen A, Hill AJ, Oikonomou G, Pham U, Hong YK, Tran S, Glass L,Sapin V, Engle J, Fraser SE, … Prober DA (2017). Genetic and neuronal regulation of sleep by neuropeptide VF. eLife, 6, e25727. doi: 10.7554/eLife.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJ,… Griffith LC. (2008). PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron, 60(4), 672–682. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muraro N, & Fernanda Ceriani M (2014). Circadian rhythms. In Dubnau J (Ed.), Behavioral Genetics of the Fly (Drosophila Melanogaster) (Cambridge Handbooks in Behavioral Genetics, pp. 104–115). Cambridge: Cambridge University Press. [Google Scholar]
- 34.Fernández MP, Berni J, & Ceriani MF (2008). Circadian remodeling of neuronal circuits involved in rhythmic behavior. PLoS Biol, 6(3):e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorostiza EA, Depetris-Chauvin A, Frenkel L, Pírez N, & Ceriani MF (2014). Circadian pacemaker neurons change synaptic contacts across the day. Curr Biol, 24(18):2161–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang X, Holy TE, & Taghert PH (2016). Synchronous Drosophila circadian pacemakers display nonsynchronous Ca(2)(+) rhythms in vivo. Science, 351(6276), 976–981. doi: 10.1126/science.aad3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaneko M, Hall JC (2000). Neuroanatomy of cells expressing clock genes in Drosophila: transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. J Comp Neurol, 422(1):66–94. doi: . [DOI] [PubMed] [Google Scholar]
- 38.Bushey D, Tononi G, & Cirelli C (2011). Sleep and synaptic homeostasis: structural evidence in Drosophila. Science, 332(6037):1576–81. doi: 10.1126/science.1202839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Vivo L, Bellesi M, Marshall W, Bushong EA, Ellisman MH, Tononi G, Cirelli C (2017). Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science. 355(6324):507–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang G, Lai CS, Cichon J, Ma L, Li W, & Gan WB (2014). Sleep promotes branch-specific formation of dendritic spines after learning. Science, 344(6188):1173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seibt J, Dumoulin MC, Aton SJ, Coleman T, Watson A, Naidoo N, & Frank MG (2012). Protein synthesis during sleep consolidates cortical plasticity in vivo. Curr Biol, 22(8):676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stahl BA, Slocumb ME, Chaitin H, DiAngelo JR, & Keene AC (2017). Sleep-Dependent Modulation of Metabolic Rate in Drosophila. Sleep. 2017 Aug 1;40(8). doi: 10.1093/sleep/zsx084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tudor JC, Davis EJ, Peixoto L, Wimmer ME, van Tilborg E, Park AJ, Poplawski SG, Chung CW, Havekes R, Huang J, Gatti E, Pierre P, … Abel T (2016). Sleep deprivation impairs memory by attenuating mTORC1-dependent protein synthesis. Science signaling, 9(425), ra41. doi: 10.1126/scisignal.aad4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Methippara MM, Alam MN, Kumar S, Bashir T, Szymusiak R, & McGinty D (2008). Administration of the protein synthesis inhibitor, anisomycin, has distinct sleep-promoting effects in lateral preoptic and perifornical hypothalamic sites in rats. Neuroscience, 151(1), 1–11. doi: 10.1016/j.neuroscience.2007.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakanishi H, Sun Y, Nakamura RK, Mori K, Ito M, Suda S,… Sokoloff, L. (1997). Positive correlations between cerebral protein synthesis rates and deep sleep in Macaca mulatta. Eur J Neurosci, 9(2):271–9. doi: 10.1111/j.1460-9568.1997.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 46.Shaw PJ, Tononi G, Greenspan RJ, & Robinson DF (2002) Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature, 417(6886):287–91. doi: 10.1038/417287a. [DOI] [PubMed] [Google Scholar]
- 47.Moreno JA, Halliday M, Molloy C, Radford H, Verity N, Axten JM,… Mallucci GR (2013). Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci Transl Med, 5(206), 206ra138. doi: 10.1126/scitranslmed.3006767. [DOI] [PubMed] [Google Scholar]
- 48.Radford H, Moreno JA, Verity N, Halliday M, & Mallucci GR (2015). PERK inhibition prevents tau-mediated neurodegeneration in a mouse model of frontotemporal dementia. Acta Neuropathol, 130(5), 633–642. doi: 10.1007/s00401-015-1487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halliday M, Radford H, Zents KAM, Molloy C, Moreno JA, Verity NC,… Mallucci GR. (2017). Repurposed drugs targeting eIF2α-P-mediated translational repression prevent neurodegeneration in mice. Brain, 140(6), 1768–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schenck CH, Boeve BF, & Mahowald MW (2013). Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. 2013 Aug;14(8):744–8. doi: 10.1016/j.sleep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 51.Ben-Zvi A, Miller EA, & Morimoto RI (2009). Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc Natl Acad Sci U S A, 106(35):14914–9. doi: 10.1073/pnas.0902882106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Solis GM, Kardakaris R, Valentine ER, Bar-Peled L, Chen AL,… Petrascheck M (2018). Translation attenuation by minocycline enhances longevity and proteostasis in old post-stress-responsive organisms. Elife, 7. pii: e40314. doi: 10.7554/eLife.40314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martínez G, Duran-Aniotz C, Cabral-Miranda F, Vivar JP, & Hetz C (2017). Endoplasmic reticulum proteostasis impairment in aging. Aging Cell, 16(4):615–623. doi: 10.1111/acel.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naidoo N, Zhu J, Zhu Y,, Fenik P, Lian J, Galante R, & Veasey S (2011). Endoplasmic reticulum stress in wake-active neurons progresses with aging. Aging Cell, 10(4):640–9. doi: 10.1111/j.1474-9726.2011.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zimmerman JE, Raizen DM, Maycock MH, Maislin G, & Pack AI (2008). A Video Method to Study Drosophila Sleep. Sleep, 31(11), 1587–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bi J, Sehgal A, Williams JA, & Wang YF (2018). Wolbachia affects sleep behavior in Drosophila melanogaster. J Insect Physiol, 107:81–88. doi: 10.1016/j.jinsphys.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 57.Wu JS, & Luo L (2006). A protocol for dissecting Drosophila melanogaster brains for live imaging or immunostaining. Nat Protoc, 1(4), 2110–2115. doi: 10.1038/nprot.2006.336. [DOI] [PubMed] [Google Scholar]
- 58.Luo W, Chen WF, Yue Z, Chen D, Sowcik M, Sehgal A, & Zheng X (2012). Old flies have a robust central oscillator but weaker behavioral rhythms that can be improved by genetic and environmental manipulations. Aging cell, 11(3), 428–438. doi: 10.1111/j.1474-9726.2012.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zars T, Wolf R, Davis R, & Heisenberg M (2000). Tissue-specific expression of a type I adenylyl cyclase rescues the rutabaga mutant memory defect: in search of the engram. Learning & memory (Cold Spring Harbor, N.Y.), 7(1), 18–31. doi: 10.1101/lm.7.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang L, Ryoo HD, Qi Y, & Jasper H (2015). PERK Limits Drosophila Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress. PLoS genetics, 11(5), e1005220. doi: 10.1371/journal.pgen.1005220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malzer E, Daly ML, Moloney A, Sendall TJ, Thomas SE, Ryder E, … Marciniak SJ (2010). Impaired tissue growth is mediated by checkpoint kinase 1 (CHK1) in the integrated stress response. Journal of cell science, 123(Pt 17), 2892–2900. doi: 10.1242/jcs.070078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bai L, Lee Y, Hsu CT, Williams JA, Cavanaugh D, Zheng X, … Sehgal A (2018). A Conserved Circadian Function for the Neurofibromatosis 1 Gene. Cell reports, 22(13), 3416–3426. doi: 10.1016/j.celrep.2018.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee DA, Andreev A, Truong TV, Chen A, Hill AJ, Oikonomou G, … Prober DA (2017). Genetic and neuronal regulation of sleep by neuropeptide VF. eLife, 6, e25727. doi: 10.7554/eLife.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The custom matlab and C+ code used to analyze Drosophila sleep data has been described pre viously [55]. This study did not generate large datasets. Further information and requests for experimental data should be directed to and be fulfilled by the Lead Contact, Nirinjini Naidoo (naidoo@pennmedicine.upenn.edu).