Abstract
Neutrophils migrate to sites of tissue damage, where they protect the host against pathogens. Often, the cost of these neutrophil defenses is collateral damage to healthy tissues. Thus, the immune system has evolved multiple mechanisms to regulate neutrophil migration. One of these mechanisms is reverse migration – the process whereby neutrophils leave the source of inflammation. In vivo, neutrophils arrive and depart the wound simultaneously – indicating that neutrophils dynamically integrate conflicting signals to engage in both forward and reverse migration. This finding is seemingly at odds with the established chemoattractant hierarchy in vitro, which places wound-derived signals at the top. Here we will discuss recent work that has uncovered key players involved in retaining and dispersing neutrophils from wounds. These findings offer the opportunity to integrate established and emerging mechanisms into a holistic model for neutrophil migration in vivo.
Keywords: chemotaxis, neutrophil, reverse migration
Introduction
Neutrophils are often the first immune cells to arrive at sites of injury and infection. There, they mount a potent defense response that must be tightly regulated to limit collateral damage to the host [1]. Indeed, excessive or inappropriate neutrophilic inflammation can tip the balance from host protection to autoimmunity and tissue damage [1]. Neutrophils and other leukocytes exhibit complex decision making within interstitial tissues in response to various cues to maintain tissue homeostasis and enable tissue repair.
An example of this complex decision making is the neutrophil response to sterile tissue injury. Until recently, neutrophil recruitment to injury sites was considered unidirectional - with neutrophils undergoing apoptosis and subsequent clearance by macrophages at the wound [2]. However, it is increasingly evident that a subset of neutrophils leave inflamed tissues and re-enter the circulation [3–6]. The reverse migration and reverse transmigration of neutrophils into the vasculature have now been described in zebrafish, mice, and humans, suggesting they play a critical role in local inflammation resolution [3–6]. Indeed, in addition to clearing apoptotic neutrophils, macrophages have also been reported to repel wound-associated neutrophils [7,8] or “cloak” sites of sterile damage, thereby limiting chemoattractant signaling and neutrophil inflammation [9•].
The mechanism underlying the prioritization between tissue damage cues and reverse migration is not fully understood. In part, this is due to the daunting complexity of the physical and chemical landscape neutrophils encounter in vivo [10,11]. Furthermore, the signals that regulate reverse migration are not well-defined. Recent work in vivo and in vitro has just begun to uncover some of the molecular players and signaling pathways that regulate reverse migration. Here, we discuss how neutrophils prioritize chemoattractant signals in vitro and more complex in vivo contexts and discuss how these mechanisms may provide insight into the complex prioritization needed to reverse migrate and resolve a local response.
Reverse neutrophil migration conundrum: The end is not always the end
Neutrophils are inherently motile cells that migrate randomly (chemokinesis) and directionally (chemotaxis) in response to numerous chemical signals [12–14]. During episodes of inflammation, a torrent of overlapping directional cues guide neutrophils toward the afflicted tissue (Figure 1A–D) [10,11]. To effectively reach their target, they must integrate and prioritize these signals [15]. Indeed, there is a clear hierarchy of recruitment signals. Early studies in vitro revealed that attractants emanating from the wound, such as formylated peptides and complement components, take precedence over long-range signals like Interleukin-8 (IL-8, also CXCL8) and Leukotriene B4 (LTB4) (Figure 1C) [15–18]. This observation led to the classification of chemoattractants by their prioritization status - with high-priority signals termed “end-target” attractants and low-priority signals designated as “intermediary” attractants [15].
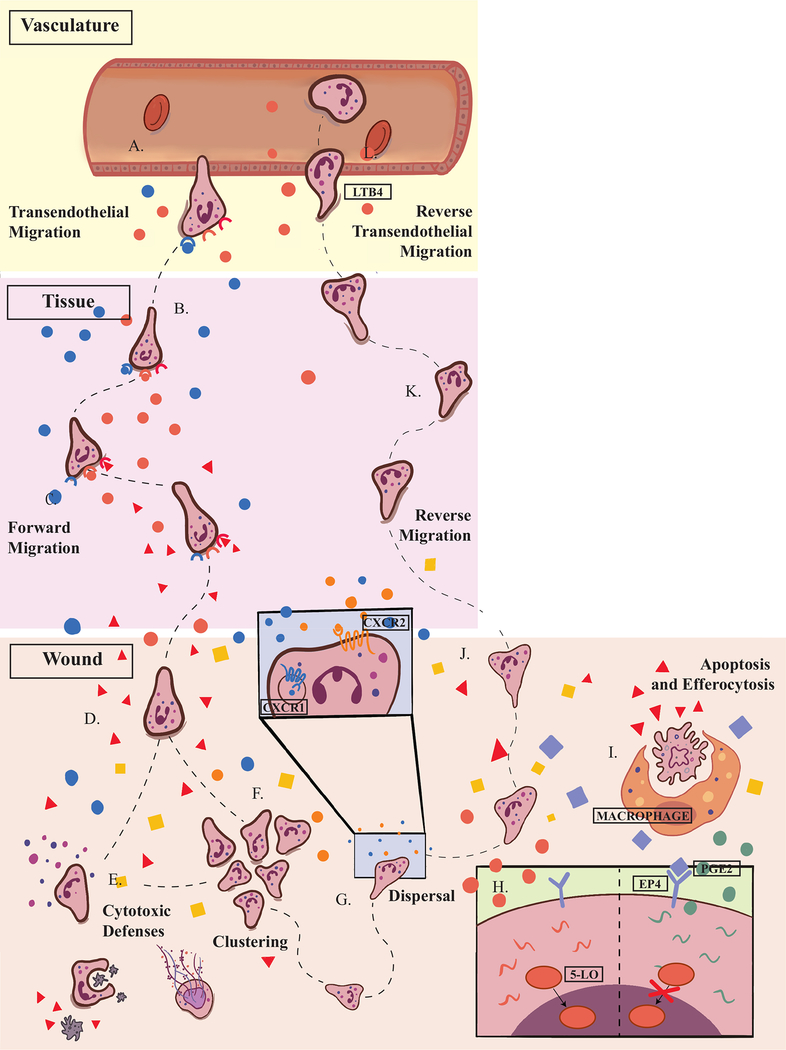
Figure 1. Neutrophils encounter complex signaling environments during forward and reverse migration in vivo.
A. When tissue damage is detected, neutrophils migrate out of the vasculature in a process called transendothelial migration B. In the tissues, neutrophils engage in multi-step navigation toward sources of intermediary chemoattractants. C. Once neutrophils detect wound-derived, end-target signals, they ignore intermediary attractants. D. In the wound, neutrophils integrate multiple signals, including recruitment and retention signals, pro-inflammatory and anti-inflammatory molecules. E. Neutrophil clustering is mediated by the CXCL1/CXCL8 signaling axis F. At the wound, neutrophils mount a cytotoxic response that includes degranulation, phagocytosis, and NET formation. G. In the wound, CXCR1 is rapidly internalized, leaving CXCR2 as the primary CXCL8 receptor. CXCR2 promotes cluster dissolution. H. Macrophages secrete PGE2, which activates neutrophil EP4 receptors preventing 5-LO translocation into the nucleus. As a result, neutrophils switch from the production of LTB4 to the production of LXA4. I. Some neutrophils undergo apoptosis and efferocytosis by macrophages. J. After overcoming recruitment and retention signals, some neutrophils are randomly dispersed from the wound microenvironment. K. Once they leave the wound, neutrophils reverse migrate back toward the vasculature. L. LTB4 mediates rTEM allowing neutrophils re-enter circulation.
CXCL8 
LTB4 
End-Target Attractant 
Retention Signal 
PGE2 
These terms were coined when the wound was considered neutrophils’ final destination, however now we know this is not true. Interestingly, in vivo, neutrophils can be seen arriving and departing the wound simultaneously (Figure 1D,J)[3] - suggesting recruitment signals are still present when neutrophils leave. It is unclear how the chemoattractant hierarchy plays out in damaged interstitial tissue highlighting the need for an updated model of prioritization that accounts for the fact that the end is not always the end.
To begin addressing this conundrum, we consider prioritization mechanisms in vitro and in vivo during the recruitment phase of inflammation and what they might tell us about the integration and prioritization of signals at the wound.
Attractant detection systems in neutrophils
Scientists first described neutrophils’ ability to migrate directionally toward chemical attractants in the late 19th century [19]. It would take almost 100 years before we began to understand the complexity and sophistication of their navigational system. In the 1970s, seminal work distinguished neutrophils’ attractant sensing mechanism from that of bacteria [12,20]. Bacteria employ temporal sensing in which chemoattractant concentration is compared over time. When higher concentrations of attractant are detected, the length of the subsequent “run” increases, resulting in biased random movement up the gradient [21]. In contrast, neutrophils can compare receptor occupancy levels across their length with astounding sensitivity [12,22]. These spatial calculations result in dynamic polarization of intracellular signaling and motility components, giving rise to neutrophils’ ability to steer toward a gradient source [23–26].
The polarization of intracellular signaling molecules presents a challenge for neutrophils that detect end-target and intermediary attractants on opposing sides. Indeed, in vitro studies using human neutrophils revealed that in competing gradients of end-target and intermediary attractants, PTEN, a negative regulator of actin polymerization, becomes uniformly distributed on the plasma membrane. In this case, end-target signals can activate an alternative motility program mediated by the MAPK, p38 – allowing them to migrate toward the end-target source [16,17].
More recently, multiple groups have observed temporal sensing by human and mouse neutrophils in vitro, which raises the possibility that differences in sensing strategies between high and low-priority chemoattractant receptors might contribute to some signals’ dominance over others [22,27–29]. Furthermore, Chandrasekaran et al. propose an intriguing model in which neutrophils initially engage in spatial sensing but require temporal gradients for persistent migration toward fMLF. The authors suggest that switching between these two sensing mechanisms supports robust recruitment while, at the same time, limiting neutrophil accumulation [22].
Receptor-level regulation of motility in forward and reverse migration
In addition to inherent differences in sensing mechanisms, chemoattractant receptors are also subject to variations in receptor-level regulation. In neutrophils, the vast majority of chemoattractants are detected by G-protein-coupled receptors (GPCRs) [30–32]. Typically, GPCR phosphorylation results in desensitization and/or receptor endocytosis [14,30–32]. While most chemoattractant receptors undergo homologous desensitization, end-target signals also impose heterologous desensitization on intermediary attractant receptors [14,33,34]. As a result, neutrophils become ignorant to intermediary signals in the presence of end-target attractants (Figure 1C) [14,35,36].
Of course, receptor inactivation is not always permanent. Internalized receptors can be degraded or separated from their ligand and recycled [37]. Trafficking dynamics vary between chemoattractant receptors. For example, in migrating neutrophil-like PLB985 cells, internalized c5a receptor 1 is degraded, while FPR1 is internalized from the cell rear and shuttled back to the plasma membrane [38]. The same study revealed that LTB4R is resistant to internalization and that mutations that affect LTB4R trafficking disrupt neutrophil migration toward fMLF, suggesting that like desensitization, receptor trafficking is subject to cross-talk.
These receptor-level regulation mechanisms can be enacted and reversed relatively quickly compared to responses that require transcription, for example. It is no surprise then that these mechanisms are implicated in inflammation resolution – where neutrophils go forward to the wound and then reverse migrate. CXCR1 and CXCR2 both bind variants of CXCL8 (IL8), have redundant roles in neutrophil recruitment but promote cell clustering and dispersal in wounds, respectively (Figure 1E,G) [39,40••]. Specifically, zebrafish CXR2 mediates neutrophil chemokinesis at the wound and subsequent reverse migration (Figure 1J) [39]. Differences in trafficking dynamics between these receptors ensure these functions happen in sequence. In the wound, activated CXCR1 is rapidly internalized, whereas CXCR2 persists on the plasma membrane [40••]. This persistence enables sustained signaling by CXCR2, which supports the dissolution of neutrophil clusters and chemokinesis (Figure 1E,G) [40••]. Indeed, in zebrafish, loss of CXCR2 results in neutrophil retention at the wound [39,40••]. Interestingly, activated CXCR2 is rapidly internalized outside of the wound microenvironment – making it a particularly compelling example of how receptor-level regulation can enable context-specific responses.
So far, we have considered two examples that point to a loss of directed motility as an essential mechanism for overcoming recruitment signals. First, a reliance on temporal sensing for continued migration toward end-target signals may limit neutrophil accumulation once the concentration of damage signals are no longer rising [22]. Second, by regulating the time and place in which chemoattractant receptors function, neutrophils can become unresponsive to signals that undermine their transition to the resolution phase [40••,41]. In agreement with a random component of the resolution phase of acute injury has been work from several groups using mathematical modeling to analyze neutrophil migration patterns away from the wound (Figure 1J) [42–44]. They found that the experimental observations of neutrophils leaving the wound are better captured by a model in which neutrophils randomly diffuse from the wound as opposed to one in which directive signals are included [42]. Importantly, neutrophil velocity and directionality are comparable in neutrophils migrating to and from a wound, suggesting reverse migration is supported by directional cues outside of the wound [3,6]. It is possible that initially, neutrophils stop responding to recruitment signals resulting in random migration and dispersal before becoming engaged by signals that lead them back to the vasculature. Identifying purported vascular homing signals is an ongoing area of investigation.
Overcoming retention signals
In vivo, where the chemical and physical landscapes are very complex, it is likely that numerous signaling events and processes coordinate to regulate reverse migration and local resolution. In addition to recruitment signals, neutrophils must also overcome retention signals to leave the wound. Of course, both continued neutrophil recruitment and retention are important until host defenses have gained adequate control of an infection or injury. Thus, when functioning properly, many retention signals likely indicate an active threat.
Recently, several studies have identified molecules that promote neutrophil retention in wounds (Figure 1D,J) [45••–49••]. These findings are of particular clinical interest as persistent neutrophilic inflammation precludes proper wound healing and is associated with a number of inflammatory conditions [1]. Hypoxia-inducible factor 1 is a transcription factor expressed in activated neutrophils and macrophages. HIF-1a is targeted for degradation by oxygen-dependent prolyl hydroxylases (PHDs) [47]. During hypoxia or in response to bacterial infections under normoxic conditions, PHD activity is suppressed, resulting in HIF-1 accumulation[50,51]. Activated HIF-1a stimulates pathways that promote neutrophil survival and defense functions. In tail transected zebrafish, neutrophils expressing constitutively active HIF-1a continuously surveil the wound even during the resolution phase suggesting that negative regulation of retention signals is an important mechanism for resolving neutrophilic inflammation [47].
Disrupting the CXCR4/CXCL12 signaling axis accelerates neutrophilic inflammation resolution by increasing reverse migration [45••]. Interestingly, CXCR4 also plays a role in retaining neutrophils in the bone marrow [52,53]. In this context, neutrophil retention is dependent on the small GTPase, Rac2, which regulates F-actin assembly and cell polarization (among other things) [52]. The finding that CXCR4 governs neutrophil retention in hematopoietic tissue (limiting inflammation) and at wounds (promoting inflammation) is interesting as it suggests its role in neutrophil retention may be more general.
On the other hand, in contrast to Rac2, which promotes F-actin assembly, sema3f was recently identified as a neutrophil retention signal that acts by inducing F-actin disassembly resulting in reduced migration velocity [49••]. Neutrophil-specific depletion of sema3f in zebrafish and Sema3f in mice accelerated neutrophil egress from tail wounds and sites of acutely inflamed lungs, respectively [49••].
Lipid signaling in reverse migration
Leukotriene B4 is a well-characterized chemoattractant that has evolved specialized roles in both forward and reverse neutrophil migration. LTB4 is one of many derivatives of arachidonic acid produced and secreted by neutrophils during inflammation [51]. Neutrophil release of LTB4 helps recruit other neutrophils by amplifying chemoattractant gradients and enhancing migration toward fMLF [51,54]. In mice, LTB4 is required for the formation of neutrophil swarms [55].
At the wound, neutrophils shift from the production of pro-inflammatory LTB4 to Lipoxin A4, an anti-inflammatory derivative of arachidonic acid (Figure 1H) [56,57]. In neutrophils, macrophage-derived Prostaglandin E2 (PGE) inhibits the translocation of 5-LO, a critical enzyme required for LTB4 synthesis, from the cytoplasm to the nucleus [57]. As a result, LXA4 may become the predominant lipid produced from arachidonic acid at wounds, and reverse migration would become the favored outcome, promoting inflammation resolution.
Intriguingly, in mice, LTB4 plays a role in reverse transendothelial migration (rTEM) - the process by which neutrophils cross the blood vessel lumen to re-enter the circulation [4,58]. Endogenously produced LTB4 promotes neutrophil rTEM by stimulating neutrophil-elastase mediated cleavage of vascular Junctional Adhesion Molecule C (JAM-C) (Figure 1L) [4,58]. It will be interesting to learn whether neutrophils revert to LTB4 production or whether LTB4 is produced by other cells near the vasculature.
Emerging mechanisms
While neutrophils’ attractant detection systems are sophisticated, there are boundaries within which they can successfully operate. For example, spatial sensing requires an adequately steep gradient (~2–5% difference in ligand concentration across the cell length) and temporal sensing fails when attractant concentrations are no longer rising [12,22,29]. Given the long distances they travel and the reliability with which neutrophils reach their targets in vivo, there must be mechanisms in place to ensure that chemoattractant fields are maintained within these bounds. In some cases, neutrophils themselves play an active role in shaping gradient fields. Neutrophils amplify chemoattractant gradients by producing and secreting LTB4, which also enhances neutrophil migration toward fMLF [38,51,54,59].
More recently, “self-generated gradients,” whereby cells create or sharpen attractant gradients by sequestering receptor-bound ligands or via attractant degradation, are emerging as an important mechanism for maintaining optimal attractant concentration [60–65••,66•] [60–62•]. Indeed, chemotaxis along self-generated gradients has been observed both in vitro and in vivo in various contexts, including nutrient acquisition, embryogenesis, and cancer cell metastasis [60,61,67]. Although this mechanism seems more applicable to collective cell migration, individual yeast cells enhance their ability to find mating partners by degrading and thereby sharpening pheromone gradients suggesting such a phenomenon is possible at the single-cell level [68]. In the context of neutrophil migration, the role of self-generated gradients remains to be determined. It is particularly intriguing to consider the role of self-generated gradients in cancer-cell dissemination from tumors as a similar mechanism may help explain neutrophil dispersal from wounds [61]. In any case, future studies on neutrophil migration should account for the role of neutrophils in gradient formation and/or degradation in design and interpretation.
Conclusion
There is a famous adage attributed to Aristotle: “The more you learn, the less you know.” Over a century of study on leukocyte migration has produced a wealth of knowledge on the fundamental principles of cellular navigation. Reductionist, in vitro approaches have allowed us to define the basic algorithms that govern signal integration in neutrophils. Through more complex studies, we have come to appreciate that neutrophil intrinsic mechanisms (e.g., dynamic regulation of attractant receptors and signaling pathways) work in concert with extrinsic signals (damage and retention signals) to ensure neutrophils carry out their functions at the right place and right time. At the same time, we recognize that in vivo, the signaling landscapes are dynamic, imprecise, and subject to randomness that can be difficult to predict or measure. This imprecision can go awry and in some cases, neutrophil reverse migration can contribute to human diseases, like lupus [69•]. We may feel like we know less, but we appreciate the complexity that inspires us to learn more.
Highlights.
Neutrophil reverse migration has emerged as a critical component of inflammation resolution
In the wound microenvironment, neutrophils must integrate and prioritize among pro-inflammatory recruitment and retention signals and anti-inflammatory dispersal signals
Recently, key molecular players and signaling pathways regulating reverse migration have provided insights into the mechanisms underlying these complex signaling decisions
In combination with what we know about prioritization during the recruitment phase of inflammation, a more holistic model of signal integration in migrating cells is emerging
Acknowledgments:
This work was supported by National Institutes of Health Grants R35 GM118027 to AH and Hematology T32 to HL07899 BRG.
Footnotes
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kolaczkowska E, Kubes P: Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013, 13:159–175. [DOI] [PubMed] [Google Scholar]
- 2.Savill J, Fadok V: Corpse clearance defines the meaning of cell death. Nature 2000, 407:784–788. [DOI] [PubMed] [Google Scholar]
- 3.Mathias JR, Perrin BJ, Liu T-X, Kanki J, Look AT, Huttenlocher A: Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J Leukoc Biol 2006, 80:1281–1288. [DOI] [PubMed] [Google Scholar]
- 4.Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, Diapouli FM, Nash GB, Chavakis T, Albelda SM, Rainger GE, et al. : The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol 2011, 12:761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckley CD, Ross EA, McGettrick HM, Osborne CE, Haworth O, Schmutz C, Stone PCW, Salmon M, Matharu NM, Vohra RK, et al. : Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J Leukoc Biol 2006, 79:303–311. [DOI] [PubMed] [Google Scholar]
- 6.Hamza B, Wong E, Patel S, Cho H, Martel J, Irimia D: Retrotaxis of human neutrophils during mechanical confinement inside microfluidic channels. Integr Biol (United Kingdom) 2014, 6:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tauzin S, Starnes TW, Becker FB, Pying Lam, Huttenlocher A: Redox and Src family kinase signaling control leukocyte wound attraction and neutrophil reverse migration. J Cell Biol 2014, 207:589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miskolci V, Klemm LC, Huttenlocher A: Cell Migration Guided by Cell–Cell Contacts in Innate Immunity. Trends Cell Biol 2021, 31:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Uderhardt S, Martins AJ, Tsang JS, Lämmermann T, Germain RN: Resident Macrophages Cloak Tissue Microlesions to Prevent Neutrophil-Driven Inflammatory Damage. Cell 2019, 177:541–555.e17.30955887 •An interesting paper that provides insight into how the immune system distinguishes between basal levels of cell death and excessive cell death associated with tissue injury. The findings demonstrate that macrophages cloak tissue lesions and prevent neutrophil inflammation.
- 10.Sarris M, Sixt M: Navigating in tissue mazes: Chemoattractant interpretation in complex environments. Curr Opin Cell Biol 2015, 36:93–102. [DOI] [PubMed] [Google Scholar]
- 11.De Oliveira S, Rosowski EE, Huttenlocher A: Neutrophil migration in infection and wound repair: Going forward in reverse. Nat Rev Immunol 2016, 16:378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zigmond SH: Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. J Cell Biol 1977, 75:606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR: Cell Migration: Integrating Signals from Front to Back. Science (80- ) 2003, 302:1704–1709. [DOI] [PubMed] [Google Scholar]
- 14.Lämmermann T, Kastenmüller W: Concepts of GPCR-controlled navigation in the immune system. Immunol Rev 2019, 289:205–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foxman EF, Campbell JJ, Butcher EC: Multistep Navigation and the Combinatorial Control of Leukocyte Chemotaxis. 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heit B, Tavener S, Raharjo E, Kubes P: An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J Cell Biol 2002, 159:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heit B, Robbins SM, Downey CM, Guan Z, Colarusso P, Miller JB, Jirik FR, Kubes P: PTEN functions to “prioritize” chemotactic cues and prevent “distraction” in migrating neutrophils. Nat Immunol 2008, 9:743–752. [DOI] [PubMed] [Google Scholar]
- 18.Kim D, Haynes CL: Neutrophil chemotaxis within a competing gradient of chemoattractants. Anal Chem 2012, 84:6070–6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearson JD: How early studies of inflammation led to our current views on the roles of vascular adhesion molecules. In Vascular Adhesion Molecules and Inflammation.. Birkhäuser Basel; 1999:1–10. [Google Scholar]
- 20.Zigmond SH: Mechanisms of sensing chemical gradients by polymorphonuclear leukocytes. Nature 1974, 249:450–452. [DOI] [PubMed] [Google Scholar]
- 21.Block SM, Segall JE, Berg HC: Impulse responses in bacterial chemotaxis. Cell 1982, 31:215–226. [DOI] [PubMed] [Google Scholar]
- 22.Chandrasekaran A, Ellett F, Jorgensen J, Irimia D: Temporal gradients limit the accumulation of neutrophils toward sources of chemoattractant. Microsystems Nanoeng 2017, 3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiner OD: Regulation of cell polarity during eukaryotic chemotaxis: The chemotactic compass. Curr Opin Cell Biol 2002, 14:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang HW, Collins SR, Meyer T: Locally excitable Cdc42 signals steer cells during chemotaxis. Nat Cell Biol 2016, 18:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graziano BR, Weiner OD: Self-organization of protrusions and polarity during eukaryotic chemotaxis. Curr Opin Cell Biol 2014, 30:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gambardella L, Vermeren S: Molecular players in neutrophil chemotaxis-focus on PI3K and small GTPases. J Leukoc Biol 2013, 94:603–612. [DOI] [PubMed] [Google Scholar]
- 27.Irimia D: Microfluidic technologies for temporal perturbations of chemotaxis. Annu Rev Biomed Eng 2010, 12:259–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aranyosi AJ, Wong EA, Irimia D: A neutrophil treadmill to decouple spatial and temporal signals during chemotaxis. Lab Chip 2015, 15:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrie Aronin CE, Zhao YM, Yoon JS, Morgan NY, Prüstel T, Germain RN, Meier-Schellersheim M: Migrating Myeloid Cells Sense Temporal Dynamics of Chemoattractant Concentrations. Immunity 2017, 47:862–874.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metzemaekers M, Gouwy M, Proost P: Neutrophil chemoattractant receptors in health and disease: double-edged swords. Cell Mol Immunol 2020, 17:433–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Futosi K, Fodor S, Mócsai A: Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol 2013, 17:638–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cotton M, Claing A: G protein-coupled receptors stimulation and the control of cell migration. Cell Signal 2009, 21:1045–1053. [DOI] [PubMed] [Google Scholar]
- 33.Tomhave ED, Richardson RM, Didsbury JR, Menard L, Snyderman R, Ali H: Cross-desensitization of receptors for peptide chemoattractants. Characterization of a new form of leukocyte regulation. J Immunol 1994, 153. [PubMed] [Google Scholar]
- 34.Ali H, Richardson RM, Haribabu B, Snyderman R: Chemoattractant receptor cross-desensitization. J Biol Chem 1999, 274:6027–6030. [DOI] [PubMed] [Google Scholar]
- 35.Lin F, Butcher EC: Modeling the Role of Homologous Receptor Desensitization in Cell Gradient Sensing. J Immunol 2008, 181:8335–8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu D, Lin F: Modeling cell gradient sensing and migration in competing Chemoattractant fields. PLoS One 2011, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borroni EM, Mantovani A, Locati M, Bonecchi R: Chemokine receptors intracellular trafficking. Pharmacol Ther 2010, doi: 10.1016/j.pharmthera.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Subramanian BC, Moissoglu K, Parent CA: The LTB4-BLT1 axis regulates the polarized trafficking of chemoattractant GPCRs during neutrophil chemotaxis. J Cell Sci 2018, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powell D, Tauzin S, Hind LE, Deng Q, Beebe DJ, Huttenlocher A: Chemokine Signaling and the Regulation of Bidirectional Leukocyte Migration in Interstitial Tissues. Cell Rep 2017, 19:1572–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Coombs C, Georgantzoglou A, Walker HA, Patt J, Merten N, Poplimont H, Busch-Nentwich EM, Williams S, Kotsi C, Kostenis E, et al. : Chemokine receptor trafficking coordinates neutrophil clustering and dispersal at wounds in zebrafish. Nat Commun 2019, 10. ••Using a zebrafish wound model and fluorescent timers, the authors reveal that neutrophil clustering and dispersal are regulated by differences in receptor trafficking between CXCR1 and CXCR2. This study also clarifies the role each receptor plays in the recruitment and resolution phases of inflammation.
- 41.Subramanian BC, Moissoglu K, Parent CA: The LTB4–BLT1 axis regulates the polarized trafficking of chemoattractant GPCRs during neutrophil chemotaxis. J Cell Sci 2018, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holmes GR, Dixon G, Anderson SR, Reyes-Aldasoro CC, Elks PM, Billings SA, Whyte MKB, Kadirkamanathan V, Renshaw SA: Drift-diffusion analysis of neutrophil migration during inflammation resolution in a zebrafish model. Adv Hematol 2012, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarris M, Masson JB, Maurin D, Van Der Aa LM, Boudinot P, Lortat-Jacob H, Herbomel P: Inflammatory Chemokines Direct and Restrict Leukocyte Migration within Live Tissues as Glycan-Bound Gradients. Curr Biol 2012, 22:2375–2382. [DOI] [PubMed] [Google Scholar]
- 44.Nourshargh S, Renshaw SA, Imhof BA: Reverse Migration of Neutrophils: Where, When, How, and Why? Trends Immunol 2016, 37:273–286. [DOI] [PubMed] [Google Scholar]
- 45. Isles HM, Herman KD, Robertson AL, Loynes CA, Prince LR, Elks PM, Renshaw SA: The CXCL12/CXCR4 Signaling Axis Retains Neutrophils at Inflammatory Sites in Zebrafish. Front Immunol 2019, 10:1784.31417560 ••Genetic depletion and pharmacological inhibition of the CXCL12/CXCR4 signaling axis accelerates neutrophilic inflammation resolution by increasing reverse migration. This paper highlights the pro-inflammatory and anti-inflammatory roles CXCR4 plays in neutrophilic inflammation.
- 46.Robertson AL, Holmes GR, Bojarczuk AN, Burgon J, Loynes CA, Chimen M, Sawtell AK, Hamza B, Willson J, Walmsley SR, et al. : A zebrafish compound screen reveals modulation of neutrophil reverse migration as an anti-inflammatory mechanism. Sci Transl Med 2014, 6:225ra29–225ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elks PM, Van Eeden FJ, Dixon G, Wang X, Reyes-Aldasoro CC, Ingham PW, Whyte MKB, Walmsley SR, Renshaw SA: Activation of hypoxia-inducible factor-1α (hif-1α) delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood 2011, 118:712–722. [DOI] [PubMed] [Google Scholar]
- 48.Ellett F, Elks PM, Robertson AL, Ogryzko NV, Renshaw SA: Defining the phenotype of neutrophils following reverse migration in zebrafish. J Leukoc Biol 2015, 98:975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Plant T, Eamsamarng S, Sanchez-Garcia MA, Reyes L, Renshaw SA, Coelho P, Mirchandani AS, Morgan JM, Ellett FE, Morrison T, et al. : Semaphorin 3F signaling actively retains neutrophils at sites of inflammation. J Clin Invest 2020, 130:3221–3237.32191647 ••This paper describes a novel role for the axon-guidance molecule, Semaphorin 3F, in retaining neutrophils at sites of tissue injury in a zebrafish. Semaphorin 3F promotes F-actin disassembly in neutrophils, resulting in slower migration.
- 50.Kaelin WG, Ratcliffe PJ: Oxygen Sensing by Metazoans: The Central Role of the HIF Hydroxylase Pathway. Mol Cell 2008, 30:393–402. [DOI] [PubMed] [Google Scholar]
- 51.Afonso P V, Janka-Junttila M, Lee YJ, McCann CP, Oliver CM, Aamer KA, Losert W, Cicerone MT, Parent CA: LTB4 Is a Signal-Relay Molecule during Neutrophil Chemotaxis. Dev Cell 2012, 22:1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deng Q, Yoo SK, Cavnar PJ, Green JM, Huttenlocher A: Dual Roles for Rac2 in Neutrophil Motility and Active Retention in Zebrafish Hematopoietic Tissue. Dev Cell 2011, 21:735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eash KJ, Means JM, White DW, Link DC: CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood 2009, 113:4711–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Subramanian BC, Majumdar R, Parent CA: The role of the LTB4-BLT1 axis in chemotactic gradient sensing and directed leukocyte migration. Semin Immunol 2017, 33:16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lämmermann T, Afonso PV, Angermann BR, Wang JM, Kastenmüller W, Parent CA, Germain RN: Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 2013, 498:371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN: Lipid mediator class switching during acute inflammation: Signals in resolution. Nat Immunol 2001, 2:612–619. [DOI] [PubMed] [Google Scholar]
- 57.Loynes CA, Lee JA, Robertson AL, Steel MJG, Ellett F, Feng Y, Levy BD, Whyte MKB, Renshaw SA: PGE2 production at sites of tissue injury promotes an anti-inflammatory neutrophil phenotype and determines the outcome of inflammation resolution in vivo. Sci Adv 2018, 4:eaar8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nourshargh S, Alon R: Leukocyte Migration into Inflamed Tissues. Immunity 2014, 41:694–707. [DOI] [PubMed] [Google Scholar]
- 59.Majumdar R, Tavakoli Tameh A, Parent CA: Exosomes Mediate LTB4 Release during Neutrophil Chemotaxis. PLoS Biol 2016, 14:e1002336. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Donà E, Barry JD, Valentin G, Quirin C, Khmelinskii A, Kunze A, Durdu S, Newton LR, Fernandez-Minan A, Huber W, et al. : Directional tissue migration through a self-generated chemokine gradient. Nature 2013, 503:285–289. [DOI] [PubMed] [Google Scholar]
- 61.Muinonen-Martin AJ, Susanto O, Zhang Q, Smethurst E, Faller WJ, Veltman DM, Kalna G, Lindsay C, Bennett DC, Sansom OJ, et al. : Melanoma Cells Break Down LPA to Establish Local Gradients That Drive Chemotactic Dispersal. PLoS Biol 2014, 12:e1001966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tweedy L, Susanto O, Insall RH: Self-generated chemotactic gradients - cells steering themselves. Curr Opin Cell Biol 2016, 42:46–51. [DOI] [PubMed] [Google Scholar]
- 63.Tweedy L, Knecht DA, Mackay GM, Insall RH: Self-Generated Chemoattractant Gradients: Attractant Depletion Extends the Range and Robustness of Chemotaxis. PLoS Biol 2016, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Susanto O, Koh YWH, Morrice N, Tumanov S, Thomason PA, Nielson M, Tweedy L, Muinonen-Martin AJ, Kamphorst JJ, Mackay GM, et al. : LPP3 mediates self-generation of chemotactic LPA gradients by melanoma cells. J Cell Sci 2017, 130:3455–3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tweedy L, Insall RH: Self-Generated Gradients Yield Exceptionally Robust Steering Cues. Front Cell. Dev Biol 2020, 8:133.32195256 •Using a combination of mathematical modeling and under agarose assays using Dictyostelium, the authors offer an experimentally supported discussion of how self-generated gradients enhance chemotaxis in challenging chemical contexts.
- 66. Tweedy L, Thomason PA, Paschke PI, Martin K, Machesky LM, Zagnoni M, Insall RH: Seeing around corners: Cells solve mazes and respond at a distance using attractant breakdown. Science (80- ) 2020, 369. ••A fun complement to reference 65, this paper challenges Dictyostelium and cancer cells with a series of complex mazes - putting their steering capabilities to the test and demonstrating how self-generated gradients enhance directional migration.
- 67.Insall R, Andrew N: Chemotaxis in Dictyostelium: how to walk straight using parallel pathways. Curr Opin Microbiol 2007, 10:578–581. [DOI] [PubMed] [Google Scholar]
- 68.Moore TI, Chou CS, Nie Q, Jeon NL, Yi TM: Robust spatial sensing of mating pheromone gradients by yeast cells. PLoS One 2008, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Skopelja-Gardner S, Tai J, Sun X, Tanaka L, Kuchenbecker JA, Snyder JM, Kubes P, Mustelin T, Elkon KB. Acute skin exposure to ultraviolet light triggers neutrophil-mediated kidney inflammation. Proc Natl Acad Sci USA 2021. 118 (3) e2019097118.33397815 ••This study shows that neutrophils reverse transmigrated from inflamed skin to the kidney following UV exposure in mouse models, with important implications to lupus.