Abstract
Coral reefs have been heavily impacted by anthropogenic stressors, such as global warming, ocean acidification, sedimentation, and nutrients. Recently, microplastics (MP) have emerged as another potential stressor that may also cause adverse impacts to coral. MP ingestion by scleractinian coral among four species, Acropora cervicornis, Montastraea cavernosa, Orbicella faveolata, and Pseudodiploria clivosa, was used to identify the relationship between calyx and MP size as it pertains to active coral ingestion. A range of MP sizes (0.231–2.60 mm) were offered to the coral species across a wide range of calyx sizes (1.33–4.84 mm). Laboratory data showed that as the mean calyx size increased, so too did the mean percent of ingestion with increasing MP size. From laboratory data, a logistic model was developed to extrapolate the range of MP sizes that can be actively ingested by coral species based on calyx size. The data and model presented here offer the first predictive approach that can be used to determine the range of MP sizes that have a high likelihood of being actively ingested by coral of various sizes, thus offering insight to possible impacts on scleractinian coral.
Keywords: microplastic, coral, polyp, ingestion, model
Graphical Abstract
1. Introduction
Plastic pollution in the environment is an emerging concern due to its ubiquity and its potential to adversely impact wildlife (Rochman et al., 2016, Law, 2017, Li et al., 2020, Huang et al. 2021a). In 2016, 335 million tons of plastic were produced (Plastics Europe, 2017) and upwards of 23 million metric tons entered aquatic habitats that year (Borelle et al., 2020). In the marine environment, microplastics (MP) (plastics <5 mm) have been found in nearly all habitats, including polar ice and deep-sea habitats (Jamieson et al., 2019, Kanhai et al., 2019, Kelly et al., 2020, Tekman et al., 2020). As MPs enter the marine environment, their hydrophobic surface attracts microbes that may colonize their surface resulting in a reduction of buoyancy and settling in the water column (Zettler et al., 2013, Kaiser et al., 2017, Wright et al., 2020). Sessile benthic organisms, such as coral, live attached to hardbottom substrata and are at risk of exposure to microplastics and the microbes attached to their surface.
Coral reef habitats are an invaluable resource that provide cultural value and shoreline protection as well as economic benefits from commercial and recreational fisheries, pharmacology, and tourism. The cornerstone of these reef habitats are the scleractinian corals. The skeletons of these coral create a three-dimensional structure that provides habitat for many other reef taxa (Graham and Nash, 2012). The energy needed by coral for growth is obtained from their endosymbiotic, photosynthesizing algae along with exogenous feeding. With respect to their exogenous feeding, coral are suspension feeders, ingesting plankton, both actively and passively, that are entrapped on their tentacles (Figure 1). In addition to plankton, corals can also ingest microplastics (Hall et al. 2015, Hankins et al., 2018, Rotjan et al., 2019, Corona et al., 2020, Hankins et al., 2021), with smaller pieces likely being inadvertently consumed (Hankins et al., 2018, Axworthy and Padilla-Gamiño, 2019). Ingested plastics may block the gastrovascular cavity of the coral polyp leaving the polyp feeling satiated or may prevent feeding on nutritious food sources, as has been seen in other taxa (McCauley and Bjornal, 1999, Xu et al., 2017, Egbeocha et al., 2018, Ory et al. 2018, de Barros et al., 2020). While large percentages of ingested MPs (64–92%) will likely be egested (Allen et al., 2017, Hankins et al., 2018, Hankins et al., 2021) by coral polyps, microplastic exposure has been shown to impact feeding, stress response, immune system, coral-host signaling, zooxanthellae photosynthetic performance, growth, and can cause bleaching and tissue necrosis (Chapron et al., 2018, Tang et al., 2018, Axworthy and Padilla-Gamiño, 2019, Reichert et al., 2019, Syakti et al., 2019, Huang et al., 2021, Lanctôt et al., 2020, Hankins et al., 2021, Huang et al. 2021b).
Figure 1.
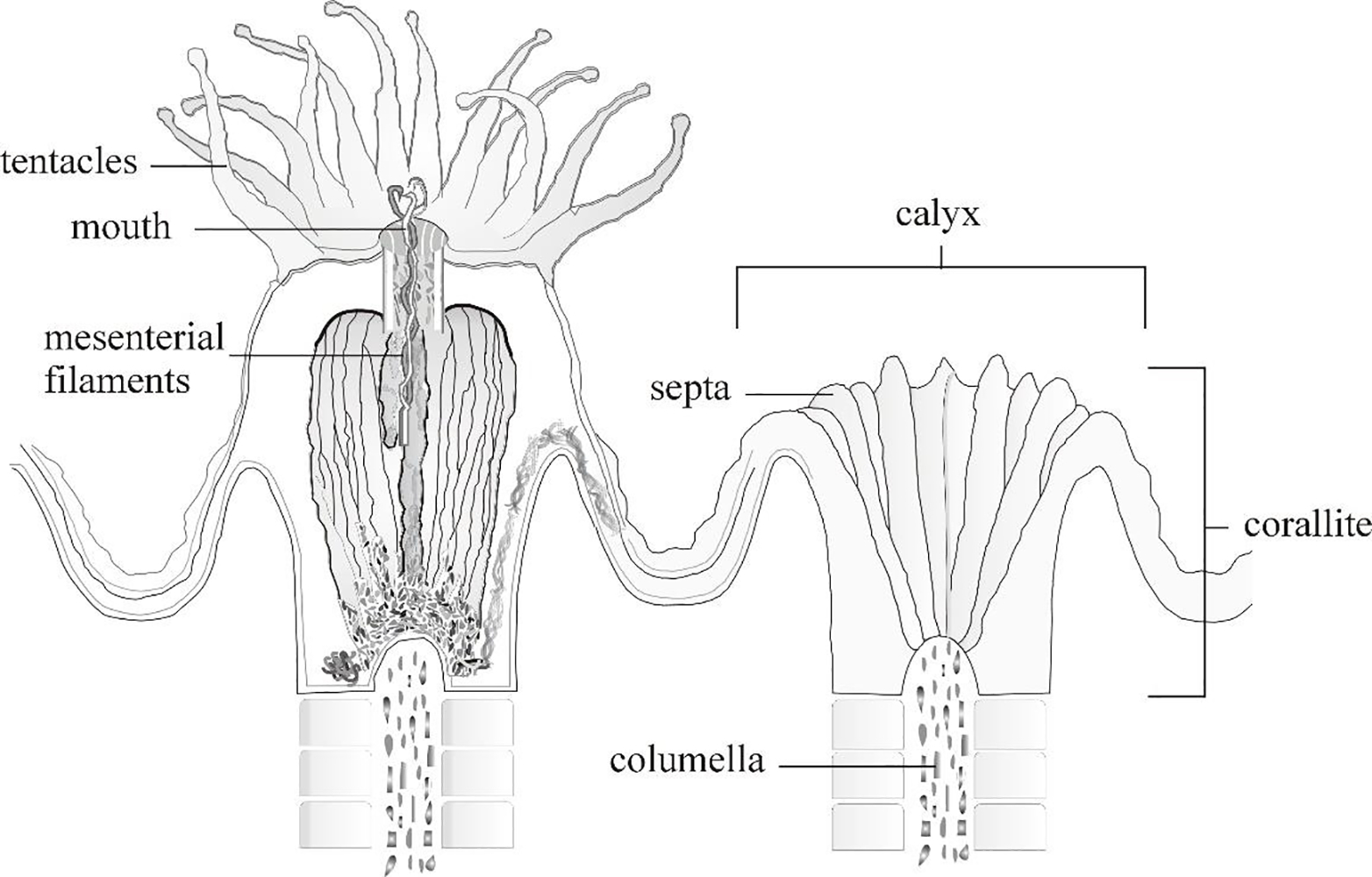
Basic anatomy of a coral showing an extended coral polyp (left) and its skeletal structure (right). From: Goreau et al., 1979, modified by C. Hankins.
Ingested MPs also have the potential to expose coral to chemical contaminants and disease. Chemicals may include those that are used in plastic production or adsorb to MPs from surrounding water (OECD, 2004, Rochman et al., 2013, Bakir et al., 2014, Mendoza and Jones, 2015). Plastic additives, such as phthalates, are a known endocrine disruptor (Lithner et al., 2011, Hermabessiere et al., 2017) and have been found in coral tissue (Saliu et al., 2019, Montano et al., 2020). Contaminants including polychlorinated biphenyls (PCBs), organochlorines, and metals have all been found on macro- and microplastic surfaces (Mato et al., 2001, Ogata et al., 2009, Ashton et al., 2010, Holmes et at., 2012, Van et al., 2012, Rochman et al., 2013) and can cause a variety of harmful effects in coral such as mortality, reduced photosynthesis, bleaching, and growth declines (Mitchelmore et al., 2007, Bielmyer et al., 2010, Biscéré et al., 2015). Additionally, pathogens such as Vibrio spp. and Hallofoliculina, have been found on microplastics (Zettler et al., 2013, Goldstein et al., 2014 Kirstein et al., 2016, Li et al., 2019). Two species of Vibrio, V. coralliilyticus and V. shiloi, have been associated with coral diseases (Kushmaro et al., 1996, Ben-Haim et al., 2003). The ciliate Hallofoliculina has been linked to skeletal eroding band disease (Antonius and Lispcomb, 2000).
Whether through physical or toxicological mechanisms, there is mounting evidence that microplastics have the potential to negatively affect corals with many studies showing species-specific responses (Mouchi et al. 2019, Reichert et al. 2019, Hierl et al. 2021, Mendrik et al. 2021). However, plastic ingestion has been predicted based on allometric measures such as buccal cavity size and body size (Fueser et al. 2019, Jâms et al. 2020). Here, we hypothesize that active coral microplastic ingestion maybe a function of both MP and calyx size, with calyces being an indicative measure of polyp size. Four coral species, Acropora cervicornis, Montastraea cavernosa, Orbicella faveolata, and Pseudodiploria clivosa, were selected based on the varying polyp sizes (Hankins et al., 2018, Hankins et al., 2021). Additionally, A. cervicornis and O. faveolata were chosen due to their importance as threatened species under the United States’ Endangered Species Act which protects imperiled species. The objective of this study was to use ingestion observations from laboratory studies for these four coral species to explore relationships between calyx (i.e., polyp size) and microplastic particle size to improve our understanding of MP ingestion behavior and biomechanics, as well as offer a predictive tool to assess potential effects of MPs on coral.
2. Material and Methods
Coral morphometric characteristics and ingestion behaviors were measured during laboratory-based experiments using microplastic spheres (MP) of various sizes in four Caribbean coral species: Monstastraea cavernosa, Orbicella faveolata, Acropora cervicornis, and Pseudodiploria clivosa. Experimental designs for ingestion experiments are described in detail in Hankins et al. (2018, 2021). In brief, these ingestion experiments were conducted in a 170 L tank containing 120 experimental chambers, made from 5.1 cm (2”) diameter polyvinyl chloride (PVC) pipe cut into ~5 cm lengths. The bottom of the experimental chambers was comprised of 118 μm Nitex® mesh, which was also attached around the circumference of the top of the chamber to ensure MPs remained within each chamber. Experimental chambers contained one fragment. Monstastraea cavernosa, O. faveolata, and P. clivosa fragments (~4 cm2) sat freely on the bottom of their chamber. Acropora cervicornis fragments (3–4 cm length) were glued onto a 2.5 cm diameter polycarbonate disc so that the fragments were oriented vertically in the chamber.
2.1. Calyx size
Each coral polyp resides in its own calyx (Figure 1). To minimize measurement variation and stress to the organism, calyces from deceased laboratory colonies were measured as a proxy for polyp size. The diameter of 100 calyces from each species were measured with dial calipers to the nearest 0.02 mm. Skeletal fragments from the experiments, which ranged in size from 2.5–4.0 cm2, were used to measure calyces for M. cavernosa, O. faveolata, and P. clivosa; with five calyces measured from each of 20 fragments for each species. Three large skeletal colonies (approx. 25 cm length × 20 cm width) of A. cervicornis from previously deceased culture stock colonies were used to measure calyces, with approximately 33 calyces measured from each colony. These measurements were limited to the calyces of A. cervicornis within 12 cm of the apical ends of the branches as these were target areas for fragments used in the Hankins et al. (2018) ingestion experiment. Calyces for A. cervicornis, O. faveolata, and M. cavernosa were measured at their widest diameter. Since the calyces of P. clivosa are not prominent and highly irregular, the length of the longest septum was measured to infer calyx size (Figure 1). The distribution of calyx diameters of the four species were assessed for normality using Shapiro test, and the average calyx size among coral species was compared using one-way analysis of variance. Tukey’s post-hoc test was used to determine which groups were significantly different from each other.
2.2. Microplastic ingestion
Five size classes of virgin, fluorescent MP spheres (obtained from Cospheric®) were offered to coral fragments (N=10 fragments per species for each of five MP sphere size classes). All spheres were polyethene and had densities of 1.002 ± 0.0006 g cc−1. The MP median size for each size class used to define treatment levels and were: (1) 0.231 mm (range = 0.212–0.250), (2) 0.462 (range = 0.425–0.500), (3) 0.925 mm (range = 0.850–1.000), (4) 1.85 mm (range = 1.7–2.0), (5) 2.6 mm (range = 2.4–2.8), and (6) a control group not exposed to MPs. To mimic chemical stimuli in coral that may feed predominantly at night, food (5–50 μm Golden Pearls® Brine Shrimp Direct, Ogden, UT) was added to seawater at a concentration of 156 mg/100 mL. The food/seawater mixture was used to apply three MPs of the same size class to a coral fragment’s polyps within the experimental chamber using a separate 1 mL transfer pipette for each fragment. One MP sphere was placed in contact with a tentacle from one polyp, such that three polyps from each fragment were fed a single MP each. Each fragment was visually monitored for up to 20 minutes to observe ingestion. MPs not ingested after 20 minutes were removed from the chamber, as described in Hankins et al. (2018, 2021). The proportion of MPs ingested by each fragment was determined from the individual MPs offered to individual polyps of the coral fragment. The control group was supplied with food in the same manner but without MP application.
2.3. Calyx and microplastic size interaction
Preliminary comparison of the proportion of MPs of size classes that were ingested by the four coral species indicated that the probability of MP ingestion may be influenced by an interaction between calyx and MP size. To visualize this relationship, we fit the data to 3-dimensional (3D) polynomial models in which the proportion of MP ingested (dependent variable) was a function of MP size (independent variable 1), and calyx size (independent variable 2). Since we did not have direct calyx size measurements for the specific polyps that ingested MPs in the laboratory experiments, each observation of polyp ingestion was associated with a calyx size that was randomly sampled without replacement from those measured on laboratory skeletons of the respective species. This assumes that: (1) the size of each calyx used in the experiments falls within the measured ranges for each species, and (2) calyx measurement error is normally distributed and approximated by the standard deviation of the measured skeletons.
Using data for all MP size categories, the proportion of MPs ingested by all species displayed a bimodal distribution with only 23 out of 200 observations exhibiting a partial response (0.33 or 0.66, i.e. ingestion of 1 (33%) or 2 (66%) of the three MPs applied) and an even distribution of 0s (N=88) and 1s (N=89). These data were fit to polynomial models of the general form:
| (EQ 1) |
Where Pingest is the proportion of MPs ingested, Dcalyx and DMP are the diameters of the calyx and MP, respectively, i and j represent the order of the polynomial and ranged from 1 to 4, and a = 0 to exclude or a = 1 to include an interaction between the two variables. A total of 64 polynomial models were fit to the data using the generalized linear model and poly functions in R studio (version 1.3.959). Of these 64 models, one subset of 32 models was fit using all data which assumed a normal distribution of errors and used the Gaussian family function. The second subset of 32 models excluded partial responses and were fit to a logistic function using a bimodal distribution. Within each subset of 32 models, 16 included the interaction term Dcalyx × DMP and 16 excluded this interaction (a = 0). The 16 models fit within or with the calyx-MP interaction were polynomials that varied by order, i. We set the highest order at 4 based on preliminary visual inspection of the data. The 32 models from each data subset (with or without partial responses) were compared using Akaike Information Criterion (AIC) to identify the best fit model for each data subset, which was then plotted to graphically represent the data in three dimensions.
There were no MPs ingested in the 0.231 mm size category by any coral species. While the models fit from all MP size ranges described above interpolates an increase in proportion ingested of MP spheres between 0.231 and 0.462 mm, it may overfit the data across the modeling space despite low AICs. To view the relationship of ingestion more clearly within the range of MP sizes that were actively ingested, we ran a second set of the 64 models described above using only the data for MP > 0.231 mm.
2.4. Potential ingestion of MP size ranges by coral
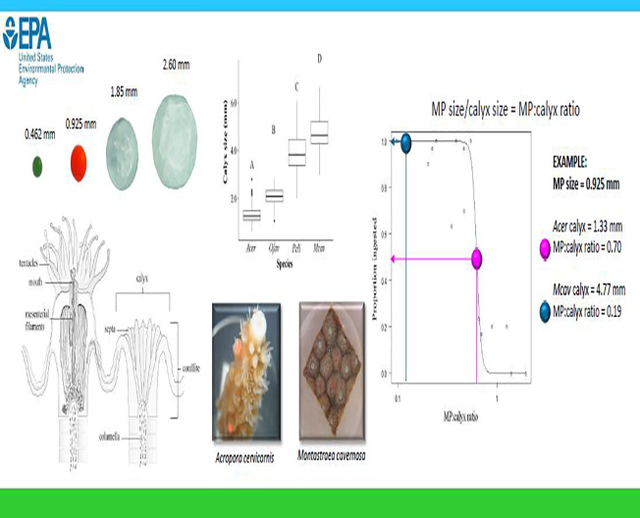
To identify the MP size range in which coral of a given calyx size are highly likely to ingest MPs, MP:calyx size ratio was first determined for each species and MP size category. For this analysis, the median size of each MP size category was divided by the average calyx size (N=100) of each coral species such that each coral species and MP size class was reduced to one ratio value. For example, all observations for which A. cervicornis (average calyx size = 1.33 mm) was offered an 0.462 mm MP were represented by the MP:calyx size the ratio of 0.35 (0.462 mm/1.33 mm). Similarly, all observations for which M. cavernosa (average calyx size 4.77 mm) were offered MPs of the median size 2.60 mm were represented by the ratio 0.54, and so on. Including all species and MP size classes, the MP:calyx size ratios ranged from 0.05 – 1.96. Excluding the smallest MP size class (0.231 mm), the MP:calyx size ratios ranged from 0.1 – 1.96. Visual inspection of the data and results of the 3D modeling described above suggested that lack of ingestion by any coral species observed with the MP 0.231 mm size class were independent of coral size and were excluded from the model fit to these data.
A logistic model with the lower and upper bounds set at 0 and 1, respectively, was fit to the data to describe the relationship between the average proportion of MPs ingested (dependent variable) and the MP:calyx size ratio (independent variable), such that:
| (EQ 2) |
Where Pingest is the proportion of MPs ingested at any MP:calyx size ratio, x. The variable e is the model inflection point associated with the 50th percentile and b is the slope of the curve. Using this model, we identify size ranges in which coral of known calyx size are most likely or unlikely to ingest MP of a particular size.
3. Results
3.1. Calyx size
Coral calyx size was significantly different among all species (ANOVA, df=3, F=760.7, p<0.00) (Figure 2), with A. cervicornis having the smallest mean calyx (1.33 mm, SD=0.39) and M. cavernosa having the largest mean calyx (4.77 mm, SD=0.73). The mean calyx size for O. faveolata and P. clivosa was 2.08 mm (SD=0.36) and 3.96 mm (SD=0.89), respectively.
Figure 2.
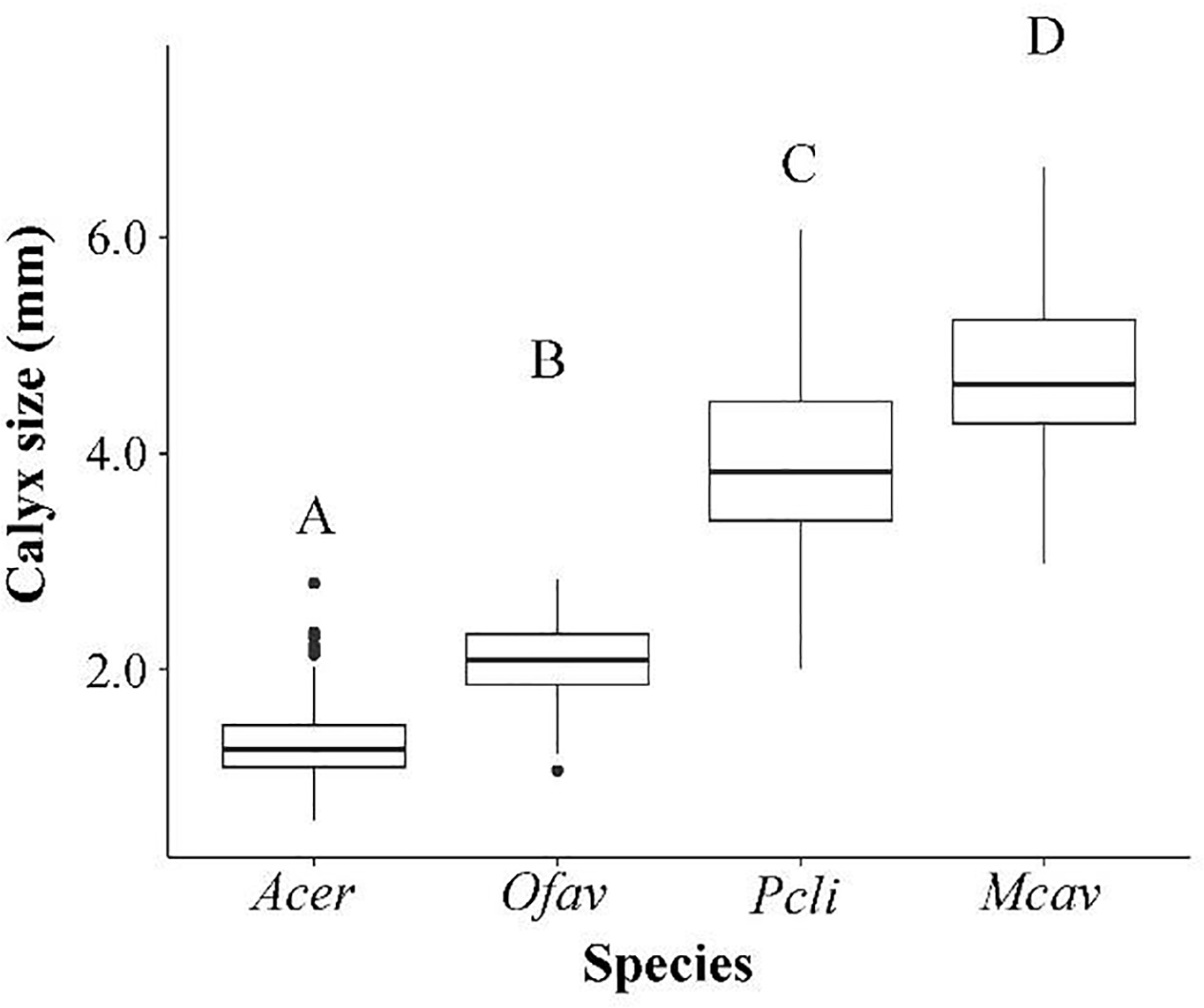
Mean calyx size for Acropora cervicornis, Orbicella faveolata, Pseudodiploria clivosa, and Montastraea cavernosa. Letters indicate significant difference between species (Tukey’s post hoc).
3.2. Microplastic Ingestion
Of the five MP size classes provided to coral fragments, none of the species ingested the 0.231 mm size class. Proportion of MPs ingested by the different coral species suggests a relationship between calyx size and MP size (Table 1). The coral species with the smallest average calyx size, A. cervicornis, did not ingest the two largest size classes, 1.85 mm or 2.60 mm, while the largest coral (M. cavernosa) ingested ≥90% of all MP size classes >0.231mm. The two intermediate coral species displayed intermediate ingestion rates that further demonstrate an increasing proportion of larger MPs ingested with increasing calyx size (Table 1).
Table 1.
Mean percent of microplastics (MPs) ingested for each species at five microplastic size classes and total mean percent ingested across all size classes. 10 fragments/species/MP size class, n = 3 MPs/size MP class/fragment
| Species | 0.231 mm | 0.462 mm | 0.925 mm | 1.85 mm | 2.60 mm | Total MPs |
|---|---|---|---|---|---|---|
|
| ||||||
| A. cervicornis | 0% (SD=0.0) | 63.30% (SD=39.9) | 16.70% (SD=28.3) | 0% (SD=0.0) | 0% (SD=0.0) | 16.0% (SD=32.5) |
| O. faveolata | 0% (SD=0.0) | 96.7% (SD=10.5) | 96.7% (SD=10.5) | 20.0% (SD=32.2) | 20.0% (SD=23.3) | 46.7% (SD=45.7) |
| P. clivosa | 0% (SD=0.0) | 100% (SD=0.0) | 100% (SD=0.0) | 70.00% (SD=39.9) | 23.30% (SD=35.3) | 58.7% (SD=46.9) |
| M. cavernosa | 0% (SD=0.0) | 100% (SD=0.0) | 90.0% (SD=31.6) | 00% (SD=0.0) | 100% (SD=0.0) | 78.0% (SD=41.9) |
3.3. Calyx and microplastic size interaction
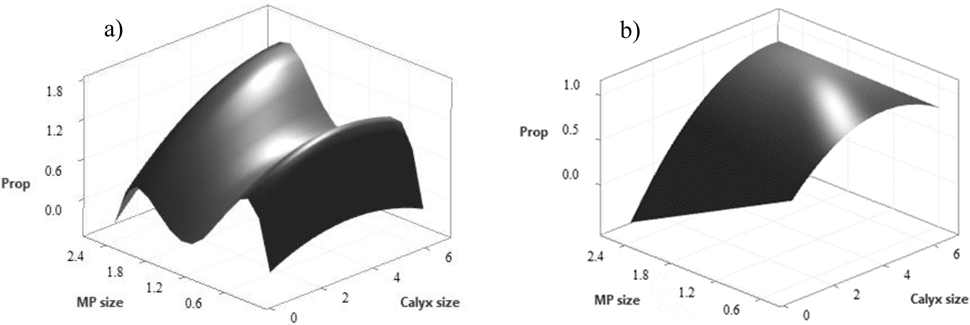
Of the 64 models fit to the dataset that included all the MP sizes with (N=32) and without (N=32) partial responses, the best fit model based on AIC was a fourth-order polynomial fit to the Gaussian family of the form: Pingest = −2.58 + 0.25 Dcalyx + 12.99 DMP − 0.03 Dcalyx2 − 17.98 DMP2 + 9.16 DMP3 − 1.56 DMP4 + 0.09 Dcalyx * DMP (Figure 3a; R2 = .72, p < 0.001, AIC = 35.7). Of the 64 models fit to the data that excluded the smallest MP size of 0.231 mm, the best fit model was a quadratic equation fit to the Gaussian family of the form Pingest = 0.47 + 0.35 Dcalyx − 0.50 DMP − 0.04 Dcalyx2 + 0.08 Dcalyx* DMP (Figure 3b; R2 = 0.62, p < 0.001, AIC = 56.6). Both models included partial response data and had significant interactions between Dcalyx and DMP.
Figure 3.
Approximated relationship between calyx size and microplastic (MP) size for proportion of MP (Prop) ingested for (a) all MP size classes 0.231–2.60 mm and (b) MP sizes classes 0.462–2.60 mm.
3.4. Potential coral ingestion of MP size ranges
The logistic model relating the proportion of MPs ingested to MP:calyx size ratios is depicted in Figure 4. The slope, b, was estimated at 18.73 and the variable e was 0.62 (EQ 2). In general, and when excluding MP = 0.231 mm, the proportion of MPs ingested decreases with increasing MP:calyx size ratio. This model identifies the upper limit of MP sizes that are ingested by coral of varying sizes at a given rate of ingestion, represented as the proportion of MP ingested, and can be used to determined MP sizes that are likely to be actively ingested by coral of a given size (Table 2). The MP:calyx size ratios for which MPs have a high likelihood of being ingested by a coral polyp are represented by proportion of ingested MP ≥ 0.75. Conversely, MPs have a low likelihood of being ingested by a coral polyp when MP:calyx size ratios are associated with low ingestion rate (proportion ingested = 0.1–0.25).
Figure 4.
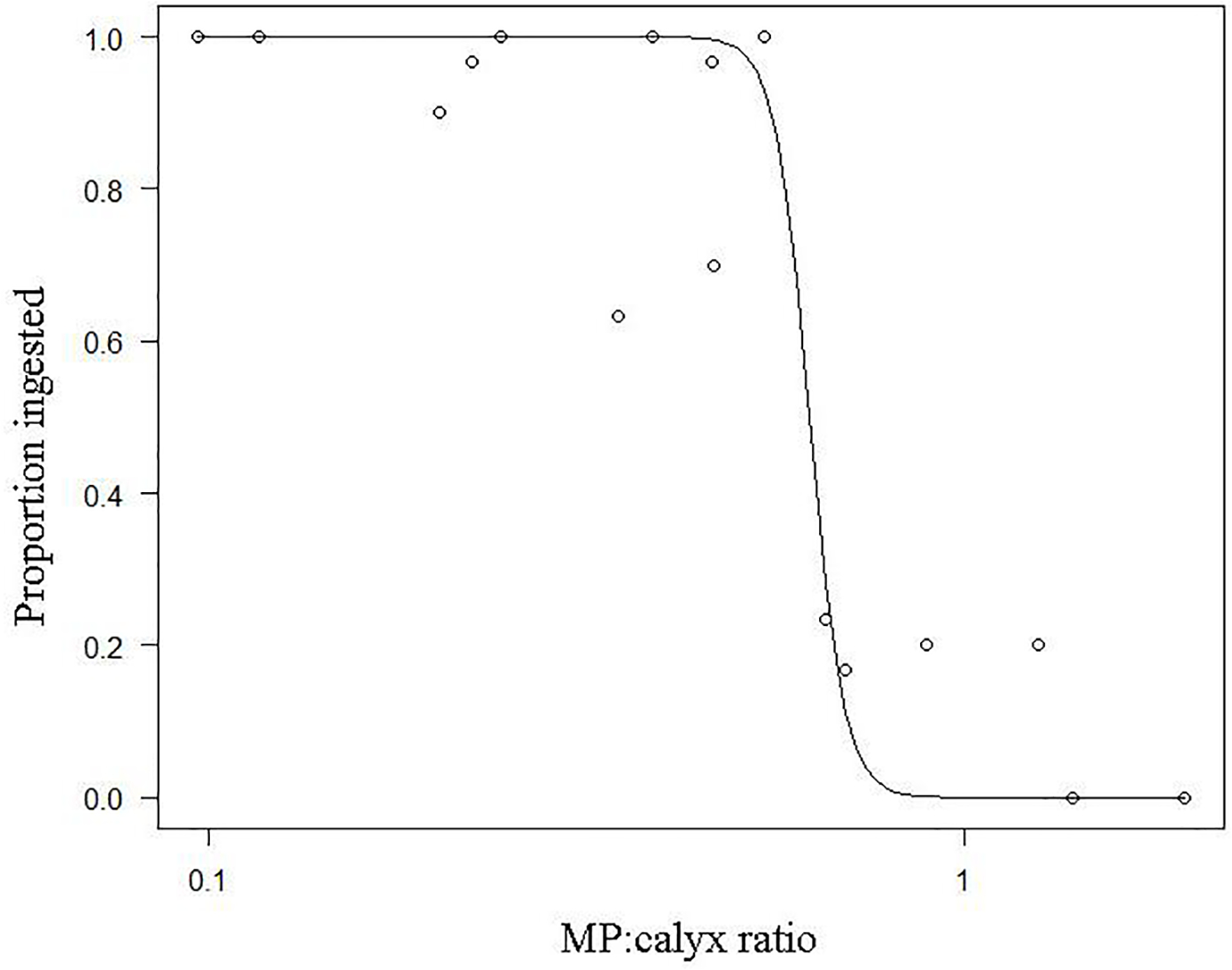
Relationship of the proportion of microbeads ingested and MP:calyx size ratios.
Table 2.
MP:calyx size ratios and 95% confidence intervals associated with various rates ofingestión, represented by the proportion of MP ingested.
| Proportion MP Ingestion | Likelihood of Ingestion | MP:calyx Ratio | 95% Confidence Interval |
|---|---|---|---|
|
| |||
| 0.10 | low | 0.70 | (0.61 – 0.81) |
| 0.25 | low | 0.66 | (0.61 – 0.72) |
| 0.50 | moderate | 0.62 | (0.57 – 0.68) |
| 0.75 | high | 0.59 | (0.50 – 0.69) |
| 0.90 | high | 0.56 | (0.43 – 0.71) |
To demonstrate this utility and estimate upper MP size limits for the species used in the present study, the MP sizes associated with ingestion rates of 0.10, 0.25, 0.50, 0.75, and 0.90 are determined by multiplying the average calyx size for each species by the MP:calyx size ratio estimated for each ingestion rate provided in Table 2. For A. cervicornis, the species with the smallest calyx, MPs of ≤ 0.78 mm are highly likely to be actively ingested, where MP > 0.88 mm are unlikely to be ingested. This is supported by the average rate of ingestion for this species listed in Table 1, in which 63% of 0.462 mm MP were ingested and 17% of 0.925 mm MP were ingested. For M. cavernosa, Table 3 identifies MP < 2.81 mm with a high likelihood of ingestion, which is demonstrated by ≥90% ingestion of MP 0.462 – 0.260mm for this species (Table 2). Based on the modeled ratios, MPs ≥3.16 mm are unlikely to be ingested by M. cavernosa, MPs < 2.98 mm have a moderate likelihood of ingestion, and MPs < 2.81 mm are highly likely to be ingested.
Table 3.
Upper limit of MP sizes ingested at various rates by four coral species, as represented by the proportion ingested. Likelihood of ingestion is associated with proportion of MP ingested.
| Species | Mean calyx size (mm) | Maximum MP size by proportion ingested (mm) | ||||
|---|---|---|---|---|---|---|
| 0.10 | 0.25 | 0.50 | 0.75 | 0.90 | ||
| low likelihood | moderate | high likelihood | ||||
|
| ||||||
| A. cervicornis | 1.33 | 0.93 | 0.88 | 0.83 | 0.78 | 0.74 |
| O. faveolata | 2.08 | 1.46 | 1.38 | 1.30 | 1.23 | 1.16 |
| P. clivosa | 3.96 | 2.78 | 2.62 | 2.47 | 2.33 | 2.20 |
| M. cavernosa | 4.77 | 3.35 | 3.16 | 2.98 | 2.81 | 2.65 |
4. Discussion
The results of this study show that the relationship of coral calyx size and MP sizes is an important determinant of potential MP ingestion by coral. The four coral species used in our studies had significantly different calyx sizes and were selected to demonstrate the relationship of coral and MP size across a wide range of calyx sizes. Since coral ingest particulate matter opportunistically, combining species that vary widely in size provides a broader perspective of MP and calyx size interactions; as the species mean calyx size increased, so too did the mean percent of ingestion with increasing MP size (Table 1). The three-dimensional models provide a view of these data as continuous variables, which is more realistic for relationships based on size. The logistic model can be used to estimate the likeliness of an MP size range to be actively ingested by any species of coral within the range of calyx sizes used in this study. The models presented here are caveated by lack of direct measurement of calyces for the polyps used in the study.
Ingestion of MP by coral polyps is analogous to gape size predation seen in many prey-predator interactions for taxa such as fish, whereby ingestion is an opportunistic function of prey size that fits within the predator’s gape. In these cases, smaller prey are more susceptible to a wider range of predation than larger prey, and predators will consume any prey smaller than a maximum size threshold (Sogard, 1997, Urban, 2007). Additionally, there is evidence of allometric relationships between ingested plastic and animal body size when compared across many different taxa (Jâms et al. 2020) as well as within taxa (Fueser at al. 2019). As demonstrated by the results here, coral will actively ingest most microplastic smaller than a maximum size threshold, but not smaller than 0.231 mm. Larger MPs (defined here as >0.925 mm) have less risk of consumption due to the coral’s small mouth gape as shown in A. cervicornis, which did not ingest any MPs from the 1.85- or 2.60 mm size classes and had a low likelihood of ingestion compared to the other, larger polyp species tested in this study. While this study demonstrates the maximum MP sizes that would potentially be actively ingested by coral with a particular calyx (i.e., polyp size), there is also a minimum size threshold in which MPs are not actively ingested. The present study identified the 0.231 MP size class as a size that is not actively ingested by any species, however, these data do not specifically identify the lower size threshold at which coral do not ingest MPs. The 3D model presented in Figure 3a interpolates ingestion potential between 0.231 and 0.462 mm; the lower threshold is likely within this size range. Additionally, the lower threshold may be indicative of the MP sizes in which coral passively feed on MPs (Hankins et al., 2018, Axworthy and Padilla-Gamiño, 2019) suggesting that smaller MPs do not elicit a tactile response from the coral tentacles.
Although microplastic ingestion is a function of calyx size for MPs >0.462 mm, MPs could impact corals without being consumed. Corals have been suggested as microplastic “sinks” in the marine environment due to MP adhesion on coral surfaces (Martin et al., 2019, Corona et al., 2020). Martin et al. (2019) investigated MP adhesion on three scleractinian coral species and found that roughly 30% of available MPs attached to the surface in two of the three species. Additionally, Corona et al. (2020) showed that MP adhesion was 40 times greater than ingestion in the mushroom coral, Danafungia scruposa. Neither study looked at effects of MP ingestion versus adhesion on coral. Acknowledging that there may be other mechanisms of MP exposure to coral that may cause adverse effects, our study only presents the likelihood of MP ingestion and does not infer impacts.
The models presented here describe MP ingestion potential as a function of the interaction between polyp and MP size. Complex relationships such as these are best visualized in 3-dimensions, but these types of models are at risk of overfitting and are less robust as prediction tools. Additionally, without direct measurements of the individual calyces and MP spheres used in the laboratory studies, we assume a slightly larger measurement error associated with these models. Multiple coral species were combined into one model, because all scleractinian coral have the same anatomy and general feeding behaviors and because phenotypic morphology within a species can vary due to environmental conditions (Foster, 1979, Todd et al., 2002, Erftemeijer et al., 2012). While models are most robust when the measurement error is minimized, the models presented here can describe the interaction of MP and coral polyp size and provide new insights into likelihood of active ingestion of MP by coral. Predictions may be altered with a more continuous distribution design rather than the one conducted in this study that resulted in four categories (0, 33, 66, and 100%). Additionally, it should be noted that some corals are opportunist feeders, therefore, ingestion of prey is often influenced by prey density and flow speeds (Helmuth and Sebens 1993, Sebens et al., 1998, Ferrier-Pages et al., 2003). In this study, we excluded these variables in the application of MPs; therefore, the likelihood of ingestion in field applications should also consider environmental parameters.
Some of the impacts of MPs on coral are species-specific (Mouchi et al., 2019, Reichert et al., 2019, Mendrik et al., 2021), however it is unknown if the responses observed may be attributed to species specificity or a response of the coral based the physical structure of its polyp size. As previously mentioned, without measurements directly from the coral used in experiments or from similar growing conditions it is difficult to discern any potential relationships between polyp size and response as there are slight morphological differences within species exposed to different environmental conditions (Foster, 1979, Todd et al., 2002, Erftemeijer et al., 2012). Generalized responses for active ingestion presented in this study are important to understand active coral MP ingestion as it relates to coral polyp size. These relationships presented here can streamline future research towards improved prevention and/or targeted mitigation.
Highlights.
Ingestion of microplastic different for coral species with different calyx sizes
Calyx size and microplastic ingestion data were used to develop model for active ingestion
Ingestion of microplastics (>0.462 mm) by coral is a function of calyx and microplastic sizes
Acknowledgements.
We would like to thank Janet Nestlerode and Juliette Chausson for manuscript review as well as the journal’s anonymous reviewers. Special thanks to Paul Soderlind for graphical assistance. We would also like to thank NOAA’s Florida Keys National Marine Sanctuary (FKNMS-2017-151) and Mote Marine Laboratory (Summerland Key, FL) for their assistance with coral collection.
Footnotes
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the U.S. EPA.
References
- Allen AS, Seymour AC, Rittschof D, 2017. Chemoreception drives plastic consumption in a hard coral. Mar. Pollut. Bull 124. DOI: 10.1016/j.marpolbul.2017.07.030. [DOI] [PubMed] [Google Scholar]
- Antonius AA,Lipscomb D, 2000. First protozoan coral-killer identified in the Indo-Pacific. Atoll Res. Bull, 481,1–21. [Google Scholar]
- Ashton K, Holmes L, Turner A, 2010. Association of metals with plastic production pellets in the marine environment. Mar. Pollut. Bull 60(11), 2050–2055. DOI: 10.1016/j.marpolbul.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Axworthy JB, Padilla-Gamiño JL, 2019. Microplastics ingestion and heterotrophy in thermally stressed corals. Sci. Rep, 18193. DOI: 10.1038/s41598-019-54698-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakir A, Rowland SJ, Thompson RC, 2014. Transport of persistent organic pollutants by microplastics in estuarine conditions. Estuar. Coast. Shelf Sci 140, 14–21. [Google Scholar]
- Ben-Haim Y, Zicherman-Keren M, Rosenburg E, 2003. Temperature-regulated bleaching and lysis of the coral Pocillopora damicornis by the novel pathogen Vibrio coralliilyticus. App. Environ. Microbiol 69(7), 4326–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielmyer GK, Grosell M, Bhagooli R, Baker AC, Langdon C, Gillette P, Capo TR, 2010. Differential effects of copper on the three species of scleractinian corals and their algal symbionts (Symbiodium spp.). Aquat. Toxicol 97(2), 125–133. DOI: 10.1016/j.aquatox.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Biscéré T, Rodlfo-Metalpa R, Lorrain A, Chauvaud L, Thébault J, Clavier J, Houlbrèque F, 2015. Responses of two scleractinian corals to cobalt pollution and ocean acidification. PloS One DOI: 10.1371/journal.pone.0122898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borelle SE, Ringma J, Law KL, Monnahan CC, Lebreton L, McGivern A, Murphy E, Jambeck J, Leonard GH, Hilleary MA, Eriksen M, Possingham HP, De Frond H, Gerber LR, Polidoro B, Tahir A, Bernard M, Mallos N, Barnes M, Rochman CM, 2020. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 369, 1515–1518. [DOI] [PubMed] [Google Scholar]
- Chapron L, Peru E, Engler A, Ghiglione JF, Meistertzheim AL, Pruski AM, Purser A, Vétion G, Galand PE, Lartaud F, 2018. Macro- and microplastics affect cold-water corals growth, feeding and behavior. Sci. Rep 8, 15299. DOI: 10.1038/s41598-018-33683-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona E, Martin C, Marasco R, Duarte CM, 2020. Passive and active removal of marine microplastics by a mushroom coral (Danafungia scruposa). Front. Mar. Sci 7, 128. DOI: 10.3389/fmars.2020.00128 [DOI] [Google Scholar]
- de Barros MSF, dos Santos Calado TC, Santos Silva A, dos Santos EV, 2020. Ingestion of plastic debris affects feeding intensity in the rocky shore crab Pachygrapsus transversus Gibbes 1850 (Brachyura: Grapsidae). Int. J. Biodivers. Conserv 12(2), 113–117. DOI: 10.5897/IJBC2020.1391. [DOI] [Google Scholar]
- Egbeocha CO, Malek S, Emenike CU, Milow P, 2018. Feasting on microplastics: ingestion by and effects of marine organisms. Aquat. Biol 27, 93–106. DOI: 10.3354/ab00701. [DOI] [Google Scholar]
- Erftemeijer PLA, Reigl B, Hoeksema BW, Todd PA, 2012. Environmental impacts of dredging and other sediment disturbances on corals: a review. Mar. Pollut. Bull 64(9), 1737–1765. [DOI] [PubMed] [Google Scholar]
- Ferrier-Pagés C, Witting J, Tambutté E, Sebens KP, 2003. Effect of natural zooplankton feeding on the tissue and skeletal growth of the scleractinian coral Stylophora pistillata. Coral Reefs 22, 229–240. [Google Scholar]
- Fueser H, Mueller M, Weiss L, Höss S, Traunspurger W, 2019. Ingestion of microplastics by nematodes depends on feeding strategy and buccal cavity size. Environ. Pollut 255, 113227. [DOI] [PubMed] [Google Scholar]
- Foster AB, 1979. Phenotypic plasticity in the reef corals Montastraea annularis (Ellis & Solander) and Siderastrea siderea (Ellis & Solander). J. Exp. Mar. Biol. Ecol 39(1), 25–54. [Google Scholar]
- Goldstein MC, Carson HS, Eriksen M, 2014. Relationship of diversity and habitat area in North Pacific plastic-associated rafting communities. Mar. Biol 161, 1441–1453. DOI: 10.1007/s00227-014-2432-8. [DOI] [Google Scholar]
- Goreau TF, Goreau NI, Goreau TJ, 1979. Corals and coral reefs. Sci. Am 241(2), 124–137. [Google Scholar]
- Graham NAJ, Nash KL, 2012. The importance of structural complexity in coral reef ecosystems. Coral Reefs 32, 315–326. [Google Scholar]
- Hall NM, Berry KLE, Rintoul L, 2015. Microplastic ingestion by scleractinian corals. Mar. Biol 162, 725–732. [Google Scholar]
- Hankins C, Duffy A, Drisco K, 2018. Scleractinian coral microplastic ingestion: Potential calcification effects, size limits, and retention. Mar. Pollut. Bull 135, 587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins C, Moso E, Lasseigne D, 2021. Microplastics impair growth in two Atlantic scleractinian coral species, Pseudodiploria clivosa and Acropora cervicornis. Environ. Pollut 275, 116649. DOI: 10.1016/j.envpol.2021.116649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmuth B, Sebens K, 1993. The influence of colony morphology and orientation to flow on particle capture by the scleractinian coral Agaricia agaricites (Linnaeus). J. Exp. Mar. Biol. Ecol 165(2), 251–278. [Google Scholar]
- Hermabessiere L, Dehault A, Paul-Pont I, Lacroix C, Jesequel R, Soudant P, Duflos G, 2017. Occurrence and effects of plastic additives on marine environments and organisms: a review. Chemosphere 182, 781–793. DOI: 10.1016/j.chemosphere.2017.05.096. [DOI] [PubMed] [Google Scholar]
- Hierl F, H.C. W, Westphal H, 2021. Scleractinian corals incorporate microplastic particles: identification from a laboratory study. Environ. Sci. Pollut Res 28, 37882–37893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes LA, Turner A, Thompson RC, 2012. Adsorption of trace metals to plastic resin pellets in the marine environment. Environ. Pollut 160, 42–48. DOI: 10.1016/j.envpol.2011.08.052. [DOI] [PubMed] [Google Scholar]
- Huang W, Song B, Liang J, Niu Q, Zeng G, Shen M, Deng J, Luo Y, Wen X, Zhang Y, 2021a. Microplastics and associated contaminants in the aquatic environment: A review on their ecotoxicological effects, trophic transfer, and potential impacts to human health. J. Hazard. Mater 405, 124187. DOI: 10.1016/j.jhazmat.2020.124187. [DOI] [PubMed] [Google Scholar]
- Huang W, Chen M, Song B, Deng J, Shen M, Chen Q, Zeng G, Liang J, 2021b. Microplastics in the coral reefs and their potential impacts on corals: a mini-review. Sci. Total Environ 762, 143112. DOI: 10.1016/j.scitotenv.2020.143112. [DOI] [PubMed] [Google Scholar]
- Jamieson AJ, Brooks LSR, Reid WDK, Piertney SB, Narayanaswamy BE, Linley TD, 2019. Microplastics and synthetic particles ingested by deep-sea amphipods in six of the deepest marine ecosystems on Earth. R. Soc. Open Sci 6. DOI: 10.1098/rsos.180667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jâms IB, Windsor FM, Poudevigne-Durance T, Ormerod SJ, Durance I, 2020. Estimating the size distribution of plastics ingested by animals. Nat Comm 11, 1594. DOI: 10.1038/s41467-020-15406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser D, Kowalski N, Waniek J, 2017. Effects of biofouling on the sinking behavior of microplastics. Environ. Res. Lett 12, 1240003. DOI: 10.1088/1748-9326/aa8e8b. [DOI] [Google Scholar]
- Kanhai LDK, Johansson C, Frias JPGL, Gardfeldt K, Thompson RC, O’Connor I, 2019. Deep sea sediments of the Arctic Central Basin: a potential sink for microplastics. Deep Sea Res. Part I Oceanog. Res. Pap 145, 137–142. DOI: 10.1016/j.dsr.2019.03.003. [DOI] [Google Scholar]
- Kirstein IV, Kirmizi S, Wichels A, Garin-Fernandez A, Erler R, Löder M, Gerdts G, 2016. Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Mar. Environ. Res 120, 1–8. [DOI] [PubMed] [Google Scholar]
- Kelly A, Lannuzel D, Rodermann T, Meiners KM, Auman HJ, 2020. Microplastic contamination in east Antarctic sea ice. Mar. Pollut. Bull 154, 111130. DOI: 10.1016/j.marpolbul.2020.111130. [DOI] [PubMed] [Google Scholar]
- Kushmaro A, Loya Y, Fine M, Rosenberg E, 1996. Bacterial infection and coral bleaching. Nature 380, 396. [Google Scholar]
- Lanctôt CM, Bednarz VN, Melvin S, Jacob H, Oberhaensli F, Swarzenski PW, Carroll Ferrier-Pagès. C., A.R., Metian M, 2020. Physiological stress response of the scleractinian coral Stylophora pistillata exposed to polyyethylene microplastics. Environ. Pollut 263, 114559. DOI: 10.1016/j.envpol.2020.114559. [DOI] [PubMed] [Google Scholar]
- Law KL, 2017. Plastics in the Marine Environment. Ann. Rev. Mar. Sci 9, 205–229. DOI: 10.1146/annurev-marin-010816-060409. [DOI] [PubMed] [Google Scholar]
- Li W, Zhang Y, Wu N, Zhao Z, Xu W, Ma Y, Niu Z, 2019. Colonization characteristics of bacterial communities on plastic debris influenced by environmental factors and polymer types in the Haihe Estuary of Bohai Bay, China. Environ. Sci. Technol 53(18), 10763–10773. DOI: 10.1021/acs.est.9b03659. [DOI] [PubMed] [Google Scholar]
- Li P, Wang X, Su M, Zou X, Duan L, Zhang H, 2020. Characteristics of plastic pollution in the environment: a review. Bull. Environ. Contam. Toxicol DOI: 10.1007/s00128-020-02820-1. [DOI] [PubMed] [Google Scholar]
- Lithner D, Larsson A, Dave G, 2011. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ 409(18), 3309–3324. [DOI] [PubMed] [Google Scholar]
- Martin C, Corona E, Mahadik GA, Duarte CM, 2019. Adhesion to coral surface as a potential sink for marine microplastic. Environ. Pollut 255, 113281. DOI: 10.1016/j.envpol.2019.113281. [DOI] [PubMed] [Google Scholar]
- Mato Y, Isobe T, Takada H, Kanehiro H, Ohtake C, Kaminuma T, 2001. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol 35, 318–324. [DOI] [PubMed] [Google Scholar]
- McCauley SJ, Bjornal KA, 1999. Conservation implications of dietary dilution from debris ingestion: sublethal effects in post-hatchling loggerhead sea turtles. Conserv. Biol 13(4), 925–929. [Google Scholar]
- Mendrik FM, Henry TB, Burdett H, Hackney CR, Waller C, Parsons DR, 2021. Species-specific impact of microplastics on coral physiology. Environ. Pollut 269, 116238. DOI: 10.1016/j.envpol.2020.116238 0269–7491. [DOI] [PubMed] [Google Scholar]
- Mendoza LMR, Jones PR, 2015. Characterisation of microplastics and toxic chemicals extracted from microplastic samples from the North Pacific Gyre. Environ. Chem 12(5), 611–617. [Google Scholar]
- Mitchelmore CL, Verde EA, Weis VM, 2007. Uptake and partitioning of copper and cadmium in the coral Pocillopora damicornis. Aquat. Toxicol 85(1), 48–56. DOI: 10.1016/j.aquatox.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Montano S, Seveso D, Maggioni D, Galli P, Corsarini S, Saliu F, 2020. Spatial variability of phthalates contamination in the reef-building corals Porites lutea, Pocillopora verrucos, and Pavona varians. Mar. Pollut. Bull 155. DOI: 10.1016/j.marpolbul.2020.111117. [DOI] [PubMed] [Google Scholar]
- Mouchi V, Chapron L, Peru E, Pruski AM, Meisterzheim AL, Vétion G, Galand PE, Lartuad F, 2019. Long-term aquaria study suggests species-specific responses of two cold-water corals to macro- and microplastic exposure. Environ. Pollut 253, 322–329. [DOI] [PubMed] [Google Scholar]
- OECD, 2004. Emission scenario document on plastic additives. Series on emission scenario documents, no. 3. Paris: Environmental directorate, OECD Environmental Health and Safety Publications. [Google Scholar]
- Ogata Y, Takada H, Mizukawa K, Hirai H, Iwasa S, Endo S, Mato Y, Saha M, Okuda K, Nakashima A, Murakami M, Zurcher N, Booyatumanondo R, Zakaria MP, Dung LQ, Gordon M, Miguez C, Suzuki S, Moore C, Karapanagioti HK, Weerts S, McClurg T, Burres E, Smith W, Van Velkenburg M, Lang JS, Lang RC, Laursen D, Danner B, Stewardson N, Thompson RC, 2009. International pellet watch: global monitoring of persistent organic pollutants (POPs) in coastal water. 1. Initial phase data on PCBs, DDTs, and HCHs. Mar. Pollut. Bull 58(10), 1437–1446. [DOI] [PubMed] [Google Scholar]
- Ory NC, Gallardo C, Lenz M, Thiel M, 2018. Capture, swallowing, and egestion of microplastics by a planktivorous juvenile fish. Environ. Pollut 240, 566–573. [DOI] [PubMed] [Google Scholar]
- Plastics Europe, 2017. Plastics – the facts 2017. An analysis of European plastics, production, demand and waste data. https://www.plasticseurope.org/application/files/5715/1717/4180/Plastics_the_facts_2017_FINAL_for_website_one_page.pdf
- Reichert J, Arnold AL, Hoogenboom MO, Schubert P, Wilke T, 2019. Impacts of microplastic on growth and health of hermatypic corals are species-specific. Environ. Pollut 254. DOI: 10.1016/j.envpol.2019.113074 0269–7491. [DOI] [PubMed] [Google Scholar]
- Rochman CM, Hoh EH, Hentschel BT, Kaye S, 2013. Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: implications for plastic marine debris. Environmental Science and Technology 47:1646–1654. DOI: 10.1021/es303700s. [DOI] [PubMed] [Google Scholar]
- Rochman CM, Anthony-Browne M, Underwood J, van Franeker J, Thompson A, Richard C, Amaral-Zettler LA, 2016. The ecological impacts of marine debris: unraveling the demonstrated evidence from what is perceived. Ecology 97(2), 302–312. DOI: 10.1890/14-2070.1. [DOI] [PubMed] [Google Scholar]
- Rotjan RD, Sharp KH, Gauthier AE, Yelton R, Lopez EMB, Carilli J, Kagan C, Urban-Rich J, 2019. Patterns, dynamics and consequences of microplastic ingestion by the temperate coral, Astrangia poculata. Proc. Royal Soc. B 286, 20190726. DOI: 10.1098/rspb.2019.0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliu F, Montano S, Leoni B, Lasagni M, Galli P, 2019. Microplastics as a threat to coral reef environments: detection of phthalate esters in neuston and scleractinian corals from the Faafu Atoll, Maldives. Mar. Pollut. Bull 142, 234–241. [DOI] [PubMed] [Google Scholar]
- Sebens KP, Grace SP, Helmuth B, Maney EJ Jr, Miles JS, 1998. Water flow and prey capture by three scleractinian corals, Madracis mirabilis, Montastrea cavernosa and Porites porites, in a field enclosure. Mar. Biol 131, 347–360. [Google Scholar]
- Sogard SM, 1997. Size-selective mortality in the juvenile stage of teleost fishes: a review. Bull. Mar. Sci 60(3), 1129–1157. [Google Scholar]
- Syakti AD, Jaya JV, Rahman A, Hidayati NV, Raza’i TS, Idris F, Trenggono M, Doumenq P, Chou LM, 2019. Bleaching and necrosis of staghorn coral (Acropora formosa) in laboratory assays: immediate impact of LDPE microplastics. Chemosphere 228, 528–535. DOI: 10.1016/j.chemosphere.2019.04.156. [DOI] [PubMed] [Google Scholar]
- Tang J, Ni X, Zhou Z, Wang L, Lin S, 2018. Acute microplastic exposure raises stress response and suppresses detoxification and immune capacities in the scleractinian coral Pocillopora damicornis. Environ. Pollut 243(A), 66–74. DOI: 10.1016/j.envpol.2018.08.045. [DOI] [PubMed] [Google Scholar]
- Tekman MB, Wekerle C, Lorenz C, Primpke S, Hasemann C, Gerdts G, Bergmann M, 2020. Tying up loose ends of microplastic pollution in the Article: distribution from the sea surface through the water column to deep-sea sediments at the HAUSGARTEN observatory. Environ. Sci. Technol 54(7), 4079–4090. DOI: 10.1021/acs.est.9b06981. [DOI] [PubMed] [Google Scholar]
- Todd PA, Sidle RC, Chou LM, 2002. Plastic coral from Singapore: 1. Coral Reefs 21, 391–392. [Google Scholar]
- Urban MC, 2007. The growth-predation risk trade-off under a growing gape-limited predation threat. Ecology 88(10), 2587–2597. DOI: 10.1890/06-1946.1 [DOI] [PubMed] [Google Scholar]
- Van A, Rochman CM, Flores EM, Hill KL, Vargas E, Vargas SA, Hoh E, 2012. Persistent organic pollutants in plastic marine debris found on beaches in San Diego, California. Chemosphere 86(3), 258–263. [DOI] [PubMed] [Google Scholar]
- Wright RJ, Erni-Cassola G, Zadjelovic V, Latva M, Christie-Oleza JA, 2020. Marine plastic debris: a new surface for microbial colonization. Environ. Sci. Technol 54, 11657–11672. [DOI] [PubMed] [Google Scholar]
- Xu XY, Lee WT, Chan AKY, Lo HS, Shin PKS, Cheung SG, 2017. Microplastic ingestion reduces energy intake in the clam Atactodea striata. Mar. Pollut. Bull 124. DOI: 10.1016/j.marpolbul.2016.12.027. [DOI] [PubMed] [Google Scholar]
- Zettler ER, Mincer TJ, Amaral-Zettler LA, 2013. Life in the “plastisphere”: microbial communities on plastic marine debris. Environ. Sci. Technol 47, 7137–7146. [DOI] [PubMed] [Google Scholar]