Abstract
Ovarian function suppression is the current pharmacotherapy of endometriosis with limited benefit and adverse effects. New therapeutic strategies other than hormonal therapy are developed based on the molecular mechanisms involved in the hypoxic and oxidative stress environments and metabolism unique to endometriosis. A literature search was performed between January 2000 and March 2021 in the PubMed database using a combination of specific terms. Endometriosis-associated metabolic changes have been organized into four hallmarks: (1) glucose uptake, (2) aerobic glycolysis, (3) lactate production and accumulation, and (4) metabolic conversion from mitochondrial oxidative phosphorylation (OXPHOS) to aerobic glycolysis. Endometriotic cells favor glycolytic metabolism over mitochondrial OXPHOS to produce essential energy for cell survival. Hypoxia, a common feature of the endometriosis environment, is a key player in this metabolic conversion, which may lead to glucose transporter overexpression, pyruvate dehydrogenase kinase 1 (PDK1) and lactate dehydrogenase kinase A (LDHA) activation, and pyruvate dehydrogenase complex inactivation. Evading mitochondrial OXPHOS mitigates excessive generation of reactive oxygen species (ROS) that may trigger cell death. Therefore, the coinactivation of LDHA and PDK1 can induce the accumulation of mitochondrial ROS by converting energy metabolism to mitochondrial OXPHOS, causing endometriotic cell death. Metabolic pattern reconstruction in endometriotic lesions is a critical factor in cell survival and disease progression. One therapeutic strategy that may avoid hormone manipulation is focused on mitigating metabolic changes that have been detected in cells/tissues from women with endometriosis.
Lay summary
The most commonly used medical therapies for endometriosis have contraceptives and other side effects associated with hormone suppression and are therefore unsuitable for women desiring pregnancy. One therapeutic strategy that may avoid hormone manipulation is focused on changing metabolic profiles that have been detected in cells/tissues from women with endometriosis. Endometriotic cells favor glycolytic metabolism over mitochondrial oxidative phosphorylation (OXPHOS) to produce essential energy for cell growth. Furthermore, the metabolic conversion from mitochondrial OXPHOS to aerobic glycolysis suppresses cell death through the reduced generation of reactive oxygen species (ROS). This unique metabolic feature of endometriosis is important for cell survival and disease progression. Thus, changing the specific metabolic switch may increase mitochondrial ROS production, causing severe oxidative stress and cell death. This review describes new treatments by changing the metabolic profiles of endometriosis.
Key Words: endometriosis, glycolysis, hypoxia, metabolism, oxidative phosphorylation, Warburg effect
Introduction
Endometriosis is an estrogen-dependent, chronic inflammatory condition that contains tissue that resembles an endometrium with one or more of the following: stromal fibroblasts, epithelial cells, immune cells, and nerves and vascular/perivascular cells in sites outside the uterine cavity (Zondervan et al. 2020, Saunders & Horne 2021). Moreover, it affects approximately 10% of all reproductive-aged women and is associated with pain and infertility (Hughes et al. 2015, Zondervan et al. 2020, Saunders & Horne 2021). The treatment choice will depend on age at diagnosis, disease stage, the patient’s symptoms, priorities and expectations, reproductive plans, safety, adverse effects incidence, tolerability, and cost (Ferrero et al. 2018). Medical endometriosis therapy should consider pain symptom control and postoperative recurrence prevention within the framework of long-term therapeutic strategies (Ferrero et al. 2018). However, the available drugs (e.g. combined oral contraceptive pills, progestins, danazol, and gonadotropin-releasing hormone (GnRH) analogs) suppress ovarian function and are not curative (Hughes et al. 2015, Ferrero et al. 2018). Thus, patients with endometriosis urgently need long-term nonhormonal therapy without affecting fertility.
Endometriosis exists in a unique inflammatory microenvironment characterized by hormonal imbalance, hypoxia, and oxidative stress (McKinnon et al. 2016, Ito et al. 2017, Lin et al. 2018). Endometriotic cells undergo genetic, epigenetic, and metabolic alterations to overcome many obstacles (e.g. adaptation and survival to harsh environments, evasion of immune defenses, and invasion of adjacent tissues; Koninckx et al. 2019). These microenvironmental changes can enhance the survival of endometriotic cells through several main pathways (e.g. phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR), mitogen-activated protein kinases (MAPK; extracellular signal-regulated kinase (ERK)1/2, p38, and c-Jun NH2-terminal kinase (JNK)), and nuclear factor-kappaB (NF-κB) signaling pathways; McKinnon et al. 2016). These kinase pathways have been evaluated as effective targets for the treatment of other diseases, especially cancer (3), and can be potential candidates for personalized endometriosis therapy (McKinnon et al. 2016). However, the current generation of these targeted therapies can induce various adverse effects (McKinnon et al. 2016). Moreover, accumulating evidence shows that endometriotic cells may survive the hypoxic environment by upgrading their metabolic properties (Atkins et al. 2019). The metabolic shift between aerobic glycolysis and oxidative phosphorylation plays a major role in the development and progression of endometriosis, and the modification of their signaling pathways can be a viable target for therapeutic intervention (Liao et al. 2015, Kobayashi et al. 2021a). The review aims to discuss the survival mechanism of endometriosis in hypoxic and oxidative stress environments and provide future perspectives on nonhormone treatment based on metabolic shifts.
Methods
Search strategy and selection criteria
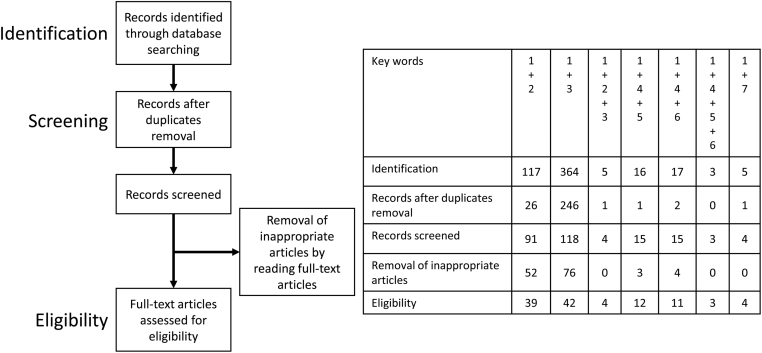
A computerized literature search was performed to identify relevant studies reported in the English language. The PubMed electronic databases published between January 2000 and March 2021 were searched, combining the keywords endometriosis, hypoxia, oxidative stress, metabolism, glycolysis, oxidative phosphorylation, and Warburg. The references of each article were searched to identify potentially relevant studies. Publications of original studies and review papers were included. Given the heterogeneity in the research theme, data from the studies were synthesized using a descriptive review design with narrative methods. Figure 1 shows that the first identification phase includes the records identified through database search. Terms in the titles and abstracts were focused in the first screening stage. During the second screening phase, duplicates were removed, and titles, abstracts, and full-text articles were read to remove inappropriate papers. The final eligibility phase included the full-text articles for analysis after excluding those for which detailed data cannot be extracted.
Figure 1.
The number of articles identified by searching for keyword combinations. This figure shows the number of articles identified by keyword combinations and the number of records identified through database searching, records after duplicate removal, records screened, removal of inappropriate articles by reading full-text articles, and full-text articles assessed for eligibility. Keywords: 1, endometriosis; 2, hypoxia; 3, oxidative stress; 4, metabolism; 5, glycolysis; 6, oxidative phosphorylation; and 7, Warburg.
Results and discussion
A review of the literature provides evidence that endometriotic cells may undergo metabolic change/adaptation to survive in extrauterine sites under conditions that may involve hypoxia and/or oxidative stress. The evidence is considered a shift in metabolic behavior under oxidative stress and hypoxia to inform the discussion of potential novel therapies. Here, three topics of endometriosis (i.e. oxidative stress and redox imbalance, hypoxic microenvironment, and metabolic reprogramming) will be discussed.
Oxidative stress and redox imbalance
Several theories have been proposed to explain the etiology of endometriosis, which includes the theories on retrograde menstruation, coelomic metaplasia, endometrial stem/progenitor cells, bone marrow stem cells, lymphatic and vascular spread, embryonic remnant differentiation or induction, and iatrogenic implantation (Zubrzycka et al. 2015). The most widely accepted is the retrograde menstruation theory. Blood containing endometrial cells is refluxed through the fallopian tubes during menstruation (Vinatier et al. 2000). Hemoglobin releases heme iron and free iron when red blood cells are hemolyzed in the peritoneal cavity or endometriotic cysts (Kobayashi et al. 2009). Hemoglobin generates superoxide radicals (O2−) when converted to methemoglobin via the autoxidation reaction (Iwabuchi et al. 2015). Free iron also generates hydroxyl radicals (OH−), a powerful reactive oxygen species (ROS), through the Fenton reaction (Iwabuchi et al. 2015). Thus, endometriotic cells are always exposed to exogenous ROS, including superoxide anion, hydroxyl radical, and peroxynitrite (ONOO−). High ROS levels induce oxidative DNA damage, methylation, and epigenetic errors (Menezo et al. 2016). Oxidative stress caused by ROS is a potential factor involved in the pathogenesis of endometriosis and may play a role in the onset and progression of this disease (Menezo et al. 2016, Ito et al. 2017). However, excessive ROS generation is also a key factor leading to cell death. Several studies have evaluated the oxidant–antioxidant balance in the blood, peritoneal fluid, follicular fluid, and tissue environment of patients with endometriosis (Santanam et al. 2002, Muscoli et al. 2003, Oner-Iyidoğan et al. 2004, Matos et al. 2009, Liu et al. 2013, Bamm et al. 2017, Chen et al. 2019). The ROS levels in both serum and follicular fluid of the endometriosis group were significantly higher than those in both serum and follicular fluid of the control group (Liu et al. 2013). The conjugated diene/triene, malondialdehyde, and oxidized low-density lipoproteins are lipid oxidation biomarkers (Santanam et al. 2002, Bamm et al. 2017). The levels of these lipid peroxidation end products were increased in both peritoneal fluid and serum of patients with endometriosis (Santanam et al. 2002, Bamm et al. 2017). Furthermore, the antioxidant capacities (e.g. superoxide dismutase (SOD) activity) were increased in endometriosis (Oner-Iyidoğan 2004, Matos et al. 2009, Chen et al. 2019). ROS suppresses SOD production, but SOD expression is upregulated in endometriosis despite ROS overproduction (Muscoli et al. 2003). Antioxidants maintain cellular redox homeostasis by eliminating ROS and protecting cells from ROS-induced damage (Chen et al. 2019). Thus, endometriotic cells can survive with oxidative stress exposure.
Hypoxic microenvironment
Endometrial fibroblasts are decidualized during pregnancy, allowing placenta formation and embryo implantation (Rytkönen et al. 2020). Placental tissue may have evolved mechanisms to tolerate hypoxic environments by expressing hypoxia-related genes such as hypoxia-inducible factor-1alpha (HIF-1α), vascular endothelial growth factor (VEGF), and transforming growth factor-beta1 (TGF-β1; Duzyj et al. 2018). Ectopic endometriotic cells also appear to inherit this property. Ectopic endometrial cells face severe hypoxic stress, but hypoxia plays a vital role in promoting pathological processes to facilitate endometriosis development (Lin et al. 2018, Lee et al. 2019, Wu et al. 2019). Under a hypoxic condition, cells undergo genetic and epigenetic modifications and evolve several survival processes, including steroidogenesis, inflammation, immune dysfunction, angiogenesis, epithelial–mesenchymal transition (EMT), and mesothelial–mesenchymal transition (MMT; Wu et al. 2019). The complex gene regulatory networks driven by the interplay between a hypoxic microenvironment and endometriotic cells allow endometriotic cells to survive (Wu et al. 2019). The effects induced by hypoxia are orchestrated by HIFs that regulate the expression of numerous genes, including VEGF, TGF-β1, PI3K/AKT, Wnt/β-catenin, and Notch (Laschke & Menger 2012, Wilson 2018, Rytkönen et al. 2020). Genes related to classical hypoxia pathways (e.g. HIF-1α, VEGF, and TGF-β1) have been extensively studied in endometriotic cells (Laschke & Menger 2012, Rytkönen et al. 2020) and adjacent peritoneal mesothelial cells (Wilson 2018). A hypoxic microenvironment stimulates endometriotic stromal cells (Dai et al. 2019) and peritoneal mesothelial cells (Lin et al. 2018) to produce and stabilize HIF-1α and promote the activation of TGF-β1/Smad and VEGF signal transduction pathways, contributing to increased cellular invasiveness, adhesiveness, cell survival, EMT, MMT, adhesion and fibrosis formation, and reduced apoptotic potential (Kasvandik et al. 2016). Endometriosis can be caused by local changes in tissues under the influence of oxidative stress and associated hypoxia. In addition, hypoxia has recently been emphasized to upregulate genes associated with glycolysis as described in the next section.
Metabolic reprogramming
The metabolic properties of endometriosis for energy acquisition are discussed in this section. In general, glycolytic conversion of glucose or fructose into adenosine 5′-triphosphate (ATP) generates energy to enable cell survival and growth (Fig. 2). Cells utilize aerobic glycolysis to derive energy from the conversion of glucose to pyruvate and then lactate, regardless of oxygen availability (Vander Heiden et al. 2009). Aerobic glycolysis produces only two ATP per one glucose molecule, whereas additional 36 ATP molecules from one glucose molecule are produced through the tricarboxylic acid (TCA) cycle and the OXPHOS machinery (Vander Heiden et al. 2009). Aerobic glycolysis is an inefficient way to generate ATP, but it is a simple mechanism with a high ATP production rate. Aerobic glycolysis is activated by the stimulation of glycolytic enzymes such as glucose transporter (GLUT; McKinnon et al. 2014, Di Tucci et al. 2018), phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3; Yi et al. 2019), pyruvate kinase M2 (PKM2; Tamada et al. 2012), pyruvate dehydrogenase kinase 1 (PDK1; Dunford et al. 2011), pyruvate dehydrogenase (PDH; Dunford et al. 2011), lactate dehydrogenase A (LDHA; Miao et al. 2013), and monocarboxylate transporter 1 (MCT-1; Halestrap 2012; Fig. 2; glycolytic pathways are surrounded by a yellow square). PFKFB3, as a key enzyme of glycolysis, positively regulates the glycolysis process (Yi et al. 2019). PKM2 is a final rate-limiting glycolysis enzyme and supports anabolic metabolism (Tamada et al. 2012). Pyruvate is converted to acetyl-coenzyme A (CoA), which is catalyzed by the PDH complex (Lapel et al. 2017). PDK1 is an enzyme that phosphorylates and deactivates PDH (Dunford et al. 2011). In addition, LDHA catalyzes the conversion of pyruvate to lactate and is considered a key checkpoint of anaerobic glycolysis (Miao et al. 2013). MCT-1 facilitates the rapid intracellular and extracellular transport of monocarboxylates (e.g. pyruvate, lactate, and the ketone bodies; Halestrap 2012). Fatty acids are transported to the mitochondria and then metabolized to acetyl-CoA by β-oxidation, which feeds the TCA cycle. Acetyl-CoA is the key starting point of the mitochondrial TCA cycle and an essential fuel for ensuring OXPHOS (Fig. 2; mitochondrial oxidative phosphorylation pathways are surrounded by a green square). However, stimulation of pyruvate flux into the mitochondrial oxidative metabolism increases ROS production, an inherent byproduct of oxidative metabolism, leading to impaired cell survival. Thus, a shift in metabolism from glycolysis to the TCA cycle/OXPHOS has not only the advantage of high energy production but also the drawback of ROS overproduction.
Figure 2.
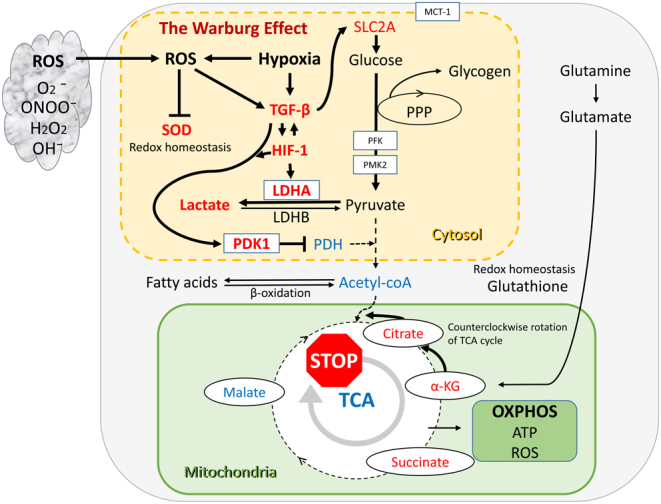
Glycolysis and mitochondrial metabolism in endometriosis. Colored boxes indicate major metabolic pathways: aerobic glycolysis (yellow box) and the TCA cycle/OXPHOS (green box). Red letters indicate increased genes, gene transcripts, enzymes, and metabolites; blue letters indicate reduced expression.
Endometriotic cells have been shown to reprogram metabolism pathways in response to various hypoxic and oxidative stress to fuel cell survival (Dunford et al. 2011, Young et al. 2014, 2016, Kasvandik et al. 2016, Lee et al. 2019). Endometriotic cells can induce metabolic conversion from oxidative phosphorylation to aerobic glycolysis to suppress ROS-mediated apoptosis. Four major steps are involved in cell metabolism: glucose uptake, glycolytic enzyme activation, lactate production and accumulation, and changes in mitochondrial function. For each step, the latest information on the metabolic alterations in endometriosis is summarized.
Enhanced glucose uptake
Glycolysis begins with glucose uptake through solute carriers of the GLUT family (McKinnon et al. 2014). Solute carrier family 2 (SLC2A) gene encodes an integral plasma membrane glycoprotein, GLUT. The expression of SLC2A3 (GLUT3), SLC2A4 (GLUT4), and SLC2A5 (GLUT5) genes and proteins in endometriotic tissues was significantly higher than that in eutopic tissues (McKinnon et al. 2014). HIF1A gene expression was higher in endometriotic lesions than in eutopic endometrium, and the HIF1A and SLC2A1 gene expression levels in the adjacent peritoneum of endometriotic lesions were higher than those in women without the disease (Di Tucci et al. 2018). An in vitro study showed that exposure of peritoneal mesothelial cells to TGF-β1 increased HIF1A and SLC2A1 mRNA expression (Di Tucci et al. 2018). Cellular glucose uptake by GLUTs is activated via the upregulation of TGF-β expression (McKinnon et al. 2014). Enhanced glucose uptake as a result of increased HIF-1 and TGF-β1 expression is a hallmark of endometriosis. Therefore, endometriosis causes metabolic reprogramming by increasing glucose uptake via the GLUT family (Fig. 2, yellow box).
Increased glycolytic capacity and lactate production
Ectopic endometriotic cells exhibit more hypoxia than their eutopic counterparts (Lee et al. 2019). Some researchers compared tissue, peritoneal fluid, follicular fluid, and blood samples from patients with endometriosis to controls and showed significant changes in glycolytic pathway-specific genes and their transcripts (HIF-1, TGF-β, LDHA, PDK1, PDH, and SOD) and metabolites (glucose and lactate), indicating a distinct glucose metabolic signature (Qi et al. 2014, Young et al. 2014, 2016, Marianna et al. 2017, Horne et al. 2019). Lactate, an essential glycolysis product, is a major metabolic fuel, energy source, and gluconeogenic precursor. Lactate concentration was positively correlated with TGF-β1 in peritoneal fluid, and both of which were significantly higher in women with endometriosis than in women without endometriosis (Qi et al. 2014, Young et al. 2014, 2016, Horne et al. 2019). TGF-β1 can induce the metabolic conversion of glucose to lactate in the endometriotic lesions and adjacent peritoneum, possibly through hypoxia-induced HIF-1α expression (Young et al. 2014, 2016). Moreover, hypoxia-induced PDK1 upregulation and increased lactate production and oxygen consumption rate in ectopic endometrial stromal cells compared to normal endometrial stromal cells (Lee et al. 2019). This is thought to be because PDK1 suppressed the conversion of pyruvate to acetyl-CoA through the inhibition of PDH activity (Dunford et al. 2011). Exposure of mesothelial cells to TGF-β1 increased the production of mRNAs encoded by glycolysis-associated genes, namely, PDK1 and LDHA (Young et al. 2014). Glycolysis-related gene LDHA was more highly expressed in endometriotic lesions than in a eutopic endometrium (Young et al. 2014). Furthermore, follicular fluid in patients with endometriosis had lower glucose levels and higher levels of lactate, pyruvate, and VEGF than those in follicular fluid in control participants (Marianna et al. 2017, Pocate-Cheriet et al. 2020). Increased glucose uptake and consumption and accumulation of lactate were common features of endometriotic cells (Qi et al. 2014). Lactate has been reported to be proangiogenic (Hunt et al. 2008). Although no experimental data using endometriotic cells exist, lactate stimulates VEGF production by tumor and endothelial cells, leading to enhanced migration and resulting in lactate-induced angiogenesis (Hirschhaeuser et al. 2011, Marianna et al. 2017). Altogether, endometriotic cells have an increased glycolytic flux, which depends on the overexpression of glycolysis-related genes or their transcripts (HIF-1α, TGF-β, GLUT, LDHA, and PDK1), resulting in lactate overproduction and accumulation (Qi et al. 2014, Young et al. 2014, 2016, Marianna et al. 2017, Horne et al. 2019). The metabolic switch of increased glycolysis in endometriosis is thought to be driven primarily by TGF-β and HIF-1α (Fig. 2, yellow box).
Metabolic conversion from TCA cycle/OXPHOS to aerobic glycolysis
Activation of aerobic glycolysis raises two possibilities. First, pyruvate is channeled into the mitochondria and converted to acetyl-CoA, and then enters the TCA cycle. Hypoxia-induced PDK1 expression results in decreased PDH activity, suppresses the conversion of pyruvate to acetyl-CoA, and accumulates pyruvate (Dunford et al. 2011, Young et al. 2014, 2016, Kasvandik et al. 2016, Lee et al. 2019). Second, LDHA promotes the conversion of pyruvate to lactate and suppresses the production of acetyl-CoA. Therefore, the conversion of pyruvate to acetyl-CoA in endometriosis may be suppressed by increased LDHA and PDK1 activity and decreased PDH activity (Young et al. 2014, 2016, Kasvandik et al. 2016) (Fig. 2, green box).
Next, reports on the concentrations of intermediate metabolites involved in glycolysis and the TCA cycle from body fluid samples in patients with endometriosis and controls were summarized. Endometriosis patients showed greater changes in levels of metabolites (e.g. glucose, lactate, citrate, alpha-ketoglutarate, succinate, and malate) compared to controls. Serum (Dutta et al. 2012) and follicular fluid (Marianna et al. 2017, Karaer et al. 2019) samples from patients with endometriosis showed elevated lactate and succinate levels and reduced glucose levels compared to controls. Metabolomics analysis revealed that citrate, alpha-ketoglutarate (α-KG), and succinate were elevated in endometriosis (Jana et al. 2013) whereas malate was decreased (Atkins et al. 2019). The cause for the elevated citrate, α-KG, and succinate in endometriosis was considered. In general, glycolysis, glutaminolysis, or fatty acid β-oxidation provides the energy and macromolecules required for cell survival. For example, cancer patients show distinctly altered metabolism involved in glycolysis, TCA cycle, glutaminolysis, and fatty acid metabolism (Zhu et al. 2017). In the event of an energy crisis, the glutaminolysis involved in the conversion of glutamine to α-KG is activated to sustain energy metabolism (DeBerardinis et al. 2007). Glutaminolysis stimulates a pathway in which citrate was formed from α-KG through reductive carboxylation of isocitrate dehydrogenase (Wise et al. 2011). Therefore, endometriotic mitochondria can produce large amounts of α-KG and citrate (Fig. 2, green box). Furthermore, glutaminolysis supports the production of glutathione, a major player in maintaining redox homeostasis (Wise & Thompson 2010). Thus, endometriosis can adapt to a unique environment by suppressing oxidative stress and enhancing its antioxidant capacity.
Surprisingly, despite survival in harsh environments, mitochondrial energy production and metabolism are reduced in endometriotic tissue compared to normal endometrial tissue (Atkins et al. 2019). Peritoneal mesothelial cells adjacent to endometriotic lesions also exhibited significantly higher glycolysis, increased lactate production, and lower mitochondrial respiration compared to those from women without the disease (Horne et al. 2019). These data suggest that endometriotic cells and adjacent peritoneal mesothelial cells are characterized by TCA cycle/OXPHOS arrest and metabolic shift to aerobic glycolysis. Endometriosis can alter cellular metabolism and can strategically reduce energy production to avoid excessive mitochondrial ROS production. The electron-producing oxidative pathway appears to be stopped in endometriosis, resulting in the lack of energy production.
Does this suggest mitochondrial dysfunction? The metabolic shift from OXPHOS to glycolysis is known as the Warburg effect and is a characteristic of many cancers (Kasvandik et al. 2016, Liberti & Locasale 2016, Atas et al. 2020). This mechanism can be driven by the TGF-β1–HIF-1α–PDK–PDH–LDHA system (Kim et al. 2006, Young et al. 2016, Wang et al. 2019, Atas et al. 2020). HIF-1 and TGF-β increased LDHA expression, promoted lactate production from pyruvate, and inhibited acetyl-CoA production from pyruvate through PDH deactivated by PDK1, consequently reducing mitochondrial energy production and ROS generation (Liao et al. 2015, Wang et al. 2019). The advantage of the Warburg effect is to suppress ROS overproduction, activate the survival signal of endometriotic cells, and thus prevent cell death (Kobayashi et al. 2021b). Like cancer cells, endometrial cells may shift energy metabolism from OXPHOS to aerobic glycolysis, suppress ROS production, and then promote survival (Liao et al. 2015, Kobayashi et al. 2021b). Alterations in the metabolic phenotype of endometriotic cells and adjacent peritoneal mesothelial cells are considered adaptations of endometriosis to the microenvironment rather than mitochondrial dysfunction.
Nonhormonal endometriosis treatment
This section discusses therapies that may alter energy metabolism, including the so-called Warburg effect. Treatment strategies for endometriosis have been divided into three categories: (1) glucose uptake suppression, (2) aerobic glycolysis suppression, and (3) metabolic switch from aerobic glycolysis to OXPHOS. Not all of the drugs described below have yielded promising preclinical outcomes for endometriosis. Some drugs that have been tested as therapies in preclinical models involving cancer cells that also exhibit altered metabolism have been considered (Table 1).
Table 1.
Summary of drugs or therapeutics tested in endometriosis and other models. This table includes target protein/metabolite, mechanism of action, in vitro/in vivo/animal experiments, results, and references.
| Target protein/metabolite |
The mechanism of action |
In vitro/in vivo/animal experiments |
Results |
References |
|---|---|---|---|---|
| (1) Suppression of glucose uptake | ||||
| Genistein | A natural isoflavone | In vitro/xenograft mouse models; hepatocellular carcinoma cells | Genistein suppressed aerobic glycolysis and induced hepatocellular carcinoma cell death | Li et al. (2017b) |
| Genistein, phlorizin, ritonavir, indinavir, STF-31, and WZB117 | A natural isoflavone; glucose transporter (GLUT and SGLT) inhibitors; HIV protease inhibitor | In vivo/in vivo/mouse model; PBMCs of patients with ulcerative colitis | The HIV protease inhibitor ritonavir suppressed glucose uptake to improve ulcerative colitis | Jodeleit et al. (2018) |
| SLC2A* | Glucose transporter | Human tissue samples | Glucose transporter SLC2A expression in ectopic endometriotic lesions is significantly higher than in eutopic endometrial tissue | McKinnon et al. (2014) |
| GZFLC* | A classic Chinese medicinal formula | Rat endometriosis model | GZFLC suppressed the expression levels of TGF-β1, GLUT4, and VEGF and inhibited the development of endometriosis | Zhou et al. (2018) |
| Atorvastatin and resveratrol* | Statin: inhibitors of hydroxymethylglutaryl-CoA reductase | Female Wistar rats/the experimental endometriosis | Effects of atorvastatin and resveratrol against the experimental endometriosis; evidence for glucose and monocarboxylate transporters | Bahrami et al. (2021) |
| (2) Suppression of aerobic glycolysis | ||||
| Genetic ablation HK2 | A family of ubiquitous exose-phosphorylating enzymes that prime glucose for intracellular utilization | Mouse models; hepatocellular carcinoma/colorectal cancer/glioblastoma, etc. | Genetic ablation of HK2 inhibited tumor growth | Ciscato et al. (2021) |
| (E)-1-(pyridin-4-yl)-3-(quinolin-2-yl)prop-2-en-1-one (PFK15) | Enzymes related to glycolysis; inhibitors of PFKFB3; glycolysis blockage by targeting PFKFB3 | In vitro/in vivo/mouse models; head and neck squamous cell carcinoma | Targeting aerobic glycolysis with PFKFB3 inhibitors suppressed tumor growth and metastasis, providing a promising strategy for cancer treatment | Li et al. (2017a) |
| Benserazide: inhibitors of PKM2 | PKM2 is an enzyme that generates pyruvate and ATP in the glycolytic pathway | In vitro/in vivo; melanoma | Benserazide blocked PKM2 enzyme activity, leading to inhibition of aerobic glycolysis; benserazide inhibited tumor cell proliferation, colony formation, invasion, and migration in vitro and in vivo models | Zhou et al. (2020) |
| Inhibitor of HSF1: KRIBB11* | A transcription factor that is rapidly induced after temperature stress and binds heat shock promoter elements | In vitro/in vivo/mouse models; endometriotic epithelial cell line (11Z) and human ESC | HSF1 promoted endometriosis development and glycolysis by upregulating PFKFB3 expression; the HSF1 inhibitor KRIBB11 abrogated endometriosis progression in vitro and in vivo | Wang et al. (2021) |
| (3) Metabolic switch from aerobic glycolysis to OXPHOS | ||||
| DCA | DCA is an anticancer agent that can reverse the glycolytic phenotype in cancer cells; a pyruvate analog; a prototypical PDK inhibitor | Several cancers | DCA inhibits mitochondrial PDK, shifted metabolism from glycolysis to glucose oxidation, decreased mitochondrial membrane potential, and increased mitochondrial H2O2; DCA decreased proliferation, induced apoptosis, and inhibited tumor growth; the orally available DCA is a promising selective anticancer agent | Bonnet et al. (2007) |
| DCA | In vitro/in vivo/rat models; breast cancer | DCA has antiproliferative properties in addition to promoting apoptosis | Sun et al. (2010) | |
| DCA | In vitro/in vivo/mouse models; multiple myeloma | DCA may be effective in multiple myeloma patients with an activated aerobic glycolytic pathway | Sanchez et al. (2013) | |
| DCA | Several cancer models; clinical administration in cancer therapy | Coadministration of DCA with conventional chemotherapy, radiotherapy, other drugs, or natural compounds may be promising for effective cancer therapy | Tataranni & Piccoli (2019) | |
| Three glycolysis inhibitors: DCA, 2-deoxyglucose, or 3-promopyruvate | In vitro; hepatocellular carcinoma HepG2 cells | The chemotherapeutic agent and glycolysis inhibitors induced oxidative stress-associated damage in HepG2 cells. | Korga et al. (2019) | |
| DCA | A phase 1 study in patients with advanced solid tumors | The phase 1 study was undertaken to assess the safety, recommended dose, and pharmacokinetic profile of oral DCA in patients with advanced solid tumors. | Chu et al. (2015) | |
| DCA | An openlabel phase II trial | The clinical trial determined the response rate, safety, and tolerability of oral DCA in patients with metastatic breast cancer and advanced-stage nonsmall cell lung cancer. Patients with previously treated advanced cancer did not benefit from oral DCA |
Garon et al. (2014) | |
| DCA | A pilot phase 2 study in patients with multiple myeloma | The pharmacokinetic profile for DCA varied from patient to patient, and the overall response rate for multiple myeloma was low | Tian et al. (2019) | |
| DCA* |
In vitro;
ectopic endometriotic stromal cells |
The PDK1 expression was upregulated in ectopic stromal cells through hypoxia-induced signals; inhibition of PDK1 activity by treatment with DCA-induced ectopic stromal cell death | Lee et al. (2019) | |
| DCA* | In vitro/in vivo/mouse models; endometriosis | Human peritoneal mesothelial cells (HPMC) in women with endometriosis exhibited metabolic conversion from OXPHOS to aerobic glycolysis due to reduced enzymatic activity of PDH compared to HPMC in disease-free women; TGF-β1 is believed to be responsible for this abnormal phenotype; treatment of endometriosis HPMC with DCA normalizes metabolism and suppresses the proliferation of ESC; oral DCA reduced endometriosis lesion size in a mouse model | Horne et al. (2019) | |
| DCA | Sepsis model: Drosophila melanogaster model of surviving sepsis infected with Staphylococcus aureus | DCA treatment was associated with improved lifespan of sepsis survivors | Bakalov et al. (2020) | |
| IQ | A sesquiterpene quinone isolated from the marine sponge Smenospongia cerebriformis; PDK1 inhibitor | Human and murine cancer cells, such as A549, DLD-1, RKO, and LLC cells | A novel candidate for anticancer therapeutics that act via the inhibition of PDK1 activity | Kwak et al. (2020) |
| Caesalpinia sappan L. (family Leguminosae)* | A herbal medicinal product used to treat gynecological symptoms, including amenorrhea; PDK1 inhibitor |
In vitro; endometriotic cells | C. sappan inhibited lactate production and phosphorylation of PDH by reducing the expression of PDK1; a novel drug candidate for treating endometriosis by inhibiting aerobic glycolysis and inducing ROS-mitochondria-mediated apoptotic cell death | Kim et al. (2021) |
| FX11 | Specific LDHA inhibitor: a small-molecule inhibitor | In vitro/human lymphoma and pancreatic cancer xenografts | FX11-induced significant oxidative stress and cancer cell death | Le et al. (2010) |
| N-hydroxy-2-carboxy-substituted indole compounds | Specific LDHA inhibitor: a small-molecule inhibitor | In vitro NMR experiments | Functional analysis of synthesized LDHA inhibitors | Granchi et al. (2011) |
| Inhibition of LDHA by either RNA interference or pharmacological agents | Inhibition of LDHA by either RNA interference or pharmacological agents | In vitro/in vivo; several cancer cells. | Review of inhibition of LDHA by either RNA interference or pharmacological agents block tumor progress in vivo | Oermann et al. (2012) |
| Inhibition of LDHA by either RNA interference or pharmacological agents | Inhibition of LDHA by either RNA interference or pharmacological agents | In vitro/in vivo; cancers including breast cancer and hepatocellular carcinoma | Review of inhibition of LDHA can block tumor growth, maintenance, and progression in vitro and in vivo | Miao et al. (2013) |
| shRNA-mediated knockdown of LDHA | Inhibition of LDHA by either RNA interference or pharmacological agents | In vitro; breast cancer MDA-MB-435 cells | shRNA-mediated knockdown of LDHA resulted in elevated mitochondrial ROS production and a concomitant decrease in cell proliferation and motility in breast cancer MDA-MB-435 cells | Arseneault et al. (2013) |
| Inhibition of LDHA by either RNA interference* | Inhibition of LDHA by either RNA interference | Immunohistochemistry of human endometriosis samples; in vitro. | Hypoxia treatment induced the expression of LDHA; silencing of LDHA expression displayed an impairment of mitochondrial function and promoted apoptosis while inhibiting migration and glycolysis | Zheng et al. (2021) |
| None | Schizophrenia | Experiments with schizophrenia brain | A significant increase in lactate in schizophrenia brain | Pruett and Meador-Woodruff (2020) |
| None | Autoimmune disease | Animal studies | Pro-inflammatory signals in autoimmune disease induced metabolic reprogramming, characterized by a shift to aerobic glycolysis | Kornberg (2020) |
*Results of preclinical studies on endometriosis.
DCA, dichloroacetate; ESC, endometrial stromal cells; FX11, 3-dihydroxy-6-methyl-7-(phenylmethyl)-4-propylnaphthalene-1-carboxylic acid; GZFLC, Gui-Zhi-Fu-Ling capsules; HK2, hexokinase 2; HSF1, heat shock factor 1; IQ, ilimaquinone; PBMCs, peripheral blood mononuclear cells; PFKFB3, phosphofructokinase-2/fructose-2,6-bisphosphatase 3; PKM2, pyruvate kinase isozyme; SLC2A, solute carrier family 2.
Glucose uptake suppression
The potent GLUT inhibitors can attenuate glycolysis and suppress the growth of various cancer cells (Reckzeh et al. 2019). GLUT and SGLT inhibitors include genistein, phlorizin, ritonavir, indinavir, STF-31, and WZB117 (Jodeleit et al. 2018). Genistein downregulates HIF-1α, inactivating GLUT1 and HK2 to suppress aerobic glycolysis (Li et al. 2017b). GLUTs were identified as off-target molecules of the HIV protease inhibitor ritonavir (Jodeleit et al. 2018). They exert antitumor effects by targeting GLUT1 via inhibiting glucose uptake in tumor cells. Recently, the glucose uptake inhibitors, which target GLUT isoforms, have also been studied for endometriotic cells. In particular, GLUT inhibitors may be an attractive target for the nonhormone-based treatment of endometriosis (McKinnon et al. 2014). The mRNA levels of GLUT1/3 and MCT1/4 were decreased in atorvastatin and resveratrol sole and simultaneous-treated groups in experimental endometriosis models (Bahrami et al. 2021). Atorvastatin did not cause significant changes during the glucose tolerance test, but coadministration of atorvastatin and resveratrol suppressed glycolysis and neovascularization (Bahrami et al. 2021). The simultaneous administration of atorvastatin and resveratrol can inhibit endometriosis development (Bahrami et al. 2021). Gui-Zhi-Fu-Ling capsules, a classic Chinese medicinal formula, may have benefits in inhibiting endometriosis development through the suppression of the expression levels of TGF-β1, GLUT4, and VEGF in a rat endometriosis model (Zhou et al. 2018). Thus, inhibition of glucose uptake may be promising therapeutic targets for endometriosis (McKinnon et al. 2014).
Aerobic glycolysis suppression
Glycolytic enzyme inhibitors such as hexokinase (HK), phosphofructokinase (PHK), and PKM2 have been preclinically studied in cancer treatment.
HK: Hexokinase, an exose-phosphorylating enzyme for aerobic glycolysis, is overexpressed in many tumor cells (Ciscato et al. 2021). Treatments with 2-deoxy-d-glucose, 3-bromopyruvate, or lonidamine inhibit the key enzyme hexokinase of glycolysis, and genetic ablation of hexokinase 2 inhibits tumor growth in mouse models (Ciscato et al. 2021).
PFK: Phosphofructokinase-1 (PFK1), a primary glycolysis enzyme, is involved in the conversion of fructose-6-phosphate to fructose-1,6-bisphosphate (Li et al. 2017a ). (E)-1-(pyridin-4-yl)-3-(quinolin-2-yl)prop-2-en-1-one (PFK15) was developed as a selective antagonist of PFK–PFKFB3 (Li et al. 2017a ). PFK15 has been demonstrated to be effective in treating head and neck squamous cell carcinoma in xenograft mouse models (Li et al. 2017a ). Furthermore, the PFKFB3 expression in endometriotic cells is known to be upregulated by heat shock factor 1 (HSF1; Wang et al. 2021). In addition, Wang et al. (2021) showed that the HSF1 inhibitor KRIBB11 suppressed endometriosis progression in a mouse model.
PKM2: The M2 splice isoform of PKM2 eventually produces pyruvate and releases energy. High PKM2 activity is associated with glycolytic capacity and tumor growth and metastasis (Zhou et al. 2020). Suppression of PKM2 expression attenuated cancer cell growth via modulating immunometabolism (Zhou et al. 2020).
Suppression of the aerobic glycolytic pathway may become a new target for endometriosis treatment, but studies are still in their infancy (Wang et al. 2021).
Metabolic switch from aerobic glycolysis to OXPHOS
The reversal of metabolism from OXPHOS to glycolysis, a metabolic characteristic of cancer cells, may be a therapeutic strategy that induces cell death through ROS overproduction by activating mitochondrial energy metabolism. The conversion of pyruvate to acetyl-CoA needs to be accelerated to reach that goal. PDH is essential for shuttling pyruvate into the mitochondria and fueling the TCA cycle. PDH activity is inhibited by PDK, and PDK inhibitors may help in activating PDH enzymatic activity. In addition, dichloroacetate (DCA) is a small-molecule pyruvate-mimetic PDK inhibitor (Bonnet et al. 2007). DCA promotes oxidative metabolism from anaerobic glycolysis to mitochondrial OXPHOS through PDH activation by PDK1 inhibition (Sun et al. 2010, Horne et al. 2019, Tataranni & Piccoli 2019). This drug was shown to reverse the PDK-induced glycolytic phenotype (Bonnet et al. 2007, Tataranni & Piccoli 2019). DCA suppressed the growth of some tumors in the field of cancer, and several preclinical studies have been reported (Bonnet et al. 2007, Sun et al. 2010, Sanchez et al. 2013, Korga et al. 2019, Tataranni & Piccoli 2019). DCA can induce cell death via excess ROS produced by OXPHOS (Tataranni & Piccoli 2019). Therefore, this drug is a promising adjuvant chemotherapeutic agent as an oxidative stress enhancer (Korga et al. 2019). For example, DCA is potentially effective against multiple myeloma in animal models (Sanchez et al. 2013). In line with this theory, novel clinical DCA studies in cancer therapy are underway (Garon et al. 2014, Chu et al. 2015, Tian et al. 2019). The phase 1 study evaluated the safety, tolerability, recommended dose, pharmacokinetics, and pharmacodynamics of oral DCA in patients with advanced solid tumors (Chu et al. 2015). DCA produced side effects, including neurotoxicity. The open-label phase II trial determined the response rate, safety, and tolerability of oral DCA in patients with metastatic breast cancer and advanced-stage nonsmall cell lung cancer (Garon et al. 2014). However, oral DCA did not confer a clinical benefit in patients with previously treated advanced cancer. In addition, the pharmacokinetic profile for DCA varied from patient to patient, and the overall response rate was low in patients with multiple myeloma (Tian et al. 2019). PDK is a druggable target and may pave the way for further approaches to cancer.
Preclinical studies have shown that selective PDK inhibition suppresses the progression of endometriosis in animal models (Horne et al. 2019, Lee et al. 2019). In vitro and in vivo studies showed that DCA reduced lactate secretion and suppressed endometrial stromal cell proliferation in coculture experiments with endometrial stromal cells and peritoneal mesothelial cells (Horne et al. 2019). In addition, DCA decreased the oxygen consumption rate of ectopic endometrial stromal cells (Lee et al. 2019). Oral DCA administration decreased peritoneal fluid lactate concentration and lesion size in a mouse model of experimental endometriosis (Horne et al. 2019). Aerobic glycolysis mediates growth promotion and resistance to apoptosis of endometriotic cells, and a metabolic shift from glycolysis to OXPHOS is considered a promising therapeutic endometriosis strategy (Kim et al. 2021). A single-arm study has begun to determine whether DCA therapy is an effective and acceptable treatment for endometriosis-related pain (Leow et al. 2021). This study provides a rationale for targeting metabolic shifts as a nonhormonal therapy for women with endometriosis.
In addition, some reports on PDK1 inhibitors such as ilimaquinone (IQ; Kwak et al. 2020) and Caesalpinia sappan L. (Kim et al. 2021) exist. IQ is a sesquiterpene quinone isolated from the marine sponge Smenospongia cerebriformis and inhibits PDK1 activity in cancer cells (Kwak et al. 2020). Moreover, C. sappan is an herbal medicinal product used to treat algomenorrhea and amenorrhea (Kim et al. 2021). Furthermore, C. sappan suppresses PDK1 expression and increases mitochondrial ROS levels, which, in turn, promotes endometrial cell apoptosis (Kim et al. 2021).
Another candidate drug is the LDHA inhibitors. However, this drug has never been used to treat endometriosis in preclinical studies. In light of previous reports, it can be speculated that the coinactivation of LDHA and PDK1 functions shifts from aerobic glycolysis to the TCA cycle/OXPHOS, causing ROS overproduction and culminating in cell death. LDHA regulates pyruvate production and thus acts as a link between glycolysis and the TCA cycle/OXPHOS (Miao et al. 2013). LDHA is elevated in many cancer types. Inhibition of LDHA activity, either by RNA interference or by pharmacological inhibitors, can block tumor growth and progression in vitro and in vivo (Oermann et al. 2012, Miao et al. 2013). Specific LDHA inhibitors include a small-molecule inhibitor 3-dihydroxy-6-methyl-7-(phenylmethyl)-4-propylnaphthalene-1-carboxylic acid (FX11; Le et al. 2010), N-hydroxy-2-carboxy-substituted indole compounds (Granchi et al. 2011), and epigallocatechin (Wang et al. 2013). LDHA siRNA or FX11 can effectively inhibit cancer growth through the shift to OXPHOS and increased intracellular ROS (Arseneault et al. 2013). From the aforementioned data, inactivating LDHA is possible to inhibit the endometriotic cell growth possibly through ROS overproduction. While revising this manuscript, an interesting paper was reported. The silencing of the LDHA expression in immortalized cells promotes apoptosis through glycolysis inhibition and mitochondrial function suppression (Zheng et al. 2021). Future studies are expected to verify the effectiveness of the combination treatment of DCA and LDHA inhibitors in endometriosis. A therapeutic strategy focusing on the shift from aerobic glycolysis to the TCA cycle/OXPHOS may be a promising nonhormonal therapy for endometriosis.
Endometriotic cells are constantly exposed to iron-derived oxidative stress and hypoxic condition, and the shift from aerobic glycolysis to the TCA cycle/OXPHOS may cause ROS overproduction, leading to cell death. Increases in glucose uptake, glycolytic reserve, and gene expression of glycolytic enzymes (HK2, PFKFB3, PKM2, LDHA, and PDK1) are associated with a compensatory decrease in mitochondrial respiration. Molecules that are directly involved in the reprogramming of mitochondrial metabolism may be therapeutic targets for endometriosis (Kobayashi et al. 2021a). In particular, the metabolic shift may be an attractive target for nonhormone-based endometriosis treatment. Treatment strategies that utilize metabolic reprogramming are implemented not only in cancer but also in sepsis (Bakalov et al. 2020), schizophrenia (Pruett & Meador-Woodruff 2020), autoimmune disease (Kornberg 2020), or mitochondrial disease (Kobayashi et al. 2021a).
Summary and conclusions
The currently available treatment options for endometriosis suppress ovarian function, but no cure currently exists. Such treatment is unsuitable for women desiring pregnancy. Therefore, studies on new drugs that do not suppress ovarian function have commenced. Several researchers focused on genes and proteins that may affect metabolic pathways to promote endometriotic cell survival and growth. The metabolism characteristic of endometriosis is significantly affected by estrogen (Kobayashi et al. 2021b). Moreover, estrogen is involved not only in hormonal action but also in various functions (e.g. mitochondrial biosynthesis and energy metabolism). Estrogen can also affect ATP production, energy conversion, ROS production, and antioxidant defense through the regulation of mitochondrial gene expression. Estrogen downstream target genes (e.g. peroxisome proliferator-activated receptor-gamma coactivator 1α), involved in mitochondrial metabolic biosynthesis, may be potential targets for nonhormonal therapy for endometriosis (Kobayashi et al. 2021b). Basic and preclinical studies are steadily progressing, although these drugs are still far from clinical application.
Endometriotic cells often reprogram their metabolic pathways to adapt to environmental challenges and facilitate survival. Endometriotic cells are essentially exposed to a hypoxic microenvironment. HIF-1- and TGF-β-mediated upregulation of LDHA and PDK1 expression induced by hypoxia and oxidative stress is an adaptive phenomenon in endometriosis (Qi et al. 2014, Young et al. 2014, 2016, Marianna et al. 2017, Horne et al. 2019). The actual balance between glycolysis and the TCA cycle/OXPHOS is regulated by glycolytic predominance (Young et al. 2014, Marianna et al. 2017, Lee et al. 2019, Reckzeh et al. 2019, Wang et al. 2019). This is supported by measurements showing local elevation of HIF-1, TGF-β, PDK1, LDHA, and lactate as well as the counterclockwise rotation of the TCA cycle (i.e. elevated levels of citrate, α-KG, and succinate; Dutta et al. 2012, Marianna et al. 2017, Karaer et al. 2019; Fig. 2). Metabolic changes in endometriosis shift from the TCA cycle/OXPHOS to aerobic glycolysis and suppress ROS overproduction for its survival.
This phenomenon is similar to the Warburg effect in cancer (Kasvandik et al. 2016, Liberti & Locasale 2016, Atas et al. 2020). Oxidative stress and hypoxia are largely involved in the development and progression of endometriosis, and functional modifications of these signaling pathways may be a viable target for endometriosis treatment (Kasvandik et al. 2016). Negative regulation of the Warburg effect can increase endogenous ROS and then induce endometriotic cell death (Lim et al. 2021). Therefore, inhibition of PDK and LDHA may be a new strategy in nonhormonal therapy for endometriosis. Currently, a few small-molecule inhibitors and natural compounds have been reported to inhibit PDK (Anwar et al. 2021) or LDHA (Wang et al. 2013) with promising oral administration (Table 1).
In conclusion, metabolic flexibility in endometriosis is the ability to adapt to environmental changes. The reverse Warburg effect could be an attractive target for developing nonhormonal treatments for endometriosis.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS), grant numbers JP16K11150, 18K09269, and 18K09234.
Author contribution statement
H K performed the literature search and collected data using the Web database. H K made a contribution to the conception of the study and also contributed to the interpretation of included research studies. The final version of the manuscript has been read and approved by H K.
References
- Anwar S, Shamsi A, Mohammad T, Islam A, Hassan MI.2021Targeting pyruvate dehydrogenase kinase signaling in the development of effective cancer therapy. Biochimica et Biophysica Acta: Reviews on Cancer 1876188568. ( 10.1016/j.bbcan.2021.188568) [DOI] [PubMed] [Google Scholar]
- Arseneault R, Chien A, Newington JT, Rappon T, Harris R, Cumming RC.2013Attenuation of LDHA expression in cancer cells leads to redox-dependent alterations in cytoskeletal structure and cell migration. Cancer Letters 338255–266. ( 10.1016/j.canlet.2013.03.034) [DOI] [PubMed] [Google Scholar]
- Atas E, Oberhuber M, Kenner L.2020The implications of PDK1-4 on tumor energy metabolism, aggressiveness and therapy resistance. Frontiers in Oncology 10583217. ( 10.3389/fonc.2020.583217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins HM, Bharadwaj MS, O’Brien Cox A, Furdui CM, Appt SE, Caudell DL.2019Endometrium and endometriosis tissue mitochondrial energy metabolism in a nonhuman primate model. Reproductive Biology and Endocrinology 17 70. ( 10.1186/s12958-019-0513-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami A, Ayen E, Razi M, Behfar M.2021Effects of atorvastatin and resveratrol against the experimental endometriosis; evidence for glucose and monocarboxylate transporters, neoangiogenesis. Life Sciences 272119230. ( 10.1016/j.lfs.2021.119230) [DOI] [PubMed] [Google Scholar]
- Bakalov V, Reyes-Uribe L, Deshpande R, Maloy AL, Shapiro SD, Angus DC, Chang CH, Le Moyec L, Wendell SG, Kaynar AM.2020Dichloroacetate-induced metabolic reprogramming improves lifespan in a Drosophila model of surviving sepsis. PLoS ONE 15 e0241122. ( 10.1371/journal.pone.0241122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamm VV, Henein MEL, Sproul SLJ, Lanthier DK, Harauz G.2017Potential role of ferric hemoglobin in MS pathogenesis: effects of oxidative stress and extracellular methemoglobin or its degradation products on myelin components. Free Radical Biology and Medicine 112494–503. ( 10.1016/j.freeradbiomed.2017.08.022) [DOI] [PubMed] [Google Scholar]
- Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet Set al. 2007A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 1137–51. ( 10.1016/j.ccr.2006.10.020) [DOI] [PubMed] [Google Scholar]
- Chen C, Zhou Y, Hu C, Wang Y, Yan Z, Li Z, Wu R.2019Mitochondria and oxidative stress in ovarian endometriosis. Free Radical Biology and Medicine 13622–34. ( 10.1016/j.freeradbiomed.2019.03.027) [DOI] [PubMed] [Google Scholar]
- Chu QS, Sangha R, Spratlin J, Vos LJ, Mackey JR, McEwan AJ, Venner P, Michelakis ED.2015A phase I open-labeled, single-arm, dose-escalation, study of dichloroacetate (DCA) in patients with advanced solid tumors. Investigational New Drugs 33603–610. ( 10.1007/s10637-015-0221-y) [DOI] [PubMed] [Google Scholar]
- Ciscato F, Ferrone L, Masgras I, Laquatra C, Rasola A.2021Hexokinase 2 in cancer: a prima donna playing multiple characters. International Journal of Molecular Sciences 22 4716. ( 10.3390/ijms22094716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Lin X, Xu W, Lin X, Huang Q, Shi L, Pan Y, Zhang Y, Zhu Y, Li Cet al. 2019MiR-210-3p protects endometriotic cells from oxidative stress-induced cell cycle arrest by targeting BARD1. Cell Death and Disease 10 144. ( 10.1038/s41419-019-1395-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB.2007Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. PNAS 10419345–19350. ( 10.1073/pnas.0709747104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Tucci C, Di Feliciantonio M, Vena F, Capone C, Schiavi MC, Pietrangeli D, Muzii L, Benedetti Panici P.2018Alpha lipoic acid in obstetrics and gynecology. Gynecological Endocrinology 34729–733. ( 10.1080/09513590.2018.1462320) [DOI] [PubMed] [Google Scholar]
- Dunford EC, Herbst EA, Jeoung NH, Gittings W, Inglis JG, Vandenboom R, LeBlanc PJ, Harris RA, Peters SJ.2011PDH activation during in vitro muscle contractions in PDH kinase 2 knockout mice: effect of PDH kinase 1 compensation. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology 300R1487–R1493. ( 10.1152/ajpregu.00498.2010) [DOI] [PubMed] [Google Scholar]
- Dutta M, Joshi M, Srivastava S, Lodh I, Chakravarty B, Chaudhury K.2012A metabonomics approach as a means for identification of potential biomarkers for early diagnosis of endometriosis. Molecular Biosystems 83281–3287. ( 10.1039/c2mb25353d) [DOI] [PubMed] [Google Scholar]
- Duzyj CM, Buhimschi IA, Laky CA, Cozzini G, Zhao G, Wehrum M, Buhimschi CS.2018Extravillous trophoblast invasion in placenta accreta is associated with differential local expression of angiogenic and growth factors: a cross-sectional study. BJOG 1251441–1448. ( 10.1111/1471-0528.15176) [DOI] [PubMed] [Google Scholar]
- Ferrero S, Evangelisti G, Barra F.2018Current and emerging treatment options for endometriosis. Expert Opinion on Pharmacotherapy 191109–1125. ( 10.1080/14656566.2018.1494154) [DOI] [PubMed] [Google Scholar]
- Garon EB, Christofk HR, Hosmer W, Britten CD, Bahng A, Crabtree MJ, Hong CS, Kamranpour N, Pitts S, Kabbinavar Fet al. 2014Dichloroacetate should be considered with platinum-based chemotherapy in hypoxic tumors rather than as a single agent in advanced non-small cell lung cancer. Journal of Cancer Research and Clinical Oncology 140443–452. ( 10.1007/s00432-014-1583-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granchi C, Roy S, Giacomelli C, Macchia M, Tuccinardi T, Martinelli A, Lanza M, Betti L, Giannaccini G, Lucacchini Aet al. 2011Discovery of N-hydroxyindole-based inhibitors of human lactate dehydrogenase isoform A (LDH-A) as starvation agents against cancer cells. Journal of Medicinal Chemistry 541599–1612. ( 10.1021/jm101007q) [DOI] [PubMed] [Google Scholar]
- Halestrap AP.2012The monocarboxylate transporter family – structure and functional characterization. IUBMB Life 641–9. ( 10.1002/iub.573) [DOI] [PubMed] [Google Scholar]
- Hirschhaeuser F, Sattler UG, Mueller-Klieser W.2011Lactate: a metabolic key player in cancer. Cancer Research 716921–6925. ( 10.1158/0008-5472.CAN-11-1457) [DOI] [PubMed] [Google Scholar]
- Horne AW, Ahmad SF, Carter R, Simitsidellis I, Greaves E, Hogg C, Morton NM, Saunders PTK.2019Repurposing dichloroacetate for the treatment of women with endometriosis. PNAS 11625389–25391. ( 10.1073/pnas.1916144116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CL, Foster WG, Agarwal SK.2015The impact of endometriosis across the lifespan of women: foreseeable research and therapeutic prospects. BioMed Research International 2015158490. ( 10.1155/2015/158490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt TK, Aslam R, Hussain Z, Beckert S.2008Lactate, with oxygen, incites angiogenesis. Advances in Experimental Medicine and Biology 61473–80. ( 10.1007/978-0-387-74911-2_9) [DOI] [PubMed] [Google Scholar]
- Ito F, Yamada Y, Shigemitsu A, Akinishi M, Kaniwa H, Miyake R, Yamanaka S, Kobayashi H.2017Role of oxidative stress in epigenetic modification in endometriosis. Reproductive Sciences 241493–1502. ( 10.1177/1933719117704909) [DOI] [PubMed] [Google Scholar]
- Iwabuchi T, Yoshimoto C, Shigetomi H, Kobayashi H.2015Oxidative stress and antioxidant defense in endometriosis and its malignant transformation. Oxidative Medicine and Cellular Longevity 2015848595. ( 10.1155/2015/848595) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana SK, Dutta M, Joshi M, Srivastava S, Chakravarty B, Chaudhury K.20131H NMR based targeted metabolite profiling for understanding the complex relationship connecting oxidative stress with endometriosis. BioMed Research International 2013329058. ( 10.1155/2013/329058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodeleit H, Al-Amodi O, Caesar J, Villarroel Aguilera C, Holdt L, Gropp R, Beigel F, Siebeck M.2018Targeting ulcerative colitis by suppressing glucose uptake with ritonavir. Disease Models and Mechanisms 11 dmm036210. ( 10.1242/dmm.036210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaer A, Tuncay G, Mumcu A, Dogan B.2019Metabolomics analysis of follicular fluid in women with ovarian endometriosis undergoing in vitro fertilization. Systems Biology in Reproductive Medicine 6539–47. ( 10.1080/19396368.2018.1478469) [DOI] [PubMed] [Google Scholar]
- Kasvandik S, Samuel K, Peters M, Eimre M, Peet N, Roost AM, Padrik L, Paju K, Peil L, Salumets A.2016Deep quantitative proteomics reveals extensive metabolic reprogramming and cancer-like changes of ectopic endometriotic stromal cells. Journal of Proteome Research 15572–584. ( 10.1021/acs.jproteome.5b00965) [DOI] [PubMed] [Google Scholar]
- Kim JW, Tchernyshyov I, Semenza GL, Dang CV.2006HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metabolism 3177–185. ( 10.1016/j.cmet.2006.02.002) [DOI] [PubMed] [Google Scholar]
- Kim BS, Chung TW, Choi HJ, Bae SJ, Cho HR, Lee SO, Choi JH, Joo JK, Ha KT.2021Caesalpinia sappan induces apoptotic cell death in ectopic endometrial 12Z cells through suppressing pyruvate dehydrogenase kinase 1 expression. Experimental and Therapeutic Medicine 21 357. ( 10.3892/etm.2021.9788) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Yamada Y, Kanayama S, Furukawa N, Noguchi T, Haruta S, Yoshida S, Sakata M, Sado T, Oi H.2009The role of iron in the pathogenesis of endometriosis. Gynecological Endocrinology 2539–52. ( 10.1080/09513590802366204) [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Hatakeyama H, Nishimura H, Yokota M, Suzuki S, Tomabechi Y, Shirouzu M, Osada H, Mimaki M, Goto YIet al. 2021aChemical reversal of abnormalities in cells carrying mitochondrial DNA mutations. Nature Chemical Biology 17335–343. ( 10.1038/s41589-020-00676-4) [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Kimura M, Maruyama S, Nagayasu M, Imanaka S.2021bRevisiting estrogen-dependent signaling pathways in endometriosis: potential targets for non-hormonal therapeutics. European Journal of Obstetrics, Gynecology, and Reproductive Biology 258103–110. ( 10.1016/j.ejogrb.2020.12.044) [DOI] [PubMed] [Google Scholar]
- Koninckx PR, Ussia A, Adamyan L, Wattiez A, Gomel V, Martin DC.2019Pathogenesis of endometriosis: the genetic/epigenetic theory. Fertility and Sterility 111327–340. ( 10.1016/j.fertnstert.2018.10.013) [DOI] [PubMed] [Google Scholar]
- Korga A, Ostrowska M, Iwan M, Herbet M, Dudka J.2019Inhibition of glycolysis disrupts cellular antioxidant defense and sensitizes HepG2 cells to doxorubicin treatment. FEBS Open Bio 9959–972. ( 10.1002/2211-5463.12628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg MD.2020The immunologic Warburg effect: evidence and therapeutic opportunities in autoimmunity. Wiley Interdisciplinary Reviews: Systems Biology and Medicine 12 e1486. ( 10.1002/wsbm.1486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak CH, Jin L, Han JH, Han CW, Kim E, Cho M, Chung TW, Bae SJ, Jang SB, Ha KT.2020Ilimaquinone induces the apoptotic cell death of cancer cells by reducing pyruvate dehydrogenase kinase 1 activity. International Journal of Molecular Sciences 21 6021. ( 10.3390/ijms21176021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapel M, Weston P, Strassheim D, Karoor V, Burns N, Lyubchenko T, Paucek P, Stenmark KR, Gerasimovskaya EV.2017Glycolysis and oxidative phosphorylation are essential for purinergic receptor-mediated angiogenic responses in vasa vasorum endothelial cells. American Journal of Physiology: Cell Physiology 312C56–C70. ( 10.1152/ajpcell.00250.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laschke MW, Menger MD.2012Anti-angiogenic treatment strategies for the therapy of endometriosis. Human Reproduction Update 18682–702. ( 10.1093/humupd/dms026) [DOI] [PubMed] [Google Scholar]
- Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV.2010Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. PNAS 1072037–2042. ( 10.1073/pnas.0914433107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Lin SC, Wu MH, Tsai SJ.2019Induction of pyruvate dehydrogenase kinase 1 by hypoxia alters cellular metabolism and inhibits apoptosis in endometriotic stromal cells. Reproductive Sciences 26734–744. ( 10.1177/1933719118789513) [DOI] [PubMed] [Google Scholar]
- Leow HW, Koscielniak M, Williams L, Saunders PTK, Daniels J, Doust AM, Jones MC, Ferguson GD, Bagger Y, Horne AWet al. 2021Dichloroacetate as a possible treatment for endometriosis-associated pain: a single-arm open-label exploratory clinical trial (EPiC) Pilot and Feasibility Studies 7 67. ( 10.1186/s40814-021-00797-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HM, Yang JG, Liu ZJ, Wang WM, Yu ZL, Ren JG, Chen G, Zhang W, Jia J.2017aBlockage of glycolysis by targeting PFKFB3 suppresses tumor growth and metastasis in head and neck squamous cell carcinoma. Journal of Experimental and Clinical Cancer Research 36 7. ( 10.1186/s13046-016-0481-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Li J, Dai W, Zhang Q, Feng J, Wu L, Liu T, Yu Q, Xu S, Wang Wet al. 2017bGenistein suppresses aerobic glycolysis and induces hepatocellular carcinoma cell death. British Journal of Cancer 1171518–1528. ( 10.1038/bjc.2017.323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao TL, Tzeng CR, Yu CL, Wang YP, Kao SH.2015Estrogen receptor-β in mitochondria: implications for mitochondrial bioenergetics and tumorigenesis. Annals of the New York Academy of Sciences 135052–60. ( 10.1111/nyas.12872) [DOI] [PubMed] [Google Scholar]
- Liberti MV, Locasale JW.2016The Warburg effect: how does it benefit cancer cells? Trends in Biochemical Sciences 41211–218. ( 10.1016/j.tibs.2015.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SA, Moon Y, Shin MH, Kim TJ, Chae S, Yee C, Hwang D, Park H, Lee KM.2021Hypoxia-driven HIF-1alpha activation reprograms pre-activated NK cells towards highly potent effector phenotypes via ERK/STAT3 pathways. Cancers 131, 904. ( 10.3390/cancers13081904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Dai Y, Xu W, Shi L, Jin X, Li C, Zhou F, Pan Y, Zhang Y, Lin Xet al. 2018Hypoxia promotes ectopic adhesion ability of endometrial stromal cells via TGF-β1/Smad signaling in endometriosis. Endocrinology 1591630–1641. ( 10.1210/en.2017-03227) [DOI] [PubMed] [Google Scholar]
- Liu F, He L, Liu Y, Shi Y, Du H.2013The expression and role of oxidative stress markers in the serum and follicular fluid of patients with endometriosis. Clinical and Experimental Obstetrics and Gynecology 40372–376. [PubMed] [Google Scholar]
- Marianna S, Alessia P, Susan C, Francesca C, Angela S, Francesca C, Antonella N, Patrizia I, Nicola C, Emilio C.2017Metabolomic profiling and biochemical evaluation of the follicular fluid of endometriosis patients. Molecular Biosystems 131213–1222. ( 10.1039/c7mb00181a) [DOI] [PubMed] [Google Scholar]
- Matos L, Stevenson D, Gomes F, Silva-Carvalho JL, Almeida H.2009Superoxide dismutase expression in human cumulus oophorus cells. Molecular Human Reproduction 15411–419. ( 10.1093/molehr/gap034) [DOI] [PubMed] [Google Scholar]
- McKinnon BD, Bertschi D, Wotzkow C, Bersinger NA, Evers J, Mueller MD.2014Glucose transporter expression in eutopic endometrial tissue and ectopic endometriotic lesions. Journal of Molecular Endocrinology 52169–179. ( 10.1530/JME-13-0194) [DOI] [PubMed] [Google Scholar]
- McKinnon BD, Kocbek V, Nirgianakis K, Bersinger NA, Mueller MD.2016Kinase signalling pathways in endometriosis: potential targets for non-hormonal therapeutics. Human Reproduction Update 22382–403. ( 10.1093/humupd/dmv060) [DOI] [PubMed] [Google Scholar]
- Menezo YJ, Silvestris E, Dale B, Elder K.2016Oxidative stress and alterations in DNA methylation: two sides of the same coin in reproduction. Reproductive Biomedicine Online 33668–683. ( 10.1016/j.rbmo.2016.09.006) [DOI] [PubMed] [Google Scholar]
- Miao P, Sheng S, Sun X, Liu J, Huang G.2013Lactate dehydrogenase A in cancer: a promising target for diagnosis and therapy. IUBMB Life 65904–910. ( 10.1002/iub.1216) [DOI] [PubMed] [Google Scholar]
- Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemermann C, Wang ZQ, Salvemini D.2003On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. British Journal of Pharmacology 140445–460. ( 10.1038/sj.bjp.0705430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oermann EK, Wu J, Guan KL, Xiong Y.2012Alterations of metabolic genes and metabolites in cancer. Seminars in Cell and Developmental Biology 23370–380. ( 10.1016/j.semcdb.2012.01.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oner-Iyidoğan Y, Koçak H, Gürdöl F, Korkmaz D, Buyru F.2004Indices of oxidative stress in eutopic and ectopic endometria of women with endometriosis. Gynecologic and Obstetric Investigation 57214–217. ( 10.1159/000076691) [DOI] [PubMed] [Google Scholar]
- Pocate-Cheriet K, Santulli P, Kateb F, Bourdon M, Maignien C, Batteux F, Chouzenoux S, Patrat C, Wolf JP, Bertho Get al. 2020The follicular fluid metabolome differs according to the endometriosis phenotype. Reproductive Biomedicine Online 411023–1037. ( 10.1016/j.rbmo.2020.09.002) [DOI] [PubMed] [Google Scholar]
- Pruett BS, Meador-Woodruff JH.2020Evidence for altered energy metabolism, increased lactate, and decreased pH in schizophrenia brain: a focused review and meta-analysis of human postmortem and magnetic resonance spectroscopy studies. Schizophrenia Research 22329–42. ( 10.1016/j.schres.2020.09.003) [DOI] [PubMed] [Google Scholar]
- Qi X, Zhang Y, Ji H, Wu X, Wang F, Xie M, Shu L, Jiang S, Mao Y, Cui Yet al. 2014Knockdown of prohibitin expression promotes glucose metabolism in eutopic endometrial stromal cells from women with endometriosis. Reproductive Biomedicine Online 29761–770. ( 10.1016/j.rbmo.2014.09.004) [DOI] [PubMed] [Google Scholar]
- Reckzeh ES, Karageorgis G, Schwalfenberg M, Ceballos J, Nowacki J, Stroet MCM, Binici A, Knauer L, Brand S, Choidas Aet al. 2019Inhibition of glucose transporters and glutaminase synergistically impairs tumor cell growth. Cell Chemical Biology 261214, .e25–1228.e25. ( 10.1016/j.chembiol.2019.06.005) [DOI] [PubMed] [Google Scholar]
- Rytkönen KT, Heinosalo T, Mahmoudian M, Ma X, Perheentupa A, Elo LL, Poutanen M, Wagner GP.2020Transcriptomic responses to hypoxia in endometrial and decidual stromal cells. Reproduction 16039–51. ( 10.1530/REP-19-0615) [DOI] [PubMed] [Google Scholar]
- Sanchez WY, McGee SL, Connor T, Mottram B, Wilkinson A, Whitehead JP, Vuckovic S, Catley L.2013Dichloroacetate inhibits aerobic glycolysis in multiple myeloma cells and increases sensitivity to bortezomib. British Journal of Cancer 1081624–1633. ( 10.1038/bjc.2013.120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santanam N, Murphy AA, Parthasarathy S.2002Macrophages, oxidation, and endometriosis. Annals of the New York Academy of Sciences 955183–19; discussion 19. ( 10.1111/j.1749-6632.2002.tb02779.x) [DOI] [PubMed] [Google Scholar]
- Saunders PTK, Horne AW.2021Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell 1842807–2824. ( 10.1016/j.cell.2021.04.041) [DOI] [PubMed] [Google Scholar]
- Sun RC, Fadia M, Dahlstrom JE, Parish CR, Board PG, Blackburn AC.2010Reversal of the glycolytic phenotype by dichloroacetate inhibits metastatic breast cancer cell growth in vitro and in vivo. Breast Cancer Research and Treatment 120253–260. ( 10.1007/s10549-009-0435-9) [DOI] [PubMed] [Google Scholar]
- Tamada M, Suematsu M, Saya H.2012Pyruvate kinase M2: multiple faces for conferring benefits on cancer cells. Clinical Cancer Research 185554–5561. ( 10.1158/1078-0432.CCR-12-0859) [DOI] [PubMed] [Google Scholar]
- Tataranni T, Piccoli C.2019Dichloroacetate (DCA) and cancer: an overview towards clinical applications. Oxidative Medicine and Cellular Longevity 20198201079. ( 10.1155/2019/8201079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian DD, Bennett SK, Coupland LA, Forwood K, Lwin Y, Pooryousef N, Tea I, Truong TT, Neeman T, Crispin Pet al. 2019GSTZ1 genotypes correlate with dichloroacetate pharmacokinetics and chronic side effects in multiple myeloma patients in a pilot phase 2 clinical trial. Pharmacology Research and Perspectives 7 e00526. ( 10.1002/prp2.526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB.2009Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 3241029–1033. ( 10.1126/science.1160809) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinatier D, Cosson M, Dufour P.2000Is endometriosis an endometrial disease? European Journal of Obstetrics, Gynecology, and Reproductive Biology 91113–125. ( 10.1016/s0301-2115(9900263-8) [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang D, Han S, Wang N, Mo F, Loo TY, Shen J, Huang H, Chen J.2013Bioactivity-guided identification and cell signaling technology to delineate the lactate dehydrogenase A inhibition effects of Spatholobus suberectus on breast cancer. PLoS ONE 8 e56631. ( 10.1371/journal.pone.0056631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lu J, Kulkarni S, Zhang W, Gorka JE, Mandel JA, Goetzman ES, Prochownik EV.2019Metabolic and oncogenic adaptations to pyruvate dehydrogenase inactivation in fibroblasts. Journal of Biological Chemistry 2945466–5486. ( 10.1074/jbc.RA118.005200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xiu J, Yang T, Ren C, Yu Z.2021HSF1 promotes endometriosis development and glycolysis by up-regulating PFKFB3 expression. Reproductive Biology and Endocrinology 19 86. ( 10.1186/s12958-021-00770-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RB.2018Hypoxia, cytokines and stromal recruitment: parallels between pathophysiology of encapsulating peritoneal sclerosis, endometriosis and peritoneal metastasis. Pleura and Peritoneum 320180103. ( 10.1515/pp-2018-0103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise DR, Thompson CB.2010Glutamine addiction: a new therapeutic target in cancer. Trends in Biochemical Sciences 35427–433. ( 10.1016/j.tibs.2010.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise DR, Ward PS, Shay JE, Cross JR, Gruber JJ, Sachdeva UM, Platt JM, DeMatteo RG, Simon MC, Thompson CB.2011Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. PNAS 10819611–19616. ( 10.1073/pnas.1117773108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MH, Hsiao KY, Tsai SJ.2019Hypoxia: the force of endometriosis. Journal of Obstetrics and Gynaecology Research 45532–541. ( 10.1111/jog.13900) [DOI] [PubMed] [Google Scholar]
- Yi M, Ban Y, Tan Y, Xiong W, Li G, Xiang B.20196-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 and 4: a pair of valves for fine-tuning of glucose metabolism in human cancer. Molecular Metabolism 201–13. ( 10.1016/j.molmet.2018.11.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young VJ, Brown JK, Maybin J, Saunders PT, Duncan WC, Horne AW.2014Transforming growth factor-β induced Warburg-like metabolic reprogramming may underpin the development of peritoneal endometriosis. Journal of Clinical Endocrinology and Metabolism 993450–3459. ( 10.1210/jc.2014-1026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young VJ, Ahmad SF, Brown JK, Duncan WC, Horne AW.2016ID2 mediates the transforming growth factor-β1-induced Warburg-like effect seen in the peritoneum of women with endometriosis. Molecular Human Reproduction 22648–654. ( 10.1093/molehr/gaw045) [DOI] [PubMed] [Google Scholar]
- Zheng J, Dai Y, Lin X, Huang Q, Shi L, Jin X, Liu N, Zhou F, Zhang S.2021Hypoxia-induced lactate dehydrogenase A protects cells from apoptosis in endometriosis. Molecular Medicine Reports 24 637. ( 10.3892/mmr.2021.12276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Ding ZM, Hardiman PJ.2018Understanding the role of Gui-Zhi-Fu-Ling-capsules (Chinese medicine) for treatment of endometriosis in the rat model: using NMR based metabolomics. Evidence-Based Complementary and Alternative Medicine 20189864963. ( 10.1155/2018/9864963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Huang Z, Su J, Li J, Zhao S, Wu L, Zhang J, He Y, Zhang G, Tao Jet al. 2020Benserazide is a novel inhibitor targeting PKM2 for melanoma treatment. International Journal of Cancer 147139–151. ( 10.1002/ijc.32756) [DOI] [PubMed] [Google Scholar]
- Zhu X, Wang K, Liu G, Wang Y, Xu J, Liu L, Li M, Shi J, Aa J, Yu L.2017Metabolic perturbation and potential markers in patients with esophageal cancer. Gastroenterology Research and Practice 20175469597. ( 10.1155/2017/5469597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondervan KT, Becker CM, Missmer SA.2020Endometriosis. New England Journal of Medicine 3821244–1256. ( 10.1056/NEJMra1810764) [DOI] [PubMed] [Google Scholar]
- Zubrzycka A, Zubrzycki M, Janecka A, Zubrzycka M.2015New horizons in the etiopathogenesis and non-invasive diagnosis of endometriosis. Current Molecular Medicine 15697–713. ( 10.2174/1566524015666150921105218) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a