Abstract
Background
Intestinal parasites are a major public health problem in the developing world and have attracted increasing levels of interest from health researchers over the past decade. Epidemiology-based studies have shown that the prevalence of intestinal parasites is high and they frequently recur in regions with poor sanitation and inadequate sewerage facilities. In this study, we determined the prevalence of intestinal parasites, their egg intensities per sample, and associated risk factors in an informal settlement.
Methods
This was a cross-sectional study conducted in three randomly selected public primary schools located in the informal settlements of Nakuru town. A total of 248 stool samples were collected from asymptomatic pupils and screened, using the Kato Katz technique, for infections caused by soil-transmitted helminths (STH). A random subset of stool samples (n=96) was also screened by polymerase chain reaction (PCR) to detect intestinal protozoa. Socio-demographic variables were collected using a pre-tested structured questionnaire; these data were analysed to identify risk factors for infection.
Results
The overall prevalence of intestinal parasites was 17.3% (43/248 pupils). The overall prevalence of both STH and intestinal protozoan parasites was 1.2% and 41.7%, respectively. The most commonly diagnosed STH infection was Trichuris trichiura (1.2%), followed by hookworms (0.4%) and Ascaris lumbricoides (0.4%). The prevalence of intestinal protozoan parasites ranged from 0% to 38.5% and included Entamoeba histolytica, Entamoeba hartmanni, Entamoeba dispar, Giardia intestinalis, and Entamoeba coli. All infections were light, with an egg intensity <100 for each of the STH infections. The prevalence of multiple infections, including intestinal protozoan parasites, was 5.2% (n=5) and 0.4% (n=1) for STH in the subset samples. Finally, our analysis identified several significant risk factors for intestinal parasitic infections, including goat rearing (p=0.046), living in a home with an earthen floor (p=0.022), the number of rooms in the household (p=0.035), and the source of food (p=0.016).
Conclusion
The low prevalence of intestinal parasites in the informal settlements of Nakuru may be attributed to improvements in hygiene and sanitation, deworming, and general good health practices that are facilitated by the Department of Public Health.
Keywords: Prevalence, risk factors, intestinal parasites, informal settlements, school-children
Introduction
Intestinal parasitic infections are a serious public health issue in developing countries despite being the most preventable and treatable disease1. It is estimated that over 3.5 billion people globally host at least one species of intestinal parasite at some point in time, leading to over 450 million disorders1. This bestows a considerable burden on health systems, particularly in the developing world where the majority of these infections occur. Intestinal parasites continue to affect human productivity by causing a variety of medical complications, including abdominal pains, anaemia, diarrhoea, delayed growth, undernutrition, reduced physical activity, and impaired cognitive development in young children2,3. Epidemiological-based surveys have shown that these parasites recur in regions with poor sanitation and hygiene facilities; for example, within informal settlements in developing countries4.
Rural-urban migration, driven by economic strain and the search for better jobs, has promoted the growth of informal settlements. Over time, these settlements have become home for a substantial number of city residents5. Such affordable housing attracts low-income earners from the cities and results in overcrowded unhygienic environments, strained basic necessities, and consequently limited government services3,6. However, poor drainage systems; the accumulation of garbage; the indiscriminate disposal of excreta, such as wraps and throws; and shared pit latrines; continue to pose an increased health risk to the inhabitants as the population increases6. The contamination of water sources, food, and the environment, by pathogens that flourish in such unkempt environments remain inevitable and infect humans via the faecal-oral route7,8. Pre-school and school-aged children inhabiting informal settlements are at a significant risk of acquiring these infections since they rarely observe hygiene, are very active, and tend to play in contaminated environments and households; furthermore, these age-groups often practice indiscriminate eating habits9. The spread of intestinal parasitic infections is further enhanced by the presence of healthy carriers who form a parasite reservoir for future infections, thus leading to continuous and persistent disease endemicity10. Recent molecular epidemiological studies have addressed the potential pathogenicity of intestinal protozoan parasites that were previously considered to be non-pathogenic for many years. For example, Entamoeba dispar, E. hartmanni, and E. moshkovskii have been recovered from patients with gastrointestinal symptoms11,12. Furthermore, E. coli has been detected in patients showing clinical symptoms but without the detection of other parasitological or bacteriological agents13. This suggests that commensal Entamoeba spp. could be pathogenic and are therefore of significant clinical importance. Consequently, there is an urgent need to reevaluate the epidemiology of intestinal protozoan parasites in terms of morbidity, particularly in geographical areas that are considered to be highly endemic. This study determined the prevalence, intensity, and risk factors associated with intestinal parasites in asymptomatic school-going children within informal settlements of Nakuru town in Kenya.
Materials and methods
Study design, setting and study subjects
This cross-sectional study was conducted in the informal settlements of Nakuru town, the fourth-largest urban centre in Kenya, covering an estimated area of 7496.52km14. Nakuru consists of fifteen wards within its locality and is categorized into low-income areas (informal settlements) with high population densities, middle-income areas with medium population densities, and high-income areas with low population densities. Over 50% of people living in Nakuru town live in informal settlements (including London, Gioto and Kaptembwa)14. The town continues to witness a tremendous increase in its population, largely due to its cosmopolitan nature, fertile agricultural hinterland, central strategic location, and opportunities for employment15. Due to a lack of space, the town boundary lies between the scenic Menengai crater to the north and Lake Nakuru to the south. Most migrants settle in the already over-crowded unplanned informal settlements, which continue to experience significant strain in terms of basic amenities.
The study participants were asymptomatic children, aged 8–13 years, from three randomly selected primary schools (Kaptembwa, Milimani and Prisons) located in informal settlements of Nakuru town. The choice of study participants was based on their age group, which was considered to be of high-risk of infection and their capability to respond to a questionnaire that aimed to determine a range of variables associated with infection. The study participants were sampled randomly; an additional cohort of children (approximately 20% of the original study population size) were included to account for pupils who failed to return samples or those who were absent from school during the sample collection period. We excluded children whose parents did not consent for their child's inclusion in the study and those who could not provide stools at the time of sample collection.
Sample and data collection
Samples were collected from children in the three selected schools in June 2018. The purpose of the study was explained to the children and their teachers prior to the issue of consent forms; this occurred 2 weeks before sample collection. Teachers helped the children to acquire consent from their parents. On the morning of sample collection, sterile Ziploc aluminium bags, labelled with a unique identity code, were issued to each of the consented participants. We then provided oral advice as to how to handle the stool bag and specimen. Each child provided a single stool sample for diagnosis. The fresh stool samples were transported to the laboratories of Langa Langa Sub-County Hospital in cool boxes immediately after collection for processing. A pretested structured questionnaire was then administered to each participant to gather demographic data and other important variables. The questionnaire also captured vital information relating to water, sanitation, and hygiene in households. The officer-in-charge of public health confirmed that they had not dewormed the school children during the 3 months before the study period (Gachahi CW, 2018, personal communication).
Laboratory screening by Kato Katz and polymerase chain reaction
Soil-transmitted helminths (STH) were detected using the Kato Katz technique and intestinal protozoan parasites were detected by the polymerase chain reaction (PCR). The Kato Katz technique involved the preparation of two thick smears from each stool sample and microscopic examination for T. trichiura, A. lumbricoides, and hookworm; this allowed us to confirm the presence of eggs and determine the intensity of infection16. The intensity of infection was defined by parasite-specific egg counts; these counts were then adjusted to eggs per gram (EPG) of faeces. Each slide was observed within 60 minutes of preparation by two independent and experienced microscopists. A laboratory supervisor randomly crosschecked 10% of the slides for quality control purposes. The intensity of infection was categorized as light, moderate or heavy in accordance with the thresholds proposed by the World Health Organization (WHO)17.
The molecular detection of intestinal protozoa parasites was performed using approximately 0.2 g of faeces from a subset of randomly selected samples (n=96) preserved in 0.8 ml DNAzol® (Molecular Research Center, Inc., OH, USA). Molecular analysis was carried out at Kenya Medical Research Institute (KEMRI) Nairobi, within the Molecular Laboratory of the Center for Microbiology Research (CMR). DNA was extracted using DNAzol® in accordance with the manufacturer's instructions. DNA was then screened for Entamoeba sp. (E. histolytica, E. hartmanni, E. dispar and E. coli) and Giardia intestinalis. The presence of Entamoeba sp. was confirmed by an initial universal nested polymerase chain reaction (PCR) followed by subsequent species-specific PCR reactions targeting the 18S ribosomal RNA subunit gene, as described previously18. Giardia intestinalis was detected via a nested PCR that targeted the glutamate dehydrogenase gene (GDH), as described previously19. All PCR products were resolved on an ethidium bromide-stained 1.5% agarose gel in Tris-acetate-ethylenediaminetetraacetic acid (TAE) buffer and the amplicons visualized under ultraviolet light. The detection and prevalence of intestinal protozoan parasites was based on band size and intensity. All data were tested for normality prior to statistical analysis.
Data management and analysis
Data were entered into a Microsoft Excel datasheet and crosschecked with the questionnaires to ensure accuracy. All statistical analyses were performed using SPSS software system, version 20 (IBM Corp., Armonk, NY, USA). Frequencies and proportions were used to describe the demographic characteristics of the study population. A comparison between the infection status of the children and risk factors for intestinal parasitic infections was performed using crosstabs. Pearson's correlation and univariate analysis were then used to determine the association between intestinal parasitic infections and risk factors. Stepwise multiple linear regression was performed for all of the analysed risk factors with regards to intestinal parasitic infections. The level of statistical significance was p<0.05.
Ethical considerations
This study was conducted in accordance with the tenets of the Declaration of Helsinki and the regulations proposed by the International Conference of Harmonization. Ethical approvals were sought from the Kenya Medical Research Institute (KEMRI) Science and Ethics Review Unit (SERU), Nakuru county health and education offices, and from respective school headteachers.
Written consent was also sought from each the guardian/parent of each participant using a consent form that clearly indicated the study purpose, any anticipated consequences of the research, the anticipated uses of the data, possible benefits and harm, data confidentiality, and the option to withdraw the participation of children at any given time. Children who tested positive for any intestinal parasite received treatment as required by the Guidelines put forward by the Ministry of Health (MoH) in Kenya.
Results
Study population characteristics
A total of 248 children, aged 8–13years with a median age of 10 years, were enrolled in this study; 56.9% of the study population were female.
Ten children did not provide stools at the time of sample collection. Two-thirds (66.5%) of the participants did not have a defined way of disposing of garbage at home; rather, garbage was left scattered in the environment.
Almost two-thirds (64.1%) of the residents used shared toilets. Approximately three-quarters of the residents (73.4%) had access to piped tap water while others used water from wells (2.1%), water vendors (8.9%), and rain-water (14.1%). The majority of the participants (56.1%) treated their drinking water, 34.3% drank boiled drinking water, and 5.7% drank untreated water. Less than a quarter of the participant's parents/guardians were unemployed (15.7%); the rest were either farmers (3.6%), formally employed (27.4%), informally employed (16.5%), or businesspersons (30.7%). Sixty-nine percent of the children had lunch prepared in their respective schools (Table 1).
Table 1:
Socio-demographic characteristics of school-going children from informal settlements in Nakuru town
| Characteristic | n | (%) |
|---|---|---|
| Age groups (years) | ||
| 8–9 | 50 | 20.2 |
| 10–11 | 167 | 67.3 |
| 12–13 | 11 | 12.5 |
| Gender | ||
| Female | 141 | 56.9 |
| Male | 107 | 43.1 |
| Occupation of the parent | ||
| Unemployed | 39 | 15.73 |
| Farmer | 9 | 3.6 |
| Formal employment | 68 | 27.4 |
| Businessman | 76 | 30.7 |
| Informal | 41 | 16.5 |
| Source of water | ||
| Piped water indoors | 43 | 17.3 |
| Piped water outdoors | 139 | 2.1 |
| Wells | 5 | 2.1 |
| Water vendors | 22 | 8.9 |
| Rain water | 35 | 14.1 |
| Type of water used for drinking | ||
| Direct from source | 14 | 5.7 |
| Commercial treatment | 139 | 56.1 |
| Boiled water | 85 | 34.3 |
| Bottled water | 1 | 0.4 |
| Means of garbage disposal | ||
| Backyard | 53 | 21.4 |
| Farm | 19 | 7.7 |
| Outside/public disposal | 165 | 66.5 |
| Collected by garbage collectors | 10 | 4.0 |
| Means of fecal waste disposal | ||
| Private toilet | 73 | 29.4 |
| Shared toilet | 159 | 64.1 |
| Flying toilet | 1 | 0.4 |
| Where the child ate | ||
| In school | 171 | 69.0 |
| Home packed lunch | 77 | 31.0 |
The prevalence and intensity of STH and intestinal protozoa
The overall prevalence of intestinal parasites was 17.3% (n=43). STH were observed in 1.2% (n=3) of the study population. The most commonly detected STH was Trichuris trichiura (1.2%; n=3) followed by hookworm (0.4%; n=1) and Ascaris lumbricoides (0.4%; n=1) (Table 2). The infection intensities were light with mean intensities of 10, 69, and 14 EPG recorded for T. trichiura, A. lumbricoides, and hookworms, respectively. With regards to the randomly selected subset of participants (n=96) screened for intestinal protozoan parasites, 41.7% (n=40) tested positive for at least one of the three parasites (E. dispar, E. coli, and G. intestinalis). The individual prevalence of E. histolytica, E. hartmanni, G. intestinalis, E. dispar, and E. coli was 0%, 0%, 4.2%, 6.3%, and 38.5%, respectively (Table 2). Multiple STH infections were observed in only one child (0.4%). In addition, from the 96 randomly selected children, multiple infections caused by intestinal protozoan parasites were recorded in 5.2% of participants (n=5) (Table 3).
Table 2:
Prevalence of intestinal parasites and intensity of soiltransmitted helminths (STH) among school-going children in informal settlements in Nakuru town (one child was positive for the three STHs)(n=number of individuals positive for the intestinal parasite)
| Soil-transmitted helminths (N=248) | n(%) | Infection intensity |
|---|---|---|
| Trichuris trichiura | 3(1.2) | Light <1000(10) |
| Ascaris lumbricoides | 1(0.4) | Light <1000(69) |
| Hookworm | 1(0.4) | Light <1000(14) |
| Intestinal Protozoa (N=96) | ||
| Entamoeba coli | 37(38.5) | |
| Entamoeba dispar | 6(6.3) | |
| Giardia intestinalis | 4(4.2) | |
| Entamoeba histolytica | 0(0) | |
| Entamoeba hartmani | 0(0) | |
Table 3:
Multiple infections prevalence in soil-transmitted helminths and intestinal protozoa parasites among school-going children in informal settlements in Nakuru town
| Co-infections | n | Prevalence (%) |
|---|---|---|
| Soil-transmitted helminths(N=248) | ||
| Trichuris trichiura, Ascaris lumbricoides, hookworm | 1 | 0.4 |
| Intestinal protozoa(N=96) | ||
| Entamoeba coli, Entamoeba dispar | 3 | 3.1 |
| Entamoeba coli, Giardia intestinalis | 1 | 1.0 |
| Entamoeba dispar, Entamoeba coli, Giardia intestinalis | 1 | 1.0 |
Risk factors associated with intestinal parasitic infections
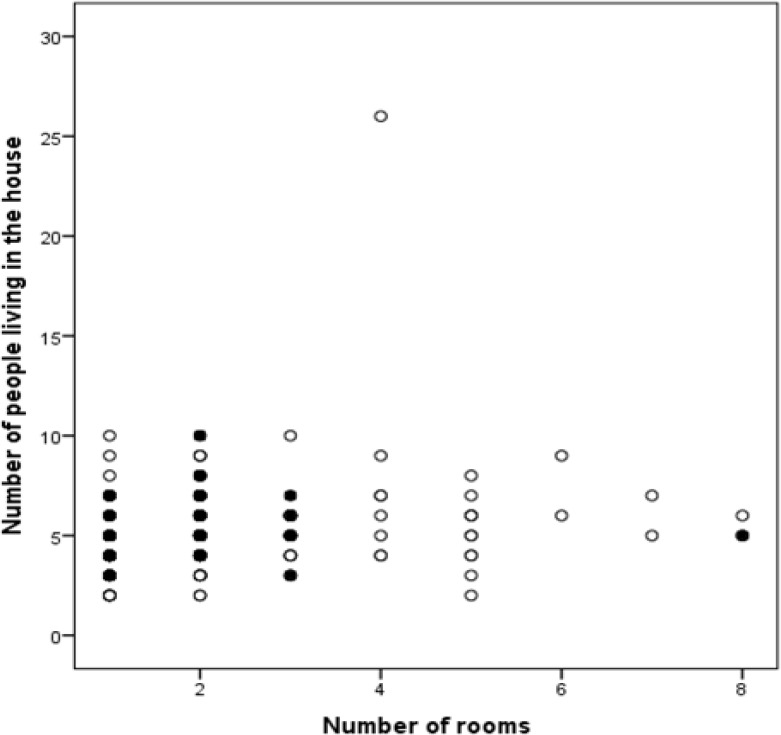
The incidence of intestinal parasites was similar when compared between females and males (16.3% and 15.9%; p=0.928). Although not statistically significant, infection rates were higher in children aged 8–9 years (20%) and those aged 12–13 years (19.4%) as compared to those 10–11 years (14.4%) (Table 4). The prevalence of intestinal parasites decreased with an increase in the number of household rooms (Figure 1) (p=0.035) while children who had lunch prepared in school had a lower infection level (12.4%) as compared to those who ate a home-packed lunch (24.7%) (p=0.016). Furthermore, the majority of children who lived in households with an earthen floor (p=0.022) and those who reared goats (p=0.046) had a higher chance of being diagnosed with STH. Subsequently, children who used rainwater for drinking were more likely to have intestinal protozoan parasite infections (p=0.052) than those who used tap water, bought water from vendors, or used water from wells. Stepwise linear multiple regression revealed a reduction in infections caused by intestinal protozoan parasites with an increase in parent employment (p=0.012), less congested households (p=0.014) and eating food prepared in school (p=0.023). Food source was shown to exert a significant influence (p=0.021) on both STH and intestinal protozoan parasites (Table 5). The number of rooms, as a major risk factor to intestinal parasitic infections, was significantly influenced by the type of household floor (p=0.002), the number of people living in the household (p=0.004), and the occupation of the parents (p=0.024) (Table 5).
Table 4:
Univariate and bivariate analysis of the different variables associated with intestinal parasitic infections (statistically significant variable sets are given in bold)
| Risk factor | Number of infections | Intestinal parasites (p-value) | STH (p-value) | Intestinal protozoan parasite (p-value) |
|---|---|---|---|---|
| Age (years) | ||||
| 8 & 9 | 10/50 (20%) | 0.407 | 0.569 | 0.737 |
| 10 & 11 | 24/167 (14.4%) | 0.282 | 0.208 | 0.269 |
| 12 & 13 | 6/31 (19.4%) | 0.603 | 0.247 | 0.218 |
| Sex | ||||
| Male | 17/107 (15.9%) | 0.928 | 0.41 | 0.728 |
| Female | 23/141 (16.3%) | |||
| Room number | ||||
| 1–3 rooms | 39/219 (17.8%) | 0.061 | 0.214 | 0.032 |
| 4–6 rooms | 0/21 (0%) | 0.035 | 0.596 | 0.082 |
| >6 rooms | 1/5 (20%) | 0.978 | 0.0001 | 0.231 |
| Water source | ||||
| Piped water indoors | 6/43 (14%) | 0.671 | 0.427 | 0.923 |
| Piped water outdoors | 27/139 (19.4%) | 0.112 | 0.427 | 0.126 |
| Well | 0/5 (0%) | 0.324 | 0.804 | 0.231 |
| Water vendors | 5/22 (22.7%) | 0.38 | 0.135 | 0.622 |
| Rain | 2/34 (5.9%) | 0.081 | 0.322 | 0.052 |
| Floor type | ||||
| Cemented | 34/217 (15.7%) | 0.961 | 0.296 | 0.755 |
| Tiles | 4/20 (20%) | 0.604 | 0.605 | 0.596 |
| Earthen | 1/11 (9.1%) | 0.463 | 0.022 | 0.785 |
| Source of food | ||||
| School | 21/169 (12.4%) | 0.016 | 0.185 | 0.015 |
| Home | 19/77 (24.7%) | |||
| Parent occupation | ||||
| Unemployed | 9/42 (21.4%) | 0.43 | 1 | 0.279 |
| Farmer | 0/7 (0%) | 1 | 1 | 1 |
| Formal employment | 12/72 (16.7%) | 0.535 | 0.865 | 0.393 |
| Businesspersons | 13/72 (18.1%) | 0.502 | 0.865 | 0.381 |
| Informal employment | 5/40 (12.5%) | 0.645 | 0.761 | 0.604 |
| Rearing of animals | ||||
| Cat rearing | 7/55 (12.7%) | 0.439 | 0.062 | 0.888 |
| Goat rearing | 1/15 (6.7%) | 0.762 | 0.046 | 0.318 |
Figure 1:
Relationship between household overcrowding and intestinal parasitic infections. The black dots represent children that were positive for intestinal parasites while the clear dots represent children who were negative for intestinal parasites.
Table 5:
Stepwise linear regression analysis of the different risk factors among school-going children in informal settlements in Nakuru town, their significance to infection and 95% CI
| Intestinal protozoa | ||||
|---|---|---|---|---|
| P-value 95% | Confidence interval | |||
| Risk factors | t | sig | Lower bound | upper bound |
| Parent occupation | −2.591 | 0.012 | −0.203 | −0.026 |
| Number of rooms | −2.547 | 0.014 | −0.025 | −0.255 |
| Source of food | −2.329 | 0.023 | −0.039 | −0.268 |
| Intestinal parasites | ||||
| Source of food | −2.333 | 0.021 | −0.289 | −0.024 |
| Room number | ||||
| Type of floor | 3.120 | 0.002 | 0.150 | 0.662 |
| Number of people living in the house | 2.876 | 0.004 | 0.037 | 0.200 |
| Parent occupation | −2.272 | 0.024 | −0.284 | −0.020 |
Discussion
Reliable estimates and updates on the status of intestinal parasites in vulnerable regions are important to guide control. Our findings with regards to intestinal parasitic infections report a 17.3% overall prevalence among school-going children from informal settlements in Nakuru town. A higher prevalence of intestinal parasites has been reported from similar settings in Thika (>48.9%)20 and Nairobi (25.6%)21, Nigeria (86.2%)22, and Pakistan (52.8%)23. These differences in prevalence could be attributed to the detection methods used, socio-economic activities, along with a number of ecological and environmental differences2. In addition, the low prevalence of infections evident in our present results may be related to a number of ongoing improvements in sanitation, hygiene, and infrastructure, that have been supported by the World Bank in the regions of Kaptembwa and Milimani (Gachahi CW, 2018, personal communication). It is also likely that public health information, provided by the Department of Health, had contributed to the low infection rates described herein. These improvements facilitated appropriate and safe methods for garbage disposal and toiletry facilities, in addition to enhanced water supply and hand washing facilities. Collectively, these improvements led to a reduction in the number of intestinal parasitic infections and transmission. The low prevalence of intestinal parasites suggests low levels of environmental contamination with the infective pathogens. The very low levels of multiple infections could be explained by the fact that these rarely remain asymptomatic and such cases may not have been present in the study's asymptomatic population. However, low infection intensities of intestinal parasites, infection duration, and the immune status of the child involved, are all factors that could influence the appearance of symptoms in infected and co-infected children24.
The prevalence of STH infections reported in urban informal settlements within Kenya range from 34% to 40.7% 3,25,26; these data are highly inconsistent with our present findings (1.2%). This discrepancy could be explained by the fact that most of the earlier studies on STH in Kenya were conducted in areas reported to be endemic for such infections. Furthermore, the prevalence of STH is known to be heavily influenced by climate (humidity, temperature, rain) and soil factors; for instance, hookworm transmission is known to peak at temperatures of approximately 40°C27.
The overall incidence of intestinal protozoan parasite infections in the present study was 41.7%; infections were caused by E. coli, E. dispar, and G. intestinalis (38.5%, 4.2%, and 6.3% respectively). The predominance of E. coli and E. dispar is common in tropical regions, although the implications of these microorganism with regards to the mechanisms underlying chronic and subclinical human diseases have yet to be fully elucidated28. Indeed, E. dispar is often misdiagnosed microscopically29 and in immunodiagnostics for E. histolytica30. Entamoeba coli has also been associated with the deposition of abdominal fat in children, suggesting long-term implications31. The prevalence of E. histolytica in this study is consistent with other studies that have previously investigated asymptomatic children in other regions of Kenya (<1%)32, Indonesia (0%)11, and Peru (0%)33. The prevalence of Giardia intestinalis reported herein was also consistent with an earlier study34 in the informal settlements of Nairobi using similar diagnostic methods.
Food and food handling hygiene is an important factor in the transmission of intestinal parasites35. Eating lunches prepared in school is known to minimize intestinal parasitic infections. Teachers and matrons are the main caregivers in schools who ensure that children observe basic hygiene practices. Home-packed lunches are subject to contamination because the food containers may not have been properly cleaned and sealed. Consequently, parents may pack leftovers and food prepared in the streets, or unwashed fruit; such practices are most unhygienic36. Food displayed in the street attracts flies that transfer cysts, eggs, and the larvae of intestinal parasites, thus contaminating the food and posing a serious threat to children37. In addition, limited water sources and the socio-economic status of the majority of inhabitants in informal settlements increases the possibility of cross-contamination of water supplies. For instance, we found that rainwater increased the likelihood of intestinal protozoan infections. The contamination of rainwater may occur from animal droppings via household roofs, collection pipes, storage tanks, from animal droppings, which form reservoirs for intestinal protozoan parasites38. Contamination could also occur during the process of transportation to households from collection tanks39. Furthermore, G. intestinalis, a predominantly water-borne protozoan, is known to survive at very low temperatures such as that found in collection tanks and is often resistant to water treatment procedures, including chlorination10.
Low socioeconomic factors are a known risk factor for intestinal parasitic infection40. As observed in our results, household overcrowding can increase the number of intestinal parasites, as reported previously in a study of children aged 0–16 years41. It is evident that programs based on reducing household overcrowding have been successful in minimizing infections41. These earlier findings are consistent with our present findings in that we also observed a significant reduction of intestinal parasitic infections in less congested households (between 4–6 rooms) and in households where the parents were employed. Overcrowding in houses increases the potential for intestinal parasites to spread from an infected individual to a healthy person by increasing transmission rates per contact40. Less overcrowded households are associated with a strain in basic amenities, including water42, thus suggesting that such chores are not performed fully or are foregone altogether. Unemployed parents may experience a strain in their budget and therefore prioritize other basic necessities at the expense of observing hygiene. It is also possible that unemployed individuals are more likely to be illiterate; this factor has been associated with increased levels of intestinal parasitic infections40. We also showed that the presence of an earthen floor in overcrowded households increased the risk of infection by intestinal parasites, thus suggesting that the transmission of intestinal parasites may involve soil-related mechanisms.
Intestinal parasites parasitize a wide range of mammalian hosts via zoonotic and anthroponomical mechanisms. An association between goat rearing and STH has not been reported previously. We detected Trichuris spp. in some samples; it is possible that the organism involved may have been Trichuris ovis, previously isolated in goat43, although the ova from T. ovis are slightly larger in diameter and are similar to T. trichiura in humans. It is important to control intestinal parasites in animals as a focused strategy if we are to reduce the number of human infections. Future studies should also investigate a possible link between STH and goat rearing.
Conclusion
In conclusion, we demonstrated a low prevalence for intestinal parasitic infections among school-going children in an informal urban setting of Nakuru town. This low prevalence could be accounted for by improvements in water, sanitation, and hygiene (WASH) practices. The current WHO recommendation for national deworming programs in schools is prevalence above 20% as children are considered to be at risk of infection. We identified risk factors that were associated with poverty; these were strongly related to hygiene and sanitation status. Thus, if overall hygiene is improved in informal settlements, there may not be a need for preventive chemotherapy. Contact with domestic animals and their waste also contributed to the number of infections recorded in this study. This suggests the need for animals to be regularly dewormed and distanced from humans as animals could readily act as potential reservoirs for intestinal parasitic infections.
References
- 1.Saki J, Khademvatan S, Foroutan-Rad M, Gharibzadeh M. Prevalence of intestinal parasitic infections in Haftkel County, southwest of Iran. Int J Infect. 2017;4(4):e15593. [Google Scholar]
- 2.Alum A, Rubino JR, Ijaz MK. The global war against intestinal parasites-should we use a holistic approach? Int J Infect Dis. 2010;14(9):e732–8. doi: 10.1016/j.ijid.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 3.Suchdev PS, Davis SM, Bartoces M, Ruth LJ, Worrell CM, Kanyi H, et al. Soil-transmitted helminth infection and nutritional status among urban slum children in Kenya. Am J Trop Med Hyg. 2014;90(2):299–305. doi: 10.4269/ajtmh.13-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coulibaly G, Ouattara M, Dongo K, Hürlimann E, Bassa FK, Koné N, et al. Epidemiology of intestinal parasite infections in three departments of south-central Côte d'Ivoire before the implementation of a cluster-randomised trial. Parasite Epidemiol Control. 2018;3(2):63–76. doi: 10.1016/j.parepi.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen LD, Raabe K, Grote U. Rural-urban migration, household vulnerability, and welfare in Vietnam. World Dev. 2015;71:79–93. [Google Scholar]
- 6.Dana T. Unhygienic living conditions and health problems: a study in selected slums of Dhaka city. Int J Sustain Dev. 2011;2(11):27–34. [Google Scholar]
- 7.Opisa S, Odiere MR, Jura WG, Karanja DM, Mwinzi PN. Faecal contamination of public water sources in informal settlements of Kisumu City, western Kenya. Water Sci Technol. 2012;66(12):2674–81. doi: 10.2166/wst.2012.503. [DOI] [PubMed] [Google Scholar]
- 8.Nyarango RM, Aloo PA, Kabiru EW, Nyanchongi BO. The risk of pathogenic intestinal parasite infections in Kisii Municipality, Kenya. BMC Public Health. 2008;8(1):237. doi: 10.1186/1471-2458-8-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinbaum L, Njenga SM, Kihara J, Boehm AB, Davis J, Null C, et al. Soil-transmitted helminth eggs are present in soil at multiple locations within households in rural Kenya. PLoS One. 2016;11(6):e0157780. doi: 10.1371/journal.pone.0157780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osman M, El Safadi D, Cian A, Benamrouz S, Nourrisson C, Poirier P, et al. Prevalence and risk factors for intestinal protozoan infections with Cryptosporidium, Giardia, Blastocystis and Dientamoeba among school children in Tripoli, Lebanon. PLoS Negl Trop Dis. 2016;10(3):e0004496. doi: 10.1371/journal.pntd.0004496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumura T, Hendarto J, Mizuno T, Syafruddin D, Yoshikawa H, Matsubayashi M, et al. Possible pathogenicity of commensal Entamoeba hartmanni revealed by molecular screening of healthy school children in Indonesia. Trop Med Health. 2019;47:7. doi: 10.1186/s41182-018-0132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parija SC, Khairnar K. Entamoeba moshkovskii and Entamoeba dispar-associated infections in Pondicherry, India. J Health Popul Nutr. 2005;23(3):292–5. [PubMed] [Google Scholar]
- 13.Kaya S, Cetin ES, Akçam Z, Kesbiç H, Demirci M. [Clinical symptoms in cases caused by Entamoeba coli and Blastocystis hominis] Turkiye Parazitol Derg. 2005;29(4):229–31. Turkish. [PubMed] [Google Scholar]
- 14.Kenya National Bureau of Statistics, Society for International Development . Exploring Kenya's Inequality: Nakuru County. Kenya National Bureau of Statistics; 2013. [Google Scholar]
- 15.Nyasani MI. Kenya's Experience on Urban Health Issues. Nakuru, Kenya: 2009. [Google Scholar]
- 16.WHO . Bench aids for the diagnosis of intestinal parasites. Geneva: World Health Organisation; 1994. [Google Scholar]
- 17.World Health Organization . Helminth control in school-age children: a guide for managers of control programmes. Geneva: World Health Organisation; 2011. [Google Scholar]
- 18.Matey EJ, Tokoro M, Nagamoto T, Mizuno T, Saina MC, Bi X, et al. Lower prevalence of Entamoeba species in children with vertically transmitted HIV infection in Western Kenya. AIDS. 2016;30(5):803–5. doi: 10.1097/QAD.0000000000001002. [DOI] [PubMed] [Google Scholar]
- 19.Hussein AI, Yamaguchi T, Nakamoto K, Iseki M, Tokoro M. Multiple-subgenotype infections of Giardia intestinalis detected in Palestinian clinical cases using a subcloning approach. Parasitol Int. 2009;58(3):258–62. doi: 10.1016/j.parint.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Ngonjo TW, Kihara JH, Gicheru M, Wanzala P, Njenga SM, Mwandawiro CS. Prevalence and intensity of intestinal parasites in school age children in Thika District, Kenya. Afr J Health Sci. 2012;21(3–4):153–60. [Google Scholar]
- 21.Mbae CK, Nokes DJ, Mulinge E, Nyambura J, Waruru A, Kariuki S. Intestinal parasitic infections in children presenting with diarrhoea in outpatient and inpatient settings in an informal settlement of Nairobi, Kenya. BMC Infect Dis. 2013;13:243. doi: 10.1186/1471-2334-13-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gyang VP, Chuang TW, Liao CW, Lee YL, Akinwale OP, Orok A, et al. Intestinal parasitic infections: current status and associated risk factors among school aged children in an archetypal African urban slum in Nigeria. J Microbiol Immunol Infect. 2019;52(1):106–113. doi: 10.1016/j.jmii.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Mehraj V, Hatcher J, Akhtar S, Rafique G, Beg MA. Prevalence and factors associated with intestinal parasitic infection among children in an urban slum of Karachi. PLoS One. 2008;3(11):e3680. doi: 10.1371/journal.pone.0003680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller SA, Rosario CL, Rojas E, Scorza JV. Intestinal parasitic infection and associated symptoms in children attending day care centres in Trujillo, Venezuela. Trop Med Int Health. 2003;8(4):342–7. doi: 10.1046/j.1365-3156.2003.01011.x. [DOI] [PubMed] [Google Scholar]
- 25.Odiere MR, Opisa S, Odhiambo G, Jura WG, Ayisi JM, Karanja DM, et al. Geographical distribution of schistosomiasis and soil-transmitted helminths among school children in informal settlements in Kisumu City, Western Kenya. Parasitology. 2011;138(12):1569–77. doi: 10.1017/S003118201100059X. [DOI] [PubMed] [Google Scholar]
- 26.Davis SM, Worrell CM, Wiegand RE, Odero KO, Suchdev PS, Ruth LJ, et al. Soil-transmitted helminths in pre-school-aged and school-aged children in an urban slum: a cross-sectional study of prevalence, distribution, and associated exposures. Am J Trop Med Hyg. 2014;91(5):1002–10. doi: 10.4269/ajtmh.14-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brooker S, Clements AC, Bundy DA. Global epidemiology, ecology and control of soil-transmitted helminth infections. Adv Parasitol. 2006;62:221–61. doi: 10.1016/S0065-308X(05)62007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fotedar R, Stark D, Beebe N, Marriott D, Ellis J, Harkness J. Laboratory diagnostic techniques for Entamoeba species. Clin Microbiol Rev. 2007;20(3):511–32. doi: 10.1128/CMR.00004-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leiva B, Lebbad M, Winiecka-Krusnell J, Altamirano I, Tellez A, Linder E. Overdiagnosis of Entamoeba histolytica and Entamoeba dispar in Nicaragua: a microscopic, triage parasite panel and PCR study. Arch Med Res. 2006;37(4):529–34. doi: 10.1016/j.arcmed.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Stauffer W, Ravdin JI. Entamoeba histolytica: an update. Curr Opin Infect Dis. 2003;16(5):479–85. doi: 10.1097/00001432-200310000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Zavala GA, Garcia OP, Campos-Ponce M, Ronquillo D, Caamano MC, Doak CM, et al. Children with moderate-high infection with Entamoeba coli have higher percentage of body and abdominal fat than non-infected children. Pediatr Obes. 2016;11(6):443–9. doi: 10.1111/ijpo.12085. [DOI] [PubMed] [Google Scholar]
- 32.Sakari SS, Mbugua AK, Mkoji GM. Prevalence of soil-transmitted helminthiases and schistosomiasis in preschool age children in Mwea division, Kirinyaga south district, Kirinyaga county, and their potential effect on physical growth. J Trop Med. 2017;2017:1013802. doi: 10.1155/2017/1013802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper MT, Searing RA, Thompson DM, Bard D, Carabin H, Gonzales C, et al. Missing the mark? A two time point cohort study estimating intestinal parasite prevalence in informal settlements in Lima, Peru. Glob Pediatr Health. 2017;4:1–8. doi: 10.1177/2333794X17739190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mbae C, Mulinge E, Guleid F, Wainaina J, Waruru A, Njiru ZK, et al. Molecular characterization of Giardia duodenalis in children in Kenya. BMC Infect Dis. 2016;16:135. doi: 10.1186/s12879-016-1436-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamau P, Aloo-Obudho P, Kabiru E, Ombacho K, Langat B, Mucheru O, et al. Prevalence of intestinal parasitic infections in certified food-handlers working in food establishments in the City of Nairobi, Kenya. J Biomed Res. 2012;26(2):84–9. doi: 10.1016/S1674-8301(12)60016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houmsou RS, Amuta EU, Olusi TA. Prevalence of intestinal parasites among primary school children in Makurdi, Benue State-Nigeria. J Infect Dis. 2010;8:97–106. [Google Scholar]
- 37.Amuta EU, Houmsou RS, Mker SD. Knowledge and risk factors of intestinal parasitic infections among women in Makurdi, Benue State. Asian Pac J Trop Med. 2010;3(12):993–6. doi: 10.1016/S1995-7645(11)60016-3. [DOI] [Google Scholar]
- 38.Leelayoova S, Siripattanapipong S, Thathaisong U, Naaglor T, Taamasri P, Piyaraj P, et al. Drinking water: a possible source of Blastocystis spp. subtype 1 infection in school children of a rural community in central Thailand. Am Soc Trop Med Hyg. 2008;79(3):401–6. [PubMed] [Google Scholar]
- 39.Singh A, Houpt E, Petri WA. Rapid diagnosis of intestinal parasitic protozoa, with a focus on Entamoeba histolytica. Interdiscip Perspect Infect Dis. 2009;2009:547090. doi: 10.1155/2009/547090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forson AO, Arthur I, Ayeh-Kumi PF. The role of family size, employment and education of parents in the prevalence of intestinal parasitic infections in school children in Accra. PloS one. 2018;13(2):e0192303. doi: 10.1371/journal.pone.0192303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baker MG, McDonald A, Zhang J, Howden-Chapman P. Infectious diseases attributable to household crowding in New Zealand: A systematic review and burden of disease estimate. Wellington: He Kainga Oranga/Housing and Health Research; 2013. [Google Scholar]
- 42.Workneh T, Esmael A, Ayichiluhm M. Prevalence of intestinal parasitic infections and associated factors among Debre Elias primary schools children, East Gojjam Zone, Amhara Region, North West Ethiopia. J Bacteriol Parasitol. 2014;5(1):1. [Google Scholar]
- 43.Gul N, Tak H. Prevalence of Trichuris spp. in small ruminants slaughtered in Srinagar District (J&K) J Parasit Dis. 2016;40(3):741–4. doi: 10.1007/s12639-014-0570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]