Abstract
We aimed to evaluate ten-year outcomes of penile prosthesis (PP) implantation for the treatment of erectile dysfunction and to assess predictors of early prosthetic infection (EPI). We identified 549 men who underwent 576 PP placements between 2008 and 2018. Univariate and multivariate analyses were used to identify potential predictors of EPI. An EPI predictive nomogram was developed. Thirty-five (6.1%) cases of EPI were recorded with an explant rate of 3.1%. In terms of satisfaction, 82.0% of the patients defined themselves as “satisfied,” while partner's satisfaction was 88.3%. Diabetes (P = 0.012), longer operative time (P = 0.032), and reinterventions (P = 0.048) were associated with EPI risk, while postoperative ciprofloxacin was inversely associated with EPI (P = 0.014). Rifampin/gentamicin-coated 3-piece inflatable PP (r/g-c 3IPP) showed a higher EPI risk (P = 0.019). Multivariate analyses showed a two-fold higher risk of EPI in diabetic patients, redo surgeries, or when a r/g-c 3IPP was used (all P < 0.03). We showed that diabetes, longer operative time, and secondary surgeries were the risk factors for EPI. Postoperative ciprofloxacin was associated with a reduced risk of EPI, while r/g-c 3IPP had higher EPI rates without an increased risk of PP explant. After further validation, the proposed nomogram could be a useful tool for the preoperative counseling of PP implantation.
Keywords: Boston, Coloplast Titan three-piece penile prosthesis, erectile dysfunction, impotence, Inhibizone, patient satisfaction, penile prosthesis, prosthesis infection, rifampin soaking
INTRODUCTION
Erectile dysfunction (ED) is defined as the inability to attain or maintain penile erection sufficient for successful vaginal intercourse.1 ED commonly affects men older than 40 years of age, with a reported prevalence of 2%–9% in men between the ages of 40 and 49 years, then increasing to 20%–40% in men aged 60–69 years, and up to 50%–100% in patients older than 70 years.2
Diabetes mellitus, hypertension, hyperlipidemia, metabolic syndrome, depression, and lower urinary tract symptoms are well-known risk factors for ED.3 In addition, a large proportion of patients suffer from iatrogenic ED, mainly due to pelvic surgery.4
Penile prosthesis (PP) implantation is considered a valuable option for ED patients who do not respond to pharmacotherapy or who prefer a permanent solution to their problem; nevertheless, the most recent American Urological Association (AUA) Guideline statement suggests considering PP, irrespective of previous attempts of medical management.3,4,5
In this context, PP has been associated with high rates of treatment-related satisfaction. One year after surgery, patients with PP reported high satisfaction in terms of prosthesis function, patients’ relationship with their partners and the outside world, as well as perceived self-image.6 Moreover, PP was found to work properly even at very long follow-up after the original implant (e.g., >10 years).7
On the other hand, this type of surgery is not devoid of potential complications.8 Most of the clinical research on this topic has mainly focused on the identification of risk factors for PP-related complications, such as ischemia of the glans penis and prosthetic infection, in order to adopt the best prevention strategies. However, a data-driven nomogram for the prediction of early prosthetic infections (EPI) is lacking.
The aim of this study was to investigate the ten-year experience of PP implantation of a tertiary referral center with the specific focus on surgical outcomes, related complications, and patient's satisfaction. Moreover, we aimed to build a predictive nomogram for postoperative infectious complications.
PATIENTS AND METHODS
In this retrospective study, we identified all men seeking medical attention for ED at a single tertiary referral center (Fundació Puigvert, Department of Andrology, University of Barcelona, Barcelona, Spain) who underwent PP implantation between January 2008 and October 2018.
Patients were assessed with a detailed medical and sexual history along with a general physical and andrological examination. Surgical procedures were performed by the same experienced surgical team. In particular, high-volume implanters (HVIs) were considered if they have placed >15 PPs in the preceding year. If surgery was conducted by a surgeon-in-training/low-volume implanter (LVI), a HVI was always present as a second operator.
A standard antibiotic prophylaxis with the first-generation cephalosporins was administered preoperatively in the absence of allergy. Antibiotic selection at patient discharge was based on physician preference and patient's specific factors, including allergies, home medications, and medical comorbidities.
Intraoperative and postoperative features and adverse events were recorded. Based on a standard internal protocol, follow-up consisted in scheduled re-evaluations at 5 days, 12 days, and 28 days after surgery and then yearly.
The diagnosis of EPI was suspected when one or more of the following signs were found between the first week after surgery and up to 9 weeks postimplant: (i) leukocytosis with or without fever accompanied by penile erythema, (ii) wound dehiscence, and (iii) discharge or skin necrosis. In patients with suspected EPI, empiric antibiotics were administered and culture swabs were collected from wound discharge. Subsequently, antibiotic therapy was adapted to culture results. Unresponsive patients to antibiotic treatment and/or with signs of sepsis or systemic symptoms (i.e., body temperature >38°C, rigors, malaise, continuous local pain, and leukocytosis >15 000 mm−3) were submitted to PP explant.
During explant procedure, all the components of the device were removed through a penoscrotal approach. The dissection was carried out using electrocautery to avoid inadvertently perforation of the implant, which could be lost in the surgical field. After removal, the surgical field was irrigated in a step-wise fashion using antibiotic solution, hydrogen peroxide solution, and povidone-iodine solution. In order to break up the bacterial biofilm, the washout was performed with high-pressure irrigation in order to maximize the antimicrobial purpose and the mechanical action. In selected cases with limited infectious process, the reservoir was not removed.
Patient satisfaction was retrospectively evaluated using the medical records of each follow-up assessment. Satisfaction was categorized as follows: “very satisfied,” if the patient was happy with the results of the operation and reported satisfactory intercourses; “not very satisfied,” if the patient reported a moderate satisfaction after surgery and an occasional use of the PP (for any reason); and “not satisfied” or “dissatisfied,” if the patient clearly expressed his dissatisfaction about the surgical outcome. Partner satisfaction was recorded and categorized as “satisfied” or “not-satisfied.” Of 552 ED men, we excluded three cases performed in a transgender setting. A convenient sample of 576 PP implantations was considered for the final analysis.
This study was conducted according to the guidelines and principles of the Declaration of Helsinki and standard ethical conduct for research involving humans; after approval of the Institutional Review Board of Fundació Puigvert, all patients signed informed consent agreeing to supply their own anonymous data for this and future studies. The study also guaranteed compliance at all times with Law 15/1999 on Protection of Personal Data (Spanish Government).
Statistical analyses
R software was used for statistical analysis (R Core Team, 2016; R Foundation for Statistical Computing, Vienna, Austria). Statistical significance for the tests was set at P = 0.05.
The Shapiro–Wilk test was applied to assess the normality of numerical variables. Continuous variables were presented as mean ± standard deviation (s.d.), while categorical variables were summarized as number (percentage).
Descriptive statistics were used to describe and to investigate potential factors associated with EPI. The statistical significance of differences in means was tested with the unpaired t-test and the Mann–Whitney U test, when appropriate. The statistical significance of differences in proportions was tested with the Pearson Chi-square test or the Fisher exact test.
Multivariate, stepwise, logistic regression9 analysis tested the association between clinical variables (diabetes, rifampin/gentamicin-coated 3-piece inflatable PP [r/g-c 3IPP], redo surgery, and patient's age) and risk of EPI. Finally, a nomogram was constructed in order to visualize and improve the understanding of the relationship between explanatory variables and the risk of EPI, particularly in terms of marginal effect and amount of probability. Package “rms” was used to obtain the final nomogram.10
RESULTS
Baseline characteristics
Mean patient's age at surgery was 60.6 (s.d.: 9.5) years. Above all, 166 (30.0%) men were current smokers and 175 (31.9%) were former smokers. Diabetes mellitus, obesity, and dyslipidemia were found in 224 (40.8%), 141 (25.7%), and 248 (45.2%) men, respectively. Among diabetic patients, 103 (18.8%) were insulin dependent and 121 (22.0%) were diet controlled.
Hypertension was found in 327 (59.6%) men, whereas cardiopathy was reported in 99 (18.0%) patients. Peyronie's disease and ischemic priapism were reported by 100 (18.2%) and 11 (2.0%) men, respectively. Previous pelvic surgery was the main cause of ED: 128 (23.3%) patients underwent radical prostatectomy, 9 (1.6%) had radical cystectomy with orthotopic neobladder, and 3 (0.5%) had radical cystectomy with ureterocutaneous vs ileal conduit diversion. Moreover, 5 (0.9%) men underwent kidney transplant, 9 (1.6%) underwent colorectal surgery, and 12 (2.2%) underwent other procedures.
Preoperative and operative variables
Table 1 depicts the surgical approach and the type of PP used among the whole cohort. Patients were usually admitted the day before surgery, and a standard antibiotic prophylaxis, first-generation cephalosporin, was administered preoperatively in 84.4% of subjects replacing it in case of allergies; 10-min standard scrub with povidone-iodine and chlorhexidine-alcohol was performed in all procedures. Antibiotic treatment was continued for the duration of the hospitalization. For Coloplast™ devices, the antibiotic dip choice was always rifampin and gentamicin; the same solution was used for wound irrigation during the procedure. At the end of surgery, a compressive “mummy wrap™” dressing was applied to the scrotum and penile shaft to minimize the risk of hematomas and to prevent local edema. Overall, the mean operative time was 83.6 (s.d.: 29.2) min. At the end of the procedure, a closed suction drainage was inserted in 281 (48.8%) cases. Surgery was performed by an LVI in 116 (20.1%) cases and mean postoperative hospital stay was 1.4 (s.d.: 1) days.
Table 1.
Types of penile prosthesis placed and surgical approach (n=576)
| Prosthesis type | Approach | Primary implant | Secondary implant | Total, n (%) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| PI | PMF | PE | ||||
| Malleable | Penoscrotal | 28 | 4 | 6 | 0 | 38 (6.6) |
| Subcoronal | 40 | 1 | 5 | 0 | 46 (8.0) | |
| Two-piece inflatable | Penoscrotal | 33 | 0 | 4 | 0 | 37 (6.4) |
| Three-piece inflatable | ||||||
| Rifampin/gentamicin-coated | Penoscrotal | 181 | 7 | 13 | 3 | 204 (35.4) |
| Rifampin/minocycline-impregnated | Infrapubic | 23 | 0 | 0 | 0 | 23 (4.0) |
| Penoscrotal | 201 | 7 | 16 | 2 | 226 (39.2) | |
In 2 cases, whereas 3-piece prosthesis was placed, the infection prevention strategy was missed. PI: previous infection; PMF: previous mechanical failure; PE: previous extrusion
A broad range of antibiotics was prescribed at discharge: amoxicillin clavulanate in 301 (52.3%) cases, ciprofloxacin in 197 (34.2%) men, cefixime in 4 (0.7%), cefuroxime in 3 (0.5%), and ceftriaxone, clindamycin, trimethoprim sulfamethoxazole in 1 (0.2%) man each. Twenty-one (3.6%) patients did not receive antibiotics at discharge. Data on the postoperative antibiotics were missed in 47 (8.2%) patients.
Intraoperative adverse events
The overall rate of intraoperative complications was low. A distal corpora perforation during dilatation was reported in two cases (0.3%). In 2 (0.3%) patients, a urethral stenosis did not allow a urinary catheter placement. In one case (0.2%), a penoscrotal 3-piece PP was unsuccessfully placed, and finally, a malleable PP was positioned with a subcoronal incision.
Postoperative adverse events
The overall rate of postoperative complication was 20.5%. Main complication features are shown in Table 2.
Table 2.
Postoperative complication after penile prosthesis implantation
| Complication | Cases (n) | Malleable (n) | 2IPP (n) | r/g-c 3IPP (n) | r/m-i 3IPP (n) | First implant (n) |
|---|---|---|---|---|---|---|
| Early infection | 35 | 5 | 0 | 21 | 9 | 27 |
| Of which explanted | 18 | 3 | 0 | 10 | 5 | 3 |
| Hematoma | 39 | 3 | 7 | 16 | 13 | 37 |
| Erosion | 12 | 2 | 2 | 6 | 2 | 7 |
| Mechanical failure | 23 | 0 | 2 | 8 | 13 | 18 |
| Pump | 7 | NA | 1 | 3 | 3 | – |
| Cylinders | 2 | 0 | 0 | 0 | 2 | – |
| Reservoir | 7 | NA | NA | 4 | 3 | – |
| Not known | 7 | 0 | 1 | 1 | 5 | – |
| Pain | 7 | 2 | 0 | 2 | 3 | 7 |
| Other | 11 | |||||
| Pulmonary edema | 1 | 1 | 0 | 0 | 0 | 1 |
| Intestinal fistula | 1 | 0 | 0 | 1 | 0 | 0 |
| AUR | 1 | 0 | 0 | 1 | 0 | 1 |
| OCD | 1 | 0 | 0 | 1 | 0 | 1 |
| Pump revision (trouble activating PP) | 1 | NA | 0 | 1 | 0 | 1 |
| Pump revision (high risk of erosion) | 1 | NA | 0 | 0 | 1 | 1 |
| Reservoir herniation | 1 | NA | NA | 1 | 0 | 1 |
| IIH | 1 | 0 | 0 | 0 | 1 | 1 |
| Orchiepididymitis | 1 | 1 | 0 | 0 | 0 | 1 |
| Fever (without local sign of infection) | 1 | 0 | 0 | 1 | 0 | 1 |
| Cylinders revision (concorde deformity) | 1 | 0 | 0 | 1 | 0 | 1 |
| Cylinders revision (for oversizing) | 1 | 0 | 0 | 1 | 0 | 1 |
2IPP: two-piece inflatable penile prosthesis; r/g-c 3IPP: rifampin/gentamicin-coated 3-piece inflatable penile prosthesis; r/m-i 3IPP: rifampin/minocycline-impregnated 3-piece inflatable penile prosthesis; AUR: acute retention of urine; OCD: obsessive-compulsive disorder; IIH: incarcerated inguinal hernia; NA: not applicable; –: not applicable
Among patients who developed hematomas, one (2.6%) required a surgical drainage, while in 2 (5.1%) patients, a re-suture was performed. In case of mechanical failure, a partial prosthesis replacement was performed in 65.2% of cases, while in one case, a 2IPP was changed with a 3IPP.
Overall, 35 (6.1%) EPI were reported, occurring within 6 weeks postoperatively in 88.5% of cases. EPI rate was 5.3% in first implants. Table 3 details the characteristics of 35 patients with EPI.
Table 3.
Early prosthetic infection: characteristics of 35 cases
| Patient number | Age (year) | Main causes of ED | Prosthesis type | First implant (yes/no) | Postoperative week to EPI | Cultured organism | Explant (yes/no) |
|---|---|---|---|---|---|---|---|
| 1 | 64 | Obesity, TS | r/g-c 3IPP | Yes | 1 | P. aeruginosa | No |
| 2 | 66 | Prostatectomy | r/g-c 3IPP | Yes | 1 | MD | No |
| 3 | 54 | ExTS, obesity, diabetes, HT | r/g-c 3IPP | Yes | 1 | E. cloacae + Achromobacter xylosoxidans | No |
| 4 | 40 | TS, diabetes | r/g-c 3IPP | Yes | 4 | Enterococcus spp. + Serratia odorifera | No |
| 5 | 75 | ExTS, obesity, diabetes, HT | r/g-c 3IPP | Yes | 7 | Staphylococcus spp. | No |
| 6 | 41 | TS, diabetes, HT, PD | r/g-c 3IPP | Yes | 3 | K. pneumoniae | No |
| 7 | 56 | TS, HT, PD | r/g-c 3IPP | Yes | 5 | K. pneumoniae | No |
| 8 | 51 | Obesity, diabetes, HT | r/g-c 3IPP | Yes | 3 | E. cloacae + Corynebacterium spp. | No |
| 9 | 54 | ExTS, diabetes, HT | r/g-c 3IPP | yes | 2 | MD | Yes |
| 10 | 62 | Diabetes, cystectomy | r/g-c 3IPP | yes | 2 | S. haemolyticus | Yes |
| 11 | 67 | MD | r/m-i 3IPP | No | 3 | E. coli | No |
| 12 | 72 | Prostatectomy | r/g-c 3IPP | No | 3 | E. coli | No |
| 13 | 70 | TS, cardiopathy, HT, diabetes | r/g-c 3IPP | No | 5 | E. faecalis | No |
| 14 | 51 | Prostatectomy, obesity, HT | r/g-c 3IPP | Yes | 1 | B. circulans + M. morganii | Yes |
| 15 | 75 | ExTS, cardiopathy, HT | Malleable | No | 5 | MD | No |
| 16 | 39 | Cardiopathy | r/m-i 3IPP | Yes | 5 | P. aeruginosa + S. marcescens | Yes |
| 17 | 58 | TS, diabetes, HT, PD | r/g-c 3IPP | Yes | 6 | K. pneumoniae | Yes |
| 18 | 60 | ExTS, diabetes, Ht | Malleable | Yes | 4 | E. coli + Candida spp. | Yes |
| 19 | 59 | ExTS, obesity, diabetes, HT, PD | r/g-c 3IPP | Yes | MD | MD | Yes |
| 20 | 73 | Diabetes, cardiopathy, HT, prostatectomy | r/m-i 3IPP | No | 2 | K. pneumoniae + E. faecium + E. coli + Bacteroides spp. | Yes |
| 21 | 55 | ExTS, diabetes, HT, depression, spinal cord injury | r/m-i 3IPP | No | 9 | E. faecium + Staphylococcus spp. | Yes |
| 22 | 67 | Cardiopathy, HT | r/g-c 3IPP | Yes | 7 | MD | Yes |
| 23 | 60 | TS, diabetes, cardiopathy, HT | r/g-c 3IPP | No | 3 | E. coli + Staphylococcus spp. | Yes |
| 24 | 57 | ExTS, obesity, diabetes, | r/m-i 3IPP | No | 5 | Streptococcus spp. | No |
| 25 | 55 | TS, obesity, cardiopathy, HT | Malleable | Yes | 3 | Enterobacter spp. | No |
| 26 | 54 | TS, diabetes, cardiopathy, HT | r/m-i 3IPP | Yes | 2 | E. coli + Bacteroides spp. | Yes |
| 27 | 37 | Priapism | r/g-c 3IPP | Yes | 6 | K. pneumoniae | No |
| 28 | 54 | ExTS, diabetes, cardiopathy, HT, kidney transplant | Malleable | Yes | 8 | K. pneumoniae | Yes |
| 29 | 64 | TS, diabetes, cardiopathy, HT, PD | r/m-i 3IPP | Yes | 4 | K. pneumoniae | Yes |
| 30 | 74 | TS, obesity, diabetes, HT, prostatectomy | r/g-c 3IPP | Yes | 5 | E. coli | No |
| 31 | 63 | Diabetes | Malleable | Yes | 2 | MD | Yes |
| 32 | 43 | Not specified medullar disorder | r/m-i 3IPP | Yes | 2 | S. epidermidis | No |
| 33 | 60 | HT, prostatectomy | r/m-i 3IPP | Yes | 2 | E. coli | Yes |
| 34 | 64 | TS, diabetes | r/g-c 3IPP | Yes | 3 | Inconclusive | Yes |
| 35 | 49 | TS, diabetes, HT, PD | r/g-c 3IPP | Yes | 2 | S. epidermidis | Yes |
EPI: early prosthetic infection; ED: erectile dysfunction; TS: tobacco smoker; exTS: former tobacco smoker; HT: hypertension; PD: Peyronie’s disease; MD: missing data; r/g-c 3IPP: rifampin/gentamicin-coated 3-piece inflatable penile prosthesis; r/m-i 3IPP: rifampin/minocycline-impregnated 3-piece inflatable penile prosthesis; P. aeruginosa: Pseudomonas aeruginosa; E. cloacae: Enterobacter cloacae; K. pneumoniae: Klebsiella pneumoniae; E. coli: Escherichia coli; E. faecium: Enterococcus faecium; S. epidermidis: Staphylococcus epidermidis; S. haemolyticus: Staphylococcus haemolyticus; E. faecalis: Enterococcus faecalis; B. circulans: Bacillus circulans; M. morganii: Morganella morganii; S. marcescens: Serratia marcescens
Ciprofloxacin was administered in 14.3% of patients, whereas 62.9% received amoxicillin clavulanate.
Cultured organisms were Escherichia coli, Staphylococcus/Streptococcus spp., and Klebsiella pneumoniae in 8 (22.8%), 7 (20.0%), and 6 (17.1%) men, respectively. A PP explant was mandatory in 18 (3.1%) patients.
Table 4 depicts descriptive statistics of the whole cohort according to the presence of EPI. Patients with EPI more frequently had a history of diabetes mellitus (P = 0.012), longer operative time (P = 0.032), and higher rates of reintervention (P = 0.048) than those without EPI. Similarly, r/g-c 3IPP showed a higher EPI risk (P = 0.019) but no increased risk of explants (P > 0.05). Moreover, patient discharged with ciprofloxacin showed a lower risk of EPI (P = 0.014).
Table 4.
Descriptive statistics of the whole population according to early prosthetic infection presence
| Variable | MD (n) | EPI group, mean±s.d. or n (%) | Non-EPI group, mean±s.d. or n (%) | P |
|---|---|---|---|---|
| Age | 0 | 58.4±10.4 | 60.8±9.4 | 0.145 |
| ASA score >2 | 274 | 10 (35.9) | 84 (31.1) | 0.618 |
| TS/exTS | 0 | 24 (68.6) | 330 (61.8) | 0.423 |
| Obesity (BMI >30 kg m−2) | 10 | 11 (31.4) | 136 (25.8) | 0.464 |
| Diabetes | 0 | 22 (62.8) | 220 (41.8) | 0.012* |
| HT | 11 | 22 (66.6) | 319 (60.5) | 0.483 |
| Dyslipidemia | 6 | 17 (48.6) | 240 (45.4) | 0.720 |
| Cardiopathy | 2 | 7 (20.0) | 99 (18.6) | 0.838 |
| Hypogonadism | 0 | 0 (0) | 11 (2.1) | – |
| Spinal cord injury | 0 | 1 (2.9) | 4 (0.7) | 0.273 |
| Immunosuppression | 0 | 1 (2.9) | 11 (2.8) | 1 |
| Previous pelvic surgery | 0 | 8 (22.9) | 170 (31.8) | 0.267 |
| Previous pelvic radiotherapy | 0 | 0 (0) | 26 (4.9) | 0.395 |
| Peyronie’s disease | 1 | 6 (17.1) | 98 (18.4) | 0.854 |
| Contextual Peyronie’s surgery | 0 | 3 (8.6) | 39 (7.3) | 0.737 |
| Priapism | 0 | 1 (2.9) | 11 (0.2) | 0.539 |
| Contextual urinary sphincter/urethral sling implantation | 0 | 0 (0) | 4 (0.7) | – |
| Postoperative antibiotic: amoxicillin clavulanate | 52 | 22 (73.3) | 277 (56.5) | 0.071 |
| Postoperative antibiotic: ciprofloxacin | 52 | 5 (16.7) | 191 (39.0) | 0.014* |
| Operation time | 0 | 95.3±31.7 | 85.5±28.9 | 0.032* |
| LVI | 2 | 10 (28.6) | 105 (19.7) | 0.204 |
| r/g-c 3IPP | 5 | 20 (58.8) | 205 (38.5) | 0.019* |
| Redo surgery | 0 | 8 (22.9) | 57 (10.7) | 0.048* |
| Drainage | 11 | 16 (47.1) | 264 (49.9) | 0.782 |
*P value according to the Mann-Whitney U test or the Fisher’s exact test as indicated. EPI: early prosthetic infection; MD: missing data; s.d.: standard deviation; ASA: American Society of Anesthesiologists; TS: tobacco smoker; BMI: body mass index; HT: hypertension; LVI: surgeon in training/low-volume implanter; r/g-c 3IPP: rifampin/gentamicin-coated 3-piece inflatable penile prosthesis; exTS: former tobacco smoker; –: not available
Table 5 shows multivariate logistic regression model predicting EPI. A history of diabetes mellitus was significantly associated with EPI (odds ratio: 2.281; P = 0.0252). In addition, the probability of EPI was almost three times greater for redo surgery than for first implant PP placement (odds ratio: 2.895, P = 0.0163). Finally, the probability of EPI was higher for patient who underwent r/g-c 3IPP placement (odds ratio: 2.38, P = 0.0177).
Table 5.
Logistic regression model
| Predictors | Estimate | s.e. | OR (95% CI) | P |
|---|---|---|---|---|
| Age | −0.031 | 0.019 | 0.969 (0.93–1.01) | 0.0966# |
| Diabetes | 0.825 | 0.368 | 2.281 (1.12–4.81) | 0.0252* |
| Reintervention | 1.063 | 0.442 | 2.895 (1.15–6.66) | 0.0163* |
| r/g-c 3IPP | 0.867 | 0.366 | 2.380 (1.17–4.97) | 0.0177* |
*Statistical significance P<0.05. #Statistical significance P<0.1. Dependent variable: early prosthetic infection. Intercept is omitted. CI: confidence interval; OR: odds ratio; r/g-c 3IPP: rifampin/gentamicin-coated 3-piece inflatable penile prosthesis; s.e.: standard error
Follow-up and patient/partner satisfaction rates
The mean follow-up was 14.6 (range: 1–67) months. Data regarding patient satisfaction were available in 40.5% of the cohort. Above all, 82.0% of the patients were satisfied, 7.3% were not very satisfied, and 10.7% were not satisfied. Satisfaction rates were 75.0%, 76.1%, and 83.6% for malleable devices, 2IPP, and 3IPP, respectively. Data of partner satisfaction were available in only 29.9% of cases. Eighty-eight point three percent of partners defined themselves as satisfied; this rate was higher considering 3IPPs (91.3%).
Early prosthetic infection predictive nomogram
Figure 1 shows the nomogram resulting from the proposed logistic model of Table 5, using EPI onset as the dependent variable and age, diabetes, reintervention, and use of r/g-c 3IPP as predictors. For simplicity and its low statistical significance, age was omitted. The nomogram was created to visualize the predicted probability for a subject with certain characteristics, if prosthesis infection would be present within 9 weeks after surgery. To summarize these results, patients with all type of diabetes, not at the first implant, and receiving a r/g-c 3IPP had the major risk of infection after surgery.
Figure 1.
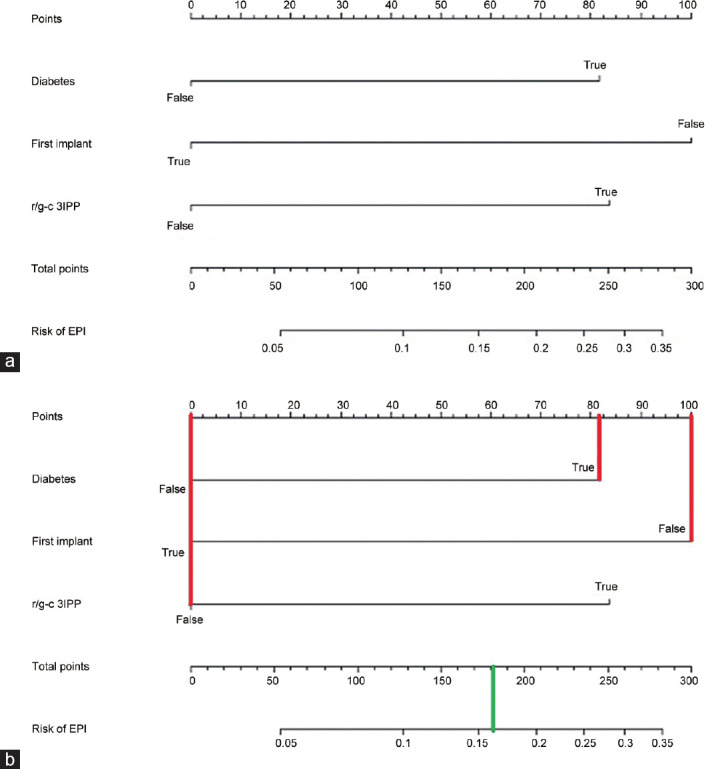
(a) Nomogram for predicting EPI onset after penile prosthesis implantation. The value for each factor (diabetes, first implant, r/g-c 3IPP) corresponds to points vertically above on the top scale. Point values are added together to determine the total points, which is directly connected to the odds of having EPI after surgery. Instructions for readers: in diabetic patients, draw a line from “Diabetes” axis straight upward to the point axis to determine how many points he receives for this comorbidity. Repeat the process for each additional variable. Sum the points for each of the predictors. Locate the final sum on the total point axis. Draw a line straight down to find the patient's probability of EPI. (b) Clinical example. Redo surgery in diabetic patient who has been scheduled for a malleable prosthesis implant: draw a vertical line (in red) for each of the variables of your patient (diabetes “Yes”= 82 points; first implant “NO” = 100 points, r/g-c 3IPP “NO” = 0 points); then you sum up the three values you read on the Points scale (82 + 100 + 0 = 182) to obtain total points. Finally, you draw a vertical line (in green) on the total points scale (182) to read the risk of EPI (0.16; 16%). EPI: early prosthetic infection; r/g-c 3IPP: rifampicin/gentamicin-coated 3-piece inflatable penile prosthesis.
DISCUSSION
In this study, we aimed to evaluate the outcomes of PP surgery at a tertiary referral center and to investigate potential risk factors for early prosthetic infections. We showed that PP implantation was an effective treatment option for men with ED with low risk of complications and high patient and partner satisfaction. Of clinical importance, our results confirmed previous studies showing that diabetes and secondary surgeries are risk factors for EPI. Of clinical importance, this is the first report that shows how different systemic and prosthetic-related strategies can significantly impact the infectious outcome of PPI implantation.
Patient satisfaction with PP is a complex and multifactorial issue that varies between the occurrence of postoperative complications, cosmetic outcome, ease of use, and partner acceptance. Numerous studies have reported high rates of patient satisfaction after PP implantation (up to 80%).11,12 In terms of implant, previous studies have shown a higher satisfaction rate for malleable devices as compared to 3IPP (86.8% vs 76%, and 54% vs 71%, respectively).13,14 On the contrary, we showed a higher overall satisfaction in patients who underwent 3IPP as compared to other devices. This difference may be explained by the different populations analyzed. Our cohort mainly included men who underwent 3IPP placement, while in other series, malleable devices were more frequently used. It is likely that the surgical expertise, which has a major impact on the rate of postoperative complication, might be the major drive for patient's satisfaction.
Infection of the prosthesis is among the most feared complication after PP surgery. Infectious complications are associated with significant impact on the economy of the healthcare system, with the cost of treatments usually exceeding the cost of the original prosthetic implant by more than six-fold.15
PP infections vary in timing, clinical presentation, and underlying microbiology. EPI, which is supposed to originate from direct infection of the device during surgery, usually emerges several weeks after surgery (ranging from 6 weeks to 8 weeks).16,17
Diabetes mellitus is the most investigated risk factor for EPI. Diabetes is a known cause of multiorgan dysfunction often affecting end arteries in organs such as the penis. While diabetes is recognized to impact postoperative wound healing and to increase the risk of infections, the physiopathology of this process is complex and likely multifactorial. In particular, decreased fibroblast proliferation and impaired neutrophil function have been found to play a significant role in diabetes-related complications.18,19 Data from the literature are controversial. While the AUA Guidelines state that there is no relevant evidence that diabetic men are at higher risk of prosthesis infection than men from the general ED population,17 the EAU Guidelines support the role of diabetes as a typical risk factor for men undergoing PP.16 Mulcahy and Carson20 found an increased rate of postoperative infections in diabetic vs nondiabetic men. In addition, a recent review reported that glucose control had a significant impact on PP infection.21 In details, a glycated hemoglobin (HbA1c) threshold level of 8.5% was found to be associated with an increased postoperative risk of IPP infection.21 Our results corroborate these findings since we showed that a history of diabetes mellitus was associated with twice higher risk of EPI.
Our results showed a three-fold higher risk of infection for redo surgeries compared to first implants. This is consistent with previous literature showing that revision surgery of PP, whether due to malfunction or infection, is at increased risk of postoperative infection. Reported rates of infection following revision surgery were as high as 10.0%–13.3%, as compared to 0.46%–2.00% of virgin cases.22,23,24,25,26 The increased incidence of postoperative infections following revision surgery is likely multifactorial with scar formation, reduced host resistance, and biofilms playing a pivotal role.
Longer operative time was also found to be associated with an increased risk of prosthetic infection.23,24,25,26,27 In this context, although the impact of surgical experience in reducing postoperative complications is widely recognized,21 we failed to find a significant association between operative time and EPI in our cohort. We believe that the operative time is subordinated to other risk factors related both to the patient and to the procedure, i.e., surgical experience, virgin or revision surgery, and fibrosis of the cavernous bodies. Moreover, our results might be influenced by the fact that, in our academic center, LVI acting as first operators is always assisted by an HVI. This finding is similar to that reported by McAbee et al.28 from an academic training center, where the rate of infections following PP placement remained low despite involvement of surgeons-in-training.
Similar to previous reports, we did not find any correlation between patient age, history of urinary diversion, history of pelvic radiotherapy, obesity, and EPI.12,24,25,26,27,28,29,30,31,32,33 Furthermore, we failed to find any association between EPI and active smoking status. Despite this, the negative effect of tobacco smoking in the immediate postoperative setting has been well documented.34 Smoking has been associated with an increased risk of infections in patients undergoing surgery, and smoking cessation has been associated with a reduced risk. In light of this, physician should encourage smoking cessation in clinical practice.
The management of EPI is challenging. Most of our patients were treated with an initial conservative approach. Habous et al.35 standardized the conservative management of localized infections and showed a complete clinical resolution of EPI in 83.8% of cases. Similar to the results from the current literature, our conservative protocol has shown a relatively high rate of success, which allowed to avoid PP explantation in more than half of the cases.
The use of postoperative antibiotics in PP surgery is a matter of debate. A recent consensus statement of 16 top prosthetic surgeons revealed that oral antibiotics administered anywhere from 5 days to 14 days after surgery was the preferred management, with a multitude of different oral antibiotics used, including quinolones, cephalosporins, penicillin, and sulfa drugs.36
Due to its good activity against Staphylococcal infection, excellent oral absorption, and activity against adherent bacteria, ciprofloxacin has been proposed in combination with other antibiotics for the prevention of infections in orthopedic and maxillofacial surgery.37,38,39,40 Of clinical importance, our results showed that the use of ciprofloxacin after surgery was associated with reduced risk of EPI. However, further large prospective studies are needed to confirm our results.
Design improvements in PP, such as antibiotic impregnation/coating, have led to a significant reduction in postoperative prosthesis infection. Since May 2001, Boston Scientific has used InhibiZone®, a device impregnation technology with minocycline/rifampin, while since August 2002, Coloplast has used a hydrophilic coating which absorbs any aqueous antibiotic solution left to surgeon discretion.41,42 Dhabuwala et al.43 compared Inhibizone-impregnated AMS penile implants with r/g-c and vancomycin/gentamicin-coated Titan Coloplast penile implants. Infection rates for Titan Coloplast penile implants coated with vancomycin/gentamicin and Inhibizone-impregnated AMS penile implants were 4.4% and 1.3%, respectively (P = 0.05). No significant difference was found in terms of infection rate among r/g-c Titan Coloplast implants (81 patients, 0 infection) and the Inhibizone-coated AMS implants group (77 patients, 1 case of infection).43 This study did not suggest superiority of r/g-c Titan Coloplast penile implants or Inhibizone-impregnated AMS penile implants but strongly suggested that all Titan Coloplast penile implants should be coated with rifampin/gentamicin solution.43 Although our study showed that r/g-c 3IPP was associated with an increased risk of EPI, no significant difference was observed in rate of explant. Therefore, a conservative management of EPI is valuable in preventing further and more severe complications. Furthermore, it could be argued that r/g-c and rifampin/minocycline-impregnated (r/m-i) 3IPP may need a different postoperative management and antibiotic regimen in order to equalize the risk of EPI.
Nomograms are common tools to estimate an event and counsel patients in clinical practice. Despite the widespread appearance of nomograms in urological and andrological research,44,45,46,47 to the best of our knowledge, this is the first nomogram with the specific aim of predicting EPI after PP implantation. This nomogram, based on simple clinical and procedural parameters, might be of clinical utility for patient's counseling toward the risk of EPI in the everyday clinical practice.
Our study should be interpreted with respect to its limitations. First, our results derived from single-center, retrospective, observational data. Second, the choice of PP to be implanted was not based on randomization but on the surgeon's preference/experience and patient's comorbidities. From an ethical point of view, we believe that such a randomization process would be challenging. In particular, if a 3IPP has been selected by the surgeon in a given case, it must be specified that it is unlikely that the r/g-c IPPs were chosen in patients at high risk of infection instead of the r/m-i IPPs, since the latter were available only in the first 5 years at our center, being replaced by the first ones in the last 5 years. Third, perioperative antibiotic treatment was not standardized. Fourth, we lacked data of HbA1c levels, which were found to be associated with EPI risk. Fifth, no standardized questionnaires were used for the assessment of partner's and patient's satisfaction.
Finally, since our study is underpowered in detecting interactions between factors, the proposed nomogram, relying only on main effects, could be an oversimplification of the actual joint effects of the considered factors. Therefore, larger prospective studies and external validation of our results are warranted.
CONCLUSIONS
PP is an effective treatment option for men with ED. This ten-year review of PP implantation in a tertiary referral center shows a very low complication rate after surgery and high patient's satisfaction. Our study confirms that diabetes, longer operative time, and secondary surgeries are risk factors for infectious complications following PP placement. Postoperative ciprofloxacin seems to reduce the risk of EPI, while r/g-c 3IPP appears to have higher risk of EPI without an increased risk of explant. We propose a data-driven nomogram for the prediction of EPI which can be used in clinical practice for better patient's counseling.
AUTHOR CONTRIBUTIONS
FP designed the study and drafted the manuscript. LB helped to draft the manuscript and analyze the data. MGS participated in the data collection. FP and RI analyzed the data. JSC, AG, AMG, ERC, and EM carried out the critical revision of the manuscript. JSG conceived of the study and participated in its design. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
The authors acknowledge and thank the employees of the paper archive of the Fundació Puigvert for their hard work and collaboration in the preparation of this article.
REFERENCES
- 1.National Institutes of Health. Consensus development conference statement. Impotence. December 7–9, 1992. Int J Impot Res. 1993;5:181–284. [PubMed] [Google Scholar]
- 2.Eardley I. The incidence, prevalence, and natural history of erectile dysfunction. Sex Med Rev. 2013;1:3–16. doi: 10.1002/smrj.2. [DOI] [PubMed] [Google Scholar]
- 3.Hatzimouratidis K, Giuliano F, Moncada I, Muneer A, Salonia A, et al. European Association of Urology Guidelines on Male Sexual Dysfunction: Erectile Dysfunction and Premature Ejaculation. Arnhem: EAU Guidelines Office; 2019. [Google Scholar]
- 4.Capogrosso P, Salonia A, Briganti A, Montorsi F. Postprostatectomy erectile dysfunction: a review. World J Mens Health. 2016;34:73–88. doi: 10.5534/wjmh.2016.34.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnett AL, Nehra A, Breau RH, Culkin DJ, Faraday MM, et al. Erectile dysfunction: AUA Guideline. J Urol. 2018;200:633–41. doi: 10.1016/j.juro.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Capogrosso P, Pescatori E, Caraceni E, Mondaini N, Utizi L, et al. Satisfaction rate at 1-year follow-up in patients treated with penile implants: data from the multicentre prospective registry INSIST-ED. BJU Int. 2019;123:360–6. doi: 10.1111/bju.14462. [DOI] [PubMed] [Google Scholar]
- 7.Chierigo F, Capogrosso P, Dehò F, Pozzi E, Schifano N, et al. Long-term follow-up after penile prosthesis implantation-survival and quality of life outcomes. J Sex Med. 2019;16:1827–33. doi: 10.1016/j.jsxm.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Scherzer ND, Dick B, Gabrielson AT, Alzweri LM, Hellstrom WJ. Penile prosthesis complications: planning, prevention, and decision making. Sex Med Rev. 2019;7:349–59. doi: 10.1016/j.sxmr.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. New York: A Wiley-Interscience Publication; 2013. pp. 1–375. [Google Scholar]
- 10.Zhang Z, Kattan MW. Drawing Nomograms with R: applications to categorical outcome and survival data. Ann Transl Med. 2017;5:211. doi: 10.21037/atm.2017.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montorsi F, Rigatti P, Carmignani G, Corbu C, Campo B, et al. AMS three-piece inflatable implants for erectile dysfunction: a long-term multi-institutional study in 200 consecutive patients. Eur Urol. 2000;37:50–5. doi: 10.1159/000020099. [DOI] [PubMed] [Google Scholar]
- 12.Palmisano F, Boeri L, Cristini C, Antonini G, Spinelli MG, et al. Comparison of infrapubic vs penoscrotal approaches for 3-piece inflatable penile prosthesis placement: do we have a winner? Sex Med Rev. 2018;6:631–9. doi: 10.1016/j.sxmr.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Minervini A, Ralph DJ, Pryor JP. Outcome of penile prosthesis implantation for treating erectile dysfunction: experience with 504 procedures. BJU Int. 2006;97:129–33. doi: 10.1111/j.1464-410X.2005.05907.x. [DOI] [PubMed] [Google Scholar]
- 14.Habous M, Tal R, Tealab A, Aziz M, Sherif H, et al. Predictors of satisfaction in men after penile implant surgery. J Sex Med. 2018;15:1180–6. doi: 10.1016/j.jsxm.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montague DK, Angermeier KW, Lakin MM. Penile prosthesis infection. Int J Impot Res. 2001;13:326–8. doi: 10.1038/sj.ijir.3900768. [DOI] [PubMed] [Google Scholar]
- 16.Swanton AR, Munarriz RM, Gross MS. Updates in penile prosthesis infections. Asian J Androl. 2020;22:28–33. doi: 10.4103/aja.aja_84_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta NK, Ring J, Trost L, Wilson SK, Köhler TS. The penoscrotal surgical approach for inflatable penile prosthesis placement. Transl Androl Urol. 2017;6:628–38. doi: 10.21037/tau.2017.07.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McManus LM, Bloodworth RC, Prihoda TJ, Blodgett JL, Pinckard RN. Agonist-dependent failure of neutrophil function in diabetes correlates with extent of hyperglycemia. J Leukoc Biol. 2001;70:395–404. [PubMed] [Google Scholar]
- 19.Barbul A, Efron DT, Kavalukas SL. Wound healing. In: Brunicardi FC, Andersen DK, Billiar TR, Dunn DL, Hunter JG, et al., editors. Schwartz's Principles of Surgery. 10th ed. New York: McGraw-Hill Education; 2014. pp. 241–72. [Google Scholar]
- 20.Mulcahy JJ, Carson CC., 3rd Long-term infection rates in diabetic patients implanted with antibiotic-impregnated versus nonimpregnated inflatable penile prostheses: 7-year outcomes. Eur Urol. 2011;60:167–72. doi: 10.1016/j.eururo.2011.01.046. [DOI] [PubMed] [Google Scholar]
- 21.Hebert KJ, Kohler TS. Penile prosthesis infection: myths and realities. World J Mens Health. 2019;37:276–87. doi: 10.5534/wjmh.180123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eid JF, Wilson SK, Cleves M, Salem EA. Coated implants and “no touch” surgical technique decreases risk of infection in inflatable penile prosthesis implantation to 0.46% Urology. 2012;79:1310. doi: 10.1016/j.urology.2011.11.076. [DOI] [PubMed] [Google Scholar]
- 23.Jarow JP. Risk factors for penile prosthetic infection. J Urol. 1996;156:402–4. doi: 10.1097/00005392-199608000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Wilson SK, Delk JR., 2nd Inflatable penile implant infection: predisposing factors and treatment suggestions. J Urol. 1995;153:659–61. [PubMed] [Google Scholar]
- 25.Wilson SK, Zumbe J, Henry GD, Salem EA, Delk JR, et al. Infection reduction using antibiotic-coated inflatable penile prosthesis. Urology. 2007;70:337–40. doi: 10.1016/j.urology.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 26.Thomalla JV, Thompson ST, Rowland RG, Mulcahy JJ. Infectious complications of penile prosthetic implants. J Urol. 1987;138:65–7. doi: 10.1016/s0022-5347(17)42991-0. [DOI] [PubMed] [Google Scholar]
- 27.Pineda M, Burnett AL. Penile prosthesis infections – a review of risk factors, prevention, and treatment. Sex Med Rev. 2016;4:389–98. doi: 10.1016/j.sxmr.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 28.McAbee KE, Pearlman AM, Terlecki RP. Infection following penile prosthesis placement at an academic training center remains low despite involvement of surgeons-in-training. Investig Clin Urol. 2018;59:342–7. doi: 10.4111/icu.2018.59.5.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loh-Doyle J, Patil MB, Nakhoda Z, Nassiri N, Yip W, et al. Three-piece inflatable penile prosthesis placement following pelvic radiation: technical considerations and contemporary outcomes. J Sex Med. 2018;15:1049–54. doi: 10.1016/j.jsxm.2018.04.634. [DOI] [PubMed] [Google Scholar]
- 30.Chung E, Solomon M, DeYoung L, Brock GB. Clinical outcomes and patient satisfaction rates among elderly male aged ≥75 years with inflatable penile prosthesis implant for medically refractory erectile dysfunction. World J Urol. 2014;32:173–7. doi: 10.1007/s00345-013-1102-7. [DOI] [PubMed] [Google Scholar]
- 31.Loh-Doyle J, Patil MB, Sawkar H, Wayne K, Boyd SD. 3-piece inflatable penile prosthesis placement following radical cystoprostatectomy and urinary diversion: technique and outcomes. J Sex Med. 2018;15:907–13. doi: 10.1016/j.jsxm.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Dubocq FM, Bianco FJ, Jr, Maralani SJ, Forman JD, Dhabuwala CB. Outcome analysis of penile implant surgery after external beam radiation for prostate cancer. J Urol. 1997;158:1787–90. doi: 10.1016/s0022-5347(01)64129-6. [DOI] [PubMed] [Google Scholar]
- 33.Gurunathan U, Ramsay S, Mitrić G, Way M, Wockner L, et al. Association between obesity and wound infection following colorectal surgery: systematic review and meta analysis. J Gastrointest Surg. 2017;21:1700–12. doi: 10.1007/s11605-017-3494-y. [DOI] [PubMed] [Google Scholar]
- 34.Osmonov D, Christopher AN, Blecher GA, Falcone M, Soave A, et al. Clinical recommendations from the European Society for sexual medicine exploring partner expectations, satisfaction in male and phalloplasty cohorts, the impact of penile length, girth and implant type, reservoir placement, and the influence of comorbidities and social circumstances. J Sex Med. 2020;17:210–37. doi: 10.1016/j.jsxm.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Habous M, Farag M, Williamson B, Laban O, Mahmoud S, et al. Conservative therapy is an effective option in patients with localized infection after penile implant surgery. J Sex Med. 2016;13:972–6. doi: 10.1016/j.jsxm.2016.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henry G, Price G, Pryor M, Greenfiel J, Jones L, et al. Observation of local clinical penile prostheses infections instead of immediate salvage rescue/removal: multicenter study with surprising results; abstract: PD20-04. J Urol. 2014;191:e612–3. [Google Scholar]
- 37.Luján S, Rogel R, Broseta E, Boronat F. Local treatment of penile prosthesis infection as alternative to immediate salvage surgery. Sex Med. 2016;4:e255–8. doi: 10.1016/j.esxm.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aboltins CA, Dowsey MM, Buising KL, Peel TN, Daffy JR, et al. Gram-negative prosthetic joint infection treated with debridement, prosthesis retention and antibiotic regimens including a fluoroquinolone. Clin Microbiol Infect. 2011;17:862–7. doi: 10.1111/j.1469-0691.2010.03361.x. [DOI] [PubMed] [Google Scholar]
- 39.Natarajan B, Balakrishnan G, Thangavelu K. Comparison of efficacy of amoxicillin versus ciprofloxacin in postsurgical management of transalveolar extraction. J Pharm Bioallied Sci. 2017;9:S187–90. doi: 10.4103/jpbs.JPBS_162_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hooton TM, Scholes D, Gupta K, Stapleton AE, Roberts PL, et al. Amoxicillin-clavulanate vs ciprofloxacin for the treatment of uncomplicated cystitis in women: a randomized trial. JAMA. 2005;293:949–55. doi: 10.1001/jama.293.8.949. [DOI] [PubMed] [Google Scholar]
- 41.Carson CC. Efficacy of antibiotic impregnation of inflatable penile prostheses in decreasing infection in original implants. J Urol. 2004;171:1611–4. doi: 10.1097/01.ju.0000118245.66976.e1. [DOI] [PubMed] [Google Scholar]
- 42.Wolter CE, Hellstrom WJ. The hydrophilic-coated inflatable penile prosthesis: 1-year experience. J Sex Med. 2004;1:221–4. doi: 10.1111/j.1743-6109.2004.04032.x. [DOI] [PubMed] [Google Scholar]
- 43.Dhabuwala C, Sheth S, Zamzow B. Infection rates of rifampin/gentamicin-coated Titan Coloplast penile implants. Comparison with inhibizone-impregnated AMS penile implants. J Sex Med. 2011;8:315–20. doi: 10.1111/j.1743-6109.2010.02068.x. [DOI] [PubMed] [Google Scholar]
- 44.Cozzi G, Musi G, Monturano M, Bagnardi V, Frassoni S, et al. Sexual function recovery after robot-assisted radical prostatectomy: outcomes from an Italian referral centre and predicting nomogram. Andrologia. 2019;51:e13385. doi: 10.1111/and.13385. [DOI] [PubMed] [Google Scholar]
- 45.Patel VR, Sandri M, Grasso AA, De Lorenzis E, Palmisano F, et al. A novel tool for predicting extracapsular extension during graded partial nerve sparing in radical prostatectomy. BJU Int. 2018;121:373–82. doi: 10.1111/bju.14026. [DOI] [PubMed] [Google Scholar]
- 46.Palmisano F, Boeri L, Fontana M, Gallioli A, De Lorenzis E, et al. Incidence and predictors of readmission within 30 days of transurethral resection of the prostate: a single center European experience. Sci Rep. 2018;8:6575. doi: 10.1038/s41598-018-25069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boeri L, Palmisano F, Preto M, Sibona M, Capogrosso P, et al. Sperm retrieval rates in non-mosaic Klinefelter patients undergoing testicular sperm extraction: what expectations do we have in the real-life setting? Andrology. 2020;8:680–7. doi: 10.1111/andr.12767. [DOI] [PubMed] [Google Scholar]


