Abstract
For infertility treatment, the selection of in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) is decided by multiplying indicators (including fallopian tube factors, semen count, and semen motility), except for sperm morphology. In this study, we conducted a retrospective analysis, from implantation to birth, over a period of 5 years. A total of 1873 couples with primary or secondary fallopian tube factors and an increased defective sperm morphology rate (DSMR) were divided into different groups to receive IVF or ICSI cycles. By comparing the outcomes, we found that the F1 group (DSMR <96%, IVF group 1) had higher cleavage rate, biochemical pregnancy rate, clinical pregnancy rate, and live birth rate than the F3 group (DSMR >98%, IVF group 3; P < 0.05). In contrast, there was no significant difference in the ICSI subgroups. Furthermore, a comparison of the outcomes between IVF and ICSI showed that the S3 group (DSMR >98%, ICSI group 3) had higher cleavage rate (P < 0.001), biochemical pregnancy rate (P < 0.05), clinical pregnancy rate (P < 0.05) and live birth rate (P < 0.05) than the F3 group. However, the ICSI subgroup had a lower two pronuclei fertilization rate than the IVF subgroup (P < 0.05). Our data suggest that the sperm morphology should also be considered when selecting IVF or ICSI combined with other semen parameters before the first assisted reproductive technologies (ART) cycle, especially for males with severe sperm defects.
Keywords: clinical outcome, in vitro fertilization, intracytoplasmic sperm injection, sperm morphology
INTRODUCTION
Infertility has become one of the major reproductive problems affecting 10%–15% of the general population worldwide. Male factors are identified in more than half of the infertile couples, so the diagnosis of male infertility and subsequent prediction of reproductive outcomes have become hot research topics.1,2,3 Traditional semen analysis, including sperm count, sperm concentration, motile sperm rate, and defective sperm morphology rate (DSMR), provides limited information for clinical management. Thus, functional diagnoses, such as acrosome reactions and DNA fragments,4,5 can be applied to improve the evaluation of male infertility. In 1986, Kruger et al.6 established strict criteria for sperm morphology, and it was reported that sperm morphology is correlated with the fertilization rate in in vitro fertilization (IVF). Subsequent publications showed significantly reduced fertilization rates with an increased DSMR.7,8,9,10 Meanwhile, the World Health Organization (WHO) updated and modified the reference value for the normal sperm morphology rate (NSMR), which decreased from 30% in 199211 to 14% in 1999,12 then to 4% in 2010.13 Due to these substantial decreases in the reference values of the NSMR, the appropriate threshold to diagnose male infertility and even predict the assisted reproductive technologies (ART) outcome is controversial.
More than 5 million people have been treated by ART to birth.14,15 Following clinical practice guidelines, the selection of IVF or intracytoplasmic sperm injection (ICSI) is decided based on sperm count and motility without regard to the sperm morphology.16,17,18 However, many studies19,20 have shown that the selection of sperm with a normal morphology by using intracytoplasmic morphologically selected sperm injection (IMSI) might be beneficial to embryonic development and the outcome of ART in couples with repeated implantation failures and severe male factor infertility. There are still conflicting data about the selection of ICSI in couples with teratozoospermia, ICSI's costs, and invasiveness to gametes.21,22,23 For medical cost analysis, Vitek et al.24 found that IVF is preferred in a single cycle, and split IVF/ICSI25 is preferred if two cycles are needed. For the analysis of invasiveness, research must be conducted in a timely manner to assess child health at birth.26
Here, we retrospectively analyzed the IVF or ICSI treatment and follow-up clinical data of infertile couples at Shanghai Ji Ai Genetics and IVF Institute , Obstetrics and Gynecology Hospital, Fudan University (Shanghai, China) from February 2011 to December 2015. We mainly analyzed the relationship between the DSMR and IVF/ICSI outcome under a stricter sperm morphology threshold than the WHO 5th manual.13 The data included preimplantation, implantation of embryo development, and birth. We aimed to discover the modified strict sperm morphology threshold to aid in the clinical outcomes of conventional IVF and ICSI for assisting couples with severely defective sperm.
PATIENTS AND METHODS
Patients
The IVF and ICSI cycles were performed at Shanghai Ji Ai Genetics and IVF Institute between January 2011 and December 2015, and then retrospectively analyzed. The inclusion criteria for females were as follows: primary or secondary fallopian tube factors leading to natural pregnancy failure, no chromosome or other genetic abnormalities, and at least one IVF or ICSI treatment cycle completed during this retrospective period. The exclusion criteria for male were azoospermia, high sperm DNA fragmentation rate, Y-chromosome microdeletion, Klinefelter's syndrome, or other reported genetic diseases. Written informed consent was obtained from patients and this study was approved by the Ethical Committee of Obstetrics and Gynecology Hospital.
Semen analysis and sperm morphology
Semen samples were obtained by masturbation on the day of oocyte collection after abstinence for 2–7 days according to the requirements of the 5th edition of the WHO Laboratory Manual for the Examination and Processing of Human Semen.13 Seminal smears were made on microscope slides and then treated according to the Papanicolaou staining protocol. Morphology was evaluated under bright-field microscopy (model 80i, Nikon, Tokyo, Japan) with a 100× objective lens, and at least 200 sperm were counted twice per slide. Based on the 95% confidence interval, the acceptable differences of twice counting values in the sperm morphology rate followed the WHO Laboratory Manual for the Examination and Processing of Human Semen (5th edition).13 The average percentages were rounded to the nearest whole number.
The couples treated by IVF or ICSI were separated into six groups as follows: IVF group 1 (F1): DSMR ≤96% (the threshold was recommended by the WHO 5th manual); IVF group 2 (F2): 96% < DSMR ≤ 98%; IVF group 3 (F3): DSMR >98%; ICSI group 1 (S1): DSMR ≤96%; ICSI group 2 (S2): 96% < DSMR ≤ 98%; and ICSI group 3 (S3): DSMR >98%.
ART protocol
Downregulation with triptorelin (Decapeptyl, Ferring GmbH, Kiel, Germany) followed by ovulation induction was the primary stimulation protocol used. According to the patients’ conditions, the initial dose of gonadotropin ranged from 150 IU to 225 IU. Oocytes were obtained by the transvaginal aspiration of follicles under B-ultrasound guidance and the serum estradiol (E2) value. All oocytes were incubated at 37°C with 5% CO2 and cultured in microdrops of human tubal fluid (HTF) medium (Life Global, Guilford, CT, USA) supplemented with 10 mg ml−1 human serum albumin under mineral oil (Cooper Surgical, Trumball, CT, USA) overlay. Then, oocytes were placed in 100 000 sperms per 50 μl drop for IVF or fertilized by ICSI. The fertilization evaluation was performed 18–20 h after IVF/ICSI treatment. The embryo evaluation was carried out on the 3rd day after fertilization. According to the number, shape, and fragment of blastomeres, embryo transfer was performed on day 3. Pregnancy tests were performed on the 15th day after embryo transfer. Clinical pregnancy was confirmed by the presence of a fetal heart on ultrasonic examination at 6–8 weeks of pregnancy.
Statistical analyses
All the outcomes in this study, including the fertilization rate, cleavage rate, pregnancy rate, sex ratio, and birth weight, were compared between groups by the Chi-square test or the Student's t-test (P < 0.05, two tails) using SPSS version 19.0 software (SPSS Inc., Cambridge, MA, USA).
RESULTS
General characteristics and preimplantation outcomes who received IVF/ICSI
These couples were divided into three groups for IVF or ICSI cycles based on the stricter sperm morphology modified on the WHO 5th manual:13 the control group (F1/S1, DSMR ≤96%), the moderate group (F2/S2, 96% < DSMR ≤ 98%), and the severe group (F3/S3, DSMR >98%). This study included data from 1873 couples who received an IVF or ICSI cycle at Shanghai Ji Ai Genetics and IVF Institute in the past 5 years (2011–2015). These couples received IVF at a mean maternal/paternal age of 33.82/33.79 years (F1 group), 34.26/34.29 years (F2 group), and 36.11/35.96 years (F3 group) or received ICSI at an average age of 33.93/33.67 years (S1 group), 33.93/33.69 years (S2 group), or 33.54/34.32 years (S3 group). The male partner received IVF with a mean sperm concentration of 53.45 × 106 ml−1 (F1 group), 51.86 × 106 ml−1 (F2 group), or 49.83 × 106 ml−1 (F3 group) or received ICSI with an average concentration of 13.47 × 106 ml−1 (S1 group), 17.06 × 106 ml−1 (S2 group), or 14.01 × 106 ml−1 (S3 group). The female partner received IVF with a mean endometrial thickness of 9.25 cm (F1 group), 8.26 cm (F2 group), or 6.65 cm (F3 group) or received ICSI with an average endometrial thickness of 8.25 cm (S1 group), 8.32 cm (S2 group), or 8.87 cm (S3 group). The details of all these parameters are listed in Table 1.
Table 1.
General parameters of the couples who participated in this study
| Parameter | DSMR ≤96% | 96% < DSMR ≤98% | DSMR >98% | |||
|---|---|---|---|---|---|---|
|
| ||||||
| F1 (n=800) | S1 (n=15) | F2 (n=554) | S2 (n=208) | F3 (n=46) | S3 (n=250) | |
| Male age (year) | 33.79±5.65 | 33.67±5.90 | 34.29±5.36 | 33.69±5.22 | 35.96±6.11 | 34.32±6.18 |
| Male BMI (kg m−2) | 24.43±3.39 | 24.02±2.45 | 24.23±3.25 | 24.52±3.31 | 24.85±3.12 | 24.52±3.33 |
| Female age (year) | 33.82±4.97 | 33.93±4.54 | 34.26±4.68 | 33.93±4.42 | 36.11±4.69 | 33.54±4.88 |
| Female BMI (kg m−2) | 21.84±4.07 | 23.47±3.15 | 21.47±2.87 | 21.38±2.74 | 21.18±2.59 | 21.69±3.10 |
| Abstinence time (day) | 7.05±2.39 | 4.93±1.44 | 5.47±2.01 | 5.78±2.15 | 4.70±1.53 | 5.69±1.77 |
| Semen volume (ml) | 2.44±0.54 | 2.00±0.84 | 2.47±0.53 | 1.85±0.54 | 2.08±0.45 | 1.91±1.33 |
| Sperm concentration (×106 ml−1) | 53.45±9.36 | 13.47±9.20 | 51.86±9.26 | 17.06±8.98 | 49.83 ±11.45 | 14.01±8.66 |
| Sperm (PR + NP), % | 54.0±6.7 | 27.9±15.0 | 51.7±6.8 | 16.4±7.2 | 46.8±5.9 | 13.7±7.0 |
| Sperm PR (%) | 37.5±12.0 | 16.3±10.4 | 35.7±10.4 | 8.0±4.6 | 31.3±6.0 | 6.4±4.5 |
| Sperm viability rate (%) | 64.3±12.1 | 37.9±15.0 | 61.7±6.9 | 26.1±7.7 | 56.8±5.9 | 22.8±8.0 |
| Endometrial thickness (cm) | 9.25±3.04 | 8.25±1.92 | 8.26±2.73 | 8.32±2.60 | 6.65±1.94 | 8.87±3.30 |
| Oocytes retrieved (n) | 10.08±6.00 | 12.92±6.09 | 10.95±6.41 | 10.51±5.81 | 4.46±4.20 | 9.96±5.63 |
| MII oocytes rate (%) | 87.8±15.4 | 75.4±17.4 | 87.1±17.5 | 82.9±19.0 | 84.6±25.2 | 80.9±19.7 |
| Fertilization number (n) | 6.85±4.34 | 8.58±4.84 | 7.53±4.75 | 7.26±4.37 | 3.32±2.94 | 6.76±4.45 |
Values are shown as the mean±s.d. DSMR: defective sperm morphology rate; F1: IVF group 1, DSMR ≤96%; F2: IVF group 2, 96% < DSMR ≤ 98%; F3: IVF group 3, DSMR >98%; S1: ICSI group 1, DSMR ≤96%; S2: ICSI group 2, 96% < DSMR ≤ 98%; S3: ICSI group 3, DSMR >98%; IVF: in vitro fertilization; ICSI: intracytoplasmic sperm injection; s.d.: standard deviation; BMI: body mass index; PR: progress motility; NP: nonprogressive motility; MII: metaphase II
For the IVF treatment subgroup, we analyzed three parameters (two pronuclei fertilization rate [PFR], cleavage rate [CR], and high-quality embryo rate [HER]). The results indicated that the cleavage rate decreased significantly (P < 0.05, Figure 1a) with increasing DSMR by comparing the severe group (F3 group, DSMR >98%) to the control group (F1, DSMR ≤96%). However, the HER and PFR were not significantly different (both P > 0.05; HER: ranging from 74.5% to 69.4%; PFR: ranging from 70.7% to 80.1%). For the ICSI treatment subgroup, the CR decreased (P > 0.05, Figure 1b) compared to the control group (S1, DSMR ≤96%), but the difference was not significant. However, neither the PFR nor the HER changed significantly.
Figure 1.
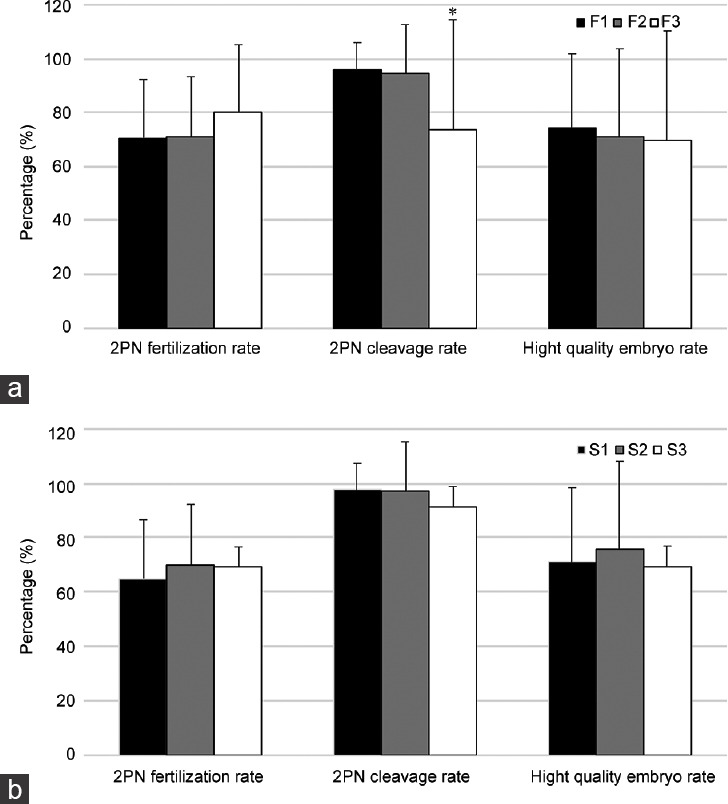
Relationship between DSMR and preimplantation embryo development. (a) Relationship between the DSMR and embryo potential originating from IVF (F3 or F2 vs F1). (b) Relationship between the DSMR and embryo potential originating from ICSI (S3 or S2 vs S1). *P < 0.05. DSMR: defective sperm morphology rate; IVF: in vitro fertilization; ICSI: intracytoplasmic sperm injection; F1: IVF group 1, DSMR ≤96%; F2: IVF group 2, 96% < DSMR ≤ 98%; F3: IVF group 3, DSMR >98%; S1: ICSI group 1, DSMR ≤96%; S2: ICSI group 2, 96% < DSMR ≤ 98%; S3: ICSI group 3, DSMR >98%.
Implantation and neonatal outcomes
Next, we analyzed 5 clinical outcomes after implantation: the biochemical pregnancy rate (BPR), clinical pregnancy rate (CPR), live birth rate (LBR), multipregnancy rate (MPR), and miscarriage rate (MR). In the IVF treatment subgroup, as the DSMR increased, the BPR, CPR, and LBR decreased significantly (all P < 0.05, Figure 2a) compared with those in the control group (F1, DSMR <96%). Conversely, the MPR of the F3 group increased from 14.8% to 17.7%, but the difference was not statistically significant (P > 0.05). A similar trend in the MR was also found in the F3 group, increasing from 2.8% to 5.9%, but the difference was not significant (P > 0.05). In the ICSI treatment subgroup, as the DSMR increased, none of the 5 clinical outcomes showed consistent changes after implantation, and none of the differences were statistically significant (all P > 0.05, Figure 2b).
Figure 2.
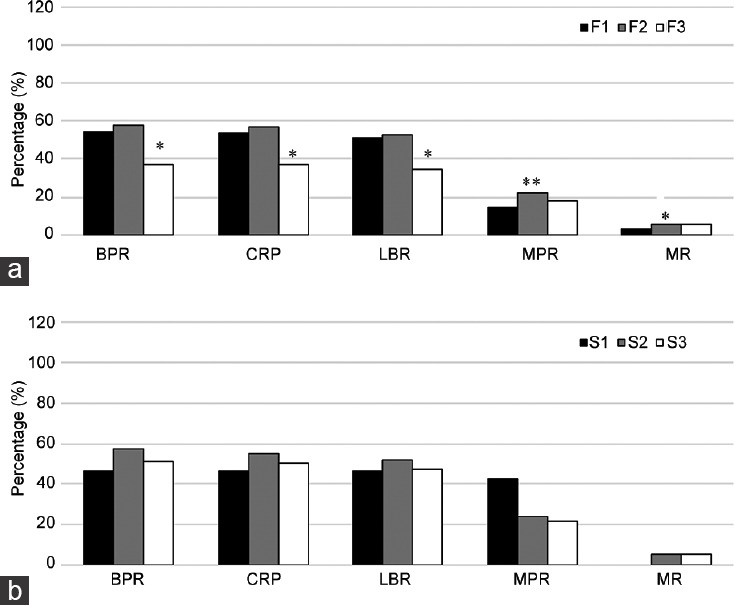
Relationship between the DSMR and IVF/ICSI outcome. (a) Relationship between the DSMR and IVF outcome (F3 or F2 vs F1). (b) Relationship between the DSMR and ICSI outcome (S3 or S2 vs S1). *P < 0.05, **P < 0.01. BPR: biochemical pregnancy rate; CPR: clinical pregnancy rate; LBR: live birth rate; MPR: multipregnancy rate; MR: miscarriage rate; DSMR: defective sperm morphology rate; IVF: in vitro fertilization; ICSI: intracytoplasmic sperm injection; F1: IVF group 1, DSMR ≤96%; F2: IVF group 2, 96% < DSMR ≤ 98%; F3: IVF group 3, DSMR >98%; S1: ICSI group 1, DSMR ≤96%; S2: ICSI group 2, 96% < DSMR ≤ 98%; S3: ICSI group 3, DSMR >98%.
We also analyzed 3 neonatal conditions after birth: the total baby sex ratio (SR = male/100 female), normal birth weight (NBW ≥2.5 kg), and healthy birth (HB: no birth-defect phenotype). In the IVF treatment subgroup, as the DSMR increased, the SR increased from 109.3% to 216.7%, but the difference was not statistically significant (P > 0.05, Figure 3a). Furthermore, neither the NBW nor the HB was significantly different. In the ICSI treatment subgroup, as the DSMR increased, the SR increased from 42.9% to 101.4% (P > 0.05, Figure 3b). A similar trend in the NBW was also found increasing from 60.0% to 82.1%, but the difference was not statistically significant (P > 0.05). Finally, the HB showed no statistically significant difference.
Figure 3.
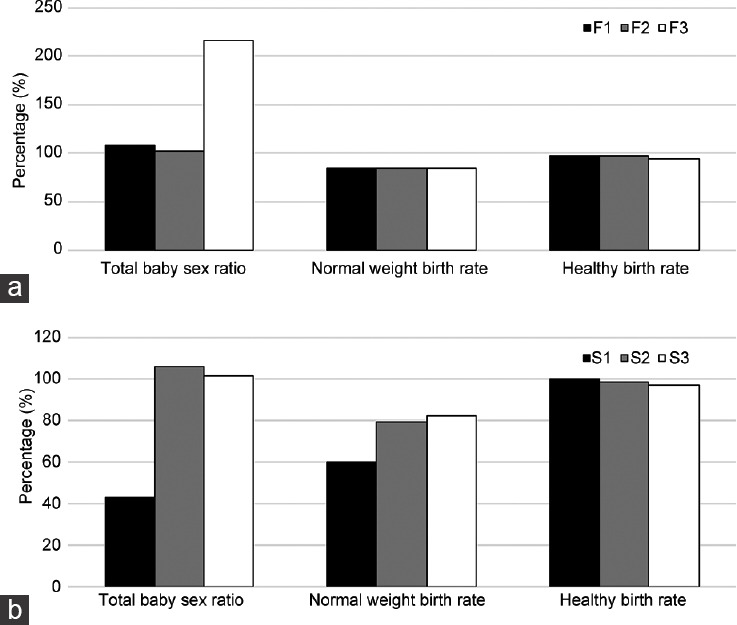
Relationship between the DSMR and newborn traits. (a) Relationship between the DSMR and newborn traits following IVF (F3 or F2 vs F1). (b) Relationship between the DSMR and newborn traits following ICSI (S3 or S2 vs S1). Total baby sex ratio = male/100 female; normal birth weight ≥2.5 kg; healthy birth: with no birth defect. DSMR: defective sperm morphology rate; IVF: in vitro fertilization; ICSI: intracytoplasmic sperm injection; F1: IVF group 1, DSMR ≤96%; F2: IVF group 2, 96% < DSMR ≤ 98%; F3: IVF group 3, DSMR >98%; S1: ICSI group 1, DSMR ≤96%; S2: ICSI group 2, 96% < DSMR ≤ 98%; S3: ICSI group 3, DSMR >98%.
Modified strict sperm morphology threshold aids in the clinical selection of IVF or ICSI
Furthermore, we conducted a direct comparison of the outcomes after IVF versus those after ICSI with respect to sperm morphology. The results showed that in the couples with a DSMR ≤96%, the PFR, MPR, and NBW were significantly better in the IVF subgroup than in the ICSI subgroup (70.7% vs 65.0%, 14.8% vs 42.9%, and 84.7% vs 60.0%, P < 0.05, Table 2). However, there were no significant differences when analyzing the other 8 indicators (CR, HER, BPR, CPR, LBR, MR, SR, and HB). In the couples with 96% < DSMR ≤ 98%, there were no significant differences when analyzing all 11 indicators (PFR, CR, HER, BPR, CPR, LBR, MPR, MR, SR, NEB, and HB).
Table 2.
Outcome indicators of the defective sperm morphology rate on clinical-assisted reproductive method selection (IVF vs ICSI)
| Indicator | DSMR ≤96% | 96% < DSMR ≤ 98% | DSMR >98% | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| F1 | S1 | P | F2 | S2 | P | F3 | S3 | P | |
| PFR (%)a, mean±s.d. | 70.7±21.6* | 65.0±16.2 | 0.042 | 71.1±22.2 | 70.1±23.2 | 0.579 | 80.1±25.1** | 69.2±23.0 | 0.004 |
| CR (%)a, mean±s.d. | 96.1±9.8 | 97.5±6.8 | 0.824 | 94.6±18.0 | 97.3±11.7 | 0.735 | 73.7±40.8 | 91.5±24.9*** | 0.000 |
| HER (%)a, mean±s.d. | 74.5±27.3 | 71.1±26.7 | 0.468 | 71.2±32.5 | 75.7±30.0 | 0.091 | 69.4±40.9 | 69.6±33.6 | 0.978 |
| BPR (%)b | 54.5 | 46.7 | 0.364 | 58.1 | 57.2 | 0.442 | 37.0 | 50.8* | 0.032 |
| CPR (%)b | 53.4 | 46.7 | 0.398 | 56.5 | 55.3 | 0.413 | 37.0 | 50.0* | 0.040 |
| LBR (%)b | 50.8 | 46.7 | 0.479 | 52.5 | 51.9 | 0.473 | 34.8 | 47.2* | 0.047 |
| MPR (%)b | 14.8 | 42.9* | 0.017 | 22.4 | 24.4 | 0.297 | 17.7 | 21.6 | 0.335 |
| MR (%)b | 2.8 | 0 | 0.661 | 5.8 | 5.2 | 0.476 | 5.9 | 5.6 | 0.509 |
| SR (%)b | 109.3 | 42.9 | 0.080 | 102.8 | 106.1 | 0.444 | 216.7 | 101.4 | 0.051 |
| NEB (%)b | 84.7* | 60.0 | 0.021 | 84.2 | 79.0 | 0.050 | 84.2 | 82.1 | 0.415 |
| HB (%)b | 98.0 | 100.0 | 0.741 | 97.0 | 98.6 | 0.159 | 94.7 | 97.2 | 0.421 |
*The difference between the two groups was statistically significant; a paried Student’s t-test or bChi-square test; *P<0.05; **P<0.01; ***P<0.001. s.d.: standard deviation; DSMR: defective sperm morphology rate; F1: IVF group 1, DSMR ≤96%; F2: IVF group 2, 96% < DSMR ≤ 98%; F3: IVF group 3, DSMR >98%; S1: ICSI group 1, DSMR ≤96%; S2: ICSI group 2, 96% < DSMR ≤ 98%; S3: ICSI group 3, DSMR >98%; IVF: in vitro fertilization; ICSI: intracytoplasmic sperm injection; PFR: two pronuclei fertilization rate; CR: two pronuclei cleavage rate; HER: high-quality embryo rate; BPR: biochemical pregnancy rate; CPR: clinical pregnancy rate; LBR: live birth rate; MPR: multipregnancy rate; MR: miscarriage rate; SR: total baby sex ratio; NEB: normal weight birth rate; HB: healthy birth rate
Most notably, our data showed that in couples with a DSMR >98%, the CR (91.5% vs 73.7%, P < 0.001), BPR (50.8% vs 37.0%, P < 0.05), CPR (50.0% vs 37.0%, P < 0.05), and LBR (47.2% vs 34.8%, P < 0.05) were higher in the ICSI subgroup than those in the IVF subgroup. However, only the PFR was significantly lower in the ICSI subgroup than that in the IVF subgroup (69.2% vs 80.1%, P < 0.05). Based on our data, sperm morphology is important for ART outcomes. For couples with severely defective sperm morphology, ICSI is a better treatment method than IVF.
DISCUSSION
Since the strict Kruger/Tygerberg criteria were established,6,9 the WHO normal sperm morphology lower limit was updated twice, changing from 14% (or a DSMR <86%) to 4% (or a DSMR <96%). Studies related to the DSMR effect on the outcomes of ART have mainly focused on the fertilization rate, cleavage rate, and pregnancy rate. Several reports showed severely reduced fertilization rates and an increased failed implantation rate in males with a DSMR >96%, but some studies indicated no obvious differences in the fertilization rate or other prognostic outcomes, such as pregnancy, in individuals with a DSMR >96%.8,15,27,28 Further follow-up data on the newborn's health condition are lacking. Our retrospective analysis of 1873 infertile couples from implant to live birth was performed over 5 years. Thus, there is relatively valuable evidence to aid in the selection of assisted reproductive technology in couples with male sperm morphology defects at their first visit to the reproductive center.
In our research on couples with an increased DSMR (≤96%, >96% and ≤98%, >98%, the same as below), those in the severe group (F3 group, DSMR >98%) had significantly lower CRs, BPRs, CPRs and LBRs than those in the control group (F1, DSMR ≤96%; P < 0.05, Figure 1a and 2a). These results indicate that a stricter standard DSMR (<98%) is strongly associated with the clinical outcome in infertile couples who choose IVF. In the ICSI treatment subgroup with an increased DSMR, the CR, BPR, CPR, and LBR decreased, but the differences were not statistically significant (all P > 0.05, Figure 1b). One explanation for this could be that the embryology laboratory was limited to the use of high-magnification microscopy (13 000×) for IMSI.19,20 During traditional ICSI manipulation, operators only subjectively (1000×) select some “normal sperm” from millions of cells with different and altered morphologies. Therefore, the outcome of fertilization, cleavage, and even live birth occurs with selected “normal sperm” that may not be representative of the sperm population within the sample, making the sperm morphology assessment irrelevant to the clinical outcome.
In ART, generally, according to clinical practice guidelines, the planned treatment is based on sperm count and motility. Nevertheless, in this study, the 11 clinical outcomes indicated that IVF treatment should be selected carefully. Based on our data, the IVF subgroup had lower CR, BPR, CPR, and LBR than the ICSI subgroup with severely defective sperm morphology (DSMR >98%) and low sperm count and motility. The other 7 clinical outcomes were not significantly different between the IVF and ICSI subgroups (Table 2). Vitek et al.24 found that IVF is preferred in a single cycle considering the medical cost, and split IVF/ICSI is preferred if two cycles are needed. Our data suggest that ICSI should be the main treatment strategy for couples with severe sperm defects (DSMR >98%), not the “first IVF cycle and second ICSI cycle” strategy.
More impressively, genetic investigations of male infertility focused on the defective sperm, including macrozoospermia, globozoospermia, and multiple morphological abnormalities of the sperm flagella (MMAF). They identified more than 30 novel genes accounting for 30% to 70% of cases.29,30 However, the gap between turning “research genes” into “diagnostic genes” still needs to be filled so that qualification tests can be applied to laboratory medicine or so the criteria can be modified to include the DSMR. Thus, the screening of potential “diagnostic genes” is not only impartment for implementing the DSMR but also for reducing the risk of transmitting genetic disorders to future offspring through ART.
AUTHOR CONTRIBUTIONS
FJ and YZ contributed to the conception and design of the study, acquisition of data, analysis and interpretation of data, and writing the manuscript. XXS contributed to the management of the clinical study. HC contributed to the acquisition of clinical data on females. FJ, YZ, and FZ contributed to drafting the article and critically reviewed the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This project was supported by the Shanghai Municipal Science and Technology Major Project (2017SHZDZX01) and State Key Laboratory of Reproductive Medicine (SKLRM-K202002). The authors would like to thank Mr. Zhi-Wen Tao of Fudan University for his assistance in data analysis and Miss Jia-Yu Deng (Judy) of Shanghai Qibao Dwight High School for her assistance in data collection; we also thank Dr. Ling-Ling Zhang of California University for her assistance in English grammar suggestions.
REFERENCES
- 1.Sharlip ID, Jarow JP, Belker AM, Lipshultz LI, Sigman M, et al. Best practice policies for male infertility. Fertil Steril. 2002;77:873–82. doi: 10.1016/s0015-0282(02)03105-9. [DOI] [PubMed] [Google Scholar]
- 2.Nallella KP, Sharma RK, Aziz N, Agarwal A. Significance of sperm characteristics in the evaluation of male infertility. Fertil Steril. 2006;85:629–34. doi: 10.1016/j.fertnstert.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 3.Brincat D, Catania S, Wismayer PS, Calleja-Agius J. Male factors in ART outcome prediction. Gynecol Endocrinol. 2015;31:1–7. doi: 10.3109/09513590.2014.984678. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Zheng Y, Zheng J, Lin J, Zhang LY, et al. The progesterone-induced sperm acrosome reaction is a good option for the prediction of fertilization in vitro compared with other sperm parameters. Andrologia. 2019;6:e13178. doi: 10.1111/and.13278. [DOI] [PubMed] [Google Scholar]
- 5.Ammar O, Tekeya O, Hannachi I, Sallem A, Mehdi M. Increased sperm DNA fragmentation in infertile men with varicocele: relationship with apoptosis, seminal oxidative stress, and spermatic parameters. Reprod Sci. 2020;28:909–19. doi: 10.1007/s43032-020-00311-6. [DOI] [PubMed] [Google Scholar]
- 6.Kruger TF, Menkveld R, Stander FS, Lombard CJ, Van der Merwe JP, et al. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil Steril. 1986;46:1118–23. doi: 10.1016/s0015-0282(16)49891-2. [DOI] [PubMed] [Google Scholar]
- 7.Check JH, Adelson HG, Schubert BR, Bollendorf A. Evaluation of sperm morphology using Kruger's strict criteria. Arch Androl. 1992;28:15–7. doi: 10.3109/01485019208987674. [DOI] [PubMed] [Google Scholar]
- 8.Van Waart J, Kruger TF, Lombard CJ, Ombelet W. Predictive value of normal sperm morphology in intrauterine insemination (IUI): a structured literature review. Hum Reprod Update. 2001;7:495. doi: 10.1093/humupd/7.5.495. [DOI] [PubMed] [Google Scholar]
- 9.Kruger TF, Coetzee K. The role of sperm morphology in assisted reproduction. Hum Reprod Update. 1999;5:172–8. doi: 10.1093/humupd/5.2.172. [DOI] [PubMed] [Google Scholar]
- 10.Ombelet W, Wouters E, Boels L, Cox A, Janssen M, et al. Sperm morphology assessment: diagnostic potential and comparative analysis of strict or WHO criteria in a fertile and a subfertile population. Int J Androl. 2010;20:367–72. doi: 10.1046/j.1365-2605.1998.00079.x. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interactions. 3th ed. Cambridge: World Health Organization; 1992. [Google Scholar]
- 12.World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interactions. 4th ed. Cambridge: World Health Organization; 1999. [Google Scholar]
- 13.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: World Health Organization; 2010. [Google Scholar]
- 14.Isidori A, Latini M, Romanelli F. Treatment of male infertility. Contraception. 2005;72:314–8. doi: 10.1016/j.contraception.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Gatimel N, Moreau J, Parinaud J, Leandri RD. Sperm morphology: assessment, pathophysiology, clinical relevance, and state of the art in 2017. Andrology. 2017;5:845–62. doi: 10.1111/andr.12389. [DOI] [PubMed] [Google Scholar]
- 16.Svalander P, Jakobsson AH, Forsberg AS, Bengtsson AC, Wikland M. The outcome of intracytoplasmic sperm injection is unrelated to 'strict criteria’ sperm morphology. Hum Reprod. 1996;11:1019–22. doi: 10.1093/oxfordjournals.humrep.a019289. [DOI] [PubMed] [Google Scholar]
- 17.Terriou P, Giorgetti C, Auquier P, Hans E, Spach JL, et al. Teratozoospermia influences fertilization rate in vitro but not embryo quality. Hum Reprod. 1997;12:1069–72. doi: 10.1093/humrep/12.5.1069. [DOI] [PubMed] [Google Scholar]
- 18.Ortega C, Verheyen G, Raick D, Camus M, Devroey P, et al. Absolute asthenozoospermia and ICSI: what are the options? Hum Reprod Update. 2011;17:684–92. doi: 10.1093/humupd/dmr018. [DOI] [PubMed] [Google Scholar]
- 19.Said TM, Land JA. Effects of advanced selection methods on sperm quality and ART outcome: a systematic review. Hum Reprod Update. 2011;65:719–33. doi: 10.1093/humupd/dmr032. [DOI] [PubMed] [Google Scholar]
- 20.Mangoli E, Khalili MA. The Beneficial role of intra cytoplasmic morphologically selected sperm injection (IMSI) in assisted reproduction. J Reprod Infertil. 2020;21:3–10. [PMC free article] [PubMed] [Google Scholar]
- 21.Verheyen G, Tournaye H, Staessen C, De Vos A, Vandervorst M, et al. Controlled comparison of conventional in-vitro fertilization and intracytoplasmic sperm injection in patients with asthenozoospermia. Hum Reprod. 1999;14:2313–9. doi: 10.1093/humrep/14.9.2313. [DOI] [PubMed] [Google Scholar]
- 22.Oehninger S, Gosden RG. Should ICSI be the treatment of choice for all cases of in-vitro conception.No, not in light of the scientific data? Hum Reprod. 2002;17:2237–42. doi: 10.1093/humrep/17.9.2237. [DOI] [PubMed] [Google Scholar]
- 23.Jain T, Gupta RS. Trends in the use of intracytoplasmic sperm injection in the United States. N Engl J Med. 2007;357:251–7. doi: 10.1056/NEJMsa070707. [DOI] [PubMed] [Google Scholar]
- 24.Vitek WS, Galárraga O, Klatsky PC, Robins JC, Carson SA, et al. Management of the first in vitro fertilization cycle for unexplained infertility: a cost-effectiveness analysis of split in vitro fertilization-intracytoplasmic sperm injection. Fertil Steril. 2013;100:1381–8. doi: 10.1016/j.fertnstert.2013.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Speyer B, O’Neill H, Saab W, Seshadri S, Cawood S, et al. In assisted reproduction by IVF or ICSI, the rate at which embryos develop to the blastocyst stage is influenced by the fertilization method used: a split IVF/ICSI study. J Assist Reprod Genet. 2019;36:647–54. doi: 10.1007/s10815-018-1358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li B, Ma Y, Huang J, Xiao X, Li L, et al. Probing the effect of human normal sperm morphology rate on cycle outcomes and assisted reproductive methods selection. PLoS One. 2014;9:e113392. doi: 10.1371/journal.pone.0113392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plachot M, Belaisch-Allart J, Mayenga JM, Chouraqui A, Tesquier L, et al. Outcome of conventional IVF and ICSI on sibling oocytes in mild male factor infertility. Hum Reprod. 2002;17:362–9. doi: 10.1093/humrep/17.2.362. [DOI] [PubMed] [Google Scholar]
- 28.Pastuszak AW, Herati AS, Eisenberg ML, Cengiz C, Langlois PH, et al. The risk of birth defects is not associated with semen parameters or mode of conception in offspring of men visiting a reproductive health clinic. Hum Reprod. 2019;34:733–9. doi: 10.1093/humrep/dez005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oehninger S, Ombelet W. Limits of current male fertility testing. Fertil Steril. 2019;111:835–41. doi: 10.1016/j.fertnstert.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Touré A, Martinez G, Kherraf ZE, Cazin C, Beurois J, et al. The genetic architecture of morphological abnormalities of the sperm tail. Hum Genet. 2020;1:21–42. doi: 10.1007/s00439-020-02113-x. [DOI] [PubMed] [Google Scholar]


