Abstract
Background:
Dacomitinib is a second-generation, irreversible epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI). Pre-clinical data suggest that intermittent pulsatile dosing of dacomitinib may result in inhibition of EGFR T790M.
Methods:
We evaluated safety, pharmacokinetics and efficacy of intermittent pulsatile dacomitinib in both molecularly unselected patients and patients with lung cancers harboring EGFR T790M (Clinical Trial Registration Number NCT01858389).
Results:
Thirty-eight patients were treated on study with pulse dacomitinib; sixteen with EGFR T790M in Cohort A and 22 who were not molecularly selected in Cohort B. One patient out of 16 patients in Cohort A had a partial response to study therapy (ORR 6.3%, 95% CI 0.2–30.2%). The median progression-free survival (PFS) in Cohort A was 2.3 months and median PFS in Cohort B was 1.6 months. The adverse event profile was similar to standard daily dose dacomitinib with the most frequent treatment-related toxicities occurring in >20% of patients being diarrhea, rash, stomatitis, nausea, dry skin, paronychia, fatigue, and decreased appetite.
Conclusion:
Intermittent pulsatile dacomitinib is safe and relatively well tolerated but is not effective in patients that harbor EGFR T790M or in unselected patients with non-small cell lung cancer.
Keywords: Dacomitinib, Non-small-cell lung cancer, T790M, Egfr, Pulsatile dosing, Tyrosine kinase inhibitors
1. Introduction
EGFR mutations in lung cancers confer response to EGFR tyrosine kinase inhibitors [1,2]. The first and second generation EGFR TKIs, erlotinib, gefitinib and afatinib, are approved as first-line treatment for patients with metastatic EGFR-mutant lung cancers [3–7]. Patients initially respond to these treatments, but then progress, on average 8–11 months after initiation of treatment. The most common mechanism of resistance to EGFR inhibitors is acquisition of a second site mutation, EGFR T790M [8]. A third generation EGFR inhibitor, osimertinib, has also recently been approved for patients with T790M mutation after failing on first or second generation EGFR inhibitors.
Dacomitinib is a second generation irreversible small molecular inhibitor of the HER family of tyrosine kinases including EGFR that has been extensively studied in non-small cell lung cancer (NSCLC). The maximum tolerated dose of dacomitinib was established to be 45 mg daily [9]. Dacomitinib was first assessed in molecularly unselected patients with previously treated advanced NSCLC. Despite promising results in earlier studies, the phase 3 confirmatory study did not show an improvement in outcomes in this population [10,11]. Dacomitinib was studied as first line treatment for clinically and molecularly selected patients [12]. In the subset of patients with EGFR-mutant lung cancers, dacomitinib was highly active with an overall response rate of 76% and an estimated median progression-free survival of 18.2 months. Severity and type of adverse of events was similar to other approved EGFR tyrosine kinase inhibitors. Results from the randomized phase 3 study comparing dacomitinib to gefitinib as first-line therapy for EGFR-mutant lung cancer, ARCHER 1050, were recently presented [13]. The median progression-free survival was 14.7 months compared to 9.2 months with gefitinib demonstrating dacomitinib’s efficacy in patients with EGFR-mutant lung cancers.
At the time of this study initiation, there was promising early data for dacomitinib in both molecularly unselected patients after failure of previous treatment as well as in patients as first-line treatment for EGFR-mutant lung cancer. The rationale for intermittent pulsatile dosing was that pulsatile doses of dacomitinib would be able to inhibit EGFR T790M-positive cells while allowing the outgrowth of EGFR T790M-negative cells, as seen in preclinical studies allowing for control of EGFR T790M positive cells. [14]. Higher doses of continuous dosing dacomitinib are limited by excessive toxicity; intermittent pulsatile dosing may be more tolerable from a toxicity standpoint and could result in sufficiently high peak concentrations to control resistant, EGFR T790M-positive, subpopulations within a tumor. For these reasons, we initiated a phase 2 study of pulsatile intermittent dacomitinib in patients with advanced non-small cell lung cancer.
2. Methods
2.1. Study design
This was a global, multicenter, open-label phase 2 study of oral intermittent dacomitinib in patients with advanced NSCLC. Two cohorts were concurrently enrolled. Cohort A included patients whose tumor had evidence of EGFR T790M. Cohort B included T790M-negative tumors that were otherwise molecularly unselected. The primary endpoint of the study was best overall response per Response Evaluation Criteria in Solid Tumors (RECIST) v 1.1 for patients in Cohort A. Secondary endpoints included characterizing the effects of dacomitinib and its metabolite concentration on the QT interval for both cohorts, additional efficacy measures including disease control rate and duration of response for Cohort A, PFS and progression free survival rate at four months for both Cohort A and Cohort B, as well as assessment of the safety and tolerability of intermittent pulse dacomitinib. All patients signed an informed consent form approved by an institutional review board.
2.2. Patient population
Patients aged > 18 years with evidence of histologically confirmed advanced NSCLC were eligible to enroll. Cohort A patients (EGFR T790M-positive) could have received any number of prior chemotherapy regimens and could have received prior reversible EGFR directed therapy. If patients were treated with prior EGFR TKI therapy, they must have demonstrated progression and could not have had any intervening treatment between progression on prior EGFR treatment and study enrollment. Patients in Cohort B (molecularly unselected) could have had 0–1 lines of systemic cytotoxic therapy, and could have received prior reversible EGFR-directed therapy. All patients must have had a performance status of 0–2 on the Eastern Cooperative Oncology Group (ECOG) scale, presence of measurable disease and adequate renal and liver function. All therapy including systemic treatment, radiation and surgery must have been completed at least 2 weeks prior to enrollment with the exception of erlotinib or gefitinib which required a washout of 3 days. Patients with evidence of mixed histology with elements of small cell or carcinoid were excluded. Patients with known leptomeningeal metastases or symptomatic brain metastases were excluded; patients with treated brain metastases that were neurologically stable and off corticosteroids for at least 2 weeks prior to study start were eligible.
2.3. Treatment
Treatment began with a lead in cycle (Cycle 0) during which patients received dacomitinib 45 mg without food every 12 h (q12 h) for six doses. Cycle 1 began the following week with patients taking dacomitinib 60 mg q12 h for six doses and cycles were repeated every two weeks. Intra-patient dose escalation beyond 60 mg was considered for patients in Cohort A provided they did not have significant toxicity after two cycles on the same dose and was done in 15 mg intervals following sponsor approval. Dose interruptions followed by dose reductions, in 15 mg intervals to a decreased dose of 30 mg occurred in response to toxicity. Dose reductions below 30 mg required sponsor approval. Patients maintained medication diaries to track treatment compliance. Treatment was discontinued for progression of disease, unacceptable side effects, non-compliance or withdrawal of informed consent.
At baseline, patients underwent review of interval medical history and physical examination, electrocardiogram (ECG), laboratory assessments and disease evaluation using CT or MRI. Women of reproductive potential underwent a pregnancy test prior to study initiation. During the lead-in cycle, time-matched ECG evaluations were done on Day 1 (prior to first dose) and Day 4 (following 6th dose). Pharmacokinetic (PK) samples were collected around the ECG evaluations on Day 4 at pre-dose, 2, 4, 6, 8 and 10 h post-dose. During Cycle 1 and any cycle where the dacomitinib dose was escalated, patients had an adverse event assessment and physical examination including vitals, ECG, laboratory assessments and a pre-dose pharmacokinetic blood sample drawn on Days 1 and 4. All other cycles required an adverse event assessment and physical examination including vitals, ECG and laboratory assessments. Tumor assessments were done at baseline and every 6 weeks for the first 3 assessments and then every 8 weeks thereafter.
2.4. Statistical analysis
2.4.1. The Fleming single stage design was used for Cohort A to test the null hypothesis
ORR was < = 1% at a significance level of 0.05. A minimum of 15 patients was required to be enrolled in Cohort A providing 80% power when true ORR was 19%. The exact test was conducted to test the null hypothesis.
The analysis of effect on QTc interval was based on a non-inferiority hypothesis testing framework. A minimum of 31 dacomitinib-treated patients, evaluable for QTc, were needed for a non-inferiority margin of 20 msec, assuming 90% power, an overall 1-sided significance level of 0.05. A random effect model was used to estimate the mean change in QTc from baseline at each post-baseline nominal time point. The intent-to-treat population included all patients enrolled and this was the population assessed for efficacy and baseline characteristics. Safety, pharmacokinetics and ECGs were assessed in all patients dosed with dacomitinib. The overall response rate was estimated with a corresponding 95% confidence interval using a method based on the binomial distribution. Time-to-event endpoints were estimated using the Kaplan-Meier method. Dacomitinib concentration–time data and ECG data were summarized using descriptive statistics.
3. Results
3.1. Patients
Between 31 July 2013 and 09 Mar 2015, 41 patients were enrolled, 16 in Cohort A and 25 in Cohort B; 38 were dosed with dacomitinib, 16 in Cohort A and 22 in Cohort B. Of the 3 patients enrolled in Cohort B that were not treated, 2 withdrew consent and 1 experienced an SAE before start of treatment. All 38 patients have discontinued study treatment and data are presented up until the end of study date, 23
September 2015. Table 1 illustrates patient demographics and baseline characteristics.
Table 1.
Clinical and molecular characteristics.
| Age, median (range) | Cohort A | Cohort B | Overall |
|---|---|---|---|
|
| |||
| N = 16 | N = 25 | N = 41 | |
|
| |||
| 56 (31–79) | 64 (47–83) | 62 (31–83) | |
| Sex (%) | |||
| Male | 4 (25) | 10 (40) | 14 (34.1) |
| Female | 12 (75) | 15 (60) | 27 (65.9) |
| Race (%) | |||
| White | 6 (37.5) | 11 (44) | 17 (41.5) |
| Black | 2 (12.5) | 2 (8) | 4 (9.8) |
| Asian | 7 (43.8) | 11 (44) | 18 (43.9) |
| Other | 1 (6.3) | 1 (4) | 2 (4.9) |
| Smoking status (%) | |||
| Never | 12 (75) | 13 (52) | 25 (61) |
| Former/Current | 4 (25) | 11 (44) | 15 (36.6) |
| Unknown | 0 | 1 (4) | 1 (2.4) |
| ECOG (%) | |||
| 0 | 7 (43.8) | 4 (16) | 11 (26.8) |
| 1 | 8 (50) | 20 (80) | 28 (68.3) |
| 2 | 1 (6.3) | 1 (4) | 2 (4.9) |
| EGFR Mutation (%) | |||
| Positive | 16 (100) | 15 (60) | |
| L858R | a | 3 (12) | |
| Exon 19 deletion | a | 9 (36) | |
| Other | a | 3 | |
| T790M | 16 (100) | 0 | |
| Negative | 0 | 10 (40) | |
The requirement for Cohort A was an EGFR T790M mutation, so specific sensitizing EGFR mutations present were not summarized.
3.2. Efficacy
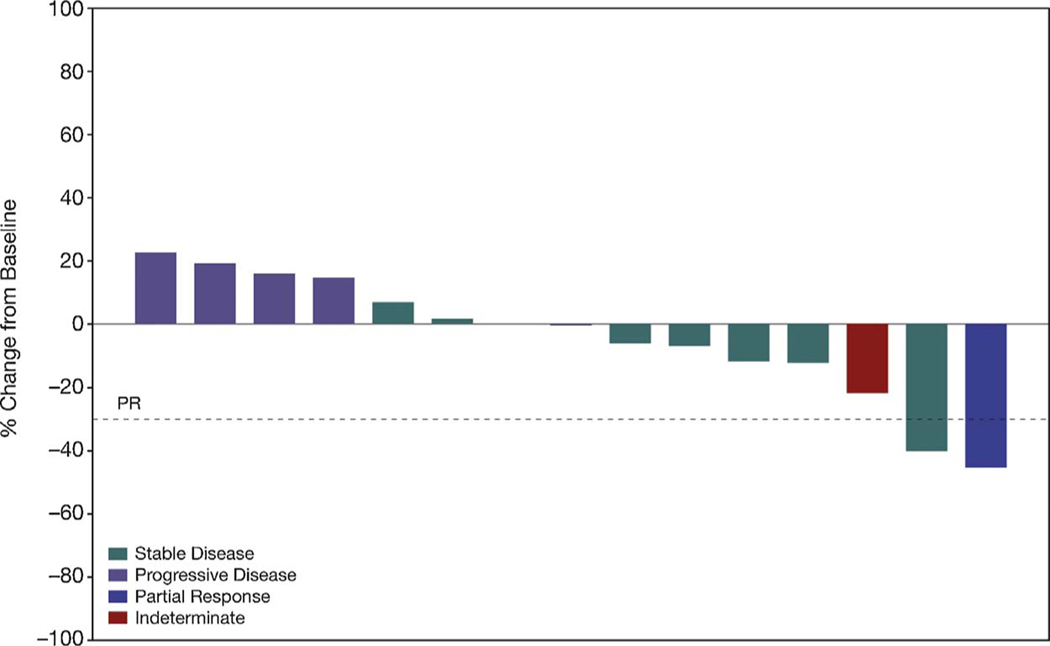
One patient out of 16 in Cohort A had an objective partial response to study therapy (ORR 6.3%, 95% CI 0.2%–30.2%; p-value = 0.15) (Fig. 1). Seven patients had a best response of stable disease; six patients had progression at their first assessment and two patients were indeterminate. The disease control rate in Cohort A was 50% (95% CI 24.7%–75.3%). The patient with the partial response had a 2.8 month duration of response. No patients in Cohort B had an objective response. The median PFS in Cohort A was 2.3 months (95% CI: 1.4–4.4 months) and median PFS in Cohort B was 1.6 months (95% CI: 1.0–3.3 months). The 4-month PFS rate was 37.5% (95% CI: 15.4%–59.8%) in Cohort A and 25.3% (95% CI: 8.6%–46.2%) in Cohort B.
Fig. 1.
Best change in target lesions in all evaluable patients in Cohort A per RECIST V1.1.
3.3. Safety
The median duration on treatment for patients was 82 days (range 11–382 days) and 39 days (11–431 days) for Cohorts A and B, respectively. The majority of patients (9 of 16) in Cohort A were dose escalated. The maximum dose escalation was to 75 mg q12 h in 5 patients, 90 mg q12 h in 3 patients and 105 mg q12 h in 1 patient.
All patients experienced at least one treatment-related adverse event. The treatment-related adverse events occurring in > 10% of subjects are reported in Table 2. The most common treatment-related adverse events included diarrhea, nausea, dry skin, rash, stomatitis, paronychia and fatigue; the majority of events were of grade 1–2 severity and manageable with standard supportive care. There were five treatment-related serious adverse events in three patients; nausea [1], diarrhea [2], and dehydration [2]. No patients discontinued study therapy due to a treatment-related adverse event, but 21% of patients had a dose reduction due to a treatment-related adverse event. Six patients died while on study within 28 days of the last dose of study medication all due to conditions unrelated to study treatment. There were no treatment-related cardiac adverse events that occurred on study. Pre-specified adverse events of special interest included paronychia, dry eye, conjunctivitis and palmar-plantar erythrodysesthesia syndrome. Six patients (15.8%) had grade 2 paronychia related to study drug. Three patients (7.9%) had conjunctivitis, one grade 2 and two grade 3, all related to study drug. One patient (2.6%) had grade 2 palmar-plantar erythrodysesthesia syndrome related to study drug.
Table 2.
Treatment-Related Treatment-Emergent Adverse Events in > 10% of Total Patients.
| Cohort A | ||||
|---|---|---|---|---|
|
| ||||
| CTCAE Term | Grade 1 | Grade 2 | Grade 3 | Total (N =16) |
|
|
||||
| n (%) | n (%) | n (%) | ||
| Diarrhea | 9 | 3 | 1 | 13 (81) |
| Rash | 6 | 1 | 1 | 8 (50) |
| Stomatitis | 4 | 1 | 2 | 7 (44) |
| Dry skin | 3 | 3 | 0 | 6 (37) |
| Nausea | 5 | 0 | 1 | 6 (37) |
| Fatigue | 3 | 1 | 1 | 5 (31) |
| Dermatitis acneiform | 0 | 4 | 0 | 4 (25) |
| Decreased appetite | 1 | 1 | 1 | 3 (19) |
| Conjunctivitis | 0 | 0 | 2 | 2 (13) |
| Dehydration | 0 | 1 | 1 | 2 (13) |
| Dysgeusia | 2 | 0 | 0 | 2 (13) |
| Elevated AST | 1 | 0 | 1 | 2 (13) |
| Elevated Alk Phos | 1 | 1 | 0 | 2 (13) |
| Maculopapular rash | 1 | 1 | 0 | 2 (13) |
| Oral pain | 2 | 0 | 0 | 2 (13) |
| Peripheral neuropathy | 1 | 0 | 1 | 2 (13) |
| Vomiting | 1 | 1 | 0 | 2 (13) |
| Xerosis | 2 | 0 | 0 | 2 (13) |
|
| ||||
| Cohort B | ||||
|
| ||||
| CTCAE Term | Grade 1 | Grade 2 | Grade 3 | Total (N = 22) |
|
| ||||
| Diarrhea | 11 | 3 | 2 | 16 (73) |
| Rash | 7 | 3 | 1 | 11 (50) |
| Nausea | 7 | 2 | 0 | 9 (41) |
| Dry skin | 5 | 2 | 0 | 7 (32) |
| Paronychia | 4 | 3 | 0 | 7 (32) |
| Fatigue | 4 | 2 | 0 | 6 (27) |
| Decreased appetite | 4 | 1 | 0 | 5 (23) |
| Pruritis | 4 | 0 | 1 | 5 (23) |
| Stomatitis | 3 | 1 | 1 | 5 (23) |
| Dermatitis acneiform | 2 | 1 | 0 | 3 (14) |
| Mucosal inflammation | 0 | 3 | 0 | 3 (14) |
Laboratory abnormalities on study treatment were nearly all grade 1 and 2 and not clinically significant. Grade 3 treatment-emergent lymphopenia was seen in five patients (13.2%) and one patient each had treatment-emergent grade 3 bilirubinemia, elevated AST, elevated ALT and hyponatremia. Grade 3 hypermagnesemia was seen in three patients as well.
3.4. Pharmacokinetic and ecg analyses
Following 45 mg q12 h dosing of dacomitinib on Days 1–4 of Cycle 0, mean values for pre-dose and maximum plasma concentrations on Day 4 (Cmax) of dacomitinib were 76.4 ng/mL and 96.8 ng/mL, respectively, and mean values for pre-dose and Cmax of metabolite, PF-05199265, were 7.1 ng/mL and 8.5 ng/mL, respectively (Table 3).
Table 3.
Summary of Descriptive Statistics for Pharmacokinetic Parameters of Plasma Dacomitinib and PF-05199265.
| N | Dacomitinib | PF-05199265 | |
|---|---|---|---|
|
|
|||
| Mean (CV%) (Median) | |||
| Cycle 0, Day4a Pre-dose (0 h) (ng/mL) |
36 | 76.4 (43) [68.4] | 7.1 (161) [4.6] |
| AUC0–10 (ng h/mL) | 33 | 793.8 (40) [728.0] | 75.0 (148) [50.8] |
| Cmax (ng/mL) | 37 | 96.8 (39) [90.7] | 8.5 (150) [5.9] |
| Cmin (ng/mL) | 37 | 71.3 (39) [65.6] | 6.8 (157) [4.5] |
| Tmaxb (hr) | 37 | 6.1 (1.3–10.9) | 5.6 (0–11.0) |
| Cycle 1, Day 1c Pre-dose (ng/mL) |
35 | 32.1 (65) [26.9] | 3.8 (83) [2.8] |
| Cycle 1, Day 4d Pre-dose (ng/mL) |
33 | 108.1 (39) [100] | 9.0 (93) [7.43] |
Note: N = the total number of patients contributing to the summary statistics.
AUC0–10 = area under the curve from time 0–10 h; Cmax = maximum plasma concentration; Cmin = minimum plasma concentration; CV% = coefficient of variation.
Following 6 doses (q12 h) of 45 mg dacomitinib.
Tmax: Median, minimum and maximum.
Following 3 day washout after 6 doses (q12 h) of 45 mg dacomitinib.
Following 5 doses (q12 h) of 60 mg dacomitinib.
The mean pre-dose concentrations of dacomitinib and PF-05199265 achieved on Day 4 of Cycle 0 in the current study following the fifth q12 h dose of 45 mg dacomitinib were comparable to those previously observed at steady state following 45 mg single oral daily dosing. The Cmax of dacomitinib on Day 4 of Cycle 0 following 6 q12 h doses of 45 mg dacomitinib were comparable to those previously observed at steady state following 45 mg daily dosing [17].
Analysis of the QT interval corrected for heart rate (QTc) using commonly used formulae, such as Bazett’s (QTcB) and Fridericia’s (QTcF) or correction derived specific for the current study population (QTcS) demonstrated that all upper limits of the 95% CI for the mean change from baseline at all Cycle 0 day 4 time points were< 10 msec indicating that a large QTc effect could be excluded at expected therapeutic concentrations of dacomitinib.
4. Discussion
This study was the first prospective study to assess pulsatile intermittent dosing of an EGFR tyrosine kinase inhibitor. The study assessed the dosing schedule in patients with lung cancers harboring EGFR T790M as well as in a separate not molecularly-defined cohort. We did not see sufficient efficacy of this treatment schedule in either cohort. Intermittent pulsatile dacomitinib is relatively well tolerated with an adverse event profile similar to standard daily dosing of dacomitinib. The majority of patients in Cohort A were able to be dose-escalated without excessive toxicity. Observations from PK and ECG data support that at exposures comparable to steady state seen with the 45 mg daily dose regimen of dacomitinib there were no effects on cardiac repolarization or a heightened risk of left ventricular ejection fraction abnormalities.
As we better understand the biology of EGFR-mutant lung cancer, we can now better discern who will benefit from EGFR-directed therapy. This study was initiated prior to the publication of the results from the SATURN [15] and IUNO [16] studies of erlotinib and the ARCHER 1009 study of dacomitinib [11] that assessed EGFR inhibitors in molecularly unselected, EGFR wild-type, patients. The majority of patients in Cohort B of this study did not have sensitizing EGFR mutations; we would now not expect dacomitinib therapy to be effective in these patients.
Despite the higher peak concentrations achieved with pulsatile dacomitinib dosing, this strategy did not translate into clinical benefit. The only other prospective study to date of pulsatile EGFR TKI, a phase 1 study of twice weekly pulse erlotinib and daily low dose erlotinib also failed to prevent emergence of EGFR T790M despite compelling preclinical data [17]. The concentration of erlotinib required to inhibit T790M was not clinically achievable despite escalating pulsatile doses. Similarly, the high peak concentrations of dacomitinib noted in preclinical studies that would be required to eradicate EGFR T790M may not be achievable in patients. While pulsatile dosing of EGFR TKIs may also result in superior central nervous system control [17,18], CNS activity was not an endpoint of this study and this data was not collected. Finally, pursuit of strategies for the use of high doses of second generation EGFR TKIs for the treatment of EGFR T790M has become less relevant with the approval of osimertinib that is effective after progression on a first or second generation EGFR TKI [19].
5. Conclusions
In conclusion, intermittent pulsatile dacomitinib is safe and well-tolerated but due to a lack of efficacy in Cohort A, this dosing regimen is not being developed further at this time. Nevertheless, dacomitinib at standard daily dosing is an effective first-line treatment for EGFR-mutant lung cancers [13,20]. In the Phase 3 ARCHER 1050 study, patients with EGFR-mutant lung cancers had a median PFS of 14.7 months on dacomitinib versus gefitinib. Dacomitinib at standard daily dosing should be considered as a new treatment option for patients with EGFR-mutant lung cancers in the first-line setting.
Acknowledgments
We thank the participating patients, as well as the investigators, research nurses, study coordinators, and operations staff.
Role funding sources
This study was supported by Pfizer Inc. Dr Yu has received research support from Astra Zeneca, Clovis Oncology, and Astellas Pharma. Dr Natale has received research support from Pfizer. Drs Giri, Sbar, Zhang, and Ms Quinn are employees of Pfizer Inc. Drs Ahn, Cho, Gerber, Giaccone, and Socinski have no conflicts of interest to declare.
Abbreviations:
- NSCLC
non small-cell lung cancer
- EGFR
epidermal growth factor receptor
- TKI
tyrosine kinase inhibitor
- PFS
progression-free survival
- ECOG
Eastern Cooperative Oncology Group
- RECIST
Response Evaluation Criteria in Solid Tumors
- ECG
electrocardiogram
References
- [1].Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. , EGF receptor gene mutations are common in lung cancers from never smokers and are associated with sensitivity of tumors to gefitinib and erlotinib, Proc. Natl. Acad. Sci. U. S. A. 101 (36) (2004) 13306–13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. , Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib, N. Engl. J. Med. 350 (21) (2004) 2129–2139. [DOI] [PubMed] [Google Scholar]
- [3].Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. , Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial, Lancet Oncol. 13 (3) (2012) 239–246. [DOI] [PubMed] [Google Scholar]
- [4].Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, et al. , Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations, J. Clin. Oncol. 31 (27) (2013) 3327–3334. [DOI] [PubMed] [Google Scholar]
- [5].Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. , Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma, N. Engl. J. Med. 361 (10) (2009) 947–957. [DOI] [PubMed] [Google Scholar]
- [6].Yang JC, Shih JY, Su WC, Hsia TC, Tsai CM, et al. , Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2):a phase 2 trial, Lancet Oncol.13 (5) (2012) 539–548. [DOI] [PubMed] [Google Scholar]
- [7].Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. , Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial, Lancet Oncol. 15 (2) (2014) 213–222. [DOI] [PubMed] [Google Scholar]
- [8].Yu H, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. , Analysis of mechanisms of acquired resistance to EGFR TKI therapy in 155 patients with EGFR-mutant lung cancers, Clin. Cancer Res. 19 (8) (2013) 2240–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Janne PA, Boss DS, Camidge DR, Britten CD, Engelman JA, Garon EB, et al. , Phase I dose-escalation study of the pan-HER inhibitor, PF299804, in patients with advanced malignant solid tumors, Clin. Cancer Res. 17 (5) (2011) 1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ramalingam SS, Blackhall F, Krzakowski M, Barrios CH, Park K, Bover I, et al. , Randomized phase II study of dacomitinib (PF-00299804), an irreversible pan-human epidermal growth factor receptor inhibitor, versus erlotinib in patients with advanced non-small-cell lung cancer, J. Clin. Oncol. 30 (27) (2012) 3337–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ramalingam SS, Janne PA, Mok T, O’Byrne K, Boyer MJ, Von Pawel J, et al. , Dacomitinib versus erlotinib in patients with advanced-stage, previously treated non-small-cell lung cancer (ARCHER 1009): a randomised, double-blind, phase3 trial, Lancet Oncol. 15 (12) (2014) 1369–1378. [DOI] [PubMed] [Google Scholar]
- [12].Janne PA, Ou SH, Kim DW, Oxnard GR, Martins R, Kris MG, et al. , Dacomitinib as first-line treatment in patients with clinically or molecularly selected advanced non-small-cell lung cancer: a multicentre, open-label, phase 2 trial, Lancet Oncol. 15 (13) (2014) 1433–1441. [DOI] [PubMed] [Google Scholar]
- [13].Dacomitinib VersusGefitinib for the First-Line Treatment of Advanced EGFR Mutation Positive Non-Small Cell Lung Cancer (ARCHER 1050): A Randomized Open-Label Phase III Trial, in: Mok TCY, Zhou X, Lee KH, Nakagawa K, et al. (Eds.), ASCO, Chicago, Illinois,2017. [Google Scholar]
- [14].Ercan D, Zejnullahu K, Yonesaka K, Xiao Y, Capelletti M, Rogers A, et al. , Amplification of EGFR T790M causes resistance to an irreversible EGFR inhibitor, Oncogene 29 (16) (2010) 2346–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczesna A, Juhasz E, et al. , Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study, Lancet Oncol. 11 (6) (2010) 521–529. [DOI] [PubMed] [Google Scholar]
- [16].Cicenas S, Geater SL, Petrov P, Hotko Y, Hooper G, Xia F, et al. , Maintenance erlotinib versus erlotinib at disease progression in patients with advanced non-small-cell lung cancer who have not progressed following platinum-based chemotherapy (IUNO study), Lung Cancer 102 (2016) 30–37. [DOI] [PubMed] [Google Scholar]
- [17].Yu SC HA, Feldman D, Liu LL, Vaitheesvaran B, Cross J, RUdin CM, Kris MG, Pao W, Michor F, Riely GJ, Ann. Oncol. 28 (2) (2016) 278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Grommes C, Oxnard GR, Kris MG, Miller VA, Pao W, Holodny AI, et al. , Pulsatile high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer, Neuro-Oncol. 13 (12) (2011) 1364–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Janne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, et al. , AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer, N. Engl. J. Med. 372 (18) (2015) 1689–1699. [DOI] [PubMed] [Google Scholar]
- [20].Janne PA, Ou SHI, Kim DW, Oxnard GR, Martins R, Kris MG, et al. , Dacomitinib as first-line treatment in patients with clinically or molecularly selected advanced non-small-cell lung cancer: a multicentre, open-label, phase 2 trial, Lancet Oncol. 15 (13) (2014) 1433–1441. [DOI] [PubMed] [Google Scholar]