SUMMARY AND CONCLUSIONS
1. A total of 14.4% of medial reticular formation (RF) cells discharged maximally in relation to movements of the facial musculature. Cells related to movement of the ipsilateral eyelids, ipsilateral lip, ipsilateral pinna, and cells related to movement of several facial regions were seen.
2. Cells related to ipsilateral eyelid closure constituted 2.2% of all RF cells recorded. Responses to stroboscopic visual stimuli causing eyelid closure were seen at a 30- to 40 ms latency.
3. Cells related to ipsilateral lip movement constituted 2.2% of all RF cells recorded.
4. Cells related to ipsilateral pinna movement constituted 7.1% of all RF cells recorded.
5. Ipsilateral eyelid, lip, and pinna movement cells were completely silent during movements restricted to the contralateral facial musculature. They discharged only during movement of the affected ipsilateral region in a single direction. They were significantly more likely than adjacent nonfacial cells to respond during the reflex head shake and significantly less likely to respond to auditory stimuli. They had little or no tonic activity during waking and sleep and no response to electric pulse stimulation of the skin.
6. Cells related to several facial movements constituted 2.9% of all RF cells recorded. Maximal discharge rates occurred during movement of the ipsilateral pinna, but smaller rate increases were also seen during movement of the ipsilateral lip and eyelid and during contralateral facial movements. In contrast to other facial cells, these cells also: a) responded during movements of ipsilateral facial regions in any direction, b) responded to somatosensory stimulation and passive movements of facial skin, c) responded to auditory and electric pulse stimulation of the skin, d) were inactive during the reflex head shake, and e) had high levels of tonic activity in both waking and sleep.
7. Each facial movement-related cell type had a different anatomical distribution within the brain stem. Cells related to eyelid, lip, and multiple facial movements formed localized clusters ventral to the abducens nucleus. Cells related to pinna movement were scattered throughout the gigantocellular tegmental field. Facial movement-related reticular cells may have premotor and proprioceptive roles in the regulation of facial movements.
INTRODUCTION
Facial movements in the cat are controlled by at least 35 muscles, 20 of which insert on portions of the auricle (3). The motoneurons controlling these muscles are contained in the nucleus of the 7th nerve. The facial nucleus is known to receive projections from the region around the 3rd nerve nucleus, the midbrain paralemniscal region, the parabrachial region, and certain reticular regions (4–7, 16). Even after section behind the colliculi, a variety of spontaneous and reflex facial movements occur (10). However, there has been no unit-recording evidence identifying cells that discharge in relation to facial movement. In the present paper we report on the discovery of several cell types located within the medial pontomedullary reticular formation (RF) that discharge in relation to facial movements. We have previously presented preliminary data on a subgroup of these cells that discharge in association with pinna movement (15).
METHODS
Methods were as reported in the companion paper (14). Topical application of Xylocaine jelly, supplemented by subdermal injections of lidocaine, was used to block somatosensory input to receptive fields of facial cells. Event markers were employed to compare facial movement to polygraphically recorded electrooculogram, neck electromyogram, and unit discharge.
RESULTS
Cells related to movement of the facial musculature (n = 45) constituted 14.7% of the total number of cells encountered. Four distinct cell types were seen. These were 1) cells related to movement of the ipsilateral eyelid, 2) cells related to movement of the ipsilateral lip region, 3) cells related to movement of the ipsilateral pinna, and 4) cells related to several facial movements. In comparison with other RF cells, as a group, the facial movement cells were less likely to have tonic activity during sleep; i.e., type 2 cells were rare (P < 0.001, χ2) and more likely to be active during the head-shake reflex (P < 0.01, χ2). However, each cell subtype within the population of facial movement-related cells had quite distinctive behavioral correlates. Table 1 lists the frequency of cell subtypes and sleep and sensory responses.
TABLE 1.
Frequency of cell subtypes and prevalence of sleep types, sensory response, and activity during head-shake reflex
| Sleep Type |
Head-Shake Reflex | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| % Total | n | 1 | 2 | 3 | Auditory | Shock | Strobe | ||
|
| |||||||||
| Eyelid | 2.2 | 1 | 67* | 0* | 33* | 0 | 0 | 33* | 86* |
| Lip | 2.2 | 1 | 57* | 14* | 29* | 0 | 0 | 0 | 50 |
| Pinna | 7.1 | 22 | 76† | 5† | 19† | 0* | 0 | 0 | 93† |
| Multiple | 2.9 | 9 | 0§ | 13§ | 88§ | 89† | 78‡ | 0 | 14§ |
| Total facial | 14.7 | 45 | 57 | 7 | 36 | 18 | 47 | 5 | 66 |
* and † Significant differences at 0.05 and 0.001 level between cell type and general RF cell population.
‡ and § Significant differences from other facial cell types at 0.05 and 0.001 level.
Eyelid movement cells
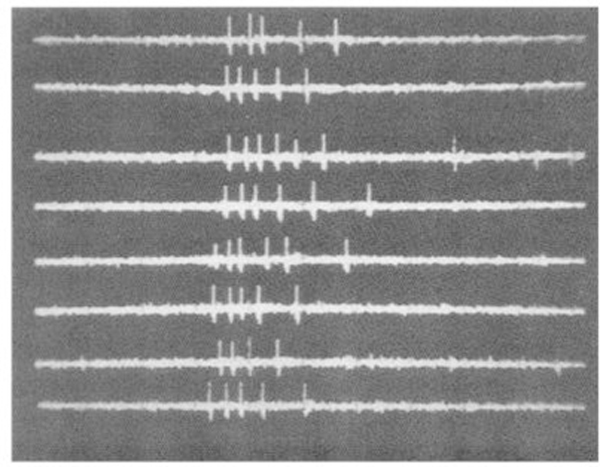
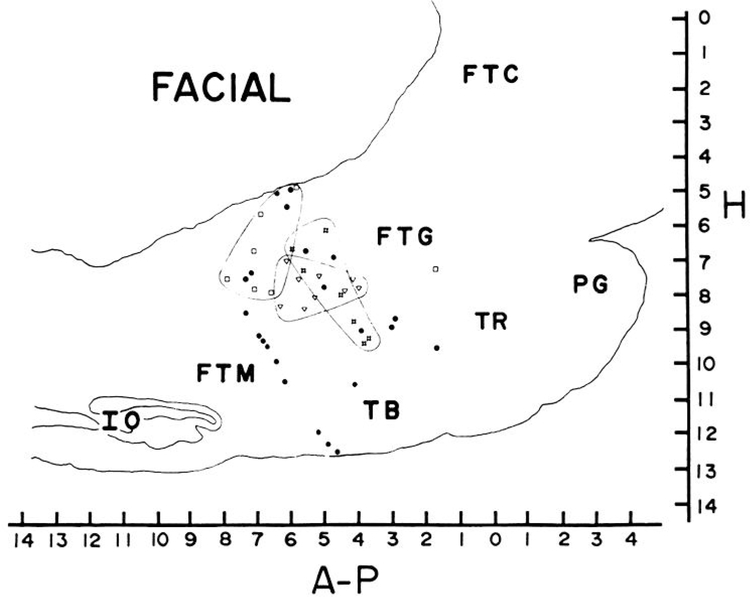
Seven cells (2.2% of the total number recorded) were related to movement of the ipsilateral eyelid. No cells recorded showed any activity during movements restricted to the contralateral eyelid. Ipsilateral eyelid movement could be reliably induced by touching the ipsilateral cornea with a cotton swab. After this stimulation had been applied once or twice, the approach of the swab was sufficient to elicit eye closure and associated unit discharge, even if no contact was made. In every case the unit activity was temporally related to the motor response and not the stimulus. Unit activity was not maintained if the eyes remained closed; rather, activity was phasically related to the closing movement of the eyelids. However, continued stimulation of the orbicular region after eye closure produced tonic discharge in association with the resulting squinting eye movement. These cells had several other common characteristics. None showed any response to auditory stimuli. Two of the cells responded to stroboscopic visual stimulation, significantly more than in the general population of recorded cells (P < 0.02, χ2). Response latency was 30–40 ms in these cells (Fig. 1). The cat was observed to close its eyelids in response to each strobe stimulus that produced unit discharge. Strobe rates greater than 60 Hz were not accompanied by blinking and did not evoke any unit discharge. Six of the seven eyelid cells were active during the head-shake reflex, significantly more (P < 0.02, χ2) than in the general population of RF cells, including head movement-related cells. This activity is presumably related to the eyelid closure that one observes during this reflex. All eyelid movement-related cells were type 1 or 3 in their sleep profiles. Tonically active type 2 cells were significantly underrepresented (P < 0.02, χ2). The absence of tonic activity during sleep, when the eyes are closed, is consistent with the phasic relation of these cells to eye closure seen in waking. Eyelid movement cells were all located 1.2 ± 0.2 mm from the midline and between P3.5 and P6 in the gigantocellular tegmental field (FTG) (Fig. 2).
FIG. 1.
Response of eyelid movement cells to strobe stimulus. Each sweep has 100 ms duration.
FIG. 2.
Anatomical distribution of facial cells. Circles, ipsilateral pinna movement cells; squares, ipsilateral lip movement cells; crossed squares, ipsilateral eyelid movement cells; triangles, nonspecific facial movement cells. Lines enclose concentrations of localized cell types. Lateralities of each cell type also differed (see text).
Ipsilateral lip movement cells
Seven cells (2.2% of the total number recorded) were related to movement of the ipsilateral lip. No cells showed any relation to movement of the contralateral lip. In five of the six cells the adequate movement was isolated to the upper lip or vibrissae, while in one, movement of both upper and lower lip was effective. As was the case with eyelid movement cells, unit discharge was related to the motor response rather than the stimulus. Manipulation of the vibrissae was ineffective unless it produced the appropriate movement of the lip. Stimulation of the skin of the lip with punctate pressure of as little as 150 mg produced a brisk response if the cat responded with movement of the ipsilatera1 lip. Conversely, even heavy pressure applied with the forefinger would not produce discharge if the appropriate contraction did not occur. Passive skin movement was in-effective. Injection of lidocaine and topical application of Xylocaine jelly to the lip and underlying tissues did not prevent unit discharge during movement in two units tested. Two cells were tested for response of 0.5-ms electric stimuli of sufficient intensity to cause twitching in the lip musculature. This stimulus did not produce unit discharge in either cell. As was the case with the eyelid cells, type 2 sleep patterns were rare in this group, while type 1 patterns were overrepresented (P < 0.02, χ2). Auditory and visual responses were absent in all of these cells. Three of the six tested cells were active during execution of the head-shake reflex, not significantly different from the general cell population. All but one of the lip movement units, which were seen in five different cats, were grouped between P6 and P8 and between Ll.2 and L2.3 (Fig. 2).
Ipsilateral pinna movement cells
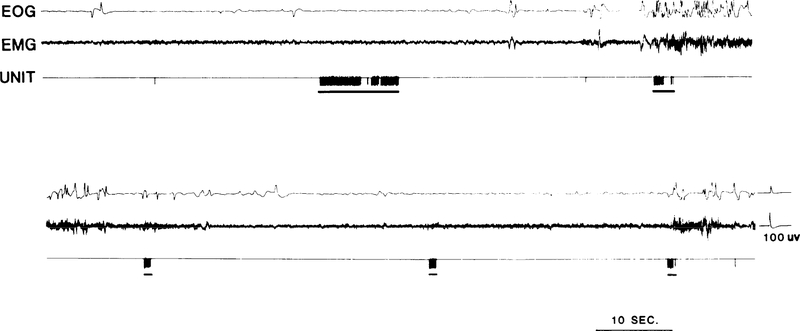
A total of 22 cells (7.1% of the total recorded) discharged in conjunction with active movement of ipsilateral pinna. No cells discharging specifically in relation to contralateral pinna movement were seen. Unit activity in pinna movement cells was identical during both pinna movement elicited by auditory stimuli and movement elicited by irritating the hairs of the auricle with a cotton swab. Detailed observation of the topography of the pinna movement correlated with unit activity was made in 14 of these cells. Of these, four related to caudal, six to ventrocaudal, and four to rostra1 movements of the pinna. All but one of the cells related to pinna movement had little or no spontaneous activity when the pinna was in the normal intermediate “resting” position. Rapid active ear rotation in the appropriate direction was accompanied by a burst of unit discharge (Fig. 3). Five cells exhibited tonic discharge if the pinna was maintained by the cat in the displaced position but the remaining cells discharged only during the movement. Vigorous pinna movements were accompanied by bursts up to 30 spikes within a 0.5-s interval, while slower movements correlated with a single spike or no discharge at all. All of these cells were tested for response to somatic stimulation. When such stimulation elicited pinna movement, discharge occurred as in the case of spontaneous movements. In the absence of detectable ear movements, 16 of the 19 tested cells had no somatic field. Of the remaining cells, one responded to light manual stroking on any portion of the ipsilateral face. Two cells responded to blunt pressure applied to the area from the lower ipsilateral jaw to the region ventral to the pinna. These same three cells were totally unresponsive to punctate stimulation of up to 8 g applied to the same area with an esthesiometer. Two cells were tested for re sponse to 0.5-ms electric pulses of sufficient intensity to cause a muscle twitch. The pulses were administered through needle electrodes on the base of the ipsilateral pinna. Neither cell responded. None of the cells responded to passive movement of the pinna by the experimenter.
FIG. 3.
Activity of pinna movement-related unit. Underline indicates when ipsilateral pinna Was retracted to caudal position. Note the independence of unit activity from eye and head movements.
These cells also shared several other common characteristics that distinguished them from adjacent RF cells. As was the case with eye movement cells and other facial cells, none of these cells responded to discrete auditory stimuli (P < 0.05, χ2). None responded to stroboscopic visual stimuli. Of the 16 tested, 13 discharged during the head-shake reflex (P < 0.001, χ2). Photographic analysis revealed that this reflex was invariably accompanied by caudoventral retraction of both pinnae. Type 2 cells were underrepresented and type 1 cells overrepresented in this group (P < 0.001, χ2), as was the case for eye movement and other specific facial movement cells.
None of the cells related to ipsilateral pinna movement were localized to the facial nucleus or nerve (15). In contrast to other cell types related to facial movement, these cells were dispersed over a very wide area, ranging from P1.5 to P8 and 0.8 to 2.3 mm lateral to the midline (Fig. 2).Subdividing cells into those related to specific pinna movements did not produce any evidence of further localization. Cells related to identical movements were as much as 6 mm apart.
Cells related to several facial movements
A total of nine cells (2.9% of total) discharged in relation to several facial movements. The relation to movement was qualitatively different from that seen in other facial cells. The defining feature of this group was that while these cells discharged at maximal rates in conjunction with movement of the ipsilateral pinna musculature, all of them also responded at slightly reduced levels during movement of the ipsilateral eyelid and the ipsilateral lips. Furthermore, while all achieved their highest rates during ipsilateral facial movements, all also responded at somewhat lower rates during active movements of the contralateral facial musculature. Still weaker responses occurred during rapid head movements. However. these cells did not change their base-line discharge rates during limb movements or movements of the caudal axial musculature.
The lack of facial movement specificity in these cells contrasted with the highly specific relations seen in all other facial cells. Not only did these cells discharge in conjunction with several movements, their relation to the movements was not directionally specific, as was the case in all other facial movement cells. For example, each of these cells accelerated during rostral, caudal, and ventral movements of the pinna.
This group of units also shared several other characteristics that distinguished them from other facial cells. Punctate stimulation of the ipsilateral face with as little as 100 mg produced unit discharge even if no movement was observed. However, the response was greatly enhanced if movement was evoked. Passive movement of either pinna in any direction produced tonic discharge similar to that occurring during active movement. This same manipulation was completely without effect in the ipsilateral pinna movement cells described above. Seven of the eight cells in this group responded to auditory stimuli, in contrast to the lack of response in other facial cells (P < 0.001, χ2). Six of the eight also responded to shock stimulation (P < 0.05, χ2). Although response latency was shortest to pulses applied to the ipsilateral facial region (6–15 ms), these cells also responded to stimulation of the torso and limbs, with an additional delay of 2–3 ms. Also in contrast to other facial cells was the lack of activity during the head-shake reflex in six of the seven cells tested (P < 0.001, χ2). Type 1 cells, which were overrepresented in the other facial cells, were significantly underrepresented in this group, while type 3 cells were overrepresented (P < 0.001, χ2). These cells were all clustered between P4 and P6.5 at 1.2 mm from the midline (Fig. 2).
DISCUSSION
Reticular facial movement-related cells were divisible into two distinct categories, those related to a single specific movement and those related to several movements. The range of motor relationships in the “specific” facial cells roughlv corresponded to the range of movements known to be produced by the contraction of individual facial muscles. Pinna movements, which are controlled by a large proportion of the facial muscles, also comprise the largest portion of facial movement cells in the RF. Facial cells related to a single movement had few sensory responses, becoming active only when stimuli induced their specific motor correlate. The presence of activity during the reflex head shake can be explained by the facial and pinna movements consistently triggered during this reflex.
These cells were generally silent in rapid eye movement (REM) sleep, at a time when most surrounding cells achieve their highest activity levels (12). The spontaneous and reflex activity and lack of sensory response in these cells are similar to those seen in neighboring RF neurons related to eye movement (14). One may hypothesize that the functional role and synaptic mechanisms underlying the behavioral relations of the specific facial movement cells are analogous to the well-studied mechanisms of eye movement interneurons. Current anatomical evidence indicates relatively few direct projections from the RF regions containing facial cells to the facial nucleus (4, 5, 7, 16). Thus, some of these cells may relay their outputs over multisynaptic pathways.
Others have reported restricted facial receptive fields to “natural” stimuli in RF cells recorded in acute paralyzed preparations (1, 2, 9). These fields correspond in size to regions effective in eliciting movements in the present group of cells. However, such movements and spontaneous movements would not have been observable in these previous preparations. These investigations may therefore have been studying the sensory stimuli effective in triggering movements in the same cell population that we are reporting on here.
Cells related to several facial movements appear to form an anatomically and behaviorally distinct population from the specific facial movement cells. Unlike the cells related to specific facial movements, they clearly receive a variety of exteroceptive and proprioceptive sensory inputs from the trigeminal, spinal, and auditory systems. The activity of these cells is qualitatively different from those of the specific movement cells in their lack of relation to the direction of movement as well as their relation to multiple movements. Their activity pattern is incompatible with any simple role in localized sensory or motor activity. Their discharge during spontaneous movement may be related to reafference discharge or facilitation of several groups of motoneurons. The presence of REM sleep activity and the absence of activity during vigorous head movement reflexes also distinguishes them from specific head movement cells.
Cells related to facial movement were distributed over a large proportion of the medial RF region studied, but there was a general localization of facial movement-related cells to RF regions rostral to the inferior olive (P8) and caudal to the level of the trochlear nucleus (P1). These cells were all rostra1 to the facial nucleus and largely medial to the facial nerve. Despite the large AP scatter of facial movement cells, the left-right segregation of subtypes was perfect. All the facial movement-related cells discharged exclusively or predominantly in relation to ipsilateral movement. The various subtypes of facial cells had different anatomical distributions within the ipsilateral RF. Cells related to pinna movement were most widely scattered. Pinna movement cells with virtually identical motor and sensory relations were separated by as much as 6 mm. In contrast, cells related to lip and to eyelid movement formed fairly localized clusters. The cells having a nonspecific relation to a number of facial movements were also sharply localized to the region ventral to the abducens nucleus. This area has been shown to have monosynaptic connections with a number of different motoneuron pools (8). Any understanding of the synaptic mechanisms mediating the observed behavioral relationships must await studies of the inputs and axonal trajectories of each cell type.
The present studies show the utility of investigating RF cells in unrestrained animals in a wide variety of behavioral situations. Several cell types appear to share certain sensory, sleep cycle, and movement characteristics. Cells sharing behavioral characteristics form clusters not otherwise apparent from histological examination of the RF. Our preliminary findings from antidromic stimulation studies indicate that each cell type may have a unique projection pattern. Recent evidence suggests that local interactions of these RF cell populations may allow synthesis of the simple reflex movements generated in brain stem systems (13). Further study of interactions between these cell groups should help reveal how this synthesis occurs.
ACKNOWLEDGMENTS
This study was supported by the Medical Research Service of the Veterans Administration, Public Health Service Grant NS14610, and National Science Foundation Grant BNS00023.
REFERENCES
- 1.AMASSIAN VE AND DEVITO RV Unit activity in reticular formation and nearby structures. J. Neurophysiol 17: 575–603, 1954. [DOI] [PubMed] [Google Scholar]
- 2.BELL C, SIERRA G, BUENDIA N, AND SEGUNDO JP Sensory properties of neurons in the mesencephalic reticular formation. J. Neurophysiol 27: 961–997, 1964. [DOI] [PubMed] [Google Scholar]
- 3.CROUCH E Text-Atlas of Cat Anatomy. Philadelphia, PA: Lea & Febiger, 1969. [Google Scholar]
- 4.HENKEL CK Afferent sources of a lateral midbrain tegmental zone associated with the pinnae in the cat as mapped by retrograde transport of horseradish peroxidase. J. Comp. Neurol. 203: 213–226, 1981. [DOI] [PubMed] [Google Scholar]
- 5.HENKEL CK AND EDWARDS SB The superior colliculus control of pinna movements in the cat: possible anatomical connections. J. Comp. Neural. 182: 763–776, 1978. [DOI] [PubMed] [Google Scholar]
- 6.HOLSTEGE G AND KUYPERS HGJM Propriobulbar fibre connections to the trigeminal, facial and hypoglossal motor nuclei. Brain 100: 239–264, 1977. [DOI] [PubMed] [Google Scholar]
- 7.PANNETON WM AND MARTIN GF Midbrain projections to the facial nucleus in the oppossum. Brain Res. 145: 355–359, 1978. [DOI] [PubMed] [Google Scholar]
- 8.PETERSON BW Reticula-motor pathways: their connections and possible roles in motor behavior. In: Integration in the Nervous System. edited by Asanuma H and Wilson VJ. Tokyo: Igaku Shoin, 1979, p. 185–204. [Google Scholar]
- 9.SEGUNDO JP, TAKENAKA T, AND ENCABO H Somatic sensory properties of bulbar reticular neurons. J. Neurophysiol. 30: 1221–1238, 1967. [DOI] [PubMed] [Google Scholar]
- 10.SHERRINGTON CS Reflexes elicitable in the cat from pinna vibrissae and jaws. J. Physiol. London 51: 404–431, 1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SIEGEL JM, BREEDLOVE SM, AND MCGINTY DJ Photographic analysis of relation between unit activity and movement. J. Neurosci. Methods 1: 159–164, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SIEGEL JM, MCGINTY DJ, AND BREEDLOVE SM Sleep and waking activity of pontine gigantocellular field neurons. Exp. Neural 56: 553–573, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SIEGEL JM, NIENHUIS R,WHEELER RL, MCGINTY DJ, AND HARPER RM Discharge pattern of reticular formation unit pairs in waking and REM sleep. Exp. Neural. 74: 875–891, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SIEGEL JM AND TOMASZEWSKI KS Behavioral organization of reticular formation: studies in the unrestrained cat. I. Cells related to axial, limb, eye, and other movements. J. Neurophysiol. 50: 696–716, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SIEGEL JM, WHEELER RL, BREEDLOVE SM, AND MCGINTY DJ Brain-stem units related to movements of the pinna. Brain Res. 202: 183–188, 1980. [PMC free article] [PubMed] [Google Scholar]
- 16.TAKEUCHI Y, NAKANO K, UEMURA M, MATSUDA K, MATSUSHIMA R, AND MIZUNO N Mesencephalic and pontine afferent fiber systems to the facial nucleus in the cat: a study using the horseradish peroxidase and silver impregnation techniques. Exp. Neural 66: 330–342, 1979. [DOI] [PubMed] [Google Scholar]