Contemporary treatments for pulmonary embolism (PE) fall into 4 broad categories: systemic anticoagulation, systemic thrombolysis (ST), catheter-directed thrombolysis (CDT), and mechanical thrombectomy (MT). In general, MT has several advantages over CDT and ST in the treatment of submassive and potentially massive PE. This article describes a promising device that enables MT, and an illustrative case is presented.
In the SEATTLE II (Submassive and Massive Pulmonary Embolism Treatment With Ultrasound Accelerated Thrombolysis Therapy) trial, which evaluated CDT, thrombolytic agents were associated with a >10% rate of bleeding complications.1 The results in an ST trial, PEITHO (Pulmonary Embolism Thrombolysis Study), were similar.2 In the SEATTLE II trial, thrombolytic agents were given to patients in intensive care for 12 to 24 hours; in the more recent OPTALYSE PE (Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Pulmonary Embolism) trial, CDT was performed in as short a time as 4 hours.3 In contrast, MT is potentially more rapid, and it is safe, effective, and performed on the catheterization laboratory table.
Early success with use of the 8F Indigo CAT 8 system (Penumbra, Inc.) for pulmonary thrombectomy prompted the EXTRACT-PE (Evaluating the Safety and Efficacy of the Indigo Aspiration System in Acute Pulmonary Embolism) trial.4 This single-arm study in 119 patients with submassive PE showed significantly reduced right ventricular-to-left ventricular ratios at 48 hours (0.43 ratio reduction; 95% CI, 0.38–0.47; P <0.0001). In addition, procedural pulmonary artery (PA) pressures were reduced in as little as 37 minutes of median overall procedure time. Adjunctive thrombolytic use was minimal (1.7% of patients) and safe (rates of pulmonary vascular injury, clinical deterioration, and major bleeding at 48 hr, all 1.7%; 30-d mortality rate, 2.5%). In the United States, this trial led to the designation of PE as an indication for use of the Indigo CAT 8 system and later the Lightning 8 and Lightning 12 Intelligent Aspiration systems (Penumbra).
Lightning 12 Technology
The fundamental components of the Lightning 12 Intelligent Aspiration system are the Engine pump, the Lightning control unit, and the CAT 12 HTORQ 12F catheter. The continuously powered engine pump (Fig. 1), which is activated by a single touch, achieves a negative suction pressure of 29 inHg, a nearly pure vacuum. When 4 solid illuminated bars on the activation switch indicate maximal suction, blood is aspirated into a disposable canister where an integrated clot-catcher strains out solid material. Device setup involves simply inserting the canister into the pump and attaching the tubing.
Fig. 1.
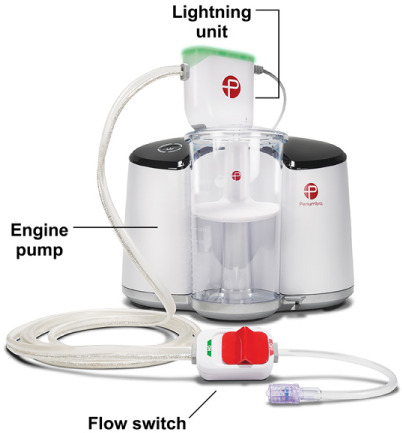
The components of the Lightning 12 Aspiration System are shown.
(Image courtesy of Penumbra, Inc.)
The Lightning 12 system includes an upgrade to Penumbra's original Dynamic Aspiration Tubing system, which had only a physician-controlled flow switch to limit aspiration and flow. Basic physics dictate that the larger the aspiration orifice and the shorter the catheter, the greater the aspiration efficiency. Without a secondary control mechanism, large catheters could cause excessive blood loss. By incorporating a dedicated microprocessor and a pressure/flow sensor system with high-frequency valves, the Lightning 12 control unit efficiently regulates aspiration. When the sensor detects open flow, the valve closes and pulses the suction pressure, indicated by an audible click and a flashing green light on the control unit. This pulsing results in substantially less blood loss: when the aspiration catheter encounters thrombus or solid material (clots), the audible clicks stop. The light turns solid green, then orange in 20 seconds after having sensed the occlusion. These auditory and visual cues help the physician focus on the procedure instead of the canister's contents. The Lightning system (currently available for the CAT 8 and CAT 12 catheters) achieves an 18:1 fluid-loss savings over the original Dynamic Aspiration Tubing, based on benchtop testing.
The final component of the Lightning 12 system is the CAT 12 catheter (Fig. 2). This unique, multipitch, laser-cut hypotube enables improved torque response from tip to base. It has an inner diameter of 0.131 inches (0.333 cm) and comes in 2 lengths (100 and 115 cm). Multiple polymer transitions increase flexibility and trackability at an atraumatic tip, with back-end support. The catheter's unique primary and secondary curve combination is highly suited for tracking PA tortuosity, including the acute angle of the left main PA. Moreover, a large Tuohy-Borst valve (diameter, 0.131 in) prevents a choke point in the aspiration system. An optional separator (SEP 12), consisting of a polymer bead attached to a robust wire, can be used to break up proximal thrombus and declot the catheter if organized thrombus is extensive. The separator is advanced through the Tuohy-Borst valve to the catheter tip under active aspiration with use of manual agitation.
Fig. 2.
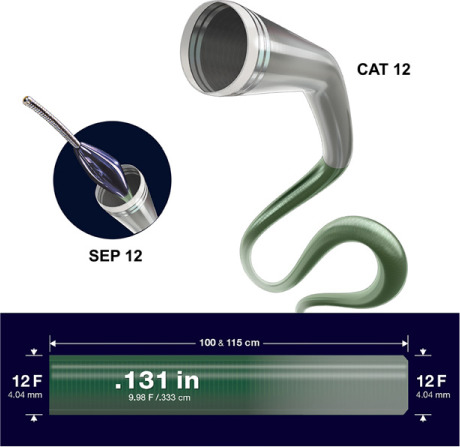
Shown are the CAT 12 catheter, the Separator 12 (SEP 12), and catheter specifications.
(Image courtesy of Penumbra, Inc.)
Aspiration Technique
When a patient with submassive or massive PE arrives at our catheterization laboratory, we obtain dual unilateral or bilateral femoral venous access with use of micropuncture 6F Pinnacle Precision sheaths (Terumo) and ultrasonographic guidance. A separate 6F pigtail catheter is deployed through one access site for pulmonary angiograms because it can provide better images than those obtained through the aspiration catheter itself, improving procedural efficiency. The diagnostic pigtail catheter is then advanced into the PA of interest, and pulmonary pressures are measured. Another pigtail catheter is advanced into the PA with support from a 0.035-in Glidewire Advantage wire (Terumo). Using a pigtail catheter enables navigation through dilated right ventricles and helps to avoid chordae that can be damaged by large sheaths. At this point, an Amplatz Super Stiff Guidewire with a short-taper 1-cm tip (Boston Scientific Corporation) is advanced through the second pigtail catheter. A sheath is then advanced into or beyond the main PA. The sheath should be at least 12F and 65 to 75 cm long. We prefer to use a 14F or 16F × 65-cm GORE DrySeal Flex Introducer Sheath (W.L. Gore & Associates, Inc.), which has a deflatable valve that enables large thrombus to be removed without shearing at the valve. After this, the 12F catheter is advanced to the face of the thrombus, and aspiration begins. If maintaining maximal contact with the thrombus is desired, it is possible to advance the catheter without a wire. After 90 seconds of occlusion, the clot can be extracted (often, intact) from the PA with use of a power aspiration–based technique.5 If the thrombus burden is large, the separator can be introduced. The polymer bead tip can be activated rapidly to separate and break up leading thrombus into smaller particles that are easily aspirated. Blindly advancing the separator too far risks perforating small-caliber vessels. When the PA is sufficiently cleared, the contralateral vessel can be targeted, often by simply manipulating the catheter and the Glidewire Advantage wire.
In general, thrombectomy is considered sufficient when peripheral PA perfusion improves and PA pressure ideally decreases. Other clinical markers, such as tachycardia and hypoxemia, may also improve.
Illustrative Case
An 82-year-old man with a history of hypertension, chronic kidney disease, and previous right nephrectomy presented at the emergency department with syncope, hypoxemia, and hypotension. His oxygen saturation was 82% on a 100% nonrebreather mask, his pulse rate was 100 to 120 beats/min, and his systolic blood pressure was 85 mmHg. A computed tomographic angiogram revealed a large saddle PE with right ventricular strain. The diagnosis was early massive PE.
Bilateral femoral venous access was obtained in standard fashion. The patient's initial PA pressure was 82/30 mmHg (mean, 50 mmHg) as measured by the pigtail catheter. We delivered a 16F × 65-cm GORE DrySeal sheath, using the technique described above. Pulmonary angiograms revealed substantial thrombus in the left main PA, extending into the lower segmental branches (Fig. 3A). We used the Lightning 12F catheter without a wire to aspirate the thrombus (Fig. 3B–C). The patient's PA pressure decreased to 58/24 mmHg (mean, 39 mmHg). Next, we cleared the right PA of substantial distal main thrombus that extended into the lower segmental branches, and then cleared a clot from the truncus arteriosus. Perfusion improved, and the patient's PA pressure decreased to 44/20 mmHg (mean, 31 mmHg). His peripheral oxygen saturation was 100% and his systolic blood pressure had returned to normal, so we ended the procedure. We closed both access sites with figure-of-8 sutures. The patient had lost approximately 350 mL of blood. He improved clinically and did not need intensive care.
Fig. 3.
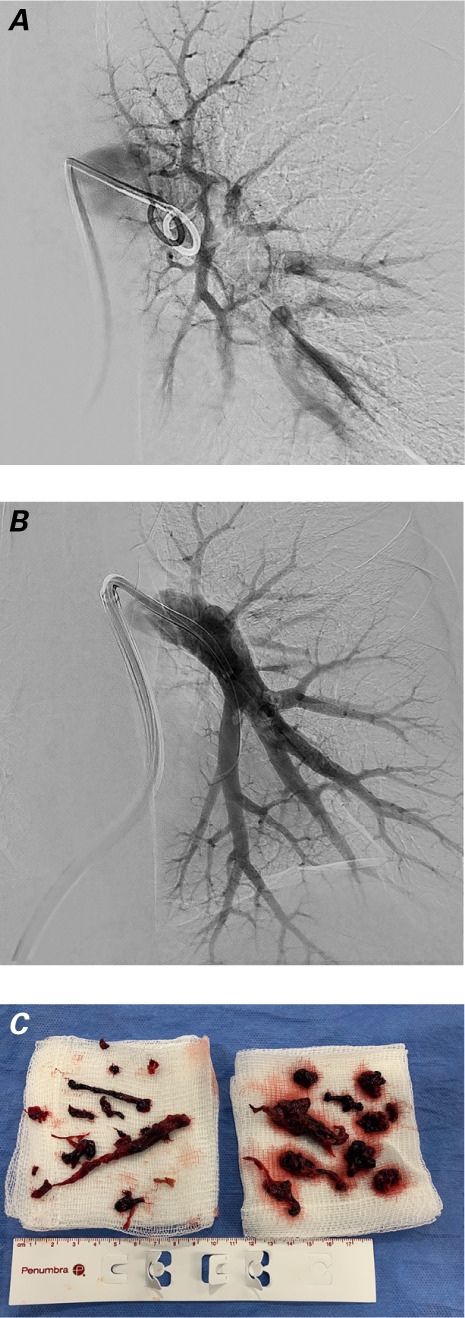
Pulmonary angiograms show the patient's left main pulmonary artery A) before and B) after aspiration thrombectomy. C) Photograph shows aspirated thrombus.
Discussion
Our case and others like it suggest that pulmonary thrombectomy with use of the Lightning 12 system is well tolerated and effective in critically ill patients. Auditory and visual feedback from the Lightning control unit eases the process. Moreover, the 12F catheter's flexibility advantageously improves maneuvers within tortuous vessels and large right ventricles.
Using larger-bore catheters and sheaths may seem feasible; however, it is unclear whether doing so is clinically beneficial, given the tradeoffs associated with larger size. Bleeding complications are usually less frequent in venous procedures, but large-bore systems can cause hematomas at access sites. A low-profile system may also reduce irritability in the heart, a problem in some patients when stiffer or larger systems are used. Finally, large or inflexible catheters may hinder the removal of distal thrombus. Perhaps an ideal system would include several types of catheters for different clot shapes, burdens, and locations.
Footnotes
Conflict of interest disclosure: Dr. Mathews consults for and has received research funding from Penumbra, Inc.
Meeting presentation: This article is based on a presentation at the Houston chapter meeting of the International Society of Endovascular Specialists, 16 December 2020.
References
- 1.Piazza G, Hohlfelder B, Jaff MR, Ouriel K, Engelhardt TC, Sterling KM et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Interv . 2015;8(10):1382–92. doi: 10.1016/j.jcin.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 2.Meyer G, Vicaut E, Danays T, Agnelli G, Becattini C, Beyer-Westendorf J et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med . 2014;370(15):1402–11. doi: 10.1056/NEJMoa1302097. [DOI] [PubMed] [Google Scholar]
- 3.Tapson VF, Sterling K, Jones N, Elder M, Tripathy U, Brower J et al. A randomized trial of the optimum duration of acoustic pulse thrombolysis procedure in acute intermediate-risk pulmonary embolism: the OPTALYSE PE trial. JACC Cardiovasc Interv . 2018;11(14):1401–10. doi: 10.1016/j.jcin.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Sista AK, Horowitz JM, Tapson VF, Rosenberg M, Elder MD, Schiro BJ et al. Indigo aspiration system for treatment of pulmonary embolism: results of the EXTRACT-PE trial. JACC Cardiovasc Interv . 2021;14(3):319–29. doi: 10.1016/j.jcin.2020.09.053. [DOI] [PubMed] [Google Scholar]
- 5.Saxon RR, Benenati JF, Teigen C, Adams GL, Sewall LE;, PRISM Trialists Utility of a power aspiration-based extraction technique as an initial and secondary approach in the treatment of peripheral arterial thromboembolism: results of the multicenter PRISM trial. J Vasc Interv Radiol . 2018;29(1):92–100. doi: 10.1016/j.jvir.2017.08.019. [DOI] [PubMed] [Google Scholar]


