1. INTRODUCTION
The behavioral correlates of activity in medial brain stem reticular formation (RF)* cells have been the subject of intensive investigation since the very first single unit recordings were performed. Early studies concentrated on the response of these cells to sensory stimuli. More recent investigations have focused on the behavioral correlates of RF unit discharge in unanesthetized animals. Many RF studies have been done by investigators interested in testing a variety of specific theories of RF function and, as a result, several independent bodies of literature on RF function have been developed. The conclusions drawn by these different groups of experiments have often been contradictory.
Most studies of RF cells have employed surgical or pharmacological paralysis, or head restraint. Only recently has it been possible to record from RF cells in unrestrained animals. These studies have shown that the majority of RF cells discharge in relation to specific head and body movements. The theme of this review will be that many of the apparent contradictions in the RF behavioral literature can be resolved if one considers the movement relations of these cells. I will not attempt to exhaustively review the enormous literature on RF activity, but will instead focus on selected behavioral investigations that have used unit recording techniques. Data relating specifically to the central gray and to monoaminergic nuclei such as the raphe and locus coeruleus, which are also commonly considered part of the brain stem reticular formation, will not be discussed, but rather the emphasis will be on the paramedial RF core. Studies in the cat will be emphasized, although when relevant, results in other species will be considered. I will first review the different kinds of behavioral studies that have been done on RF neurons and then attempt to show how the findings of these studies can be integrated.
2. BEHAVIORAL CORRELATES OF RF UNIT DISCHARGE
2.1. Sensory
Most medial RF neurons respond to one or more sensory modalities. One of the most potent sensory inputs comes from the vestibular system. Stimulation of the vestibular nerve produced EPSPs or IPSPs in 75% of medial reticular neurons160. More than half of the medial pontomedullary reticular neurons received monosynaptic input from the vestibular nuclei158. Ten per cent of pontine reticular neurons were found to respond to lateral head tilt223.
Somatosensory responses are seen in neurons located throughout the medial RF. Seventy-seven per cent of medial reticular formation neurons responded to natural somatic stimuli201. This study included medial reticular formation areas caudal to P8. Similar findings have been made in more anterior regions3,18,208. Response latency to electrical stimulation of receptive fields ranges from 12 to 80 msec3. Spinal cord neurons responsive to a variety of somatic stimuli have been shown to project directly to medial RF areas66. Specific responses to vaginal and other sexual stimuli have been demonstrated throughout the brain stem reticular formation90,183-185. Unit responses to vaginal stimulation are increased during estrous183,186. RF units responsive to bladder stimulation have been reported at the pontomesencephalic junction (APO)33. Thermosensitive neurons have been seen in midbrain RF regions from APO to A4 in the cat55 and also in the medulla oblongata114,115. Seventy per cent of medullary neurons, 72% of caudal midbrain units, and 18 % of rostral midbrain units are thermosensitive55,115. At least 27% of reticular formation units, recorded largely in the vicinity of the nucleus gigantocellularis in decerebrate cats, respond to proprioceptive input, as evidenced by their response to vibration of the gastrocnemius-soleus muscle170.
Auditory responses in RF cells have been noted in several studies3,7,96. Between 23 and 40% of RF cells responded to discrete auditory stimuli196,208.
Visual responses in RF cells are most common in anterior regions, especially in the mesencephalic reticular formation18,65,82. Faingold and Caspary65 have shown that a convulsant drug can cause previously unresponsive RF units to respond to visual stimuli. Cells in the anterior pontine nuclei have been shown to have specific visual receptive fields72.
Motokizawa140 has identified olfactory responses in 58% of the reticular formation neurons tested. His explorations were limited to the mesencephalic reticular formation.
Groves et al.82 have systematically mapped the anterior–posterior representation of sensory signals in the rat. Their work reveals differences in the anatomical distribution of different sensory modalities. However, there is considerable overlap between regions and even within neurons. As many as 57% of RF neurons respond to more than one sensory modality208, but the convergence is clearly not unlimited31,111,127,196,244 or random160. The role of the RF in sensory integration is the subject of a recent review192.
2.2. Pain
Casey43,44 studied the discharge of NGC cells in cats trained to cross a barrier in order to escape painful electrical stimulation of the superficial radial nerve. He found that NGC unit discharge rates increased as stimulation intensities were raised to levels eliciting escape behavior. NGC cells respond to noxious stimuli applied to either spinal or trigeminal systems153 and to injections of bradykinin which induced nociceptive reactions26. Most of these cells were also found to discharge in relation to natural noxious stimuli42,113. Discharge in 57% of the neurons in NGC of the cat was related to noxious skin stimuli or escape behavior47. NGC neurons have also been reported to respond to presumably noxious gallbladder distension152. Similarly, units in the rostral mesencephalic reticular formation were found to respond to nociceptive stimuli12,183,245, although other workers have contended that these cells respond primarily to non-noxious stimuli145. Cells in the medial reticular formation of the caudal medulla oblongata also responded to nociceptive stimuli38,243. Thus, cells responding to pain have been reported throughout the medial RF from A6.5 to P1 and from P4 to P18.3 in the cat. The region between P1 and P4 has not been systematically explored. Responses to painful stimuli were of long duration, often lasting several seconds, and including periods of silence and reciprocal discharge in neighboring neurons7,26,44,113,245. Procedures which reduce pain responses, such as morphine administration and stimulation of the periaqueductal gray or of peripheral nerves, are effective in reducing both inhibitory and excitatory RF responses to noxious stimuli153,226,243. The activation of RF neurons by painful stimuli has been interpreted as indicating that medial reticular formation cells were specifically concerned with the sensory and motivational aspects of pain38,43-47,245. The criteria for identifying neurons involved in pain perception are carefully discussed in a recent review176.
2.3. Conditioning and habituation
Several workers have recorded the activity of reticular formation neurons during conditioning. Buchwald et al.37 systematically observed multiple unit activity in RF, sensory and motor systems during classical conditioning of a hindlimb flexion response. They conclude that ‘activation of the conditioned stimulus and reticular systems are primary events in conditioning.’ Yoshii and Ogura244 have studied classical conditioning of cells in the NRPC-NGC region. They find that 46% of cells are influenced by this procedure. Pragay et al.174, working in monkeys, report that 67% of the task-related RF units studied appeared to be involved in central processes underlying attention in visual discrimination tasks. Sparks and Travis216,218 studied RF unit activity in monkeys during a discriminative bar press task. They classified RF units into four different groups. Each group showed a distinctive pattern of changes in firing rate during the task. They mention in passing that a few RF units seemed to be related to head or eye movement. Most, but not all, of these units were described as being near the oculomotor nuclei. They note the need for careful observation and control of physiological variables, since in well-trained subjects consistent unit responses may ‘be related to small undetected components of a stereotyped behavior pattern rather than to cognitive or psychological variables.’
Most RF neurons show a progressive decrease in their response to repeated stimuli. This ‘habituation’ process has been studied by several investigators18,81,82,147,161,194,200,201. The Scheibels194 report that 75% of bulbar and pontine cells show habituation to repeated sciatic nerve stimulation. Bell et al.18 report that the same percentage of midbrain cells show habituation. After a 30–60 sec period without stimulation the habituated response returns, although subsequent habituation progresses more rapidly. Groves et al.81 have reported a progressive increase of response, or ‘sensitization’ in some reticular formation cells to certain stimuli. Peterson et al.161 have recently published a quantitative study of habituation in pontomedullary cells. They conclude that these cells display ‘most of the parametric features of behavioral habituation’ as defined by Thompson and Spencer228.
Analysis of the properties of habituation manifested in RF cells suggests that the habituation process does not result from postsynaptic inhibition of RF neurons themselves but rather from alterations in the pathway between sensory receptors and RF neurons. The evidence supporting this conclusion includes: (1) a PRF neuron habituated to one stimulus will respond normally to a different stimulus81,161,194. In some cases a neuron may even show habituation to one stimulus and sensitization to another; (2) there is no evidence of IPSPs in reticular formation neurons during habituation200; and (3) there is no decrease in the spontaneous activity of RF neurons during habituation161.
Changes in unit activity during conditioning and habituation have been reported for virtually all medial RF areas from A4 to P12.
2.4. Arousal and complex behavioral states
In 1949 Moruzzi and Magoun139 reported that MRF stimulation in intact cats produced a long-lasting cortical desynchrony. This classic observation has been repeatedly confirmed. Further work developed and refined the concept of an ascending reticular activating system contributing to both behavioral and electroencephalo-graphic arousal. Arousal came to be viewed as a separate dimension of behavior that energized the organism’s interaction with the environment125. The advent of unit recording techniques allowed investigators to explore the RF for the neural substrate of these phenomena.
An early study by Machne et al.121 reported a marked increase in the activity of most MRF units in response to arousal caused by either sciatic nerve or brain stem stimulation. They also reported that arousal disrupts temporal correlations between the discharge of adjacent neurons thus ‘desynchronizing’ them. Schlag197 and Schlag and Balvin198 reported similar correlations between RF activity and cortical EEG. Podvoll and Goodman168 and Bambridge and Gijsbers10 reported a strong positive correlation between behavioral arousal and the level of MRF and PRF multiple unit activity (MUA) in the unrestrained cat. Beyer et al.28 extended these observations by reporting decreased MRF multiple unit activity during ‘relaxation behavior’ induced by petting, feeding, grooming and similar activities. Single unit studies in unrestrained cats have reported correlations between arousal and level of MRF activity94,103,128. A subclass of ‘attention’ units responding only to novel stimuli was also reported. The proportion of such units is unknown94.
More detailed behavioral analyses have been directed at further defining the motivational variables responsible for relations between RF activity and arousal. A study by Phillips and Olds165 manipulated drive states in rats by food or water deprivation and observed unit activity in rats that were trained to remain still. They found that MRF activity was greatest when rats were signalled to expect the reinforcement for which they were most strongly motivated. A similar paradigm was used by Olds et al.146. They found a marked acceleration in activity in every MRF unit as reinforcement became imminent. They conclude that the MRF activity increase is specifically related to anticipation of reward. This conclusion can be contrasted with the findings of Vertes and Miller234 and Best et al.27 (in rats), and Umemoto et al.231 (in cats). These workers studied the activity of pontine and mesencephalic units during the conditioned emotional response (CER), using a procedure similar to the one used by Olds et al. In this behavioral paradigm, a previously neutral stimulus signals an unavoidable shock. These studies reported an increased firing rate in RF units during anticipation of the shock. The increased RF activity was hypothesized to relate to fear of the shock. Umemoto et al. reported that 68% of encountered neurons discharged specifically in relation to the CER. Best et al. reported that 85% of recorded cells increased their discharge rate in relation to the CER.
Another behavioral approach to the study of RF neurons has been the study of elicited fighting behavior. In these experiments midbrain RF and central gray units were recorded while cats or rats were induced to fight1,2,173. In the cat, 80% of midbrain cells increased their discharge during fighting behavior. Ten per cent of midbrain cells were found to fire almost exclusively during fighting behavior and therefore ‘had the characteristics expected for cells mediating affective defense behavior’1. Most of these cells were located in the central gray, the area most thoroughly explored. However, a few were located in adjacent regions of the MRF. A more recent study done on rats173 did not report cells discharging only during affective behavior, but did find that 19% of all midbrain cells studied and 4 of 32 cells (12.5 %) in midbrain RF regions adjacent to central gray, discharged predominantly during fighting. The careful behavioral observation procedures employed led the authors to conclude that these cells participate in the integration of fighting behavior. However, it was also noted that the data do not compel this conclusion and that ‘some subtle aspect of the sensory stimulus presented by the opponent rat or some subtle aspect of the motor response of the subject conceivably could account for the maximal firing during the fight sequence’173.
Several recent studies have pointed out that traditional concepts of arousal level can not adequately explain changes in RF activity. Goodman and Mann74 studied MRF MUA during natural sleep, waking and anesthesia. Their goal was to determine if the loss of consciousness and pain response in anesthesia could be explained by a parallel decrease of MRF MUA. They found that different anesthetics could produce anesthesia of similar depth even though MRF MUA differed significantly. Even more striking was their observation that surgical anesthesia could be achieved even though MRF MUA was no lower than that seen in normal slow wave sleep. They conclude that ‘the characteristics of spontaneous unit firing at reticular … sites cannot be used to differentiate between wakefulness, sleep and anesthesia’. Similar results were found using single unit techniques197.
Other studies, while reporting RF cells related to arousal, have also noted that some MRF cells show correlations with more specific behavioral patterns. Thus, Komisaruk and Olds108 reported on a cell that fired rapidly during any form of locomotion but not when the alert rat was standing still. Malmo and Malmo126 reported a significant correlation between midbrain MUA and body movement. Vertes233 reports a significant increase in pontine unit activity during movement detected by an unshielded ‘free wire’ attached to the headplug.
Schwartzbaum199 studied MRF MUA in rats during a variety of behavioral situations. He found that midbrain MUA covaried with behavioral state, progressively increasing as the rat shifted from slow wave sleep to stationary waking, to ‘face-washing’ and lapping behavior, to more vigorous movement categories. He found that MUA activity during sensory stimulation and conditioned emotional response remained correlated with the movement category elicited by the stimulus. He concludes that MRF activity relates to the ‘extensive and intensive properties of movement, although the data would not rule out other more specific relationships’. These findings are compatible with either MRF modulation by the sensory feedback from movements or MRF participation in the facilitation of movement.
It is worth noting that a few recent studies have also detected general relations between movement and MUA activity in forebrain areas71,126,199. Thus, many of the considerations discussed here in connection with RF activity may apply to more rostral regions.
Studies of correlations between RF activity and arousal have explored rat midbrain regions most thoroughly. Cat studies have extended from approximately A4 to P5. The relations reported have generally not been found to be localized to specific portions of the area investigated.
2.5. REM sleep
Decerebrate cats show both rapid eye movement (REM) sleep and slow wave sleep (SWS)88,100,236. Lesions within the brain stem RF can disrupt REM sleep14,15,40,41,84,100,101,129,188. Therefore, many studies have recorded from RF units to determine if any particular subgroup of cells showed activity changes consistent with a role in the generation of REM sleep.
Hobson and McCarley and their co-workers have reported, in a series of studies, that cells in the pontine FTG field discharge at much higher rates in REM sleep than in either waking or SWS. During waking and SWS ‘most FTG neurons either showed very low discharge rates (< 1 impulse per second) or were silent’ while during REM sleep they discharged in rapid bursts130. Therefore, the ratio of REM sleep rates to waking and slow wave sleep rates or ‘selectivity’ was very high. It was concluded that because of this high selectivity ‘FTG units are at present the best candidates’ for REM sleep generator neurons89.
Pompeiano and Hoshino have conducted a series of studies that parallel the studies of Hobson and McCarley91-93,171,172. They investigated unit discharge in the PRF of decerebrated cats during the cataplectic state induced by the anticholinesterase, eserine sulfate. The loss of motor tone and the eye movement bursts of this state are reminiscent of REM sleep, although there are important differences in eye movement pattern and level of consciousness between the cataplectic state and REM sleep92,93,135. Their results are similar to those of Hobson and McCarley in finding that the discharge of cells in the FTG region is highly selective for the cataplectic state.
The Hobson and McCarley studies were performed in cats adapted to head restraint. The Hoshino and Pompeiano studies were done on the acute decerebrate preparation, which does not show spontaneous movements. Therefore, it appeared possible that the apparent selectivity of these cells for REM sleep was a function of the abnormal waking immobility of these preparations, a possibility discussed in early studies89.
We have investigated these cells in unrestrained cats during both waking and sleep131,132,207,208,212. We found three cell types in the FTG region. Type 1 had no spontaneous activity during quiet waking and sleep, discharging only during movements. Type 2 had high rates of tonic activity during both quiet waking and slow wave sleep which further increased during waking movement and REM sleep. Type 3 had low activity rates during quiet waking and slow wave sleep, but discharged in bursts during both waking movements and REM sleep. Units with augmented discharge restricted to REM sleep were not observed. All FTG cells discharged rapidly during specific waking movements at rates exceeding mean REM sleep rates. FTG units’ REM sleep discharge rates were significantly, and positively, correlated with their rates during waking movement, i.e. cells that discharged at high rates in REM sleep tended to have relatively high rates during active waking212. We also studied these same cell types while cats adapted to head restraint. This procedure greatly reduced motor activity and correlated FTG unit discharge. We concluded that the restraint, by reducing waking discharge rates, would make these cells appear to be selective for REM sleep. Our results are consistent with a role for FTG units in the motor activation common to waking and REM sleep, but not with an ‘executive’ role in REM sleep generation. Vertes233 has reported congruent results in the rat. These findings, and their relationship to theories of sleep control, have been discussed in detail elsewhere206.
Single and multiple unit studies have also been made in anterior midbrain areas A2–A59,57,94,103,109,116,128,134,240 and in the nucleus reticularis pontis oralis-caudalis (RPO-RPC) region A1–P4206,213. This latter area has been described as the RF area crucial for REM sleep41,100. Virtually all medial RF cells within this region (A2–P4) showed a marked increase in discharge rate as the animal shifted from non-REM sleep into REM sleep. This rate enhancement, similar to that seen in pontine regions, is largely due to discharge bursts correlated with the eye movements and muscle twitches of REM sleep. A similar unit activity increase was observed during active waking periods. The same three cell types described in the pontine FTG field are seen in the RPO-RPC region206.
A recent study by Netick et al.142 has reported the observation of 4 cells which concentrated their discharge in REM sleep. These cells were located in the medullary RF at about P9.5, L1.6. However, these cells were recorded in head-restrained preparations and may therefore appear to be selective for REM sleep by virtue of the reduction in waking rate caused by head restraint, as was the case in the PRF units. Therefore, we recorded from this region in unrestrained cats206,211. We saw the same 3 cell types in this area that we found in RPO-RPC and in the pontine gigantocellular area. Waking discharge related to motor activity, as was the case in the more anterior regions. Several cells in this area discharged at high rates when the cat held particular postures. If the cat shifted into a different position, the discharge rate was greatly reduced.
We saw no cells which discharged selectively in REM sleep, as Netick et al.142 reported. However, two of the posture-related cells discharged at a low rate in quiet waking and SWS, showed a tonic rate increase during the SWS–REM sleep transition and maintained this rate throughout REM sleep. Therefore, the sleep and quiet waking behavior of these cells is strikingly similar to that of the cells seen by Netick et al. Since these workers were recording from cats which were adapted to head restraint, they would not have been able to observe unit activity in a variety of postures. Therefore, it appears likely that the REM sleep-selective cells they observed are similar to the posture-related cells which we have seen. If this is the case, then the REM sleep-related acceleration can be understood as reflecting the motor activation of REM sleep. Just as cells which are phasically active in waking show phasic activity in REM sleep, these cells, which discharge tonically while postures are maintained in waking, also show tonic REM sleep activity. It is also possible that medullary RF cells with discharge selective for REM sleep are rare and have not yet been encountered in the unrestrained cat.
The region explored in these studies lies from 0.3 to 3.0 mm lateral to the midline and between A2 and P13. More than 72% of the cells in this area discharge in REM sleep and virtually all discharge at high rates during waking movements206-208,212.
2.6. Eye movement
Studies in the head-restrained monkey have demonstrated relationships between the discharge of medial RF cells and eye movement. In these experiments, the animals are typically trained to track a visual target or are allowed to spontaneously explore their environment while RF unit activity is recorded and correlated with EOG activity. A variety of cell types, including pause units which stop discharging during eye movements, long and medium lead burst units which begin discharging up to 20 msec before eye movements, and tonic units, which discharge in relation to eye position, have been reported in both midbrain and pontine regions39,52,68,85,106,120. Intermediate cell types also occur and other cell type classification systems have been developed217. It has been reported that ‘almost every unit’ encountered in the medial pontine region relates to eye movement52,53. The relationship of brain stem neuronal activity and eye movements was the subject of a recent symposium8.
Anatomical studies in the monkey have shown that eye movement cells are widespread but are not homogeneously distributed throughout the reticular formation, being concentrated in dorsomedial regions120. The distribution of types of eye movement cells from the midline to 3 mm lateral to the midline has been mapped106. In the anterior–posterior plane, these cells extend from at least 3.75 mm anterior to 3.75 mm posterior to the center of the abducens nucleus. Since the monkey brain stem is oriented at a more acute angle to the stereotaxic vertical plane than the cat brain stem, this would correspond to an area beginning several mm posterior to the cat’s abducens nucleus in ventral RF regions. Eye movement cell types similar to those seen in the monkey have been found in dorsomedial positions of the cat RF between P3.5 and P9.5. The RF region around the abducens nucleus was the most intensively studied. Quantitative data on the percentage of cells with eye movement relations was not presented86. Cat RF activity has also been shown to precede the eye movements of REM sleep167. A variety of cell types related to eye movements have been seen in the vicinity of the cat abducens nucleus73,175,177,178. Cells related to nystagmus-induced eye movements have been observed in the pons of the rabbit50,58 and guinea pig6. It has been concluded that discharge in pontine reticular formation cells is ‘predominantly related to eye movement’51.
2.7. Respiration
The relationship of RF unit activity to respiration has been extensively studied in acute preparations. Typically, respiratory related units are sought in cats that are paralyzed with Flaxedil and artificially ventilated. Units related to respiration are detected by visual observation of periodicities in discharge rate, or more frequently, by computer analysis of temporal relations in unit firing. Units related to respiration have been found throughout the RF. The proportion of units that are related to respiration ranges from 21% in the MRF to 36% in the PRF of the cat25. Inspiratory and expiratory units are concentrated in distinct anatomical areas. Although many units related to respiration are in lateral RF regions, there are also large numbers in medial areas235. RF units related to respiration can be found from P2.5 to P1124,235.
2.8. Locomotion
The neural circuitry underlying locomotion has been extensively studied in the cat (see reviews by Grillner76; Shik and Orlovskii204; Wetzel and Stuart238). It has been found that stimulation of a circumscribed midbrain region produced rhythmic stepping behavior in a decerebrate cat placed on a treadmill79 and speeds locomotion in the intact cat225. This midbrain locomotor region (MLR) has been localized to the area ventral to the inferior colliculus and predominantly dorsal to the brachium conjunctivum, in the vicinity of the nucleus cuneiformis79,224.
Since there are no direct connections between the MLR and the spinal cord, and since spinal cord areas containing reticular projections show a marked activity increase during locomotion, it was hypothesized that reticulospinal pathways might form part of the system producing both spontaneous and MLR-induced locomotion148. Orlovskii has, therefore, studied the activity of reticulospinal neurons during locomotion. In his preparation, the head and spinal cord of the cat are fixed to a stereotaxic frame and the limbs rest on a treadmill. In this way he has been able to record intracellularly during MLR-induced locomotion. High and low decerebrate cats are used. Reticulospinal neurons were identified by antidromic stimulation from the L1 level of the spinal cord.
Orlovskii found that there are monosynaptic projections from the MLR to reticulospinal neurons. Furthermore, it was shown that 69% of reticulospinal neurons were activated during MLR-induced locomotion and that half of the activated neurons showed rhythmic rate modulation related to the limb movements of locomotion148,149. In high decerebrate cats it was possible to observe the same relationships during spontaneous treadmill locomotion149. It was concluded that the ‘transfer phase’ of locomotor movement was the most active point for reticulospinal cells, but that individual cells varied widely in their relation to movement. Orlovskii also observed reticulospinal neurons during locomotion induced after cerebellar removal. Under these conditions 62% of the neurons were activated during locomotion but only 8% of these activated cells showed frequency modulation in step with locomotion150. Both cerebellar removal and decerebration lower the spontaneous discharge rates of RF neurons.
Orlovskii recorded from neurons in the area bounded by H.C. planes P5 to P15 and L0.5 to L1.5149. This includes mainly medial regions of the nucleus gigantocellularis and the nucleus paramedianus. Sixty-nine per cent of reticulospinal neurons were found to be activated by locomotion. Similar modulation with locomotion has been observed in most cells in the nucleus reticularis tegmenti pontis246. It has been hypothesized that the RF’s role in locomotion is to facilitate and readjust locomotor activity whose basic rhythm is generated in the spinal cord76.
2.9. Specific movements
With only a few exceptions, the studies mentioned above have employed animals whose heads and bodies were restrained. Siegel and McGinty208 studied PRF activity (P4–P8) in unrestrained cats. We reported that, of 35 cells studied, we were able to identify consistent, individually distinctive motor correlates of discharge in 32 cells (91%). These included head and neck (n=21), ear (n=3), forepaw (n=1), scapula (n=2), tongue (n=2) and facial (n=3) movements. Each cell responded only during a single kind of movement. For example, cells related to ear movements did not increase discharge during head movements and vice versa. Furthermore, a given cell did not discharge in relation to all head movements or all tongue movements; each cell related to directionally specific movements. For example, cellular discharge was found to be related to turning the head right, or moving the tongue out and to the left.
While natural stimuli could repeatedly elicit unit activity in the unrestrained unanesthetized cat, the duration and intensity of unit discharge was not closely related to the eliciting stimulus. For example, stimulation applied by lightly placing a cotton swab in the concha of one of the ears produced intense discharge in the three PRF ‘ear movement’ cells. However, the discharge normally continued with undiminished rate for 1–10 sec after the swab was removed from the ear, ending abruptly with a change in the ear’s position. Rhythmic movement of the stimulus did not produce rhythmic discharge in the unit. Furthermore, in most PRF units we observed discharges at rates equal to or exceeding those induced by our most effective stimuli during small, spontaneous movements.
Obviously, spontaneous behaviors produce a variety of sensory stimuli. In order to determine whether or not stimuli generated by motor activity were responsible for observed unit activity, randomly selected cells responding to vestibular, auditory, visual, or localized somatic stimuli were subjected to a procedure designed to eliminate or reduce all identified sensory inputs. Vestibular stimuli were eliminated by an atraumatic head restraint system. The receptive fields of somatic cells were locally anesthetized with injections of lidocaine supplemented by topical application of lidocaine jelly. Visual input was eliminated by placing the cat in a light-tight box. Auditory input was attenuated in responsive cells by blocking the ear canals with wax. Unit rates were not reduced by these procedures. Phasic unit discharge continued to be correlated with motor activity visible in the EMG. We concluded that PRF unit activity is more closely related to motor output than to sensory input208. Studies in curarized cats demonstrate that proprioceptive input can be eliminated without preventing high rates of PRF discharge17,113. Thus, modulation of muscle spindle, tendon and joint receptor input does not appear to be required for PRF activation. Further, work in our laboratory has shown that cats rewarded for increasing discharge in PRF units rapidly learn to make repetitive directionally specific movements in order to gain more reinforcements34. Photographic analysis of the relationship between movements and unit discharge has enabled us to describe the specific movement vectors related to unit activity (Siegel et al., in press). This analysis has demonstrated that body movement onset occurs within 50 msec of the onset of PRF unit discharge. Recent studies have found similar movement relations in cells in the NRPO and NRPC (A1–P3) and in caudal medullary regions (P8–P14)206.
3. OVERVIEW OF RF BEHAVIORAL RELATIONS
3.1. Anatomical considerations
How can one integrate the various behavioral relations reviewed above into a coherent picture of the functional role of RF neurons? The most obvious approach is to acknowledge the complexity and heterogeneity of RF behavioral relations and conclude that many disparate cell types occur within it, being concentrated in distinct anatomical regions. However, as one can readily see in Fig. 1, the different cell types are not segregated at different A–P levels. There is a nearly complete anatomical overlap of all functional groupings. Lateralities (0.5–2.5 mm from midline) and horizontal levels (0–10 mm ventral to stereotaxic zero) are also similar. This overlap is even more striking when one realizes that the limits of the distributions sketched do not, in most cases, represent points beyond which cells in a particular functional grouping could not be found. Rather they represent the limits of exploration, i.e. there is no reason to think that relations between RF cells and locomotion could not be found rostral to P5, or that relations between RF cells and pain could not be found between P4 and P1.
Fig. 1.
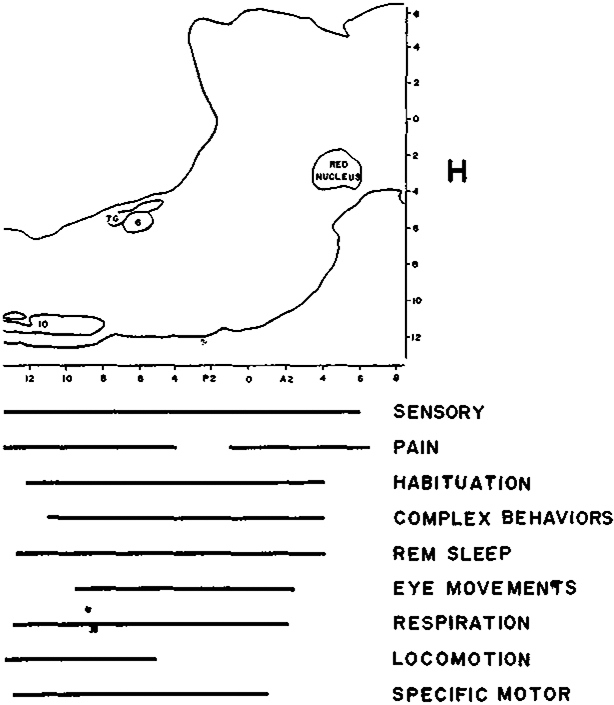
Anatomical distribution of cell types within the reticular formation. Figure shows sagittal view of the brain stem of the cat. Bars at bottom indicate the anterior–posterior distribution of cells identified as having specific behavioral functions. Cells with different behavioral properties are not localized in different RF areas. G, abducens nucleus; 7G, genu of the facial nerve; I.O., inferior olive.
3.2. Cell selection procedures
A way of dealing with the anatomical overlap of cell types is to postulate that each group of investigators has selected cells with particular behavioral correlates while ignoring the other cell types in the same area. Clearly this could be the case in Adams’ study1 where the cells of interest were found by eliciting fighting behavior. Since these cells were relatively inactive at other times, other investigators might never have noticed them. Similarly, Hobson et al.89 searched for REM sleep-selective cells in REM sleep, and one might wonder if the failure of others131,132,208,212,233 to observe such cells resulted from these experimenters searching for cells during waking. However, the cells that Hobson et al. report on, unlike Adams’ cells, were not silent in waking. Their mean waking rates equalled or exceeded those found by these other investigators during quiet waking. For example, Hobson et al.89 report a mean waking rate of 6 spikes/sec while Siegel et al.212 report mean waking FTG rates ranging from a minimum of 0 spikes/sec to a maximum of 49 spikes depending on the cell type and the activity level of the cat. The average REM sleep rate of 17.8 spikes/sec reported in unrestrained cats212 is also close to Hobson et al.’s89 mean of 17.4 spikes/sec. Therefore there is no reason to believe that REM sleep-selective cells were missed when units were located in the waking animal. RF cells without spontaneous activity in waking and sleep have recently been described207. However these cells do not appear to have qualitatively different behavioral relationships from cells with higher spontaneous activity rates. The vast majority of RF cells recorded in the studies reviewed above were not found during specific behaviors. Normally cells were first detected by observing their spontaneous activity and then studied in relation to behavior. Therefore the behavioral techniques used to locate cells are not a major factor causing discrepant results.
3.3. Microelectrode techniques
Another approach to integrating these findings is to attribute the varying results to differences in microelectrode techniques. However, a careful study of this issue did not reveal any difference in unit discharge correlates when one switched from 1 μm glass electrodes to 10 μm steel electrodes127. Multiple unit electrodes have reported the same behavioral relations as single unit electrodes168,199. Most findings have been replicated with several different unit recording techniques. For example, response habituation, with a similar time course, has been seen with glass intracellular electrodes of tip diameter less than 1 μm200, metal electrodes with tip diameter 2–4 μm194 and with microwire electrodes with 32 μm tip diameter208. Conversely, similar unit recording techniques have been used in reports finding a variety of behavioral relations in the same reticular areas. For example, tungsten electrodes less than 7 μm at the tip have been used in studies linking reticular activity to pain17,38, REM sleep91, respiration25, sexual behavior185, polysensory responses, habituation81, and olfactory responses140.
The RF area has a great number of large cells36 and a common observation in RF studies is that a large percentage of the cells have ‘giant’ spikes and large signal-to-noise ratios. Many cells can be recorded while microelectrodes are moved over a distance of greater than 100 μm and some can be recorded for as much as 500 μm241. The preponderance of large cells, which can be recorded with all unit techniques, minimizes the effect of sampling bias. While one would expect small cells to be under-represented, this would be true in virtually all of the studies reviewed above.
3.4. Conclusion: Different RF studies are observing the same cell populations
Another explanation is that all of the cell subtypes co-exist in the same areas. However, if one calculates the percentage of encountered cells that have been reported to have each behavioral function (Table I), it is clear that this is an arithmetic impossibility. The different cell type percentages add up to far more than 100%. For example, some studies89,130 report that virtually all pontomedullary cells discharge specifically in REM sleep while others39,120 report that between 70% and 90% of these cells relate specifically to eye movements and others12,38,47 report that 57–88% of these cells relate specifically to the sensory and motivational aspects of pain. Therefore, we are forced to conclude that different RF studies are investigating the same populations. The same cells that one group of studies are describing as sensory integrators are being described by another group of studies as specifically concerned with the sensory and motivational aspects of pain, by a third group as being primarily related to the conditioning and habituation process, by a fourth group as relating primarily to reward, by a fifth group as relating primarily to fear, by a sixth group as being candidates for the role of the executive neurons of REM sleep, by a seventh group as predominantly related to eye movements, by an eighth group as being respiratory neurons, by a ninth group as being related to the transfer phase of locomotion, and by a tenth group as being related to arousal.
TABLE I. A partial list of the behavioral functions attributed to RF cells.
Cat stereotaxic coordinates were estimated from figures or taken from the authors’ description. General anatomical terms were used in rat and monkey studies and in cat studies where precise AP levels were not available. Third column lists percentage of encountered cells having function listed in column one.
| Function or stimulus | Cat AP stereotaxic coordinate or anatomical designation |
Percentage of encountered cells |
Reference |
|---|---|---|---|
| Vestibular | P15–A0 | 75 | Peterson et al., 1975 |
| Somatosensory | P18.5–P8.5 | 77 | Segundo et al., 1967 |
| P8–P3 | 71 | Siegel and McGinty, 1977 | |
| A2–A4 | 68 | Bell et al., 1964 | |
| Auditory | P8–P3 | 40 | Siegel and McGinty, 1977 |
| BRF, MRF | 23 | Scheibel et al., 1955 | |
| Olfactory | APO–A6 | 58 | Motokizawa, 1974 |
| Pain | P18.3–P12.1 | 82 | Burton, 1968 |
| P11–P6 | 57 | Casey et al., 1974 | |
| A0–A3 | 88 | Barnes, 1976 | |
| Habituation | BRF | 75 | Scheibel and Scheibel, 1965 |
| A2–A4 | 75 | Bell et al., 1964 | |
| Reinforcement | MRF† | 100 | Olds et al., 1969 |
| Fear | MRF† | 85 | Best et al., 1973 |
| MRF | 68 | Umemoto et al., 1970 | |
| REM sleep | P7–A0 | 100 | Hobson et al., 1974 |
| P9–P3 | 72 | Siegel et al., 1977 | |
| Eye movements | MRF* | 70 | Buttner et al., 1977 |
| PRF* | 92 | Luschei and Fuchs, 1972 | |
| Locomotion | P15–P5 | 69 | Orlovskii, 1970 (ref. 149) |
| Respiration | PRF | 36 | Bertrand et al., 1973 |
| MRF | 21 | Bertrand et al., 1973 | |
| Specific movements | P8–P3 | 91 | Siegel and McGinty, 1977 |
Monkey studies.
Rat studies.
One might hypothesize that individual RF cells can have several of these often contradictory functions. This hypothesis would require a mechanism that would somehow change the function of most RF cells in different behavioral situations, and simultaneously mask their other functional modes of operation. Since most studies have employed the same behavioral situation (i.e. an intact immobilized cat or rat presented with stimuli) it is difficult to imagine what cues control this switching mechanism and why most investigators only see one cell type (e.g. fear cells or reward cells, or REM sleep cells, etc.). Such an hypothesis is non-reductionistic and does not readily lend itself to scientific test. The alternative is to seek some underlying relationship that can explain the participation of these cells in so many different behaviors.
3.5. A behavioral hypothesis explaining RF unit discharge relations
Is there a model of RF unit function that can encompass the findings reviewed above? I propose the following hypothesis: that discharge in most RF cells is primarily related to the excitation of specific muscle groups. It is hypothesized that individual cells would have their own distinctive muscle relations. For example, one cell might relate to extending the tongue (genioglossus muscle), another to turning the head (e.g. cleidomastoideus, biventer cervicis, complexus), another to fixating the back (e.g. thoracic and lumbar interspinales). More complex relations are possible. For example, a single unit might function to facilitate muscles involved in head turning and forelimb muscles supporting the head in its new position. RF control would be mediated by excitation or inhibition of motor neurons innervating each muscle in the group. The relationship of RF cells to simple movements would be responsible for the variety of complex behavioral relations attributed to RF cells. This hypothesis provides a framework in which to integrate previous results without the necessity of attributing complex or multiple behavioral properties to RF units.
The ascending projections of RF cells might serve to relay a ‘collateral discharge’ for skeletal muscles to forebrain areas. This discharge would be similar to that which has been thought to accompany eye movements. The collateral discharge would: (1) allow perceptual constancy by compensating for sensory changes induced by body movements, (2) facilitate sensory channels to speed the acquisition of information needed to guide the movement, and (3) allow higher neuronal mechanisms to interact with movement patterns.
The RF has long been thought to be involved in motor control. In their pioneering stimulation studies, Magoun and Rhines124,182 demonstrated motor facilitation and inhibition from RF stimulation. Related studies, briefly discussed below, have developed this concept. The emphasis in these studies was on the generality of the motor effects mediated by the RF. Thus, for example, it was shown that both flexors and extensors at all levels of the body were inhibited by stimulation applied to a wide area in the medullary RF99,124. The present concept differs from these previous formulations by hypothesizing a highly specific RF relation of individual RF cells to movements in particular muscles or muscle groups.
This hypothesis is aimed at integrating the behavioral findings surveyed above. As will be reviewed in the next section there are a variety of established synaptic mechanisms that can mediate this relationship. RF cells receive inputs from many ‘higher’ motor areas and send axons to ventral spinal cord areas. RF cells can exert mono- and disynaptic influence on motoneurons. Critical tests of the hypothesis proposed here would require precise behavioral experiments to determine the strength and stability of RF–movement linkage in a variety of situations, and coordinated physiological studies to clarify the central and peripheral mechanisms underlying observed relationships.
4. RELATED FINDINGS
4.1. Anatomical and physiological data
The hypothesis that RF units relate to the excitation of specific muscle groups is consistent with a variety of anatomical and physiological findings. Ingram et al.97, in one of the early studies making use of the Horsley–Clarke stereotaxic apparatus, beautifully demonstrated what they described as the ‘tegmental response’. This response could be elicited in the cat by electrical stimulation of the reticular formation from midbrain to caudal pontine levels, and consisted of ‘a curving of the head, neck and trunk toward the side stimulated, flexion of the ipsilateral forelimb and extension of the contralateral forelimb, with varying movements of the hind limbs’. Stimulation of these areas in anesthetized or decerebrated cats produced non-reciprocal inhibition or excitation in a wide range of muscles and widespread changes in reflex excitability222. According to the present hypothesis these non-specific effects may be a consequence of the anatomical proximity of groups of neurons with a variety of behavioral correlates. This proximity would cause suprathreshold electrical ‘macro-stimulation’ to recruit groups of adjacent units, thereby producing more generalized spinal cord effects. Sprague and Chambers220 used finely graded electrical stimulation of both anesthetized and unanesthetized cats to further define the motor properties of the RF. They did not find pure excitatory and inhibitory effects when using liminal stimulation. Instead they emphasized that stimulation at all levels of the RF produced reciprocal changes in flexor and extensor muscles. Stimulation of the mesencephalic RF has been shown to affect gamma motoneurons5 and alter reflex excitability141.
Torvik and Brodal229, investigating retrograde changes in cats after spinal cord lesion, found that more than half of the pontine and medullary cells send projections into the ventral, motor regions of the spinal cord. All pontine fibers descend homolaterally, while some medullary fibers also descend contralaterally. Nyberg-Hansen144, using silver impregnation methods, noted that pontine and medullary reticulospinal fibers descend in the ventral part of the lateral funiculus and end in laminae 7, 8 and 9 of the ventral spinal cord. Similar results were reported by Petras164 who emphasized the contributions of the NGC to the spinal cord. Direct spinal projections from midbrain RF tegmental regions have been demonstrated in primates48, but not in the cat. Brodal35 reported extensive reticular projections to the cerebellum. Anatomically localized RF projections to the oculomotor nuclei have been defined using horseradish peroxidase, autoradiographic and electrophysiological techniques75,122,180. Basbaum et al.13 have mapped the projections of rostral medullary RF cells using [3H]leucine injections. They conclude that NGC ‘projects primarily to ‘motor’ related sites’ including cranial nerve nuclei and ventral spinal cord areas. Spinal cord terminals were predominantly ipsilateral with major projections to Rexed’s lamina VII and smaller projections to VIII and IX. Horseradish peroxidase studies have shown similar reticulospinal projection patterns in the primate107. Golgi preparations in the cat have shown RF fiber terminations on internuncials and on motoneuron dendrites195. The terminations of the cat’s reticulospinal system contrast with the more dorsal termination of corticospinal fibers from motor cortex, which end in layers 5, 6 and 7 chiefly or exclusively on internuncials36.
Recent studies have used electrophysiological techniques to investigate the connections of RF units. Magni and Willis123 extended the caudal limits of reticulospinal projections beyond those described using anatomical techniques by antidromically exciting reticular neurons from sacral levels of the cord. Individual RF neurons branch extensively in the ventral horn, often innervating both sides of the cord and extending over several levels162. The number of RF neurons projecting caudally is far greater than the number projecting rostrally, the ratio being approximately four to one60. Grillner and Lund77,78 conclude that a pathway producing monosynaptic EPSPs on flexor motoneurons in lumbar spinal cord regions originates from medullary and pontine reticulospinal cells, which send their axons through the medial longitudinal fasciculus (mlf). A disynaptic RF pathway mediated by propriospinal neurons and ending on hindlimb alpha motoneurons has been demonstrated electrophysiologically203. Disynaptic IPSPs mediated by a ventral reticulospinal pathway have been reported in decerebrate cats99. Stimulation of the pontomedullary reticular formation can produce monosynaptic EPSPs and IPSPs in neck motoneurons163. Back motoneurons receive monosynaptic EPSPs and polysynaptic IPSPs from pontomedullary RF regions while limb motoneurons receive EPSP’s from PRF stimulation but do not respond to medullary RF stimulation166. Orlovskii148 found that pontine and medullary reticulospinal neurons receive monosynaptic projections from the mesencephalic tegmentum. Thus, MRF neurons have disynaptic access to spinal cord motor areas4.
Inputs into the RF are also suggestive of motor functions. RF neurons receive monosynaptic projections from the cerebellum. The synaptic relations between RF neurons and spinal cord and cerebellum have been the subject of several detailed studies32,60-62,70,79,98,117,118,230. The RF also receives extensive projections from the tectum96,105. Magni and Willis123 and Peterson et al.159 all report that the precruciate, or motor, cortex is the most effective cortical site for evoking EPSPs in RF neurons. RF unit response to repetitive precruciate stimulation does not habituate, as does RF response to peripheral sensory stimuli161. They conclude that reticulospinal neurons are not well suited to function as relays for repetitive sensory inputs, but may serve as a motor relay. Direct projections to RF areas from motor cortex and from orbital cortex, which has specific motor inhibitory properties, have been demonstrated with anatomical and physiological techniques136,143,187,191.
Physiological investigations of the RF have left many fundamental questions regarding its motor role unanswered. These include: (1) do individual RF cells exert bilaterally symmetrical, relatively non-specific effects on motoneuron pools; (2) are RF cells activated synchronously during movements; (3) do RF units discharge in relation to specific movements, and (4) is the relationship of a given RF cell to movement dependent on the means by which the movement is elicited? For example, do some RF cells mediate only movements in response to painful stimuli, while others mediate movements during either exploration or REM sleep, or conditioning or sexual behavior? Our behavioral findings and hypothesis suggest an affirmative answer to question 3 and negative answers to the remaining questions, at least for the majority of RF cells. Further work is needed to explain the synaptic mechanisms mediating the observed behavioral relations and to determine the frequency and anatomical distribution of cells related to various specific movements.
4.2. Phylogenetic data
In evaluating the functional role of mammalian RF cells, it is instructive to consider findings on the RF of other vertebrate classes. The medial RF of the most primitive vertebrates, the cyclostomes, contains a number of giant cells, the Muller and Mauthner cells. These are the principal source of descending control, running the whole length of the spinal cord155. Like mammalian reticulospinal cells, these neurons receive convergent sensory inputs and make monosynaptic connection with motoneurons202. Rovainen189 defined Muller cells in the sea lamprey (Petromyzon marinus) as the cells of origin of 8 pairs of large uncrossed axons in the medial longitudinal fasciculus of the spinal cord. Two pairs of Mauthner cells were defined by their location near the eighth nerve and by their decussating axons extending into the cord. Other large reticulospinal elements were also identified. High frequency stimulation of individual Muller or Mauthner axons produced stimulation bound movements of the neck, posterior body, dorsal fins and tail. Rovainen concludes that these cells form a descending system producing the withdrawal, swimming and coiling movements of the lamprey.
The medial RF of teleost fish contains a pair of giant Mauthner cells. The neurobiology of the Mauthner cell is the subject of a recent book64. Discharge in these cells, produced by electrical or natural stimuli, has been shown to produce non-graded tail flips in the lungfish239. These cells are thought to carry the motor output of a startle response system56. In other respects, the reticular formation of fish resembles that of cyclostomes, although fish have more reticulospinal cells202. Stimulation of the medial midbrain tegmentum in the carp (Cyprinus carpio) produces stimulus-bound swimming movements104 and lesions of this region produce abnormalities in swimming behavior in the goldfish157.
Elasmobranchs do not have Muller and Mauthner cells, but have other large medial reticular cells. Restieaux and Satchell181 have described reticular cells up to 250 μm in length in the dogfish (Squalus lebruni). Like large reticular cells in other vertebrate orders, they receive a variety of sensory inputs and project to the spinal cord. Restieaux and Satchell conclude that these cells are ‘well suited to mediate turning movements towards or away from biologically meaningful stimuli’.
Mauthner cells are absent from the brains of adult tetrapods with the exception of urodeles (salamanders and newts). Other amphibia, such as the frog, while lacking specialized Muller and Mauthner cells, have well developed reticulospinal systems155. The frog’s reticulospinal cells are the largest neurons in its brain and make monosynaptic connections with spinal cord motoneurons. These connections are concentrated in cervical segments. Vestibulo- and tectospinal tracts play only a secondary role in motor control in amphibia202.
The medial medullary RF of reptiles is more highly differentiated than the amphibian RF and ‘can be regarded as the homologue of the gigantocellular pontomedullary RF in mammals’. RF neurons in turtles have excitatory, monosynaptic projections to motoneurons at lumbar levels of the cord. Stimulation of rubro- and vestibulospinal tracts produces smaller effects on motoneuron firing probability than reticular stimulation202.
Thus the brain stem RF of more primitive vertebrate orders contains large or giant medially located cells as does the mammalian RF. Like their mammalian counterparts, many of these cells respond to a variety of sensory stimuli, project monosynaptically to spinal cord motoneurons and have generally low levels of spontaneous activity. In advanced vertebrate classes there is a trend towards somewhat smaller and more numerous reticular cells although even in mammals there are only 5000–6000 giant RF neurons229. Synaptic inputs and outputs of RF cells are similar in all classes. Physiological and behavioral evidence suggests that RF activity in primitive vertebrates is closely coupled to movements, and in the most primitive vertebrate classes they are clearly the predominant source of motor control. These findings complement the hypothesized relationship of mammalian RF cells to motor function.
4.3. Summary
While the anatomical, physiological and phylogenetic findings are consistent with our hypothesis, they do not in and of themselves compel one to view the RF as a motor control system in mammals. RF cells with bifurcating axons and ascending projections reaching cortical levels19,69,123,193,232 and innervating sensory nuclei214,215 have been described. The widely ramifying RF projections can support a large number of functional hypotheses. However, the anatomical and physiological data, considered in the context of the behavioral studies reviewed here, make the motor hypothesis a plausible one. The discussion that follows shows the utility of our hypothesis in integrating previous behavioral findings on RF units.
5. RECONSIDERATION OF BEHAVIORAL CORRELATES OF RF UNIT DISCHARGE FROM STANDPOINT OF OUR BEHAVIORAL HYPOTHESIS
5.1. Sensory stimuli and pain
In the unrestrained behaving cat, only a small proportion of RF unit activity was related to identified sensory stimuli; small spontaneous movements produce far more intense discharge than even the most effective stimuli208. The brief, short latency response to discrete sensory stimuli parallels the motor startle response to such stimuli. Sensory inputs such as somatic stimulation on a particular side of the body or auditory stimulation from a particular point in space might be expected to bring the same groups of muscles into play in orientation responses, thereby resulting in polysensory receptive fields. RF unit responses to thermal stimuli may reflect thermoregulatory motor responses such as postural adjustments and changes in motor tone.
Noxious stimuli and such stimuli as vaginal probing would be expected to elicit vigorous movement (including lordosis responses185) and escape attempts. The temporal relations to the stimulus and the often prolonged discharges are consistent with this explanation. Changes in unit activity lasting for 20 sec after noxious stimuli and ‘wind-up’ phenomenon, which are characterized by progressive increases in the intensity of neuronal response that are not temporarily related to individual presentation of the stimulus, have a time course similar to the expected motor responses to noxious stimuli. Similarly, unit response latencies of up to 3 sec (ref. 7) are difficult to explain as sensory responses but might well relate to a portion of the motor response to the stimulus, such as attempted escape. Since many of these studies were performed in animals in which muscle activity was prevented by Flaxedil, the motor correlates of unit responses could not be observed. However, it is reasonable to assume that a flaxedilized animal would attempt to withdraw a part of the body to which noxious stimulation had been applied and that cells related to such movements would discharge during the attempt. Conversely, procedures that are known to have analgesic effects, such as morphine administration, would be expected to reduce motor response to pain. Therefore, our hypothesis is consistent with findings that RF responses to noxious stimuli are reduced by such procedures. Finally, our observation that all PRF cells fired at their highest rates during spontaneous behaviors208 is inconsistent with a PRF role in pain mechanisms since, by definition, an animal will not voluntarily inflict noxious stimulation on itself. Discharge rates during small movements greatly exceeded discharge in response to strong electric shock.
5.2. Conditioning and habituation
Bell et al.18 have cautioned that although RF units do show phenomena resembling habituation ‘even if one were to record the discharges of a neuron whose responsiveness changes corresponded to those in a behavioral act or EEG shift one might still know little more concerning the genesis of the latter since the recorded unit might simply be in the motor pathway of the event in question’. A similar view has been presented by Groves and Lynch80. Consistent with this idea are findings from an analysis of the time course of RF activity during the learning of an appetitive classically conditioned task110. This study demonstrated that RF unit activity tended to be temporally related to orientation responses.
In our studies208 we report a close relationship between specific movements resulting from a stimulus and the changes in PRF unit activity during repetitive stimulation. For example, two PRF ‘neck dorsoflexion’ cells increased discharge when any object was rapidly brought toward the cat’s eyes. This stimulus elicited a behavioral flinch response. When after 3 or 4 repetitions of the stimulus the flinch response stopped, the unit acceleration also stopped, despite the fact that the cat continued to observe the approaching object. Three of the 19 FTG cells tested showed no response decrement with stimulus repetition. Two of these discharged during tongue extensions which could be induced by pushing the tongue to the rear of the oral cavity. (These ‘tongue cells’ were in the pontine RF, several mm away from the hypoglossal nucleus.) Another unit fired during head shaking which could be triggered by placing a foreign object in either ear. In these three cases the behavioral response to the stimuli also showed no decrement with stimulus repetition. All units which exhibited response decrement with repeated sensory stimulation failed to show rate decrement during repetitive motor activities such as ‘grooming’. The ‘habituation’ seen in PRF cells can be likened to the habituation of response that one can record in a peripheral muscle unit. Existing data on RF activity during conditioning and habituation processes are consistent with the hypothesis that discharge in individual RF cells relates to activity in specific muscle groups.
5.3. Arousal and complex behavioral states
As outlined above, a variety of relations between RF unit activity and complex behavior have been reported. Several of these relationships seem contradictory, such as RF unit relations to both fear and anticipation of reward. The hypothesis of a relationship of RF unit discharge to activity in specific muscle groups is able to reconcile these contradictions. Emotional states in animals are closely correlated with motor activity. It is difficult to imagine fearful or reward anticipation situations that would not correlate with changes in muscle tone, or overt movements.
RF cells related to certain muscle groups might be expected to show better correlations with some complex behavioral states simply because certain muscle groups show better correlations with these states. For example, RF units related to ear flattening or back arching might show somewhat specific relations with fear states, while paw muscle tone might correlate with preparation for operant responses and consummatory behavior. Activity in postural muscles might be expected to correlate with ‘arousal’ but be relatively difficult to correlate with specific movements if only visual observation was employed. However, it would be expected that most muscle groups would show some activity with most active behavioral states. Therefore, one would predict that discharge in most RF cells would correlate with arousal and relaxation and anticipation of reward and fear and fighting behavior, as has been reported.
Multiunit techniques have been used in most studies relating RF activity to arousal. By averaging the activity of several motor units, and by integrating this averaged data, these techniques obscure specific movement relations. While some studies have related integrated MUA to arousal, others have emphasized relations between RF activity and the intensity of movement. In many cases movement relations have been detected with a ‘free wire’ which responds non-specifically to any cable movement, thus making it difficult to detect directionally specific movement relations. The general relations between RF activity and movement are consistent with the hypothesis that discharge in individual RF cells is related to the activation of muscle groups. The hypothesized relation of RF discharge to activity in specific muscle groups provides a framework for encompassing previous results without the necessity for attributing complex properties to these cells.
5.4. REM sleep
RF discharge in REM sleep may be a correlate of the motor activation of this state. Other motor centers also have increased discharge in both REM sleep and waking movement63,112,156. REM sleep motor activation can be seen most dramatically after lesions of the pontomesencephalic tegmentum. These lesions disrupt the peripheral atonia of REM sleep producing an animal which engages in intense motor behavior during a state otherwise resembling REM sleep84,101. The motor behavior of these animals during REM sleep is episodic with quiet periods separating intense movement bursts. In intact animals, periodic twitching of facial and distal limb musculature and eyes are the hallmark of REM sleep, although the full expression of this motor activation is prevented by the postsynaptic inhibition of motor neurons169. In younger animals the contractions are so intense that this state is often labeled active sleep. RF units show burst-pause discharge patterns that are similar in time course to the episodic motor activation of REM sleep. This is clearly seen when one compares the mean 10 sec maximum rate for PRF cells during REM sleep of 63.8 spikes/sec, with their mean 10 sec minimum REM sleep rate of 0.8 spikes/sec. REM sleep discharge rates were positively correlated with rates during waking movement212.
High rates of firing are readily observed in RF cells recorded in unrestrained animals. PRF cells on the average reach 250% of their mean sleep rates during waking212. Thus, high rates of firing in RF cells are not linked uniquely to the REM sleep state and are not sufficient for its triggering. The activity pattern of RF cells across the sleep cycle is not consistent with an executive role for these cells in the triggering of REM sleep but is consistent with the hypothesis of RF relation to the contraction of specific muscle groups.
5.5. Eye movement
The hypothesis that RF activity relates to muscle activity is of course consistent with the findings discussed above that RF activity relates to eye movements. Most eye movement cells have been found to be related to eye movement in specific directions and hence to activity in specific extraocular muscles. A smaller percentage of RF eye movement-related cells do not relate to directionally specific movements. For example, ‘pauser’ cells that cease firing before eye movements in any direction and burster cells that fire before saccades in several directions have been reported120. An important challenge is to determine whether the relation of discharge in such cells is specific to eye movements, and if cells can be found with similar relation to activity in other groups of somatic muscles.
The only substantive difference of interpretation regarding eye movement cells concerns whether or not they are the ‘predominant’ cell type in the feline PRF. In our explorations we found that only a small percentage of RF cells were specifically related to eye movements208,210, although we systematically tested for eye movement relations in every cell we encountered. Most of the eye movement-related cells we found were at the border between the PRF and abducens nuclei. ‘The gigantocellular tegmental field (FTG) units that we identified as head movement cells, the most common cell type, all showed intense discharge without any eye movement. Conversely, rapid eye movements and maintained eye positions in both the horizontal and vertical planes without accompanying unit discharge were observed in each of these cells. Since head movements tend to be associated with eye movements these cells do show a general correlation with eye movements. Cells specifically related to eye movement may exist in the PRF86, but clearly they are not the predominant cell type in the FTG area, which comprises most of the PRF.
‘Several other findings illustrate the lack of relationship between unit activity in most FTG cells and eye movement. (a) During adaptation to head restraint, FTG unit firing decrement correlated closely with decrease in neck electromyogram208,212, not electrooculogram (EOG). (b) Most cells habituated to rapid head acceleration in conjunction with changes in neck muscle tone. However, EOG response to such stimulation does not habituate. (c) Operant conditioning of increased firing rate in those FTG cells which appeared to discharge in relation to head movement was accompanied by repetitive head movements. In no case did we observe a conditioned increase in unit firing correlated only with increased eye movements. (d) During REM sleep many of these cells discharge in long intense bursts. This firing does not result from increased numbers of eye movemens89,207. (e) Many FTG cells were found to be entirely unrelated to head or eye movement. We have observed cells which discharge in close relationship to directionally specific tongue movements. Other cells exhibited activity related to facial musculature and to specific postures207,208. It would be difficult to reconcile such findings with the claim that FTG cells relate predominantly to eye movement.
‘Eye and head movements are normally coordinated and therefore it should not be surprising that eye movement relations have been found in cells related to head and other movements. Indeed, as Bizzi et al.30 have pointed out, neck muscles are activated prior to eye muscles in coordinated movements. Neck muscle activation can also be detected in head-restrained animals. Most studies of eye movement relations in PRF cells have been performed in head-restrained animals. Therefore, correlations between activity in head movement cells and EOG in these preparations might speciously suggest that these cells were triggering eye movements’210 (copyright 1978, AAAS).
It is likely that there are substantial species differences in the proportion of RF cells related to eye movement. Monkeys and man, which explore their visual world with frequent eye movements, would be expected to devote a higher proportion of their RF to this function than cats, which are inclined to use their more mobile heads in visual exploration.
Although they do not appear to be the predominant cell type, eye movement cells clearly do exist in the feline RF. According to our hypothesis we can see the eye movement-related RF cells as a subpopulation of the RF’s muscle-related cells. They appear to be generally localized to dorsomedial RF regions. The precise documentation of RF eye movement relations can serve as a model for the future quantitative study, in unrestrained animals, of motor relations in other RF areas.
5.6. Respiration
Respiration involves not only the use of the diaphragm and intercostal muscles, but also a variety of ‘accessory’ muscles. Accessory musculature can include not only facial, jaw and tongue musculature, but also skeletal musculature moving the head, neck, chest and back. Indeed, many proximal muscle groups are involved in intense respiration. Sophisticated computer techniques for detecting respiratory-related units, applied to flaxedilized animals, allow one to detect respiratory-related units that would be missed by casual visual observations in behaving animals. Thus, the high percentage of respiratory related units in flaxedilized cats is not inconsistent with the absence of reports of such cells in the vast majority of RF unit recordings in behaving animals. RF respiratory related units can be seen as relating to the facilitation of groups of skeletal muscles with primary and accessory respiratory function.
5.7. Locomotion
The discharge of RF cells during locomotion is entirely consistent with what is known about the behavioral relations of these cells in unrestrained cats. Thus one would expect that the large numbers of head movement cells that have been described would discharge rhythmically during the rhythmic activation of muscles facilitating vertical head movements during locomotion. RF cells related to static postural mechanisms206 would be expected to show a non-rhythmic enhancement of discharge during induced locomotion. The reduction in the number of rhythmically activated cells in preparations with cerebellar removal150 could be related to the poorer modulation of locomotion in these ataxic preparations.
6. DISCUSSION
By hypothesizing that most RF cells have the relatively simple functional role of controlling small groups of muscles, I do not mean to imply that they do not have roles in complex behavioral functions. Several investigations have shown that chronically maintained cats and rats with brain stem transections at mesencephalic and even pontine levels exhibit a wide range of behaviors11,16,49,119,242. Chronically maintained decerebrate rats242, and cats decerebrated at a young age29 are able to eat solid foods, lap milk, show auditory reflexes, proprioceptive hopping responses, effective defense reactions with appropriate vocalizations, climbing, and prey pursuit behavior. They showed a high level of spontaneous activity and have nearly normal righting reflexes and locomotion. Clearly the brain stem contains the basic machinery for the control of behavior.
Lesion and stimulation studies support the findings of the transection studies in finding a wide variety of complex behavioral effects as a result of manipulations of the RF. For example, lesions have been reported to produce loss of REM sleep and arousal functions41,100,102, paralysis of eye movement54, analgesia83, deficits in lordosis behavior137, hypokinesis and changes in emotionality221. Conversely, reticular formation stimulation has been reported to enhance REM sleep67,138, and produce eye movements95, escape behavior and EEG arousal45,190, postural and motor reflex changes77,97,220, changes in speed of locomotion225, and increases in specific complex behaviors21-23,133,237. While it is beyond the scope of this paper to review the histological differences between the various lesion and recording sites, it can be said that, as was the case with the unit recording studies, there is a considerable anatomical overlap of the RF areas producing these effects. The main determinant of the experimental outcome seems to be the techniques used and the experimenters’ observational emphasis.
How can we reconcile our hypothesis that individual RF units are related to excitation of small groups of muscles, with the complexity of the behaviors mediated by the brain stem? There are several possible explanations for this apparent paradox.
(1). Complex behaviors are mediated by other brain stem structures.
Lateral reticular structures, the central gray, monoaminergic reticular nuclei such as the raphe and locus coeruleus, cranial nerve nuclei, pontine nuclei and tectal structures have not been discussed. These areas might have more important roles in organizing higher order behavioral functions than medial reticular cells. Another possibility is that RF cells related to ‘complex’ behaviors are located in the medial RF but are rare or are in areas that have not yet been explored.
(2). Complex behaviors are mediated by smaller cells within the RF.
As pointed out above, unit studies of the RF have focused on the larger cells in this region. While large cells are well suited for rapid conduction of neural outputs, smaller cells might be effective in the organization of long behavioral sequences. Electrical stimulation and lesions would affect the activity of these cells while single unit recording studies would tend to miss them. While the sampling problem is perhaps more severe in studies using fine wire techniques, conventional microelectrodes also are subject to sampling bias. The behavioral and physiological correlates of these small cells, and especially any differences between small and large cells, may hold the key to many of the mysteries of RF function. Intracellular staining techniques that allow one to correlate neuronal size and morphology with other measures would be useful in exploring this possibility.
(3). Complex behaviors are emergent properties of nerve nets.
While it is tempting to explain complex behaviors by looking for cells correlated with specific behaviors, this anthropomorphic approach does not necessarily ‘explain’ anything. The extension of the concept of neural ‘centers’ to the single cell, stretches an already tenuous concept beyond the breaking point. In order to understand the neural mechanisms underlying complex behavior, reductionistic principles must be applied.
In 1952 Sperry219 presented a model of complex behavioral control utilizing such principles. His thesis was that the understanding of the neural control of complex behavior could best be achieved if one views the brain as “a mechanism for governing motor activity… overt behavior, upon analysis, we find to be constituted almost entirely of patterns of muscular contraction. It follows that the principal function of the nervous system is the coordinated innervation of the musculature. Its fundamental anatomical plan and working principles are understandable only on these terms… If any scheme or plan at all is evident in the complicated fiber association and nuclear interconnections of the brain, it is a design patterned throughout for governing excitation of the ‘final common (motor) pathways’.”.
When one considers the views of Sperry and other behaviorists, that motor activity is not merely the output of the brain, but is also integrally involved in neural processing, our hypothesis of reticular relation to specific movement patterns no longer seems inconsistent with the behavioral roles of the RF. Neural control need not be mediated by single cells relating primarily to complex behaviors or behavioral states. Instead, it may be seen to be coded in patterns of excitation within the neural networks interconnecting cells, each of which has specific motor correlates. The behaviors that result are emergent properties of the network not manifest in the activity of any of its constituent neurons. This behaviorist view may or may not apply to forebrain areas, but the available evidence does suggest such an interpretation of medial RF functioning.
We hope that our hypothesis, by providing an explicit experimental challenge for RF researchers to meet in distinguishing movement-related unit discharge from more complex behavioral relations, will help stimulate research in this area. The further understanding of the RF will help reveal the operational principals of the brain stem regions which contain the basic mechanisms controlling behavior.
7. GENERAL SUMMARY
Studies of the behavioral correlates of activity in reticular formation cells, usually performed in restrained animals, have found units whose discharge relates to sensory stimuli, pain and escape behavior, conditioning and habituation, arousal, complex motivational states, REM sleep, eye movements, respiration and locomotion. Units with these different behavioral correlates were found in the same anatomical areas. Most studies report that a large proportion of encountered cells related to the behavior being studied. If one adds up the reported percentages, the total far exceeds 100%. Therefore it appears that many investigators are looking at the same cells and reaching very different conclusions about their behavioral roles. On the basis of observations in unrestrained cats, it is hypothesized that discharge in most RF cells is primarily related to the excitation of small groups of muscles. This hypothesis can parsimoniously explain many previous observations on the behavioral correlates of these cells, and is consistent with anatomical, physiological and phylogenetic studies of the reticular formation. The hypothesized simplicity of reticular formation unit function is contrasted with the complexity of the behavioral functions mediated by the RF, and the implications of this contrast discussed.
ACKNOWLEDGEMENTS
I thank Dr. Dennis McGinty for advice, encouragement and many valuable discussions. This work was supported by the Medical Research Service of the Veterans Administration and National Institutes of Health Research Grant NS14610.
Footnotes
Several terms are commonly used to describe portions of the medial brain stem reticular formation (RF). These include bulbar reticular formation (BRF), pontine reticular formation (PRF), paramedian pontine reticular formation (PPRF), nucleus gigantocellularis (NGC), nucleus reticularis parvocellularis, nucleus reticularis pontis caudalis (NRPC), nucleus reticularis pontis oralis (NRPO), midbrain reticular formation (MRF), and gigantocellular tegmental field (FTG). While some of these structures can be histologically distinguished227, sharp divisions between nuclear fields cannot be made195 and in common usage the anatomical locations they describe overlap. Thus, a location that one worker will describe as NRPC, is described as NRPO by a second, NGC by a third, and FTG by a fourth. This has impeded communication and contributed to a lack of integration in some of the RF literature. For this reason, in the following discussion I will attempt where possible to describe cat Horsley–Clarke (H.C.) coordinates, or anatomical landmarks, locating each of the areas studied.
REFERENCES
- 1.Adams DB, The activity of single cells in the midbrain and hypothalamus of the cat during affective defense behavior, Arch. ital. Biol, 106 (1968) 243–269. [PubMed] [Google Scholar]
- 2.Adams DB, Cells related to fighting behavior recorded from mid-brain central grey neuropil of cat, Science, 159 (1968) 894–896. [DOI] [PubMed] [Google Scholar]
- 3.Amassian VE and Devito RV, Unit activity in reticular formation and nearby structures, J. Neurophysiol, 17 (1954) 575–603. [DOI] [PubMed] [Google Scholar]
- 4.Anderson ME, Yoshida M and Wilson VS, Tectal and tegmental influences on cat forelimb and hindlimb motorneurons, J. Neurophysiol, 35 (1972) 462–470. [DOI] [PubMed] [Google Scholar]
- 5.Appleberg B and Jenskog T, Mesencephalic fusimotor control, Exp. Brain Res, 15 (1972) 97–112. [DOI] [PubMed] [Google Scholar]
- 6.Azzena GB, Giretti ML and Deriu PL, Convergence of labyrinthine and cerebral nystag-mogenic impulses on brain stem units, Exp. Neurol, 19 (1967) 401–411. [DOI] [PubMed] [Google Scholar]
- 7.Bach-y-Rita P, Convergent and long-latency unit responses in the reticular formation of the cat, Exp. Neurol, 9 (1964) 327–344. [DOI] [PubMed] [Google Scholar]
- 8.Baker R and Berthoz A (Eds.), Control of Gaze by Brain Stem Neurons, Developments in Neuroscience, Vol. 1, Elsevier/North-Holland Biomedical Press, Amsterdam, 1977, 510 pp. [Google Scholar]
- 9.Balzano E and Jeannerod M, Activite multi-unitaire de structures sous-corticales pendant le cycle veille-sommeil chez le chat, Electroenceph. clin. Neurophysiol, 28 (1970) 136–145. [DOI] [PubMed] [Google Scholar]
- 10.Bambridge B and Gijsbers K, The role of tonic neural activity in motivational processes, Exp. Neurol, 56 (1977) 370–385. [DOI] [PubMed] [Google Scholar]
- 11.Bard P and Macht MB, The behavior of chronically decerebrate cats. In Wolstenholme GEW and O’Connor CM (Eds.), Neurological Basis of Behavior, Churchill, London, 1958, pp. 55–75. [Google Scholar]
- 12.Barnes KL, A quantitative investigation of somatosensory coding in single cells of the cat mesencephalic reticular formation, Exp. Neurol, 50 (1976) 180–193. [DOI] [PubMed] [Google Scholar]
- 13.Basbaum AI, Clanton Ch. H. and Fields HL, Three bulbospinal pathways from the rostral medulla of the cat: an autoradiographic study of pain modulating systems, J. comp. Neurol, 178 (1978) 209–224. [DOI] [PubMed] [Google Scholar]
- 14.Batini C, Magni F, Palestini M, Rossi GF and Zanchetti A, Neural mechanisms underlying the enduring EEG and behavior activation in the midpontine pretrigeminal cat, Arch. ital. Biol, 97 (1959)13–25. [Google Scholar]
- 15.Batini C, Moruzzi G, Palestini M, Rossi GF and Zanchetti A, Persistent patterns of wakefulness in the pretrigeminal midpontine preparation, Science, 128 (1958) 30–32. [DOI] [PubMed] [Google Scholar]
- 16.Bazett HC and Penfield WG, A study of the Sherrington decerebrate animal in the chronic as well as the acute condition, Brain, 45 (1922) 185–265. [Google Scholar]
- 17.Becker DP, Gluck H, Nulsen FE and Jane JA, An inquiry into the neurophysiological basis for pain, J. Neurosurg, 30 (1969) 1–13. [DOI] [PubMed] [Google Scholar]
- 18.Bell C, Sierra G, Buendia N and Segundo JP, Sensory properties of neurons in the mesencephalic reticular formation, J. Neurophysiol, 27 (1964) 961–997. [DOI] [PubMed] [Google Scholar]
- 19.Bentivoglio M, Macchi G, Rossini P and Tempesta E, Brain stem neurons projecting to neocortex: a HRP study in the cat, Exp. Brain Res, 31 (1978) 489–498. [DOI] [PubMed] [Google Scholar]
- 20.Berman AL, The Brain Stem of the Cat, University of Wisconsin Press, Madison, Wisc., 1968, 175 pp. [Google Scholar]
- 21.Berntson GG, Attack, grooming, and threat elicited by stimulation of the pontine tegmentum in cats, Physiol. Behav, 11 (1973) 81–87. [DOI] [PubMed] [Google Scholar]
- 22.Berntson GG and Hughes HC, Medullary mechanisms for eating and grooming behaviors in the cat, Exp. Neurol, 44 (1974) 255–265. [DOI] [PubMed] [Google Scholar]
- 23.Berntson GG and Micco DJ, Organization of brainstem behavioral systems, Brain Res. Bull, 1 (1976) 471–483. [DOI] [PubMed] [Google Scholar]
- 24.Bertrand F and Hugelin A, Respiratory synchronizing function of nucleus parabrachialis medialis: pneumotaxic mechanisms, J. Neurophysiol, 34 (1971) 189–207. [DOI] [PubMed] [Google Scholar]
- 25.Bertrand F, Hugelin A and Vibert JF, Quantitative study of anatomical distribution of respiration related neurons in the pons, Exp. Brain Res, 16 (1973) 383–399. [DOI] [PubMed] [Google Scholar]
- 26.Besson JM, Guilbaud G and Lombard MC, Effects of bradykinin intra-arterial injection into the limbs upon bulbar and reticular unit activity, Advanc. Neurol, 4 (1974) 207–215. [Google Scholar]
- 27.Best PJ, Mays LE and Olmstead CE, Activity of midbrain reticular formation units during conditioned freezing. In Phillips MI (Ed.), Brain Unit Activity During Behavior, Charles C. Thomas, 1973, pp. 155–164. [Google Scholar]
- 28.Beyer C, Almanza J, de la Torre L and Guzman-Flores C, Brain stem multi-unit activity during ‘relaxation’ behavior in the female cat, Brain Research, 29 (1971) 213–222. [DOI] [PubMed] [Google Scholar]
- 29.Bignall KE and Schramm L, Behavior of chronically decerebrated kittens, Exp. Neurol, 42 (1974) 519–531. [DOI] [PubMed] [Google Scholar]
- 30.Bizzi E, Kalil RE and Tagliasco V, Eye–head coordination in monkeys: evidence for centrally patterned organization, Science, 173 (1971) 452–454. [DOI] [PubMed] [Google Scholar]
- 31.Bowsher D, Mallart A, Petit D and Albe-Fessard D, A bulbar relay to the centre median, J. Neurophysiol, 31 (1968) 288–300. [DOI] [PubMed] [Google Scholar]
- 32.Bowsher D and Westman J, The gigantocellular reticular region and its spinal afferents: a light and electron microscope study in the cat, J. Anat. (Lond.), 106 (1970) 23–36. [PMC free article] [PubMed] [Google Scholar]
- 33.Bradley WE and Conway CJ, Bladder representation in the pontine-mesencephalic reticular formation, Exp. Neurol, 16 (1966) 237–249. [DOI] [PubMed] [Google Scholar]
- 34.Breedlove SM, McGinty DJ and Siegel JM, Operant conditioning of FTG units, Physiol. Behav, (1979) in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brodal A, Reticulo-cerebellar connections in the cat, J. comp. Neurol, 98 (1953) 113–153. [DOI] [PubMed] [Google Scholar]
- 36.Brodal A, Neurological Anatomy in Relation to Clinical Medicine, Oxford University Press, London, 1969, 807 pp. [Google Scholar]
- 37.Buchwald JS, Halas ES and Schramm S, Changes in cortical and subcortical unit activity during behavioral conditioning, Physiol. Behav, 1 (1966) 11–22. [Google Scholar]
- 38.Burton H, Somatic sensory properties of caudal bulbar reticular neurons in the cat (Felis domestica), Brain Research, 11 (1968) 357–372. [DOI] [PubMed] [Google Scholar]
- 39.Buttner U, Buttner-Ennever JA and Henn V, Vertical eye movement related unit activity in the rostral mesencephalic reticular formation of the alert monkey, Brain Research, 130 (1977) 239–252. [DOI] [PubMed] [Google Scholar]
- 40.Candia O, Rossi GF and Sekino T, Brain stem structures responsible for the electroencephalo-graphic patterns of desynchronized sleep, Science, 155 (1967) 720–722. [DOI] [PubMed] [Google Scholar]
- 41.Carli G and Zanchetti A, A study of pontine lesions suppressing deep sleep in the cat, Arch. ital. Biol, 103 (1965) 751–788. [PubMed] [Google Scholar]
- 42.Casey KL, Somatic stimuli, spinal pathways, and size of cutaneous fibers influencing unit activity in the medial medullary reticular formation, Exp. Neurol, 25 (1969) 35–56. [DOI] [PubMed] [Google Scholar]
- 43.Casey KL, Somatosensory responses of bulboreticular units in awake cat: relation to escape-producing stimuli, Science, 173 (1971) 77–80. [DOI] [PubMed] [Google Scholar]
- 44.Casey KL, Responses of bulboreticular units to somatic stimuli eliciting escape behavior in the cat, Int. J. Neurosci, 2 (1971) 15–28. [DOI] [PubMed] [Google Scholar]
- 45.Casey KL, Escape elicited by bulboreticular stimulation in the cat, Int. J. Neurosci, 2 (1971) 29–34. [DOI] [PubMed] [Google Scholar]
- 46.Casey KL and Keene JJ, Unit analysis of the effects of motivating stimuli in the awake animal: pain and self stimulation. In Phillips MI (Ed.), Brain Unit Activity During Behavior, C. C. Thomas, 1973, pp. 115–129. [Google Scholar]
- 47.Casey KL, Keene JJ and Morrow T, Bulboreticular and medial thalamic unit activity in relation to aversive behavior and pain, Advanc. Neurol, 4 (1974) 197–205. [Google Scholar]
- 48.Castiglioni AJ, Gallaway MC and Coulter JD, Spinal projections from the midbrain in monkeys, J. comp. Neurol, 178 (1978) 329–346. [DOI] [PubMed] [Google Scholar]
- 49.Chambers WW, Seigel MS, Liu JC and Liu CN, Thermoregulatory responses of decerebrate and spinal cats, Exp. Neurol, 42 (1974) 282–299. [DOI] [PubMed] [Google Scholar]
- 50.Chatelier G and Leitner LM, Localisation d’unites synchrones du nystagmus dans le rhombencephale du lapin, J. Physiol. (Paris), 54 (1962) 312. [PubMed] [Google Scholar]
- 51.Cohen B, Pontine reticular formation neurons and motor activity, Science, 199 (1978) 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen B and Henn V, Unit activity in the pontine reticular formation associated with eye movements, Brain Research, 46 (1972) 403–410. [DOI] [PubMed] [Google Scholar]
- 53.Cohen B and Henn V, The origin of quick phases of nystagmus in the horizontal plane, Bibl. Ophthal. (Basel), 82 (1972) 36–55. [PubMed] [Google Scholar]
- 54.Cohen B, Komatsuzaki A and Bender MB, Electrooculographic syndrome in monkeys after pontine reticular formation lesions, Arch. Neurol. (Chic.), 18 (1968) 78–92. [DOI] [PubMed] [Google Scholar]
- 55.Cronin MJ and Baker MA, Thermosensitive neurons in the cat, Brain Research, 128 (1977) 461–472. [DOI] [PubMed] [Google Scholar]
- 56.Diamond J, The Mauthner cell. In Hoar WW and Randall DJ (Eds.), Fish Physiology, Vol. 5, Academic Press, New York, 1971, pp. 265–346. [Google Scholar]
- 57.Drucker-Colin RR, Bernal-Pedraza JG, Diaz-Mitoma FJ and Zamora-Quezada J, Oscillatory changes in multiple unit activity during rapid eye movement sleep, Exp. Neurol, 57 (1977) 331–341. [DOI] [PubMed] [Google Scholar]
- 58.Duensing F and Schaffer KP, Die neuronenaktivität in der formatio reticularis des rhombencephalons beim vestibularen nystagmus, Arch. Psychiat, 196 (1957) 265–290. [DOI] [PubMed] [Google Scholar]
- 59.Eccles JC, Nicoll RA, Rantucci T, Taborikova H and Willey TJ, Topographic studies on medial reticular nucleus, J. Neurophysiol, 39 (1976) 109–118. [DOI] [PubMed] [Google Scholar]
- 60.Eccles JC, Nicoll RA, Taborikova H and Willey TJ, Medial reticular neurons projecting rostrally, J. Neurophysiol, 38 (1975) 531–538. [DOI] [PubMed] [Google Scholar]
- 61.Engberg I, Lundberg A and Ryall RW, Reticulospinal inhibition of transmission in reflex pathways, J. Physiol. (Lond.), 194 (1968) 201–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Engberg I, Lundberg A and Ryall RW, Reticulospinal inhibition of interneurons, J. Physiol. (Lond.), 194 (1968) 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Evarts EV, Temporal patterns of discharge of pyramidal tract neurons during sleep and waking in the monkey, J. Neurophysiol, 27 (1964) 152–171. [DOI] [PubMed] [Google Scholar]
- 64.Faber DS and Korn H (Eds.), Neurbiology of the Mauthner Cell, Raven Press, New York, 1978, 290 pp. [Google Scholar]
- 65.Faingold CL and Caspary DM, Changes in reticular formation unit response patterns associated with pentylenetetrazol-induced enhancement of sensory evoked responses, Neuropharmacology, 16 (1977) 143–147. [DOI] [PubMed] [Google Scholar]
- 66.Fields HL, Clanton CH and Anderson SD, Somatosensory properties of spinoreticular neurons in the cat, Brain Research, 120 (1977) 49–66. [DOI] [PubMed] [Google Scholar]
- 67.Frederickson CJ and Hobson JA, Electrical stimulation of the brain stem and subsequent sleep, Arch. ital. Biol, 108 (1970) 564–576. [PubMed] [Google Scholar]
- 68.Fuchs AF and Luschei ES, Unit activity in the brainstem related to eye movement: possible inputs to the motor nuclei, Bibl. Ophthal, (Basel), 82 (1972) 17–27. [PubMed] [Google Scholar]
- 69.Fuller JH, Brain stem reticular units: some properties of the course and origin of the ascending trajectory, Brain Research, 83 (1975) 349–367. [DOI] [PubMed] [Google Scholar]
- 70.Gallager DW and Pert A, Afferents to brain stem nuclei (brain stem raphe, nucleus reticularis pontis caudalis and nucleus gigantocellularis) in the rat as demonstrated by microiontophoretically applied horseradish peroxidase, Brain Research, 144 (1978) 257–275. [DOI] [PubMed] [Google Scholar]
- 71.Gijsbers KJ and Melzack R, Multiunit changes in the visual system of the freely moving cat, Exp. Neurol, 35 (1972) 165–178. [DOI] [PubMed] [Google Scholar]
- 72.Glickstein M, Stein J and King RA, Visual input to the pontine nuclei, Science, 178 (1972) 1110–1111. [DOI] [PubMed] [Google Scholar]
- 73.Gogan P, Gueritaud JP, Horcholle-Bossavit G and Tyc-Dumont S, Inhibitory nystagmic interneurons. Physiological and anatomical identification within the abducens nucleus, Brain Research, 59 (1973) 410–416. [DOI] [PubMed] [Google Scholar]
- 74.Goodman JS and Mann PEG, Reticular and thalamic multiple unit activity during wakefulness, sleep, and anesthesia, Exp. Neurol, 19 (1967) 11–24. [DOI] [PubMed] [Google Scholar]
- 75.Graybriel AM, Direct and indirect preoculomotor pathways of the brainstem: an autoradiographic study of the pontine reticular formation in the cat, J. comp. Neurol, 175 (1977) 37–78. [DOI] [PubMed] [Google Scholar]
- 76.Grillner S, Locomotion in vertebrates: central mechanisms and reflex interaction, Physiol Rev., 55 (1975) 247–304. [DOI] [PubMed] [Google Scholar]
- 77.Grillner S and Lund S, A descending pathway with monosynaptic action on flexor motorneurons, Experientia (Basel), 22 (1966) 390. [DOI] [PubMed] [Google Scholar]
- 78.Grillner S and Lund S, The origin of a descending pathway with monosynaptic action on flexor motorneurons, Acta physiol. scand, 74 (1968) 274–284. [DOI] [PubMed] [Google Scholar]
- 79.Grillner S and Shik ML, On the descending control of the lumbosacral spinal cord from the ‘mesencephalic locomotor region’, Acta physiol. scand, 87 (1973) 320–333. [DOI] [PubMed] [Google Scholar]
- 80.Groves PM and Lynch GS, Mechanisms of habituation in the brain stem, Psychol. Rev, 79 (1972) 237–244. [DOI] [PubMed] [Google Scholar]
- 81.Groves PM, Miller SW and Parker MV, Habituation and sensitization of neuronal activity in the reticular formation of the rat, Physiol. Behav, 8 (1972) 589–593. [DOI] [PubMed] [Google Scholar]
- 82.Groves PM, Miller SW, Parker MV and Rebec GV, Organization by sensory modality in the reticular formation of the rat, Brain Research, 54 (1973) 207–224. [DOI] [PubMed] [Google Scholar]
- 83.Halpern BP and Halverson JD, Modification of escape from noxious stimuli after bulbar reticular formation lesions, Behav. Biol, 11 (1974) 215–229. [DOI] [PubMed] [Google Scholar]
- 84.Henley K and Morrison AR, A re-evaluation of the effects of lesions of the pontine tegmentum and locus coeruleus on phenomena of paradoxical sleep in the cat, Acta neurobiol. exp, 34 (1974) 215–232. [PubMed] [Google Scholar]
- 85.Henn V and Cohen B, Coding of information about rapid eye movements in the pontine reticular formation of alert monkeys, Brain Research, 108 (1976) 307–325. [DOI] [PubMed] [Google Scholar]
- 86.Hikosaka O and Kawakami T, Inhibitory reticular neurons related to the quick phase of vestibular nystagmus — their location and projection, Exp. Brain Res, 27 (1977) 377–396. [DOI] [PubMed] [Google Scholar]
- 87.Hirano T, Best P and Olds J, Units during habituation, discrimination learning and extinction, Electroenceph. clin. Neurophysiol, 28 (1970) 127–135. [DOI] [PubMed] [Google Scholar]
- 88.Hobson JA, The effects of chronic brain stem lesions on cortical and muscular activity during sleep and waking in the cat, Electroenceph. clin. Neurophysiol, 19 (1965) 41–62. [DOI] [PubMed] [Google Scholar]
- 89.Hobson JA, McCarley RW, Pivik T and Freedman R, Selective firing by cat pontine brain stem neurons in desynchronized sleep, J. Neurophysiol, 37 (1974) 497–511. [DOI] [PubMed] [Google Scholar]
- 90.Hornby JB and Rose JD, Responses of caudal brainstem neurons to vaginal and somatosensory stimulation in the rat and evidence of genital nociceptive interactions, Exp. Neurol, 51 (1976) 363–376. [DOI] [PubMed] [Google Scholar]
- 91.Hoshino K and Pompeiano O, Selective discharge of pontine neurons during the postural atonia produced by an anticholinesterase in the decerebrate cat, Arch. ital. Biol, 114 (1976) 244–277. [PubMed] [Google Scholar]
- 92.Hoshino K, Pompeiano O, Magherini PC and Mergner T, Oscillatory activity of pontine neurons related to the regular occurrence of REM bursts in the decerebrate cat, Brain Research, 116 (1976) 125–130. [DOI] [PubMed] [Google Scholar]
- 93.Hoshino K, Pompeiano O, Magherini PC and Mergner T, The oscillatory system responsible for the oculomotor activity during the bursts of REM, Arch. ital. Biol, 114 (1976) 278–309. [PubMed] [Google Scholar]
- 94.Huttenlocher PR, Evoked and spontaneous activity in single units of medial brain stem during natural sleep and waking, J. Neurophysiol, 24 (1961) 451–468. [Google Scholar]
- 95.Hyde JE and Eliasson SG, Brainstem induced eye movements in cats, J. comp. Neurol, 108 (1957) 139–172. [DOI] [PubMed] [Google Scholar]
- 96.Ingle D and Sprague SM, Sensorimotor function of the midbrain tectum, Neurosci. Res. Progr. Bull, 13 (1975) 169–288. [PubMed] [Google Scholar]
- 97.Ingram WR, Ranson SW, Hannett FI, Zeiss FR and Terwilliger EH, Results of stimulation of the tegmentum with the Horsley-Clark stereotaxic apparatus, Arch. Neurol. Psychiat. (Chic.), 28 (1932) 513–541. [Google Scholar]
- 98.Ito M, Udo M and Mano N, Long inhibitory and excitatory pathways converging onto cat reticular and deiters neurons and their relevance to reticulofugal axons, J. Neurophysiol, 33 (1970) 210–226. [DOI] [PubMed] [Google Scholar]
- 99.Jankowsky E, Lund S, Lundberg A and Pompeiano O, Inhibitory effects evoked through ventral reticulo spinal pathways, Arch. ital. Biol, 106 (1968) 124–140. [PubMed] [Google Scholar]
- 100.Jouvet M, Recherches sur les structures nerveuses et les méchanismes responables des différentes phases du sommeil physiologique, Arch. ital. Biol, 100 (1962) 125–206. [PubMed] [Google Scholar]
- 101.Jouvet M and Delorme F, Locus coeruleus et sommeil paradoxal, C. R. Soc. Biol. (Paris), 159 (1965) 895–899. [Google Scholar]
- 102.Jouvet M and Mounier D, Effects des lésions de la formation réticulée pontique sur le sommeil de chat, C.R. Soc. Biol. (Paris), 154 (1960) 2301–2305. [PubMed] [Google Scholar]
- 103.Kasamatsu T, Maintained and evoked unit activity in the mesencephalic reticular formation of the freely moving cat, Exp. Neurol, 28 (1970) 450–470. [DOI] [PubMed] [Google Scholar]
- 104.Kashin SM, Feldman AG and Orlovsky GN, Locomotion of fish evoked by electrical stimulation of the brain, Brain Research, 82 (1974) 41–47. [DOI] [PubMed] [Google Scholar]
- 105.Kawamura K, Brodal A and Hoddevik G, The projection of the superior colliculus onto the reticular formation of the brainstem: an experimental anatomical study in the cat, Exp. Brain Res, 19 (1974) 1–19. [DOI] [PubMed] [Google Scholar]
- 106.Keller EL, Participation of medial pontine reticular formation in eye movement generation in monkey, J. Neurophysiol, 37 (1974) 316–332. [DOI] [PubMed] [Google Scholar]
- 107.Kneisley LW, Biber MP, and La Vail JH, A study of the origin of brain stem projections to monkey spinal cord using the retrograde transport system, Exp. Neurol, 60 (1978) 116–139. [DOI] [PubMed] [Google Scholar]
- 108.Komisaruk BR and Olds J, Neuronal correlates of behavior in freely moving rats, Science, 161 (1968) 810–812. [DOI] [PubMed] [Google Scholar]
- 109.Koranyi L, Whitmoyer DI and Sawyer CH, Effect of thyrotropin-releasing hormone, lutenizing hormone-releasing hormone and somatostatin on neuronal activity of brain stem reticular formation and hippocampus in the female rat, Exp. Neurol, 57 (1977) 807–816. [DOI] [PubMed] [Google Scholar]
- 110.Kornblith C and Olds J, Unit activity in the brain stem reticular formation of the rat during learning, J. Neurophysiol, 36 (1973) 489–501. [DOI] [PubMed] [Google Scholar]
- 111.Kubo T, Matsunaga T and Yasumasa H, Convergence of visual and vestibular inputs on pontine reticular formation of the rabbit, Brain Research, 147 (1978) 177–182. [DOI] [PubMed] [Google Scholar]
- 112.Lamarre Y, Filion M and Cordeau JP, Neuronal discharges of the ventrolateral nucleus of the thalamus during sleep and wakefulness in the cat. I. Spontaneous activity, Exp. Brain Res, 12 (1971) 480–498. [DOI] [PubMed] [Google Scholar]
- 113.Le Blanc HJ and Gatipon GB, Medial bulboreticular response to peripherally applied noxious stimuli, Exp. Neurol, 42 (1974) 264–273. [DOI] [PubMed] [Google Scholar]
- 114.Lee HK and Chai CY, Temperature-sensitive neurons in the medulla oblongata of the cat, Brain Research, 104 (1976) 163–165. [DOI] [PubMed] [Google Scholar]
- 115.Lee HK, Chai CY, Chung PM and Chen CC, Medullary unit responses to changes in local and hypothalamic temperatures in the cat, Brain Res. Bull, 2 (1977) 375–380. [DOI] [PubMed] [Google Scholar]
- 116.Le Moal M and Cardo B, Ondes lentes rhythmiques et activité multi-unitaire dans de tegmentum ventral pendant le cycle veille-sommeil chez le rat, Electroenceph. clin. Neurophysiol, 39 (1975) 183–192. [DOI] [PubMed] [Google Scholar]
- 117.Llinas R and Terzuolo CA, Mechanisms of supraspinal actions upon spinal cord activities. Reticular inhibitory mechanisms on alpha-extensor motoneurons, J. Neurophysiol, 27 (1964) 579–591. [DOI] [PubMed] [Google Scholar]
- 118.Llinas R, and Terzuolo CA, Mechanisms of supraspinal actions upon spinal cord activities. Reticular inhibitory mechanisms upon flexor motoneurons, J. Neurophysiol, 28 (1965) 413–422. [DOI] [PubMed] [Google Scholar]
- 119.Lovick TA, The behavioral repertoire of precolliculal decerebrate rats, Proc. Physiol. Soc, 226 (1972) 4–6. [PubMed] [Google Scholar]
- 120.Luschei ES and Fuchs AF, Activity of brain stem neurons during eye movements of alert monkeys, J. Neurophysiol, 35 (1972) 445–461. [DOI] [PubMed] [Google Scholar]
- 121.Machne X, Calma I and Magoun WH, Unit activity of central cephalic brain stem in EEG arousal, J. Neurophysiol, 18 (1955) 547–558. [DOI] [PubMed] [Google Scholar]
- 122.Maciewicz RJ, Eagen K, Kaneko CRS and Highstein SM, Vestibular and medullary brain stem afferents to the abducens nucleus in the cat, Brain Research, 123 (1977) 229–240. [DOI] [PubMed] [Google Scholar]
- 123.Magni F and Willis WD, Identification of reticular formation neurons by intracellular recording, Arch. ital. Biol, 101 (1963) 681–702. [PubMed] [Google Scholar]
- 124.Magoun HW and Rhines R, An inhibitory mechanism in the bulbar reticular formation, J. Neurophysiol, 9 (1946) 165–171. [DOI] [PubMed] [Google Scholar]
- 125.Malmo RB, Activation: a neuropsychological dimension, Psychol. Rev, 66 (1959) 367–386. [DOI] [PubMed] [Google Scholar]
- 126.Malmo HP and Malmo RB, Movement-related forebrain and midbrain multiple unit activity in rats, Electroenceph. clin. Neurophysiol, 42 (1977) 501–509. [DOI] [PubMed] [Google Scholar]
- 127.Mancia M, Mechelse K and Mollica A, Microelectrode recording from midbrain reticular formation in the decerebrate cat, Arch. ital. Biol, 95 (1957) 110–119. [Google Scholar]
- 128.Manohar S, Noda H and Adey WR, Behavior of mesencephalic reticular neurons in sleep and wakefulness, Exp. Neurol, 34 (1972) 140–157. [DOI] [PubMed] [Google Scholar]
- 129.Markand ON and Dyken ML, Sleep abnormalities in patients with brain stem lesions, Neurology (Minneap.), 26 (1976) 769–776. [DOI] [PubMed] [Google Scholar]
- 130.McCarley RW and Hobson JA, Single neuron activity in cat gigantocellular tegmental field: selectivity of discharge in desynchronized sleep, Science, 174 (1971) 1250–1252. [DOI] [PubMed] [Google Scholar]
- 131.McGinty DJ, Harper RM and Fairbanks MK, Neuronal unit activity and the control of sleep states. In Advanc. in Sleep Res, Vol. 1, 1974, pp. 173–216. [Google Scholar]
- 132.McGinty DJ and Siegel JM, Neuronal activity patterns during rapid eye movement sleep: relation to waking patterns. In Drucker-Colin RR and McGaugh JL (Eds.), Neurobiology of Sleep and Memory, Academic Press, 1977, pp. 135–158. [Google Scholar]
- 133.Micco DS, Complex behaviors elicited by stimulation of the dorsal pontine tegmentum in rats, Brain Research, 75 (1974) 172–176. [DOI] [PubMed] [Google Scholar]
- 134.Mink WD, Best PJ and Olds J, Neurons in paradoxical sleep and motivated behavior, Science, 158 (1967) 1335–1337. [DOI] [PubMed] [Google Scholar]
- 135.Mitler M and Dement W, Catalectic-like behavior in cats after micro-injection of carbachol in the pontine reticular formation, Brain Research, 68 (1974) 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mizuno N, Sauerland EK and Clemente CD, Projections from the orbital gyrus in the cat. I. To brain stem structures, J. comp. Neurol, 133 (1968) 463–476. [DOI] [PubMed] [Google Scholar]
- 137.Modianos DT and Pfaff O, Brain stem and cerebellar lesions in female rats. II. Lordosis reflex, Brain Research, 106 (1976) 47–56. [DOI] [PubMed] [Google Scholar]
- 138.Monti JM, Effect of recurrent stimulation of the brain stem reticular formation on REM sleep in cats, Exp. Neurol, 28 (1970) 484–493. [DOI] [PubMed] [Google Scholar]
- 139.Moruzzi G and Magoun HW, Brain stem reticular formation and activation of the EEG, Electroenceph. clin. Neurophysiol, 1 (1949) 455–473. [PubMed] [Google Scholar]
- 140.Motokizawa F, Electrophysiological studies of olfactory projection to the mesencephalic reticular formation, Exp. Neurol, 44 (1974) 135–144. [DOI] [PubMed] [Google Scholar]
- 141.Nakamura Y, Goldberg LJ, Chandler SH and Chase MH, Intracellular analysis of trigeminal motoneuron activity during sleep in the cat, Science, 199 (1978) 204–207. [DOI] [PubMed] [Google Scholar]
- 142.Netick A, Orem J and Dement W, Neuronal activity specific to REM sleep and its relationship to breathing, Brain Research, 120 (1977) 197–207. [DOI] [PubMed] [Google Scholar]
- 143.Newman PP and Wolstencroft JH, Medullary responses to stimulation of orbital cortex, J. Neurophysiol, 22 (1959) 516–523. [DOI] [PubMed] [Google Scholar]
- 144.Nyberg-Hansen R, Sites and mode of termination of reticulo-spinal fibers in the cat: an experimental study with silver impregnation methods, J. comp. Neurol, 124 (1965) 71–100. [DOI] [PubMed] [Google Scholar]
- 145.Nyquist JK and Greenhoot JH, A single neuron analysis of mesencephalic reticular formation responses to high intensity cutaneous input in cats, Brain Research, 70 (1974) 157–164. [DOI] [PubMed] [Google Scholar]
- 146.Olds J, Mink WD and Best PJ, Single unit patterns during anticipatory behavior, Electroenceph. clin. Neurophysiol, 26 (1969) 144–158. [DOI] [PubMed] [Google Scholar]
- 147.Olds J, Disterhoft JF, Segal M, Kornblith CL and Hirsh R, Learning centers of rat brain mapped by measuring latencies of conditioned unit responses. In Phillips MI (Ed.), Brain Unit Activity During Behavior, Charles C. Thomas, 1973, pp. 314–346. [Google Scholar]
- 148.Orlovskii GN, Connexions of the reticulospinal neurones with the ‘locomotor sections’ of the brain stem, Biophysics, 15 (1970) 178–187. [Google Scholar]
- 149.Orlovskii GN, Work of the reticulo-spinal neurones during locomotion., Biophysics, 15 (1970) 761–771. [PubMed] [Google Scholar]
- 150.Orlovskii GN, Influence of the cerebellum on the reticulo-spinal neurons during locomotion, Biophysics, 15 (1970) 928–936. [Google Scholar]
- 151.Orlovskii GN and Pavlova GA, Vestibular responses of neurons of the descending tracts during locomotion, Neurophysiology, 4 (1972) 241–245. [PubMed] [Google Scholar]
- 152.Pavlasek J, Gokin AP and Duda P, Visceral pain: responses of the reticular formation neurons to gallbladder distension, J. Physiol. (Paris), 73 (1977) 335–346. [PubMed] [Google Scholar]
- 153.Pearl GS and Anderson KV, Effect of high-frequency peripheral nerve and dorsal column stimulation on neuronal responses in feline nucleus reticularis gigantocellularis after nociceptive electrical stimulation, Exp. Neurol, 57 (1977) 307–321. [DOI] [PubMed] [Google Scholar]
- 154.Pearl GS and Anderson KV, Response patterns of cells in the feline caudal nucleus reticularis gigantocellularis after noxious trigeminal and spinal stimulation, Exp. Neurol, 58 (1978) 231–241. [DOI] [PubMed] [Google Scholar]
- 155.Pearson R and Pearson L, The Vertebrate Brain, Academic Press, London, 1976, 744 pp. [Google Scholar]
- 156.Pellet J, Tardy MF, Harlay F, Dubrocard S and Gilhodes JC, Activite spontanée des céllules de Purkinje chez le chat chronique: étude statistique des spikes complexes, Brain Research, 81 (1974) 75–96. [DOI] [PubMed] [Google Scholar]
- 157.Peter RE, Effects of midbrain tegmentum and diencephalon lesions on swimming and body orientation in goldfish, Exp. Neurol, 57 (1977) 922–927. [DOI] [PubMed] [Google Scholar]
- 158.Peterson BW and Abzug C, Properties of projections from vestibular nuclei to medial reticular formation in the cat, J. Neurophysiol, 38 (1975) 1421–1435. [DOI] [PubMed] [Google Scholar]
- 159.Peterson BW, Anderson ME and Filion M, Responses of pontomedullary reticular neurons to cortical, tectal, and cutaneous stimuli, Exp. Brain Res, 21 (1974) 19–44. [DOI] [PubMed] [Google Scholar]
- 160.Peterson BW, Filion M, Felpel LP and Abzug C, Responses of medial reticular neurons to stimulation of the vestibular nerve, Exp. Brain Res, 22 (1975) 335–350. [Google Scholar]
- 161.Peterson BW, Franck JI, Pitts NG and Daunton NG, Changes in responses of medial pontomedullary reticular neurons during repetitive cutaneous, vestibular, cortical, and tectal stimulation, J. Neurophysiol, 39 (1976) 564–581. [DOI] [PubMed] [Google Scholar]
- 162.Peterson BW, Maunz RA, Pitts NG and Mackel RG, Patterns of projection and branching of reticulospinal neurons, Exp. Brain Res, 23 (1975) 333–351. [DOI] [PubMed] [Google Scholar]
- 163.Peterson BW, Pitts NG, Fukushima K and Mackel RG, Reticulospinal excitation and inhibition of neck motoneurons, Exp. Brain Res, 32 (1978) 471–489. [DOI] [PubMed] [Google Scholar]
- 164.Petras JM, Cortical, tectal and tegmental fiber connections in the spinal cord of the cat, Brain Research, 6 (1967) 275–324. [DOI] [PubMed] [Google Scholar]
- 165.Phillips MI and Olds J, Unit activity: motivation-dependent responses from midbrain neurons, Science, 165 (1969) 1269–1271. [DOI] [PubMed] [Google Scholar]
- 166.Pitts NG, Fukushima K and Peterson BW, Reticulospinal action on cervical, thoracic and lumbar motorneurons, Neurosci. Abstr, 3 (1977) 276. [Google Scholar]
- 167.Pivik RT, McCarley RW and Hobson JA, Eye movement associated discharge in brain stem neurons during desynchronized sleep, Brain Research, 121 (1977) 59–76. [DOI] [PubMed] [Google Scholar]
- 168.Podvoll EM and Goodman SJ, Averaged neural electrical activity and arousal, Science, 155 (1967) 223–225. [DOI] [PubMed] [Google Scholar]
- 169.Pompeiano O, Mechanisms of sensorimotor integration during sleep, Progr. Physiol. Psychol, 3 (1970) 1–179. [Google Scholar]
- 170.Pompeiano O and Barnes CD, Responses of brain stem reticular neurons to muscle vibration in the decerebrate cat, J. Neurophysiol, 34 (1971) 709–724. [DOI] [PubMed] [Google Scholar]
- 171.Pompeiano O and Hoshino K, Central control of posture: reciprocal discharge by two pontine neuronal groups leading to suppression of decerebrate rigidity, Brain Research, 116 (1976) 131–138. [DOI] [PubMed] [Google Scholar]
- 172.Pompeiano O and Hoshino K, Tonic inhibition of dorsal pontine neurons during the postural atonia produced by an anticholinesterase in the decerebrate cat, Arch. ital. Biol, 114 (1976) 310–340. [PubMed] [Google Scholar]
- 173.Pond FJ, Sinnamon HM and Adams DB, Single unit recording in the midbrain of rats during shock-elicited fighting behavior, Brain Research, 120 (1977) 469–484. [DOI] [PubMed] [Google Scholar]
- 174.Pragay EB, Mirsky AF, Ray CL, Turner DF and Mirsky CV, Neuronal activity in the brain stem reticular formation during performance of a ‘go-no-go’ visual attention task in the monkey, Exp. Neurol, 60 (1978) 83–95. [DOI] [PubMed] [Google Scholar]
- 175.Precht W, Richter A and Grippo J, Responses of neurons in cats abducens nuclei to horizontal angular acceleration, Pflügers Arch. ges. Physiol, 309 (1969) 285–309. [DOI] [PubMed] [Google Scholar]
- 176.Price DD and Dubner R, Neurons that subserve the sensory-discriminative aspects of pain, Pain, 3 (1977) 307–338. [DOI] [PubMed] [Google Scholar]
- 177.Reinhart RJ and Zuber BL, Cellular activation patterns in the abducens nucleus during horizontal nystagmus in the cat, Brain Research, 15 (1969) 284–287. [DOI] [PubMed] [Google Scholar]
- 178.Reinhart RJ and Zuber BL, Abducens nerve signals controlling saccadic eye movements in the cat, Brain Research, 34 (1971) 331–344. [DOI] [PubMed] [Google Scholar]
- 179.Reinoso-Suarez F, Topographischer Hirnatlas der Katze, für Experimental–Physiologische Unter-suchungen, E. Merck, Darmstadt, 1961, 75 pp. [Google Scholar]
- 180.Remmel RS, Pola S and Skinner RD, Pontomedullary reticular projections into the region of the ascending medial longitudinal fasciculus in cat, Exp. Brain Res, 32 (1978) 31–37. [DOI] [PubMed] [Google Scholar]
- 181.Restieaux NJ and Satchell GH, A unitary study of the reticulomotor system of the dogfish, Squalus lebruni (Valliant), J. comp. Neurol, 109 (1958) 391–415. [DOI] [PubMed] [Google Scholar]
- 182.Rhines R and Magoun HW, Brain stem facilitation of cortical motor response, J. Neurophysiol, 9 (1946) 219–229. [DOI] [PubMed] [Google Scholar]
- 183.Rose JD, Responses of midbrain neurons to genital and somatosensory stimulation in estrous and anestrous cats, Exp. Neurol, 49 (1975) 639–652. [DOI] [PubMed] [Google Scholar]
- 184.Rose JD, Response properties and anatomical organization of pontine and medullary units responsive to vaginal stimulation in the cat, Brain Research, 97 (1975) 79–93. [DOI] [PubMed] [Google Scholar]
- 185.Rose JD, Midbrain and pontine unit responses to lordosis — controlling forms of somatosensory stimuli in the female golden hamster, Exp. Neurol, 60 (1978) 499–508. [DOI] [PubMed] [Google Scholar]
- 186.Rose JD and Michael RP, Facilitation by estradiol of midbrain and pontine unit responses to vaginal and somatosensory stimulation in the squirrel monkey (Saimiri sciureus), Exp. Neurol, 58 (1978) 46–58. [DOI] [PubMed] [Google Scholar]
- 187.Rossi GF and Brodal A, Corticofugal fibres to the brain-stem reticular formation, an experimental study in the cat, J. Anat. (Lond.), 90 (1956) 42–63. [PMC free article] [PubMed] [Google Scholar]
- 188.Roussel B, Pujol JF and Jouvet M, Effets des lésions du tegmentum pontique sur les états de sommeil chez le rat, Arch. ital. Biol, 114 (1976) 188–209. [PubMed] [Google Scholar]
- 189.Rovainen CM, Physiological and anatomical studies on large neurons of central nervous system of the sea lamprey (Petromyzon marinus). I. Muller and Mauthner cells, J. Neurophysiol, 30 (1967) 1000–1023. [DOI] [PubMed] [Google Scholar]
- 190.Ruth RE and Rosenfeld JP, Tonic reticular activating system. Relationship to aversive brain stimulation effects, Exp. Neurol, 57 (1977) 41–56. [DOI] [PubMed] [Google Scholar]
- 191.Sauerland EK, Nakamura Y and Clemente CD, The role of the lower brain stem in cortically induced inhibition of somatic reflexes in the cat, Brain Research, 6 (1967) 164–180. [DOI] [PubMed] [Google Scholar]
- 192.Scheibel AB, The brain stem reticular core and sensory function. In Handbook of Physiology, 2nd edn., 1979, in press. [Google Scholar]
- 193.Scheibel ME and Scheibel AB, On circuit patterns of the brain stem reticular core, Ann. N. Y. Acad. Sci, 89 (1961) 856–857. [DOI] [PubMed] [Google Scholar]
- 194.Scheibel ME and Scheibel AB, The response of reticular units to repetitive stimulus, Arch. ital. Biol, 103 (1965) 279–299. [PubMed] [Google Scholar]
- 195.Scheibel ME and Scheibel AB, Anatomical basis of attention mechanisms in vertebrate brains. In Quarton GC, Melnechuk T, and Schmitt FO (Eds.), The Neurosciences, Rockefeller University Press, New York, 1967, pp. 577–602. [Google Scholar]
- 196.Scheibel ME, Scheibel A, Mollica A and Moruzzi G, Convergence and interaction of afferent impulses on single units of reticular formation, J. Neurophysiol, 18 (1955) 309–331. [DOI] [PubMed] [Google Scholar]
- 197.Schlag J, L’Activité Spontanée des Céllules du Système Nerveaux Central, Arscia, Bruxelle, 1959. [Google Scholar]
- 198.Schlag J and Balvin R, Background activity in the cerebral cortex and reticular formation in relation with the electroencephalogram, Exp. Neurol, 8 (1963) 203–219. [Google Scholar]
- 199.Schwartzbaum JS, Interrelationship of multiunit activity of the midbrain reticular formation and lateral geniculate nucleus, thalamocortical arousal, and behavior in rats, J. comp. Physiol, 89 (1975) 131–157. [DOI] [PubMed] [Google Scholar]
- 200.Segundo JP, Takenaka T and Encabo H, Electrophysiology of bulbar reticular neurons, J. Neurophysiol, 30 (1967) 1194–1220. [DOI] [PubMed] [Google Scholar]
- 201.Segundo JP, Takenaka T and Encabo H, Somatic sensory properties of bulbar reticular neurons, J. Neurophysiol, 30 (1967) 1221–1238. [DOI] [PubMed] [Google Scholar]
- 202.Shapovalov AI, Evolution of neuronal systems of suprasegmental motor control, Neurophysiology, 4 (1972) 346–359. [PubMed] [Google Scholar]
- 203.Shapovalov AI and Kozhandy VM, Disynaptic brainstem propriospinal projections to mammalian motoneurons, Neuroscience, 3 (1978) 105–108. [Google Scholar]
- 204.Shik ML and Orlovsky GN, Neurophysiology of locomotor automatism, Physiol. Rev, 56 (1976) 465–501. [DOI] [PubMed] [Google Scholar]
- 205.Siegel J, Saxton PM and Hageman T, Descending projections to the region of the gigantocellular tegmental field of the medulla oblongata, Neurosci. Abstr, 3 (1977) 71. [Google Scholar]
- 206.Siegel JM, Unit activity in the reticular formation and REM sleep. In Drucker-Colin R, Shkurovich M and Sterman MB (Eds.), The Functions of Sleep, Academic Press, New York, 1979, 31–71. [Google Scholar]
- 207.Siegel JM and McGinty DJ, Brainstem neurons without spontaneous unit discharge, Science, 193 (1976) 240–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Siegel JM and McGinty DJ, Pontine reticular formation neurons: relationship of discharge to motor activity, Science, 196 (1977) 678–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Siegel JM and McGinty DJ, Pontine FTG units: l Sensory responses, Sleep Res., 6 (1977) 32. [Google Scholar]
- 210.Siegel JM and McGinty DJ, Pontine reticular formation neurons and motor activity, Science, 199 (1978) 207–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Siegel JM, Wheeler RL and McGinty DJ, Activity of medullary reticular formation neurons in the unrestrained cat during waking and sleep, Brain Research, (1979) in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Siegel JM, McGinty DJ and Breedlove SM, Sleep and waking activity of pontine gigantocellular field neurons, Exp. Neurol, 56 (1977) 553–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Siegel JM, McGinty DJ and Breedlove SM, Sleep and waking activity of nucleus reticularis pontis oralis-caudalis cells, Sleep Res., 7 (1978) 51. [Google Scholar]
- 214.Sotgiu ML and Margnelli M, Electrophysiological identification of pontomedullary reticular neurons directly projecting into dorsal column nuclei, Brain Research, 103 (1976) 443–453. [DOI] [PubMed] [Google Scholar]
- 215.Sotgiu ML and Marini G, Reticulo-cuneate projections as revealed by horseradish peroxidase axonal transport, Brain Research, 128 (1977) 341–345. [DOI] [PubMed] [Google Scholar]
- 216.Sparks DL and Travis RP Jr., Patterns of reticular unit activity observed during the performance of a discriminative task, Physiol. Behav, 3 (1968) 961–967. [Google Scholar]
- 217.Sparks DL and Travis RP Jr., Firing patterns of reticular formation neurons during horizontal eye movements, Brain Research, 33 (1971) 477–481. [DOI] [PubMed] [Google Scholar]
- 218.Sparks DL and Travis RP Jr., Firing patterns of extrapyramidal and reticular neurons of the alert monkey. In Phillips MI (Ed.), Brain Unit Activity During Behavior, Charles C. Thomas. 1973, pp. 288–301. [Google Scholar]
- 219.Sperry RW, Neurology and the mind–brain problem, Amer. Scientist, 40(1952) 291–312. [Google Scholar]
- 220.Sprague JM and Chambers WW, Control of posture by reticular formation and cerebellum in the intact, anesthetized and unanesthetized cat, Amer. J. Physiol, 176 (1954) 52–64. [DOI] [PubMed] [Google Scholar]
- 221.Sprague JM, Levitt M, Robson K, Liu CN, Stellar E and Chambers WW, A neuro-anatomical and behavioral analysis of the syndromes resulting from midbrain lemniscal and reticular lesions in the cat, Arch. ital. Biol, 101 (1963) 225–295. [PubMed] [Google Scholar]
- 222.Sprague JM, Schreiner LH, Lindsley DB and Magoun HW, Reticulo-spinal influences on stretch reflexes, J. Neurophysiol, 11 (1948) 501–507. [DOI] [PubMed] [Google Scholar]
- 223.Spyer KM, Ghelarducci B and Pompeiano O, Gravity responses of neurons in main reticular formation, J. Neurophysiol, 37 (1974) 705–721. [DOI] [PubMed] [Google Scholar]
- 224.Steeves JO, Jordan LM and Lake N, The close proximity of catecholamine-containing cells to the ‘mesencephalic locomotor region’ (MLR), Brain Research, 100 (1975) 663–670. [DOI] [PubMed] [Google Scholar]
- 225.Sterman MB and Fairchild MD, Modification of locomotor performance by reticular formation and basal forebrain stimulation in the cat: evidence for reciprocal systems, Brain Research, 2 (1966) 205–217. [DOI] [PubMed] [Google Scholar]
- 226.Sun CL and Gatipon GB, Effects of morphine sulfate on medial bulboreticular response to peripherally applied noxious stimuli, Exp. Neurol, 52 (1976) 1–12. [DOI] [PubMed] [Google Scholar]
- 227.Taber E, The cytoarchitecture of the brain stem of the cat. I. Brain stem nuclei of the cat, J. comp. Neurol, 116 (1971) 27–70. [DOI] [PubMed] [Google Scholar]
- 228.Thompson RF and Spencer WA, Habituation: a model phenomenon for the study of neuronal substrates of behavior, Psychol. Rev, 173 (1966) 16–43. [DOI] [PubMed] [Google Scholar]
- 229.Torvik A and Brodal A, The origin of reticulospinal fibers in the cat, Anat. Rec, 128 (1957) 113–137. [DOI] [PubMed] [Google Scholar]
- 230.Udo M and Mano N, Discrimination of different spinal monosynaptic pathways converging onto reticular neurons, J. Neurophysiol, 33 (1970) 227–238. [DOI] [PubMed] [Google Scholar]
- 231.Umemoto M, Murai Y, Kodama M and Kido R, Neuronal discharge patterns in conditioned emotional response, Brain Research, 24 (1970) 347–351. [DOI] [PubMed] [Google Scholar]
- 232.Valverde F, Reticular formation of the pons and medulla oblongata. A Golgi study, J. comp. Neurol, 116 (1961) 71–99. [DOI] [PubMed] [Google Scholar]
- 233.Vertes RP, Selective firing of rat pontine gigantocellular neurons during movement and REM sleep, Brain Research, 128 (1977) 146–152. [DOI] [PubMed] [Google Scholar]
- 234.Vertes RP and Miller NE, Brain stem neurons that fire selectively to a conditioned stimulus for shock, Brain Research, 103 (1976) 229–242. [DOI] [PubMed] [Google Scholar]
- 235.Vibert JF, Bertrand F, Denavit-Saubie M and Hughelin A, Three dimensional representation of bulbo-pontine respiratory networks architecture from unit density maps, Brain Research, 114 (1976) 227–244. [DOI] [PubMed] [Google Scholar]
- 236.Villablanca J, Behavioral and polygraphic study of ‘sleep’ and ‘wakefulness’ in chronic decerebrate cats, Electroenceph. clin. Neurophysiol, 21 (1966) 562–577. [DOI] [PubMed] [Google Scholar]
- 237.Waldbillig RJ, Attack, eating, drinking, and gnawing elicited by electrical stimulation of rat mesencephalon and pons, J. comp. Physiol. Psychol, 89 (1975) 200–212. [DOI] [PubMed] [Google Scholar]
- 238.Wetzel MC and Stuart DG, Ensemble characteristics of cat locomotion and its neural control, Progr. Neurobiol, 7 (1976) 1–98. [DOI] [PubMed] [Google Scholar]
- 239.Wilson DM, Function of giant Mauthner’s neurons in the lungfish, Science, 129 (1959) 841–842. [DOI] [PubMed] [Google Scholar]
- 240.Winters WD, Mori K, Spooner CE and Kado RT, Correlation of reticular and cochlear multiple unit activity with auditory evoked responses during wakefulness and sleep. I. Electroenceph. clin. Neurophysiol, 23 (1967) 539–545. [DOI] [PubMed] [Google Scholar]
- 241.Wolstencroft JH, Reticulospinal neurons, J. Physiol. ( Lond.), 174 (1964) 91–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Woods JW, Behavior of chronic decerebrate rats, J. Neurophysiol, 27 (1964) 635–644. [DOI] [PubMed] [Google Scholar]
- 243.Yokota T and Hashimoto S, Periaqueductal gray and tooth pulp afferent interaction on units in caudal medulla oblongata, Brain Research, 117 (1976) 508–512. [DOI] [PubMed] [Google Scholar]
- 244.Yoshii N, Ogura H, Studies on the unit discharge of brain-stem reticular formation in the cat, Med. J. Osaka Univ, 11 (1960) 1–17. [Google Scholar]
- 245.Young DW, and Gottschaldt KM, Neurons in the rostral mesencephalic reticular formation of the cat responding specifically to noxious mechanical stimulation, Exp. Neural, 51 (1976) 628–636. [DOI] [PubMed] [Google Scholar]
- 246.Zangger P, Schultz W, The activity of cells of nucleus reticularis tegmenti pontis during spontaneous locomotion in the decorticate cat, Neurosci. Lett, 7 (1978) 95–99. [DOI] [PubMed] [Google Scholar]


