Abstract
All Doberman pinschers and Labrador retrievers homozygous for a mutation of the hypocretin (orexin) receptor-2 (hcrtr2) gene develop narcolepsy under normal conditions. Degenerative changes and increased display of major histocompatibility complex class II antigens have been linked to symptom onset in genetically narcoleptic Doberman pinschers. This suggests that the immune system may contribute to neurodegenerative changes and narcoleptic symptomatology in these dogs. We therefore attempted to alter the course of canine genetic narcolepsy, as an initial test of principle, by administering a combination of three immunosuppressive and anti-inflammatory drugs chosen to suppress the immune response globally. Experimental dogs were treated with a combination of methylprednisolone, methotrexate and azathioprine orally starting within 3 weeks after birth, and raised in an environment that minimized pathogen exposure. Symptoms in treated and untreated animals were quantified using the food elicited cataplexy test (FECT), modified FECT and actigraphy. With drug treatment, time to cataplexy onset more than doubled, time spent in cataplexy during tests was reduced by more than 90% and nighttime sleep periods were consolidated. Short-term drug administration to control dogs did not reduce cataplexy symptoms, demonstrating that the drug regimen did not directly affect symptoms. Treatment was stopped at 6 months, after which experimental animals remained less symptomatic than controls until at least 2 years of age. This treatment is the first shown to affect symptom development in animal or human genetic narcolepsy. Our findings show that hcrtr2 mutation is not sufficient for the full symptomatic development of canine genetic narcolepsy and suggest that the immune system may play a role in the development of this disorder.
Keywords: Narcolepsy, Cataplexy, Sleep, REM, Immunosuppression, Genetic disorder, Hypocretin, Orexin, Dog, Canine
Introduction
Narcolepsy is a disabling disorder characterized by excessive daytime sleepiness and disrupted nighttime sleep. Most narcoleptics also experience episodes of motor paralysis during wakefulness, called cataplexy, usually triggered by the sudden onset of strong emotions, and some experience episodes of motor paralysis at sleep onset or offset, called sleep paralysis. Hypnagogic hallucinations and the abnormal occurrence of rapid eye movement (REM) sleep at sleep onset also accompany the disorder. Narcolepsy affects approximately one in 2000 individuals (Aldrich, 1998; Scammell, 2003).
Canine narcoleptics have symptoms and drug responses that closely parallel those seen in human narcolepsy with cataplexy (Mignot et al., 1992; Mitler, 1976; Siegel et al., 1991). It has been shown that mutations in the hypocretin receptor-2 (hcrtr2) gene are the cause of inherited narcolepsy in Doberman pinschers and Labrador retrievers (Lin et al., 1999). In contrast, in most human cases, no mutation is present in either the preprohypocretin gene or the known Hcrt receptor genes (Peyron et al., 2000). Instead, 80–90% of the hypocretin (Hcrt) cells in the hypothalamus are lost in most human cases (Thannickal et al., 2000a,b), and preprohypocretin mRNA in the hypothalamus is undetectable (Peyron et al., 2000). It is hypothesized that autoimmune processes cause the disorder in humans (Honda et al., 1984).
It is also possible that the immune system contributes to the development of genetic narcolepsy. Symptoms are not present at birth in genetically narcoleptic dogs, but develop gradually over 4–6 months (Riehl et al., 1998), in all dogs. Hcrt cell somas are not lost in canine genetic narcolepsy (Thannickal et al., 2000a,b); however, during symptom onset, degenerative changes occur in the amygdala, hypothalamus and septal nucleus (Siegel et al., 1999), regions with major roles in sleep control and in the integration of emotions that can trigger cataplexy (Gulyani et al., 2002), and which either contain or are innervated by Hcrt cells (Nambu et al., 1999; Peyron et al., 1998). Increased major histocompatibility complex class II (MHC-II) expression by microglia is also seen during symptom onset, indicating immune system activation (Tafti et al., 1996). Once symptoms have developed, dogs vary in the severity of their symptomatology, even within litters. In addition, peritoneal macrophages are depolarized by Hcrt (Ichinose et al., 1998), suggesting a direct link between Hcrt and the immune system.
Because immunological factors may be important in canine genetic narcolepsy, we have assessed the effects of three widely used immunosuppressive an anti-inflammatory medications, chosen to suppress the immune system globally, on the development of the disorder, as an initial test of principle. We treated dogs with methylprednisolone, azathioprine and methotrexate from soon after birth until 6 months of age. Methylprednisolone, a corticosteroid, reduces the numbers of circulating T and B cells, inhibits T- and B-cell activation and proliferation and can inhibit antibody synthesis. Azathioprine inhibits T- and B-cell proliferation, by inhibiting DNA synthesis. Methotrexate reduces inflammation and antibody synthesis, by blocking folic acid-dependent pathways necessary for DNA synthesis (Roitt et al., 2003). In combination, these drugs potently suppress a wide array of immune functions. We tested the hypothesis that treatment with these drugs would delay symptom onset and produce a permanent reduction of symptom severity.
Materials and methods
Animals
Five litters of genetically narcoleptic (nine females, five males) and one litter of normal Doberman pinscher dogs (for actigraphy studies; four females, two males) were studied. Seven narcoleptic dogs (two males, five females) were treated, and seven narcoleptic dogs (three males, four females) were not treated and served as controls. All procedures were approved by the Animal Research Committee of the University of California at Los Angeles and by the Animal Studies Committee of the Sepulveda Veterans Administration Greater Los Angeles Healthcare System, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23), revised 1996.
All dogs were bred by artificial insemination. They were weaned between 3 and 6 weeks of age. All dogs were raised indoors in a clean environment. Their rooms were cleaned and disinfected daily, by washing the floor and all other accessible areas with a 1.8% bleach solution. Shoe covers, garment covers, hair covers, gloves and masks were worn by all caretakers to minimize dogs’ exposure to pathogens. Because treated dogs could not be vaccinated due to their immunosuppressed status, untreated dogs were also left unvaccinated.
Drug regime
The first litter was treated starting at 21 days of age; all subsequent litters were treated starting at 3 days of age. Treated dogs were given methylprednisolone (1.0 mg/kg PO, 1×/day at 7 AM; Rugby Laboratories, Inc., Rockville Centre, NY), azathioprine (1.0 mg/kg PO, 2×/day, in AM and PM; Glaxo Wellcome Inc., Research Triangle Park, NC) and methotrexate (0.3 mg/kg PO, 1×/week; Mylan Pharmaceuticals Inc., Morgantown, WV). Dogs were weighed daily until 2 months of age, and twice weekly thereafter, to determine correct dosages. For dogs up to 3 weeks of age, drugs were given by nasogastric feeding tube, in the form of ground pills dissolved or suspended in water. Untreated littermates were given the same volumes of water by nasogastric feeding tube whenever their littermates were given drugs. For dogs older than 3 weeks of age, drugs were given as pills in balls of food; untreated dogs were given balls of food not containing pills. Treated dogs were closely monitored behaviorally and physically for indications of toxicity or illness. Blood was drawn once per week, for blood tests to monitor the health of the treated animals. Complete blood counts, differentials and chemistry panels were used to provide an index of immunosuppression or toxicity and to alert us to secondary infections. Lymphocyte counts ranged from 450 to 2000/μl and averaged 1200/μl, which is approximately 20% of normal levels for young dogs (Bounous, 1995). Growth was diminished relative to littermates, which is a well-known consequence of corticosteroid administration (Goldman and Bennett, 2000). Dogs were treated as necessary for infections. Indications of liver toxicity sometimes required temporary reductions in dosage of medications, which were restored when liver function returned to acceptable levels, as is also the practice in managing immunosuppressive treatments in humans. When dogs reached 6 months of age, azathioprine and methotrexate were discontinued, and methylprednisolone was tapered by 20% every 2 weeks, until it was discontinued at 9 months of age.
Four untreated narcoleptic dogs were given short-term drug treatments, using the same medications at the same dosages as were given to treated dogs, to determine the direct effects of these drugs on cataplexy. One 3-month-old dog (female) was treated for 3 days, and later, at age 6 months, was treated for 2 weeks. Three 4-month-old dogs (one male, two female) were treated for 5 days.
Cataplexy testing
Animals were tested starting between 6 and 8 weeks of age. The first litter of immunosuppressed and control dogs was tested 6 days per week. Subsequent litters were tested three times per week. The tests were administered between 5 PM and 6 PM for all litters; treated and untreated littermates were tested in randomized order.
The food elicited cataplexy test (FECT) measures the time it takes to consume a fixed amount of food arranged in a pattern on the floor. A non-symptomatic animal eats the food in a minimal amount of time. However, a symptomatic animal has cataplectic attacks elicited by food consumption. These attacks interrupt food consumption, increasing the time taken to complete the FECT. The FECT was done by placing six balls of 1/3 cup of food in a straight line, at 12-cm intervals, and allowing the animal to make its way through all of the food. The food used for the tests was a 50%/50% mixture of Eukanuba Large Breed Puppy dry chow (IAMS, Dayton, OH) and Pedigree Puppy canned food (Kal Kan Foods, Vernon, CA).
The modified FECT, or mFECT, was done by placing a bowl of food in front of the animal and allowing the animal to eat. This test duplicated the usual feeding situation the animals experienced, with the addition of canned food. The same amount and mixture of food was used for this test as for the FECT. The dog was observed for 10 min, starting with the introduction of the food bowl. Cataplexy time was scored when any of the following criteria were present: falling, collapse of the fore or hind limbs, sagging posture with minimized movements and/or swaying, interruption of eating caused by jaw hypotonia, drooping eyelids and drooping tail. The amount of time spent in cataplexy during the test was recorded.
Four dogs were videotaped over a 2-week period during all cataplexy testing sessions so that the scoring could be compared to scoring of the videotaped test by another researcher blind to the treatment condition of the animal.
A videotape was made of the first litter at 2.5 months of age, showing FECTs and play behavior.
Actigraphy
The activity of dogs was monitored continuously for 24 h/day with collar-mounted actigraphs (Actiwatch, Mini Mitter, Sundriver, OR) while the animals remained in their usual housing. Three treated narcoleptic, three untreated narcoleptic and six normal dogs, age 5 months, were monitored for 9 days. Data were downloaded to a personal computer with an inductively coupled Actiwatch reader and further analyzed by a program of our design, which was used to score sleep. Prior work, in which narcoleptic and normal dogs were monitored simultaneously by polygraphic and actigraphic means, demonstrated that noninvasive actigraphic monitoring alone is sufficient to determine sleep and waking periods (John et al., 2001; Wu et al., 2002). A reduction in activity below a threshold of 10 actigraphic units in each 1 min epoch was used to identify the onset and duration of sleep bouts. The numbers of sleep bouts of different lengths in the dark period were analyzed by computer.
Data analysis
Ages at cataplexy onset in treated vs. untreated narcoleptic dogs were compared by t test with Bonferroni correction. Means ± SEM are reported. FECT and mFECT data were analyzed by ANOVA, and t tests with Bonferroni correction were used to determine the significance of differences between the cataplexy scores of treated and untreated animals at given ages. To analyze the results of short-term drug treatment, t tests with Bonferroni correction were used to compare FECT or mFECT scores before, during, or after treatment. The distributions of sleep bout length derived from actigraphic data were analyzed by ANOVA, with Tukey’s post hoc test to determine whether, for a given sleep bout length, the numbers of sleep bouts were significantly different between pairs of treatment groups. Mean sleep bout length was analyzed by t test with Bonferroni correction. Means ± SEM are reported for mean sleep bout length.
Results
Drug treatment delayed cataplexy onset
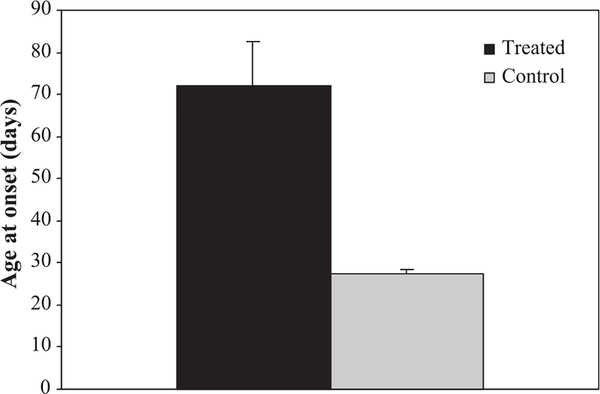
Drug treatment more than doubled the time from birth to cataplexy onset (Fig. 1). The mean age of onset of cataplexy was 27.4 ± 2.5 days for the untreated dogs and 72.0 ± 27.7 days for the treated dogs. The difference between the groups was highly significant (F = 17.9; df = 1,12; P < 0.001).
Fig. 1.
Drug treatment more than doubled the age of cataplexy onset. Seven narcoleptic dogs (two males, five females) were treated with a combination of methylprednisolone, azathioprine and methotrexate. Seven narcoleptic dogs (three males, four females) were not treated and served as controls. Drug treatment started at 3 weeks of age for the first litter and at 3 days of age for the subsequent four litters. The difference between the groups was significant (F = 17.9; df = 1,12; P < 0.001; t test with Bonferroni correction). Means ± SEM are shown.
Drug treatment reduced severity of cataplexy
Cataplexy reaches its peak severity by 4–6 months of age in the genetically narcoleptic dog (Riehl et al., 1998); thus, dogs were tested up to 6 months of age, and treatment of the experimental animals was discontinued and tapered starting at that age. For all litters, the amount of cataplexy was greatly reduced in the immunosuppressed dogs (F = 33.4; df = 1,725; P < 0.001; ANOVA).
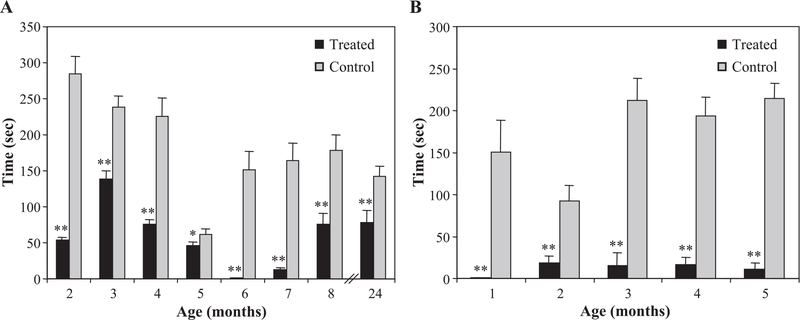
Treated dogs in the first litter had significantly less cataplexy than did their littermates up to 6 months of age, as measured by FECT time (F = 137.0; df = 1,343; P < 0.001; Fig. 2A). The first litter was tested 6 days per week. Perhaps as a result of this repetition, the dogs increasingly learned to delay cataplexy until after food consumption, and this post-feeding cataplexy, often of long duration, was therefore not being quantified according to standard FECT scoring rules. When narcoleptic dogs are tested less frequently, FECT scores typically increase up to 4–6 months of age (Riehl et al., 1998); here, untreated dogs’ scores decreased over time. Even so, the treated animals had substantially less cataplexy each month than their untreated littermates (P < 0.001, ages 2–4 months; P < 0.05, age 5 months; t test with Bonferroni correction). Because of this unanticipated learning effect, however, we changed the test to the mFECT (see Materials and methods) at 6 months of age. After switching to the mFECT, this learning effect was absent (Fig. 2A).
Fig. 2.
Drug treatment reduced cataplexy severity. (A) Treated dogs in the first litter had significantly less cataplexy than did their littermates up to 6 months of age, as measured by FECT time (F = 137.0; df = 1,343; P < 0.001). From 6 months of age onwards, dogs were tested by mFECT. Significant differences in cataplexy symptoms remained at 2 years of age (t = 3.436; df = 50; P = 0.001), long after medications had been withdrawn, indicating that the reduction in symptoms is permanent and not dependent upon the presence of the immunosuppressive drugs. (B) For subsequent litters, time spent in cataplexy during mFECT tests during all months up to 6 months of age was significantly and markedly reduced for treated dogs compared to controls (F = 145.6; df = 1,330; P < 0.001). Means ± SEM are shown; *P < 0.05, **P < 0.001, t test with Bonferroni correction.
Subsequent litters were tested by mFECT rather than FECT. Cataplexy intensity as assessed in this way was markedly reduced by the immunosuppression regimen (Fig. 2B). Time spent in cataplexy during mFECT tests during all months up to 6 months of age was significantly and markedly reduced for treated dogs compared to controls (F = 145.6; df = 1,330; P < 0.001); differences were significant for each month (P < 0.001, ages 1, 3, 4, 5 months; P = 0.001, age 2 months).
FECT and mFECT scores did not reflect observer bias. Scoring of FECTs and mFECTs by researchers blind to the treatment condition of the dogs deviated by less than 6% on average from scores by researchers regularly testing dogs.
The behavior of treated vs. untreated littermates during an FECT can be seen in the video. The normal play behavior of the treated dogs is also shown (see Video).
The first litter was studied again at 2 years of age, long after medications had been withdrawn. Significant differences in cataplexy symptoms remained at 2 years of age (t = 3.436; df = 50; P = 0.001; Fig. 2A), indicating that the reduction in symptoms is permanent and not dependent upon the presence of the immunosuppressive drugs.
Short-term treatment did not reduce cataplexy
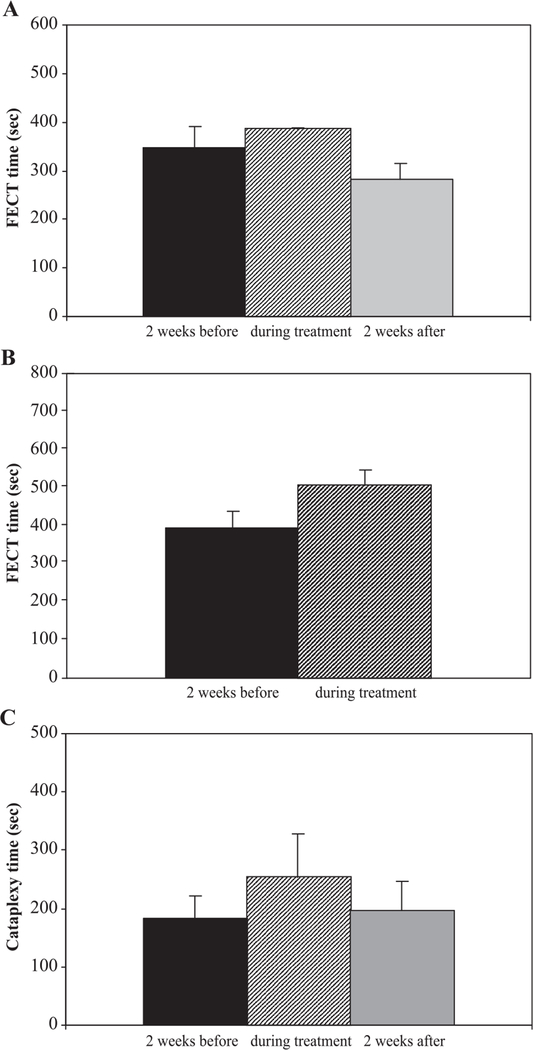
We considered the possibility that the reduction of symptoms during treatment was mediated by a direct effect of the treatment drugs on symptoms, rather than by long-term effects that prevented the development of symptoms. To determine the acute effect of our drug regimen on cataplexy, one of the otherwise untreated narcoleptic dogs was administered the same drugs at the same dosages as were being given to treated littermates, for a 3-day period at 3 months of age, and for a 2-week period at 6 months of age (Figs. 3A,B). These transient treatments did not reduce cataplexy; instead, cataplexy was nonsignificantly increased during treatment, as measured by FECT.
Fig. 3.
Short-term drug administration did not reduce cataplexy, demonstrating that the reduction of symptoms during treatment was not mediated by a direct effect of the treatment drugs on symptoms. (A) FECT times were not reduced during a 3-day treatment of an otherwise untreated narcoleptic dog, age 3 months, from the first litter. Drugs were administered at the same dosages as were given to treated dogs. (B) FECT times were not reduced when the same dog was treated for a 2-week period at 6 months of age. (C) Time spent in cataplexy during mFECTs was not reduced for three transiently treated narcoleptic dogs from subsequent litters. These otherwise untreated dogs underwent a 5-day course of immunosuppression at 4.5 months of age, receiving drugs at the same dosages as were given to treated littermates. Means ± SEM are shown.
Three of the otherwise untreated narcoleptic dogs from subsequent litters underwent a 5-day course of immunosuppression at 4.5 months of age, using the same doses being given to their littermates. As in the first litter, these treatments did not reduce cataplexy symptoms, as measured by mFECT (Fig. 3C).
Drug treatment lengthened nighttime sleep bouts
To assess the effect of immunosuppressive treatment on sleep fragmentation, we used actigraphy to quantify the duration and distribution of sleep periods non-invasively. We studied the sleep patterns of treated and untreated littermates at 5 months of age, together with those of normal dogs of the same age and breed.
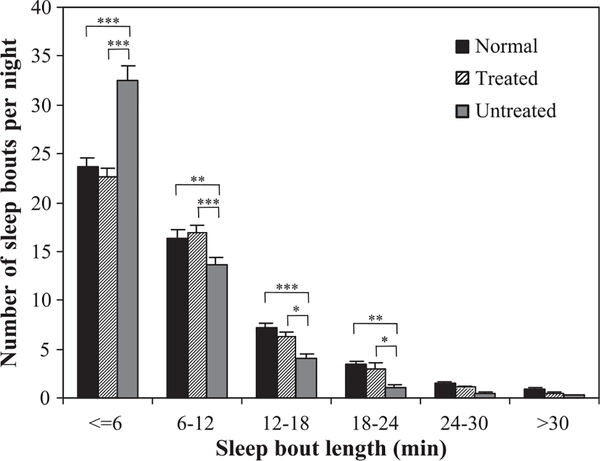
In treated dogs, nighttime sleep was less fragmented than in untreated narcoleptic dogs, and was similar to that of normal dogs. Untreated dogs had more short sleep bouts (≤6 min) and fewer long sleep bouts (6–12 min, 12–18 min, 18–24 min, 24–30 min, >30 min) than did treated or normal dogs. The sleep bout length distributions of normal and treated narcoleptic dogs both differed significantly from that of untreated narcoleptic dogs (normal vs. untreated: F = 22.1; df = 5,474; P < 0.001; treated vs. untreated: F = 27.0; df = 5,312; P < 0.001; ANOVA), but not from each other (Fig. 4).
Fig. 4.
Drug treatment lengthened nighttime sleep bouts as measured by actigraphy. The activity of dogs was monitored continuously for 24 h/day with collar-mounted actigraphs while the animals remained in their usual housing. Actigraphs were worn for 9 days by three treated narcoleptic dogs, three untreated narcoleptic dogs and six normal dogs, age 5 months. Untreated dogs had more short sleep bouts (≤6 min) and fewer long sleep bouts (6–12 min, 12–18 min, 18–24 min, 24–30 min, >30 min) than did treated or normal dogs. The sleep bout length distributions of normal and treated narcoleptic dogs both differed significantly from that of untreated narcoleptic dogs (normal vs. untreated: F = 22.1; df = 5,474; P < 0.001; treated vs. untreated: F = 27.0; df = 5,312; P < 0.001; ANOVA), but not from each other. Values shown are means ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, Tukey’s test.
Mean sleep bout durations were also increased by 31% in treated narcoleptic dogs compared to littermate controls (t = 5.157; df = 52; P < 0.001). The average sleep bout length was 9.1 ± 1.2 min for treated dogs, as compared to 7.4 ± 1.1 min for untreated narcoleptic dogs and 9.9 ± 1.5 min for normal dogs.
Discussion
We have treated genetically narcoleptic dogs with immunosuppressive medications, starting 3–21 days after birth. These medications are commonly used in the treatment of many human disorders, including autoimmune diseases and graft-versus-host disease, and the doses we gave have been used clinically, although usually for shorter durations (Goldman and Bennett, 2000). Drug treatment more than doubled the time to cataplexy onset and profoundly reduced symptom severity. This reduction in severity was not due to acute effects of the immunosuppressive medications. Symptoms remained diminished beyond the period of treatment, suggesting that protection during a period of vulnerability prevents symptom development permanently.
Canine genetic narcolepsy is a disorder with 100% penetrance. Our findings demonstrate that the presence of the hcrtr2 mutation alone is not sufficient for the full development of genetic narcolepsy. Instead, the results of our drug treatment indicate that some combination of environmental and developmental factors participate in the genesis of symptoms in genetically narcoleptic dogs.
In prior work, reported in abstract form, cyclosporin A was administered to young genetically narcoleptic dogs in the hope that immunosuppression could reduce dogs’ symptoms (Kilduff et al., 1987). Dogs given high doses of the medication had less cataplexy than did littermate controls. However, the immunosuppressed dogs were behaviorally depressed, so that the authors of this study were uncertain whether this reduction in symptoms simply reflected the dogs’ malaise rather than any true amelioration of disease. Two dogs administered a lower dose of cyclosporin A did not clearly have less cataplexy than did their untreated littermates. There were several significant differences between this exploratory study and the current work. Dogs in the Kilduff et al. study were treated starting at 39 days of age, after most axonal degeneration in narcoleptic dogs has already occurred (Siegel et al., 1995) and after up-regulated MHC-II expression in microglia can be observed in these dogs (Tafti et al., 1996). In contrast, we treated dogs starting at 3 or 21 days of age, before significant neurodegeneration has been observed. The treated dogs in our study were not significantly behaviorally depressed; treated dogs that showed little cataplexy in testing would often play vigorously following tests. Our drug regimen also differed from that used in the Kilduff et al. study. Cyclosporin A primarily interferes with lymphokine production by T cells and hence T-cell proliferation and function, but also interferes with B-cell proliferation in certain circumstances and can affect antigen presentation by some cell types (Roitt et al., 2003). The drugs we gave to dogs had a broader range of effects. Whereas the results of the prior study were ambiguous, our drug treatment produced very large and long lasting differences in symptom development between treated and untreated littermates.
Because one significant effect of these drugs is immunosuppression, our results suggest that the immune system may be involved in the full expression of genetic narcolepsy. The hcrtr2 mutation in genetically narcoleptic dogs may produce its effects not only via the direct effects of the resulting loss of functional Hcrtr2 on Hcrt signal transduction, but perhaps also by initiating immunological and degenerative changes. Reduced Hcrt transmission, due to a loss of Hcrtr2 function, could result in mutant hcrtr2 expressing neurons receiving insufficient stimulus to maintain fully normal function or synaptic connections. In addition, the absence of Hcrtr2 function might cause such cells to develop abnormally to the extent that they would elicit an immune response. The immune system could then be recruited to damage or destroy such cells and to remove any debris. Although such neurons would be functioning suboptimally before damage were to occur, their loss, or partial loss, and any subsequent formation of abnormal connections, could result in worsening symptoms. Immunosuppression, by preventing this pathological neuronal damage, could allow these neurons to carry out some functions despite the absence of functional Hcrtr2. Because macrophages have a functional receptor for Hcrt (Ichinose et al., 1998), it is also possible that the hcrtr2 mutation could cause a dysregulation of central immunological processes, which could lead to neurodegeneration and an intensification of symptoms over time.
The scenario outlined above would not be entirely without precedent. The immune system has been shown to play a role in the development of other genetic disorders. In the cerebral form of adrenoleukodystrophy, inflammatory demyelination eventually results in death. Populations of oligodendrocytes and microglia are specifically affected by the disorder; perhaps surprisingly, oligodendrocytic death appears to be due primarily to CD8+ T-cell-mediated cytolysis, rather than apoptosis (Ito et al., 2001). In inherited dystrophin-deficient myopathies, including Duchenne muscular dystrophy in humans, pathological changes in muscle are caused in part by the activities of CD8+ and CD4+ T lymphocytes and other immune cells (Spencer and Tidball, 2001).
The medications used in this study have effects other than immunosuppression, however, and the reduction in symptoms that we observed could be mediated by drug-induced changes in the brain that do not involve the immune system. For example, manipulation of monoaminergic systems produces some of the most potent pharmacological effects on cataplexy. Neonatal administration of glucocorticoids is known to cause changes in monoamine synthesis, turnover and concentrations in the rat brain, with some changes seen early on and other changes observed in adulthood (Bakker et al., 2001). Serotonin concentrations in brain regions containing serotonergic cell bodies and projections are slightly decreased in rats up to 3 weeks of age treated neonatally (Yuwiler et al., 1978). Such a decrease, if it occurs in dogs, could be expected to exacerbate cataplexy symptoms mildly (Mignot et al., 1993). In contrast, brain serotonin concentrations are elevated in adult rats treated neonatally (Bakker et al., 2001), which, if true in dogs, should improve symptoms in adult animals to a limited extent (Mignot et al., 1993). Glucocorticoid effects on norepinephrine signaling are less clear, although evidence from prenatal glucocorticoid treatment of rats suggests that brain norepinephrine levels and turnover could be reduced throughout a significant portion of pre-adult life (Bakker et al., 2001; Bian et al., 1993). This would be expected to result in worsened cataplexy symptoms (Wu et al., 1999). Thus, methylprednisolone, a glucocorticoid, could affect cataplexy by modulating monoaminergic systems, although it is unclear whether methylprednisolone administration would be expected to improve or worsen cataplexy. Treatment with methotrexate may decrease brain serotonin synthesis (Fredrikson et al., 1994; Johnston et al., 1986; Silverstein and Johnston, 1986; Silverstein et al., 1986), which should worsen cataplexy. No effects of azathioprine on brain monoamines have been reported. Overall, it is not certain whether the drugs used in this study would be expected to have had a significant long-term effect on the cataplexy symptoms of dogs in this study via effects on monoaminergic systems; short-term drug administration had no effect on cataplexy. As for effects of these drugs on nighttime sleep consolidation, methylprednisolone administration would be expected to have reduced sleep continuity in treated dogs, in contrast with the improved sleep continuity we observed; elevated levels of glucocorticoids, whether in Cushing’s disease or depression or from exogenous administration, are known to increase sleep latency and wake time (Steiger, 2002). No effects of methotrexate or azathioprine on sleep have been reported.
It has been shown that single injections of thalidomide, an immunomodulatory and anti-inflammatory drug (Barnhill and McDougall, 1982; Matthews and McCoy, 2003), aggravates cataplexy in adult hcrtr2 mutant dogs (Kanbayashi et al., 1996). However, the neurodegenerative process that we have observed peaks when animals are 2–3 months of age, with no indication of ongoing degeneration in adult, symptomatic dogs (Siegel et al., 1999). Acute treatment with thalidomide could not, therefore, influence the underlying degenerative process. Thalidomide has hypnotic properties, increasing both REM and non-REM sleep times (Cohen, 1960; Kanbayashi et al., 1996). The hypnotic effects of this drug, rather than its anti-inflammatory properties, may account for its aggravation of cataplexy.
Most human cases of narcolepsy are not caused by genetic mutations in any of the known Hcrt system genes (Peyron et al., 2000). Instead, approximately 85–95% of all narcoleptics with cataplexy possess the human leukocyte antigen DQB1*0602, a gene that is present in 20–30% of the normal population (Honda et al., 1984; Mignot, 1998). Furthermore, there is a lack of concordance in the majority of human identical twin pairs in which one member has narcolepsy (Douglass et al., 1989; Montplaisir and Poirier, 1987; Pollmacher et al., 1990; Partinen et al., 1994). A report also exists of sudden onset of severe narcolepsy with cataplexy one week after a fly bite that caused a large local reaction and neurological disturbances (Montplaisir and Poirier, 1988). These facts, together with the finding of extensive gliosis in human narcoleptic brains (Thannickal et al., 2000a,b), suggest the possibility that the immune system may be responsible for damage to the Hcrt system or its input or output mechanisms in most cases (Siegel, 1999). While it is unlikely that any immune system involvement in the pathogenesis of human narcolepsy would be identical in nature to any immunological involvement in canine genetic narcolepsy, it is possible that some processes could be shared in common. Immunosuppressive treatment very early in the development of human narcolepsy could produce a reduction of symptoms in humans as well.
Supplementary Material
Acknowledgments
We thank Mark Kang and Kristi Alderman for their invaluable help. We are also grateful to Alexandra Arias, Sepehr Bady, Joe Czapkowicz, Thuy Hoang, Bob Nienhuis, Ndaisha Slaughter, Tina Thammasatit and Clint Winkler for their assistance. This work was supported by the Medical Research Service of the Department of Veterans Affairs and PHS grants NS14610, HL41370 and MH6410.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi: 10.1016/j.expneurol.2004.04.006.
References
- Aldrich MS, 1998. Diagnostic aspects of narcolepsy. Neurology 50, S2–S7. [DOI] [PubMed] [Google Scholar]
- Bakker JM, van Bel F, Heijnen CJ, 2001. Neonatal glucocorticoids and the developing brain: short-term treatment with life-long consequences? Trends Neurosci. 24, 649–653. [DOI] [PubMed] [Google Scholar]
- Barnhill RL, McDougall AC, 1982. Thalidomide: use and possible mode of action in reactional lepromatous leprosy and in various other conditions. J. Am. Acad. Dermatol. 7, 317–323 (JID—7907132). [DOI] [PubMed] [Google Scholar]
- Bian X, Seidler FJ, Slotkin TA, 1993. Fetal dexamethasone exposure interferes with establishment of cardiac noradrenergic innervation and sympathetic activity. Teratology 47, 109–117. [DOI] [PubMed] [Google Scholar]
- Bounous D, 1995. Hematology of normal dogs and cats and responses to disease. In: Hoskins J (Ed.), Veterinary Pediatrics: Dogs and Cats from Birth to Six Months. Saunders WB, Philadelphia, pp. 337–353. [Google Scholar]
- Cohen S, 1960. Sleep regulation with thalidomide. Am. J. Psychiatry 116, 1030–1031 (JID—0370512). [DOI] [PubMed] [Google Scholar]
- Douglass AB, Harris L, Pazderka F, 1989. Monozygotic twins concordant for the narcoleptic syndrome. Neurology 39, 140–141. [DOI] [PubMed] [Google Scholar]
- Fredrikson M, Hursti TJ, Steineck G, Furst CJ, Borjesson S, Peterson C, 1994. Delayed chemotherapy-induced nausea is augmented by high levels of endogenous noradrenaline. Br. J. Cancer 70, 642–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L, Bennett J (Eds.), 2000. Cecil Textbook of Medicine. W.B. Saunders, Philadelphia. [Google Scholar]
- Gulyani S, Wu M-F, Nienhuis R, John J, Siegel JM, 2002. Cataplexy-related neurons in the amygdala of the narcoleptic dog. Neuroscience 112, 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y, Doi Y, Juji T, Satake M, 1984. Narcolepsy and HLA: positive DR2 as a prerequisite for the development of narcolepsy. Folia Psychiatr. Neurol. Jpn. 38, 360. [Google Scholar]
- Ichinose M, Asai M, Sawada M, Sasaki K, Oomura Y, 1998. Induction of outward current by orexin-B in mouse peritoneal macrophages. FEBS Lett. 440, 51–54. [DOI] [PubMed] [Google Scholar]
- Ito M, Blumberg BM, Mock DJ, Goodman AD, Moser AB, Moser HW, Smith KD, Powers JM, 2001. Potential environmental and host participants in the early white matter lesion of adreno-leukodystrophy: morphologic evidence for CD8 cytotoxic T cells, cytolysis of oligodendrocytes, and CD1-mediated lipid antigen presentation. J. Neuropathol. Exp. Neurol. 60 (10), 1004–1019 (Oct.). [DOI] [PubMed] [Google Scholar]
- John J, Wu MF, Maidment NT, Siegel JM, 2001. Developmental changes in hypocretin-1 (orexin-A) level in canine narcolepsy. Soc. Neurosci. Abstr. 27, 8.2. [Google Scholar]
- Johnston MV, Silverstein FS, Greenberg H, Knutsen CA, Chandler W, Phillips T, Ensminger WD, 1986. Effects of intraventricular methotrexate on cerebrospinal fluid monoamine metabolites in rhesus monkeys. Cancer Treat. Rep. 70, 1205–1209. [PubMed] [Google Scholar]
- Kanbayashi T, Nishino S, Hishikawa Y, Dement WC, Mignot E, 1996. Thalidomide, a hypnotic with immune modulating properties, increases cataplexy in canine narcolepsy. Neuroreport 12, 1881–1886 ((GENERIC) Ref Type: Journal (Full)). [DOI] [PubMed] [Google Scholar]
- Kilduff TS, Radde L, Burtch M, Steinman L, Dement WC, 1987. Immunosuppression with cyclosporine as a treatment for canine narcolepsy. Sleep Res. 16, 369. [Google Scholar]
- Lin L, Faraco J, Kadotani H, Rogers W, Lin X, Qui X, de Jong P, Nishino S, Mignot E, 1999. The REM sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor gene. Cell 98, 365–376. [DOI] [PubMed] [Google Scholar]
- Matthews SJ, McCoy C, 2003. Thalidomide: a review of approved and investigational uses. Clin. Ther. 25, 342–395 (JID—7706726). [DOI] [PubMed] [Google Scholar]
- Mignot E, 1998. Genetic and familial aspects of narcolepsy. Neurology, S16–S22. [DOI] [PubMed] [Google Scholar]
- Mignot E, Guilleminault C, Dement WC, Grumet C, 1992. Genetically determined animal models of narcolepsy, a disorder of REM sleep. In: Driscoll D (Ed.), Genetically Determined Animal Models of Neurobehavioral Dysfunction. Birkhauser Boston, Cambridge, pp. 554–557. [Google Scholar]
- Mignot E, Renaud A, Nishino S, Arrigoni J, Guilleminault C, Dement WC, 1993. Canine cataplexy is preferentially controlled by adrenergic mechanisms: evidence using monoamine selective uptake inhibitors and release enhancers. Psychopharmacology (Berl.) 113, 76–82. [DOI] [PubMed] [Google Scholar]
- Mitler MM, 1976. Toward an animal model of narcolepsy-cataplexy. In: Guilleminault C, Dement WC, Passouant P (Eds.), Narcolepsy. Spectrum Publications, New York, pp. 387–409. [Google Scholar]
- Montplaisir J, Poirier G, 1987. Narcolepsy in monozygotic twins. Neurology 37, 1089–1092. [DOI] [PubMed] [Google Scholar]
- Montplaisir J, Poirier G, 1988. HLA in narcolepsy in Canada. In: Honda Y, Juji T (Eds.), HLA in Narcolepsy. Springer-Verlag, Berlin, pp. 97–107. [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K, 1999. Distribution of orexin neurons in the adult rat brain. Brain Res. 827, 243–260. [DOI] [PubMed] [Google Scholar]
- Partinen M, Hublin C, Kaprio J, Koskenvuo M, Guilleminault C, 1994. Twin studies in narcolepsy. Sleep 17, S13–S16. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, De Lecea L, Heller HC, Sutcliffe JG, Kilduff TS, 1998. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 18, 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E, 2000. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat. Med. 6, 991–997. [DOI] [PubMed] [Google Scholar]
- Pollmacher T, Schulz H, Geisler P, Kiss E, Albert ED, Schwarz-fischer F, 1990. DR-2-positive monozygotic twins discordant for narcolepsy. Sleep 13, 336–343. [DOI] [PubMed] [Google Scholar]
- Riehl J, Nishino S, Cederberg R, Dement WC, Mignot E, 1998. Development of cataplexy in genetically narcoleptic dobermans. Exp. Neurol. 152, 292–302. [DOI] [PubMed] [Google Scholar]
- Roitt I, Brostoff J, Male D, 2003. Immunology. Mosby, New York. [Google Scholar]
- Scammell TE, 2003. The neurobiology, diagnosis, and treatment of narcolepsy. Ann. Neurol. 53, 154–166. [DOI] [PubMed] [Google Scholar]
- Siegel JM, 1999. Narcolepsy: a key role for hypocretins (orexins). Cell 98, 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, Fahringer H, Paul R, Shiromani P, Dement WC, Mignot E, Chiu C, 1991. Neuronal activity in narcolepsy: identification of cataplexy related cells in the medial medulla. Science 262, 1315–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, Fahringer H, Gulyani S, Mignot E, Switzer RC, 1995. Evidence for localized neuronal degeneration in the narcoleptic dog. Soc. Neurosci. Abstr. 21, 1235. [Google Scholar]
- Siegel JM, Nienhuis R, Gulyani S, Ouyang S, Wu MF, Mignot E, Switzer RC, Cornford M, 1999. Neuronal degeneration in canine narcolepsy. J. Neurosci. 19, 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein FS, Johnston MV, 1986. A model of methotrexate encephalopathy: neurotransmitter and pathologic abnormalities. J. Child Neurol 1, 351–357. [DOI] [PubMed] [Google Scholar]
- Silverstein FS, Hutchinson RJ, Johnston MV, 1986. Cerebrospinal fluid biogenic amine metabolites in children during treatment for acute lymphocytic leukemia. Pediatr. Res. 20, 285–291. [DOI] [PubMed] [Google Scholar]
- Spencer MJ, Tidball JG, 2001. Do immune cells promote the pathology of dystrophin-deficient myopathies? Neuromuscul. Disord. 11 (6–7), 556–564 (Sept.). [DOI] [PubMed] [Google Scholar]
- Steiger A, 2002. Sleep and the hypothalamo-pituitary-adrenocortical system. Sleep Med. Rev. 6 (2), 125–138 (Apr.). [DOI] [PubMed] [Google Scholar]
- Tafti M, Nishino S, Aldrich MS, Liao W, Dement WC, Mignot E, 1996. Major histocompatibility class II molecules in the CNS: increased microglial expression at the onset of narcolepsy in canine model. J. Neurosci. 16, 4588–4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM, 2000a. Reduced number of hypocretin neurons in human narcolepsy. Neuron 27, 469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal TC, Nienhuis R, Ramanathan L, Gulyani S, Turner K, Chestnut B, Siegel JM, 2000b. Preservation of hypocretin neurons in genetically narcoleptic dogs. Sleep 23, A296. [Google Scholar]
- Wu MF, Gulyani S, Yao E, Mignot E, Phan B, Siegel JM, 1999. Locus coeruleus neurons: cessation of activity during cataplexy. Neuroscience 91, 1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MF, John J, Maidment N, Lam HA, Siegel JM, 2002. Hypocretin release in normal and narcoleptic dogs after food and sleep deprivation, eating, and movement. Am. J. Physiol. Regul. Integr. Comp. Physiol 283 (5), R1079–R1086 (Nov.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuwiler A, Simon M, Bennett B, Plotkin S, Wallace R, Brammer G, Ulrich R, 1978. Effect of neonatal corticoid treatment on tryptophan and serotonin metabolism. Endocrinol. Exp 12, 21–31. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.