Abstract
Cataplexy is the pathognomonic symptom of narcolepsy, and is the sudden uncontrollable onset of skeletal muscle paralysis or weakness during wakefulness. Cataplexy is incapacitating because it leaves the individual awake but temporarily either fully or partially paralyzed. Occurring spontaneously, cataplexy is typically triggered by strong positive emotions such as laughter and is often underdiagnosed owing to a variable disease course in terms of age of onset, presenting symptoms, triggers, frequency and intensity of attacks. This disorder occurs almost exclusively in patients with depletion of hypothalamic orexin neurons. One pathogenetic mechanism that has been hypothesized for cataplexy is the activation, during wakefulness, of brainstem circuitry that normally induces muscle tone suppression in rapid eye movement sleep. Muscle weakness during cataplexy is caused by decreased excitation of noradrenergic neurons and increased inhibition of skeletal motor neurons by γ-aminobutyric acid-releasing or glycinergic neurons. The amygdala and medial prefrontal cortex contain neural pathways through which positive emotions probably trigger cataplectic attacks. Despite major advances in understanding disease mechanisms in cataplexy, therapeutic management is largely symptomatic, with antidepressants and γ-hydroxybutyrate being the most effective treatments. This Review describes the clinical and pathophysiological aspects of cataplexy, and outlines optimal therapeutic management strategies.
Introduction
Cataplexy is defined as sudden involuntary muscle weakness or paralysis during wakefulness, typically triggered by strong emotions, and is the pathognomonic symptom of narcolepsy with cataplexy—a sleep disorder that affects 0.06% of the adult population.1,2 In addition to cataplexy, narcolepsy is characterized by sleep paralysis, sleep-onset rapid eye movement (REM) periods, hypnagogic hallucinations, and fragmented night-time sleep.3,4 Cataplexy is virtually exclusive to patients with narcolepsy, and is the optimal behavioural biomarker of this disease.2–4 Excessive daytime sleepiness (EDS) is usually the presenting symptom of narcolepsy, and cataplexy often develops within 1 year of birth and persists for life, although some patients report a delay between EDS and the onset of cataplexy of more than 5 years.5 The age of onset of narcolepsy ranges from early childhood (with 5% of patients in the prepubertal stage) to the fifth decade, with a bimodal distribution that peaks at 15 years and 35 years of age.5 Patients with narcolepsy have difficulty in executing daily activities, socializing and maintaining personal relationships mainly due to cataplexy and EDS, and are estimated to experience a quality of life that is comparable or inferior to that of patients with epilepsy or sleep apnoea.6,7
Cataplexy has been identified in a range of species, including humans, horses, dogs and mice.8,9 Genetic studies of cataplexy in dogs and mice indicate that loss of functional orexin or mutations in the genes encoding orexin receptors underlie the pathophysiology.10–13 Humans with narcolepsy and cataplexy have a marked decrease in orexin levels in cerebrospinal fluid (CSF), together with a decreased number of orexin neurons in postmortem brain tissue.2,14–16 The close associations of narcolepsy or cataplexy with HLA-DQB1*06:02, polymorphisms in the T-cell receptor α and P2RY11 genes, and the pandemic anti-H1N1 vaccination, suggest that the loss of orexin neurons might have an autoimmune origin.17–23
This Review is timely because cataplexy is still an under-recognized symptom of narcolepsy—a disease that is currently underdiagnosed, especially in children. In Europe, the delay between the onset of symptoms and a correct diagnosis is about 10 years, due to insufficient awareness and understanding of the condition among clinicians and individuals.24 Considering that the onset of narcolepsy is mainly in the second decade of life, and the condition can remain untreated for a further 10 years, many patients are affected during the most important period in their education and/or career. To overcome these consequences of narcolepsy and cataplexy, early diagnosis and treatment are essential to best improve patient quality of life.
Features of cataplexy
Cataplexy can be difficult to diagnose, as the symptoms vary not only between patients but also within individuals. For example, different cataplexy phenotypes exist in terms of age of onset, presenting symptoms (that is, the muscles affected), triggers (for example, laughter versus anger), frequency and severity, and the frequency of attacks often decreases with time.4,25,26 Cataplectic attacks range from partial muscle weakness to complete paralysis, but are always bilateral, even if one side of the body is more affected than the other. These attacks are debilitating for patients because they leave the affected individual awake but either fully or partially paralyzed. Cataplexy affects all skeletal muscles apart from the diaphragm and extraocular muscles, but its greatest effect is on facial and neck muscles. Typically, the result is dysarthria, twitching of the facial muscles, jaw tremor, head dropping or jaw dropping, dropping of objects, and/or buckling of the knees (Supplementary Video 1 online). Extreme muscle weakness in the knees, arms and shoulders is also common. 50% of patients with cataplexy experience both partial muscle weakness and complete paralysis, whereas 30% experience only partial paralysis.25,27 Injury during cataplexy is uncommon because most patients ‘feel’ the onset of muscle weakness and are able to sit or lie down. In rare instances, however, a cataplectic attack may result in fractures or bruises to the patient, and might be dangerous in certain settings (for example, during swimming).
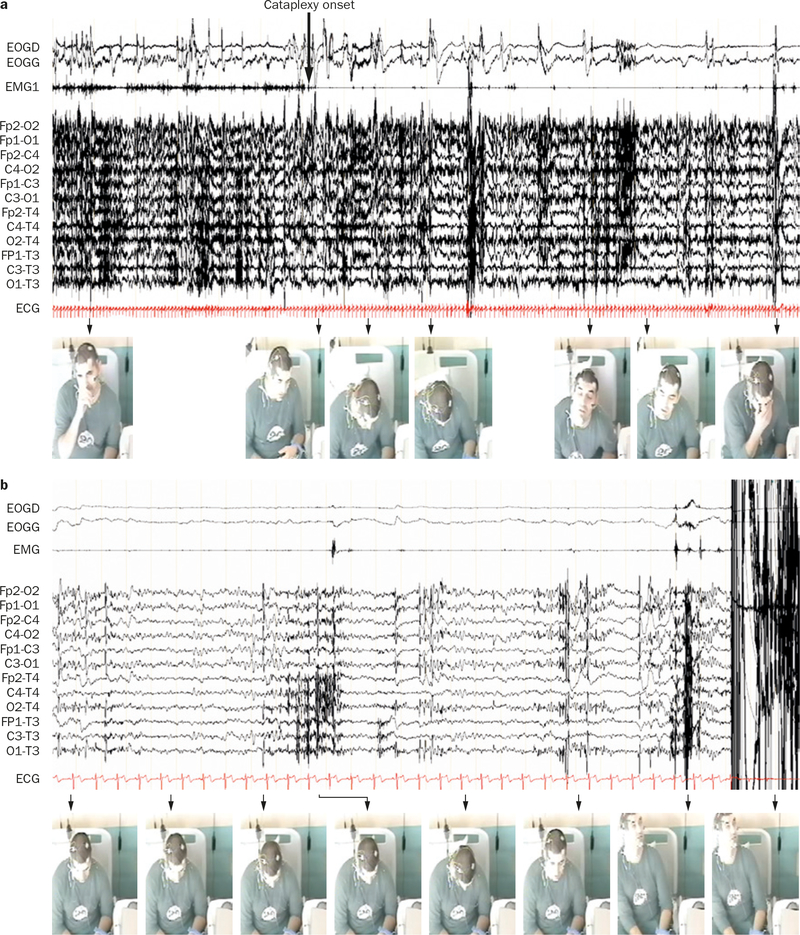
During a cataplectic attack, patients remain conscious and are able to remember what happened to them before, during and after the cataplectic episode.28 Some patients with narcolepsy report hypnagogic hallucinations during attacks, and some patients enter into REM sleep, but this is rare.29 Skeletal muscle tone is reduced or absent during a cataplectic episode. A study of the neurophysiology of cataplexy indicates waxing and waning of postural muscle tone during cataplexy attacks that progresses along muscle groups rostro-caudally. Most episodes are accompanied by reduced heart rate and EEG desynchronization (Figure 1).29,30
Figure 1 |.
Video-polysomnographic recording of a patient during a cataplectic attack with loss of muscle tone. Video clips were taken sequentially over a period of a | 2 min and b | 30 s. The patient presents with sustained loss of muscle tone that alternates with brief enhanced EMG activity leading to a flapping up-and-down motion of the body segments. These movements were reported as voluntary by the patient, who was trying to fight against the repetitive postural losses. The patient was fully conscious during the entire episode. Note that the EEG is characterized by low voltage frequencies (alpha and theta) and a decrease in heart rate during the brief suppressions of EMG activity. Abbreviations: ECG, electrocardiogram; EMG, electromyogram; EOGD, right electrooculogram; EOGG, left electrooculogram. Written consent for publication was obtained from the patient.
The duration of an attack varies from several seconds to several minutes, and in rare instances it lasts for hours—a condition known as status cataplecticus.2 The frequency of cataplectic attacks in patients varies from fewer than one episode per year to several episodes per day. Many patients with narcolepsy report that sleep loss and fatigue worsen the frequency of cataplectic attacks, but studies have not shown a clear link between sleep patterns (total sleep time, sleep efficiency, percentage of sleep stages, periodic leg movements and REM behaviour disorder), EDS and the severity of cataplexy.31 Cataplexy persists throughout life, although the frequency of attacks might decrease with age.31 Men often experience a higher number of cataplectic attacks than do women.31
Near the time of disease onset, children with narcolepsy often display abnormal motor behaviour that does not meet the classic definition of cataplexy.31 Some children display a complex array of ‘negative’ (that is, hypotonic) and ‘active’ movements (for example, jaw opening with tongue protrusion, closure of eyelids and dyskinetic–dystonic movements) that can occur without any obvious emotional triggers.32 These symptoms, however, decrease over a 3 year period and evolve into the classic cataplectic attacks described.33
In clinical practice, cataplexy is mostly diagnosed on the basis of the patient’s history. Cataplexy is often documented in verbal reports, videos taken by the patient’s family or, in some patients, after cataplectic episodes that occur in the presence of a physician. The clinical description of cataplectic attacks should be precise to enable classification as ‘typical’ or ‘clear-cut’, and should include triggering factors, muscles that are affected, duration and frequency of attacks. In patients with a potential differential diagnosis, the term ‘atypical’ cataplexy should be used and an assay of orexin levels in CSF used to verify the diagnosis of orexin deficiency or narcolepsy.
Trigger factors
In the International Classification of Sleep Disorders, narcolepsy is classified as type 1 or type 2.34 According to this classification, type 1 narcolepsy (narcolepsy with cataplexy) is defined as EDS that persists for at least 3 months, plus at least two of the following: clear-cut cataplexy, a positive result on the Multiple Sleep Latency Test (that is, time elapsed from the start of a daytime nap period to the first signs of sleep of ≤8 min, and two or more sleep-onset REM periods) or low levels of orexin in CSF. Type 2 narcolepsy (narcolepsy without cataplexy, which as an entity remains controversial with unknown prevalence) is diagnosed as EDS that persists for at least 3 months and a positive result on the Multiple Sleep Latency Test, in the presence of normal levels of orexin. The presence of atypical cataplexy is sometimes reported in type 2 narcolepsy.
More than 90% of patients with narcolepsy and cataplexy present with low levels of orexin (<110 pg/ml) in CSF, which undoubtedly stem from the loss of approximately 90% of orexin-expressing neurons.2,14,15 By contrast, more than 80% of individuals who are healthy or have atypical cataplexy have normal levels of orexin.35 Importantly, a postmortem study of a patient who exhibited narcolepsy without cataplexy indicated loss of 33% of orexin-positive cells, largely in the posterior hypothalamus.36 This finding suggests that narcolepsy with cataplexy only ensues when a patient loses almost all their orexin-positive cells.14,15,36
The association of H1N1 virus infection or anti-H1N1 vaccination with narcolepsy or narcolepsy with cataplexy is well-established.21,23,37 For example, a substantial spike in newly diagnosed cases of narcolepsy or narcolepsy with cataplexy in children and adolescents who were exposed to the H1N1 virus or the vaccine has been recorded.21,23,37 A study of patients with narcolepsy revealed the presence of CD4+ T cells that were reactive to orexin and might also be reactive to a similar epitope on the H1N1 virus.38 The presence of high titres of antibodies against streptolysin O and Tribbles homologue 2 (TRB-2, a protein that is abundant in orexin neurons) near the onset of narcolepsy also suggests an autoimmune basis for the disease and the potential for immunotherapy by generating crossreactive antibodies.39,40 However, autoantibodies against TRB-2 in mice and other species might be a consequence rather than a cause of orexin neuron damage.
A link between cataplexy and emotion
A cataplectic attack is generally triggered by strong positive emotions such as excited laughter, repartee (for example, making a clever remark), elation, or surprise (for example, unexpectedly meeting a friend).25 Infrequently, they are associated with negative emotions such as frustration or anger or, even more rarely, by stress, fear or physical effort.2,25,41 Although a certain intensity of positive emotion is required to trigger a catapletic attack, nearly half of all patients experience spontaneous attacks that have no identifiable trigger.25,32,42
Benign muscle weakness, especially in the lower limbs, often occurs in healthy people when they laugh, hence the expression ‘weak with laughter’.43 This muscle weakness is linked to suppression of the Hoffmann reflex, which occurs during normal laughter in healthy individuals.44 Orexin neurons are active in the response to strong emotions; therefore, loss of orexin-positive neurons in patients who have narcolepsy or narcolepsy with cataplexy hypothetically destabilizes the motor control system within the brainstem such that positive emotions trigger severe muscle weakness or total motor paralysis.8,45
Evidence exists that patients with cataplexy have altered neuronal responses to positive emotions. For example, neurophysiological data show that processing of humorous stimuli is temporally disturbed in patients with narcolepsy or narcolepsy and cataplexy.46 Neuroimaging studies show that patients with narcolepsy have a reduced threshold for neuronal activation in the amygdala (a brain region that has a key role in the regulation of emotional activity) in response to both humorous and reward stimuli compared with controls.47,48 In addition, functional neuroimaging studies describe changes in brain perfusion49 and glucose metabolism50 during cataplexy in humans. A PET study revealed increased metabolic activity during cataplexy in the bilateral precentral and postcentral gyri and primary somatosensory cortex, and a marked decrease in activity in the hypothalamus.50 A study using singlphoton emission CT indicated hyperperfusion in the right amygdala, bilateral cingulate gyri, basal ganglia, thalamus, premotor cortex, sensorimotor cortex, right insula, and brainstem, and hypoperfusion in the prefrontal cortex and occipital lobe, during cataplexy.49,50 Abnormal functioning of the amygdala during cataplexy might stem from orexin deficiency, because the release of orexin from neurons is maximal when healthy individuals are experiencing positive emotions.51 Animal studies also indicate that cataplexy is associated with abnormal function of the amygdala. Postmortem data show marked axonal degeneration in the amygdala of narcoleptic dogs, and electrophysiological recordings demonstrate that individual cells in the amygdala have increased firing rates during cataplexy.52,53
Animal models
Genetic studies in narcoleptic dogs and mice have provided valuable insights into the pathophysiology of cataplexy. Genetic deletion of Hcrt, which encodes orexin, in mice and the consequent degeneration of orexin-expressing neurons induces a behavioural phenotype that recapitulates the cardinal features of human narcolepsy, including cataplexy, sleepiness and disturbed REM sleep.10,11,13 In dogs, introduction of exon skipping into the Hcrt-R2 gene causes a narcoleptic phenotype, including cataplexy.12,53 These findings not only corroborate human data showing that narcolepsy or narcolepsy and cataplexy is the result of abnormal functioning of the orexin system (Figure 2), but also suggest that the orexin system is important in promoting arousal, controlling REM sleep, and regulating postural muscle tone.45,53
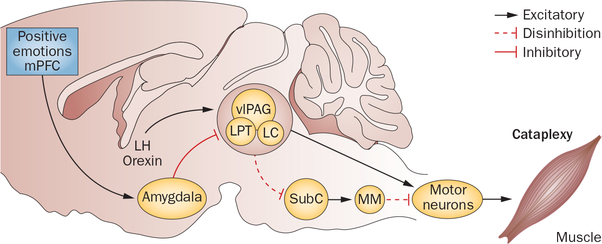
Figure 2 |.
Hypothetical circuits and pathways controlling cataplexy in the rodent brain. Activation during wakefulness of neural circuits involved in REM sleep paralysis is thought to underlie cataplexy, and is probably triggered by a two-part brainstem circuit—the SubC and MM connection. Glutamatergic neurons in the SubC trigger REM paralysis by activating GABAergic or glycinergic cells in the MM, which in turn project to and inhibit skeletal motor neurons. When a positive emotion is experienced, GABAergic neurons in the CeA switch on and inhibit cells in the LC, vlPAG and LPT. The LC–vlPAG–LPT circuit normally prevents muscle paralysis during wakefulness by suppressing the activity of SubC neurons. GABAergic CeA neurons inhibit neurons in the LC–vlPAG–LPT circuit, which in turn disinhibits the SubC to motor neuron circuit, triggering muscle paralysis and cataplexy. Muscle paralysis in cataplexy is also enabled by loss of noradrenergic input from LC neurons, which are inhibited during cataplexy. In healthy individuals, orexin-expressing neuronal activity cancels out the inhibitory effect of amygdalar neurons. Abbreviations: CeA, central amygdala; GABA, γ-aminobutyric acid; LC, locus coeruleus; LH, lateral hypothalamus; LPT, lateral pontine tegmentum; MM, medial medulla; mPFC, medial prefrontal cortex; REM, rapid eye movement; SubC, subcoereulus; vlPAG, ventrolateral periaqueductal grey.
Cataplectic attacks in Hcrt–/– mice seem remarkably similar to those in human cataplexy (Table 1). Attacks are characterized by the rapid onset of skeletal muscle paralysis during wakefulness, resulting in abrupt postural collapse that terminates purposeful behaviour (Supplementary Video 2 online).8 Mice seem to be awake during attacks, because they respond to visual stimuli, and their EEG activity is similar to the spectrum of waking EEG activity seen during cataplectic episodes in children.13,54 Most cataplectic attacks in Hcrt–/– mice range from 15 s to 2 min, with a mean duration of about 60 s, similar to human cataplexy. Cataplectic attacks end with rapid restoration of muscle tone and resumption of normal waking behaviour, as they do in patients with narcolepsy.
Table 1 |.
Cataplexy in humans and animal models
| Features | Human | Mouse* | Dog‡ |
|---|---|---|---|
| Behavioural | Postural collapse, jaw sagging, weak knees | Postural collapse, falling prone or onto their sides | Postural collapse, weakness |
| Level of consciousness | Awake (memory of episode) | Probably awake (response to visual stimuli intact) | Awake (response to visual stimuli intact) |
| Triggers | Strong positive emotions (for example, laughter, joking, elation) | Emotionally rewarding behaviours (for example, eating palatable food, running, social interaction) | Emotionally rewarding behaviours (for example, eating palatable food, running, social interaction) |
| Duration of cataplectic attach | Brief (seconds to minutes) | Brief (seconds to minutes) | Brief (seconds to minutes) |
| Cortical EEG | Mixture of waking and REM-sleep-like EEG | Mixture of waking and REM-sleep-like EEG | Mixture of waking and REM-sleep-like EEG |
| Muscle tone | Muscle paralysis or weakness; loss of EMG activity | Muscle paralysis; loss of EMG activity | Muscle paralysis; loss of EMG activity |
| Therapy | Suppressed by monoamine reuptake blockers (for example, antidepressants) and GHB | Suppressed by monoamine reuptake blockers (for example, antidepressants) and GHB | Suppressed by monoamine reuptake blockers (for example, antidepressants) but no response to GHB |
Hcrt−/− mouse model.
Disruption of Hcrtr2.
Abbreviations: EMG, electromyogram; GHB, γ-hydroxybutyrate; REM, rapid eye movement.
As in humans with narcolepsy, cataplectic attacks in narcoleptic dogs and mice can be triggered by positive emotional stimuli. In narcoleptic dogs, cataplexy is triggered by palatable foods, play or sex, and in narcoleptic mice it is triggered by reward stimuli such as social reunion, running in wheels and palatable food (Supplementary Video 3 online).55,56 The frequency of cataplectic attacks is significantly increased when Hcrt–/– mice are given access to running wheels and chocolate, which are both reward stimuli for mice.41,57 EEG recordings in narcoleptic mice show that cataplexy begins with a brief phase of wakefulness, followed by high-amplitude irregular theta activity and then by short 1–2 s bursts of high-amplitude, regular (~7 Hz), hypersynchronous paroxysmal theta activity.54 Intracranial EEG recordings also show that this activity involves the medial prefrontal cortex, a region associated with reward-driven motor impulses.54 Interestingly, hypersynchronous paroxysmal theta activity (~4 Hz) is also observed at the onset of cataplexy in children with narcolepsy. These bursts of activity might represent medial prefrontal cortical activity, but the clinical relevance of this finding is unclear.54
Neurobiology
A longstanding hypothesis in sleep medicine is that cataplexy results from the intrusion of REM sleep paralysis into wakefulness.2,4,58 This idea stems from the observation that cataplexy and REM sleep paralysis have a common neural mechanism.59 For example, tricyclic antidepressants, which are used to alleviate cataplexy, also suppress REM sleep, and rapid withdrawal of these drugs causes a rebound of either cataplexy or REM sleep.2,60 Deep tendon and monosynaptic Hoffmann reflex activity are absent during both cataplexy and REM sleep.2,4,44 Neuroimaging studies of patients with narcolepsy and electrophysiological recordings from isolated cells in narcoleptic dogs show that the brainstem circuitry involved in REM sleep might have similar activity during both REM sleep and cataplectic episodes.49,52 The underlying cause of clinical cataplexy is a reduction in skeletal motor neuron activity, which results from increased inhibitory and reduced excitatory signalling in the brain.41,58 Inhibitory signals are produced by γ-aminobutyric acid-releasing (GABAergic) and glycinergic neurons in the medial medulla, which are intensely activated during cataplexy and REM sleep, but not during normal waking.55,61,62 Simultaneously, pontine grey neurons, which are responsible for atonia both in cataplexy and in REM sleep, activate GABAergic neurons, which in turn inhibit noradrenergic neurons in the locus coeruleus (Figure 2).63 The cessation of firing of noradrenergic neurons stops the release of noradrenaline to motor neurons and results in their disfacilitation.8 The two processes cause reduced motor neuron activity and a decrease in, or elimination of, tone in the postural muscles (Figure 2).58,64
The close association between the occurrence of cataplexy and orexin deficiency in patients with narcolepsy and animal models of narcolepsy suggests that orexin has a key role in the pathophysiology of cataplexy.2 The strength of the excitatory projections from orexin neurons to the noradrenergic neurons in the locus coeruleus is thought to prevent cataplexy in healthy individuals.65,66 In patients with narcolepsy or narcolepsy and cataplexy, however, orexin deficiency reduces normal levels of noradrenergic neuronal activity, which closely correlates with cataplectic attacks in Hcrt–/– mice, and dogs with narcolepsy.8,66 Drugs that increase noradrenaline levels in the CNS are effective in alleviating cataplexy in humans, dogs and mice.8,26,65 A study demonstrated that the frequency of cataplectic attacks was reduced when orexin receptors were restored to serotonergic neurons in the dorsal raphe of mice lacking orexin receptors, which suggests the serotonin signalling system could also be involved in cataplexy.67 Previous work has shown that activity of serotonergic neurons in the dorsal raphe does not change during cataplexy, in contrast to noradrenergic neurons in the locus coeruleus.65,68
Orexin A and orexin B are two different peptides produced by 70,000–80,000 neurons in the healthy hypothalamus in humans. Orexin neurons not only strongly innervate and directly excite noradrenergic, dopaminergic, serotonergic, histaminergic and cholinergic neurons, but also modulate the release of glutamate and other amino acid transmitters.69,70 Behavioural studies revealed that orexin is released at high levels during active waking, at intermediate or low levels in quiet but alert waking periods and during REM sleep, and at minimal levels in non-REM sleep.71,72 Electrophysiological recording of neuronal unit activity in narcoleptic dogs shows that most of the brain regions involved in the generation of REM sleep atonia are also involved in episodes of cataplexy.52,55,68,73 Thus, at the neuronal level, these findings support the concept of cataplexy as an intrusion of REM sleep paralysis into wakefulness. Cataplexy is not identical to REM sleep, however, the main difference being the maintenance of consciousness. Preservation of activity of histaminergic neurons during cataplexy but not in REM sleep suggests a function for histamine in maintaining wakefulness during cataplectic episodes.66 Orexin neuron activity and orexin release are absent during conditions of quiet waking and drowsiness, but the cessation of this neuronal activity is not sufficient enough to cause cataplexy under normal physiological conditions; thus, the absence of orexin neurons in narcolepsy or narcolepsy and cataplexy might be associated with other anatomical and physiological changes in the brain, perhaps secondary to orexin malfunction.74–76 Accordingly, two studies of histaminergic neurons in human narcolepsy or narcolepsy and cataplexy indicated an increase in numbers of these neurons, but this result differs from results in animal models.77,78 The relationship between changes in histaminergic neuronal numbers, cataplexy and other symptoms of narcolepsy across different species is unclear.
The amygdala has an important role in the processing of emotional stimuli79 and, therefore, might also be important in triggering cataplexy. Clinical and basic research studies show that changes in neuronal activity in the amygdala are associated with cataplexy. Functional neuroimaging shows increased activity in the amygdala while patients watch humorous photographic images, and electrophysiological recordings from isolated cells in narcoleptic dogs demonstrate that activity of certain amygdalar neurons is closely associated with cataplectic attacks.52,80 Another study indicates that bilateral lesions of the amygdala significantly reduce the frequency of cataplectic attacks in Hcrt–/– mice.41 A population of GABAergic neurons in the amygdala innervates the locus coeruleus, lateral pontine tegmentum (LPT) and ventrolateral periaqueductal grey (vlPAG), the functions of which are to support muscle tone during wakefulness.41 Lesions in the LPT and vlPAG in rodents cause sporadic bouts of muscle paralysis during wakefulness that resemble cataplectic attacks, and inactivity of locus coeruleus neurons is associated with muscle atonia during cataplexy.8,66,81,82 In patients experiencing positive emotions, therefore, GABAergic neurons in the amygdala might become active and in turn inhibit the activity of cells in the locus coeruleus, LPT and vlPAG that would normally maintain waking postural tone.
The medial prefrontal cortex (mPFC) also has a role in triggering cataplexy. Ingestion of palatable foods (for example, chocolate), which trigger cataplexy in Hcrt–/– mice, also activates neurons in the mPFC, and inhibition of mPFC neurons markedly reduces cataplectic attacks associated with positive emotional stimuli.83 In addition, neurons in the mPFC innervate the amygdala and lateral hypothalamus, which contain neurons that are active during cataplexy and might innervate brainstem regions involved in the regulation of muscle tone.
Treatment
The inhibitory effect of various antidepressants on the adrenergic system is supported by in vivo and in vitro studies.84 The dopaminergic system is involved in the regulation of cataplexy via the D2-like receptor in mouse models of narcolepsy. The frequency of cataplectic attacks in these mice increases after D2-like receptor activation, and decreases after receptor blockade.85 Cholinergic systems are also thought to be important in the regulation of cataplexy in animal models, with stimulation of the cholinergic system severely aggravating canine cataplexy.86
The effectiveness of drugs used to treat cataplexy is difficult to evaluate, as the methods employed to assess the frequency and intensity of attacks—for example, recall history, scale, diaries or video recordings—vary from one study to another. Some patients might exhibit a decrease in frequency and severity of cataplectic attacks with disease duration. Behavioural measures such as cognitive behavioural therapy might be of interest for some patients to enable them to either control their emotion or learn to avoid situations that trigger cataplectic attacks, but this approach is not usually sufficiently effective to be considered as a recommended treatment.
Antidepressants
Antidepressants and γ-hydroxybutyrate (GHB, also known as sodium oxybate) are reportedly the most effective drugs to treat cataplexy (Table 2).87 Neither tricyclic agents nor selective serotonin reuptake inhibitors (SSRIs) or selective norepinephrine reuptake inhibitors (SNRIs) are approved by the European Medicines Agency or the FDA for the treatment of cataplexy in children or adults. This practice is based only on expert opinion, as no studies demonstrating efficacy and safety of these drugs for this indication have been carried out.
Table 2 |.
Therapies for cataplexy
| Mode of action | Treatment | Dose |
|---|---|---|
| First line | ||
| GABAB agonist that modulates dopamine neurotransmission | GHB | 6–9 g per night* |
| Norepinephrine and serotonin reuptake inhibitor | Venlafaxine | 37.5–300.0 mg per day |
| Selective serotonin reuptake inhibitors | Fluoxetine Citalopram |
20–60 g per day 20–40 mg per day |
| Tricyclic antidepressants | Clomipramine Protriptyline |
10–20 mg per day 5–10 mg per day |
| Second line | ||
| Tricyclic antidepressants | Clomipramine Protriptyline |
30–100 mg per day 20–80 mg per day |
| Norepinephrine and serotonin reuptake inhibitor | Duloxetine | 30–120 mg per day |
| Norepinephrine reuptake inhibitors | Reboxetine Atomoxetine |
2–10 mg per day 2–10 mg per day |
| Third line | ||
| Tricyclic, anorectic, nonamphetamine stimulant | Mazindol | 1–4 mg per day |
| Monoamine oxidase inhibitor | Selegiline | 20–40 mg per day |
| Future therapies | ||
| GABAB agonist | GHB slow-release | 6–9 g per night* |
| NA | Immune-based therapy at disease onset | Intravenous immunoglobulin, plasmapheresis or monoclonal antibodies |
| NA | Orexin, orexin agonists or orexin-expressing cell transplantation | NA |
GHB is the first and only medication indicated for cataplexy.
Abbreviations: GABAB, γ-aminobutyric acid type B receptor; GHB, γ-hydroxybutyrate; NA, not applicable.
Tricyclic agents, which were the first drugs used to treat cataplexy,87,88 are nonspecific monoamine reuptake inhibitors that increase the availability of serotonin, noradrenaline and, in some cases, dopamine.88 Some tricyclic agents also have anticholinergic properties, which might contribute to their anticataplectic effect. Clomipramine is the most widely used tricyclic agent to treat cataplexy.87 Often, these agents have an effect on cataplexy within 48 h at doses below those required to treat depression, but tolerance frequently develops. Cataplexy rebound, or status cataplecticus, which is defined by an increase in the number of attacks and the severity of cataplexy, typically occurs if antidepressant intake, especially tricyclic agents, is interrupted or abruptly halted.
Monoamine oxidase inhibitors (MAOIs) increase the availability of the monoamine neurotransmitters, for example, dopamine, noradrenaline and serotonin. Studies have indicated that MAOIs (specifically, selegiline hydrochloride) strongly suppress REM sleep and reduce the frequency of cataplectic attacks, but these drugs are now rarely used because they are associated with serious adverse effects.87
SSRIs (fluoxetine, paroxetine and citalopram), although less effective than tricyclic antidepressants in decreasing the frequency of cataplectic attacks, are widely used for this purpose, and the frequency of associated adverse effects is lower than for tricyclic agents. SSRIs, and SNRIs (such as venlafaxine, duloxetine and milnacipran), are the antidepressants most widely used to treat cataplexy, particularly venlafaxine as it is effective within 48 h.87 Due to the short duration of action of venlafaxine, the extended-release form seems to be preferable, starting at a low dose (37.5 mg) but higher doses are often needed (75–300 mg). Venlafaxine is not recommended for treatment in pregnant women with narcolepsy, but has an acceptable tolerance profile for use in children.89,90 The differential efficacy of venlafaxine on either ‘negative’ (hypotonia) or ‘active’ motor components during cataplexy is unknown. Other SNRIs, such as duloxetine and milnacipran, or noradrenaline reuptake inhibitors, such as viloxazine, reboxetine, atomoxetine and bupropion, seem to be promising treatments in decreasing the frequency of cataplectic attacks, are well tolerated, and have a mild stimulant effect.91,92
γ-Hydroxybutyrate
GHB is a natural metabolite of γ-aminobutyrate and functions as a neurotransmitter at the GHB receptor (GHBR; also known as solute carrier family 52, riboflavin transporter, member 2) at physiological concentrations and as a GABA receptor agonist at pharmacological concentrations, and also modulates dopaminergic signalling.93
GHB is effective at reducing both the frequency and intensity of cataplectic attacks, as well as restoring nocturnal sleep continuity and reducing EDS in patients with narcolepsy or narcolepsy and cataplexy.93–95 Despite a half-life of only 40–60 min, its clinical benefit persists well beyond this period, and with nightly use the benefit is significant after 4 weeks, highest after 8 weeks, and maintained during long-term therapy.94,95 GHB also has an acceptable tolerance profile for treatment of children; however, as for venlafaxine, its relative efficacy on negative versus active components of cataplexy is unknown.89 It can also be used with antidepressant or stimulant therapy, but should not be used in conjunction with alcohol.95 Unlike antidepressants, interruption of treatment with GHB does not result in a rebound of cataplexy. One major issue with the use of GHB is its nonmedical use, as it is sometimes used in athletes for performance enhancement owing to its metabolic effects. Safety data and clinical experience of GHB therapy indicate that the potential for misuse is low in patients with narcolepsy.95,96,97
Effects of stimulants
Drugs that increase adrenergic and dopaminergic signalling, such as amphetamines, methylphenidate hydrochloride and mazindol (but not modafinil), also decrease the frequency of cataplectic attacks. Although rarely used in practice, mazindol is a tricyclic, anorectic, nonamphetamine that is a very effective stimulant (half-life 10 h) and is also effective in the treatment of cataplexy.95 A careful cardiological follow-up is required with mazindol and amphetamines. Mazindol has less potential for misuse and development of tolerance than amphetamines in patients with narcolepsy.
Future therapeutic management
Orexin deficiency underlies the pathophysiology of cataplexy; therefore, orexin replacement therapy could be an effective strategy. In humans, the use of orexin-based treatment has been disappointing; however, there has been some success with this approach in treating cataplexy in dogs.98,99 Intraventricular delivery of orexin A has potential efficacy, but is probably inappropriate for long-term therapy. Intranasal delivery to bypass the blood–brain barrier is a noninvasive method to deliver orexin to the brain. This method has been shown to improve cognition and olfactory performance and stabilize sleep in rhesus monkeys and patients with narcolepsy or narcolepsy and cataplexy, but has not been tested for its effects on cataplexy alone.100–103 Synthetic orexin receptor agonists might be another treatment option. Transplantation of orexin neurons might, theoretically, provide a cure for patients with narcolepsy, even if the results of neuronal transplantation in other diseases have been disappointing, with graft rejection and low survival rates of the implant.104,105
The activation of histaminergic neurons by an inverse agonist of the histamine H3 receptor, which is presynaptic and enhances histamine release, is a promising therapy. One of these compounds, pitolisant, improved wakefulness in normal animals, blocked abnormal transition from wakefulness to REM sleep in Hcrt–/– mice, and decreased sleepiness and might have the potential to treat cataplectic attacks in patients with narcolepsy.106,107
Finally, on the basis of the immune-mediated hypothesis for the loss of orexin neurons, we suggest that immunotherapies at disease onset might modify the long-term disease outcome if the ‘autoimmune’ process that targets orexin neurons is not too advanced and can be partially reversed. Corticosteroids, intravenous immunoglobulin, plasmapheresis, immunoadsorption and alemtuzumab have all been tested in the treatment of cataplexy, with variable efficacy.108–112 In one patient who had undetectable levels of orexin in the CSF, intravenous immunoglobulin treatment only 15 days after disease onset resulted in clinical improvement of cataplexy and biological normalization of CSF orexin A levels.113 At the onset of narcolepsy, high doses of immunomodulators might downregulate T-cell functions and pathogenic cytokines and interfere with autoantigen recognition through HLA-DQB1*06:02 expression during induction therapy.114 Well-designed trials of immunotherapies in patients at the onset of disease are needed; however, an improved understanding of the pathophysiology of orexin neuron loss is also required to develop effective treatment strategies.
Conclusions
Cataplexy is the pathognomonic symptom of narcolepsy, but is underdiagnosed as a symptom as it varies phenotypically in terms of age of onset, affected muscle group, trigger factors, frequency and intensity. Cataplexy results from the inappropriate activation during wakefulness of the brainstem circuits that normally generate muscle atonia during REM sleep. The pathological intrusion of REM sleep paralysis into wakefulness occurs almost exclusively when orexin neurons are depleted. Neurons expressing orexin normally serve to drive and synchronize the activity of monoaminergic and cholinergic neuronal systems. The loss of an excitatory noradrenergic drive onto motor neurons underlies the loss of postural muscle tone during cataplexy. Involvement of the amygdala and medial prefrontal cortex is highlighted by the triggering of cataplectic attacks by emotional stimuli and processing thereof.
To overcome the consequences of narcolepsy and cataplexy, early diagnosis and treatment of patients are essential. Despite a major advance in our understanding of the neurobiology of narcolepsy–cataplexy, there is no cure. Current therapeutic management is only symptomatic, with widespread use of antidepressants and GHB to reduce the frequency of cataplectic attacks. The discovery of orexin deficiency in humans has led to a new diagnostic test for narcolepsy and might lead to orexin replacement therapy. Future therapeutic targets must be focused on immunotherapies at early stages in the disease to prevent the loss of orexin neurons and disease progression.
Supplementary Material
Key points.
Cataplexy is the pathognomonic symptom of narcolepsy, and is characterized by sudden involuntary loss of skeletal muscle tone during wakefulness, typically triggered by strong positive emotions
The pathogenesis of cataplexy in human narcolepsy involves degeneration of orexin neurons in the hypothalamus; genetically induced orexin deficiency causes cataplexy in both mice and dogs
Cataplexy is thought to result from activation during wakefulness of the sleep circuitry involved in rapid eye movement sleep
Reduced noradrenergic and increased inhibitory input to motor neurons causes muscle weakness or paralysis during cataplexy; positive emotions trigger cataplexy through neuronal pathways in the amygdala and medial prefrontal cortex
γ-Hydroxybutyrate (GHB) and antidepressants are effective treatments for cataplexy, but most treatments (excluding GHB) are used ‘off-label’
Novel and experimental treatments to manage cataplexy are required, including orexin replacement therapy and immune-based therapies
Review criteria.
Articles were identified from publications listed in English on PubMed from January 1970 to October 2013. The following keywords were used alone and in combination: “narcolepsy”,“cataplexy”,“REM sleep”,“atonia”, “motoneuron”, “amygdala”,“emotions”,“hypocretin”, “orexin”,“dog”,“mouse”,“mice”,“brainstem”,“locus coeruleus”,“noradrenergic”,“arousal”and“neurobiology”. Publications were also identified through the authors’ collections of scientific literature.
Footnotes
Competing interests
Y.D. declares that he has received speaker honoraria and support for travel to meetings and has participated on the advisory boards for the following companies: UCB Pharma, JAZZ and Bioprojet. The other authors declare no competing interests.
Supplementary information is linked to the online version of the paper at www.nature.com/nrneurol.
Contributor Information
Yves Dauvilliers, Department of Neurology, INSERM U1061, University of Montpellier, Montpellier 34295, France.
Jerome M. Siegel, Neurobiology Research 151A3, University College Los Angeles, Veterans Administration Greater Los Angeles Healthcare System, 16111 Plummer Street, North Hills, CA 91343, USA
Regis Lopez, Department of Neurology, INSERM U1061, University of Montpellier, Montpellier 34295, France.
Zoltan A. Torontali, Centre for Brain Sciences, Department of Cell and Systems Biology, University of Toronto, Toronto, ON M4X 1H7, Canada
John H. Peever, Centre for Brain Sciences, Department of Cell and Systems Biology, University of Toronto, Toronto, ON M4X 1H7, Canada
References
- 1.American Academy of Sleep Medicine. The international classification of sleep disorders, revised. Diagnostic and coding manual. European Society of Sleep Technology [online], http://www.esst.org/adds/ICSD.pdf (2001).
- 2.Dauvilliers Y, Arnulf I & Mignot E Narcolepsy with cataplexy. Lancet 369, 499–511 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Overeem S, Mignot E, van Dijk JG & Lammers GJ Narcolepsy: clinical features, new pathophysiologic insights, and future perspectives. J. Clin. Neurophysiol. 18, 78–105 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Dauvilliers Y, Billiard M & Montplaisir J Clinical aspects and pathophysiology of narcolepsy. Clin. Neurophysiol. 114, 2000–2017 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Dauvilliers Y et al. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology 57, 2029–2033 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Daniels E, King MA, Smith IE & Shneerson JM Health-related quality of life in narcolepsy. J. Sleep Res. 10, 75–81 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Beusterien KM et al. Health-related quality of life effects of modafinil for treatment of narcolepsy. Sleep 22, 757–765 (1999). [DOI] [PubMed] [Google Scholar]
- 8.Burgess CR & Peever JH A noradrenergic mechanism functions to couple motor behavior with arousal state. Curr. Biol. 23, 1719–1725 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Siegel JM Functional implications of sleep development. PLoS Biol. 3, e178 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chemelli RM et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98, 437–451 (1999). [DOI] [PubMed] [Google Scholar]
- 11.Hara J et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 30, 345–354 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Lin L et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 98,365–376 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Willie JT et al. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of non-REM and REM sleep regulatory processes. Neuron 38, 715–730 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Peyron C et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat. Med. 6, 991–997 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Thannickal TC et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron 27, 469–474 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mignot E et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch. Neurol. 59,1553–1562 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Mignot E et al. Complex HLA-DR and -DQ interactions confer risk of narcolepsy-cataplexy in three ethnic groups. Am. J. Hum. Genet. 68, 686–699 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faraco J et al. ImmunoChip study implicates antigen presentation to T cells in narcolepsy. PLoS Genet. 9, el003270 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallmayer J et al. Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nat. Genet. 41, 708–711 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kornum BR et al. Common variants in P2RY11 are associated with narcolepsy. Nat. Genet. 43, 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dauvilliers Y et al. Increased risk of narcolepsy in children and adults after pandemic H1N1 vaccination in France. Brain 136,2486–2496 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Hor H et al. Genome-wide association study identifies new HLA class II haplotypes strongly protective against narcolepsy. Nat. Genet. 42, 786–789. [DOI] [PubMed] [Google Scholar]
- 23.Partinen M et al. Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PLoS ONE 7, e33723 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luca G et al. Clinical, polysomnographic and genome-wide association analyses of narcolepsy with cataplexy: a European Narcolepsy Network study. J. Sleep Res. 22,482–495 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Overeem S et al. The clinical features of cataplexy: a questionnaire study in narcolepsy patients with and without hypocretin-1 deficiency. Sleep Med. 12,12–18. [DOI] [PubMed] [Google Scholar]
- 26.Nishino S & Mignot E Narcolepsy and cataplexy. Handb. Clin. Neurol. 99, 783–814 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Sturzenegger C & Bassetti CL The clinical spectrum of narcolepsy with cataplexy: a reappraisal. J. Sleep Res. 13,395–406 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Mayer G The neurophysiology of cataplexy [German]. Nervenarzt 76,1464–1469 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Vetrugno R et al. Behavioural and neurophysiological correlates of human cataplexy: a video-polygraphic study. Clin. Neurophysiol. 121,153–162 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Billiard M, Besset A & Cadilhac J in Sleep/Wake Disorders: Natural History, Epidemiology and Long-Term Evolution (eds Guilleminault C & Lugaresi E) 171–185 (Raven Press, 1983). [Google Scholar]
- 31.Mattarozzi K et al. Clinical, behavioural and polysomnographic correlates of cataplexy in patients with narcolepsy/cataplexy. Sleep Med. 9, 425–433 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Plazzi G et al. Complex movement disorders at disease onset in childhood narcolepsy with cataplexy. Brain 134, 3480–3492 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pizza F et al. Clinical and polysom nographic course of childhood narcolepsy with cataplexy. Brain 136, 3787–3795 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American Academy of Sleep Medicine. The International Classification of Sleep Disorders —Third Edition (ICSD-3). AASM Resource Library [online], (2014). [Google Scholar]
- 35.Andlauer O et al. Predictors of hypocretin (orexin) deficiency in narcolepsy without cataplexy. Sleep 35, 1247–1255F (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thannickal TC, Nienhuis R & Siegel JM Localized loss of hypocretin (orexin) cells in narcolepsy without cataplexy. Sleep 32, 993–998 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han F et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann. Neurol. 70, 410–417 (2011). [DOI] [PubMed] [Google Scholar]
- 38.De la Herran-Arita AK et al. CD4+ T cell autoimmunity to hypocretin/orexin and crossreactivity to a 2009 H1N1 influenza A epitope in narcolepsy. Sci. Transl. Med. 5, 216ra176 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Aran A et al. Elevated anti-streptococcal antibodies in patients with recent narcolepsy onset. Sleep 32, 979–983 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cvetkovic-Lopes V et al. Elevated Tribbles homolog 2-specific antibody levels in narcolepsy patients. J. Clin. Invest. 120, 713–719 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burgess CR, Oishi Y, Mochizuki T, Peever JH & Scammell TE Amygdala lesions reduce cataplexy in orexin knock-out mice. J. Neurosci. 33, 9734–9742 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plazzi G et al. Narcolepsy with cataplexy associated with holoprosencephaly misdiagnosed as epileptic drop attacks. Mov. Disord. 25, 780–782. [DOI] [PubMed] [Google Scholar]
- 43.Overeem S, Lammers GJ & van Dijk JG Weak with laughter. Lancet 354, 838 (1999). [DOI] [PubMed] [Google Scholar]
- 44.Overeem S, Reijntjes R, Huyser W, Lammers GJ & van Dijk JG Corticospinal excitability during laughter: implications for cataplexy and the comparison with REM sleep atonia. J. Sleep Res. 13, 257–264 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Siegel JM & Boehmer LN Narcolepsy and the hypocretin system—where motion meets emotion. Nat. Clin. Pract. Neurol. 2, 548–556 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mensen A, Poryazova R, Schwartz S & Khatami R Humor as a reward mechanism: event-related potentials in the healthy and diseased brain. PLoS ONE 9, e85978 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reiss AL et al. Anomalous hypothalamic responses to humor in cataplexy. PLoS ONE 3, e2225 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ponz A et al. Abnormal activity in reward brain circuits in human narcolepsy with cataplexy. Ann. Neurol. 67, 190–200 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Hong SB, Tae WS & Joo EY Cerebral perfusion changes during cataplexy in narcolepsy patients. Neurology 66, 1747–1749 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Dauvilliers Y et al. A brain PET study in patients with narcolepsy-cataplexy. J. Neurol. Neurosurg. Psychiatry 81, 344–348 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Blouin AM et al. Human hypocretin and melanin-concentrating hormone levels are linked to emotion and social interaction. Nat. Commun. 4, 1547 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gulyani S, Wu MF, Nienhuis R, John J & Siegel JM Cataplexy-related neurons in the amygdala of the narcoleptic dog. Neuroscience 112, 355–365 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siegel JM Narcolepsy: a key role for hypocretins (orexins). Cell 98, 409–412 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vassalli A et al. Electroencephalogram paroxysmal theta characterizes cataplexy in mice and children. Brain 136, 1592–1608 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Siegel JM et al. Neuronal activity in narcolepsy: identification of cataplexy-related cells in the medial medulla. Science 252, 1315–1318 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Espana RA, McCormack SL, Mochizuki 1 & Scammell TE Running promotes wakefulness and increases cataplexy in orexin knockout mice. Sleep 30,1417–1425 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clark EL, Baumann CR, Cano G, Scammell TE & Mochizuki T Feeding-elicited cataplexy in orexin knockout mice. Neuroscience 161, 970–977 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peever J Control of motoneuron function and muscle tone during REM sleep, REM sleep behavior disorder and cataplexy/narcolepsy. Arch. Hal. Biol. 149,454–466 (2011). [DOI] [PubMed] [Google Scholar]
- 59.Luppi PH et al. The neuronal network responsible for paradoxical sleep and its dysfunctions causing narcolepsy and rapid eye movement (REM) behavior disorder. Sleep Med. Rev. 15,153–163 (2011). [DOI] [PubMed] [Google Scholar]
- 60.Ristanovic RK, Liang H, Hornfeldt CS & Lai C Exacerbation of cataplexy following gradual withdrawal of antidepressants: manifestation of probable protracted rebound cataplexy. Sleep Med. 10, 416–421 (2009). [DOI] [PubMed] [Google Scholar]
- 61.Kodama I, Lai YY & Siegel JM Changes in inhibitory amino acid release linked to pontineinduced atonia: an in vivo microdialysis study. J. Neurosci. 23,1548–1554 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lai YY & Siegel JM Medullary regions mediating atonia. J. Neurosci. 8, 4790–4796 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mileykovskiy BY, Kiyashchenko LI, Kodama I, Lai YY & Siegel JM Activation of pontine and medullary motor inhibitory regions reduces discharge in neurons located in the locus coeruleus and the anatomical equivalent of the midbrain locomotor region. J. Neurosci. 20, 8551–8558 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brooks PL & Peever JH Identification of the transmitter and receptor mechanisms responsible for REM sleep paralysis. J. Neurosci. 32, 9785–9795 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu MF et al. Locus coeruleus neurons: cessation of activity during cataplexy. Neuroscience 91,1389–1399 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.John J, Wu MF, Boehmer LN & Siegel JM Cataplexy-active neurons in the hypothalamus: implications for the role of histamine in sleep and waking behavior. Neuron 42,619–634 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hasegawa E, Yanagisawa M, Sakurai T & Mieda M Orexin neurons suppress narcolepsy via 2 distinct efferent pathways. J. Clin. Invest. 124, 604–616 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu MF et al. Activity of dorsal raphe cells across the sleep-waking cycle and during cataplexy in narcoleptic dogs. J. Physiol. 554, 202–215(2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peyron C et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 18, 9996–10015 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peever JH, Lai YY & Siegel JM Excitatory effects of hypocretin-1 (orexin-A) in the trigeminal motor nucleus are reversed by NMDA antagonism. J. Neurophysioi. 89,2591–2600 (2003). [DOI] [PubMed] [Google Scholar]
- 71.Lee MG, Hassani OK & Jones BE Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J. Neurosci. 25, 6716–6720 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mileykovskiy BY, Kiyashchenko LI & Siegel JM Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron 46, 787–798 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siegel JM et al. Activity of medial mesopontine units during cataplexy and sleep-waking states in the narcoleptic dog. J. Neurosci. 12, 1640–1646 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siegel JM et al. Neuronal degeneration in canine narcolepsy. J. Neurosci. 19, 248–257 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu MF, Nienhuis R, Maidment N, Lam HA & Siegel JM Role of the hypocretin (orexin) receptor 2 (Hcrt-r2) in the regulation of hypocretin level and cataplexy. J. Neurosci. 31, 6305–6310 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grimaldi D, Silvani A, Benarroch EE & Cortelli P Orexin/hypocretin system and autonomic control: new insights and clinical correlations. Neurology 82, 271–278 (2014). [DOI] [PubMed] [Google Scholar]
- 77.John J et al. Greatly increased numbers of histamine cells in human narcolepsy with cataplexy. Ann. Neurol. 74, 786–793 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Valko PO et al. Increase of histaminergic tuberomammillary neurons in narcolepsy. Ann. Neurol. 74, 794–804 (2013). [DOI] [PubMed] [Google Scholar]
- 79.LeDoux J The amygdala. Curr. Biol. 17, R868–R874 (2007). [DOI] [PubMed] [Google Scholar]
- 80.Schwartz S et al. Abnormal activity in hypothalamus and amygdala during humour processing in human narcolepsy with cataplexy. Brain 131, 514–522 (2008). [DOI] [PubMed] [Google Scholar]
- 81.Lu J, Sherman D, Devor M & Saper CB A putative flip-flop switch for control of REM sleep. Nature 441, 589–594 (2006). [DOI] [PubMed] [Google Scholar]
- 82.Kaur S et al. Hypocretin-2 saporin lesions of the ventrolateral periaquaductal gray (vIPAG) increase REM sleep in hypocretin knockout mice. PLoS ONE 4, e6346 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Covington HE 3rd et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J. Neurosci. 30,16082–16090 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nishino S & Mignot E Pharmacological aspects of human and canine narcolepsy. Prog. Neurobiol. 52,27–78 (1997). [DOI] [PubMed] [Google Scholar]
- 85.Burgess CR, Tse G, Gillis L & Peever JH Dopaminergic regulation of sleep and cataplexy in a murine model of narcolepsy. Sleep 33, 1295–1304 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reid MS et al. Neuropharmacological characterization of basal forebrain cholinergic stimulated cataplexy in narcoleptic canines. Exp. Neurol. 151, 89–104 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Billiard M et al. EFNS guidelines on management of narcolepsy. Eur. J. Neurol. 13, 1035–1048 (2006). [DOI] [PubMed] [Google Scholar]
- 88.Parkes JD & Schachter M Clomipramine and clonazepam in cataplexy. Lancet 2,1085–1086 (1979). [DOI] [PubMed] [Google Scholar]
- 89.Aran A et al. Clinical and therapeutic aspects of childhood narcolepsy-cataplexy: a retrospective study of 51 children. Sleep 33, 1457–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ratkiewicz M & Splaingard M Treatment of cataplexy in a three-year-old using venlafaxine. J. Clin. Sleep Med. 9,1341–1342 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Larrosa O, de la Llave Y, Bario S, Granizo JJ & Garcia-Borreguero D Stimulant and anticataplectic effects of reboxetine in patients with narcolepsy: a pilot study. Sleep 24, 282–285 (2001). [DOI] [PubMed] [Google Scholar]
- 92.Niederhofer H Atomoxetine also effective in patients suffering from narcolepsy? Sleep 28, 1189 (2005). [DOI] [PubMed] [Google Scholar]
- 93.Pistis M et al. γ-hydroxybutyric acid (GHB) and the mesoaccumbens reward circuit: evidence for GABAB receptor-mediated effects. Neuroscience 131, 465–474 (2005). [DOI] [PubMed] [Google Scholar]
- 94.[No authors listed]. A randomized, double blind, placebo-controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsy. Sleep 25, 42–49 (2002). [PubMed] [Google Scholar]
- 95.Alshaikh MK et al. Sodium oxybate for narcolepsy with cataplexy: systematic review and meta-analysis. J. Clin. Sleep Med. 8, 451–458 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang YG, Swick TJ, Carter LP, Thorpy MJ & Benowitz NL Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem): abuse, misuse, dependence, and diversion. J. Clin. Sleep Med. 5, 365–371 (2009). [PMC free article] [PubMed] [Google Scholar]
- 97.Wang YG, Swick TJ, Carter LP, Thorpy MJ & Benowitz NL Sodium oxybate: updates and correction to previously published safety data. J. Clin. Sleep Med. 7, 415–416 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kastin AJ & Kerstrom V Orexin A but not orexin B rapidly enters brain from blood by simple diffusion. J. Pharmacol. Exp. Ther. 289, 219–223 (1999). [PubMed] [Google Scholar]
- 99.John J, Wu MF & Siegel JM Systemic administration of hypocretin-1 reduces cataplexy and normalizes sleep and waking durations in narcoleptic dogs. Sleep Res. Online 3, 23–28 (2000). [PMC free article] [PubMed] [Google Scholar]
- 100.Deadwyler SA, Porrino L, Siegel JM & Hampson RE Systemic and nasal delivery of orexin-A (hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. J. Neurosci. 27, 14239–14247 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baier PC et al. Olfactory dysfunction in patients with narcolepsy with cataplexy is restored by intranasal orexin A (hypocretin-1). Brain 131, 2734–2741 (2008). [DOI] [PubMed] [Google Scholar]
- 102.Baier PC et al. Effects of intranasal hypocretin-1 (orexin A) on sleep in narcolepsy with cataplexy. Sleep Med. 12, 941–946 (2011). [DOI] [PubMed] [Google Scholar]
- 103.Weinhold SL et al. The effect of intranasal orexin-A (hypocretin-1) on sleep, wakefulness and attention in narcolepsy with cataplexy. Behav. Brain Res. 262, 8–13 (2014). [DOI] [PubMed] [Google Scholar]
- 104.Kantor S et al. Orexin gene therapy restores the timing and maintenance of wakefulness in narcoleptic mice. Sleep 36, 1129–1138 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arias-Carrion O et al. Transplantation of hypocretin neurons into the pontine reticular formation: preliminary results. Sleep 27, 1465–1470 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dauvilliers Y et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 12, 1068–1075 (2013). [DOI] [PubMed] [Google Scholar]
- 107.Lin JS et al. An inverse agonist of the histamine H3 receptor improves wakefulness in narcolepsy: studies in orexin–/– mice and patients. Neurobiol. Dis. 30, 74–83 (2008). [DOI] [PubMed] [Google Scholar]
- 108.Dauvilliers Y, Carlander B, Rivier F, Touchon J & Tafti M Successful management of cataplexy with intravenous immunoglobulins at narcolepsy onset. Ann. Neurol. 56, 905–908 (2004). [DOI] [PubMed] [Google Scholar]
- 109.Lecendreux M, Maret S, Bassetti C, Mouren MC & Tafti M Clinical efficacy of high-dose intravenous immunoglobulins near the onset of narcolepsy in a 10-year-old boy. J. Sleep Res. 12, 347–348 (2003). [DOI] [PubMed] [Google Scholar]
- 110.Plazzi G et al. Intravenous high-dose immunoglobulin treatment in recent onset childhood narcolepsy with cataplexy. J. Neurol. 255, 1549–1554 (2008). [DOI] [PubMed] [Google Scholar]
- 111.Chen W, Black J, Call P & Mignot E Late-onset narcolepsy presenting as rapidly progressing muscle weakness: response to plasmapheresis. Ann. Neurol. 58, 489–490 (2005). [DOI] [PubMed] [Google Scholar]
- 112.Donjacour CE & Lammers GJ A remarkable effect of alemtuzumab in a patient suffering from narcolepsy with cataplexy. J. Sleep Res. 21, 479–480 (2012). [DOI] [PubMed] [Google Scholar]
- 113.Dauvilliers Y, Abril B, Mas E, Michel F & Tafti M Normalization of hypocretin-1 in narcolepsy after intravenous immunoglobulin treatment. Neurology 73, 1333–1334 (2009). [DOI] [PubMed] [Google Scholar]
- 114.Dauvilliers Y et al. Cerebrospinal fluid and serum cytokine profiles in narcolepsy with cataplexy: a case–control study. Brain Behav. Immun. 37, 260–266 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.