ABSTRACT
Pneumococcal disease is a serious public health problem worldwide and an important cause of morbidity and mortality among children and adults in developing countries. Although vaccination is among the most effective approaches to prevent and control pneumococcal diseases, approved vaccines have limited protective effects. We developed a pneumococcal protein-polysaccharide conjugate vaccine that is mediated by the noncovalent interaction between biotin and streptavidin. Biotinylated type IV capsular polysaccharide was incubated with a fusion protein containing core streptavidin and Streptococcus pneumoniae virulence protein and relied on the noncovalent interaction between biotin and streptavidin to prepare the protein-polysaccharide conjugate vaccine. Analysis of vaccine efficacy revealed that mice immunized with the protein-polysaccharide conjugate vaccine produced antibodies with high potency against virulence proteins and polysaccharide antigens and were able to induce Th1 and Th17 responses. The antibodies identified using an opsonophagocytic assay were capable of activating the complement system and promoting pathogen elimination by phagocytes. Additionally, mice immunized with the protein-polysaccharide conjugate vaccine and then infected with a lethal dose of Streptococcus pneumoniae demonstrated induced protective immunity. The data indicated that the pneumococcal protein-polysaccharide (biotin-streptavidin) conjugate vaccine demonstrated broad-spectrum activity applicable to a wide range of people and ease of direct coupling between protein and polysaccharide. These findings provide further evidence for the application of biotin-streptavidin in S. pneumoniae vaccines.
KEYWORDS: Streptococcus pneumoniae, protein-polysaccharide conjugate vaccine, pneumococcal surface adhesin A, pneumococcal surface protein A, streptavidin, biotin
INTRODUCTION
Streptococcus pneumoniae is a Gram-positive bacterium and a widely distributed conditional pathogen in humans, who are its only host (1). Changes in the colonization environment of the host, such as decreased body resistance and respiratory virus infection, can promote S. pneumoniae-related pneumococcal diseases, such as sinusitis, otitis media, bacteremia, and bacterial meningitis (2). Individuals at risk of S. pneumoniae infection include infants, young children, the elderly, and people with underlying diseases (3). PPV23 and PCV13 are two commercially available pneumococcal vaccines; however, because the capsular polysaccharide is a T cell-independent antigen, PPV23 cannot induce protective immunity in infants and children <2 years of age (4). PCV13 overcomes this problem by connecting polysaccharides with protein carriers to change antigen type, but the process is complex and expensive; therefore, the vaccine is not popular worldwide (5). Importantly, there are >90 serotypes of S. pneumoniae, of which PPV23 and PCV13 only cover a small portion. However, although vaccination with existing vaccines can reduce the S. pneumoniae carrier rate of the serotype covered by vaccines in community children, the decrease in vaccine-serotype disease was accompanied by an increase in disease caused by nonvaccine serotypes. Thus, there is a need for the development of vaccines using proteins, such as pneumococcal surface adhesin A (PsaA) and pneumococcal surface protein A (PspA), which are candidates for pneumococcal vaccine development (6). The protein antigens on the surface of S. pneumoniae are not restricted by serotype and have demonstrated good immunogenicity and effective immune protection. A group from our laboratory constructed two recombinant proteins as follows: (i) PsaA-PspA23, which contains the highly-conserved PsaA protein, the N-terminal α-helix region of PspA2, and the complementarity-determining region of PspA3; and (ii) PspA4, which contains the N-terminal α-helix region and the proline-rich region of PspA4. We previously reported that PsaA-PspA23 and PspA4 are good immunogens when used alone or in combination (7).
The capsular polysaccharide of S. pneumoniae is combined with virulence protein to improve vaccine immunogenicity. In PCV, the carrier protein CRM197 is covalently linked to capsular polysaccharides of different serotypes, respectively, and then mixed to make a polysaccharide-conjugate vaccine. This process is complicated, and there are only 13 polysaccharide conjugates. In this study, we fused the virulence protein of S. pneumoniae and streptavidin and expressed them to form a protein carrier in the conjugate vaccine that can be incubated with any biotinylated polysaccharide in a certain proportion through noncovalent interactions. The process allows completion of the indirect combination of protein and polysaccharide, thereby making it easier to add polysaccharides of different serotypes to the vaccine. Additionally, biotin is a small-molecule, water-soluble vitamin with an esterophilic heterocycle capable of specifically binding to avidin and a hydrophilic carboxylic acid chain that reacts with many other groups (8–10). Choosing the appropriate biotin or derivative compound allows the biotinylation of amines and carboxyl groups for use in different fields of study (11–13).
The interaction between biotin and avidin is one of the strongest noncovalent interactions (14), with the stability of this interaction promoting its use in biochemical detection (11), affinity purification (15), and drug delivery (16, 17). Streptavidin, which is derived from Streptomyces avidinii, is a nonglycosylated tetrameric protein comprising subunits with biotin-binding sites (18–20). Sano et al. (21) designed and expressed two recombinant core streptavidins with the T7 expression system using BL21(DE3)(pLysE) cells (Stv-25 and Stv-13), both of which have biological activities similar to those of natural streptavidin, except that Stv-13 has higher structural stability. In 6 M guanidine hydrochloride (pH 1.5), the biotin-binding capacity of Stv-13 was maintained at >80% compared with that of Stv-25 and natural core streptavidin (∼20%). Therefore, in the present study, we fused the Stv-13 sequence with PsaA-PspA23 and PspA4 to obtain two fusion proteins (PsaA-PspA23-SA and PspA4-SA), followed by their combination with biotinylated pneumococcal polysaccharides.
Given the unclear antigen composition of whole-cell vaccines, they have been largely replaced by noncellular or subunit vaccines, which have reduced vaccine-specific effects following immunization. The composition of the vaccine presented in this study is the purified pneumococcal virulence protein and capsular polysaccharide, which activates antigen-specific T cell immunity and compensates for the limited antigen diversity and low immunogenicity of subunit vaccines. The protein-polysaccharide conjugate vaccine based on biotin-streptavidin has the following advantages. First, it does not exclusively rely on S. pneumoniae polysaccharides, as the introduction of virulence proteins expands the scope of protection and improves upon the low coverage of serotypes in commercially available vaccines. Second, PsaA and PspA are thymus-dependent antigens with immune memory, and individuals with incomplete B-cell development can also be protected by relying on T cells (22). Finally, the biotin-streptavidin interaction is strong and stable, and they can only be conjugated by coincubation. Moreover, it is easier to add polysaccharides of different serotypes to the vaccine as compared with covalent coupling. Furthermore, application of biotin and streptavidin to vaccines makes protein-polysaccharide binding more efficient and convenient (23, 24).
RESULTS
Preparation of protein-polysaccharide conjugate vaccines.
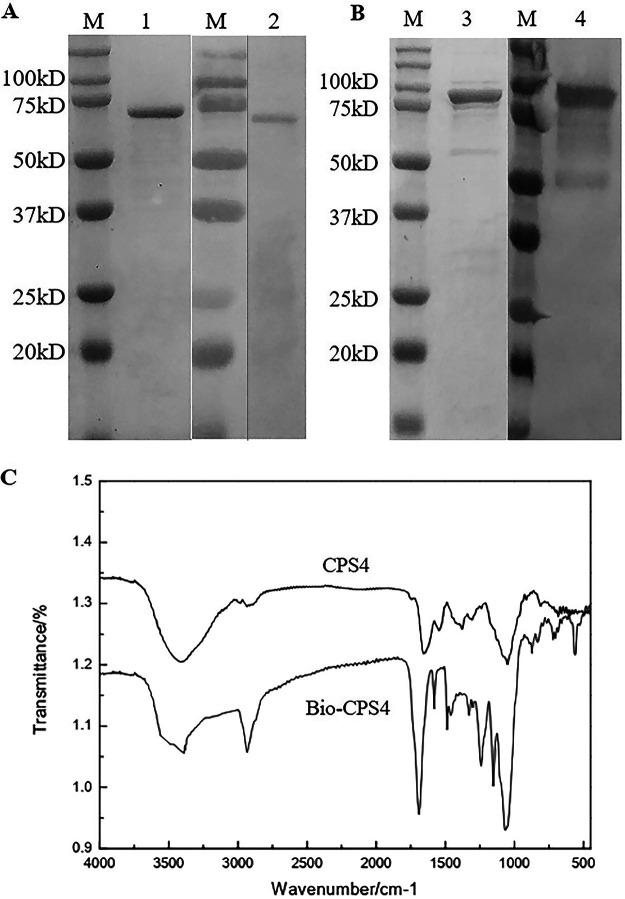
The core streptavidin fragment (SA) with higher stability and stronger biotin-binding ability than full-length streptavidin was individually fused with PsaA-PspA23 and PspA4, and a histidine tag was added to their N termini. PspA4-SA and PsaA-PspA23-SA were purified with a nickel column (Fig. 1A and B), and their sizes were 75 kDa and 100 kDa, respectively.
FIG 1.
Preparation of the fusion protein and biotinylated polysaccharides. Identification and recognition of PspA4-SA (A) and PsaA-PspA23-SA (B) by SDS-PAGE and Western blotting. (C) Infrared spectrogram of polysaccharides and biotinylated polysaccharides.
The capsular polysaccharide of S. pneumoniae type IV was biotinylated and characterized by infrared spectroscopy (25–27). We observed a broad and strong absorption peak at ∼3,400 cm−1 to 3,200 cm−1 defined as VO-H, whereas the absorption peak at 1,200 cm−1 to 1,030 cm−1 was defined as VC-O. These absorption peaks are generally characteristic of polysaccharides. Figure 1C shows that the width of the hydroxyl peak of biotinylated CPS4 (Bio-CPS4) was smaller than that of the polysaccharide before reaction with biotin, indicating that the number of hydroxyl groups in Bio-CPS4 decreased. Additionally, Bio-CPS4 showed a strong peak near 1,690 cm−1, which indicated the stretching vibration peak of the carbon-nitrogen double bond and the formation of a cyanate ester. These results support the hypothesis that there was a reaction between biotin and the polysaccharide.
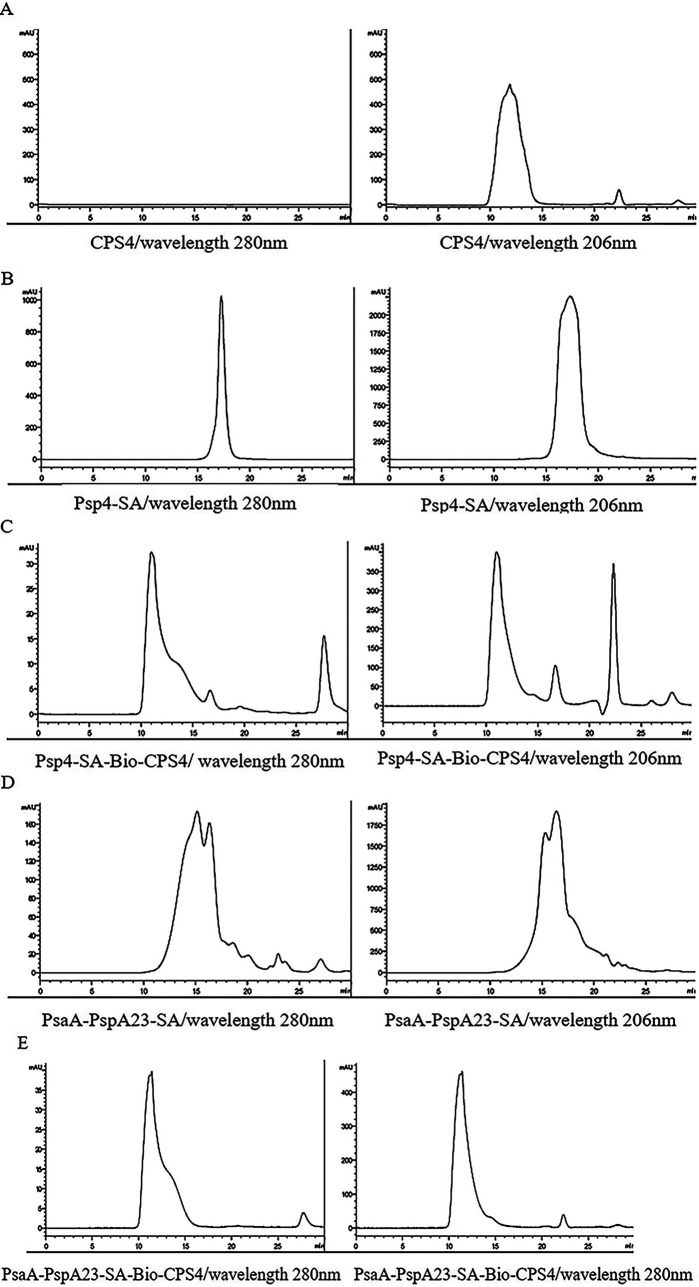
The protein-polysaccharide conjugates were then generated via interaction between biotin and streptavidin for characterization using high-performance liquid chromatography (HPLC) (28–30). The peak of the protein-polysaccharide conjugate sample appeared earlier at a wavelength of 280 nm (Fig. 2C), indicating that its molecular weight was higher than that of the protein sample (Fig. 2B). Additionally, CPS4 had no absorption peak at 280 nm (Fig. 2A), indicating that the earlier peak time of the protein-polysaccharide conjugates was due to the coupling of the protein with macromolecules. These results suggested that PspA4-SA successfully combined with Bio-CPS4. Similarly, we observed that PsaA-PspA23-SA coupled with type IV polysaccharide increased in weight, resulting in an advanced peak time at 280 nm (compare Fig. 2A, D, and E). Furthermore, anthrone assays determined the content of free polysaccharides separated by sodium deoxycholate precipitation at 26.15%.
FIG 2.
Characterization of proteins, polysaccharides, and protein-polysaccharide conjugates by HPLC. HPLC characterization of CPS4 (A), PspA4-SA (B), PspA4-SA-Bio-CPS4 (C), PsaA-PspA23-SA (D), and PsaA-PspA23-SA-Bio-CPS4 (E) at OD280 and OD206. The maximum absorption peak was observed at OD280 for proteins and OD206 for polysaccharides.
The protein-polysaccharide conjugate vaccine induces stronger humoral immunity than the protein vaccine.
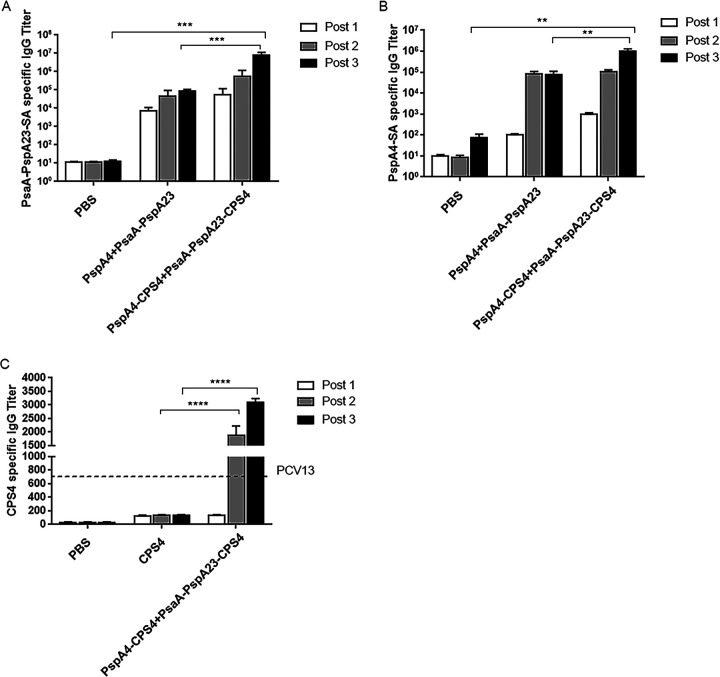
We then evaluated both antigen-specific IgG antibody levels and IgG antibody typing. We observed similar protein-specific antibody titers after the second and third immunizations in the protein group (Fig. 3A and B). We detected high antibody titers in the protein-polysaccharide conjugate group after the second immunization; however, titers after the third immunization increased significantly, with the protein-specific antibody titer of the conjugate group higher than that of the protein and phosphate-buffered saline (PBS) groups, suggesting that the conjugate vaccine induced stronger humoral immunity than the protein vaccine. These findings suggested that the assembly of pneumococcal polysaccharides and virulence proteins significantly improved the immunogenicity of the polysaccharides. We found that control mice immunized three times with CPS4 induced undetectable levels of CPS4-specific IgG, whereas mice immunized with a conjugate produced high levels anti-CPS4 IgG (Fig. 3C). Specifically, after three immunizations, the anti-CPS4 IgG titer in the conjugate-immunized mice was 17-fold higher than that in mice receiving uncoupled CPS4 and 5-fold higher than that in mice receiving three immunizations with PCV13.
FIG 3.
Induction of antigen-specific antibody responses in serum. PsaA-PspA23-SA- (A), PspA4-SA- (B), and CPS4-specific (C) IgG titers. Post 1, post 2, and post 3 represent the specific antibody titers after the first, second, and third immunizations, respectively. The dashed line indicates the anti-CPS4 IgG titer in serum from mice receiving three immunizations with PCV13. *, P < 0.05; **, P < 0.01; ***, P < 0.001. ns, not significant.
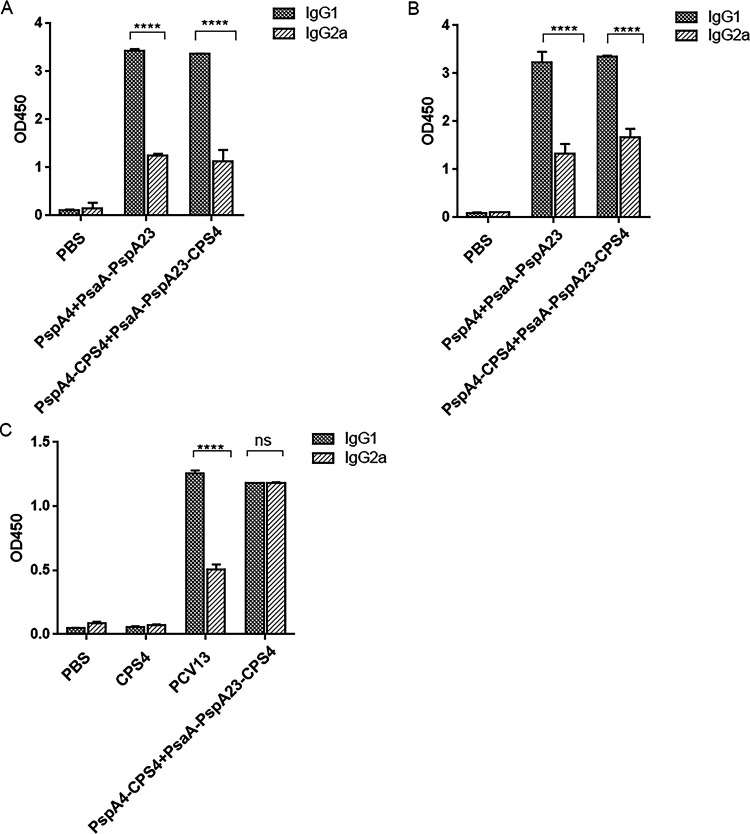
We then determined the Th1 and Th2 bias of the antibodies by comparing the optical density at 450 nm (OD450) values of the antigen-specific IgG1 and IgG2a antibodies at the same dilution ratio. The protein-specific IgG1 in the serum of the protein-polysaccharide conjugate vaccine group was significantly higher than IgG2a (Fig. 4A and B), whereas levels of polysaccharide-specific IgG1 were similar to those of IgG2a in the protein-polysaccharide conjugate vaccine group (Fig. 4C). These results showed that although the protein-polysaccharide conjugate induced high levels of antibodies, it also induces a certain degree of cellular immunity as a supplement to the immunogenic effect.
FIG 4.
Detection of vaccine-induced specific antibody types. PsaA-PspA23-SA- (A), PspA4-SA- (B), and CPS4-specific (C) IgG1 and IgG2a titers. *, P < 0.05; **, P < 0.01; ***, P < 0.001. ns, not significant.
Immunization with the protein-polysaccharide conjugate vaccine induces cellular immunity to protein antigens.
We then evaluated the ability of the protein-polysaccharide conjugate vaccine to elicit antigen-specific cellular responses, including Th1 and Th17 responses, using PsaA-PspA23 and PspA4 as target antigens. Irrespective of whether the stimulant protein was PsaA-PspA23 or PspA4, the level of tumor necrosis factor alpha (TNF-α) and interleukin 17A (IL-17A) induced by the protein-polysaccharide conjugate vaccine was higher than that in the protein and PBS groups (Fig. 5), indicating that the protein-polysaccharide conjugate vaccine induced a strong cellular immune response.
FIG 5.
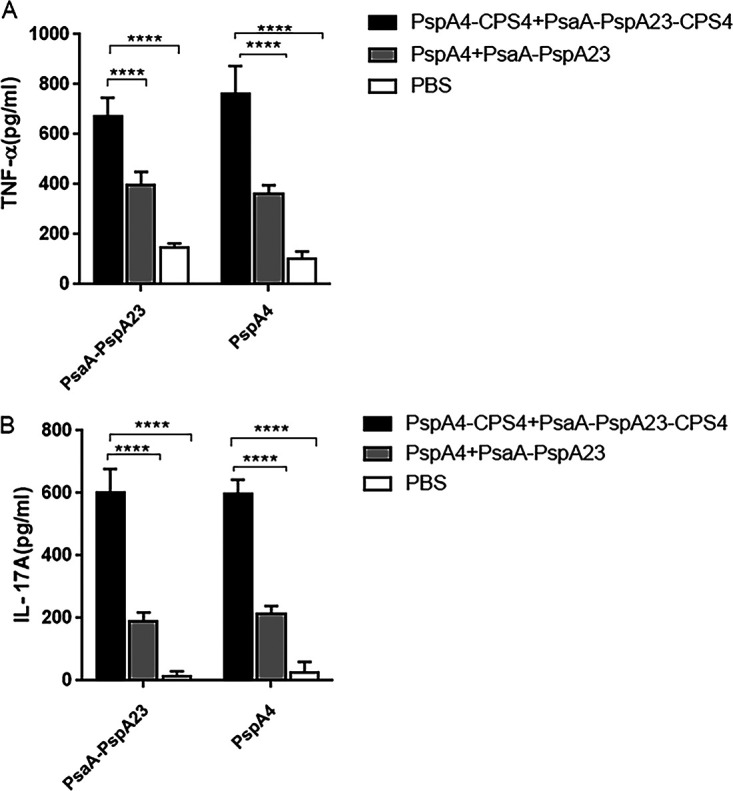
Determination of Th1 and Th17 cell-mediated immune responses in mice. Fourteen days after the third immunization, splenocytes were harvested from mice and stimulated with PsaA-PspA23 and PspA4. TNF-α (A) and IL-17A (B) levels in the supernatant were determined after 48 h. Open bars represent the PBS group, gray bars represent the protein vaccine, and black bars represent the protein-polysaccharide conjugate vaccine. *, P < 0.05; **, P < 0.01; ***, P < 0.001. ns, not significant.
Activity of antibodies induced by protein-polysaccharide conjugate vaccine in vitro.
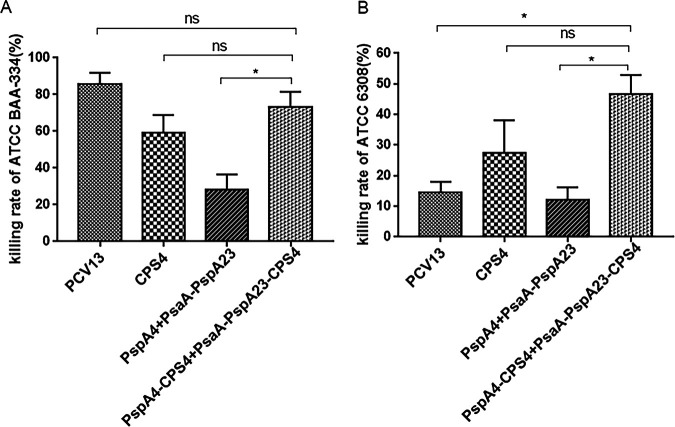
Differentiated HL60 phagocytes recognize the C3 component attached to the surface of S. pneumoniae and activate the complement system after incubation with serum to form an antigen-antibody complex with its own C3 receptor, leading to phagocytosis and pathogen elimination (31). Therefore, we compared the number of surviving S. pneumoniae cells in the conjugate group with that in the control group in order to calculate the killing rate (equivalent to detecting the ability of serum antibodies to capture antigens). The killing rate of antibodies induced by the protein-polysaccharide conjugate vaccine against ATCC BAA334 was lower than that of the antibodies induced by PCV13 but still considerably higher than that observed in the protein and polysaccharide groups (Fig. 6A). Because the ATCC 6308 strain (serotype 8) was not covered by the vaccine serotype in each group, the killing rate of antibodies in the protein-polysaccharide conjugate vaccine group was <50%, whereas that of antibodies induced by PCV13 against ATCC 6308 was <20% (Fig. 6B).
FIG 6.
Opsonophagocytic killing of pneumococcus mediated by serum from mice immunized with the protein-polysaccharide conjugate vaccine. S. pneumoniae ATCC BAA334 (A) and S. pneumoniae ATCC 6308 (B). *, P < 0.05; **, P < 0.01; ***, P < 0.001. ns, not significant.
Immunization with the protein-polysaccharide conjugate vaccine protects mice against fatal pneumococcal challenge.
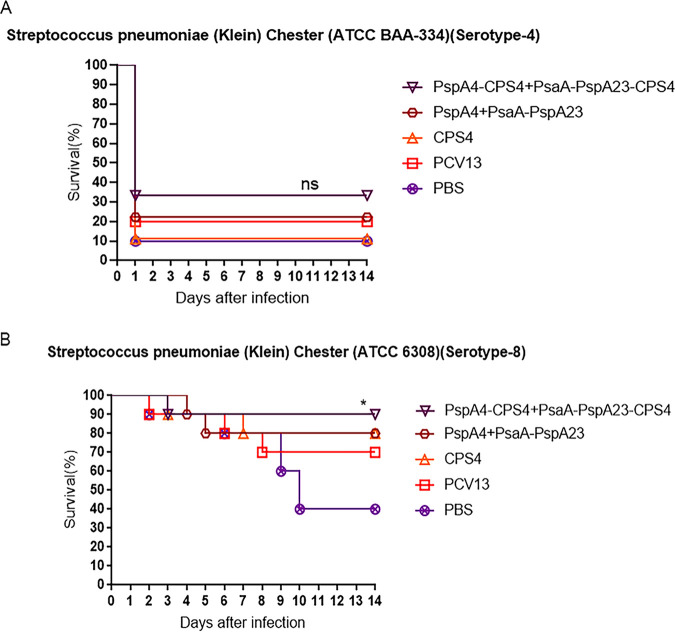
The protective effect of vaccines is an important criterion for determining vaccine efficacy. None of the groups presented good protection against the lethal dose of S. pneumoniae ATCC BAA334 (Fig. 7A). The survival rate of the protein-polysaccharide conjugate group was 35%, and that of the commercial vaccine PCV13-immunized group was 20%, although the survival rate of the protein-polysaccharide conjugate group was higher than that of the negative control group. Notably, the survival rate of the protein-polysaccharide conjugate group reached 90% after administration of a lethal dose of S. pneumoniae ATCC 6308, with this rate higher than that of the protein-immunized, polysaccharide-immunized, and PBS groups (Fig. 7B). These findings indicated that the protein-polysaccharide conjugated vaccine provided more effective and cross-reactive protection to the immunized mice than the protein vaccine.
FIG 7.
Determination of protection against a lethal intranasal pneumococcal challenge. Two weeks after the third immunization, mice were intranasally challenged with a 90% lethal dose of S. pneumoniae ATCC BAA-334 (A) or ATCC6308 (B). Survival rates were monitored for 14 days (n = 10/group). *, P < 0.05; **, P < 0.01; ***, P < 0.001; log-rank test. ns, not significant.
DISCUSSION
In this study, we purified PspA4-SA and PsaA-PspA23-SA on a nickel column to obtain highly pure fusion proteins. Additionally, we synthesized biotinylated polysaccharides (Bio-CPS4) by reacting biotin-PEG3-amine with type IV capsular polysaccharides. The activation time, pH, and feed ratio are important factors to consider when cyano-4-dimethylaminopyridine tetrafluoroborate (CDAP) activates the free hydroxyl groups of type IV polysaccharides, as this significantly effects the degree of polysaccharide biotinylation and potentially leads to a low binding rate with streptavidin-fusion protein (32, 33). In the present study, we used a streptavidin monomer and not complete streptavidin, and the degree of polysaccharide biotinylation was unknown; therefore, we set the feeding ratio (wt/wt) of the streptavidin-fusion protein and biotinylated polysaccharides at 1:1 (34). In future research, the feed ratio of the protein and polysaccharide should be systematically explored to reduce experimental cost. Importantly, reducing free proteins or polysaccharides will likely substantially improve the effectiveness of the vaccine.
We evaluated the effectiveness of the protein-polysaccharide conjugate vaccine by determining the titer, antigen-specific antibody typing in serum, cytokine secretion, opsonizing phagocytic activity of the antibodies, and the survival rate of mice after pathogen challenge. The protein-specific IgG titer in serum from mice immunized with the protein–polysaccharide conjugate vaccine was higher than that of mice immunized with the protein vaccine. Therefore, it is reasonable to conclude that the conjugate vaccine (containing 25% of the amount of free polysaccharides) induced an effective immune response. Additionally, the results suggest that the relationship between protein and polysaccharide is not based on adhesion but rather a noncovalent interaction. We believe that the protective effect observed in this study was mainly due to the antibodies produced by humoral immunity, which agrees with previous reports that antibodies against capsular polysaccharides exhibit sufficient protective effects. Specifically, the cellular immunity generated after inoculation with the conjugate vaccine supplemented the protective effect, and the capsule effectively induced phagocytic activity after binding with the corresponding antibodies (35). Furthermore, another study showed that IL-17A accelerates the clearance of S. pneumoniae and resists bacterial colonization (36).
In the opsonophagocytic assay (OPA), the killing rate of the conjugate vaccine against S. pneumoniae ATCC 6308, the serotypes of which were not covered by the conjugate vaccine, was higher than that of the PCV13 group, indicating that use of PsaA and PspA as carriers effectively improved the broad performance of the vaccine. We speculate that this method is currently unsuitable for evaluating mouse-derived protein serum antibodies owing to the low killing rate of the protein vaccine against S. pneumoniae ATCC BAA-334 and ATCC 6308. Additionally, a previous study suggested that PspC− strains should be used when evaluating the effect of PspA on complement deposition because the presence of the choline-binding protein PspC can interfere with complement deposition and bind to factor H to inhibit activation of the alternative pathway (37).
To evaluate the protection rate and broad performance of the vaccine, we used lethal doses of S. pneumoniae ATCC BAA334 (serotype 4; covered by PCV13) and S. pneumoniae ATCC 6308 (serotype 8; beyond the coverage of PCV13) to challenge mice. The results showed that mice immunized with the protein-polysaccharide conjugate vaccine (PspA4-CPS4+PsaA-PspA23-CPS4) presented a 90% survival rate, which was higher than that of the PCV13 group treated with a lethal dose of S. pneumoniae ATCC 6308. This indicated that the conjugate vaccine exerted cross-protection, which can be attributed to the protein components of the conjugate vaccine. Under infection by S. pneumoniae ATCC BAA334, the survival rate following PCV13 immunization was only 20%. This might be due to the use of the lethal dose, which reduced the protective performance of PCV13. Similarly, we also observed low survival rates in the other groups, as that of the protein-polysaccharide conjugate group was only 35%. One explanation might be related to the immune pathway. Use of part of the protein component (PspA4+PspA2) in a protein-polysaccharide conjugate for mucosal immunization resulted in a survival rate after infection with S. pneumoniae ATCC BAA334 of 80%, which was higher than that generated by PspA4+PsaA-PspA23. Therefore, we speculated that the main antibody produced by subcutaneous immunity was IgG, which is the main driving force against S. pneumoniae ATCC 6308, whereas IgA produced by mucosal immunity is effective against S. pneumoniae ATCC BAA334, resulting in a lower survival rate in subcutaneously immunized mice following ATCC BAA334 infection. Moreover, the gradual death of mice in each group infected with ATCC BAA334 within 24 h was likely related to the site of invasion and pathogenesis, although the pathogenicity of different S. pneumoniae serotypes remains unclear. Furthermore, we cannot rule out the possibility that these findings were a consequence of the high-dose challenge. Nevertheless, the survival rate of the group immunized with the protein-polysaccharide conjugate was higher than that of the negative control group, indicating that the conjugate vaccine exerted a protective effect against a lethal dose of this strain.
In summary, we described a novel pneumococcal protein-polysaccharide conjugate vaccine with a streptavidin-biotin bridge. Although this study has several limitations, it provides evidence supporting further research on the application of strong noncovalent streptavidin-biotin interactions to enhance pneumococcal vaccines.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
S. pneumoniae ATCC BAA334 (serotype 4; PspA family 2, clade 3) and ATCC 6308 (serotype 8; PspA family 1, clade 1) were cultured in Todd-Hewitt broth (THB) medium supplemented with 10% fetal bovine serum (FBS) (Gibco, Gaithersburg, MD, USA) and 0.5% yeast extract (Solarbio, Beijing, China) at 37°C and 5% CO2 for challenge. For quantification, S. pneumoniae at different serial dilutions was cultured on Columbia blood plates at 37°C and 5% CO2 overnight before counting the bacterial cells.
Assembly of the pneumococcal protein-polysaccharide conjugate vaccine.
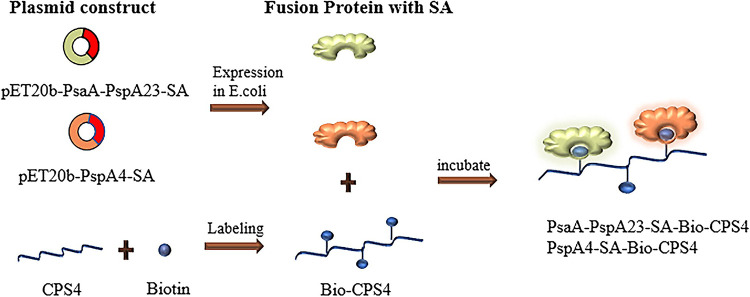
The sequence of the core streptavidin was selected and expressed as a fusion with PsaA-PspA23 and PspA4 to obtain the fusion proteins PsaA-PspA23-SA and PspA4-SA, respectively, and then purified using a nickel column. Additionally, cyano-4-dimethylaminopyridine tetrafluoroborate (CDAP) activates polysaccharides with hydroxyl groups to form cyanate esters that react with biotin-PEG3-amine at appropriate pH levels to yield biotinylated polysaccharides (34), which are then characterized by infrared spectroscopy. Protein-polysaccharide conjugates were assembled by incubation with biotinylated CPS4 (Bio-CPS4) and the two fusion proteins at 4°C overnight, and they were characterized using a TSK-GEL G4000 PWxl column (TOSOH, Japan). The flow chart of the process is shown in Fig. 8. The concentration of PsaA-PspA23-SA-CPS4 and PspA4-SA-CPS4 was measured using a bicinchoninic acid protein assay kit (Beyotime Biotechnology, Beijing, China). Free polysaccharides were separated by sodium deoxycholate precipitation (38–40), and CPS4 concentration was determined using an anthrone assay (41–43).
FIG 8.
Flow chart of the construction of the protein-polysaccharides conjugate vaccines. The pET20b-PspA4-SA and pET20b-PsaA-PspA23-SA plasmids were first constructed, expressed in Escherichia coli, and purified to obtain recombinant proteins. Activated biotin was reacted with type IV capsular polysaccharide to generate biotinylated polysaccharide. The recombinant protein containing the core streptavidin was incubated with the biotinylated capsular polysaccharide, and the virulence protein was indirectly coupled with the capsular polysaccharide via the noncovalent interaction of biotin with streptavidin.
Animals and immunization.
Female BALB/c mice (6 to 8 weeks old) were purchased from Liaoning Changsheng Biotechnology (Benxi City, China) and subcutaneously immunized three times at 14-week intervals with 100 μL of antigens, 100 μL phosphate-buffered saline (PBS) as the negative control, and 50 μL of PCV13 as the positive control. Antigens including protein-polysaccharide conjugates (PsaA-PspA23-SA-CPS4+PspA4-SA-CPS4), CPS4, and proteins (PsaA-PspA23-SA+PspA4-SA) were diluted to different concentrations with PBS and then mixed with aluminum hydroxide (final concentration, 0.1 mg/mL) for overnight adsorption at 4°C. The protein control group was prepared with 5 μg of PsaA-PspA23-SA and 20 μg of PspA4-SA in 100 μL PBS, and the polysaccharide control group comprised 3.08 μg of CPS4. After mixing multiple ligation products, the inoculation dose of the protein-polysaccharide conjugate vaccine was the same for the protein and polysaccharide control groups.
Antibody measurements.
Blood samples were obtained at 2 weeks after the first, second, and third immunizations for antibody analysis. Antigen-specific IgG, IgG1, and IgG2a levels in serum were measured using an enzyme-linked immunosorbent assay (ELISA) in 96-well plates coated with 1 μg of CPS4 or 0.5 μg of protein antigens per well. To determine antibody titer, the diluted serum or horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (1:10,000) (Dingguo Biology, China) was added to the plates, and the samples were incubated for 1 h at 25°C. For antibody typing, serum (1:100), goat anti-mouse IgG1 or IgG2a (1:1,000), and HRP-conjugated rabbit anti-goat IgG (1:4,000) were used to detect the corresponding antibody levels in serum samples. After terminating the reaction, the optical density was measured at 450 nm (OD450).
Measurement of cytokine levels.
The spleens of mice in the protein-polysaccharide conjugate, protein, and PBS groups were obtained 2 weeks after the third immunization for cytokine analysis. Briefly, red blood cells were removed after grinding the spleen samples, and 5 × 106 isolated cells in RPMI 1640 medium (HyClone Laboratories, Logan, UT, USA) supplemented with 10% FBS were added to each well of a 24-well plate. Cells from each mouse were stimulated with 5 μg of PsaA-PspA23, PspA4, or positive control ConA for 48 h, with an equal volume of RPMI 1640 medium used as the negative control. The supernatant was then collected, and the secretion of cytokines Th1 and Th17 was detected according to the manufacturer’s instructions (BioLegend, San Diego, CA, USA). Data were analyzed using LEGENDplex software (BioLegend).
Opsonophagocytic assay.
For the OPA (44–47), HL-60 cells were grown in RPMI 1640 medium supplemented with 0.8% N,N-dimethylformamide (Thermo Fisher Scientific, Waltham, MA, USA) and 20% FBS at a density of 5 × 105/mL for differentiation over 5 days. The cells were then collected, oligo binding buffer (OBB) (8 mL sterile water, 1 mL Hanks’ balanced salt solution [Gibco], 1 mL 1% gelatin [Sigma-Aldrich], and inactivated 530 μL FBS) was used to adjust the cell density to 1 × 107/mL, and the concentration of S. pneumoniae ATCC BAA334 or ATCC 6308 was adjusted to 5 × 104 CFU/mL. The bacterial suspension (10 μL) and 20 μL of sera were added to a 96-well plate, with the control wells filled with 20 μL OBB instead of sera. The plate was then incubated at 25°C for 30 min, after which 40 μL of cells (1 × 107/mL) and 10 μL of complement were added and incubated for 90 min. The reaction was terminated in an ice bath, and the liquid in each well was diluted 10- and 100-fold. We then added 100 μL of the dilution solution to the Columbia blood plate, which was incubated overnight in a 5% CO2 incubator at 37°C. Subsequently, the number of colonies was recorded and the killing rate calculated.
Streptococcus challenge.
Fourteen days after the third immunization, mice were intranasally infected with S. pneumoniae ATCC BAA334 at 9 × 106 CFU/mouse and ATCC 6308 at 0.8 × 102 CFU/mouse. The survival status of the mice was observed for 14 days, during which the data were recorded and analyzed.
Statistical analysis.
A two-way analysis of variance was used to determine differences between the protein-polysaccharide conjugate and other groups. The results are expressed as the mean ± standard error of the mean. Statistical analyses were performed using GraphPad Prism software (v.7.0; GraphPad Software, La Jolla, CA, USA), with a P value of <0.05 considered statistically significant.
ACKNOWLEDGMENTS
We acknowledge the financial support of the Science and Technology Project of Jilin Province, China (no. 20200404112YY).
We report no conflicts of interest. The authors are also responsible for the contents of this article.
Contributor Information
Yongge Wu, Email: yongge_wu@163.com.
Nancy E. Freitag, University of Illinois at Chicago
REFERENCES
- 1.Steel HC, Cockeran R, Anderson R, Feldman C. 2013. Overview of community-acquired pneumonia and the role of inflammatory mechanisms in the immunopathogenesis of severe pneumococcal disease. Mediators Inflamm 2013:490346. 10.1155/2013/490346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinberger DM, Trzciński K, Lu Y-J, Bogaert D, Brandes A, Galagan J, Anderson PW, Malley R, Lipsitch M. 2009. Pneumococcal capsular polysaccharide structure predicts serotype prevalence. PLoS Pathog 5:e1000476. 10.1371/journal.ppat.1000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldman C, Anderson R. 2014. Review: current and new generation pneumococcal vaccines. J Infect 69:309–325. 10.1016/j.jinf.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence H, Pick H, Baskaran V, Daniel P, Rodrigo C, Ashton D, Edwards-Pritchard RC, Sheppard C, Eletu SD, Litt D, Fry NK, Rose S, Trotter C, McKeever TM, Lim WS. 2020. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against vaccine serotype pneumococcal pneumonia in adults: a case-control test-negative design study. PLoS Med 17:e1003326. 10.1371/journal.pmed.1003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marra F, Vadlamudi NK. 2019. Efficacy and safety of the pneumococcal conjugate-13 valent vaccine in adults. Aging Dis 10:404–418. 10.14336/AD.2018.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu J, Li B, Chen X, Lu J, Wang D, Gu T, Kong W, Wu Y. 2018. Comparison of immunogenicity and protection of two pneumococcal protein vaccines based on PsaA and PspA. Infect Immun 86:e00916-17. 10.1128/IAI.00916-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu J, Sun T, Wang D, Dong Y, Xu M, Hou H, Kong FT, Liang C, Gu T, Chen P, Sun S, Lv X, Jiang C, Kong W, Wu Y. 2015. Protective immune responses elicited by fusion protein containing PsaA and PspA fragments. Immunol Invest 44:482–496. 10.3109/08820139.2015.1037956. [DOI] [PubMed] [Google Scholar]
- 8.Bratthauer GL. 2010. The avidin-biotin complex (ABC) method and other avidin-biotin binding methods. Methods Mol Biol 588:257–270. 10.1007/978-1-59745-324-0_26. [DOI] [PubMed] [Google Scholar]
- 9.Said HM. 2012. Biotin: biochemical, physiological and clinical aspects. Subcell Biochem 56:1–19. 10.1007/978-94-007-2199-9_1. [DOI] [PubMed] [Google Scholar]
- 10.Attwood PV, Wallace JC. 2002. Chemical and catalytic mechanisms of carboxyl transfer reactions in biotin-dependent enzymes. Acc Chem Res 35:113–120. 10.1021/ar000049+. [DOI] [PubMed] [Google Scholar]
- 11.Piketty ML, Polak M, Flechtner I, Gonzales-Briceno L, Souberbielle JC. 2017. False biochemical diagnosis of hyperthyroidism in streptavidin-biotin-based immunoassays: the problem of biotin intake and related interferences. Clin Chem Lab Med 55:780–788. 10.1515/cclm-2016-0606. [DOI] [PubMed] [Google Scholar]
- 12.Lotierzo M, Tse Sum Bui B, Florentin D, Escalettes F, Marquet A. 2005. Biotin synthase mechanism: an overview. Biochem Soc Trans 33:820–823. 10.1042/BST0330820. [DOI] [PubMed] [Google Scholar]
- 13.Lesch HP, Kaikkonen MU, Pikkarainen JT, Yla-Herttuala S. 2010. Avidin-biotin technology in targeted therapy. Expert Opin Drug Deliv 7:551–564. 10.1517/17425241003677749. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto T, Aoki K, Sugiyama A, Doi H, Kodama T, Shimizu Y, Kanai M. 2015. Design and synthesis of biotin analogues reversibly binding with streptavidin. Chem Asian J 10:1071–1078. 10.1002/asia.201500120. [DOI] [PubMed] [Google Scholar]
- 15.Sano T, Vajda S, Cantor CR. 1998. Genetic engineering of streptavidin, a versatile affinity tag. J Chromatogr B Biomed Sci Appl 715:85–91. 10.1016/S0378-4347(98)00316-8. [DOI] [PubMed] [Google Scholar]
- 16.Le Q, Nguyen V, Park S. 2019. Recent advances in the engineering and application of streptavidin-like molecules. Appl Microbiol Biotechnol 103:7355–7365. 10.1007/s00253-019-10036-5. [DOI] [PubMed] [Google Scholar]
- 17.Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, Yu T, Vunjak-Novakovic G. 2015. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 37:194–207. 10.1016/j.biomaterials.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dundas CM, Demonte D, Park S. 2013. Streptavidin-biotin technology: improvements and innovations in chemical and biological applications. Appl Microbiol Biotechnol 97:9343–9353. 10.1007/s00253-013-5232-z. [DOI] [PubMed] [Google Scholar]
- 19.Luong JHT, Male KB, Glennon JD. 2019. Biotin interference in immunoassays based on biotin-strept(avidin) chemistry: an emerging threat. Biotechnol Adv 37:634–641. 10.1016/j.biotechadv.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Sedlak SM, Bauer MS, Kluger C, Schendel LC, Milles LF, Pippig DA, Gaub HE. 2017. Monodisperse measurement of the biotin-streptavidin interaction strength in a well-defined pulling geometry. PLoS One 12:e0188722. 10.1371/journal.pone.0188722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sano T, Pandori MW, Chen X, Smith CL, Cantor CR. 1995. Recombinant core streptavidins. A minimum-sized core streptavidin has enhanced structural stability and higher accessibility to biotinylated macromolecules. J Biol Chem 270:28204–28209. 10.1074/jbc.270.47.28204. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Li B, Yu J, Zhang Y, Mo Z, Gu T, Kong W, Zhang Y, Wu Y. 2019. Comparison of four adjuvants revealed the strongest protection against lethal pneumococcal challenge following immunization with PsaA-PspA fusion protein and AS02 as adjuvant. Med Microbiol Immunol 208:215–226. 10.1007/s00430-019-00579-9. [DOI] [PubMed] [Google Scholar]
- 23.Deshpande NU, Jayakannan M. 2018. Biotin-tagged polysaccharide vesicular nanocarriers for receptor-mediated anticancer drug delivery in cancer cells. Biomacromolecules 19:3572–3585. 10.1021/acs.biomac.8b00833. [DOI] [PubMed] [Google Scholar]
- 24.Komarova BS, Orekhova MV, Tsvetkov YE, Beau R, Aimanianda V, Latge JP, Nifantiev NE. 2015. Synthesis of a pentasaccharide and neoglycoconjugates related to fungal alpha-(1→3)-glucan and their use in the generation of antibodies to trace Aspergillus fumigatus cell wall. Chemistry 21:1029–1035. 10.1002/chem.201404770. [DOI] [PubMed] [Google Scholar]
- 25.Yang W, Wang M, Ma L, Li H, Huang L. 2014. Synthesis and characterization of biotin modified cholesteryl pullulan as a novel anticancer drug carrier. Carbohydr Polym 99:720–727. 10.1016/j.carbpol.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Cheng M, Ma D, Zhi K, Liu B, Zhu W. 2018. Synthesis of biotin-modified galactosylated chitosan nanoparticles and their characteristics in vitro and in vivo. Cell Physiol Biochem 50:569–584. 10.1159/000494169. [DOI] [PubMed] [Google Scholar]
- 27.Cho E, Kwon C, Lee S, Tahir MN, Park S, Jung S. 2014. Biotinylation of the rhizobial cyclic beta-glucans and succinoglycans crucial for symbiosis with legumes. Carbohydr Res 389:141–146. 10.1016/j.carres.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Yang H, Wu Y, Gan C, Yue T, Yuan Y. 2016. Characterization and antioxidant activity of a novel polysaccharide from Pholidota chinensis Lindl. Carbohydr Polym 138:327–334. 10.1016/j.carbpol.2015.11.071. [DOI] [PubMed] [Google Scholar]
- 29.Arvaniti A, Karamanos NK, Dimitracopoulos G, Anastassiou ED. 1994. Isolation and characterization of a novel 20-kDa sulfated polysaccharide from the extracellular slime layer of Staphylococcus epidermidis. Arch Biochem Biophys 308:432–438. 10.1006/abbi.1994.1061. [DOI] [PubMed] [Google Scholar]
- 30.Bonato PS, de Abreu LR, de Gaitani CM, Lanchote VL, Bertucci C. 2000. Enantioselective HPLC analysis of propafenone and of its main metabolites using polysaccharide and protein-based chiral stationary phases. Biomed Chromatogr 14:227–233. . [DOI] [PubMed] [Google Scholar]
- 31.Melin M, Jarva H, Siira L, Meri S, Kayhty H, Vakevainen M. 2009. S. pneumoniae capsular serotype 19F is more resistant to C3 deposition and less sensitive to opsonophagocytosis than serotype 6B. Infect Immun 77:676–684. 10.1128/IAI.01186-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lees A, Barr JF, Gebretnsae S. 2020. Activation of soluble polysaccharides with 1-cyano-4-dimethylaminopyridine tetrafluoroborate (CDAP) for use in protein-polysaccharide conjugate vaccines and immunological reagents. III Optimization of CDAP activation. Vaccines (Basel) 8:777. 10.3390/vaccines8040777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lees A, Nelson BL, Mond JJ. 1996. Activation of soluble polysaccharides with 1-cyano-4-dimethylaminopyridinium tetrafluoroborate for use in protein-polysaccharide conjugate vaccines and immunological reagents. Vaccine 14:190–198. 10.1016/0264-410X(95)00195-7. [DOI] [PubMed] [Google Scholar]
- 34.Zhang F, Lu Y-J, Malley R. 2013. Multiple antigen-presenting system (MAPS) to induce comprehensive B- and T-cell immunity. Proc Natl Acad Sci USA 110:13564-9. 10.1073/pnas.1307228110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Guo X, Guo M, Chen X, Li B, Yu J, Gu T, Kong W, Wu Y. 2019. Combined prime-boost immunization with systemic and mucosal pneumococcal vaccines based on Pneumococcal surface protein A to enhance protection against lethal pneumococcal infections. Immunol Res 67:398–407. 10.1007/s12026-019-09107-6. [DOI] [PubMed] [Google Scholar]
- 36.Lu YJ, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, Kolls JK, Srivastava A, Lundgren A, Forte S, Thompson CM, Harney KF, Anderson PW, Lipsitch M, Malley R. 2008. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog 4:e1000159. 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jarva H, Janulczyk R, Hellwage J, Zipfel PF, Bjorck L, Meri S. 2002. S. pneumoniae evades complement attack and opsonophagocytosis by expressing the pspC locus-encoded Hic protein that binds to short consensus repeats 8–11 of factor H. J Immunol 168:1886–1894. 10.4049/jimmunol.168.4.1886. [DOI] [PubMed] [Google Scholar]
- 38.Zhu R, Zhou S, Peng W, Huang Y, Mirzaei P, Donohoo K, Mechref Y. 2018. Enhanced quantitative LC-MS/MS analysis of N-linked glycans derived from glycoproteins using sodium deoxycholate detergent. J Proteome Res 17:2668–2678. 10.1021/acs.jproteome.8b00127. [DOI] [PubMed] [Google Scholar]
- 39.Arnold U, Ulbrich-Hofmann R. 1999. Quantitative protein precipitation from guanidine hydrochloride-containing solutions by sodium deoxycholate/trichloroacetic acid. Anal Biochem 271:197–199. 10.1006/abio.1999.4149. [DOI] [PubMed] [Google Scholar]
- 40.Yu L, Wolin MJ. 1972. Separation of the primary dehydrogenase from the cytochromes of the nicotinamide adenine dinucleotide (reduced form) oxidase of Bacillus megaterium. J Bacteriol 109:59–68. 10.1128/jb.109.1.59-68.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leyva A, Quintana A, Sanchez M, Rodriguez EN, Cremata J, Sanchez JC. 2008. Rapid and sensitive anthrone-sulfuric acid assay in microplate format to quantify carbohydrate in biopharmaceutical products: method development and validation. Biologicals 36:134–141. 10.1016/j.biologicals.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Song RQ, Nan TG, Yuan Y, Jin Y, Yang Q, Zhang M, Hu KY. 2019. Study on polysaccharide content and monosaccharide composition of Polyporus umbellatus from different production areas. Zhongguo Zhong Yao Za Zhi 44:3608–3614. (In Chinese.) 10.19540/j.cnki.cjcmm.20190701.103. [DOI] [PubMed] [Google Scholar]
- 43.Yu Y, Ye H, Wu D, Shi H, Zhou X. 2019. Chemoenzymatic quantification for monitoring unpurified polysaccharide in rich medium. Appl Microbiol Biotechnol 103:7635–7645. 10.1007/s00253-019-10042-7. [DOI] [PubMed] [Google Scholar]
- 44.Nolan KM, Bonhomme ME, Schier CJ, Green T, Antonello JM, Murphy RD. 2020. Optimization and validation of a microcolony multiplexed opsonophagocytic killing assay for 15 pneumococcal serotypes. Bioanalysis 12:1003–1020. 10.4155/bio-2020-0024. [DOI] [PubMed] [Google Scholar]
- 45.Sterrett S, Peng BJ, Burton RL, LaFon DC, Westfall AO, Singh S, Pride M, Anderson AS, Ippolito GC, Schroeder HW, Jr, Nahm MH, Krishna Prasad A, Goepfert P, Bansal A. 2020. Peripheral CD4 T follicular cells induced by a conjugated pneumococcal vaccine correlate with enhanced opsonophagocytic antibody responses in younger individuals. Vaccine 38:1778–1786. 10.1016/j.vaccine.2019.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramachandran G, Boyd MA, MacSwords J, Higginson EE, Simon R, Galen JE, Pasetti MF, Levine MM, Tennant SM. 2016. Opsonophagocytic assay to evaluate immunogenicity of nontyphoidal salmonella vaccines. Clin Vaccine Immunol 23:520–523. 10.1128/CVI.00106-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi W, Kim JG, Beom SH, Hwang JE, Shim HJ, Cho SH, Shin MH, Jung SH, Chung IJ, Song JY, Bae WK. 2020. Immunogenicity and optimal timing of 13-valent pneumococcal conjugate vaccination during adjuvant chemotherapy in gastric and colorectal cancer: a randomized controlled trial. Cancer Res Treat 52:246–253. 10.4143/crt.2019.189. [DOI] [PMC free article] [PubMed] [Google Scholar]