ABSTRACT
Plasmodium falciparum cysteine-rich protective antigen (CyRPA) is a conserved component of an essential erythrocyte invasion complex (RH5/Ripr/CyRPA) and a target of potent cross-strain parasite-neutralizing antibodies. While naturally acquired human RH5 antibodies have been functionally characterized, there are no similar reports on CyRPA. Thus, we analyzed the parasite-neutralizing activity of naturally acquired human CyRPA antibodies. In this regard, CyRPA human antibodies were measured and purified from malaria-infected plasma obtained from patients in central India and analyzed for their parasite neutralizing activity via in vitro growth inhibition assays (GIA). We report that, despite being susceptible to antibodies, CyRPA is a highly conserved antigen that does not appear to be under substantial immune selection pressure, as a very low acquisition rate for anti-CyRPA antibodies was reported in malaria-exposed Indians. We demonstrate for the first time that the small amounts of natural CyRPA antibodies exhibited functional parasite-neutralizing activity and that a CyRPA-based vaccine formulation induces highly potent antibodies in rabbits. Importantly, the vaccine-induced CyRPA antibodies exhibited a robust 50% inhibitory concentration (IC50) of 21.96 μg/ml, which is comparable to the IC50 of antibodies against the leading blood-stage vaccine candidate, reticulocyte-binding-like homologous protein 5 (RH5). Our data support CyRPA as a unique vaccine target that is highly susceptible to immune attack but is highly conserved compared to other leading candidates such as MSP-1 and AMA-1, further substantiating its promise as a leading blood-stage vaccine candidate.
KEYWORDS: Plasmodium falciparum, malaria vaccine, RH5, CyRPA, naturally acquired immunity, growth inhibition assay
INTRODUCTION
Malaria remains a major global health challenge, accounting for 200 million cases and around 0.4 million deaths each year, primarily in children under 5 years of age (1). The disease severity and clinical manifestations are a result of the parasite’s blood-stage life cycle (2, 3). Several merozoite antigens have been identified to play a critical role during erythrocyte invasion, of which Plasmodium falciparum reticulocyte-binding-like homologous protein 5 (RH5) has emerged as a key invasion determinant that plays an essential role during P. falciparum erythrocyte invasion (4, 5). Antibodies targeting RH5 or its erythrocyte receptor, basigin, exhibit potent parasite neutralization in vitro and in vivo (6–10). RH5-based vaccines were found to be safe and immunogenic in a phase 1a controlled human malaria infection-based clinical trial (10–12).
The molecular redundancy associated with the highly rapid P. falciparum erythrocyte invasion process presents a major hurdle to achieving high antibody titers for efficacious neutralization that is unlikely to be overcome by a vaccine based on a single antigen like RH5 (13, 14). Thus, a promising approach to circumvent the problem is to develop a multicomponent malaria vaccine that targets multiple parasite antigens and generate a highly robust, synergistic, strain-transcending antibody response that is effective at low concentrations and is able to abrogate the highly rapid process of erythrocyte invasion.
In this regard, P. falciparum cysteine-rich protective antigen (CyRPA), an essential parasite antigen, has shown promise as a potential blood-stage malaria vaccine candidate (15, 16). The antigen forms an essential multiprotein complex with RH5 and RH5-interacting protein (Ripr), thereby facilitating binding of RH5 to basigin (16–18). CyRPA lacks erythrocyte binding activity, but antibodies targeting CyRPA mediated highly potent in vitro parasite neutralization across worldwide parasite strains and exhibited a synergistic growth-inhibitory effect with RH5 antibodies (16, 19, 20). The inhibitory potential of these antibodies is attributed to their ability to prevent CyRPA interaction with Ripr and RH5, leading to the abrogation of the complex and downstream signaling events (17, 18, 21, 22). Importantly, passively transferred CyRPA monoclonal antibodies significantly reduced the parasite load in an immunocompromised P. falciparum mouse model, demonstrating the in vivo efficacy of CyRPA antibodies (15, 20). The exact functional role of CyRPA is unknown, but the antigen is suggested to mediate Ca2+-mediated signaling events required for the downstream invasion steps. Super-resolution microscopy combined with conditional knockouts of CyRPA and Ripr showed that the binding of the trimeric complex, RH5/Ripr/CyRPA to basigin is necessary for triggering Ca2+ release and subsequent formation of the tight junction complex (17).
Additionally, in a recent immune-epidemiological study, a positive correlation was observed between naturally acquired human CyRPA antibodies and protection from subsequent malaria infection (23). We recently demonstrated that antibody combinations based on CyRPA (RH5 and MSP-119) exhibit highly potent neutralization of P. falciparum clinical isolates (24). Furthermore, CyRPA exhibited complete sequence conservation in >200 worldwide P. falciparum clinical isolates (24). Together, these studies strongly support CyRPA as a highly promising blood-stage vaccine target.
Growth inhibition assay (GIA) is a well-established in vitro assay that is widely used to assess the vaccine potential of merozoite antigens by analyzing the efficacy of merozoite-specific antibodies in inhibiting erythrocyte invasion by P. falciparum (9, 13, 25, 26). Importantly, GIA activity has been used as a mechanistic correlate of protection against malaria. In this regard, vaccine induced RH5 antibodies were demonstrated to exhibit GIA activity, which correlated with its ability to impart in vivo protection against malaria in a nonhuman primate model and in controlled human malaria infection (CHMI) trials (12, 27). Additionally, naturally acquired antibodies against RH5 and MSP-119 have been demonstrated to neutralize the parasite in GIA, thus substantiating to their ability to provide in vivo protection from malaria (28–30).
CyRPA has been shown to elicit cross-strain-neutralizing antibodies in small animals against diverse P. falciparum strains (15, 16). However, there is no previous study that has characterized the functional growth inhibitory activity of naturally acquired CyRPA-specific human antibodies, which would help understand whether functional antibody responses are induced by natural P. falciparum infections that could potentially be augmented by a vaccine or vice versa. To address this important question, we carried out the present study, in which we showed that CyRPA-specific human antibodies, purified from plasma of malaria-infected individuals, were functionally active and exhibited parasite neutralization in GIA. We further demonstrated that the functional parasite-neutralizing activity of CyRPA antibodies can be boosted upon CyRPA vaccination. Our study, for the first time, provides experimental evidence of a protective role of CyRPA-specific human antibodies and lends support for the inclusion of CyRPA as a component of a next-generation combination blood-stage malaria vaccine.
(This work was previously presented at the World Malaria Day Symposium 2021 [Virtual Scientific Symposium] held by the Johns Hopkins Malaria Research Institute, Baltimore, Maryland, USA.)
RESULTS
Production and characterization of full-length CyRPA.
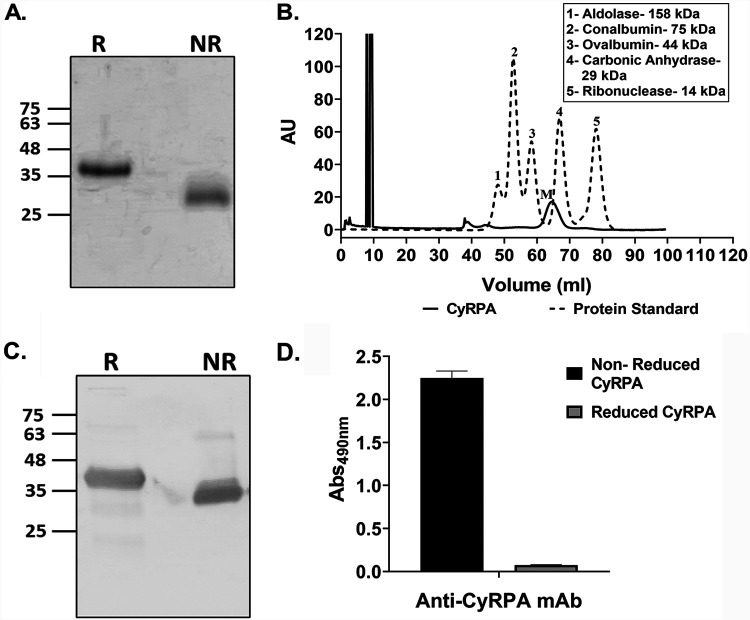
The full-length CyRPA (R31 to E362; 3D7) gene was codon optimized and expressed with a C-terminal 6×His tag as inclusion bodies in Escherichia coli. CyRPA inclusion bodies were solubilized and enriched by immobilized metal affinity chromatography (IMAC) under denaturing conditions, followed by refolding. The refolded protein was finally purified to homogeneity by ion exchange chromatography (Fig. 1A). Size exclusion chromatography (SEC) confirmed the monomeric state of the purified protein (Fig. 1B). The identity and conformation of the purified protein were confirmed by immunoblotting and enzyme-linked immunosorbent assay (ELISA) using anti-6×His monoclonal antibody (MAb) (Fig. 1C) and a conformation-specific CyRPA MAb (c10), respectively (Fig. 1D).
FIG 1.
Production of recombinant full-length CyRPA in Escherichia coli. (A) Purified CyRPA was analyzed on an 12% SDS-PAGE gel with Coomassie brilliant blue under reducing and nonreducing conditions. The resulting shift between the nonreduced and reduced CyRPA was due to faster migration of the former because of its folded, globular structure, which confirmed the successful refolding of CyRPA. (B) The monomeric state of the purified CyRPA was confirmed by its analysis through size exclusion chromatography (SEC) on a Superdex 75 (16/600) instrument. The size exclusion chromatography (SEC) elution profile showed that CyRPA was eluted at a volume corresponding to its monomeric state (M), as evident by the overlap of its elution profile with the protein standards, in which the CyRPA peak lies close to the peak corresponding to 29-kDa carbonic anhydrase. (C) Purified CyRPA was detected in immunoblots with anti-6×His monoclonal antibody to confirm its identity. (D) The conformation of purified CyRPA was confirmed in enzyme-linked immunosorbent assay (ELISA) by the ability of a conformation-specific anti-CyRPA monoclonal antibody (c10) (15, 43) to recognize CyRPA only in the nonreduced state and not in the reduced form. R, reduced; NR, nonreduced; AU, arbitrary unit(s); Abs490nm, absorbance at 490 nm.
CyRPA reactivity in malaria-infected human plasma.
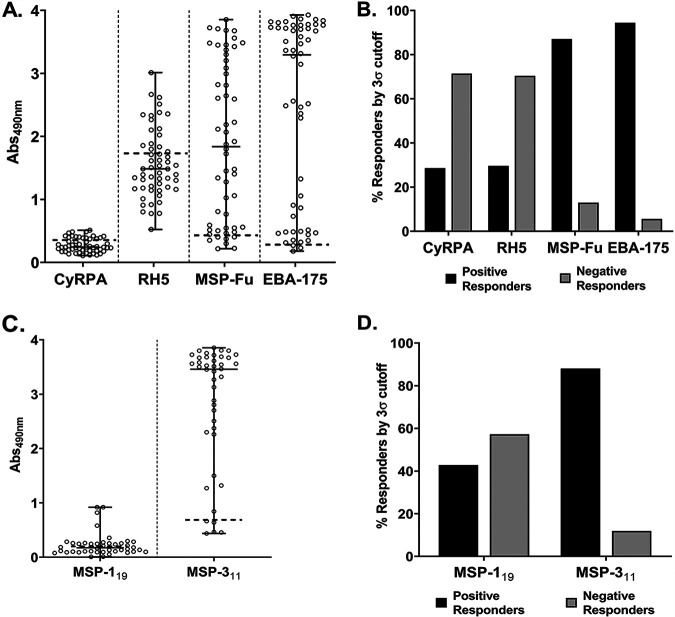
We measured antibody titers against CyRPA in the plasma of 54 malaria-infected individuals from a region in central India where malaria is endemic (Balaghat, Madhya Pradesh) by ELISA at a plasma dilution of 1:200. Samples from five malaria-naive individuals were used as negative controls and to calculate the cutoff optical density (OD) for defining the positively and negatively responding plasma samples. A plasma sample with an OD above the cutoff was described as a positive responder and those with ODs below the cutoff were described as negative responders. The total number of positive responders was used to calculate their percentage (the positivity rate).
The immune reactivity of CyRPA was low, which is consistent with previous reports (15, 19, 23). Around 30% of plasma samples reacted positively to CyRPA, with the response in range of ∼0.37 to 0.47 ELISA OD units (Fig. 2A and B). For comparison, immunogenicity of other major merozoite antigens, namely, EBA-175, RH5, and MSP-Fu (chimera of MSP-119 plus MSP-3) (31, 32), was also analyzed in these plasma samples. Consistent with earlier reports, EBA-175 and MSP-Fu (MSP-119 and MSP-3 were both immunogenic [Fig. 2C and D]) reacted strongly with the human plasma, exhibiting high positivity rates of 94% and 87%, respectively, whereas only 30% of samples were positive for RH5, similar to the positivity rate for CyRPA (Fig. 2A and B).
FIG 2.
Detection of naturally acquired antibody response against CyRPA. (A) Antibody response against CyRPA and other key merozoite antigens, namely RH5, MSP-Fu, and EBA-175, were analyzed in plasma from 54 malaria-infected individuals obtained from a region in central India where malaria is endemic, Balaghat (Madhya Pradesh), by enzyme-linked immunosorbent assay (ELISA). CyRPA exhibited detectable levels of antibodies, with absolute ELISA values ranging from ∼0.1 to 0.48 optical density (OD) units. However, MSP-Fu and EBA-175 exhibited high immunogenicity, with values ranging from 0.29 to 3.8 and 0.17 to 3.9 ELISA OD units, respectively. Similarly, RH5 had higher ELISA OD unit values (0.5 to 3.0) than CyRPA. Note that the recombinantly produced F2 domain region of EBA-175 was used in ELISA to analyze the immunogenicity against EBA-175. (B) Among 54 plasma samples, only ∼28% showed a positive response against CyRPA. Similarly, RH5 was positively detected in only ∼30% samples. On the other hand, EBA-175 and MSP-Fu showed high seropositivity, with ∼94% and ∼87% samples, respectively, exhibiting a positive response. (C) The immunogenicity of individual components of MSP-Fu, MSP-119, and MSP-311 was also analyzed in the malaria-infected plasma samples. MSP-311 exhibited a high antibody response, with ELISA OD values ranging from 0.4 to 3.8 units. MSP-119 antibody response was low compared to that of MSP-311 antibody, ranging between 0.01 and 0.9 ELISA OD units. (D) Percent seropositivity of MSP-119 and MSP-311 shows that around 43% of individuals were positive for MSP-119-specific antibodies, while ∼88% of plasma samples exhibited a positive response for MSP-311. The mean OD of control malaria-naive samples, 5, plus 3× the standard deviation was taken as a cutoff and was used to calculate the percentages of positive and negative responders. An ELISA OD value above the cutoff was described as a positive response, and a value below the cutoff was considered a negative response. (A and C) Graph showing median values and ranges; a horizontal dashed line indicates the cutoff.
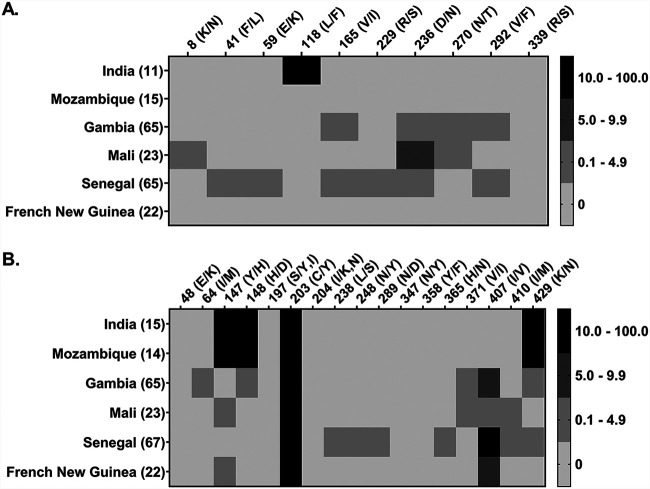
The fact that the malaria-infected plasma samples had detectable levels of CyRPA antibody only suggests that the antigen could be under low immune selection pressure. Furthermore, in our recently published study, analyses of CyRPA sequences in P. falciparum clinical isolates obtained from the same group of malaria-infected individuals whose plasma were used in this study identified only one single-nucleotide polymorphism (SNP) (L118F) in two clinical isolates (RM09 and DP11) (24). Besides, CyRPA was completely conserved in >200 clinical isolates from other regions worldwide. In contrast, despite RH5 exhibiting a similar immunogenicity profile to that of CyRPA, with ∼30% positivity, the antigen exhibited a higher level of sequence variation, with 5 SNPs observed in the same set of clinical isolates used for CyRPA sequence analysis. Global analysis of SNPs in RH5 in >200 field isolates also revealed that the antigen is relatively less conserved than CyRPA (24). A comparison of the SNPs and their frequencies for CyRPA and RH5 is shown in Fig. 3. While further investigations are needed to correctly understand the effect of CyRPA and RH5 sequence polymorphism on their immunogenicity, the present data, along with previous reports (15, 24, 33, 34), indicate a stronger balancing selection pressure on RH5 than that on CyRPA, suggesting that the latter is less prone to acquire sequence polymorphism.
FIG 3.
Comparative analysis of sequence polymorphism in CyRPA and RH5. Based on a recent study by our group (24), we generated a map to show a head-to-head comparison of sequence polymorphism in CyRPA (A) and RH5 (B) in P. falciparum clinical isolates (201 isolates for CyRPA and 206 isolates for RH5) from 6 countries where malaria is endemic, including India. The Indian samples were from the same batch of individuals whose plasma were analyzed in the present study. Overall, CyRPA (A) was observed to have 10 polymorphic amino acid positions. However, only one single-nucleotide polymorphism (SNP) at position 118 (L118F) in two Indian clinical isolates was found to be significant (frequency ≥ 10%); it is shown in a black square in the map. All other SNPs were present at much lower frequencies, below 10%, and hence do not seem to be the target of balancing selection. On the other hand, RH5 (B) was observed to have 17 polymorphic sites. A total of 5 SNPs (Y147H, H148D, C203Y, I407V, and K429N) at 5 amino acid positions with >10% frequency across the six countries were observed in RH5. Out of the 5 SNPs, 4 were present in the clinical isolates of Indian origin. These data strongly suggest that CyRPA is highly conserved and is under lower immune selection pressure than RH5. Black and gray color coding are used to show the frequency distribution of SNPs in each country. Different shades of gray represent different frequencies of <10%. A frequency of ≥10% is depicted in a black square. The left x axis in the maps shows the country name, and the number in brackets represents the number of P. falciparum clinical isolates analyzed. The top y axis in the maps shows the amino acid position and the type of amino acid change arising due to the presence of an SNP. The 3D7 gene sequence was used as a reference for identifying SNPs.
Naturally acquired human CyRPA-specific antibodies inhibit erythrocyte invasion by P. falciparum merozoites.
We and others have previously reported highly potent cross-strain parasite neutralization activity of CyRPA antibodies, raised in small animals, in in vitro GIA (16, 19, 35). In a separate study from our laboratory, we found that CyRPA elicits highly potent parasite-neutralizing antibodies that, in combination with other antibodies (MSP-119 and RH5), strongly inhibited erythrocyte invasion by P. falciparum clinical isolates (24). However, there is no report on the parasite neutralizing activity of CyRPA human antibodies induced during natural malaria infections. To this end, we purified CyRPA-specific human antibodies from the pooled human plasma and tested them for their functional activity in GIA. Due to low immunogenicity in the plasma samples, the final yield of CyRPA antibodies was not high, only achieving a final concentration of 0.5 mg/mL. The final purified antibodies were specific for CyRPA and did not cross-react with other antigens (MSP-Fu), as confirmed through ELISA (Fig. 4).
FIG 4.
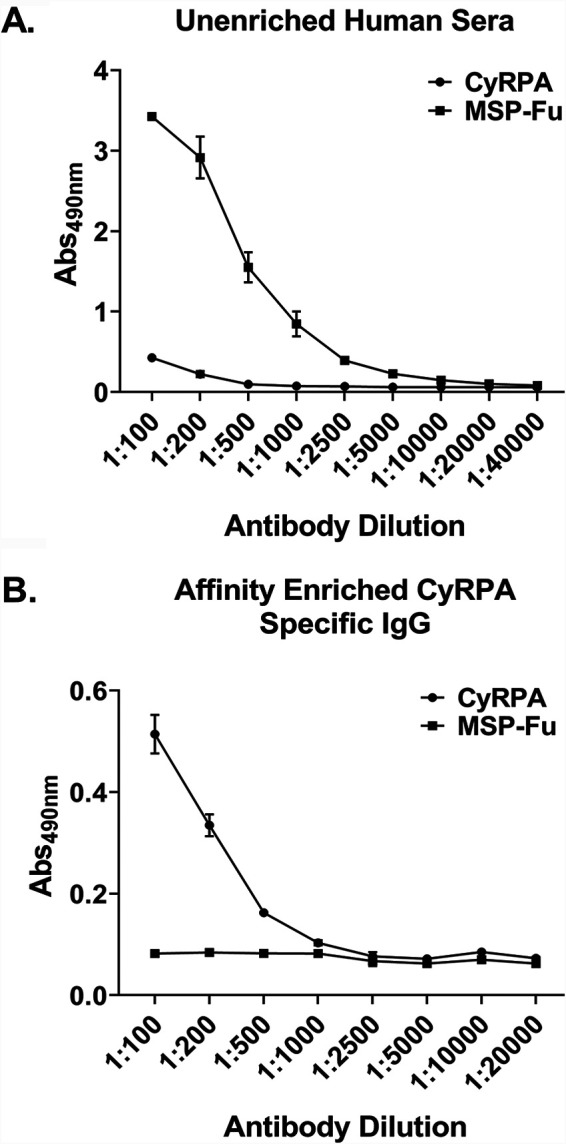
Specificity of purified naturally acquired human antibodies against CyRPA. (A) Plasma samples from the malaria-infected individuals were pooled, and antibody response against CyRPA and MSP-Fu (chimera of MSP-119 plus MSP-3) was assessed by ELISA before affinity purification of CyRPA-specific human antibodies. CyRPA and MSP-Fu both showed an immunogenic response in the pooled plasma before enrichment. In comparison, MSP-Fu exhibited a much stronger response than CyRPA, as shown in Fig. 2. (B) Affinity-purified CyRPA-specific naturally acquired human antibodies were assessed through ELISA by their ability to recognize CyRPA and MSP-Fu. In the affinity-purified antibody sample, the antibodies detected only CyRPA and failed to cross-react with MSP-Fu, thus confirming the specificity of the affinity-purified naturally acquired CyRPA human antibodies.
We next performed GIA with the affinity-purified CyRPA human antibodies. Due to the limited yield of purified IgG, the GIA was performed only to a maximum IgG concentration of 250 μg/mL in a one-cycle assay against a single P. falciparum laboratory clone, 3D7. It is noteworthy that at the relatively low concentration of 250 μg/ml, the human CyRPA-specific antibodies exhibited significant parasite-neutralizing activity, achieving a maximum inhibition of ∼44% (Fig. 5). The GIA activity of naturally acquired CyRPA antibodies not only provides evidence about their functionality but also gives support to the idea that these antibodies can give protection from malaria reinfection during natural exposure, since GIA has been suggested by many studies to be a surrogate marker of in vivo protection (12, 27).
FIG 5.
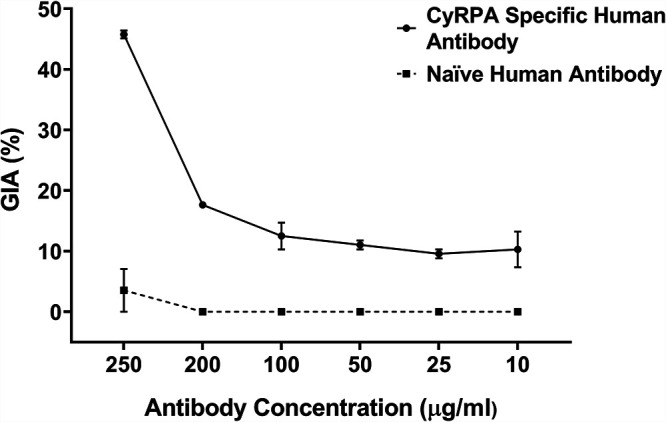
Invasion inhibitory potential of affinity purified CyRPA specific naturally acquired human antibodies. The purified CyRPA specific human antibodies were tested in the standard in vitro growth inhibition assay (GIA) in a one-cycle assay against 3D7. The graph shows GIA with CyRPA-specific human antibody (black line) and naive human antibody (dashed line), calculated against a nonantibody control. A dose-dependent inhibitory response was observed, and a maximum inhibition of ∼44% was achieved at 250 μg/mL IgG concentration. Data represent the average of two independent experiments conducted in duplicate. Error bars represent the standard error between the two independent GIA experiments.
Vaccine induced CyRPA antibodies mediate highly potent parasite neutralization.
We next studied whether the efficacy of CyRPA antibodies can be further augmented through CyRPA vaccination. We therefore immunized rabbits with CyRPA-CFA/IFA formulation, which induced high antibody titer on day 70 postpriming (Fig. 6A). The day 70 serum was used to affinity purify CyRPA-specific IgG using CyRPA-coupled CNBr-Sepharose beads. The affinity purification resulted in an 11-fold enrichment of affinity purified CyRPA-specific antibodies compared to the unenriched serum (Fig. 6A).
FIG 6.
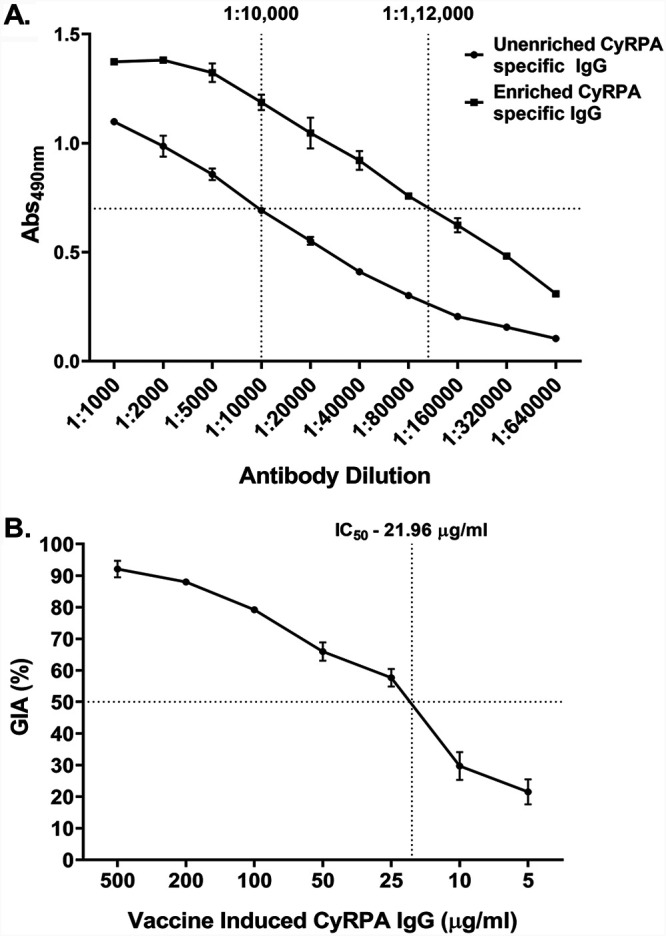
Parasite neutralization activity of vaccine-induced anti-CyRPA antibodies. (A) Serum from CyRPA-immunized rabbit was used to affinity purify CyRPA-specific antibodies. The affinity-purified antibodies were enriched 11-fold, confirmed by comparison of OD values between enriched and unenriched anti-CyRPA rabbit IgG though ELISA. The fold enrichment was determined by identifying the antibody dilution of the unenriched and enriched sera that gives the same OD value for both, as shown by dashed lines. (B) Enriched, affinity-purified anti-CyRPA IgG from rabbit was tested for parasite neutralizing activity using the in vitro GIA against 3D7 in a one-cycle assay. A highly potent dose-dependent inhibition was observed that reached >90% at 500 μg/mL IgG concentration. The 50% inhibitory concentration (IC50; the IgG concentration required to induce 50% growth inhibition), calculated using nonlinear least-squares regression (described in Materials and Methods) (44), was observed to be 21.96 μg/mL and is indicated by dashed lines. Data represent the average of two independent experiments conducted in duplicate. Error bars represent the standard error between the two independent GIA experiments.
The vaccine-induced CyRPA antibodies were tested in GIA at various concentrations in a one cycle assay against 3D7. The antibodies exhibited highly potent dose-dependent parasite invasion inhibition, with a >90% inhibition observed at the highest tested IgG concentration, 500 μg/ml. (Fig. 6B). The most advanced and potent blood-stage vaccine candidate, RH5, has been reported to induce parasite-neutralizing antibodies that exhibit an IC50 of ∼10 to 15 μg/ml (13). We calculated the IC50 of the affinity-purified anti-CyRPA vaccine-induced rabbit antibodies to be 21.96 μg/ml, which is comparable to that for RH5 antibodies (Fig. 6B). Thus, these results suggest that the immunogenicity and potency of CyRPA antibodies may be boosted by immunizing against the antigen in a vaccine formulation.
DISCUSSION
Erythrocyte invasion is responsible for malaria pathogenesis and disease severity (2, 3). Importantly, repeated exposure to blood-stage parasites over a time period leads to the development of clinical immunity, known as naturally acquired immunity (36), that is primarily directed against the antigens on infected red blood cells (RBCs) and merozoite surface antigens like EMP-1, AMA-1, MSP-1, erythrocyte-binding antigens (EBAs), and reticulocyte-binding-like homologous (RH) proteins, thus making them promising vaccine targets (28, 37–39). We have recently identified a novel antigen combination (RH5 plus CyRPA plus MSP-119) that elicits potent parasite-neutralizing antibodies against P. falciparum laboratory clones and clinical isolates exhibiting diverse invasion phenotypes. While RH5 and MSP-119 have also been demonstrated to elicit invasion-inhibitory antibodies during natural malaria exposure, no such report exists for CyRPA. Here, we show that naturally acquired CyRPA human antibodies exhibit parasite-neutralizing activity that can be further augmented by a CyRPA-based vaccine.
We found that CyRPA induced humoral response in regions where malaria is endemic, but, as reported earlier (15, 19, 23), the immunogenicity of CyRPA was low in these plasma samples compared to that of other key merozoite antigens like MSP-1, MSP-3, EBA-175, etc., which exhibited high antibody titers. Several possible reasons might explain the low natural immunogenicity of CyRPA, ranging from its intracellular localization to its specific spatial positioning in the multiprotein complex RH5/Ripr/CyRPA during erythrocyte invasion. (15, 19, 23). CyRPA is localized inside micronemes and is expressed on the merozoite surface only prior to erythrocyte invasion (15–17), which is an extremely rapid process lasting for only <1 min (14). Thus, a short duration and a time-specific surface expression may restrict the generation of a strong immune response against the antigen. Second, CyRPA does not bind directly to erythrocyte surface but functions as a part of a ternary complex involving RH5 and Ripr on the merozoite surface, such that CyRPA is positioned in between the two proteins (18). Therefore, the formation of this complex may also sterically hinder and limit the exposure of CyRPA to the immune system, thus contributing to its low immunogenicity. A possible implication of a low immunogenic response against CyRPA could be that the antigen would remain silent to immune selection pressure and therefore less likely to accumulate polymorphisms as a strategy to evade host immunity. It was interesting to observe that, despite being part of the same multiprotein complex and eliciting a similar immunogenic response, RH5 exhibited a greater level of polymorphism than CyRPA. We believe that a high level of antigenic polymorphism in RH5 might be a key adaptation required by the parasite to respond to various basigin polymorphisms or to its varied expression levels observed on RBCs in different malaria populations where malaria is endemic or to avoid immune detection during erythrocyte invasion (6, 8, 40). However, further investigations will be needed to ascertain these hypotheses.
We demonstrated that naturally acquired CyRPA antibodies exhibited substantial in vitro parasite erythrocyte invasion inhibition, up to 44% at 250 μg/ml. However, compared to the invasion-inhibitory activity of naturally acquired antibodies against other essential (RH5 and AMA-1) or nonessential (RH2) merozoite antigens, which has been reported to be ∼60% (RH5/RH2) and ∼80% (AMA-1) at 100 μg/mL concentration (29, 41), naturally acquired CyRPA human antibodies exhibited moderate inhibition. We reasoned, as explained earlier, that the specific positioning of CyRPA between RH5 and Ripr in the multiprotein complex (RH5/Ripr/CyRPA) may not only affect its immunogenicity but also restrict exposure of functionally relevant epitopes required for eliciting a potent neutralizing antibody response. In support of this hypothesis, it was observed that immunization of the entire RH5/Ripr/CyRPA complex resulted in an ∼7.5-fold decrease in antibody titer against CyRPA compared to titers elicited against individually immunized CyRPA. Further, the antibodies raised against the ternary complex were less efficacious at inhibiting parasite erythrocyte invasion than antibodies raised against CyRPA alone (35). These findings clearly indicate a negative effect of the RH5/Ripr/CyRPA complex on CyRPA immunogenicity, which consequently could have resulted in lower efficacy of CyRPA-specific antibodies. In addition, analysis of the mechanism of invasion inhibition by a monoclonal CyRPA antibody identified that it acted by abrogating CyRPA/RH5 interaction (21, 35). The binding site of the monoclonal antibody did not exactly overlap the RH5 binding region of CyRPA but was located nearby. There is a high possibility that the RH5-CyRPA interaction not only masks the CyRPA interface region from the human immune response but mediates a similar effect on nearby regions through steric hinderance. A recent cryo-electron microscopy (cryo-EM) structure of RH5/Ripr/CyRPA complex mapped the binding regions of RH5 and Ripr on CyRPA, and it is now crucial to study and compare the efficacy of antibodies targeting these binding regions against that of those targeting the nonbinding regions to get a better understanding of the effect of the RH5/Ripr/CyRPA complex in modulating the host immune response against CyRPA.
Earlier studies have shown that antibodies induced by RH5 vaccination exhibited a highly potent invasion inhibition (IC50, ∼10 to 15 μg/m:) (13) compared to that exhibited by the naturally acquired RH5 human antibodies (IC50, ∼55 μg/mL and ∼110 μg/mL) (29, 30). Our study also shows a highly robust efficacy of CyRPA antibodies induced through vaccination compared to that of those acquired during natural malaria exposure. The vaccine-induced CyRPA antibodies (rabbit) exhibited highly potent parasite neutralization activity, with >90% inhibition at 500 μg/ml. More importantly, we observed a very robust IC50 value of 21.96 μg/mL, which was comparable to the IC50 reported for RH5 antibodies. Besides, the strong efficacy of vaccine-induced CyRPA antibodies also supports our hypothesis that the potency of CyRPA antibodies may be highly compromised when raised through RH5/Ripr/CyRPA coimmunization rather than individual CyRPA immunization; however, a head-to-head comparison to demonstrate this effect is yet to done. On the other hand, in a recent study, an Oxford group reported a moderate efficacy of CyRPA antibodies (IC50, 330 to 470 μg/mL) (33), which is lower than our observation reported here. However, this discrepancy may be attributable to differences in the design of the recombinant antigen and the expression systems used in both studies. We produced full-length CyRPA with a C-terminal 6×His tag in E. coli, while the Oxford group used a mammalian expression system to produce CyRPA antigen with a C-terminal rat CD4, d3+4 domain.
The vaccine-induced CyRPA antibodies exhibited greater potency than that observed with antibodies targeting the other important parasite antigens and the major vaccine targets AMA-1 and MSP-142, which were shown to be around 70 μg/mL and 210 μg/mL, respectively. In the multistep erythrocyte invasion process, MSP-1 participates in the initial attachment, while AMA-1 mediates the tight junction formation. Considering the kinetic constraints associated with highly rapid erythrocyte invasion process, a promising strategy to develop an effective blood-stage vaccine would be to target the key antigens involved at different steps of invasion. In a recently published study, our group identified a triple antigen combination based on RH5, CyRPA, and MSP-1 (MSP-119) that elicits potent cross-strain parasite-neutralizing antibodies against P. falciparum lab clones and clinical isolates (24). Our findings here provide further support for the clinical development of an RH5-, CyRPA-, and MSP-119-based combination. Furthermore, it would be interesting to check combination of these blood-stage antigens with those of other stages (such as CSP and Pfs230) in an approach to generate a multistage-targeting malaria vaccine. Advancements in the development of novel adjuvant platforms such as glucopyranosyl lipid adjuvant (GLA), Adjuvant Systems (AS), liposomes, etc., would further aid in the identification of the ideal vaccine/adjuvant combination that could generate a potent and durable humoral and cell-mediated immune response.
In summary, we demonstrate here for the first time that naturally acquired CyRPA-specific human antibodies are functionally active, exhibiting parasite neutralization activity in the in vitro GIA. We demonstrated that, due to low immunogenicity coupled with a low level of sequence polymorphism, CyRPA is under much lesser balancing selection relative to that on RH5. Moreover, we showed that vaccine-induced CyRPA-specific IgG exhibited highly robust parasite neutralization activity, comparable to the IC50 of the leading blood-stage vaccine candidate, RH5. Our study provides strong evidence for the inclusion of CyRPA in a potent, broadly neutralizing multicomponent blood-stage malaria vaccine.
MATERIALS AND METHODS
Sample collection and ethics approval.
P. falciparum–infected blood samples were collected from patients residing in the regions of Balaghat district (Madhya Pradesh) where malaria is endemic by the National Institute of Research in Tribal Health (NIRTH) (42). Door-to-door fever surveys were carried out in the villages of Balaghat, and blood samples were collected from patients with fever or history of fever within the last 2 weeks, after due consent of the patient or guardian. P. falciparum infection was confirmed by detection of P. falciparum-specific HRP-2 antigen (SD Bioline Malaria Antigen Pf/Pv; Bio Standard Diagnostics, India) and by microscopic examination of Giemsa-stained blood smears. Patients were treated for P. falciparum per the drug policy of the National Vector Borne Disease Control Programme. All ethical approvals were obtained from the institutional ethical review committees of the concerned institutes per guidelines laid out by the Indian Council of Medical Research (ICMR), Government of India.
Blood samples were transported to the laboratory within a few hours, and plasma samples were obtained after centrifugation of the blood samples. The plasma samples obtained from the regions of Balaghat where malaria is endemic were pooled for affinity-based antibody purification.
For a negative control, blood samples were collected from consenting healthy volunteers from regions where malaria is not endemic who had no history of travelling to regions where malaria is endemic in the last few years. Plasma was obtained after centrifugation at 2,200 to 2,500 rpm for 15 min at 4°C.
Production of recombinant full-length P. falciparum cysteine-rich protective antigen.
A gene sequence corresponding to full-length CyRPA (R31 to E362) sequence of the parasite clone 3D7 was codon optimized (Gene Art; Life Technologies) and subcloned into pET-24b upstream to a 6×His tag-encoding sequence (Novagen). CyRPA-pET24b plasmid construct was transformed into E. coli BL21(DE3) cells for recombinant protein expression, which was observed in inclusion bodies. These inclusion bodies were washed and solubilized in 6 M guanidine hydrochloride, followed by enrichment of the protein by immobilized metal affinity chromatography (IMAC) (Ni-nitrilotriacetic acid [NTA]) under denaturing conditions. The IMAC-purified protein eluates were concentrated and refolded in a Tris-based buffer (pH 8.0) supplemented with sucrose and arginine under redox conditions (glutathione reduced and glutathione oxidized) by a rapid dilution method at 4°C overnight. In the last step of purification, the refolded protein was dialyzed and subjected to ion exchange chromatography to obtain homogenous monomeric fraction. The monomeric state of the protein was confirmed by running reduced and nonreduced SDS-PAGE, as well as by size exclusion chromatography (Superdex 75 [16/600]; Cytiva Life Sciences). MSP-Fu, RH5, and EBA-175 F2 were produced as described previously (9, 26, 31, 32).
Western blotting and enzyme-linked immunosorbent assay.
Western blotting was done as described previously (9). Briefly, purified protein was transferred onto the nitrocellulose membrane for 2 h in Tris-glycine buffer. The membrane was blocked with 5% skim milk in 1× phosphate-buffered saline (PBS) for 2 h, followed by incubation either with anti-6×His monoclonal antibody (MAb) (1:1,000; G-Biosciences) or anti-CyRPA MAb c10 (15, 43) (15 μg/mL), both raised in mice, for 1 h. The membrane was then incubated with horseradish peroxidase (HRP)-conjugated anti-mouse secondary IgG (1:3,000; Sigma) for 1 h. The Western blot was developed using the substrate 3,3′-diaminobenzidine (DAB) (1 mg/mL; Sigma) in the presence of hydrogen peroxide. After each step, the membrane was washed 3 times with 1× PBST (1× PBS plus 0.05% Tween 20) (and also followed by 1× PBS after a secondary antibody incubation step). All steps were performed at room temperature. For Western blotting in the nonreduced state, the antigen was not treated with β-mercaptoethanol and was not heated.
The method used for performing ELISA was same as that previously reported (26). Briefly, 200 ng of antigen was coated in a 96-well microtiter plate (Nunc Maxisorp) in carbonate-bicarbonate buffer (pH 8.3) at 4°C overnight. The next day, plates were blocked with 2% skim milk prepared in 1× PBS at 37°C for 2 h. Plates were washed three times with 1× PBST, and individual human serum samples (1:200) or CyRPA MAb c10 (15 μg/mL) were added and incubation done at 37°C for 1 h. Plates were washed three times with 1×PBST and incubated with HRP-conjugated anti-human (1:10,000; Sigma) or anti-mouse (1:3,000; Sigma) secondary antibody for 1 h at 37°C. Plates were washed three times with 1× PBST and twice with 1× PBS, followed by development of the reaction using the substrate O-phenylenediamine dihydrochloride (OPD; Sigma) prepared in phosphate-citrate buffer (1 mg/mL; Sigma), in the presence of hydrogen peroxide. After 20 min of incubation with the substrate, the reaction was stopped by adding 1 M sulfuric acid, followed by measurement of optical density (OD) at 490 nm in a microplate reader (Molecular Devices). In ELISA with human plasma samples, a sample was said to be a positive responder if its OD value was greater than the mean of the ODs of malaria-naive plasma samples plus three times the standard deviation of their mean. Any sample with a value below this was described as a negative responder For ELISA in the reduced state, the antigen was pretreated with 5% β-mercaptoethanol and heated before coating, as reported previously (32). All subsequent steps were as described above.
Affinity purification of antigen-specific antibodies.
To purify CyRPA-specific human antibodies, recombinant CyRPA protein was first coupled to activated cyanogen bromide (CNBr)-Sepharose beads (Sigma) in carbonate-bicarbonate buffer at pH 8.3 at 4°C for an overnight period. The CyRPA-coupled CNBr beads were incubated with pooled human sera at 4°C for an overnight period. The next day, beads were washed extensively with 1× PBS, and CyRPA-specific antibodies were eluted using 0.1 M glycine (pH 2.7) and the pH adjusted with 1 M Tris-HCl (pH 9.0). The purified antibodies were concentrated, buffer exchanged against RPMI 1640, and their integrity was confirmed by running reducing and nonreducing SDS-PAGE. The same protocol was used to purify CyRPA-specific antibodies from rabbit sera. Normal human IgG from malaria-naive plasma samples were purified in the same manner as that described above, except that instead of CNBr-Sepharose beads, protein G-Sepharose beads (Cytiva Life Sciences) were used for capturing the IgG.
In vitro parasite growth inhibition assay.
The growth inhibition assay (GIA) was performed per a protocol described previously (26). Briefly, P. falciparum 3D7 clone was synchronized using Percoll and sorbitol treatments. Synchronized late-stage schizont parasites maintained at 2% hematocrit were incubated with control and purified antigen-specific antibodies (human or rabbit) at an initial parasitemia of 0.3%. Parasites were stained with ethidium bromide after a single cycle of invasion (after 40 to 44 h), and parasitemia was measured by flow cytometry. GIA activity was calculated with respect to erythrocyte invasion of parasite in the presence of control human IgG (from malaria naive individuals) or rabbit preimmune IgG. GraphPad Prism software version 8.0 was used to calculate the IC50 using the nonlinear least-squares regression method (44). A four-parameter sigmoidal dose-response curve was fitted to the relationship between log10[CyRPA-specific IgG] and GIA (%), constraining the top of the curve to 100% GIA and the bottom of the curve to 0% GIA.
Statistical analysis.
GraphPad Prism software version 8.0 was used to perform statistical analysis wherever needed. The Mann-Whitney U test was used to calculate statistical significance in human immunogenicity analysis. A P value of <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
We thank Louis Miller (NIH) for providing the P. falciparum laboratory-adapted clones, Gerd Pluschke for providing CyRPA monoclonal antibody, and JNU Central Laboratory Animal Resources for their technical assistance.
This work was supported by the following grants: Vaccine Grand Challenge Program Department of Biotechnology (to D.G. and V.S.C.); GLUE program (ICGEB, NIRTH), Department of Biotechnology (to D.G., V.S.C., P.B., and N.S.). S.Y.M., S.L.U., and H.S. are the recipients of the Senior Research Fellowship of the Department of Biotechnology, Government of India. A.S. is the recipient of the Senior Research Fellowship of the Indian Council of Medical Research (ICMR), Government of India. K.C. is the recipient of the Senior Research Fellowship of the University Grants Commission (UGC), Government of India.
We declare that we have no conflicts of interest.
Contributor Information
Virander Singh Chauhan, Email: viranderschauhan@gmail.com.
Jeroen P. J. Saeij, UC Davis School of Veterinary Medicine
REFERENCES
- 1.World Health Organization (WHO). 2020. World malaria report 2020. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Cowman AF, Healer J, Marapana D, Marsh K. 2016. Malaria: biology and disease. Cell 167:610–624. doi: 10.1016/j.cell.2016.07.055. [DOI] [PubMed] [Google Scholar]
- 3.Miller LH, Good MF, Milon G. 1994. Malaria pathogenesis. Science 264:1878–1883. doi: 10.1126/science.8009217. [DOI] [PubMed] [Google Scholar]
- 4.Baum J, Chen L, Healer J, Lopaticki S, Boyle M, Triglia T, Ehlgen F, Ralph SA, Beeson JG, Cowman AF. 2009. Reticulocyte-binding protein homologue 5—an essential adhesin involved in invasion of human erythrocytes by Plasmodium falciparum. Int J Parasitol 39:371–380. doi: 10.1016/j.ijpara.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Hayton K, Gaur D, Liu A, Takahashi J, Henschen B, Singh S, Lambert L, Furuya T, Bouttenot R, Doll M, Nawaz F, Mu J, Jiang L, Miller LH, Wellems TE. 2008. Erythrocyte binding protein PfRH5 polymorphisms determine species-specific pathways of Plasmodium falciparum invasion. Cell Host Microbe 4:40–51. doi: 10.1016/j.chom.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crosnier C, Bustamante LY, Bartholdson SJ, Bei AK, Theron M, Uchikawa M, Mboup S, Ndir O, Kwiatkowski DP, Duraisingh MT, Rayner JC, Wright GJ. 2011. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature 480:534–537. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams AR, Douglas AD, Miura K, Illingworth JJ, Choudhary P, Murungi LM, Furze JM, Diouf A, Miotto O, Crosnier C, Wright GJ, Kwiatkowski DP, Fairhurst RM, Long CA, Draper SJ. 2012. Enhancing blockade of Plasmodium falciparum erythrocyte invasion: assessing combinations of antibodies against PfRH5 and other merozoite antigens. PLoS Pathog 8:e1002991. doi: 10.1371/journal.ppat.1002991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wanaguru M, Liu W, Hahn BH, Rayner JC, Wright GJ. 2013. RH5-basigin interaction plays a major role in the host tropism of Plasmodium falciparum. Proc Natl Acad Sci USA 110:20735–20740. doi: 10.1073/pnas.1320771110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy KS, Pandey AK, Singh H, Sahar T, Emmanuel A, Chitnis CE, Chauhan VS, Gaur D. 2014. Bacterially expressed full-length recombinant Plasmodium falciparum RH5 protein binds erythrocytes and elicits potent strain-transcending parasite-neutralizing antibodies. Infect Immun 82:152–164. doi: 10.1128/IAI.00970-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douglas AD, Baldeviano GC, Lucas CM, Lugo-Roman LA, Crosnier C, Bartholdson SJ, Diouf A, Miura K, Lambert LE, Ventocilla JA, Leiva KP, Milne KH, Illingworth JJ, Spencer AJ, Hjerrild KA, Alanine DGW, Turner AV, Moorhead JT, Edgel KA, Wu Y, Long CA, Wright GJ, Lescano AG, Draper SJ. 2015. A PfRH5-based vaccine is efficacious against heterologous strain blood-stage Plasmodium falciparum infection in Aotus monkeys. Cell Host Microbe 17:130–139. doi: 10.1016/j.chom.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Payne RO, Silk SE, Elias SC, Miura K, Diouf A, Galaway F, De Graaf H, Brendish NJ, Poulton ID, Griffiths OJ, Edwards NJ, Jin J, Labbé GM, Alanine DGW, Siani L, Di Marco S, Roberts R, Green N, Berrie E, Ishizuka AS, Nielsen CM, Bardelli M, Partey FD, Ofori MF, Barfod L, Wambua J, Murungi LM, Osier FH, Biswas S, McCarthy JS, Minassian AM, Ashfield R, Viebig NK, Nugent FL, Douglas AD, Vekemans J, Wright GJ, Faust SN, Hill AVS, Long CA, Lawrie AM, Draper SJ. 2017. Human vaccination against RH5 induces neutralizing antimalarial antibodies that inhibit RH5 invasion complex interactions. JCI Insight 2:e96381. doi: 10.1172/jci.insight.96381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minassian AM, Silk SE, Barrett JR, Nielsen CM, Miura K, Diouf A, Loos C, Fallon JK, Michell AR, White MT, Edwards NJ, Poulton ID, Mitton CH, Payne RO, Marks M, Maxwell-Scott H, Querol-Rubiera A, Bisnauthsing K, Batra R, Ogrina T, Brendish NJ, Themistocleous Y, Rawlinson TA, Ellis KJ, Quinkert D, Baker M, Lopez Ramon R, Ramos Lopez F, Barfod L, Folegatti PM, Silman D, Datoo M, Taylor IJ, Jin J, Pulido D, Douglas AD, de Jongh WA, Smith R, Berrie E, Noe AR, Diggs CL, Soisson LA, Ashfield R, Faust SN, Goodman AL, Lawrie AM, Nugent FL, Alter G, Long CA, Draper SJ. 2021. Reduced blood-stage malaria growth and immune correlates in humans following RH5 vaccination. Med (N Y) 2:701–719.e19. doi: 10.1016/j.medj.2021.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Draper SJ, Sack BK, King CR, Nielsen CM, Rayner JC, Higgins MK, Long CA, Seder RA. 2018. Malaria vaccines: recent advances and new horizons. Cell Host Microbe 24:43–56. doi: 10.1016/j.chom.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss GE, Gilson PR, Taechalertpaisarn T, Tham WH, de Jong NWM, Harvey KL, Fowkes FJI, Barlow PN, Rayner JC, Wright GJ, Cowman AF, Crabb BS. 2015. Revealing the sequence and resulting cellular morphology of receptor-ligand interactions during Plasmodium falciparum invasion of erythrocytes. PLoS Pathog 11:e1004670. doi: 10.1371/journal.ppat.1004670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dreyer AM, Matile H, Papastogiannidis P, Kamber J, Favuzza P, Voss TS, Wittlin S, Pluschke G. 2012. Passive immunoprotection of Plasmodium falciparum-infected mice designates the CyRPA as candidate malaria vaccine antigen. J Immunol 188:6225–6237. doi: 10.4049/jimmunol.1103177. [DOI] [PubMed] [Google Scholar]
- 16.Reddy KS, Amlabu E, Pandey AK, Mitra P, Chauhan VS, Gaur D. 2015. Multiprotein complex between the GPI-anchored CyRPA with PfRH5 and PfRipr is crucial for Plasmodium falciparum erythrocyte invasion. Proc Natl Acad Sci USA 112:1179–1184. doi: 10.1073/pnas.1415466112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volz JC, Yap A, Sisquella X, Thompson JK, Lim NTY, Whitehead LW, Chen L, Lampe M, Tham WH, Wilson D, Nebl T, Marapana D, Triglia T, Wong W, Rogers KL, Cowman AF. 2016. Essential role of the PfRh5/PfRipr/CyRPA complex during Plasmodium falciparum invasion of erythrocytes. Cell Host Microbe 20:60–71. doi: 10.1016/j.chom.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Wong W, Huang R, Menant S, Hong C, Sandow JJ, Birkinshaw RW, Healer J, Hodder AN, Kanjee U, Tonkin CJ, Heckmann D, Soroka V, Søgaard TMM, Jørgensen T, Duraisingh MT, Czabotar PE, de Jongh WA, Tham WH, Webb AI, Yu Z, Cowman AF. 2019. Structure of Plasmodium falciparum Rh5-CyRPA-Ripr invasion complex. Nature 565:118–121. doi: 10.1038/s41586-018-0779-6. [DOI] [PubMed] [Google Scholar]
- 19.Bustamante LY, Powell GT, Lin YC, Macklin MD, Cross N, Kemp A, Cawkill P, Sanderson T, Crosnier C, Muller-Sienerth N, Doumbo OK, Traore B, Crompton PD, Cicuta P, Tran TM, Wright GJ, Rayner JC. 2017. Synergistic malaria vaccine combinations identified by systematic antigen screening. Proc Natl Acad Sci USA 114:12045–12050. doi: 10.1073/pnas.1702944114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamborrini M, Hauser J, Schäfer A, Amacker M, Favuzza P, Kyungtak K, Fleury S, Pluschke G. 2020. Vaccination with virosomally formulated recombinant CyRPA elicits protective antibodies against Plasmodium falciparum parasites in preclinical in vitro and in vivo models. NPJ Vaccines 5:9. doi: 10.1038/s41541-020-0158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Xu Y, Wong W, Thompson JK, Healer J, Goddard-Borger ED, Lawrence MC, Cowman AF. 2017. Structural basis for inhibition of erythrocyte invasion by antibodies to Plasmodium falciparum protein CyRPA. Elife 6:e21347. doi: 10.7554/eLife.21347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Favuzza P, Guffart E, Tamborrini M, Scherer B, Dreyer AM, Rufer AC, Erny J, Hoernschemeyer J, Thoma R, Schmid G, Gsell B, Lamelas A, Benz J, Joseph C, Matile H, Pluschke G, Rudolph MG. 2017. Structure of the malaria vaccine candidate antigen CyRPA and its complex with a parasite invasion inhibitory antibody. Elife 6:e20383. doi: 10.7554/eLife.20383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valmaseda A, Macete E, Nhabomba A, Guinovart C, Aide P, Bardají A, Bassat Q, Nhampossa T, Maculuve S, Casellas A, Quintó L, Sanz S, Jiménez A, Feng G, Langer C, Reiling L, Reddy KS, Pandey A, Chitnis CE, Chauhan VS, Aguilar R, Aponte JJ, Dobaño C, Beeson JG, Gaur D, Menéndez C, Alonso PL, Mayor A. 2018. Identifying immune correlates of protection against Plasmodium falciparum through a novel approach to account for heterogeneity in malaria exposure. Clin Infect Dis 66:586–593. doi: 10.1093/cid/cix837. [DOI] [PubMed] [Google Scholar]
- 24.Singh H, Mian SY, Pandey AK, Krishna S, Anand G, Reddy KS, Chaturvedi N, Bahl V, Hans N, Shukla MM, Bassat Q, Mayor A, Miura K, Bharti PK, Long C, Singh N, Chauhan VS, Gaur D. 2020. Antibody combinations targeting the essential antigens CyRPA, RH5 and MSP-119 potently neutralize Plasmodium falciparum clinical isolates from India and Africa. J Infect Dis 223:1953–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miura K, Zhou H, Diouf A, Moretz SE, Fay MP, Miller LH, Martin LB, Pierce MA, Ellis RD, Mullen GED, Long CA. 2009. Anti-apical-membrane-antigen-1 antibody is more effective than anti-42-kilodalton-merozoite-surface-protein-1 antibody in inhibiting Plasmodium falciparum growth, as determined by the in vitro growth inhibition assay. Clin Vaccine Immunol 16:963–968. doi: 10.1128/CVI.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandey AK, Reddy KS, Sahar T, Gupta S, Singh H, Reddy EJ, Asad M, Siddiqui FA, Gupta P, Singh B, More KR, Mohmmed A, Chitnis CE, Chauhan VS, Gaur D. 2013. Identification of a potent combination of key Plasmodium falciparum merozoite antigens that elicit strain-transcending parasite-neutralizing antibodies. Infect Immun 81:441–451. doi: 10.1128/IAI.01107-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Douglas AD, Baldeviano GC, Jin J, Miura K, Diouf A, Zenonos ZA, Ventocilla JA, Silk SE, Marshall JM, Alanine DGW, Wang C, Edwards NJ, Leiva KP, Gomez-Puerta LA, Lucas CM, Wright GJ, Long CA, Royal JM, Draper SJ. 2019. A defined mechanistic correlate of protection against Plasmodium falciparum malaria in non-human primates. Nat Commun 10:1953. doi: 10.1038/s41467-019-09894-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.John CC, O’Donnell RA, Sumba PO, Moormann AM, de Koning-Ward TF, King CL, Kazura JW, Crabb BS. 2004. Evidence that invasion-inhibitory antibodies specific for the 19-kDa fragment of merozoite surface protein-1 (MSP-1 19) can play a protective role against blood-stage Plasmodium falciparum infection in individuals in a malaria endemic area of Africa. J Immunol 173:666–672. doi: 10.4049/jimmunol.173.1.666. [DOI] [PubMed] [Google Scholar]
- 29.Patel SD, Ahouidi AD, Bei AK, Dieye TN, Mboup S, Harrison SC, Duraisingh MT. 2013. Plasmodium falciparum merozoite surface antigen, PfRH5, elicits detectable levels of invasion-inhibiting antibodies in humans. J Infect Dis 208:1679–1687. doi: 10.1093/infdis/jit385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran TM, Ongoiba A, Coursen J, Crosnier C, Diouf A, Huang CY, Li S, Doumbo S, Doumtabe D, Kone Y, Bathily A, Dia S, Niangaly M, Dara C, Sangala J, Miller LH, Doumbo OK, Kayentao K, Long CA, Miura K, Wright GJ, Traore B, Crompton PD. 2014. Naturally acquired antibodies specific for Plasmodium falciparum reticulocyte-binding protein homologue 5 inhibit parasite growth and predict protection from malaria. J Infect Dis 209:789–798. doi: 10.1093/infdis/jit553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta PK, Mukherjee P, Dhawan S, Pandey AK, Mazumdar S, Gaur D, Jain SK, Chauhan VS. 2014. Production and preclinical evaluation of Plasmodium falciparum MSP-1 19 and MSP-3 11 chimeric protein, PfMSP-Fu 24. Clin Vaccine Immunol 21:886–897. doi: 10.1128/CVI.00179-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazumdar S, Mukherjee P, Yazdani SS, Jain SK, Mohmmed A, Chauhan VS. 2010. Plasmodium falciparum merozoite surface protein 1 (MSP-1)-MSP-3 chimeric protein: immunogenicity determined with human-compatible adjuvants and induction of protective immune response. Infect Immun 78:872–883. doi: 10.1128/IAI.00427-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Illingworth JJ, Alanine DG, Brown R, Marshall JM, Bartlett HE, Silk SE, Labbé GM, Quinkert D, Cho JS, Wendler JP, Pattinson DJ, Barfod L, Douglas AD, Shea MW, Wright KE, de Cassan SC, Higgins MK, Draper SJ. 2019. Functional comparison of blood-stage Plasmodium falciparum malaria vaccine candidate antigens. Front Immunol 10:1254. doi: 10.3389/fimmu.2019.01254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bustamante LY, Bartholdson SJ, Crosnier C, Campos MG, Wanaguru M, Nguon C, Kwiatkowski DP, Wright GJ, Rayner JC. 2013. A full-length recombinant Plasmodium falciparum PfRH5 protein induces inhibitory antibodies that are effective across common PfRH5 genetic variants. Vaccine 31:373–379. doi: 10.1016/j.vaccine.2012.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Healer J, Wong W, Thompson JK, He W, Birkinshaw RW, Miura K, Long CA, Soroka V, Søgaard TMM, Jørgensen T, de Jongh WA, Weir C, Svahn E, Czabotar PE, Tham WH, Mueller I, Barlow PN, Cowman AF. 2019. Neutralising antibodies block the function of Rh5/Ripr/CyRPA complex during invasion of Plasmodium falciparum into human erythrocytes. Cell Microbiol 21:e13030. doi: 10.1111/cmi.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. 2008. Immunity to malaria: more questions than answers. Nat Immunol 9:725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 37.McCallum FJ, Persson KEM, Fowkes FJI, Reiling L, Mugyenyi CK, Richards JS, Simpson JA, Williams TN, Gilson PR, Hodder AN, Sanders PR, Anders RF, Narum DL, Chitnis C, Crabb BS, Marsh K, Beeson JG. 2017. Differing rates of antibody acquisition to merozoite antigens in malaria: implications for immunity and surveillance. J Leukoc Biol 101:913–925. doi: 10.1189/jlb.5MA0716-294R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osier FH, Mackinnon MJ, Crosnier C, Fegan G, Kamuyu G, Wanaguru M, Ogada E, McDade B, Rayner JC, Wright GJ, Marsh K. 2014. New antigens for a multicomponent blood-stage malaria vaccine. Sci Transl Med 6:247ra102–247ra102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bull PC, Abdi AI. 2016. The role of PfEMP1 as targets of naturally acquired immunity to childhood malaria: prospects for a vaccine. Parasitology 143:171–186. doi: 10.1017/S0031182015001274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plenderleith LJ, Liu W, MacLean OA, Li Y, Loy DE, Sundararaman SA, Bibollet-Ruche F, Learn GH, Hahn BH, Sharp PM. 2018. Adaptive evolution of RH5 in ape Plasmodium species of the Laverania subgenus. mBio 9:e02237-17. doi: 10.1128/mBio.02237-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miura K, Zhou H, Moretz SE, Diouf A, Thera MA, Dolo A, Doumbo O, Malkin E, Diemert D, Miller LH, Mullen GED, Long CA. 2008. Comparison of biological activity of human anti-apical membrane antigen-1 antibodies induced by natural infection and vaccination. J Immunol 181:8776–8783. doi: 10.4049/jimmunol.181.12.8776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh N, Chand SK, Bharti PK, Singh MP, Chand G, Mishra AK, Shukla MM, Mahulia MM, Sharma RK. 2013. Dynamics of forest malaria transmission in Balaghat district, Madhya Pradesh, India. PLoS One 8:e73730. doi: 10.1371/journal.pone.0073730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dreyer AM, Beauchamp J, Matile H, Pluschke G. 2010. An efficient system to generate monoclonal antibodies against membrane-associated proteins by immunisation with antigen-expressing mammalian cells. BMC Biotechnol 10:87. doi: 10.1186/1472-6750-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Douglas AD, Williams AR, Illingworth JJ, Kamuyu G, Biswas S, Goodman AL, Wyllie DH, Crosnier C, Miura K, Wright GJ, Long CA, Osier FH, Marsh K, Turner AV, Hill AVS, Draper SJ. 2011. The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody. Nat Commun 2. doi: 10.1038/ncomms1615. [DOI] [PMC free article] [PubMed] [Google Scholar]