Abstract
Monoterpenoids are C10 isoprenoids and constitute a large family of natural products. They have been used as ingredients in food, cosmetics, and therapeutic products. Many monoterpenoids such as linalool, geraniol, limonene, and pinene are volatile and can be found in plant essential oils. Conventionally, these bioactive compounds are obtained from plant extracts by using organic solvents or by distillation method, which are costly and laborious if high-purity product is desired. In recent years, microbial biosynthesis has emerged as alternative source of monoterpenoids with great promise for meeting the increasing global demand for these compounds. However, current methods of production are not yet at levels required for commercialization. Production efficiency of monoterpenoids in microbial hosts is often restricted by high volatility of the monoterpenoids, a lack of enzymatic activity and selectivity, and/or product cytotoxicity to the microbial hosts. In this review, we summarize advances in microbial production of monoterpenoids over the past 3 years with particular focus on the key metabolic engineering strategies for different monoterpenoid products. We also provide our perspective on the promise of future endeavors to improve monoterpenoid productivity.
Keywords: Monoterpenoids, Metabolic engineering, Microbial production, Biotransformation, Escherichia coli, Saccharomyces cerevisiae
Introduction
Isoprenoids (or terpenoids) are the largest class of natural products, which includes more than 50,000 structurally diverse compounds with a wide range of useful functions. All isoprenoid molecules larger than hemiterpenoids share two common precursors, isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) (Farasat et al., 2014; Lange et al., 2000). Hemiterpenoids are derived from either IPP or DMAPP. To synthesize larger isoprenoids, IPP and DMAPP are condensed into linear polyprenyl phosphate precursors, which are usually cyclized to produce diverse terpene skeletons. Extensive decoration of these terpene backbones gives rise to thousands of multifunctional isoprenoids (McCaskill & Croteau, 1997; Zebec et al., 2016). The most widely adopted classification system of resultant isoprenoids is based on the number of their constituent isoprene units; for example, monoterpenoids for the 10 carbon molecules, sesquiterpenoids for the 15 carbons, diterpenoids for the 20 carbons, and so forth.
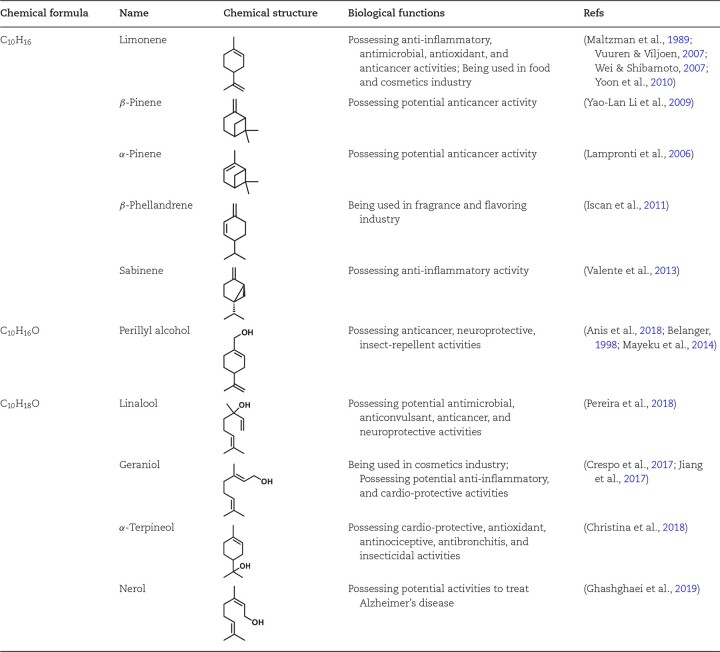
Recently, monoterpenes and their derivatives have drawn particular attention because some can be used as fragrances, insect repellants, pharmaceuticals, or flavoring agents (Bicas et al., 2011; Lorenzo & Eugenio, 2011; Vickers et al., 2014) (Table 1). Many monoterpenoids are volatile, which increases their potentials to serve as signaling molecules in the air. Notably, plants emit massive amounts of these volatiles as secondary metabolites to kill pathogens, attract pollinators, or reduce abiotic stress (Kesselmeier & Staudt, 1999; Pichersky & Gershenzon, 2002; Sharkey & Singsaas, 1995; Souliman et al., 2019; Vaughn & Spencer, 1993). To date, the global supply of monoterpenoids heavily relies on isolation from essential oils that are extracted or distilled from plants. These methods face sustainability challenges because of the low yield and high purification cost of the final product, due to the very low isoprenoid content of plants, as well as complex plant material matrix (De Luca et al., 2012; Krivoruchko & Nielsen, 2015). Another disadvantage of plant extraction methods is large fluctuation of plant harvest volumes largely caused by climate and plant disease outbreaks. An alternative route to producing monoterpenoids is by chemical synthesis. However, the structure of isoprenoids is often complex with diverse chiral centers, which makes the chemical synthesis of such isoprenoids exceptionally difficult. Additionally, the excessive use of organic solvents in organic synthesis paths raises environmental and health concerns (Gao et al., 2020; Lei et al., 2021; Chao-Jun Li & Trost, 2008; Xie et al., 2019). Hence, there is a need to develop more sustainable and more cost-effective methods for the manufacturing of desired monoterpenoid products.
Table 1.
Structure and Functions of the Key Monoterpenoids Produced by Engineered Microbes
Biosynthesis of monoterpenoids in microbial hosts using fermentation is such an emerging solution. Other than potentially providing a scalable and efficient production platform, a biocatalytic process is also typically characterized by high enantioselectivity, which is critical to maintain bioactivity of the (chiral) target products. So far, product titers of such processes are still low for many monoterpenoids, partly due to their cytotoxicity and/or loss through evaporation.
Metabolic engineers have explored various microbial hosts and used pathway optimization and process engineering to address these limitations. This review provides a brief overview of monoterpenoid biosynthesis and summarizes the advances in microbial production of volatile monoterpenoids in the past 3 years (Table 2). The review is concluded with a perspective on promising directions of future endeavors.
Table 2.
Monoterpenoids Production in Microorganisms
| Products | Hosts | Substrate | Titer/Content | Scale | Reference |
|---|---|---|---|---|---|
| Linalool | Escherichia coli | Glycerol | 100 mg/l (R)-linalool | 250 ml shake flask | (Wang et al., 2020) |
| 1,027 mg/l (R)-linalool | 1.3 l bioreactor | ||||
| E. coli | Glycerol | 278 mg/l (R)-linalool | 250 ml shake flask | (Jing Wu et al., 2021) | |
| 1,523 mg/l (R)-linalool | 1.3 l bioreactor | ||||
| Pantoea ananatis | Glucose | 5,600 mg/l (S)-linalool and 3,710 mg/l (R)-linalool | Test tube | (Hoshino et al., 2020) | |
| E. coli | Glucose | 63 mg/l | 500 ml shake flask | (Kong et al., 2020) | |
| Saccharomyces cerevisiae | Sucrose | 23 mg/l | 2 l fermenter | (Yaoyao Zhang et al., 2020) | |
| S. cerevisiae | Glucose | 53 mg/l | 250 ml shake flask | (Zhou et al., 2020) | |
| S. cerevisiae | Glucose | 81 mg/l | Shake flask (50 ml media) | (Zhou et al., 2021) | |
| E. coli | Glycerol | 284 mg/l | Glass screw capped vials (2 ml reaction volume) | (Wilkes et al., 2020) | |
| Geraniol | E. coli | Glycerol | 600 mg/l | 25 ml shake flask | (Clomburg et al., 2019) |
| Azospirillum basilence | Glycerol | 0.2 mg/l | – | (Mishra et al., 2020) | |
| S. cerevisiae | 1.8% galactose with 0.2% glucose | 43 mg/l | Culture tubes (3 ml media) | (Yee et al., 2019) | |
| E. coli | Glycerol | 120 mg/l | Glass screw capped vials (2 ml reaction volume) | (Wilkes et al., 2020) | |
| E. coli | Glycerol | 2,124 mg/l | Shake flask | (Wang et al., 2021) | |
| S. cerevisiae | Glucose | 2.75 mg/l | – | (Gerke et al., 2020) | |
| Limonene | S. cerevisiae | Alcoholic beverage | 62 mg/l | – | (Hu et al., 2020) |
| S. cerevisiae | Ethanol | 918 mg/l | 250 ml shake flask | (Si Cheng et al., 2019) | |
| S. cerevisiae | Galactose | 166 mg/l | Shake flask | (Ignea et al., 2019) | |
| S. cerevisiae | Glucose | 76 mg/l | Shake flask (25 ml media) | (Peng et al., 2018) | |
| S. cerevisiae | Ethanol | 2,230 mg/l | Shake flask (50 ml media) | (Xue Zhang et al., 2021) | |
| Yarrowia lipolytica | Glycerol (citrate as an auxiliary carbon source) | 165 mg/l | 1.5 l bioreactor | (Bo-Qian Cheng et al., 2019) | |
| E. coli | Glycerol | 500 mg/l | 25 ml shake flask | (Clomburg et al., 2019) | |
| E. coli | Glucose | 1,290 g/l | 250 ml shake flask | (Jihua Wu et al., 2019) | |
| E. coli | Glycerol | 3,600 mg/l | 3.1 l bioreactor (1 l media) | (Rolf et al., 2020) | |
| Y. lipolytica | 70% of waste cooking oil | 3 mg/l (D)-limonene | 250 ml shake flask | (Pang et al., 2019) | |
| 3 mg/l (L)-limonene | |||||
| E. coli lysates | Glucose | 90 mg/l | 1.5 ml Eppendorf tube (25 µl total volume) | (Dudley et al., 2019) | |
| Pinene | E. coli | Sucrose | 150 mg/l | Shake flask (50 ml reaction volume) | (Niu et al., 2019) |
| E. coli | Glucose | 105 mg/l | Shake flask (10 ml reaction volume) | (Bao et al., 2019) | |
| E. coli lysates | Glucose | 1,256 mg/l | 1.5 ml Eppendorf tube (25 µL total volume) | (Niu et al., 2020) | |
| α-Terpineol | S. cerevisiae | Glucose | 22 mg/l | 5 l bioreactor | (Chuanbo Zhang et al., 2019) |
| Sphingobium sp. | Limonene | 240,000 mg/l | 250 ml conical flask (50 ml working volume) | (Molina et al., 2019) | |
| Perillyl alcohol | E. coli | Glucose | 87 mg/l | 5 l bioreactor | (Sun et al., 2021) |
| β-Phellandrene | Synechocystis sp. | – | 24 mg/g DCW | 1 l bioreactor | (Betterle & Melis, 2019) |
| Nerol | E. coli | Glucose | 2 mg/l | Shake flask | (Zong et al., 2019) |
| E. coli | Glucose | 967 mg/l | Shake flask (50 ml reaction volume) | (Lei, Qiu, Wu, et al., 2021) | |
| Meyerozyma guilliermondii | Glucose | 3 mg/l | – | (Mo et al., 2021) | |
| Citronellol | S. cerevisiae | Glucose | 6,370 mg/l | 5 l bioreactor | (Rongsheng Li et al., 2021) |
| Borneol | E. coli | Glucose | 87 mg/l | Shake flask (50 ml reaction volume) | (Lei, Qiu, Wu, et al., 2021) |
| Sabinene | S. cerevisiae | Ethanol | 155 mg/l | Shake flask (50 ml reaction volume) | (Jia et al., 2020) |
| S. cerevisiae | Galactose | 113 mg/l | Shake flask | (Ignea et al., 2019) |
Biosynthetic Pathways of Monoterpene-based Compounds
Conventionally, biosynthesis of isoprenoids mainly relies on either of the two natural pathways: the eukaryotic mevalonate (MVA) pathway and the methylerythritol phosphate (MEP) pathway that typically exists in prokaryotes and plant plastids (Goldstein & Brown, 1990; Rohmer, 1999). Specifically, the MVA pathway starts with the condensation of two acetyl-CoA into acetoacetyl-CoA, which is transformed into IPP in five steps; the MEP pathway is initiated by the condensation of glyceraldehyde 3-phosphate and pyruvate.
There is also one novel two-step pathway developed recently to synthesize IPP and DMAPP from petroleum-derived isopentenols (prenol and isoprenol) (Chatzivasileiou et al., 2019). This isopentenol utilization pathway (IUP) is completely decoupled from the central metabolism and has been successfully used to synthesize a few isoprenoids efficiently. Prenol could also be synthesized by using acetyl-CoA as building blocks (Clomburg et al., 2019) offering a different route for isoprenoid synthesis from renewable feedstocks. This synthetic pathway shares two steps with the MVA pathway until hydroxymethylglutaryl-CoA, which is transformed into prenol in four steps. Regardless of the upstream pathway adopted for IPP and DMAPP synthesis, the downstream monoterpenoid biosynthesis is mostly derived from geranyl diphosphate (GPP), which is synthesized via the head-to-tail condensation of these two C5 backbones. Geranyl diphosphate could be cyclized into diverse structures by respective monoterpene synthases in just one step. For example, biosynthesis of linalool, geraniol, limonene, pinene, phellandrene, and sabinene has been successfully demonstrated in conventional hosts—S. cerevisiae and E. coli—and non-conventional species such as cyanobacteria Synechocystis sp. PCC 6803 by expressing corresponding synthases discovered in bacteria or plant species. With the development of engineering tools and characterization of novel enzymes, the monoterpenoid product spectrum in microbial hosts will be further expanded.
Approaches
Microbial monoterpenoid production is potentially restricted by insufficient precursor supply, imbalanced metabolic fluxes, lack of appropriate cofactors, and/or low enzymatic activity/specificity. In addition, cytotoxicity caused by monoterpenoids can have a dose-dependent effect on the producing organisms, which undoubtedly impacts efforts for titer improvement (Erdogan & Ozkan, 2017; Miller & Borden, 2000). As a result, various approaches have been proposed to address these challenges. For example, availability of GPP was increased by substrate channeling, downregulation of GPP-consuming reactions, pathway compartmentation to shelter the GPP pool, and/or recruiting alternative node—neryl diphosphate (NPP)—for synthesizing some monoterpenoids. Besides modulating biosynthetic routes, another approach examined improvement of monoterpene synthase activity and promising results were obtained by screening enzymes from different species, construction of fusion enzymes, and/or protein-directed evolution. Additionally, medium and process optimization has also substantially improved monoterpenoid production. To overcome monoterpenoid cytotoxicity, conventional organisms were engineered to improve their robustness and new hosts with natural high tolerance have also been isolated. In this section, these recent efforts are summarized according to the target product of each study.
Linalool
Linalool is an acyclic monoterpenoid with a pair of enantiomers present in essential oils of many plant species (Ilc et al., 2016; Raguso, 2016). Compared with (S)-linalool, (R)-linalool has attracted greater research interest because it exhibits outstanding bioactivity, such as anticonvulsant, antimicrobial, anti-inflammatory, and neuroprotective properties (de Sousa et al., 2010; Pereira et al., 2018; Sampaio et al., 2012). Biosynthesis of linalool is specifically catalyzed by a linalool synthase (LIS) from GPP, the linear C10 polyprenyl precursor.
In S. cerevisiae, monoterpenoid biosynthesis is typically carried out in both the cytoplasm and inside organelles. For example, Zhang et al. constructed a linalool-producing S. cerevisiae by placing the entire linalool pathway in both mitochondria and the cytoplasm (Zhang et al., 2020). The resultant strain showed ∼20-fold improvement in linalool titer compared with the strain harboring only the cytoplasmic pathway. Two mechanisms may be responsible for the observed large titer increase. The first one is abundant availability of acetyl-CoA in mitochondria, which pushed the flux into the MVA pathway (acetyl-CoA is the substrate of the pathway). The second mechanism is that metabolites derived from the mitochondrial MVA pathway may be more efficiently directed toward linalool synthesis: GPP is the precursor of linalool and is synthesized from IPP and DMAPP by using ERG20, which catalyzes both GPP production and consumption (to be converted to farnesyl diphosphate [FPP]); when ERG20 is located in mitochondria, the produced FPP cannot be consumed to synthesize ergosterol (whose biosynthesis is located in the cytoplasm), thus reducing the driving force of GPP consumption.
To conserve more GPP in the cytoplasm for linalool synthesis, site-directed mutagenesis of ERG20 was carried out in the above strain to downregulate the undesired GPP consumption activity. Twofold increase in linalool production level was observed in the resultant strain carrying ERG20F96W-N127W. Linalool titer was further improved to 23 mg/l by co-expressing the LIS and ERG20 F96W-N127W in both cytoplasm and mitochondria, together with carbon source optimization (Zhang et al., 2020).
Enhancing the catalytic activity of LIS should significantly affect its production level, especially in strains with sufficient precursor supply. To this end, several LISss were evaluated in S. cerevisiae. A truncated version of LIS from Mentha citrata (t67OMcLIS) led to a linalool titer of 25 mg/l in shake-flask culture. Directed evolution of the same enzyme was performed and a mutant library was created by using error-prone PCR. Introducing the positive mutant t67OMcLISE343D/E352H together with the previously constructed ERG20 F96W-N127W further increased linalool titer to 53 mg/l in S. cerevisiae in shake-flask culture (Zhou et al., 2020). However, this was accompanied by a dramatic decrease in biomass, probably caused by the high metabolic burden of the dual MVA pathway or the excessive protein expression. To circumvent this limitation, the researchers employed a combinatorial modulation strategy instead of simple overexpression of multiple genes in a follow-up study. Expression level of t67OMcLISE343D/E352H was increased (∼2.4-fold) by fusing a SKIK tag to its N-terminus. Afterwards, RIDD and RIAD peptide tags were introduced to the C-terminus of ERG20 F96W-N127W and SKIK-t67OMcLISE343D/E352H, respectively. Expressing the generated ERG20 F96W-N127W and t67OMcLISE343D/E352H enhanced conversion efficiency of GPP to linalool, resulting in a 42% titer improvement. Combining these two approaches with downregulation of native ERG20 led to the highest reported linalool titer (81 mg/l) in S. cerevisiae shake-flask culture (Zhou et al., 2021).
Linalool synthesis in E. coli could also be limited by insufficient supply of GPP, which can be consumed by further condensation with IPP (producing FPP, catalyzed by endogenous farnesyl diphosphate synthase [ispA]) and dephosphorylation (producing geraniol, catalyzed by endogenous alkaline phosphatase [PhoA]) (Liu et al., 2015). Recently, 15 mg/l of linalool was synthesized in shake-flask E. coli cultures by expressing GPP synthase (GPPS) from Abies grandis (AgGPPS) together with LIS from Actinidia arguta. Combined with overexpression of IPP isomerase (IDI), linalool titer was increased to 63 mg/l (Kong et al., 2020). To improve linalool synthase activity, LISs from different organisms were screened and the one from Streptomyces clavuligerus (bLIS) showed best catalytic activity toward GPP. The expression level of bLIS was upregulated by modifying ribosome binding site (RBS) sequences, leading to a 2.8-fold higher linalool titer. Subsequently, fusion tags were used to enhance the availability of soluble bLIS, which further increased linalool production level. Co-expression of the modified bLIS and N-terminus truncated AgGPPS improved (R)-linalool titer to 100 mg/l (Wang et al., 2020). However, among the three proteins (EcIDI, AgGPPS, and bLIS) expressed in the recombinant strain, bLIS showed lowest catalytic activity. Three peptidyl ligands GBD, SH3, and PDZ were attached to the C-termini of EcIDI, AgGPPS, and bLIS, respectively. Linkers were used to assemble these protein–protein interaction domains to construct the synthetic protein scaffold, which co-localized the three enzymes in cytosol and led to higher conversion rate of IPP, DMAPP, and GPP into linalool. By manipulating ratio of the scaffolds associated with each enzyme, linalool titer was improved to 187 mg/l from 100 mg/l in shake-flask culture. The linalool production level was further pushed to 278 mg/l after optimizing the culturing conditions, such as adjusting induction temperature, inducer concentration, and carbon-source concentration. When the culture was scaled up to bioreactor level in the same study, a (R)-linalool titer of approximately 1,523 mg/l was obtained, which is the highest reported linalool titer in E. coli culture to date (Wu et al., 2021).
A final optimization strategy is to control carbon partition between cell growth and product (linalool) synthesis. This can be achieved by regulating flux distribution at the acetyl-CoA branch point, as this is a key intermediate participating in both the tricarboxylic acid (TCA) cycle (critical for cell growth) and the MVA pathway, which supplies the flux for isoprenoid synthesis. When the MVA pathway draws carbon flux for linalool production, it is important to prevent starvation of competing growth-critical pathways as well as optimally balance metabolite and energy demands for both functions. Hoshino et al. adopted a phosphate-sensing (PhoR/PhoB) system from E. coli, and successfully separated the cell growth phase of Pantoea ananatis from the subsequent product-formation phase. With phosphate-starvation induced promoters, when phosphorylated isoprenoid intermediates were depleted, more carbon flux in the engineered strain was directed at the acetyl-CoA node toward the MVA pathway (Nitta et al., 2020). This strategy was combined with screening for better linalool synthases to construct superior linalool-producing strains. Synthases from A. arguta (AaLINS) and Streptomyces clavuligerus (bLIS) were selected for (S)-linalool and (R)-linalool biosynthesis, respectively. Co-expression of an ispA mutant from E. coli together with either of these two synthases resulted in 5.60 g/l (S)-linalool or 3.71 g/l (R)-linalool in test tube culture (Hoshino et al., 2020). P. ananatis is capable of growing at an acidic pH and more tolerant of various inhibitory molecules (Hara et al., 2012). Hence, this extraordinary performance might be partially due to the robustness of the host system. If the improved linalool performance is indeed due to higher tolerance of P. ananatis to monoterpenoid toxicity, this result underlines the importance of host selection to successful engineering of microbes for toxic compound production.
Geraniol
Geraniol is an acyclic monoterpenoid that is found in essential oils from different plants. The rose-like scent made it a desirable ingredient in fragrances and cosmetics. Geraniol also has validated pharmacological properties, such as inhibiting tumor cell growth and possessing cardio-protective effect (Burke et al., 1997; Chen & Viljoen, 2010; Crespo et al., 2017; Yu et al., 1995).
Similar to linalool biosynthesis, S. cerevisiae and E. coli are the most commonly investigated microbial hosts for geraniol biosynthesis. Here too, the compartmentalization strategy was effective in improving geraniol biosynthesis in S. cerevisiae. A strain engineered such that all enzymes involved in geraniol biosynthesis were targeted to mitochondria, exhibited a sixfold titer improvement relative to a strain expressing the enzymes in the cytosol (Yee et al., 2019). Likewise, peroxisomal localization of GPPS and geraniol synthase (GES) could also be beneficial.
On the other hand, systematic metabolic engineering strategies such as selection of key enzymes (GPPS and GES) and background E. coli strains are effective for improving geraniol production. Combination of these tools led to a 46-fold improvement in geraniol titer (to 964 mg/l) in recombinant E. coli from heterologous MVA pathway. Fusion tag evolution engineering was used to optimize the expression level of eukaryotic GES. A high-throughput screening method was established by fusing enhanced green fluorescence protein (eGFP) to the C-terminal of GES (fusion tags were fused to its N-terminal), which enabled transduction of protein concentration into detectable fluorescence signals. Variants were generated by substituting the third to fifth codons of the selected fusion tag. Compared with the strain harboring the original fusion tag, the best-performed variants produced 2,124 mg/l geraniol in shake-flask culture, which is the highest titer to date (Wang et al., 2021).
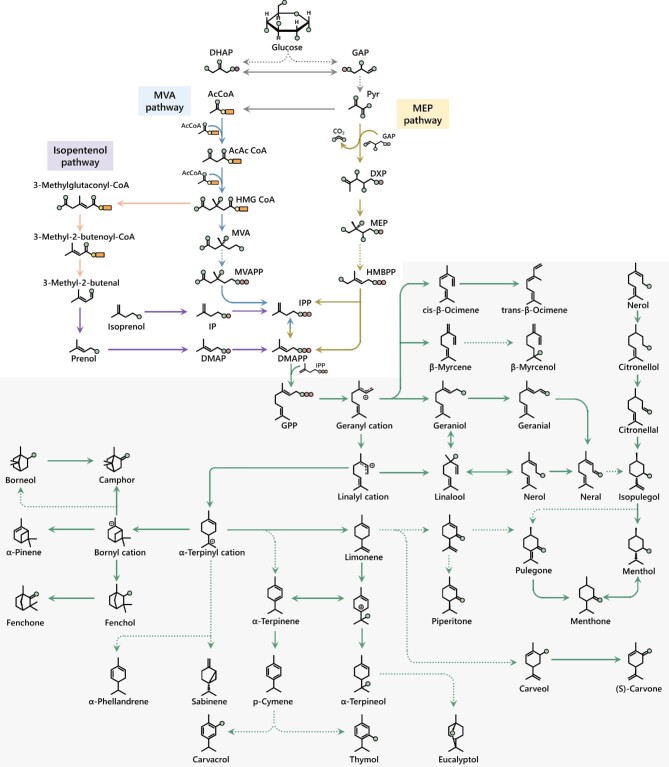
Recently, a new isoprenoid biosynthetic pathway was designed for the synthesis of geraniol from central metabolism (Fig. 1). A key intermediate of this pathway was prenol. The authors initially established the lower segment of the pathway, which converted 3 g/l prenol into 2 g/l geranoids (geraniol and isomers/derivatives). Besides prenol, the upper segment could be constructed such that prenol is derived from acetyl-CoA, which was synthesized from glycerol through central metabolism. The complete pathway produced 600 mg/l geranoids from 40 g/l glycerol in E. coli (Clomburg et al., 2019).
Fig. 1.
An overview of biosynthetic pathways for monoterpenoids. Each monoterpenoid is derived from isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). There are three pathways to synthesize IPP and DMAPP: the mevalonate (MVA) pathway, the methylerythritol phosphate (MEP) pathway, and the isopentenol pathway. DHAP, dihydroxyacetone phosphate; AcCoA, acetyl CoA; AcAcCoA, acetoacetyl CoA; HMG-CoA, β-hydroxy-β-methylglutaryl-CoA; MVAPP, mevalonate 5-diphosphate; GAP, glyceraldehyde 3-phosphate; Pyr, pyruvate; DXP, 1-deoxy-D-xylulose 5-phosphate; HMBPP, 4-hydroxy-3-methyl-but-2-enyl diphosphate; GPP, geranyl diphosphate.
Nonconventional hosts have also been used for geraniol synthesis. A recent study used Azospirillum brasilense—a nonphotosynthetic carotenoid-overproducing species—to synthesize geraniol with the expression of a heterologous synthase (Mishra et al., 2020). Due to its ability to synthesize carotenoids (also members of the isoprenoid family), A. brasilense has a relatively efficient native pathway to synthesize the key precursors IPP/DMAPP. Unlike photosynthetic carotenoid-producing bacteria, A. brasilense does not require carotenoids for cell growth. Hence, almost all the metabolic flux could be channeled to desired geraniol biosynthesis with appropriate engineering strategies. Although the production level (0.2 mg/l) was lower than that in major industrial workhorses, this study explored the potential of new microbial hosts to synthesize monoterpenoids.
Limonene
Limonene is a cyclic monoterpene abundant in essential oils derived from citrus fruit or mint. It often occurs as a pair of enantiomers and is widely used in food and cosmetic formulations. Limonene has also exhibited antimicrobial and anticancer properties that are intriguing and worth exploring further (Felipe et al., 2017; Haag et al., 1992; Vuuren & Viljoen, 2007).
Limonene is also derived from GPP and its synthesis shares with linalool and geraniol the same challenges in redirecting flux toward the desired product. To improve limonene production, the cytoplasmic GPP pool could be sheltered by downregulating FPP synthase (ERG20) with a combinatorial approach. A weak degron (K3K15) was fused to the N-terminus of ERG20 based on the degron-mediated degradation mechanism, repressing the synthesis of FPP and thus ergosterol. Ergosterol responsive promoter (PERG1), which would be upregulated when the ergosterol content is too low, was used to guide the expression of ERG20. This combinatorial strategy, which minimized ERG20 activity while maintaining cell growth, led to 76 mg/l limonene in S. cerevisiae (Peng et al., 2018).
In another study, optimization of carbon source (glycerol vs. glucose) and inducer concentration resulted in 368 mg/l limonene produced by an engineered E. coli strain that carried a heterologous MVA pathway. To further improve limonene production, a carbon-limited exponential feed strategy was adopted to prolong cells’ exponential growth phase in bioreactor fermentation. With additional trace element supplementation and the use of an inert organic phase (DINP), a higher biomass concentration was achieved, led to production of 3.6 g/l limonene (Rolf et al., 2020).
Alternatively, a synthetic orthogonal pathway could be adopted for monoterpenoid synthesis. The isomeric substrate that cannot be used for FPP synthesis (NPP) can be efficiently recognized by engineered monoterpenoid synthases. Limonene titer in the S. cerevisiae strain expressing the engineered NPP-specific limonene synthase (H570Y) reached 166 mg/l in semibatch shake-flask cultivation, which was 7.8 times that in GPP-only cells equipped with wild-type limonene synthase (Ignea et al., 2019). Likewise, when heterologous NPP synthase (NPPS) and N-terminus truncated limonene synthase were expressed in S. cerevisiae, a sixfold improvement in limonene titer was achieved in fed-batch fermentation (to 918 mg/l) (Cheng et al., 2019). To further improve limonene synthesis, the authors enhanced the pyruvate dehydrogenase bypass by overexpressing a reported acetyl-CoA synthetase variant (SeACSL641P) in a follow-up study. In parallel, among the selected candidates, deletion of CIT2 (citrate synthase, involved in acetyl-CoA catabolism) was the most effective in limonene production improvement. When the SeACSL641P expression cassette was inserted to CIT2 sites, the resulting S. cerevisiae harboring NPPS and limonene synthase produced 1.45 g/l limonene. With higher biomass in shake-flask culture, the final limonene titer reached 2.23 g/l (Zhang et al., 2021). Another strategy, whereby site-directed mutation to key enzymes of the MVA pathway was combined with engineering RBS of enzymes in the upstream MVA pathway, led to successful construction of an efficient limonene-producing E. coli. Using fed-batch bioreactor operation to culture the strain co-expressing NPPS and limonene synthase increased the titer to 1.29 g/l (Wu et al., 2019).
There have been also notable advances made in other host system. More specifically, medium optimization significantly facilitated limonene synthesis in Yarrowia lipolytica (Cheng et al., 2019); the potential of converting wastes (e.g., waste cooking oil and lignocellulosic hydrolysate) into limonene was evaluated as well (Pang et al., 2019; Yao et al., 2020).
Pinene
Pinene is a bicyclic monoterpene with two structural isomers, α-pinene and β-pinene, which could be derived from GPP. Besides attractive biological properties such as antiproliferative or anticarcinogenic potential, pinene is also a promising candidate for jet fuel (Harvey et al., 2010; Lampronti et al., 2006; Li et al., 2009; Raman et al., 2016).
To improve the production level of α-pinene, initial efforts focused on tolerance enhancement of the producing organism. An evolved E. coli strain was obtained after adaptive laboratory evolution and overexpression of genes encoding the efflux pumps. An E. coli–E. coli co-culture system was developed to modularize the upstream MVA pathway and downstream pinene biosynthetic pathway. Metabolic burden caused by excessive protein expression could thus be alleviated in the co-culture system. Additionally, modular optimization could be achieved by fine-tuning the co-culture composition. Combination of these approaches led to production of 167 mg/l α-pinene (Niu et al., 2018).
Usually, genes encoding rate-limiting enzymes are frequently incorporated into plasmids and introduced to E. coli for high-level production of target compounds. However, cellular response such as homologous recombination may inactivate gene expression through mutation, deletion, and/or insertion, especially when the microorganisms must function under high metabolic burden caused by complex pathway and/or toxic product. Integrating the expression cassettes into the genome was found to alleviate this issue and improve pinene production (Bao et al., 2019).
Another useful approach is to synthesize pinene in cell-free system by using crude lysates of E. coli. Optimization of the experimental parameters including medium composition, cofactor levels, and enzyme concentrations resulted in production of 1,256 mg/l pinene from glucose (Niu et al., 2020).
Nerol
Nerol is an acyclic monoterpenoid alcohol that is widely used in cosmetics, agricultural, or pharmaceutical industries because of its fragrance and antifungal effect (Ghashghaei et al., 2019; Lapczynski et al., 2008; Li et al., 2021). It is an isomer of geraniol, and can be synthesized from GPP or NPP catalyzed by nerol synthase.
The first attempts to synthesize nerol from glucose in E. coli were implemented via the NPP bypass. Co-expression of nerol synthase and truncated NPP synthase (tNPPS) led to 0.053 mg/l nerol. Overexpression of genes involved in the MVA pathway and ERG10 (encoding conversion of acetyl-CoA to acetoacetyl-CoA) enhanced carbon flux toward nerol by 30-fold in shake-flask culture (to 1.6 mg/l) (Zong et al., 2019).
To improve nerol production level, metabolic and protein engineering strategies were coupled to enhance de novo nerol biosynthesis in E. coli from glucose. The NPP was employed as the linear C10 precursor. The strain harboring tNPPS and nerol synthase (GmNES) produced 55 mg/l nerol. When GmNES was replaced by an endogenous Nudix hydrolase (NudJ, a phosphatase), nerol titer was improved to 262 mg/l by enhancing dephosphorylation of NPP. Optimization of induction conditions and initial glucose concentration further boosted nerol titer to 967 mg/l in shake-flask culture, which is the highest reported nerol titer in microbes to date (Lei et al., 2021).
Finally, other nonconventional species such as Meyerozyma guilliermondii (a probiotic species with outstanding stress tolerance) was also used to produce nerol from various carbon sources (Mo et al., 2021).
Other Monoterpenoids
Besides the aforementioned compounds, advances have also been made in the synthesis of other monoterpenoids. Recently, Sun et al. reported the engineering of E. coli to synthesize (R)-perillyl alcohol in the first demonstration of de novo synthesis of this compound from glucose in recombinant E. coli (Sun et al., 2021). Limonene hydroxylase from Bacillus stearothermophilus (LHBS) and P-cymene monooxygenase hydroxylase/monooxygenase reductase (CymA) were compared for producing (R)-perillyl alcohol (Chang et al., 1995; Niu et al., 2018). When supplemented with 272 mg/l (R)-limonene, the recombinant E. coli carrying CymA produced 87 mg/l of (R)-perillyl alcohol and was selected as the parental strain for subsequent de novo synthesis of (R)-perillyl alcohol from glucose. With overexpression of the heterologous MVA pathway, 12.5 mg/l of (R)-perillyl alcohol could be produced in shake-flask culture. When scaled up in 5 l bioreactor in the same study, (R)-perillyl alcohol titer was improved to ∼87 mg/l at an OD600 level of 108. It is noteworthy that the concentration of acetate and perillyl aldehyde during fed-batch fermentation was 8 g/l and 45 mg/l, which might exert pressure on the microorganism and negatively affect the production level of (R)-perillyl alcohol. Further optimization to reduce carbon flux toward these toxic by-products will benefit (R)-perillyl alcohol production.
Conventional pathway engineering was applied to S. cerevisiae for α-terpineol synthesis. Overexpression of rate-limiting enzymes and introduction of fusion constructs led to production of 22 mg/l α-terpineol (Zhang et al., 2019). Given that Sphingobium sp. could effectively synthesize α-terpineol from (R)-limonene (Bicas et al., 2010), a high-level biotransformation platform was established accordingly. Optimal fermentation conditions were determined by using Plackett–Burman experimental design and Central Composite Design, and yielded 240 g/(liter of organic phase) of α-terpineol from 350 g/(liter of organic phase) limonene after 96 h in 4.8 l bioreactor (Molina et al., 2019). Specifically, agitation speed (mechanical stirring) was found to be one of the key parameters, suggesting mass transfer limitation. Moreover, improving aqueous/organic ratio to 3 boosted total α-terpineol production level by 30%. It is also noteworthy that Sphingobium sp. can degrade various aromatic or chloroaromatic chemicals at high concentrations. The robustness of Sphingobium sp. was a key factor in obtaining the high product titer during the biotransformation process.
Likewise, β-phellandrene was produced in cyanobacteria Synechocystis sp. PCC 6803, and a substantial yield improvement was observed when GPPS and β-phellandrene synthase were expressed as a fusion protein (Betterle & Melis, 2019). Localization of GPP pools in both peroxisome and mitochondria was explored in S. cerevisiae to improve sabinene production (final titer: 155 mg/l, shake flask scale) (Jia et al., 2020).
Li et al. reported production of citronellol (an acyclic monoterpenoid) in S. cerevisiae. Citronellol can by synthesized from geraniol when the OYE2 gene was expressed. To improve the precursor supply for citronellol production, the authors fused the truncated GES to the N-terminus of a negative variant of ERG20 (ERG20*). The native promoter of ERG20 was replaced with ERG7 promoter to redirect the metabolic flux from steroid synthesis to monoterpenoids production. Additional copies of the fused GES-ERG20* was introduced into the recombinant yeast cell. The resulting strain produced 358 mg/l citronellol in shake-flask culture. The final citronellol titer was 6.37 g/l in fed-batch bioreactor fermentation at higher biomass and with longer cultivation time (Li et al., 2021).
Borneol, a bicyclic monoterpenoid alcohol, could be synthesized from bornyl diphosphate (BPP, derived from NPP, which is catalyzed by bornyl diphosphate synthase [BPPS]). Endogenous phosphatases in E. coli were hypothesized to dephosphorylate BPP to form borneol. By co-expressing tNPPS and BPPS, 4 mg/l borneol was produced from glucose in E. coli. Site-directed mutagenesis was applied to BPPS, and improved borneol titer to 10 mg/l. Further overexpression of nudJ (encoding an endogenous Nudix hydrolases, NudJ) increased the titer to 28 mg/l. Optimization of induction conditions and initial glucose concentration further boosted borneol production level to 87 mg/l in shake-flask culture, which is the highest borneol titer in microbial hosts to date (Lei et al., 2021).
Perspectives
Despite many impressive advances in monoterpenoids research, titers of most products remain low. A key focus of efforts to address bottlenecks in the downstream biosynthetic pathways should be the catalytic efficiency of the different monoterpenoid synthases.
For those monoterpenoids whose synthases have been characterized, it has been determined that many of these enzymes are not expressed well in the new hosts or show low activity because of, among other factors, misfolding issues. New computational tools would be invaluable in aiding selection of better natural variants and guiding their laboratory evolution. Better understanding of how these enzymes work in their native hosts will also be critical. High enzyme activity might be achieved by co-expressing their native helper proteins (including chaperones) and/or targeting the enzymes to subcellular compartments that have similar pH and co-factor concentrations to their native working environment.
It will be intriguing to expand the spectrum of monoterpenoids that can be synthesized in microbial host cells. So far, synthases or functionalizing enzymes have not been discovered for many valuable monoterpenoids. This is primarily a computational effort as the space of possible synthases is huge and can only be probed computationally. To this end, better algorithms are needed to shortlist promising enzyme candidates. In parallel, lower DNA synthesis cost and faster screening methods will also allow evaluating a larger number of enzyme candidates.
Volatility of monoterpenoids is a unique property of this class of molecules, which poses special challenges for their production. Biphasic fermentation is often employed, in which an organic overlay in situ extracts and traps monoterpenoids. This approach, however, requires costly and laborious downstream product purification, due to the similarity of physical properties between monoterpenoids and the organic extractant. Using closed system to produce volatile molecules or using gas stripping to remove them from the fermentation present interesting options. Besides schemes for their production, monoterpenoid volatility also creates problems for the accurate measurement of culture parameters, as well as fermentation monitoring and control.
It is critical to maintain sufficient supply of precursors by minimizing the resource competition with cell growth. High cell density plays an important role in high-titer monoterpenoid production, but accumulation of cytotoxic monoterpenoids would hamper cell growth. Hence, separation of the cell growth phase and production phase may be useful to improving synthesis of monoterpenoids.
In addition, it seems that monoterpenoid production in E. coli and S. cerevisiae is often restricted by toxicity, as many of these products possess antimicrobial features. In this regard, exploiting nonconventional hosts with greater tolerance to monoterpenoid concentration would be a promising direction. Some of these potential hosts can grow well at high solvent concentrations or under extreme pH. To date, many nonconventional hosts are not genetically tractable, which increases the difficulty of engineering these organisms for monoterpenoid biosynthesis. As such, it would be very desirable to develop suitable genetic tools to the genetic modulation of these nonconventional hosts.
Contributor Information
Yurou Liu, Department of Chemical and Biomolecular Engineering, National University of Singapore, Singapore 117585, Singapore; Disruptive & Sustainable Technologies for Agricultural Precision, Singapore-MIT Alliance for Research and Technology, Singapore 138602, Singapore.
Xiaoqiang Ma, Department of Chemical and Biomolecular Engineering, National University of Singapore, Singapore 117585, Singapore.
Hong Liang, Department of Chemical and Biomolecular Engineering, National University of Singapore, Singapore 117585, Singapore; Disruptive & Sustainable Technologies for Agricultural Precision, Singapore-MIT Alliance for Research and Technology, Singapore 138602, Singapore.
Gregory Stephanopoulos, Disruptive & Sustainable Technologies for Agricultural Precision, Singapore-MIT Alliance for Research and Technology, Singapore 138602, Singapore; Department of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Kang Zhou, Department of Chemical and Biomolecular Engineering, National University of Singapore, Singapore 117585, Singapore; Disruptive & Sustainable Technologies for Agricultural Precision, Singapore-MIT Alliance for Research and Technology, Singapore 138602, Singapore.
Funding
None declared.
Conflict of Interest
The authors declare no conflict of interest.
Data Availability
All of the data used to support the claims of this work have been presented in the form of figures and/or tables, which are available in this manuscript.
References
- Anis E., Zafeer M. F., Firdaus F., Islam S. N., Fatima M., Mobarak Hossain M. (2018). Evaluation of phytomedicinal potential of perillyl alcohol in an in vitro Parkinson's Disease model. Drug Development Research, 79(5), 218–224. 10.1002/ddr.21436. [DOI] [PubMed] [Google Scholar]
- Bao S.-H., Zhang D.-Y., Meng E. (2019). Improving biosynthetic production of pinene through plasmid recombination elimination and pathway optimization. Plasmid, 105, 102431. 10.1016/j.plasmid.2019.102431. [DOI] [PubMed] [Google Scholar]
- Belanger J. T. (1998). Perillyl alcohol: applications in oncology. Alternative Medicine Review, 3(6), 448–457. https://europepmc.org/abstract/MED/9855569. [PubMed] [Google Scholar]
- Betterle N., Melis A. (2019). Photosynthetic generation of heterologous terpenoids in cyanobacteria. Biotechnology and Bioengineering, 116(8), 2041–2051. 10.1002/bit.26988. [DOI] [PubMed] [Google Scholar]
- Bicas J. L., Fontanille P., Pastore G. M., Larroche C. (2010). A bioprocess for the production of high concentrations of R-(+)-α-terpineol from R-(+)-limonene. Process Biochemistry, 45(4), 481–486. 10.1016/j.procbio.2009.11.007. [DOI] [Google Scholar]
- Bicas J. L., Neri-Numa I. A., Ruiz A. L. T. G., De Carvalho J. E., Pastore G. M. (2011). Evaluation of the antioxidant and antiproliferative potential of bioflavors. Food and Chemical Toxicology, 49(7), 1610–1615. 10.1016/j.fct.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Burke Y. D., Stark M. J., Roach S. L., Sen S. E., Crowell P. L. (1997). Inhibition of pancreatic cancer growth by the dietary isoprenoids farnesol and geraniol. Lipids, 32(2), 151. 10.1007/s11745-997-0019-y. [DOI] [PubMed] [Google Scholar]
- Chang H. C., Gage D. A., Oriel P. J. (1995). Cloning and expression of a limonene degradation pathway from Bacillus stearothermophilus in Escherichia coli. Journal of Food Science, 60(3), 551–553. 10.1111/j.1365-2621.1995.tb09824.x. [DOI] [Google Scholar]
- Chatzivasileiou A. O., Ward V., Edgar S. M., Stephanopoulos G. (2019). Two-step pathway for isoprenoid synthesis. Proceedings of the National Academy of Sciences, USA, 116(2), 506. 10.1073/pnas.1812935116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Viljoen A. M. (2010). Geraniol — a review of a commercially important fragrance material. South African Journal of Botany, 76(4), 643–651. 10.1016/j.sajb.2010.05.008. [DOI] [Google Scholar]
- Cheng B.-Q., Wei L.-J., Lv Y.-B., Chen J., Hua Q. (2019). Elevating limonene production in oleaginous yeast Yarrowia lipolytica via genetic engineering of limonene biosynthesis pathway and optimization of medium composition. Biotechnology and Bioprocess Engineering, 24(3), 500–506. 10.1007/s12257-018-0497-9. [DOI] [Google Scholar]
- Cheng S., Liu X., Jiang G., Wu J., Zhang J., Lei D., Yuan Y.-J., Qiao J., Zhao G.-R. (2019). Orthogonal engineering of biosynthetic pathway for efficient production of limonene in Saccharomyces cerevisiae. ACS Synthetic Biology, 8(5), 968–975. 10.1021/acssynbio.9b00135. [DOI] [PubMed] [Google Scholar]
- Christina K., Nurhayat T., Gerhard B. (2018). α-Terpineol, a natural monoterpene: a review of its biological properties. Open Chemistry, 16(1), 349–361. 10.1515/chem-2018-0040. [DOI] [Google Scholar]
- Clomburg J. M., Qian S., Tan Z., Cheong S., Gonzalez R. (2019). The isoprenoid alcohol pathway, a synthetic route for isoprenoid biosynthesis. Proceedings of the National Academy of Sciences, USA, 116(26), 12810. 10.1073/pnas.1821004116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo R., Wei K., Rodenak-Kladniew B., Mercola M., Ruiz-Lozano P., Hurtado C. (2017). Effect of geraniol on rat cardiomyocytes and its potential use as a cardioprotective natural compound. Life Sciences, 172, 8–12. 10.1016/j.lfs.2017.01.008. [DOI] [PubMed] [Google Scholar]
- De Luca V., Salim V., Atsumi S. M., Yu F. (2012). Mining the biodiversity of plants: a revolution in the making. Science, 336(6089), 1658. 10.1126/science.1217410. [DOI] [PubMed] [Google Scholar]
- de Sousa D. P., Nóbrega F. F. F., Santos C. C. M. P., de Almeida R. N. (2010). Anticonvulsant activity of the linalool enantiomers and racemate: investigation of chiral influence. Natural Product Communications, 5(12), 1847–1851. . [DOI] [PubMed] [Google Scholar]
- Dudley Q. M., Nash C. J., Jewett M. C. (2019). Cell-free biosynthesis of limonene using enzyme-enriched Escherichia coli lysates. Synthetic Biology, 4(1), ysz003. 10.1093/synbio/ysz003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdogan A., Ozkan A. (2017). Investigatıon of antioxıdative, cytotoxic, membrane-damaging and membrane-protective effects of the essentıal oil of Origanum majorana and its oxygenated monoterpene component linalool in human-derived Hep G2 cell line. Iranian Journal of Pharmaceutical esearch: IJPR, 16(Suppl), 24–34. 10.22037/IJPR.2017.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farasat I., Kushwaha M., Collens J., Easterbrook M., Guido M., Salis H. M. (2014). Efficient search, mapping, and optimization of multi-protein genetic systems in diverse bacteria. Molecular Systems Biology, 10(6), 731. 10.15252/msb.20134955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felipe L. d. O., Oliveira A. M. d., Bicas J. L. (2017). Bioaromas – perspectives for sustainable development. Trends in Food Science & Technology, 62, 141–153. 10.1016/j.tifs.2017.02.005. [DOI] [Google Scholar]
- Gao Q., Wang L., Zhang M., Wei Y., Lin W. (2020). Recent advances on feasible strategies for monoterpenoid production in Saccharomyces cerevisiae. Frontiers in Bioengineering and Biotechnology, 8, 609800–609800. 10.3389/fbioe.2020.609800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke J., Frauendorf H., Schneider D., Wintergoller M., Hofmeister T., Poehlein A., Zebec Z., Takano E., Scrutton N. S., Braus G. H. (2020). Production of the fragrance geraniol in peroxisomes of a product-tolerant Baker's yeast. Frontiers in Bioengineering and Biotechnology, 8, 1097. 10.3389/fbioe.2020.582052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei S., Ghobeh M., Yaghmaei P. (2019). The effect of nerol on behavioral, biochemical and histological parameters in male Wistar Alzheimer's rats. Biomacromolecular Journal, 5(1), 12–22. https://www.bmmj.org/article_37221.html. [Google Scholar]
- Goldstein J. L., Brown M. S. (1990). Regulation of the mevalonate pathway. Nature, 343(6257), 425–430. 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Haag J. D., Lindstrom M. J., Gould M. N. (1992). Limonene-induced regression of mammary carcinomas. Cancer Research, 52(14), 4021. https://cancerres.aacrjournals.org/content/52/14/4021.abstract. [PubMed] [Google Scholar]
- Hara Y., Kadotani N., Izui H., Katashkina J. I., Kuvaeva T. M., Andreeva I. G., Golubeva L. I., Malko D. B., Makeev V. J., Mashko S. V., Kozlov Y. I. (2012). The complete genome sequence of Pantoea ananatis AJ13355, an organism with great biotechnological potential. Applied Microbiology and Biotechnology, 93(1), 331–341. 10.1007/s00253-011-3713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey B. G., Wright M. E., Quintana R. L. (2010). High-density renewable fuels based on the selective dimerization of pinenes. Energy & Fuels, 24(1), 267–273. 10.1021/ef900799c. [DOI] [Google Scholar]
- Hoshino Y., Moriya M., Matsudaira A., Katashkina J. I., Nitta N., Nishio Y., Usuda Y. (2020). Stereospecific linalool production utilizing two-phase cultivation system in Pantoea ananatis. Journal of Biotechnology, 324, 21–27. 10.1016/j.jbiotec.2020.09.021. [DOI] [PubMed] [Google Scholar]
- Hu Z., Li H., Weng Y., Li P., Zhang C., Xiao D. (2020). Improve the production of d-limonene by regulating the mevalonate pathway of Saccharomyces cerevisiae during alcoholic beverage fermentation. Journal of Industrial Microbiology and Biotechnology, 47(12), 1083–1097. 10.1007/s10295-020-02329-w. [DOI] [PubMed] [Google Scholar]
- Ignea C., Raadam M. H., Motawia M. S., Makris A. M., Vickers C. E., Kampranis S. C. (2019). Orthogonal monoterpenoid biosynthesis in yeast constructed on an isomeric substrate. Nature Communications, 10(1), 3799. 10.1038/s41467-019-11290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilc T., Parage C., Boachon B., Navrot N., Werck-Reichhart D. (2016). Monoterpenol oxidative metabolism: role in plant adaptation and potential applications. Frontiers in Plant Science, 7, 509. 10.3389/fpls.2016.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscan G., Kırımer N., Demirci F., Baser K. H. C. (2011). Microbial transformation of beta-phellandrene. Planta Medica, 77(12), 1278–1278. 10.1055/s-0031-1282262. [DOI] [Google Scholar]
- Jia H., Chen T., Qu J., Yao M., Xiao W., Wang Y., Li C., Yuan Y. (2020). Collaborative subcellular compartmentalization to improve GPP utilization and boost sabinene accumulation in Saccharomyces cerevisiae. Biochemical Engineering Journal, 164, 107768. 10.1016/j.bej.2020.107768. [DOI] [Google Scholar]
- Jiang K., Zhang T., Yin N., Ma X., Zhao G., Wu H., Qiu C., Deng G. (2017). Geraniol alleviates LPS-induced acute lung injury in mice via inhibiting inflammation and apoptosis. Oncotarget, 8(41), 71038–71053. 10.18632/oncotarget.20298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesselmeier J., Staudt M. (1999). Biogenic volatile organic compounds (VOC): an overview on emission, Physiology and Ecology. Journal of Atmospheric Chemistry, 33(1), 23–88. 10.1023/A:1006127516791. [DOI] [Google Scholar]
- Kong S., Fu X., Li X., Pan H., Guo D. (2020). De novo biosynthesis of linalool from glucose in engineered Escherichia coli. Enzyme and Microbial Technology, 140, 109614. 10.1016/j.enzmictec.2020.109614. [DOI] [PubMed] [Google Scholar]
- Krivoruchko A., Nielsen J. (2015). Production of natural products through metabolic engineering of Saccharomyces cerevisiae. Current Opinion in Biotechnology, 35, 7–15. 10.1016/j.copbio.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Lampronti I., Saab A. M., Gambari R. (2006). Antiproliferative activity of essential oils derived from plants belonging to the Magnoliophyta division. International Journal of Oncology, 29(4), 989–995. 10.3892/ijo.29.4.989. [DOI] [PubMed] [Google Scholar]
- Lange B. M., Rujan T., Martin W., Croteau R. (2000). Isoprenoid biosynthesis: the evolution of two ancient and distinct pathways across genomes. Proceedings of the National Academy of Sciences, USA, 97(24), 13172. 10.1073/pnas.240454797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapczynski A., Foxenberg R. J., Bhatia S. P., Letizia C. S., Api A. M. (2008). Fragrance material review on nerol. Food and Chemical Toxicology, 46(11), S241–S244. 10.1016/j.fct.2008.06.062. [DOI] [PubMed] [Google Scholar]
- Lei D., Qiu Z., Qiao J., Zhao G.-R. (2021). Plasticity engineering of plant monoterpene synthases and application for microbial production of monoterpenoids. Biotechnology for Biofuels, 14(1), 147. 10.1186/s13068-021-01998-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei D., Qiu Z., Wu J., Qiao B., Qiao J., Zhao G.-R. (2021). Combining metabolic and monoterpene synthase engineering for de novo production of monoterpene alcohols in Escherichia coli. ACS Synthetic Biology, 10(6), 1531–1544. 10.1021/acssynbio.1c00081. [DOI] [PubMed] [Google Scholar]
- Li C.-J., Trost B. M. (2008). Green chemistry for chemical synthesis. Proceedings of the National Academy of Sciences, USA, 105(36), 13197. 10.1073/pnas.0804348105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Wang K., Wang D., Xu L., Shi Y., Dai Z., Zhang X. (2021). Production of plant volatile terpenoids (rose oil) by yeast cell factories. Green Chemistry, 23(14), 5088–5096. 10.1039/D1GC00917F. [DOI] [Google Scholar]
- Li X., Liu M., Huang T., Yang K., Zhou S., Li Y., Tian J. (2021). Antifungal effect of nerol via transcriptome analysis and cell growth repression in sweet potato spoilage fungi Ceratocystis fimbriata. Postharvest Biology and Technology, 171, 111343. 10.1016/j.postharvbio.2020.111343. [DOI] [Google Scholar]
- Li Y.-L., Yeung C.-M., Chiu L. C. M., Cen Y.-Z., Ooi V. E. C. (2009). Chemical composition and antiproliferative activity of essential oil from the leaves of a medicinal herb, Schefflera heptaphylla. Phytotherapy Research, 23(1), 140–142. 10.1002/ptr.2567. [DOI] [PubMed] [Google Scholar]
- Liu W., Zhang R., Tian N., Xu X., Cao Y., Xian M., Liu H. (2015). Utilization of alkaline phosphatase PhoA in the bioproduction of geraniol by metabolically engineered Escherichia coli. Bioengineered, 6(5), 288–293. 10.1080/21655979.2015.1062188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo C., Eugenio A. (2011). Use of terpenoids as natural flavouring compounds in food industry. Recent Patents on Food, Nutrition & Agriculture, 3(1), 9–16. 10.2174/2212798411103010009. [DOI] [PubMed] [Google Scholar]
- Maltzman T. H., Hurt L. M., Elson C. E., Tanner M. A., Gould M. N. (1989). The prevention of nitrosomethylurea-induced mammary tumors by d-limonene and orange oil. Carcinogenesis, 10(4), 781–783. 10.1093/carcin/10.4.781. [DOI] [PubMed] [Google Scholar]
- Mayeku W. P., Omollo N. I., Odalo O. J., Hassanali A. (2014). Chemical composition and mosquito repellency of essential oil of Conyza newii propagated in different geographical locations of Kenya. Medical and Veterinary Entomology, 28(3), 253–256. 10.1111/mve.12039. [DOI] [PubMed] [Google Scholar]
- McCaskill D., Croteau R. (1997). Prospects for the bioengineering of isoprenoid biosynthesis. Advances in Biochemical Engineering/Biotechnology, 55, 107–146. 10.1007/BFb0102064. [DOI] [PubMed] [Google Scholar]
- Miller D. R., Borden J. H. (2000). Dose-dependent and species-specific responses of pine bark beetles (coeoptera: scolytidae) to monoterpenes in association with phermones. The Canadian Entomologist 132(2), 183–195. 10.4039/Ent132183-2. [DOI] [Google Scholar]
- Mishra S., Pandey P., Dubey A. P., Zehra A., Chanotiya C. S., Tripathi A. K., Mishra M. N. (2020). Engineering a carotenoid-overproducing strain of Azospirillum brasilense for heterologous production of geraniol and amorphadiene. Applied and Environmental Microbiology, 86(17), e00414–00420. 10.1128/AEM.00414-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo X., Cai X., Hui Q., Sun H., Yu R., Bu R., Yan B., Ou Q., Li Q., He S., Jiang C. (2021). Whole genome sequencing and metabolomics analyses reveal the biosynthesis of nerol in a multi-stress-tolerant Meyerozyma guilliermondii GXDK6. Microbial Cell Factories, 20(1), 4. 10.1186/s12934-020-01490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina G., Pessôa M. G., Bicas J. L., Fontanille P., Larroche C., Pastore G. M. (2019). Optimization of limonene biotransformation for the production of bulk amounts of α-terpineol. Bioresource Technology, 294, 122180. 10.1016/j.biortech.2019.122180. [DOI] [PubMed] [Google Scholar]
- Nitta N., Tajima Y., Katashkina J. I., Yamamoto Y., Onuki A., Rachi H., Kazieva E., Nishio Y. (2020). Application of inorganic phosphate limitation to efficient isoprene production in Pantoea ananatis. Journal of Applied Microbiology, 128(3), 763–774. 10.1111/jam.14521. [DOI] [PubMed] [Google Scholar]
- Niu F.-X., He X., Wu Y.-Q., Liu J.-Z. (2018). Enhancing production of pinene in Escherichia coli by using a combination of tolerance, evolution, and modular co-culture engineering. Frontiers in Microbiology, 9, 1623–1623. 10.3389/fmicb.2018.01623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu F.-X., Huang Y.-B., Ji L.-N., Liu J.-Z. (2019). Genomic and transcriptional changes in response to pinene tolerance and overproduction in evolved Escherichia coli. Synthetic and Systems Biotechnology, 4(3), 113–119. 10.1016/j.synbio.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu F.-X., Huang Y.-B., Shen Y.-P., Ji L.-N., Liu J.-Z. (2020). Enhanced production of pinene by using a cell-free system with modular cocatalysis. Journal of Agricultural and Food Chemistry, 68(7), 2139–2145. 10.1021/acs.jafc.9b07830. [DOI] [PubMed] [Google Scholar]
- Pang Y., Zhao Y., Li S., Zhao Y., Li J., Hu Z., Zhang C., Xiao D., Yu A. (2019). Engineering the oleaginous yeast Yarrowia lipolytica to produce limonene from waste cooking oil. Biotechnology for Biofuels, 12(1), 241. 10.1186/s13068-019-1580-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng B., Nielsen L. K., Kampranis S. C., Vickers C. E. (2018). Engineered protein degradation of farnesyl pyrophosphate synthase is an effective regulatory mechanism to increase monoterpene production in Saccharomyces cerevisiae. Metabolic Engineering, 47, 83–93. 10.1016/j.ymben.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Pereira I., Severino P., Santos A. C., Silva A. M., Souto E. B. (2018). Linalool bioactive properties and potential applicability in drug delivery systems. Colloids and Surfaces B: Biointerfaces, 171, 566–578. 10.1016/j.colsurfb.2018.08.001. [DOI] [PubMed] [Google Scholar]
- Pichersky E., Gershenzon J. (2002). The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Current Opinion in Plant Biology, 5(3), 237–243. 10.1016/S1369-5266(02)00251-0. [DOI] [PubMed] [Google Scholar]
- Raguso R. A. (2016). More lessons from linalool: insights gained from a ubiquitous floral volatile. Current Opinion in Plant Biology, 32, 31–36. 10.1016/j.pbi.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Raman V., Sivasankaralingam V., Dibble R., Sarathy S. M. (2016). α-pinene - a high energy density biofuel for SI engine applications. SAE Technical Paper. 10.4271/2016-01-2171. [DOI] [Google Scholar]
- Rohmer M. (1999). The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Natural Product Reports, 16(5), 565–574. 10.1039/A709175C. [DOI] [PubMed] [Google Scholar]
- Rolf J., Julsing M. K., Rosenthal K., Lütz S. (2020). A gram-scale limonene production process with engineered Escherichia coli. Molecules (Basel, Switzerland), 25(8), 1881. 10.3390/molecules25081881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio L. d. F. S., Maia J. G. S., de Parijós A. M., de Souza R. Z., Barata L. E. S. (2012). Linalool from rosewood (Aniba rosaeodora ducke) oil inhibits adenylate cyclase in the retina, contributing to understanding its biological activity. Phytotherapy Research, 26(1), 73–77. 10.1002/ptr.3518. [DOI] [PubMed] [Google Scholar]
- Sharkey T. D., Singsaas E. L. (1995). Why plants emit isoprene. Nature, 374(6525), 769–769. 10.1038/374769a0. [DOI] [Google Scholar]
- Souliman R., Bouayed J., Joshi R. K. (2019). Limonene: natural monoterpene volatile compounds of potential therapeutic interest. American Journal of Essential Oils and Natural Products, 7(4), 01–10. https://www.essencejournal.com/archives/2019/7/4/A/7-4-2. [Google Scholar]
- Sun C., Dong X., Zhang R., Xie C. (2021). Effectiveness of recombinant Escherichia coli on the production of (R)-(+)-perillyl alcohol. BMC Biotechnology, 21(1), 3. 10.1186/s12896-020-00662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente J., Zuzarte M., Gonçalves M. J., Lopes M. C., Cavaleiro C., Salgueiro L., Cruz M. T. (2013). Antifungal, antioxidant and anti-inflammatory activities of Oenanthe crocata L. essential oil. Food and Chemical Toxicology, 62, 349–354. 10.1016/j.fct.2013.08.083. [DOI] [PubMed] [Google Scholar]
- Vaughn S. F., Spencer G. F. (1993). Volatile monoterpenes as potential parent structures for new herbicides. Weed Science, 41(1), 114–119. 10.1017/S0043174500057672. [DOI] [Google Scholar]
- Vickers C. E., Bongers M., Liu Q., Delatte T., Bouwmeester H. (2014). Metabolic engineering of volatile isoprenoids in plants and microbes. Plant, Cell & Environment, 37(8), 1753–1775. 10.1111/pce.12316. [DOI] [PubMed] [Google Scholar]
- Vuuren S. F. V., Viljoen A. M. (2007). Antimicrobial activity of limonene enantiomers and 1,8-cineole alone and in combination. Flavour and Fragrance Journal, 22(6), 540–544. 10.1002/ffj.1843. [DOI] [Google Scholar]
- Wang X., Chen J., Zhang J., Zhou Y., Zhang Y., Wang F., Li X. (2021). Engineering Escherichia coli for production of geraniol by systematic synthetic biology approaches and laboratory-evolved fusion tags. Metabolic Engineering, 66, 60–67. 10.1016/j.ymben.2021.04.008. [DOI] [PubMed] [Google Scholar]
- Wang X., Wu J., Chen J., Xiao L., Zhang Y., Wang F., Li X. (2020). Efficient biosynthesis of R-(−)-linalool through adjusting the expression strategy and increasing GPP supply in Escherichia coli. Journal of Agricultural and Food Chemistry, 68(31), 8381–8390. 10.1021/acs.jafc.0c03664. [DOI] [PubMed] [Google Scholar]
- Wei A., Shibamoto T. (2007). Antioxidant activities and volatile constituents of various essential oils. Journal of Agricultural and Food Chemistry, 55(5), 1737–1742. 10.1021/jf062959x. [DOI] [PubMed] [Google Scholar]
- Wilkes J., Scott-Tucker A., Wright M., Crabbe T., Scrutton N. S. (2020). Exploiting single domain antibodies as regulatory parts to modulate monoterpenoid production in E. coli. ACS Synthetic Biology, 9(10), 2828–2839. 10.1021/acssynbio.0c00375. [DOI] [PubMed] [Google Scholar]
- Wu J., Cheng S., Cao J., Qiao J., Zhao G.-R. (2019). Systematic optimization of limonene production in engineered Escherichia coli. Journal of Agricultural and Food Chemistry, 67(25), 7087–7097. 10.1021/acs.jafc.9b01427. [DOI] [PubMed] [Google Scholar]
- Wu J., Wang X., Xiao L., Wang F., Zhang Y., Li X. (2021). Synthetic protein scaffolds for improving R-(−)-linalool production in Escherichia coli. Journal of Agricultural and Food Chemistry, 69(20), 5663–5670. 10.1021/acs.jafc.1c01101. [DOI] [PubMed] [Google Scholar]
- Xie S.-S., Zhu L., Qiu X.-Y., Zhu C.-S., Zhu L.-Y. (2019). Advances in the metabolic engineering of Escherichia coli for the manufacture of monoterpenes. Catalysts, 9(5), 433. 10.3390/catal9050433. [DOI] [Google Scholar]
- Yao F., Liu S.-C., Wang D.-N., Liu Z.-J., Hua Q., Wei L.-J. (2020). Engineering oleaginous yeast Yarrowia lipolytica for enhanced limonene production from xylose and lignocellulosic hydrolysate. FEMS Yeast Research, 20(6), foaa046. 10.1093/femsyr/foaa046. [DOI] [PubMed] [Google Scholar]
- Yee D. A., DeNicola A. B., Billingsley J. M., Creso J. G., Subrahmanyam V., Tang Y. (2019). Engineered mitochondrial production of monoterpenes in Saccharomyces cerevisiae. Metabolic Engineering, 55, 76–84. 10.1016/j.ymben.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon W.-J., Lee N. H., Hyun C.-G. (2010). Limonene suppresses lipopolysaccharide-induced production of nitric oxide, prostaglandin E2, and pro-inflammatory cytokines in RAW 264.7 macrophages. Journal of Oleo Science, 59(8), 415–421. 10.5650/jos.59.415. [DOI] [PubMed] [Google Scholar]
- Yu S. G., Hildebrandt L. A., Elson C. E. (1995). Geraniol, an inhibitor of mevalonate biosynthesis, suppresses the growth of hepatomas and melanomas transplanted to rats and mice. The Journal of Nutrition, 125(11), 2763–2767. 10.1093/jn/125.11.2763. [DOI] [PubMed] [Google Scholar]
- Zebec Z., Wilkes J., Jervis A. J., Scrutton N. S., Takano E., Breitling R. (2016). Towards synthesis of monoterpenes and derivatives using synthetic biology. Current Opinion in Chemical Biology, 34, 37–43. 10.1016/j.cbpa.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Zhang C., Li M., Zhao G.-R., Lu W. (2019). Alpha-terpineol production from an engineered Saccharomyces cerevisiae cell factory. Microbial Cell Factories, 18(1), 160. 10.1186/s12934-019-1211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Liu X., Meng Y., Zhang L., Qiao J., Zhao G.-R. (2021). Combinatorial engineering of Saccharomyces cerevisiae for improving limonene production. Biochemical Engineering Journal, 176, 108155. 10.1016/j.bej.2021.108155. [DOI] [Google Scholar]
- Zhang Y., Wang J., Cao X., Liu W., Yu H., Ye L. (2020). High-level production of linalool by engineered Saccharomyces cerevisiae harboring dual mevalonate pathways in mitochondria and cytoplasm. Enzyme and Microbial Technology, 134, 109462. 10.1016/j.enzmictec.2019.109462. [DOI] [PubMed] [Google Scholar]
- Zhou P., Du Y., Fang X., Xu N., Yue C., Ye L. (2021). Combinatorial modulation of linalool synthase and farnesyl diphosphate synthase for linalool overproduction in Saccharomyces cerevisiae. Journal of Agricultural and Food Chemistry, 69(3), 1003–1010. 10.1021/acs.jafc.0c06384. [DOI] [PubMed] [Google Scholar]
- Zhou P., Du Y., Xu N., Yue C., Ye L. (2020). Improved linalool production in Saccharomyces cerevisiae by combining directed evolution of linalool synthase and overexpression of the complete mevalonate pathway. Biochemical Engineering Journal, 161, 107655. 10.1016/j.bej.2020.107655. [DOI] [Google Scholar]
- Zong Z., Hua Q., Tong X., Li D., Wang C., Guo D., Liu Z. (2019). Biosynthesis of nerol from glucose in the metabolic engineered Escherichia coli. Bioresource Technology, 287, 121410. 10.1016/j.biortech.2019.121410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the data used to support the claims of this work have been presented in the form of figures and/or tables, which are available in this manuscript.