Abstract
Classic physiology divides all neural cells into excitable neurons and nonexcitable neuroglia. Neuroglial cells, chiefly responsible for homeostasis and defense of the nervous tissue, coordinate their complex homeostatic responses with neuronal activity. This coordination reflects a specific form of glial excitability mediated by complex changes in intracellular concentration of ions and second messengers organized in both space and time. Astrocytes are equipped with multiple molecular cascades, which are central for regulating homeostasis of neurotransmitters, ionostasis, synaptic connectivity, and metabolic support of the central nervous system. Astrocytes are further provisioned with multiple receptors for neurotransmitters and neurohormones, which upon activation trigger intracellular signals mediated by Ca2+, Na+, and cyclic AMP. Calcium signals have distinct organization and underlying mechanisms in different astrocytic compartments thus allowing complex spatiotemporal signaling. Signals mediated by fluctuations in cytosolic Na+ are instrumental for coordination of Na+ dependent astrocytic transporters with tissue state and homeostatic demands. Astroglial ionic excitability may also involve K+, H+, and Cl−. The cyclic AMP signalling system is, in comparison to ions, much slower in targeting astroglial effector mechanisms. This evidence review summarizes the concept of astroglial intracellular excitability.
Keywords: astrocyte, astrocytic processes, calcium signaling, sodium signaling, ionic signaling, astroglial excitability
The Concept of Excitability
The concept of physiological excitability and the definition of excitable and nonexcitable tissues was formulated by Albrecht von Haller, who exposed different organs or their parts to injury, by squeezing and stinging, by sprinkling with cold, hot, or corrosive substances or by electrocuting. Analyzing responses to such interrogations, von Haller proposed to classify all organs into sensible (sensibilis) and irritable (irritabilis) ones.1 In addition to the tissues that actively responded to various manipulations, von Haller also noticed a third type of organs and tissues, which were neither sensible nor irritable; he named this type of tissue the Zellgewebsfaser or cell tissue fiber that came together to form the Zellgewebe (“cellular tissue”). This was an inert tissue, forming a filling or basic substance that surrounds and covers all components of the organism being in a way a predecessor of the connective tissue of Rudolf Virchow.
Although the cellular theory had not yet been established by the time of von Haller’s work the notion of the cell as an elementary living entity has been considered; the term being invented by Robert Hook in the 1650s.2 The first description of brain cells was made by Marcello Malpighi who described the cortical tissue as being formed from many globules or “little glands”3; similar structures were also observed by Antonie van Leeuwenhoek.4 The first detailed description of brain elementary structures were made by Emanuel Swedenborg in 1740s, who envisaged the nervous tissue as made from functionally independent globules or cerebellulas (minute brains) that are connected by nerve fibers, which receive sensations from or project motor impulses into the peripheral organs. Swedenborg described these structures as the substrates for brain function. He wrote “From each cortical gland proceeds a single nerve fiber; this is carried down into the body, in order that it may take hold of some part of a sensation or produce some action.”5 The first documented drawing of nerve cells (known as “globules” or “kugeln” both denoting spheres) from the microscopic observations were made by Christian Gottfried Ehrenberg6 and Jan Evangelista Purkinje7; while the term “nerve cell” was introduced by Robert Bently Todd8 in 1845.
A pupil and assistant of Purkinje, Gabriel Gustav Valentin was the first to contemplate two types of nervous elements, which he called nervous masses. One of these masses representing “the creative, active, higher principle” while the second “the receiving and guiding, passive, lower principle.”9 The active substance was represented by spherical elements (Kugeln der Belegungsmassen) and nerve fibers (Primitivfasern), whereas the passive substance was defined as intermediate substance (Zellgewebescheide) made from fibers and threads. The ideas about specific brain connective tissue were further developed by Carl von Rokitansky,10 and Rudolf Virchow who, in 1856 defined the connective tissue of the brain as “…connective substance forms in the brain, the spinal cord and the higher sensory nerves a type of putty (neuroglia), in which the nervous elements are embedded.”11 Although Virchow most likely considered neuroglia as an acellular bona fide connective tissue, glial cells have been visualized and identified by many neuroanatomists12 and their roles have been considered by physiologists. Many active contributions of neuroglia to numerous brain functions have been proposed, these range from interfacing the vasculature with brain parenchyma, thereby regulating local hyperemia, to control over synaptic transmission and brain states, such as the sleep-wake transition.13–16 These considerations changed the role of glia from being regarded as simply a connective tissue to an active counterpart of neurons in executing brain functions.
The advent of electrophysiology and intracellular recordings led to a detailed characterization of electrical excitability of nerves, muscle, and neurons. The very first observation of experimentally evoked muscle contractions were made in the 1660s by Jan Swammerdam, who designed the classic frog neuromuscular preparation.17,18 The discovery of animal electricity was made in 1780–1790s by Luigi Galvani working together with his wife Lucia Galeazzi and his nephew Giovanni Aldini. Galvani recorded electrical excitation of the nerve–muscle preparation, described the relationship between stimulus intensity and muscle contraction, defined the refractory period, and above all demonstrated the propagating wave of excitation through nerve and muscle, known to us as the action potential.19,20
Some 150 years later, the seminal discoveries of Hodgkin and Huxley provided the first quantitative description of the ionic conductance changes underlying the action potential,21 while the emergence of patch-clamp techniques22 and molecular cloning23 identified structural and functional properties of ion channels and established mechanisms underlying electrical excitability of neurons. The first electrophysiological recordings from glial cells in vivo, in organotypic cultures, in isolated optic nerve preparations from amphibians or in the isolated ganglionic chain of the leech24–27 revealed the passive properties of the membranes of these cells as well as the inability of glia to generate action potentials. These experiments also found that glial cells respond with small (several mV) depolarizations to neuronal activity or to some neurotransmitters; all these responses were attributed to originate from K+ accumulation in the extracellular space.
When the technique for making purified glial cell cultures was developed28 and these cultured cells were interrogated with microelectrodes and patch-clamp approaches the functional expression of neurotransmitter receptors was discovered.29,30 Subsequent experiments found that glial cells are capable of expressing virtually every type of neurotransmitter receptor in existence, and moreover in vivo expression of these receptors was tightly regulated by the neurochemical environment: the neuroglial receptor pattern is tailored to neurotransmitters operating in a particular brain region.31 When cultured neuroglial cells were probed with Ca2+indicators, it turned out that chemical or mechanical stimulation of glia almost invariably triggered complex cytoplasmic Ca2+ signals, which, in a form of Ca2+ waves, could propagate over long distances through the gap-junction connected glial syncytium.32–35 Thus, the concept of calcium excitability of neuroglia was developed.36 Ensuing years brought further advancement in the understanding of astroglial excitability, as it turned out that stimulation of astrocytes is associated with substantial Na+ fluxes that generate cytoplasmic Na+ signals as well as with highly organized changes in cytosolic second messengers such as InsP3 and cAMP; the former being linked to Ca2+ signaling whereas the latter being connected with numerous intracellular enzymatic cascades and influenced by Ca2+. Consequently, a coherent concept of intracellular astroglial excitability is in need of definition.
Astroglial Intracellular Excitability
Appearance of the central nervous system (CNS), which emerged early in evolution, was accompanied by division of neural cells into neuron, which represent executive arm responsible for sensory input, information processing, and initiation of peripheral responses and homeostatic and defensive neuroglia. Neuroglial cells of the brain and the spinal cord are classified into macroglia (the cells of neuroepithelial origin further subdivided into astrocytes and oligodendroglia) and microglia, which are scions of fetal macrophages invading the CNS early in the development. Neurons are universally considered as the only excitable cells of the nervous system; they generate fast action potentials, which are conducted over large distances and initiate neurotransmitter release responsible for synaptic connectivity. Nonetheless, fast signaling (in addition to relatively slow ones) does also occur in glial cells, which respond to physiological stimulation with transient fluctuations in their ionic content; these ionic signals are the substrate for rapid stimulus-induced glial excitability.
Astrocytes are the principal homeostatic cells of the CNS, which constantly adapt operation of elaborated homeostatic molecular cascades to neuronal activity and brain state. Astrocytes control CNS homeostasis at many levels. First and foremost astrocytes are responsible for CNS ionostasis—the ionic composition of the interstitial fluid, which are tightly associated with changes in brain state, such as sleep and arousal.37 Astroglial cells are fundamental for uptake and catabolism of the principal neurotransmitters including glutamate, noradrenaline (NA), GABA, glycine, and adenosine38–42; astrocytes also supply neurons with neurotransmitter precursors such as glutamine or l-serine.43,44 Astrocytes provide neurons with energy substrates45 and contribute to regulation of capillary blood flow and local functional hyperemia.46,47 They also provide for water transport from the perivascular space thus supporting the operation of glymphatic system,48 astrocytes sustain the blood–brain barrier,49 and participate in the defense of the CNS through mounting reactive astrogliosis.50 Furthermore, astrocytes act as baroreceptors to sense cerebral perfusion and control systemic circulation.51 All these functions and processes need to be coordinated with neural activity, which stipulates the existence of sophisticated signaling underlying astroglial activation in various physiological and pathological contexts; this activation is the result of astroglial excitability.
Sensing the neural tissue environment involves the stimulation of astroglial membrane receptors. Activation of these receptors does not trigger regenerative transmembrane depolarization, instead it produces changes in intracellular ion activity reflecting changes in free ion concentration ([ion]i), which regulate astroglial physiological activity. Similarly to neurons, astrocytes, are activated in response to sensory stimulation; numerous experiments in vivo in anesthetized and awake animals have demonstrated synchronous cytosolic [Ca2+]i transients engulfing groups of astrocytes in the sensory cortex.52,53 Synaptic transmission is similarly associated with activation of astrocytes: synaptically released glutamate induces local astroglial Ca2+ signals originating from endoplasmic reticulum (ER) Ca2+ release and/or from Ca2+ entry across the plasmalemma54,55; at the same time glutamate is taken up into astrocytes by Na+-dependent transporters, generating a massive Na+ influx, which triggers cytosolic Na+ signals.56,57 These ionic signals in turn affect various intracellular sensors, which regulate astroglial homeostasis pathways and astroglial morphological plasticity.58,59
Changes in the state of the brain—arousal, stress, concentration, behavior - are associated with activation of locus coeruleus (LC), which represents the prime neuronal plexus localized in the brain stem; projections of the LC neurons synchronously release NA in various brain and spinal cord regions. In the adult human brain, the LC consists of only around 50 000 neurons60; these neurons deliver ∼70% of all NA in the CNS.61 The hallmark of LC-mediated activities include arousal, attention, memory formation, sleep regulation, emotional balance, and cognitive control, all depending on NA-mediated morphologic neuroplasticity and metabolic support.62,63
Astrocytes are major targets of NA in the CNS; mature astrocytes express adrenoceptors of both α and β varieties while the density of adrenoceptors in astrocytic processes seems to be significantly higher than in neurons.64 The action of NA on astroglia results in the activation of fast ionic signals and much slower stimulus-response signaling associated with changes in the concentration of the second messenger 3′,5′-cyclic adenosine monophosphate (cAMP), triggering downstream enzymatic cascades, which regulate numerous processes, including the control of gene transcription, needed for astroglial plasticity during learning and memory.65
Ionic Excitability of Astroglia
Maintenance of cellular ionic homeostasis is one of the most fundamental conditions for life; all living organisms on planet Earth are keeping the ionic composition of cytosol and organelles under tight control at the expense of considerable energy. Ionic gradients between the extracellular space and the cytosol are driving ion fluxes. These ionic fluxes originate from opening of ion channels following for example an environmental stress in unicellular organisms or due to release of chemical messengers in multicellular ones. Thus, from the very beginning of life, ions evolved as dynamic intracellular signalers coupling extrinsic challenges to intracellular processes. Conceptually, living cells are constantly balancing the preservation of their ionic composition with generation of ionic fluctuations organized in space and time. To achieve this steady-state, evolution selected transport systems moving ions along and against concentration gradients. In essence, all changes in the cytosolic concentration of any ion, di- or monovalent, can regulate/modulate various cellular events, and hence may act as second messengers in biological systems. Ionic signalingis shaped by dynamic interactions of diffusion (ion movement along an electrochemical gradient) and primary or secondary ion transport (often against electrochemical gradients), which requires energy. All these molecular cascades are in operation in astrocytes.
Astroglial Calcium Signaling
It is universally acknowledged that an increase in the Ca2+ concentration acts as a ubiquitous physiological signal, operating in most (if not in all) cells and tissues. Changes in the Ca2+ concentration in various cellular compartments trigger or regulate a wide variety of cellular processes. The Ca2+ homoeostatic and signaling system involves relatively few molecular elements (Ca2+-permeable ion channels, Ca2+ pumps, Ca2+ solute carrier transporters, and Ca2+ buffers) which, by operating in concert, shape Ca2+ signals in the cytosol and in the organelles while at the same time preventing life-endangering Ca2+ overloads. Changes in [Ca2+]i are sensed by numerous Ca2+-binding proteins, which translate Ca2+ signals into cellular activity.
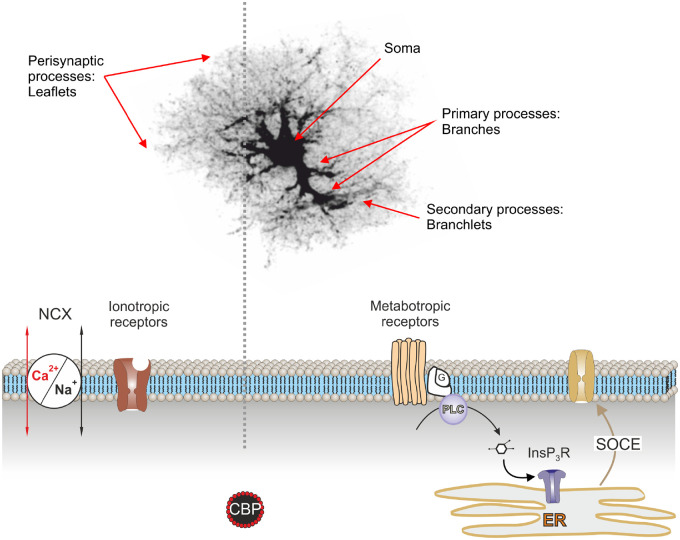
Astroglial Ca2+ signaling is characterized by a complex spatiotemporal organization, which reflects the elaborate astrocyte architecture. Furthermore, different types of astrocytes seemingly have distinct [Ca2+]i dynamics with idiosyncratic underlying mechanisms. The morphological compartments of protoplasmic astrocytes (which are probably the most studied class of astroglia) are represented by (i) soma; (ii) main processes also known as branches; (iii) secondary to tertiary processes designated as branchlets; (iv) peripheral parenchymal and perisynaptic processes known as leaflets; and (v) perivascular processes, which terminate with end feet plastering blood vessels (Figure 1 66,67). All these parts have distinct sizes (with soma being ∼10–15 µm, while primary processes ∼2–5 µm in diameter, an end feet size being in the 2–3 µm range, the branchlets having sub-micrometer diameters and leaflets representing structures with a thickness of ∼100 nm) and different organelle compositions. The perisynaptic leaflets are flat terminal processes with high surface-to-volume ratio and devoid of organelles.68 The terminal branchlets, however, may possess miniature mitochondria.69 These morphological arrangements are associated with distinct mechanisms of Ca2+ signal generation and distinct [Ca2+]i dynamics in different astroglial compartments.
Figure 1.
Morphofunctional Organization of Ca2+ Signaling Compartments in Protoplasmic Astrocyte. Morphological compartments of protoplasmic astrocyte66,67 are represented by (1) soma; (2) main processes also known as branches; (3) secondary to tertiary processes designated as branchlets; (4) peripheral parenchymal and perisynaptic processes known as leaflets; and (5) perivascular processes, which terminate with end feet plastering blood vessels; these latter are not shown on the figure. Calcium signaling in soma, branches, and branchlets are mainly associated with Ca2+ release from the ER with subsequent SOCE. This Ca2+ release is mediated by InsP3 receptors (InsP3R); InsP3 is synthesized by phospholipase C (PLC) linked to G-protein metabotropic receptors. Calcium signaling in the leaflets is associated with Ca2+ entry through ionotropic receptors (NMDA glutamate receptors or P2X purinoceptors) or Ca2+-permeable channels (such as, for example, TRPA1 channels). Plasmalemmal Ca2+ influx can also be mediated by the NCX operating in the reverse mode.
Numerous lines of evidence have demonstrated that Ca2+ signaling in distal processes develop independently from the soma and are often confined to leaflets or branchlets; these signals emerge as local micro- (or even nano-) domains of elevated [Ca2+]i. Focal [Ca2+]i transients can either be spontaneous, with no association with neuronal activity,70–73 or local Ca2+ signals can result from neuronal activity and stimulation of astroglial receptors.74–76 As a rule, Ca2+ signals in the peripheral processes of protoplasmic astrocytes are shorter in duration than in the soma77,78 and are dominated (Figure 1) by plasmalemmal Ca2+ influx through Ca2+ permeable ionotropic receptors55,79 or transient receptor potential (TRP) channels80,81 or reversed Na+/Ca2+ exchanger (NCX).82,83 Calcium signals in the fine astrocytic branchlets appear more frequently than in the thicker branches; these local Ca2+ events in branchlets and branches can be amplified by Ca2+ released from the ER and mitochondria. The higher surface-to-volume ratio of branchlets allows larger plasma membrane Ca2+ influx and hence larger [Ca2+]i fluctuations.73 As a result, local [Ca2+]i fluctuations more frequently reach the threshold for Ca2+-induced Ca2+ release through InsP3 receptors. Hence, loss of fine astrocytic branchlets in pathological conditions such as epilepsy can be linked to reduced astrocytic Ca2+ activity.84
Somatic Ca2+ signals in protoplasmic astrocytes, as well as [Ca2+]i transients in the primary processes are larger in amplitude and slower, are often synchronized with neighboring astrocytes (for example within the confines of a barrel in the somatosensory cortex53) and are originating from stimulation of metabotropic receptors and InsP3-induced Ca2+ release from the ER (Figure 1)53,85,86 that is associated with a consequent activation of store-operated Ca2+ entry (SOCE).81,87 Genetic deletion of the InsP3 receptor type II (the predominant astroglial InsP3 receptor) eliminates Ca2+ signals in the soma and in the primary processes, leaving [Ca2+]i dynamics in branchlets and leaflets very much undisturbed or only partially suppressed.88–91
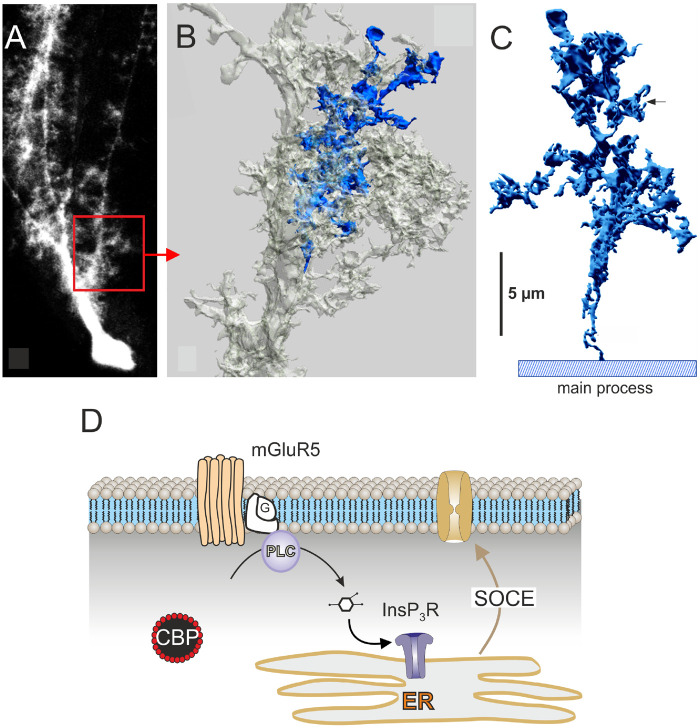
This type of segregated Ca2+ signalling (ER-based Ca2+ release in soma and primary processes vs. plasmalemmal Ca2+ influx in leaflets) does not operate in all types of astrocytes. For example, in Bergmann glial cells (radial astrocytes in the cerebellum) Ca2+ signalling microdomains are associated with specific morphological structures—the appendages. These appendages emanate from the primary radially oriented processes of the Bergmann glial cells; each appendage contains mitochondria and projects leaflets that contact 50–70 synapses formed by axons of granular neurons (Figure 2A–C). Activation of parallel fibers triggers localized Ca2+ signals in these appendages; the [Ca2+]i transients originate from activation of metabotropic receptors (mGluR5, P2Y purinoceptors, ETB endothelin receptors, α1-adrenoceptors, and H1 histamine receptors) and InsP3 receptor-mediated Ca2+ release, with subsequent activation of SOCE.54,92–95 In neocortical astrocytes ryanodine receptor-mediated [Ca2+]i-induced Ca2+ release was shown to substantially contribute to α1-adrenoceptor-mediated Ca2+ signals96; conversely, this mechanism is absent in hippocampal astrocytes.97 Spontaneous [Ca2+]i dynamics in the peripheral fine branchlets of cortical mouse astrocytes (examined in culture, in slices, and in vivo) were reported to originate from mitochondrial Ca2+ release through the flickering mitochondrial permeability transition pore.98 Local Ca2+ signals in branchlets (which possess ER) may involve combination of InsP3-induced Ca2+ release and plasmalemmal Ca2+ entry. Spatial restriction of [Ca2+]i increases could result from local mitochondria, which act as powerful Ca2+ buffers99 and can localize [Ca2+]i increases in astroglial processes.75 Another mechanism for functional compartmentalization of Ca2+ signals can be associated with plasmalemma-ER junctions that have been described in cultured primary astrocytes; these junctions are rich in InsP3 receptors, SERCA pumps, and NCX being thus a substrate for focal Ca2+ signaling.100 These examples form the mass of evidence demonstrating the diversity of astroglial Ca2+ signaling, which most likely changes depending on the physiological context, astrocyte morphology, age, and environmental settings.
Figure 2.
Formation of Ca2+ Microdomain in the Perisynaptic Appendages of Cerebellar Bergmann Glial Cells. Reconstruction of an appendage is based on electron microscopic data. (A) Fluorescence light micrograph of a dye-injected Bergmann glial cell is shown; the red square corresponds to the portion that was reconstructed from consecutive ultrathin sections. (B) One of the lateral appendages (marked in blue), arising directly from main process. (C) The same appendage is shown in isolation and at higher magnification. (D) Calcium signaling in the appendages of Bergmann glial cells is mediated solely through metabotropic receptors (mGluR5 or P2Y purinoceptors), which stimulate induced synthesis of InsP3 with subsequent InsP3-induced Ca2+ release from the ER and secondary SOCE. Modified from Ref. Grosche et al.54
Data on astroglial [Ca2+]i dynamics in vivo, in awake animals, remain rather limited. It seems that sensory stimulation triggers large and global (ie, pan-cellular) [Ca2+]i elevations controlled mainly by noradrenergic stimulation of α1 adrenoceptors.53,65,101 This cascade underlies a pan-cortical massive and spreading astroglial [Ca2+]i increase in response to transcranial direct current stimulation.102 Arousal, attention, and vigilant state trigger global and widespread astroglial Ca2+ signals evoked by acetylcholine release from projections of the nucleus basalis of Meynert; these [Ca2+]i responses are mediated through muscarinic ACh receptors and involve InsP3-induced Ca2+ release.103,104 Whether astrocytes in the in vivo setting communicate through propagating Ca2+ waves, which were characterized in detail in vitro and in brain slices,32,105 remains to be determined.
In summary, astrocytes possess a complex and spatially diverse Ca2+ signalling machinery that relies on several Ca2+ mobilizing pathways associated with ER Ca2+ release (mainly InsP3 receptor type II) and plasmalemmal Ca2+ entry through channels and the reversed NCX. Deciphering the targets for the Ca2+ signals in physiological and pathological contexts remains a pressing task. The remarkable heterogeneity of astroglial Ca2+ signaling is most likely linked to the extensive adaptive potential of astrocytes, which may tailor Ca2+ signalling toolkits to meet a multitude of challenges.
How Ca2+ signals translate into astroglial functional responses and how to find the physiological targets of [Ca2+]i fluctuations remain largely unanswered questions. Similarly to other cells, astroglial Ca2+ signals regulate gene expression and provide for excitation-metabolic coupling; formation of [Ca2+]i microdomains in astroglial branchlets immobilize mitochondria thus securing local metabolic support106 and Ca2+ signals may trigger astrocyte morphological plasticity.58,59 Astroglial Ca2+ signalling is implicated in secretion, both exocytotic and nonvesicular.107,108 Astrocytes are coupled to the regulation of functional hyperaemia109,110 through releasing vasodilators and vasoconstrictors, the secretion of which was initially linked to Ca2+ signals.46,47 Subsequent experiments however questioned this paradigm by demonstrating that suppression of astroglial InsP3-mediated Ca2+ signalling does not affect increases in local blood flow in response to sensory stimulation.111–113 Subsequently, astrocyte-vascular coupling was linked to extra-fast [Ca2+]i transients occurring in the end feet shortly before vasodilatation.77,78 At the same time, the astrocytes were proposed to provide Ca2+-dependent slow tonic regulation of the vascular tone through continuous release of prostaglandins.114 Astroglial Ca2+ signals were also reported to undergo changes in sleep, while suppression of astroglial [Ca2+]i dynamics by knocking out the InsP3 receptor type II affects slow-wave sleep with an increase in the number of micro-arousals, abnormal brain rhythms, and an increased frequency of slow-wave sleep state transitions and sleep spindles.115
In a pathological context, Ca2+ signaling controls reactive astrogliosis, the archetypal astrocytic defensive program. Challenge of astrocytes with damage-associated molecular patterns, such as ATP or β-amyloid triggers [Ca2+]i rises,116–119 which initiate astroglial reactivity. Inhibition of astrocyte Ca2+ signaling by genetic ablation of InsP3 receptors120 or by pharmacological agents121 inhibits reactive astrogliosis.
Astroglial Sodium Signaling
Changes in [Na+]i are of particular significance for astrocytic homeostatic function, because the absolute majority of plasmalemmal transporters engaged in maintaining various aspects of molecular homeostasis are driven by transmembrane Na+ gradient. These transporters not only utilize Na+ gradients; their operation produces Na+ fluxes thus transporters being simultaneously the sensors and modifiers of [Na+]i. The resting [Na+]i in astrocytes lies in the range of 15–20 mM,81,122,123 which is about twice that of neurons. Stimulation of astrocytes by neurotransmitters or by neuronal activity triggers substantial (up to 10–20 mM) increases in [Na+]i, which may last for tens of seconds.56,82,122,124,125 These [Na+]i transients were shown to spread in the form of propagating [Na+]i waves through individual cells (from processes to soma125) and into adjacent cells through gap junctions, thus creating intercellular [Na+]i waves.126,127 In appearance therefore astroglial [Na+]i dynamics is quite similar to [Ca2+]i changes. The presence of complex [Na+]i fluctuations together with the existence of numerous Na+-dependent molecules (or Na+-sensors) led to the concept of astroglial Na+ signaling.128,129
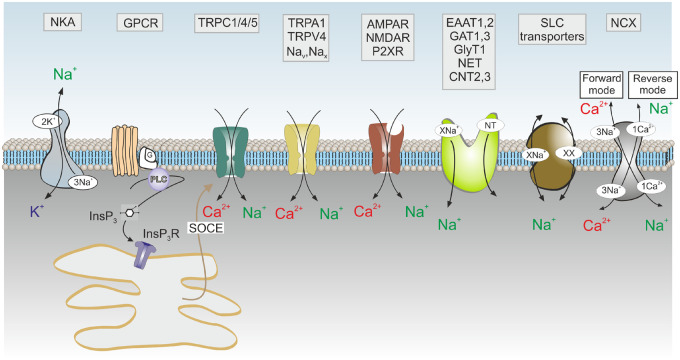
Despite overt similarity between [Ca2+]i and [Na+]i dynamics in astrocytes, the underlying mechanisms are quite distinct. Astrocytic Na+ signals rely on plasmalemmal Na+ movements only, as no Na+ storing structures exist in the cells (Figure 3). Plasmalemmal Na+ entry is mediated by cationic channels, which include ionotropic receptors and TRP channels. All these channels have considerable Na+ permeability; the pCa/pNa for P2X and NMDA receptors and TRP channels varies between 2 and >5, but given the high Na+ concentration in the interstitial fluids, Na+ fluxes through these channels are predominant.55,81 Astrocytes in the subfornical organ possess a specific Na+ channel, classified as Nax channels (which were initially cloned from astrocytes131) that are activated by increases in extracellular Na+ above 140 mM. These channels allow subfornical astrocytes to monitor blood Na+ concentration and contribute to systemic regulation of Na+ homeostasis.132 Expression of voltage-gated Nav1.2, Nav1.3, Nav1.5, and Nav1.6 channels in astrocytes has been detected at both mRNA and protein levels; however, their functional relevance remains to be tested.133
Figure 3.
Membrane Molecular Pathways of Na+ Signaling in Astrocytes. Influx of Na+ occurs though (1) Na+-permeable channels, which include ionotropic receptors (AMPAR, NMDAR, P2XR - AMPA, NMDA glutamate receptors and ionotropic purinoceptors); channels of the TRP family (TRPC1/4/5 channels that operate as a part of store-operated Ca2+ entry and hence generate Na+ influx in response to the depletion of ER Ca2+ stores; as well as TRPA and TRPV channels); voltage-dependent Nav channels and [Na+]o-activated Nax channels; (2) through Na+-dependent SLC transporters that include excitatory amino acid transporters EAAT1,2, GABA transporters GAT 1,3, glycine transporters GlyT, NA transporters NET or concentrative adenosine transporters CNT2/3. The main pathway for Na+ exit is provided by Na+-K+ pump, NKA. The Na+-Ca2+ exchanger NCX fluctuates between forward and reverse mode and couples Na+ and Ca2+ signaling. Modified from Verkhratsky et al.130
The second route for Na+ entry is associated with Na+-dependent transporters of which Na+-dependent neurotransmitter transporters contribute the most. These include excitatory amino acid transporters types 1 and 2 (EAAT1/SLC1A3 and EAAT2/SLC1A2134,135); GABA transporters type 1 and 3 (GAT-3/SLC6A1 and GAT-3/SLC6A12136); glycine transporters type 1 (GlyT1/SLC6A9137); NA and dopamine transporters (NET/SLC6A2 and DAT/SLC6A3138) and Na+-coupled neutral amino acid transporters SNAT3/SLC38A3 and SNAT5/SLC38A5.139 All these transporters are of paramount importance for neurotransmitter homeostasis and neurotransmission maintenance. In addition, Na+ fluxes are created by homeostatic transporters such as Na+-K+-Cl− co-transporter NKCC1/SLC12A2, Na+-dependent d-glucose transporter SGLT1/SLC5A1 or Na+-dependent vitamin C transporter SVCT2/SLC23A2.140–142
Extrusion of Na+ from astrocytes is mediated solely by Na+/K+ ATPase (NKA). Inhibition of NKA in cultured astroglia by ouabain or by removal of extracellular K+ results in an increase in [Na+]i up to 30–40 mM within ∼5 min.122,143,144 This reveals a substantial basal Na+ influx into astrocytes mediated probably by all types of Na+ permeable channels and possibly transporters such as Na+-H+ exchanger (NHE) or Na+-dependent bicarbonate symporter (NBCe1). The NKA in astrocytes incorporates the α2 subunit; which is not expressed in neurons (which possess α 1 and α 3 subunits). As a result, the affinity of astroglial NKA to K+ is substantially lower than in neurons. The EC50 for K+ for astroglial NKA composed from α2/β1 subunits is ∼3.6 mM, while EC50 for K+ in neuronal NKA (formed by α1/β1, α1/β2, α3/β1, or α3/β2 subunits) varies between 0.25 and 0.65 mM.140 Thus, differences in structure determine NKA function: at physiological levels of interstitial [K+] (∼3–3.5 mM) the neuronal NKA K+ binding sites are fully saturated; whereas for astrocytic NKA half of the K+ binding sites remain unoccupied. Consequently, an increase in interstitial [K+] activates astroglial NKA, which is the main mechanism for extracellular K+ sensing and buffering. Neuronal NKA is activated solely by an increase in [Na+]i. The NKA-dependent transport of K+ and Na+ in astrocytes maintains ion gradients critical for operation of homoeostatic transporters; in essence, the NKA acts as the master regulator of astroglial homoeostatic physiology. Increases in NKA transport, which accompany neuronal activity (to buffer K+ or to expel excessive Na+ entering the cell in the course of glutamate uptake) are also linked to astroglial l-lactate production and hence are central for the operation of astrocyte-to-neurone-lactate shuttle (ANLS).45 Operation of astroglial NKA is regulated by β-adrenoceptors145 and possibly by endogenous ouabain-like molecules.146 Normal operation of astroglial NKA is needed for learning,147 whereas loss-of-function mutations in the α2 subunit is associated with familial hemiplegic migraine type 2.148,149
The second key player of Na+ signaling is represented by the NCX; astrocytes express all three subtypes of this exchanger (NCX1/SLC8A1, NCX2/SLC8A2, and NCX3/SLC8A3). These subtypes are quite similar from the functional point of view, exchanging Na+ and Ca2+ with a 3:1 stoichiometry.150,151 The reversal potential of the astrocytic NCX is quite close to the resting membrane potential and hence even minor changes in [Na+]i or small depolarizations turn NCX into the reverse mode when it brings Ca2+ into the cell in exchange for Na+; in this mode, the NCX generates a [Ca2+]i rise while accelerating recovery to resting [Na+]i.83,152,153 Conversely, when [Ca2+]i rises the NCX is forced into the forward mode in which it assists the recovery of [Ca2+]i transients while producing Na+ influx.123 Thus, the NCX acts as a central molecule linking Ca2+ and Na+ signaling.
Similar to [Ca2+]i dynamics, astrocytic Na+ signals may be confined to microdomains. Such local subcellular [Na+]i transients have been characterized in Bergmann glia and in hippocampal protoplasmic astrocytes.57,124 The molecular mechanisms behind such localizations remain unknown; apart from the plasmalemma localized channels and transporters, there is no evidence for cytosolic Na+ buffers/binding sites, which may account for the localization of [Na+]i rises. The Na+ transporters in the plasmalemma endowed with Na+ binding sites may act as some sort of highly localized and relatively immobile Na+ buffers. Alternatively, Na+ (and other cations) may be trapped in tiny leaflets by binding sites associated with the inner side of the plasma membrane.154 Besides forming microdomains, Na+ signals can propagate from cell to cell by diffusion through gap junctions; the speed of these waves in [Na+]i may reach 100–150 mm/s.125,155
There are surprisingly large varieties of Na+ sensors, which act as effectors of Na+ signals. The larger class of molecules governed by [Na+]i is represented by the SLC membrane transporters, which fulfill an astroglial homoeostatic function. Changes in [Na+]i may affect not only the efficacy of transports but also change their operational direction. The reversal is well documented for NCX (see above) and can also occur to some other transporters, such as, for example, GABA or glycine transporters, which have been shown to reverse in physiological settings following an increase in [Na+]i.156–158 Increases in [Na+]i may translate to various biochemical and cellular responses though action on enzymes; in an astroglial context Na+ regulates glutamine synthetase thus affecting availability of glutamine for the glutamate (GABA)-glutamine shuttle.159 Cytoplasmic Na+ ions are also known to modulate or open various types of ion channels, such as, for example, Na+-dependent K+ channels or Kir4.1 inward rectifying K+ channels.160,161 Nonetheless in astrocytes the SLC transporters remain the main target; astroglial Na+ signaling therefore was proposed as a mechanism for rapid tuning of astroglial homeostatic cascades to neuronal activity.128
Other Ions in Astroglial Excitability
Chloride
Chloride, the major inorganic anion in the living tissues, is a likely contributor to astroglial ionic excitability. There are multiple indications for signaling role of intracellular Cl−. Changes in [Cl−]i regulate plasmalemmal channels (for instance Slo-2 K+ channels160 or TRPM7 channels162) and transporters (such as Na+/ transporter NBCe1-B163 or Na+/H+ exchanger HNE164); furthermore [Cl−]i affects the activity of G proteins.165,166 Another signaling cascade directly regulated by [Cl−]i is associated with WNK (With No lysine [K]) serine/threonine protein kinases.167,168 Finally, dynamic changes in [Cl−]i contribute to the regulation of several fundamental cellular processes such as cell differentiation and death.169,170
At the same time, Cl− is the central ion for mediating inhibitory currents in neural cells, and hence fluctuations in [Cl−] in the interstitial fluid are of paramount importance for balancing neurotransmission. Experiments on cultured astrocytes demonstrated that astrocytes maintain high [Cl−]i ranging between 20 and 50 mM, which corresponds to ECl = −35 mV.171–173 These data have not been universally confirmed in experiments in situ in acute brain slices. In Bergmann glial cells of the cerebellum Cl− imaging indeed revealed high [Cl−]i of around 50 mM in newborn and 35 mM in mature mice.174 In contrast probing astrocytes in acute hippocampal slices with gramicidine-based perforated patch-clamp estimated much lower [Cl−]i at 3–4 mM.175 Certainly mapping astroglial [Cl−]i in vivo is of pressing importance; as it may reveal either regional or state-dependent differences.
Astrocytic Cl− homeostasis depends on Cl− diffusion through several sets of anion channels that include (i) GABAA and glycine receptors; (ii) inwardly rectifying chloride channels ClC-1, -2, and -3; (iii) Ca2+-dependent Cl− channels; (iv) anion channels of the Bestrophin (Best) family and by (v) volume-regulated anion channels VRAC or SWELL1.176–180 All these channels mediate Cl− efflux or influx depending on the [Cl−]i; at the same time molecular mechanism(s) for Cl− accumulation into astrocytes remains to be identified. The only known Cl− accumulating transporter, Na+/K+/Cl− co-transporter NKCC1/SLC12A1, has been frequently identified in astrocytes in culture, however, whether NKCC1 operates in situ or in vivo remains controversial.140,181 Operation of several other transporters (such as GABA transporters and EAATs) is also associated with Cl− fluxes.174,182 The sensors for Cl− signaling in astrocytes are yet to be fully characterized; the role of astrocytes as a source for Cl− to maintain inhibitory transmission has been proposed172 and demonstrated in hippocampal slices.183
Potassium
Life on Earth is believed to have emerged around four billion years ago in a Na+-rich Primordial Ocean. Surprisingly, the cytoplasm of most cells has high K+ and low Na+ concentrations. Several hypotheses explaining this phenomenon have been developed. For example, protocells could have emerged in the K+ enriched vents at the bottom of the ocean; or they may have appeared in the inland basins molded from K+ rich clay and filled with rainwater.184 Be this as it may, K+ plays a vital role in cellular life. High [K+]i is required for protein synthesis and sets the cell membrane potential, while K+ efflux repolarizes the cell membrane following action potentials, excitatory postsynaptic potentials, and dendritic spikes in neurons.
Hence, neuronal activity is associated with substantial K+ fluxes across astroglial membranes. Astrocytes remove excess K+ at the peak of neuronal activity and then return K+ back to restore neuronal ionic gradients; in pathology, astrocytes are capable of redistributing K+ through the syncytial networks. Notably, most of the K+ removed by astrocytes from the synaptic cleft during neuronal activity comes from K+ efflux through ionotropic glutamate receptors, predominantly of NMDA type.185–187 Accumulation of K+ into astrocytes is mainly mediated by NKA (discussed in the previous section), while K+ efflux is mediated by inwardly rectifying K+ channels.140 This scenario implies emergence of short-lived K+ microdomains in perisynaptic astroglial processes, but whether these domains exist remains to be experimentally seen. The mechanisms of formation of K+ microdomains are similarly unknown. Recently, the role of intramembrane negative charges preventing K+ diffusion154 has been suggested. Whether dynamic changes in astroglial [K+]i have a signaling role and directly modulate cellular functions similarly needs to be tested. One testable possibility is that K+-mediated depolarization can affect voltage-dependent steps of glutamate transporter cycle, hence, affecting glutamate uptake.188
Protons
Neuronal activity is accompanied by a transient decrease in astroglial [H+]i. This phenomenon is known as “depolarization-induced alkalinization” and results in accumulation of H+ in the extracellular space.189 Astroglial alkalinization is linked to activation of the Na+/ transporter NBCe1/SLC4A4. This transporter contributes to regulation of astroglial metabolism through stimulation of cAMP production and subsequent increase in glycolysis.190 Another metabolic pathway controlled by H+ is represented by phosphofructokinase; activation of the latter is perceived as a key step in stimulation of astrocyte-neuronal lactate shuttle.191
Astroglial cAMP Excitability
The discovery of the first second messenger cAMP is linked to the studies of glycogen regulation. Under the mentorship of Carl Ferdinand Cori, who won a Nobel Prize in 1947 for identifying the mechanism of glycogen metabolism, Earl Wilbur Sutherland revealed that the action of adrenaline on glycogen degradation is mediated by cAMP.192,193 For this discovery, Sutherland was awarded the 1971 Nobel Prize in Physiology or Medicine. Unlike the technology for measuring cellular Ca2+, which emerged from two chemical inventions: a new family of calcium chelators with high affinity for Ca2+194and a method for trapping such substances inside intact cells by means of nonpolar ester derivatives,194 the methods to measure [cAMP]i at cellular level appeared much later. The cAMP indicators are based on the fluorescence resonance energy transfer (FRET), a quantum-mechanical, nonradiant, transfer of energy from the excited state of a donor fluorophore to the ground state of a neighboring acceptor chromophore or fluorophore. The acceptor must absorb light at roughly the same wavelengths as the donor emits and if the donor and acceptor are located within <10 nm distance from each other, FRET may occur.195 Although the very first cAMP FRET sensors were available already in 1991,196 their usage was hindered by the need to inject FRET holoprotein nanosensors into individual cells, which prevented a wider application. The problem was solved by utilizing the green fluorescent proteins (GFPs) from jellyfish, engineering smaller FRET constructs which are introduced into cells via plasmid transfection. Cyclic AMP exerts its cytoplasmic effects via cAMP-binding proteins including cAMP-dependent protein kinase (PKA), cAMP-gated ion channels, and isoforms of exchange protein directly activated by cAMP (Epac). Full length proteins or only cAMP-binding domains of these target proteins, for example using Epac, together with variants of GFPs, were used to make the FRET nanosensors.197–199
Given the relatively complex design of cAMP nanosensor, it is not surprising that the first single-cell measurements of [cAMP]i in astrocytes emerged only recently.200 In these experiments the expression of the FRET-based cAMP sensor, Epac1-camps, utilizing a single chain cAMP binding domain of the Epac1 protein,198 revealed a uniform distribution of the nanosensor fluorescence throughout the cytosol, but was excluded from the nucleus, indicating that [cAMP]i may be homogeneously distributed at rest in the cytoplasm, yielding levels from 0.1 to several µM of [cAMP]i.201 While there is evidence that in microglia cAMP may accumulate at cell processes,202 this needs to be further addressed in astrocytes.
Stimulation of astrocytes with adrenaline at 29 nM induced a half-maximal increase in [cAMP]i, consistent with the action of β-adrenergic receptors.200 The increase in [cAMP]i was characterized by a monoexponential rise to a plateau with a time-constant of ∼15 s, much slower than the agonist-induced increases in [Ca2+]i in astrocytes.203–205 The steady-state level of [cAMP]i represents the balance between the production of cAMP by adenylyl cyclases (AC) and its enzymatic degradation by phosphodiesterases.206 Unlike in other cells, where oscillations in [cAMP]i were recorded and were considered to be due to an interaction with Ca2+ signaling,207 measurements in astrocytes failed to detect such oscillations.208
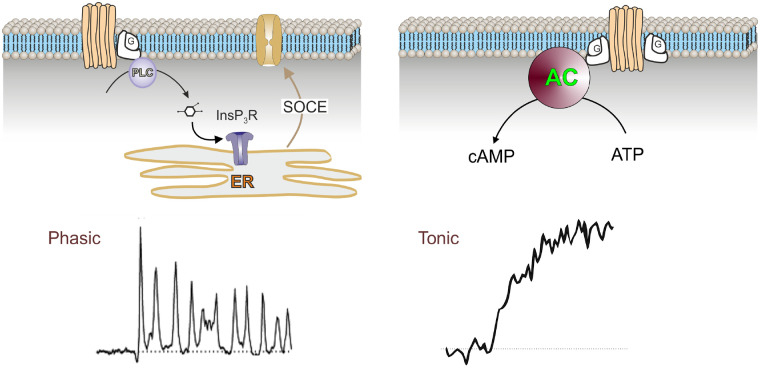
However, despite the fact that cAMP and Ca2+ signaling operate in different time domains in astrocytes, there is an interaction between these pathways.203 Both pathways are activated by G-protein coupled receptors. While the elevation in [cAMP]i is tonic, lasting several minutes, the swift changes in [Ca2+]i are phasic, often exhibiting oscillations (Figure 4 203,208). This dichotomy in kinetics of Ca2+ and cAMP signals was recently confirmed also in vivo,65 demonstrating that the two signaling mechanisms drive downstream cellular processes with distinct temporal characteristics.
Figure 4.
Distinct Temporal Dynamics of cAMP and Ca2+-Excitability in Astrocytes. Activation of astrocytic metabotropic receptors coupled to Gq proteins leads to phasic oscillations in intracellular Ca2+ levels (left), while the activation of metabotropic receptors coupled to Gs proteins leads to tonic long-lasting increase in cAMP-dependent PKA activity without oscillations (right). Cyclic AMP is produced by AC from ATP. PLC, phospholipase C; InsP3R, receptor. Modified from Horvat et al.202
The cross-talk between the cAMP- and Ca2+-signaling in astrocytes, reflects a mode of optimization of cellular responses upon receptor activation. The molecular mechanisms underlying the cross-talk between the Ca2+ and cAMP responses in astrocytes in health and disease remain to be studied. However, as observed in other cell types, Ca2+ may modulate the activity of the ACs and PDEs, through calmodulin, while cAMP-dependent signaling may affect Ca2+ transport mechanisms and may regulate gene expression via cAMP/PKA, therefore affecting the production of proteins of the Ca2+ signaling cascades. Moreover, Ca2+ oscillation frequency appears to determine gene transcription,209,210 thus the cAMP-mediated regulation of Ca2+ oscillations may alter astroglial gene expression.
Astroglial glycogen represents an energy reserve, which is used during increased activity to support many CNS functions, including memory formation and consolidation.211 When astrocytes are stimulated, for example by NA,212 this results in an increased glucose uptake, glycogenolysis, and glycolysis with l-lactate as the end glycolytic product despite the normal oxygen levels (ie, aerobic glycolysis, known also as the Warburg effect). Glycogen-derived l-lactate exits astrocytes through monocarboxylate transporters (MCTs) 1 and 4 and/or yet unidentified ion channels213 to enter neurons through the MCT2, where it is used in oxidative metabolism (ie, astrocyte-neurone-lactate-shuttle hypothesis45). Moreover, l-lactate can also act as an extracellular signal where it binds to l-lactate metabotropic receptors or to yet unknown receptors.214–216
Aerobic glycolysis together with glycogenolysis is regulated in astrocytes by a variety of receptors on the surface of astrocytes that are linked to intracellular Ca2+- and/or cAMP-pathways, such as ARs and purinoreceptors. Upon stimulation of LC neurons, NA is released, with subsequent activation of metabotropic adrenoceptors and increases in astrocytic [Ca2+]i and [cAMP]i.53,65 The contribution of Ca2+ and cAMP as second messengers to the regulation of aerobic glycolysis and glycogenolysis in astrocytes remains unclear and even controversial. It is thought that aerobic glycolysis and glycogenolysis are primarily elevated through the cAMP-dependent pathway in astrocytes,217,218 although there is evidence that Ca2+ signals might also be involved.219
In conclusion, astroglial noradrenergic signaling, involving Ca2+ and cAMP regulates many cellular processes affecting the function of astrocytes and neighboring neurons in health and disease. This intracellular excitability provides regulatory clues in distinct space and time domains, which underlies the capacity of adapting to dynamic and life-long changes that occur during the function of the CNS in health and disease.
Recapitulation
Astrocytes are an indispensable part of the nervous tissue, which together with neurons and other neural cells produce a cellular fabric responsible for brain function. Homoeostatic cascades in the astrocytes, which support the most fundamental functional properties of the CNS, are tightly correlated with neuronal activity and tissue demands. This coordination is a function of astroglial excitability mediated through spatiotemporal fluctuations of intracellular ions and second messengers.
Funding
A.S. work was supported by Russian Science Foundation (grant number 20-14-00241); R.Z. is supported by grants from the Slovenian Research Agency (P3 310, J36790, J3 9266, J3 7605), CIPKEBIP, COST Nanonet, COST Mouse Ageing, and COST CM1207 – GLISTEN.
Conflict of Interest Statement
None declared.
References
- 1.von Haller A. Commentarii Societatis Regiae Scientiarum Gottingensis. Gottingen: Vadenhoek, 1753. [Google Scholar]
- 2.Hooke R. Micrographia. UK: Folio Society, 2017:1655. [Google Scholar]
- 3.Malpighi M. In de Viscerum Structura Exercitatio Anatomica. Bologna: Typographia Iacobi Montij, 1666. [Google Scholar]
- 4.Shapiro S. Antony van Leeuwenhoek; a review of his life and work. J Biol Photogr Assoc 1955;23(2–3):49–57. [PubMed] [Google Scholar]
- 5.Swedenborg E. The Brain, Considered Anatomically, Physiologically and Phylosopically (translated and edited by Tafel R. L.) in 4 volumes. London: James Speirs, 1882. [Google Scholar]
- 6.Ehrenberg CG. Beobachtungeiner Auffallenden Bisher Unerkannten Strukfurdes Seelenorgans Bei Menschen und Thieren. Berlin: Königlichen Akademie der Wissenschchaft, 1836. [Google Scholar]
- 7.Purkinje JE. Oper Omnia. Prague, Chec Republic: Purkynova Spolecnost, 1837:3. [Google Scholar]
- 8.Todd RB. The Descriptive and Physiological Anatomy of the Brain, Spinal Cord, Ganglions and Their Coverings. London: Sherwood, Gilbert and Piper, 1845. [Google Scholar]
- 9.Valentin G. Über den verlauf und die letzten enden der nerven. Nova Acta. 1836;18:51–240. [Google Scholar]
- 10.Rokitansky K. Über das auswachsen der bindegewebs-substanzen und die beziehung desselben zur entzündung. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften Mathematisch-Naturwissenschaftliche Classe Wien 1854;13:122–140. [Google Scholar]
- 11.Virchow R. Ueber das granulirte ansehen der wandungen der gehirnventrikel. In: Virchow R, ed. Gesammelte Abhandlungen zur Wissenschaftlichen Medicin. Frankfurt, Germany: Meidinger Sohn & Comp, 1856. [Google Scholar]
- 12.Chvatal A, Verkhratsky A. An early history of neuroglial research. Personalities. Neuroglia 2018;1:245–281. [Google Scholar]
- 13.Golgi C. Opera Omnia. Milano: Hoepli, 1903. [Google Scholar]
- 14.Lugaro E. Sulle funzioni della nevroglia. Riv Pat Nerv Ment 1907;12:225–233. [Google Scholar]
- 15.Ramón y Cajal S. Algunas Conjeturas Sobre el Mechanismoanatomico de la Ideacion, Asociacion y Atencion. Madrid, Spain: Imprenta y Libreria de Nicolas Moya, 1895. [Google Scholar]
- 16.Schleich CL. Psychophysik des Natürlichen und Künstlichen Schlafes. Berlin: Julius Springer, 1894. [Google Scholar]
- 17.Swammerdam J. The Book of Nature (Biblia naturae). London: C.G. Seyfert, 1758. [Google Scholar]
- 18.Cobb M. Timeline: exorcizing the animal spirits: Jan Swammerdam on nerve function. Nat Rev Neurosci 2002;3(5):395–400. [DOI] [PubMed] [Google Scholar]
- 19.Galvani L. De viribus electricitatis in motu musculari commentarius. Bon Sci Art Inst Acad Comm 1791;7:363–418. [Google Scholar]
- 20.Galvani L. Opere Edite ed Inedite del Professore Luigi Galvani Raccolte e Pubblicate Dall’Accademia Delle Science Dell’Istituto di Bologna. Bologna: Dall’Olmo, 1841. [Google Scholar]
- 21.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 1952;117(4):500–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neher E, Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature 1976;260(5554):799–802. [DOI] [PubMed] [Google Scholar]
- 23.Noda M, Ikeda T, Suzuki H, et al. Expression of functional sodium channels from cloned cDNA. Nature 1986;322(6082):826–828. [DOI] [PubMed] [Google Scholar]
- 24.Orkand RK, Nicholls JG, Kuffler SW. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol 1966;29(4):788–806. [DOI] [PubMed] [Google Scholar]
- 25.Tasaki I, Chang JJ. Electric response of glia cells in cat brain. Science 1958;128(3333):1209–1210. [DOI] [PubMed] [Google Scholar]
- 26.Hild W, Chang JJ, Tasaki I. Electrical responses of astrocytic glia from the mammalian central nervous system cultivated in vitro. Experientia 1958;14(6):220–221. [DOI] [PubMed] [Google Scholar]
- 27.Kuffler SW, Potter DD. Glia in the leech central nervous system: physiological properties and neuron-glia relationship. J Neurophysiol 1964;27(2):290–320. [DOI] [PubMed] [Google Scholar]
- 28.Morrison RS, de Vellis J. Growth of purified astrocytes in a chemically defined medium. Proc Natl Acad Sci USA 1981;78(11):7205–7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowman CL, Kimelberg HK. Excitatory amino acids directly depolarize rat brain astrocytes in primary culture. Nature 1984;311(5987):656–659. [DOI] [PubMed] [Google Scholar]
- 30.Kettenmann H, Backus KH, Schachner M. Aspartate, glutamate and gamma-aminobutyric acid depolarize cultured astrocytes. Neurosci Lett 1984;52(1–2):25–29. [DOI] [PubMed] [Google Scholar]
- 31.Verkhratsky A, Nedergaard M. Physiology of Astroglia. Physiol Rev 2018;98(1):239–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 1990;247(4941):470–473. [DOI] [PubMed] [Google Scholar]
- 33.Enkvist MO, Holopainen I, Akerman KE. Glutamate receptor-linked changes in membrane potential and intracellular Ca2+ in primary rat astrocytes. Glia 1989;2(6):397–402. [DOI] [PubMed] [Google Scholar]
- 34.Dave V, Gordon GW, McCarthy KD. Cerebral type 2 astroglia are heterogeneous with respect to their ability to respond to neuroligands linked to calcium mobilization. Glia 1991;4(5):440–447. [DOI] [PubMed] [Google Scholar]
- 35.McCarthy KD, Salm AK. Pharmacologically-distinct subsets of astroglia can be identified by their calcium response to neuroligands. Neuroscience 1991;41(2–3):325–333. [DOI] [PubMed] [Google Scholar]
- 36.Verkhratsky A, Orkand RK, Kettenmann H. Glial calcium: homeostasis and signaling function. Physiol Rev 1998;78(1):99–141. [DOI] [PubMed] [Google Scholar]
- 37.Ding F, O'Donnell J, Xu Q, et al. Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science 2016;352(6285):550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schousboe A, Svenneby G, Hertz L. Uptake and metabolism of glutamate in astrocytes cultured from dissociated mouse brain hemispheres. J Neurochem 1977;29(6):999–1005. [DOI] [PubMed] [Google Scholar]
- 39.Inazu M, Takeda H, Matsumiya T. Functional expression of the norepinephrine transporter in cultured rat astrocytes. J Neurochem 2003;84(1):136–144. [DOI] [PubMed] [Google Scholar]
- 40.Hertz L, Wu PH, Schousboe A. Evidence for net uptake of GABA into mouse astrocytes in primary cultures–its sodium dependence and potassium independence. Neurochem Res 1978;3(3):313–323. [DOI] [PubMed] [Google Scholar]
- 41.Adams RH, Sato K, Shimada S, et al. Gene structure and glial expression of the glycine transporter GlyT1 in embryonic and adult rodents. J Neurosci 1995;15(3 Pt 2):2524–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng L, Huang R, Yu AC, et al. Nucleoside transporter expression and function in cultured mouse astrocytes. Glia 2005;52(1):25–35. [DOI] [PubMed] [Google Scholar]
- 43.Rothman DL, De Feyter HM, Maciejewski PK, Behar KL. Is there in vivo evidence for amino acid shuttles carrying ammonia from neurons to astrocytes? Neurochem Res 2012;37(11):2597–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang JH, Wada A, Yoshida K, et al. Brain-specific Phgdh deletion reveals a pivotal role for L-serine biosynthesis in controlling the level of D-serine, an N-methyl-D-aspartate receptor co-agonist, in adult brain. J Biol Chem 2010;285(53):41380–41390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 1994;91(22):10625–10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zonta M, Angulo MC, Gobbo S, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci 2003;6(1):43–50. [DOI] [PubMed] [Google Scholar]
- 47.Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 2004;431(7005):195–199. [DOI] [PubMed] [Google Scholar]
- 48.Iliff JJ, Lee H, Yu M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 2013;123(3):1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alvarez JI, Dodelet-Devillers A, Kebir H, et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 2011;334(6063):1727–1731. [DOI] [PubMed] [Google Scholar]
- 50.del Río-Hortega P, Penfield WG. Cerebral cicatrix: the reaction of neuroglia and microglia to brain wounds. Bull J Hopkins Hosp 1927;41:278–303. [Google Scholar]
- 51.Marina N, Christie IN, Korsak A, et al. Astrocytes monitor cerebral perfusion and control systemic circulation to maintain brain blood flow. Nat Commun 2020;11(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bekar LK, He W, Nedergaard M. Locus coeruleus α-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb Cortex 2008;18(12):2789–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding F, O'Donnell J, Thrane AS, et al. α1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium 2013;54(6):387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grosche J, Matyash V, Moller T, et al. Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat Neurosci 1999;2(2):139–143. [DOI] [PubMed] [Google Scholar]
- 55.Palygin O, Lalo U, Verkhratsky A, Pankratov Y. Ionotropic NMDA and P2X1/5 receptors mediate synaptically induced Ca2+ signalling in cortical astrocytes. Cell Calcium 2010;48(4):225–231. [DOI] [PubMed] [Google Scholar]
- 56.Kirischuk S, Kettenmann H, Verkhratsky A. Membrane currents and cytoplasmic sodium transients generated by glutamate transport in Bergmann glial cells. Pflugers Arch 2007;454(2):245–252. [DOI] [PubMed] [Google Scholar]
- 57.Langer J, Rose CR. Synaptically induced sodium signals in hippocampal astrocytes in situ. J Physiol 2009;587(Pt 24):5859–5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Molotkov D, Zobova S, Arcas JM, Khiroug L. Calcium-induced outgrowth of astrocytic peripheral processes requires actin binding by Profilin-1. Cell Calcium 2013;53(5–6):338–348. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka M, Shih PY, Gomi H, et al. Astrocytic Ca2+ signals are required for the functional integrity of tripartite synapses. Mol Brain 2013;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mouton PR, Pakkenberg B, Gundersen HJ, Price DL. Absolute number and size of pigmented locus coeruleus neurons in young and aged individuals. J Chem Neuroanat 1994;7(3):185–190. [DOI] [PubMed] [Google Scholar]
- 61.Feinstein DL, Kalinin S, Braun D. Causes, consequences, and cures for neuroinflammation mediated via the locus coeruleus: noradrenergic signaling system. J Neurochem 2016;139(Suppl 2):154–178. [DOI] [PubMed] [Google Scholar]
- 62.Dong JH, Wang YJ, Cui M, et al. Adaptive activation of a stress response pathway improves learning and memory through Gs and beta-Arrestin-1-regulated lactate metabolism. Biol Psychiatry 2017;81(8):654–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao V, Suzuki A, Magistretti PJ, et al. Astrocytic β2-adrenergic receptors mediate hippocampal long-term memory consolidation. Proc Natl Acad Sci USA 2016;113(30):8526–8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aoki C. β-adrenergic receptors: astrocytic localization in the adult visual cortex and their relation to catecholamine axon terminals as revealed by electron microscopic immunocytochemistry. J Neurosci 1992;12(3):781–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oe Y, Wang X, Patriarchi T, et al. Distinct temporal integration of noradrenaline signaling by astrocytic second messengers during vigilance. Nat Commun 2020;11(1):471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci 2015;18(7):942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gavrilov N, Golyagina I, Brazhe A, et al. Astrocytic coverage of dendritic spines, dendritic shafts, and axonal boutons in hippocampal neuropil. Front Cell Neurosci 2018;12:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patrushev I, Gavrilov N, Turlapov V, Semyanov A. Subcellular location of astrocytic calcium stores favors extrasynaptic neuron-astrocyte communication. Cell Calcium 2013;54(5):343–349. [DOI] [PubMed] [Google Scholar]
- 69.Derouiche A, Haseleu J, Korf HW. Fine astrocyte processes contain very small mitochondria: glial oxidative capability may fuel transmitter metabolism. Neurochem Res 2015;40(12):2402–2413. [DOI] [PubMed] [Google Scholar]
- 70.Kanemaru K, Sekiya H, Xu M, et al. In vivo visualization of subtle, transient, and local activity of astrocytes using an ultrasensitive Ca2+ indicator. Cell Rep 2014;8(1):311–318. [DOI] [PubMed] [Google Scholar]
- 71.Nett WJ, Oloff SH, McCarthy KD. Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. J Neurophysiol 2002;87(1):528–537. [DOI] [PubMed] [Google Scholar]
- 72.Shigetomi E, Kracun S, Sofroniew MV, Khakh BS. A genetically targeted optical sensor to monitor calcium signals in astrocyte processes. Nat Neurosci 2010;13(6):759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu YW, Gordleeva S, Tang X, et al. Morphological profile determines the frequency of spontaneous calcium events in astrocytic processes. Glia 2019;67(2):246–262. [DOI] [PubMed] [Google Scholar]
- 74.Panatier A, Vallee J, Haber M, et al. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 2011;146(5):785–798. [DOI] [PubMed] [Google Scholar]
- 75.Di Castro MA, Chuquet J, Liaudet N, et al. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat Neurosci 2011;14(10):1276–1284. [DOI] [PubMed] [Google Scholar]
- 76.Srinivasan R, Huang BS, Venugopal S, et al. Ca2+ signaling in astrocytes from Ip3r2-/- mice in brain slices and during startle responses in vivo. Nat Neurosci 2015;18(5):708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lind BL, Brazhe AR, Jessen SB, Tan FC, Lauritzen MJ. Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo. Proc Natl Acad Sci USA 2013;110(48):E4678–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Otsu Y, Couchman K, Lyons DG, et al. Calcium dynamics in astrocyte processes during neurovascular coupling. Nat Neurosci 2015;18(2):210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lalo U, Palygin O, North RA, Verkhratsky A, Pankratov Y. Age-dependent remodelling of ionotropic signalling in cortical astroglia. Aging Cell 2011;10(3):392–402. [DOI] [PubMed] [Google Scholar]
- 80.Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci 2012;15(1):70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reyes RC, Verkhratsky A, Parpura V. TRPC1-mediated Ca2+ and Na+ signalling in astroglia: differential filtering of extracellular cations. Cell Calcium 2013;54(2):120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kirischuk S, Kettenmann H, Verkhratsky A. Na+/Ca2+ exchanger modulates kainate-triggered Ca2+ signaling in Bergmann glial cells in situ. FASEB J 1997;11(7):566–572. [DOI] [PubMed] [Google Scholar]
- 83.Ziemens D, Oschmann F, Gerkau NJ, Rose CR. Heterogeneity of activity-induced sodium transients between astrocytes of the mouse hippocampus and neocortex: mechanisms and consequences. J Neurosci 2019;39(14):2620–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Plata A, Lebedeva A, Denisov P, et al. Astrocytic atrophy following status epilepticus parallels reduced Ca2+ activity and impaired synaptic plasticity in the rat hippocampus. Front Mol Neurosci 2018;11:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Porter JT, McCarthy KD. GFAP-positive hippocampal astrocytes in situ respond to glutamatergic neuroligands with increases in [Ca2+]i. Glia 1995;13:101–112. [DOI] [PubMed] [Google Scholar]
- 86.Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci 1996;16(16):5073–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Toth AB, Hori K, Novakovic MM, et al. CRAC channels regulate astrocyte Ca2+ signaling and gliotransmitter release to modulate hippocampal GABAergic transmission. Sci Signal 2019;12(582). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Petravicz J, Boyt KM, McCarthy KD. Astrocyte IP3R2-dependent Ca2+ signaling is not a major modulator of neuronal pathways governing behavior. Front Behav Neurosci 2014;8:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haustein MD, Kracun S, Lu XH, et al. Conditions and constraints for astrocyte calcium signaling in the hippocampal mossy fiber pathway. Neuron 2014;82(2):413–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Petravicz J, Fiacco TA, McCarthy KD. Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci 2008;28(19):4967–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Agulhon C, Fiacco TA, McCarthy KD. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science 2010;327(5970):1250–1254. [DOI] [PubMed] [Google Scholar]
- 92.Kirischuk S, Kirchhoff F, Matyash V, Kettenmann H, Verkhratsky A. Glutamate-triggered calcium signalling in mouse bergmann glial cells in situ: role of inositol-1,4,5-trisphosphate-mediated intracellular calcium release. Neuroscience 1999;92(3):1051–1059. [DOI] [PubMed] [Google Scholar]
- 93.Kirischuk S, Moller T, Voitenko N, Kettenmann H, Verkhratsky A. ATP-induced cytoplasmic calcium mobilization in Bergmann glial cells. J Neurosci 1995;15(12):7861–7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kirischuk S, Tuschick S, Verkhratsky A, Kettenmann H. Calcium signalling in mouse Bergmann glial cells mediated by alpha1-adrenoreceptors and H1 histamine receptors. Eur J Neurosci 1996;8(6):1198–1208. [DOI] [PubMed] [Google Scholar]
- 95.Tuschick S, Kirischuk S, Kirchhoff F, et al. Bergmann glial cells in situ express endothelinB receptors linked to cytoplasmic calcium signals. Cell Calcium 1997;21(6):409–419. [DOI] [PubMed] [Google Scholar]
- 96.Pankratov Y, Lalo U. Role for astroglial α1-adrenoreceptors in gliotransmission and control of synaptic plasticity in the neocortex. Front Cell Neurosci 2015;9:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Beck A, Nieden RZ, Schneider HP, Deitmer JW. Calcium release from intracellular stores in rodent astrocytes and neurons in situ. Cell Calcium 2004;35(1):47–58. [DOI] [PubMed] [Google Scholar]
- 98.Agarwal A, Wu PH, Hughes EG, et al. Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron 2017;93(3):587–605 e587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tinel H, Cancela JM, Mogami H, et al. Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca2+ signals. EMBO J 1999;18(18):4999–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lencesova L, O'Neill A, Resneck WG, Bloch RJ, Blaustein MP. Plasma membrane-cytoskeleton-endoplasmic reticulum complexes in neurons and astrocytes. J Biol Chem 2004;279(4):2885–2893. [DOI] [PubMed] [Google Scholar]
- 101.Paukert M, Agarwal A, Cha J, et al. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 2014;82(6):1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Monai H, Ohkura M, Tanaka M, et al. Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nat Commun 2016;7:11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen N, Sugihara H, Sharma J, et al. Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes. Proc Natl Acad Sci USA 2012;109(41):E2832–E2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Takata N, Mishima T, Hisatsune C, et al. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J Neurosci 2011;31(49):18155–18165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schipke CG, Haas B, Kettenmann H. Astrocytes discriminate and selectively respond to the activity of a subpopulation of neurons within the barrel cortex. Cereb Cortex 2008;18(10):2450–2459. [DOI] [PubMed] [Google Scholar]
- 106.Jackson JG, Robinson MB. Reciprocal regulation of mitochondrial dynamics and calcium signaling in astrocyte processes. J Neurosci 2015;35(45):15199–15213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nagai J, Rajbhandari AK, Gangwani MR, et al. Hyperactivity with disrupted attention by activation of an astrocyte synaptogenic cue. Cell 2019;177(5):1280–1292e1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu T, Sun L, Xiong Y, et al. Calcium triggers exocytosis from two types of organelles in a single astrocyte. J Neurosci 2011;31(29):10593–10601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mosso A. Sulla circolazione del sangue nel cervello dell'uomo. Mem Real Acc Lincei 1880;5:237–358. [Google Scholar]
- 110.Roy CS, Sherrington CS. On the regulation of the blood-supply of the brain. J Physiol (Lond) 1890;11:85–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bonder DE, McCarthy KD. Astrocytic Gq-GPCR-linked IP3R-dependent Ca2+ signaling does not mediate neurovascular coupling in mouse visual cortex in vivo. J Neurosci 2014;34(39):13139–13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nizar K, Uhlirova H, Tian P, et al. In vivo stimulus-induced vasodilation occurs without IP3 receptor activation and may precede astrocytic calcium increase. J Neurosci 2013;33(19):8411–8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takata N, Nagai T, Ozawa K, et al. Cerebral blood flow modulation by Basal forebrain or whisker stimulation can occur independently of large cytosolic Ca2+ signaling in astrocytes. PLoS One 2013;8(6):e66525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rosenegger DG, Tran CH, Wamsteeker Cusulin JI, Gordon GR. Tonic local brain blood flow control by astrocytes independent of phasic neurovascular coupling. J Neurosci 2015;35(39):13463–13474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bojarskaite L, Bjornstad DM, Pettersen KH, et al. Astrocytic Ca2+ signaling is reduced during sleep and is involved in the regulation of slow wave sleep. Nat Commun 2020;11(1):3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bianco F, Colombo A, Saglietti L, et al. Different properties of P2X7 receptor in hippocampal and cortical astrocytes. Purinergic Signal 2009;5(2):233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abramov AY, Canevari L, Duchen MR. Calcium signals induced by amyloid β peptide and their consequences in neurons and astrocytes in culture. Biochim Biophys Acta 2004;1742(1–3):81–87. [DOI] [PubMed] [Google Scholar]
- 118.Ronco V, Grolla AA, Glasnov TN, et al. Differential deregulation of astrocytic calcium signalling by amyloid-β, TNFα, IL-1β and LPS. Cell Calcium 2014;55(4):219–229. [DOI] [PubMed] [Google Scholar]
- 119.Grolla AA, Sim JA, Lim D, et al. Amyloid-β and Alzheimer's disease type pathology differentially affects the calcium signalling toolkit in astrocytes from different brain regions. Cell Death Dis 2013;4:e623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kanemaru K, Kubota J, Sekiya H, et al. Calcium-dependent N-cadherin up-regulation mediates reactive astrogliosis and neuroprotection after brain injury. Proc Natl Acad Sci USA 2013;110(28):11612–11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alberdi E, Wyssenbach A, Alberdi M, et al. Ca2+-dependent endoplasmic reticulum stress correlates with astrogliosis in oligomeric amyloid beta-treated astrocytes and in a model of Alzheimer's disease. Aging Cell 2013;12(2):292–302. [DOI] [PubMed] [Google Scholar]
- 122.Rose CR, Ransom BR. Intracellular sodium homeostasis in rat hippocampal astrocytes. J Physiol 1996;491(Pt 2):291–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Reyes RC, Verkhratsky A, Parpura V. Plasmalemmal Na+/Ca2+ exchanger modulates Ca2+-dependent exocytotic release of glutamate from rat cortical astrocytes. ASN Neuro 2012;4(1):00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bennay M, Langer J, Meier SD, Kafitz KW, Rose CR. Sodium signals in cerebellar Purkinje neurons and Bergmann glial cells evoked by glutamatergic synaptic transmission. Glia 2008;56:1138–1149. [DOI] [PubMed] [Google Scholar]
- 125.Langer J, Gerkau NJ, Derouiche A, et al. Rapid sodium signaling couples glutamate uptake to breakdown of ATP in perivascular astrocyte endfeet. Glia 2017;65(2):293–308. [DOI] [PubMed] [Google Scholar]
- 126.Langer J, Stephan J, Theis M, Rose CR. Gap junctions mediate intercellular spread of sodium between hippocampal astrocytes in situ. Glia 2012;60(2):239–252. [DOI] [PubMed] [Google Scholar]
- 127.Bernardinelli Y, Magistretti PJ, Chatton JY. Astrocytes generate Na+-mediated metabolic waves. Proc Natl Acad Sci USA 2004;101(41):14937–14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kirischuk S, Parpura V, Verkhratsky A. Sodium dynamics: another key to astroglial excitability? Trends Neurosci 2012;35(8):497–506. [DOI] [PubMed] [Google Scholar]
- 129.Rose CR, Verkhratsky A. Principles of sodium homeostasis and sodium signalling in astroglia. Glia 2016;64:1611–1627. [DOI] [PubMed] [Google Scholar]
- 130.Verkhratsky A, Untiet V, Rose CR. Ionic signalling in astroglia beyond calcium. J Physiol 2020;598(9):1655–1670. [DOI] [PubMed] [Google Scholar]
- 131.Gautron S, Dos Santos G, Pinto-Henrique D, et al. The glial voltage-gated sodium channel: cell- and tissue-specific mRNA expression. Proc Natl Acad Sci USA 1992;89(15):7272–7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shimizu H, Watanabe E, Hiyama TY, et al. Glial Nax channels control lactate signaling to neurons for brain [Na+] sensing. Neuron 2007;54(1):59–72. [DOI] [PubMed] [Google Scholar]
- 133.Pappalardo LW, Samad OA, Black JA, Waxman SG. Voltage-gated sodium channel Nav 1.5 contributes to astrogliosis in an in vitro model of glial injury via reverse Na+/Ca2+ exchange. Glia 2014;62(7):1162–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron 1997;19(6):1297–1308. [DOI] [PubMed] [Google Scholar]
- 135.Zerangue N, Kavanaugh MP. Flux coupling in a neuronal glutamate transporter. Nature 1996;383(6601):634–637. [DOI] [PubMed] [Google Scholar]
- 136.Minelli A, DeBiasi S, Brecha NC, Zuccarello LV, Conti F. GAT-3, a high-affinity GABA plasma membrane transporter, is localized to astrocytic processes, and it is not confined to the vicinity of GABAergic synapses in the cerebral cortex. J Neurosci 1996;16(19):6255–6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zafra F, Aragon C, Olivares L, et al. Glycine transporters are differentially expressed among CNS cells. J Neurosci 1995;15(5 Pt 2):3952–3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pacholczyk T, Blakely RD, Amara SG. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature 1991;350(6316):350–354. [DOI] [PubMed] [Google Scholar]
- 139.Todd AC, Marx MC, Hulme SR, Broer S, Billups B. SNAT3-mediated glutamine transport in perisynaptic astrocytes in situ is regulated by intracellular sodium. Glia 2017;65(6):900–916. [DOI] [PubMed] [Google Scholar]
- 140.Larsen BR, Assentoft M, Cotrina ML, et al. Contributions of the Na+/K+-ATPase, NKCC1, and Kir4.1 to hippocampal K+ clearance and volume responses. Glia 2014;62(4):608–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vega C, R. Sachleben L J, Gozal D, Gozal E. Differential metabolic adaptation to acute and long-term hypoxia in rat primary cortical astrocytes. J Neurochem 2006;97(3):872–883. [DOI] [PubMed] [Google Scholar]
- 142.Salazar K, Martinez F, Perez-Martin M, et al. SVCT2 expression and function in reactive astrocytes is a common event in different brain pathologies. Mol Neurobiol 2018;55(7):5439–5452. [DOI] [PubMed] [Google Scholar]
- 143.Golovina V, Song H, James P, Lingrel J, Blaustein M. Regulation of Ca2+ signaling by Na+ pump α2 subunit expression. Ann N Y Acad Sci 2003;986:509–513. [DOI] [PubMed] [Google Scholar]
- 144.Illarionava NB, Brismar H, Aperia A, Gunnarson E. Role of Na,K-ATPase α1 and α2 isoforms in the support of astrocyte glutamate uptake. PLoS One 2014;9(6):e98469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hajek I, Subbarao KV, Hertz L. Acute and chronic effects of potassium and noradrenaline on Na+, K+-ATPase activity in cultured mouse neurons and astrocytes. Neurochem Int 1996;28(3):335–342. [DOI] [PubMed] [Google Scholar]
- 146.Kala G, Kumarathasan R, Peng L, Leenen FH, Hertz L. Stimulation of Na+,K+-ATPase activity, increase in potassium uptake, and enhanced production of ouabain-like compounds in ammonia-treated mouse astrocytes. Neurochem Int 2000;36(3):203–211. [DOI] [PubMed] [Google Scholar]
- 147.Gibbs ME, Ng KT. Counteractive effects of norepinephrine and amphetamine on quabain-induced amnesia. Pharmacol Biochem Behav 1977;6(5):533–537. [DOI] [PubMed] [Google Scholar]
- 148.Capuani C, Melone M, Tottene A, et al. Defective glutamate and K+ clearance by cortical astrocytes in familial hemiplegic migraine type 2. EMBO Mol Med 2016;8(8):967–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stoica A, Larsen BR, Assentoft M, et al. The α2β2 isoform combination dominates the astrocytic Na+/K+ -ATPase activity and is rendered nonfunctional by the α2.G301R familial hemiplegic migraine type 2-associated mutation. Glia 2017;65(11):1777–1793. [DOI] [PubMed] [Google Scholar]
- 150.Kimura J, Miyamae S, Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol 1987;384:199–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Levy LM, Warr O, Attwell D. Stoichiometry of the glial glutamate transporter GLT-1 expressed inducibly in a Chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. J Neurosci 1998;18(23):9620–9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Paluzzi S, Alloisio S, Zappettini S, et al. Adult astroglia is competent for Na+/Ca2+ exchanger-operated exocytotic glutamate release triggered by mild depolarization. J Neurochem 2007;103(3):1196–1207. [DOI] [PubMed] [Google Scholar]
- 153.Wade JJ, Breslin K, Wong-Lin K, et al. Calcium microdomain formation at the perisynaptic cradle due to NCX reversal: a computational study. Front Cell Neurosci 2019;13:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Breslin K, Wade JJ, Wong-Lin K, et al. Potassium and sodium microdomains in thin astroglial processes: a computational model study. PLoS Comput Biol 2018;14(5):e1006151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Moshrefi-Ravasdjani B, Hammel EL, Kafitz KW, Rose CR. Astrocyte sodium signalling and panglial spread of sodium signals in brain white matter. Neurochem Res 2017;42(9):2505–2518. [DOI] [PubMed] [Google Scholar]
- 156.Heja L, Nyitrai G, Kekesi O, et al. Astrocytes convert network excitation to tonic inhibition of neurons. BMC Biol 2012;10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Unichenko P, Dvorzhak A, Kirischuk S. Transporter-mediated replacement of extracellular glutamate for GABA in the developing murine neocortex. Eur J Neurosci 2013;38(11):3580–3588. [DOI] [PubMed] [Google Scholar]
- 158.Shibasaki K, Hosoi N, Kaneko R, Tominaga M, Yamada K. Glycine release from astrocytes via functional reversal of GlyT1. J Neurochem 2017;140(3):395–403. [DOI] [PubMed] [Google Scholar]
- 159.Benjamin AM. Influence of Na+, K+, and Ca2+ on glutamine synthesis and distribution in rat brain cortex slices: a possible linkage of glutamine synthetase with cerebral transport processes and energetics in the astrocytes. J Neurochem 1987;48(4):1157–1164. [DOI] [PubMed] [Google Scholar]
- 160.Yuan A, Santi CM, Wei A, et al. The sodium-activated potassium channel is encoded by a member of the Slo gene family. Neuron 2003;37(5):765–773. [DOI] [PubMed] [Google Scholar]
- 161.Kucheryavykh YV, Antonov SM, Shuba YM, et al. Sodium Accumulated in Glia during Glutamate Transport Increases Polyamine Dependent Block of Kir4.1 Channels. Programme No 23605/C15 2012 Society for Neuroscience. New Orleans, LA: Society for Neuroscience, 2012. [Google Scholar]
- 162.Yu H, Zhang Z, Lis A, Penner R, Fleig A. TRPM7 is regulated by halides through its kinase domain. Cell Mol Life Sci 2013;70(15):2757–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shcheynikov N, Son A, Hong JH, et al. Intracellular Cl- as a signaling ion that potently regulates Na+/ transporters. Proc Natl Acad Sci USA 2015;112(3):E329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Aharonovitz O, Kapus A, Szaszi K, et al. Modulation of Na+/H+ exchange activity by Cl. Am J Physiol Cell Physiol 2001;281(1):C133–141. [DOI] [PubMed] [Google Scholar]
- 165.Higashijima T, Ferguson KM, Sternweis PC. Regulation of hormone-sensitive GTP-dependent regulatory proteins by chloride. J Biol Chem 1987;262(8):3597–3602. [PubMed] [Google Scholar]
- 166.Dinudom A, Komwatana P, Young JA, Cook DI. Control of the amiloride-sensitive Na+ current in mouse salivary ducts by intracellular anions is mediated by a G protein. J Physiol 1995;487 (Pt 3):549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Piala AT,, Moon TM, Akella R, et al. Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal 2014;7(324):ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Terker AS, Zhang C, Erspamer KJ, et al. Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int 2016;89(1):127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Poulsen KA, Andersen EC, Hansen CF, et al. Deregulation of apoptotic volume decrease and ionic movements in multidrug-resistant tumor cells: role of chloride channels. Am J Physiol Cell Physiol 2010;298(1):C14–25. [DOI] [PubMed] [Google Scholar]
- 170.Dezaki K, Maeno E, Sato K, Akita T, Okada Y. Early-phase occurrence of K+ and Cl- efflux in addition to Ca2+ mobilization is a prerequisite to apoptosis in HeLa cells. Apoptosis 2012;17(8):821–831. [DOI] [PubMed] [Google Scholar]
- 171.Bekar LK, Walz W. Intracellular chloride modulates A-type potassium currents in astrocytes. Glia 2002;39(3):207–216. [DOI] [PubMed] [Google Scholar]
- 172.Kettenmann H, Backus KH, Schachner M. γ-Aminobutyric acid opens Cl- channels in cultured astrocytes. Brain Res 1987;404(1–2):1–9. [DOI] [PubMed] [Google Scholar]
- 173.Kimelberg HK. Active accumulation and exchange transport of chloride in astroglial cells in culture. Biochim Biophys Acta 1981;646(1):179–184. [DOI] [PubMed] [Google Scholar]
- 174.Untiet V, Kovermann P, Gerkau NJ, et al. Glutamate transporter-associated anion channels adjust intracellular chloride concentrations during glial maturation. Glia 2017;65(2):388–400. [DOI] [PubMed] [Google Scholar]
- 175.Ma BF, Xie MJ, Zhou M. Bicarbonate efflux via GABAA receptors depolarizes membrane potential and inhibits two-pore domain potassium channels of astrocytes in rat hippocampal slices. Glia 2012;60(11):1761–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Blanz J, Schweizer M, Auberson M, et al. Leukoencephalopathy upon disruption of the chloride channel ClC-2. J Neurosci 2007;27(24):6581–6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Park H, Han KS, Oh SJ, et al. High glutamate permeability and distal localization of Best1 channel in CA1 hippocampal astrocyte. Mol Brain 2013;6:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Park H, Oh SJ, Han KS, et al. Bestrophin-1 encodes for the Ca2+-activated anion channel in hippocampal astrocytes. J Neurosci 2009;29(41):13063–13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Parkerson KA, Sontheimer H. Biophysical and pharmacological characterization of hypotonically activated chloride currents in cortical astrocytes. Glia 2004;46(4):419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yang J, Vitery MDC, Chen J, et al. Glutamate-releasing SWELL1 channel in astrocytes modulates synaptic transmission and promotes brain damage in stroke. Neuron 2019;102(4):813–827 e816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kelly T, Rose CR. Ammonium influx pathways into astrocytes and neurones of hippocampal slices. J Neurochem 2010;115(5):1123–1136. [DOI] [PubMed] [Google Scholar]
- 182.Kavanaugh MP, Arriza JL, North RA, Amara SG. Electrogenic uptake of γ-aminobutyric acid by a cloned transporter expressed in Xenopus oocytes. J Biol Chem 1992;267(31):22007–22009. [PubMed] [Google Scholar]
- 183.Egawa K, Yamada J, Furukawa T, Yanagawa Y, Fukuda A. Cl- homeodynamics in gap junction-coupled astrocytic networks on activation of GABAergic synapses. J Physiol 2013;591(16):3901–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mulkidjanian AY, Bychkov AY, Dibrova DV, Galperin MY, Koonin EV. Origin of first cells at terrestrial, anoxic geothermal fields. Proc Natl Acad Sci USA 2012;109(14):E821–E830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lebedeva A, Plata A, Nosova O, Tyurikova O, Semyanov A. Activity-dependent changes in transporter and potassium currents in hippocampal astrocytes. Brain Res Bull 2018;136:37–43. [DOI] [PubMed] [Google Scholar]
- 186.Shih PY, Savtchenko LP, Kamasawa N, et al. Retrograde synaptic signaling mediated by K+ efflux through postsynaptic NMDA receptors. Cell Rep 2013;5(4):941–951. [DOI] [PubMed] [Google Scholar]
- 187.Sibille J, Pannasch U, Rouach N. Astroglial potassium clearance contributes to short-term plasticity of synaptically evoked currents at the tripartite synapse. J Physiol 2014;592(1):87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Grewer C, Rauen T. Electrogenic glutamate transporters in the CNS: molecular mechanism, pre-steady-state kinetics, and their impact on synaptic signaling. J Membr Biol 2005;203(1):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chesler M, Kraig RP. Intracellular pH transients of mammalian astrocytes. J Neurosci 1989;9(6):2011–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Choi HB, Gordon GR, Zhou N, et al. Metabolic communication between astrocytes and neurons via bicarbonate-responsive soluble adenylyl cyclase. Neuron 2012;75(6):1094–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ruminot I, Gutierrez R, Pena-Munzenmayer G, et al. NBCe1 mediates the acute stimulation of astrocytic glycolysis by extracellular K +. J Neurosci 2011;31(40):14264–14271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rall TW, Sutherland EW. The regulatory role of adenosine-3', 5'-phosphate. Cold Spring Harb Symp Quant Biol 1961;26:347–354. [DOI] [PubMed] [Google Scholar]
- 193.Sutherland EW. Studies on the mechanism of hormone action. Science 1972;177(4047):401–408. [DOI] [PubMed] [Google Scholar]
- 194.Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry 1980;19(11):2396–2404. [DOI] [PubMed] [Google Scholar]
- 195.Tsien RY. Indicators based on fluorescence resonance energy transfer (FRET). Cold Spring Harb Protoc 2009;2009(7):pdb top57. [DOI] [PubMed] [Google Scholar]
- 196.Adams SR, Harootunian AT, Buechler YJ, Taylor SS, Tsien RY. Fluorescence ratio imaging of cyclic AMP in single cells. Nature 1991;349(6311):694–697. [DOI] [PubMed] [Google Scholar]
- 197.DiPilato LM, Cheng X, Zhang J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc Natl Acad Sci USA 2004;101(47):16513–16518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nikolaev VO, Bunemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem 2004;279(36):37215–37218. [DOI] [PubMed] [Google Scholar]
- 199.Ponsioen B, Zhao J, Riedl J, et al. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep 2004;5(12):1176–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Vardjan N, Kreft M, Zorec R. Dynamics of β-adrenergic/cAMP signaling and morphological changes in cultured astrocytes. Glia 2014;62(4):566–579. [DOI] [PubMed] [Google Scholar]
- 201.Lasic E, Lisjak M, Horvat A, et al. Astrocyte specific remodeling of plasmalemmal cholesterol composition by ketamine indicates a new mechanism of antidepressant action. Sci Rep 2019;9(1):10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bernier LP, Bohlen CJ, York EM, et al. Nanoscale surveillance of the brain by microglia via cAMP-regulated filopodia. Cell Rep 2019;27(10):2895–2908; e2894. [DOI] [PubMed] [Google Scholar]
- 203.Horvat A, Zorec R, Vardjan N. Adrenergic stimulation of single rat astrocytes results in distinct temporal changes in intracellular Ca(2+) and cAMP-dependent PKA responses. Cell Calcium 2016;59(4):156–163. [DOI] [PubMed] [Google Scholar]
- 204.Kreft M, Stenovec M, Rupnik M, et al. Properties of Ca2+-dependent exocytosis in cultured astrocytes. Glia 2004;46(4):437–445. [DOI] [PubMed] [Google Scholar]
- 205.Pangrsic T, Potokar M, Haydon PG, Zorec R, Kreft M. Astrocyte swelling leads to membrane unfolding, not membrane insertion. J Neurochem 2006;99(2):514–523. [DOI] [PubMed] [Google Scholar]
- 206.Baillie GS. Compartmentalized signalling: spatial regulation of cAMP by the action of compartmentalized phosphodiesterases. FEBS J 2009;276(7):1790–1799. [DOI] [PubMed] [Google Scholar]
- 207.Willoughby D, Cooper DM. Ca2+ stimulation of adenylyl cyclase generates dynamic oscillations in cyclic AMP. J Cell Sci 2006;119(Pt 5):828–836. [DOI] [PubMed] [Google Scholar]
- 208.Vardjan N, Horvat A, Anderson JE, et al. Adrenergic activation attenuates astrocyte swelling induced by hypotonicity and neurotrauma. Glia 2016;64(6):1034–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature 1998;392(6679):933–936. [DOI] [PubMed] [Google Scholar]
- 210.Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature 1998;392(6679):936–941. [DOI] [PubMed] [Google Scholar]
- 211.Harris RA, Lone A, Lim H, et al. Aerobic glycolysis is required for spatial memory acquisition but not memory retrieval in mice. eNeuro 2019;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Prebil M, Vardjan N, Jensen J, Zorec R, Kreft M. Dynamic monitoring of cytosolic glucose in single astrocytes. Glia 2011;59(6):903–913. [DOI] [PubMed] [Google Scholar]
- 213.Sotelo-Hitschfeld T, Niemeyer MI, Mächler P, et al. Channel-mediated lactate release by K+-stimulated astrocytes. J Neurosci 2015;35(10):4168–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.de Castro Abrantes H, Briquet M, Schmuziger C, et al. The lactate receptor HCAR1 modulates neuronal network activity through the activation of galpha and gbetagamma subunits. J Neurosci 2019;39(23):4422–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mosienko V, Rasooli-Nejad S, Kishi K, et al. Putative receptors underpinning L-lactate signalling in locus coeruleus. Neuroglia 2018;1(2):365–380. [Google Scholar]
- 216.Vardjan N, Chowdhury HH, Horvat A et al. Enhancement of astroglial aerobic glycolysis by extracellular lactate-mediated increase in cAMP. Front Mol Neurosci 2018;11:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pellerin L, Stolz M, Sorg O, et al. Regulation of energy metabolism by neurotransmitters in astrocytes in primary culture and in an immortalized cell line. Glia 1997;21(1):74–83. [DOI] [PubMed] [Google Scholar]
- 218.Sorg O, Magistretti PJ. Characterization of the glycogenolysis elicited by vasoactive intestinal peptide, noradrenaline and adenosine in primary cultures of mouse cerebral cortical astrocytes. Brain Res 1991;563(1–2):227–233. [DOI] [PubMed] [Google Scholar]
- 219.Ververken D, Van Veldhoven P, Proost C, Carton H, De Wulf H. On the role of calcium ions in the regulation of glycogenolysis in mouse brain cortical slices. J Neurochem 1982;38(5):1286–1295. [DOI] [PubMed] [Google Scholar]