ABSTRACT
Chlamydia trachomatis is a leading infectious cause of infertility in women due to its induction of lasting pathology such as hydrosalpinx. Chlamydia muridarum induces mouse hydrosalpinx because C. muridarum can both invade tubal epithelia directly (as a first hit) and induce lymphocytes to promote hydrosalpinx indirectly (as a second hit). In the current study, a critical role of CD8+ T cells in chlamydial induction of hydrosalpinx was validated in both wild type C57BL/6J mice and OT1 transgenic mice. OT1 mice failed to develop hydrosalpinx partially due to the failure of their lymphocytes to recognize chlamydial antigens. CD8+ T cells from naive C57BL/6J mice rescued the ability of recipient OT1 mice to develop hydrosalpinx when naive CD8+ T cells were transferred at the time of infection with Chlamydia. However, when the transfer was delayed for 2 weeks or longer after the Chlamydia infection, naive CD8+ T cells no longer promoted hydrosalpinx. Nevertheless, CD8+ T cells from mice immunized against Chlamydia still promoted significant hydrosalpinx in the recipient OT1 mice even when the transfer was delayed for 3 weeks. Thus, CD8+ T cells must be primed within 2 weeks after Chlamydia infection to be pathogenic, but, once primed, they can promote hydrosalpinx for >3 weeks. However, Chlamydia-primed CD4+ T cells failed to promote chlamydial induction of pathology in OT1 mice. This study optimized an OT1 mouse-based model for revealing the pathogenic mechanisms of Chlamydia-specific CD8+ T cells.
KEYWORDS: Gastrointestinal Chlamydia, CD8+ T cells, hydrosalpinx, two-hit hypothesis, Chlamydia, gastrointestinal colonization, hydrosalpinx
INTRODUCTION
Sexually transmitted Chlamydia trachomatis is known to ascend to the upper genital tract, causing sequelae like adhesion/fibrosis/hydrosalpinx in women (1–4). However, the precise mechanisms by which C. trachomatis induces lasting pathology in the upper genital tract remains unclear. A mouse model with Chlamydia muridarum infection in the genital tract has been used for investigating the pathogenic mechanisms of C. trachomatis because the C. muridarum-infected mice frequently develop long-lasting tubal fibrosis/hydrosalpinx (5–9). The mouse model-based studies have revealed pathogenic determinants in both Chlamydia (10–15) and the host (16–19). However, these findings cannot fully explain why lasting tubal fibrosis is maintained after tubal infection is cleared. In vivo imaging of C. muridarum-infected mice (20) unexpectedly revealed that genital C. muridarum spreads to the gastrointestinal (GI) tract and colonizes there for long periods (21). The chlamydial spread to the GI tract correlated with chlamydial pathogenicity in the genital tract because C. muridarum mutants that are attenuated in inducing hydrosalpinx are also defective in spreading to the GI tract (22, 23). This and other correlative observations led to the proposal of a two-hit model for explaining the development of long-lasting tubal fibrosis (24). The two-hit model emphasized the contributions of both tubal infection by the ascending chlamydial organisms in the genital tract (as a first hit) and tubal fibrotic responses exacerbated by Chlamydia-specific lymphocytes (as a second hit) to the induction and maintenance of hydrosalpinx.
By taking advantage of the failure of C. muridarum mutants to induce hydrosalpinx and spread to the GI tract, a coinoculation experiment was designed to test the two-hit hypothesis (25). It was found that intravaginal inoculation of CBA/1J mice with a C. muridarum mutant alone failed to induce hydrosalpinx, although the mutant was able to ascend the GI tract and infect tubal epithelial cells (10, 13). The lack of hydrosalpinx correlated with reduced or absent spreading of the mutant chlamydial organisms to the GI tract (22, 23, 26). Further, intragastric coinoculation with the wild type C. muridarum successfully rescued the ability of C. muridarum mutants to induce hydrosalpinx while the gastrointestinal C. muridarum alone failed to induce hydrosalpinx, demonstrating that GI Chlamydia can promote the pathogenicity of mutant Chlamydia in the genital tract. The fact that GI C. muridarum did not spread to or contaminate the genital tract lumen (25, 27) suggested that GI C. muridarum might promote the pathogenicity of genital Chlamydia by an indirect mechanism. CD8+ T cells, which have been shown to promote chlamydial pathogenicity (16, 17), were recently evaluated for their ability to act a second hit to promote hydrosalpinx in mice intravaginally inoculated with a Chlamydia mutant (28). It was found that CD8+ T cells were both necessary and sufficient for GI Chlamydia to promote hydrosalpinx in the CBA/1J mouse model.
In the current study, we used an OT1 mouse model to further characterize the pathogenic CD8+ T cells. The OT1 transgenic mice could only develop the first hit without suffering from the second hit following an intravaginal infection with a wild type C. muridarum. All T cell receptors (TCRs) of OT1 mice are genetically engineered to recognize the H-2Kb-restricted ovalbumin peptide SIINFEKL only (29) and, thus, unable to recognize chlamydial antigens, resulting in the lack of Chlamydia-induced lymphocytes as the second hit. Indeed, OT1 mice failed to develop significant hydrosalpinx after intravaginal infection with wild type C. muridarum (30, 31). Nevertheless, wild type C. muridarum inoculated intravaginally may still ascend to the upper genital tract and cause the first hit in the oviduct, including processing chlamydial antigens and presenting MHC class I-restricted chlamydial epitopes to T cells. Consistently, adoptive transfer of CD8+ T cells from naive C57BL/6j mice to OT1 mice at the time when the OT1 mice were intravaginally inoculated with wild type Chlamydia successfully rescued hydrosalpinx development (17). We further optimized the OT1 mouse model to characterize the pathogenic CD8+ T cells. It was found that when the transfer of naive CD8+ T cells to OT1 mice was delayed for 2 weeks after the OT1 mice were intravaginally infected with wild type Chlamydia, there was no significant hydrosalpinx, although a simultaneous transfer did rescue hydrosalpinx development. These observations suggest that the donor CD8+ T cells have to be primed in the recipient OT1 mice during the first 2 weeks after chlamydial infection to become pathogenic. Interestingly, transfer with gastrointestinal Chlamydia-primed CD8+ T cells promoted hydrosalpinx to OT1 mice even in the 4th week of chlamydial infection. Thus, the primed CD8+ T cells can act as a second hit to continuously promote hydrosalpinx for >3 weeks after chlamydial infection. The difference in the time of adoptive transfer for promoting hydrosalpinx between the naive and immunized CD8+ T cells can be utilized to evaluate the hydrosalpinx-promoting capabilities of CD8+ T lymphocytes induced by Chlamydia under different conditions. It was found that Chlamydia-primed CD8+ T cells but not CD4+ T cells (prepared from the same donor mice) promoted hydrosalpinx in the recipient OT1 mice when the transfer was delayed by 2 weeks. Thus, we optimized an adoptive transfer to OT1 recipient mouse approach to characterize the pathogenic CD8+ T cells. Because OT1 mice share the C57BL/6J background, which is the background that most genetically modified mouse lines are developed, the OT1 mouse approach may provide a promising platform for further revealing the mechanisms by which Chlamydia-specific CD8+ T lymphocytes promote chlamydial pathogenicity in the female upper genital tract.
RESULTS
Chlamydial induction of hydrosalpinx is dependent on CD8+ T cells capable of recognizing chlamydial antigens.
A critical role of CD8+ T cells in chlamydial induction of hydrosalpinx was validated in female C57BL/6J mice using an antibody depletion of CD8+ T cell approach (Fig. 1). Mice treated with a rat anti-CD8 antibody but not rat IgG significantly reduced hydrosalpinx in response to intravaginal infection with wild type C. muridarum. Because the anti-CD8 antibody treatment did not significantly alter the course of chlamydial shedding from either vaginal or rectal swabs, the above observation demonstrated an essential role of CD8+ T cells in mediating chlamydial pathogenicity. Furthermore, OT1 transgenic mice with TCRs engineered to recognize a H-2Kb-restricted ovalbumin epitope only failed to develop significant hydrosalpinx following C. muridarum infection. Because OT1 mice still have CD8+ T cells but without the ability to recognize chlamydial antigens, the above observation suggests that the pathogenic role of CD8+ T cells is dependent on their ability to recognize chlamydial antigens. This hypothesis is supported by the finding that the adoptive transfer of CD8+ T cells from wild type C57BL/6J mice successfully rescued the ability of OT1 mice to develop hydrosalpinx (Fig. 2). The donor CD8+ T cells might be primed by chlamydial infection in the recipient OT1 mice to recognize chlamydial antigens and promote hydrosalpinx.
FIG 1.
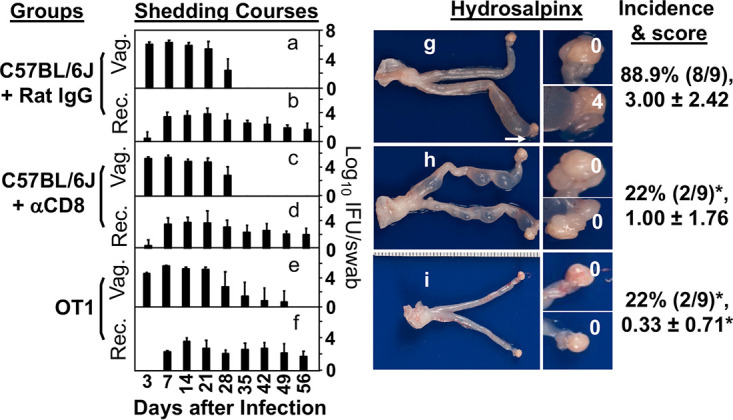
Effect of CD8+ T cell depletion and genetic engineering of T cell receptors (TCRs) on chlamydial infection and induction of hydrosalpinx. Female C57BL/6J mice treated with rat IgG (a, b, and g) or rat anti-CD8 antibody (αCD8; c, d, and h) or with TCRs engineered to recognize a H-2Kb-restricted ovalbumin epitope only (OT1; e, f, and i) were intravaginally inoculated with Chlamydia and then monitored for live organism shedding from both vaginal (a, c, and e) and rectal (b, d, and f) swabs. The infectious titers were expressed as log10 inclusion forming units (IFUs) per swab (y-axis) on different days after intravaginal infection as indicated along the x-axis. On day 56 after infection, all mice were sacrificed to observe hydrosalpinx macroscopically (g to i). Only one genital tissue image is presented from each group with the vagina on the left and oviduct/ovary on the right. Hydrosalpinx was marked with a white arrow. The magnified oviduct/ovary images were marked with hydrosalpinx scores. Both hydrosalpinx incidence and severity scores from each group were listed on the right of the corresponding images. Note that anti-CD8 antibody treatment or TCR engineering prevented 7 out of 9 mice from developing hydrosalpinx. *, P < 0.05. Fisher’s Exact for comparing hydrosalpinx incidence and Wilcoxon rank sum for comparing hydrosalpinx severity scores. Although there were no significant differences in shedding courses (Wilcoxon for comparing AUC), the shedding titers of the OT1 mice on day 35 were considered significantly higher than those of the C57BL/6J mice (P = 0.052). Data were acquired from 2 or 3 independent experiments (n = 9 per group).
FIG 2.
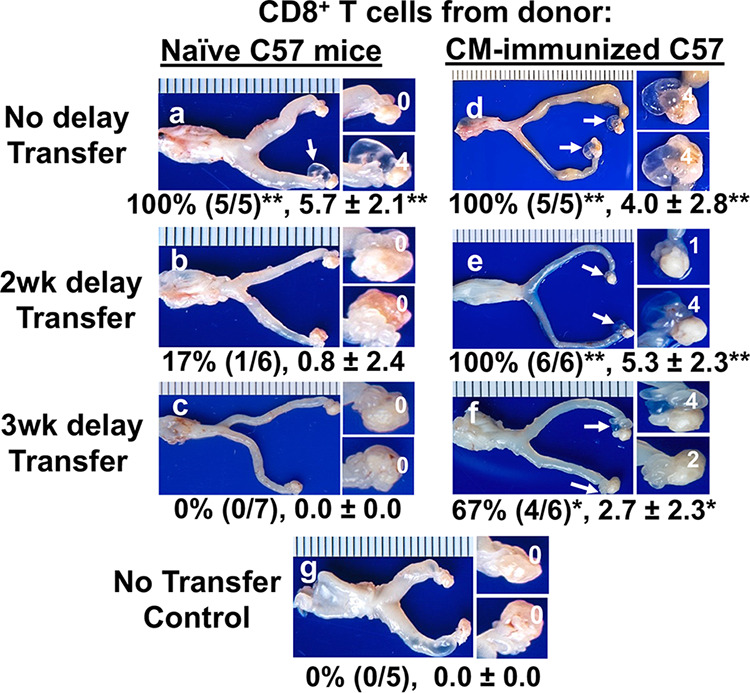
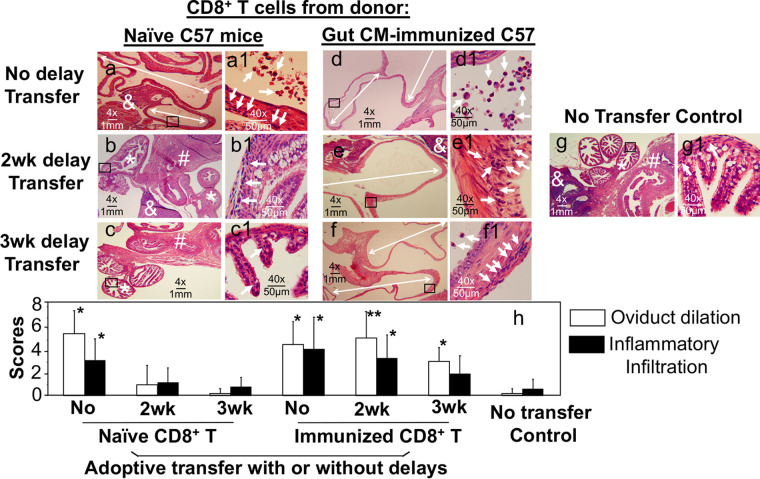
Effect of adoptive transfer with CD8+ T cells from naive versus immunized C57BL/6J mice on chlamydial induction of hydrosalpinx in the recipient OT1 mice. OT1 female mice were adoptively transferred with CD8+ T cells from naive (a to c) or immunized (d to f) donor C57BL/6J mice on the same day (no delay transfer; a and d), 2 weeks (2wk delay; b and e), or 3 weeks (3wk delay; c and f) after the recipient OT1 mice were intravaginally infected with Chlamydia as indicated on the left. The donor C57BL/6J mice were immunized with or without Chlamydia for 10 days following intragastric inoculation before splenic CD8+ T cells were harvested and purified as the donor cells. A group of recipient OT1 mice who did not receive the CD8+ T cell transfer was set as the control (g). All recipient mice were sacrificed on day 56 after the intravaginal infection for macroscopically observing hydrosalpinx in the upper genital tract. Only one genital tissue image was presented from each group with the vagina on the left and oviduct/ovary on the right. Hydrosalpinx was marked with a white arrow. The magnified oviduct/ovary images with hydrosalpinx scores (as indicated with white numbers) were displayed on the right of the corresponding whole genital tract images. Hydrosalpinx incidence and severity scores from each group are listed below the corresponding group image. Note that, although the recipient OT1 mice alone failed to develop hydrosalpinx, transfer of CD8+ T cells significantly promoted hydrosalpinx when the donor CD8+ T cells were from immunized mice (regardless of the delays in transfer) or naive mice when the transfer occurred simultaneously with the intravaginal infection but not when the transfer was delayed for 2 or 3 weeks (wks). *, P < 0.05 and **, P < 0.01. Fisher’s Exact test was used for comparing hydrosalpinx incidences and Wilcoxon rank sum for hydrosalpinx scores between the no transfer control group and each experimental group. Data were acquired from 2 or 3 independent experiments (n = 5 to 7).
Naïve CD8+ T cells have to be primed within 2 weeks of chlamydial infection to promote hydrosalpinx while Chlamydia-primed CD8+ T cells can promote hydrosalpinx for more than 3 weeks after chlamydial infection.
The naive donor CD8+ T cells must be primed by chlamydial infection in OT1 mice to differentiate into effector cells and promote hydrosalpinx. To determine the time range during which naive CD8+ T cells are primed to become pathogenic, transfer of the naive CD8+ T cells was delayed for 2 or 3 weeks after the recipient OT1 mice were infected with Chlamydia (Fig. 2). It was found that a 2-week delay significantly reduced the ability of the donor CD8+ T cells to promote hydrosalpinx, with only 1 out of 6 OT1 mice developing hydrosalpinx. After a 3-week delay, none of the 7 OT1 mice developed hydrosalpinx. Thus, a delay of 2 weeks or longer was sufficient to prevent the naive donor CD8+ T cells from becoming pathogenic, indicating that naive CD8+ T cells have to be primed within 2 weeks after chlamydial infection to become pathogenic.
To further determine whether the 2-week period was required for priming the naive CD8+ T cells alone or for both priming the naive CD8+ T cells (priming phase) and promoting hydrosalpinx by the primed CD8+ T cells (effector phase), CD8+ T cells from Chlamydia-immunized mice were used as donor cells (Fig. 2). Because CD8+ T cells induced in mice with chlamydial colonization in the gastrointestinal tract were recently shown to promote hydrosalpinx (28), CD8+ T cells isolated from the spleen and draining lymph node from mice intragastrically inoculated with Chlamydia for 10 days were used as the Chlamydia-immunized donor CD8+ T cells. When the immunized CD8+ T cells were transferred to the recipient OT1 mice 2 weeks after chlamydial infection, all OT1 mice developed hydrosalpinx, indicating that the primed donor CD8+ T cells are fully capable of promoting hydrosalpinx 2 weeks after chlamydial infection. When the transfer was delayed for 3 weeks such that it was carried out on day 21 after chlamydial infection, 67% of OT1 mice still developed significant hydrosalpinx, indicating that the Chlamydia-primed CD8+ T cells can promote significant hydrosalpinx in OT1 mice even in the fourth week after chlamydial infection. These observations revealed the time requirement for priming the pathogenic CD8+ T cells (within 2 weeks) versus the time required for the primed pathogenic CD8+ T cells to promote hydrosalpinx (>3 weeks).
To validate the above observations, we also evaluated the histopathology in these mice (Fig. 3). Both oviduct dilation scores and inflammatory infiltration scores consistently supported the conclusions based on the hydrosalpinx observation. Finally, the adoptive transfer with either naive or immunized CD8+ T cells under various transfer schemes did not significantly alter Chlamydia infection courses (Fig. 4).
FIG 3.
Effect of naive versus immunized donor CD8+ T cells on chlamydial induction of oviduct dilation and inflammatory infiltration in recipient OT1 mice. Groups of the recipient OT1 female mice with CD8+ T cells adoptively transferred and intravaginally infected with Chlamydia as described in the Fig. 2 caption were monitored for oviduct dilation and inflammatory infiltration under microscopy using the same genital tissues. After H&E staining, tissue sections made from the recipient OT1 mouse genital tissues were first examined for the overall appearance of the oviduct tissues under a 4× objective lens (a to g). Representative normal oviduct tissues were labeled with a white star (*), ovary with “&”, and uterine horn tissue with “#” while dilated oviducts were indicated with the white double arrowhead lines. Random views of oviduct tissues (as indicated with a black box) were further examined for inflammatory infiltration under a 40× subjective lens and representative images were displayed on the right side of the corresponding 4× images. Inflammatory infiltrates were indicated with white arrows (a1 to g1). Both the oviduct dilation (h; open bar) and inflammatory infiltration (h; solid bar) scores were semi-quantitatively measured as described in the Materials and Methods. Note that, although the recipient OT1 mice alone (without receiving any transfer) failed to develop significant oviduct dilation or inflammatory infiltration (g and g1), transfer of CD8+ T cells significantly promoted both oviduct dilation and inflammatory infiltration under certain conditions, including delayed transfer of immunized CD8+ T cells and no delay transfer of naive CD8+ T cells. *, P < 0.05 and **, P < 0.01. Wilcoxon rank sum for comparing dilation and inflammatory scores between the control group and each experimental group. Data were acquired from 2 or 3 independent experiments (n = 5 to 7).
FIG 4.
Effect of naive versus immunized donor CD8+ T cells on chlamydial infection courses in both genital and gastrointestinal tracts of the recipient OT1 mice. Groups of the recipient OT1 female mice adoptively transferred with CD8+ T cells under various transfer conditions (as listed on the left) and intravaginally infected with Chlamydia as described in the Fig. 2 caption were monitored for chlamydial infection courses in both genital (Vag.; a to g) and gastrointestinal (Rec., a1 to g1) tracts on days 3 and 7 and weekly thereafter as indicated along the x-axis. There were no significant differences in shedding courses between any group of mice receiving adoptive transfer (a to f or a1 to f1) and the no transfer control group (g or g1). P > 0.05, Wilcoxon rank sum (AUC). Data were acquired from 2 or 3 independent experiments (n = 5 to 7).
The sorter-purified Chlamydia-primed CD8+ T cells but not CD4+ T cells can promote hydrosalpinx in OT1 mice.
The experiments described above showed that, with a 2-week delay, adoptive transfer of naive CD8+ T cells can no longer promote significant hydrosalpinx while the transfer of Chlamydia-immunized CD8+ T cells can still promote 100% of the recipient mice to develop hydrosalpinx. Thus, the 2-week delayed adoptive transfer can be utilized to compare the capacity of lymphocytes induced under different conditions or from different sources to promote hydrosalpinx. The sorter-purified immunized CD4+ T cells versus CD8+ T cells from the same donor mice were compared for their ability to promote hydrosalpinx using the 2-week delay transfer scheme (Fig. 5). It was found that the transfer of either CD4+ or CD8+ T cells failed to significantly affect the live chlamydial shedding courses in the recipient OT1 mice. However, transfer of the immunized CD8+ T cells promoted all 5 recipient OT1 mice to develop hydrosalpinx, but the transfer of the immunized CD4+ T cells only promoted 1 of the 6 recipient OT1 mice to develop hydrosalpinx. Because both CD4+ and CD8+ T cells were sorter-purified from the same donor C57BL/6J mice (CD45.1+) that were intragastrically inoculated with Chlamydia for 10 days, the above observations demonstrated that only the gastrointestinal Chlamydia-induced CD8+ T cells but not CD4+ T cells can function as an efficient second hit to promote hydrosalpinx in the OT1 recipient mice. When the donor T cells were monitored in the recipient mice (Fig. 6), it was found that both donor cell subsets were maintained at significant levels in the peripheral blood of the recipient mice.
FIG 5.
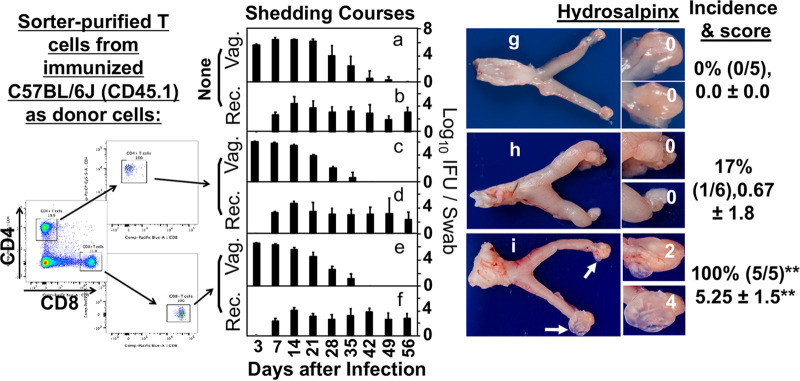
Chlamydial shedding courses and hydrosalpinx development in OT1 mice adoptively transferred with sorter-purified T cells from Chlamydia-immunized C57BL/6J mice. OT1 female mice were adoptively transferred without (None; a, b, and g) or with sorter-purified CD4+ T cells (c, d, and h) or CD8+ T cells (e, f, and i) from Chlamydia-immunized C57BL/6J mice 2 weeks after OT1 mice were intravaginally infected with Chlamydia. All mice were monitored for live organism shedding from both vaginal (Vag.; a, c, and e) and rectal (Rec.; b, d, and f) swabs and the titers were expressed as log10 IFUs per swab (y-axis) on different days after infection (x-axis). On day 56 after infection, all mice were sacrificed for observing hydrosalpinx (g to i). Only one gross image of the genital tract tissue was presented for each group and both hydrosalpinx incidence and severity scores from each group were listed on the right of the corresponding images as described in the Fig. 1 caption. Note that transfer of immunized CD8+ T cells but not CD4+ T cells with a delay of 2 weeks promoted significant hydrosalpinx. There were no significant differences in shedding courses (AUC). Data were acquired from 2 independent experiments (n = 5 or 6).
FIG 6.
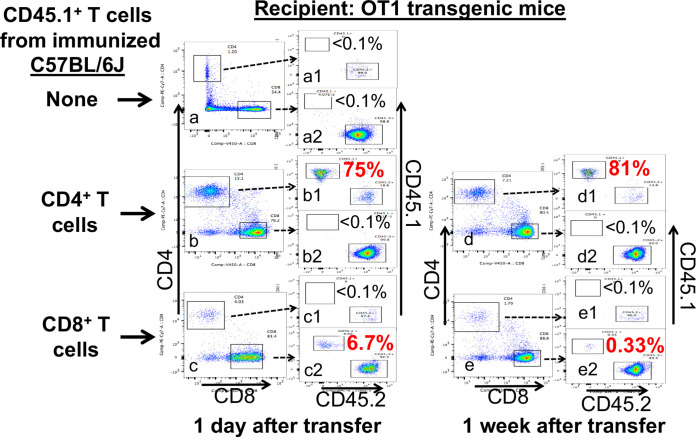
Detection of CD45.1+ donor cells in the OT1 recipient mice. The three groups of recipient OT1 mice were intravaginally infected with Chlamydia and 2 weeks later adoptively transferred without (a) or with donor CD4+ T cells (b and d) or CD8+ T cells (c and e) harvested from the immunized CD45.1+ C57BL/6J mice as described in the Fig. 5 caption. All mice were monitored for the presence of donor CD45.1+ T cells in the peripheral blood 1 day (a to c) or 1 week (d and e) after the first transfer. Peripheral mononuclear cells were gated for CD4 and CD8 first followed by quantitating CD45.1+ versus CD45.2+ cells from each population (a1 to e1 for CD4; a2 to e2 for CD8). Note that CD45.1+ donor CD4+ T cells continuously dominated while CD45.1+ donor CD8+ T cells maintained a significant presence in the peripheral blood of the recipient mice. Flow plots shown were generated from one mouse sample from each group.
DISCUSSION
The mouse intravaginal infection with C. muridarum model (5, 7, 9, 10, 13, 14, 32) has been extensively used for investigating the pathogenic mechanisms of C. trachomatis (2, 3, 33). Upon intravaginal inoculation or sexual transmission, Chlamydia may both ascend to the upper genital tract to cause tubal epithelial damage (as a first hit) and induce pathogenic immune responses (as a second hit) to convert the initial damage-triggered tissue repair responses into excessive and pathological fibrosis in the oviduct. The two-hit hypothesis emphasizes the contributions of both the first and second hits to chlamydial pathogenicity (24). In the current study, we further characterized CD8+ T cells as a second hit to promote chlamydial pathogenicity in the upper genital tract. First, we validated that CD8+ T cells play an essential role in chlamydial induction of hydrosalpinx and that the pathogenicity of CD8+ T cells is dependent on their recognition of chlamydial antigens. Although OT1 mice failed to develop hydrosalpinx in response to chlamydial infection, adoptive transfer of CD8+ T cells from naive C57BL/6J was sufficient for rescuing the ability of OT1 mice to develop hydrosalpinx. Second, the rescuing efficiency was significantly reduced when the transfer was delayed for 2 or 3 weeks, indicating that pathogenic CD8+ T cells have to be primed within the first 2 weeks of the chlamydial infection. The caveat is the lack of statistically significant differences in the hydrosalpinx rate and severity between mice without transfer, and we cannot exclude the possibility that the delayed transfer of naive CD8+ T cells may still contribute to the chlamydial induction of hydrosalpinx in the mice with the delayed transfer due to the small sample size. Third, transfer of the Chlamydia-immunized CD8+ T cells still significantly promoted hydrosalpinx in OT1 mice 2 or 3 weeks after chlamydial infection, indicating that pathogenic CD8+ T cells may continuously promote hydrosalpinx beyond 3 weeks after the initial chlamydial infection. It is worth noting that under the same delayed transfer schedules, only the immunized, but not naive CD8+ T cells, significantly promoted the induction of hydrosalpinx by the chlamydial infection. Finally, the distinct transferring time requirements for naive versus immunized CD8+ T cells to promote hydrosalpinx in OT1 mice have provided a platform for further evaluating the pathogenic mechanisms of Chlamydia-primed CD8+ T lymphocytes. When gastrointestinal Chlamydia-induced CD8+ and CD4+ T cells were independently transferred to OT1 mice 2 weeks after chlamydial infection, only the immunized CD8+ T cells but not CD4+ T cells significantly promoted hydrosalpinx. These observations demonstrated that pathogenic CD8+ T cells are most efficiently primed within 2 weeks after chlamydial infection, but, once primed, they can continuously promote hydrosalpinx for >3 weeks. Clearly, the optimized OT1 mouse model can be used to further reveal the mechanisms by which Chlamydia-primed pathogenic CD8+ T lymphocytes act as a second hit to promote hydrosalpinx in mice with the genital Chlamydia-triggered first hit.
The essence of the two-hit model is that chlamydial induction of long-lasting hydrosalpinx in the upper genital tract is dependent on both the first hit caused directly by the chlamydial invasion of tubal epithelial cells and the second hit caused indirectly by Chlamydia-induced lymphocyte responses (24). Following intravaginal inoculation, C. muridarum ascends to the oviduct to induce tubal inflammation (34) that may clear the chlamydial infection and damage tubal epithelia, which triggers tissue repairing responses that include transient fibrosis. These events are collectively defined as the first hit. In many cases, mouse tubal fibrosis continues despite the clearance of the initial chlamydial infection, leading to oviduct lumen occlusion/hydrosalpinx/infertility (5, 7, 9). Extensive efforts have been made to understand why the fibrosis/adhesion continues in the upper genital tract in the absence of microbial infection. Using the C. muridarum induction of hydrosalpinx mouse model, it was found that some host responses, such as CD8+ T cells, induced by C. muridarum are essential for chlamydial induction of hydrosalpinx (16, 17, 30), although nonspecific CD8+ T cells may downregulate hydrosalpinx development (31, 35). However, these previous experiments failed to address whether the CD8+ T cells were induced by Chlamydia in the genital tract or the GI tract. An adoptive transfer approach can be used to compare the ability of Chlamydia-induced CD8+ T cells from different sources to act as a second hit to promote hydrosalpinx in recipient mice. The recipient mice must bear the Chlamydia-induced first hit but without developing hydrosalpinx. When CBA/1J mice were used as the recipient mice, a chlamydial mutant was used for inducing the first hit (25) because intravaginal inoculation with wild type Chlamydia alone was sufficient for inducing robust hydrosalpinx (9). The finding that the transfer of CD8+ T cells induced by Chlamydia in the GI tract, but not the genital tract, of donor mice successfully rescued the CBA/1J recipient mice to develop hydrosalpinx (28) provided the first direct experimental evidence for demonstrating that gastrointestinal Chlamydia-induced CD8+ T cells can act as a second hit for promoting hydrosalpinx.
An advantage of the OT1 transgenic mouse model is that it can be used for evaluating whether Chlamydia-induced CD8+ T cells can act as a second hit to promote the first hit caused by wild type Chlamydia to develop into the long-lasting hydrosalpinx. This is because OT1 mice, with their TCRs engineered to recognize the H-2Kb-restricted ovalbumin peptide SIINFEKL, cannot develop Chlamydia-specific CD8+ T cells as a second hit, thus only allowing the first hit to occur in response to intravaginal inoculation with wild type Chlamydia. Consequently, OT1 mice failed to develop significant hydrosalpinx following intravaginal infection with wild type Chlamydia (30) (see Fig. 1) and transfer of wild type CD8+ T cells was sufficient for rescuing OT1 mice to develop hydrosalpinx (17) (see Fig. 2). In the current study, we further optimized the OT1 mouse model for evaluating the pathogenic roles of Chlamydia-induced CD8+ T lymphocytes in promoting chlamydial pathogenicity. It was found that a delay of 2 weeks was sufficient for fully preventing naive CD8+ T cells from becoming pathogenic effectors, but this delay did not affect the ability of the Chlamydia-induced or primed CD8+ T cells to promote hydrosalpinx in the recipient OT1 mice. Thus, this optimized 2-week delay of transfer to OT1 mouse scheme can be used to compare donor CD8+ T cells induced by Chlamydia under different conditions for their ability to act as a second hit to promote hydrosalpinx. It was found that the gastrointestinal Chlamydia-induced CD8+ T cells but not CD4+ T cells promoted chlamydial pathogenesis in the OT1 recipient mice that were intravaginally inoculated with wild type Chlamydia.
The OT1 mouse model optimized in the current study provided a productive platform for further revealing the mechanisms by which the pathogenic CD8+ T cells promote hydrosalpinx in the mouse oviduct because OT1 mice can accept CD8+ T cells from C57BL/6J mice, which are the mouse strain from which most of the genetically modified mouse lines are created. The recent finding that CD8+ T cells induced by Chlamydia in the GI tract, but not the genital tract, were able to promote chlamydial induction of hydrosalpinx (25, 28) is consistent with the concept that GI tract infections can induce fibrosis-promoting lymphocytes (36–38). Mice deficient in either tumor necrosis factor receptor 1 (TNFR1) (39) or interleukin 13 (IL-13) (40) significantly reduced chlamydial induction of hydrosalpinx. IL-13+ CD8+ T cells, which are known to promote fibrosis in other systems (41–43), have been isolated from Chlamydia-infected mice (44). It will be interesting to test whether IL-13 is required for Chlamydia-induced CD8+ T cells to promote hydrosalpinx in the optimized OT1 mouse model.
It is worth emphasizing that the concept that chlamydial antigen-specific CD8+ T cells can promote chlamydial pathogenicity (as reported previously [17] and in the current study) does not necessarily contradict our recent report that nonspecific CD8+ T cells suppress chlamydial pathogenicity (31). The above concept and report were because Chlamydia failed to induce hydrosalpinx in OT1 mice. It is likely that the lack of Chlamydia-specific CD8+ T cells and inhibition of hydrosalpinx by nonspecific CD8+ T cells contribute to the resistance of OT1 mice to chlamydial induction of hydrosalpinx. The CD8+ T cells from OT1 mice were found to suppress chlamydial pathogenicity in both the CD8 knockout (KO) and C57BL/6J mice (31). However, when Chlamydia-specific CD8+ T cells were provided as donor cells to OT1 mice, the OT1 mice became susceptible to chlamydial pathogenicity. Thus, we hypothesize that the endogenous nonspecific CD8+ T cells in the OT1 mice may not be able to efficiently target the Chlamydia-specific CD8+ T donor cells for inhibition. The question of why the nonspecific CD8+ T cells from OT1 mice inhibited chlamydial pathogenicity in both CD8 KO and C57BL/6J mice, but not in OT1 mice themselves, with Chlamydia-specific donor CD8+ T cells adoptively transferred is still under investigation. Regardless of the mechanisms, our current finding that a 2-week delay in the transfer can selectively allow the immunized CD8+ T cells to promote chlamydial induction of hydrosalpinx in OT1 mice has provided a model system for us to further characterize the pathogenic CD8+ T cells.
We are aware that Chlamydia may be able to induce hydrosalpinx by multiple mechanisms while the two-hit hypothesis with CD8+ T cells as a second hit may only represent one of the many mechanisms. We recently reported that mice genetically deficient in CD8+ T cells were still able to develop hydrosalpinx via a CD4+ T cell-dependent mechanism (45). However, the adoptive transfer of Chlamydia-specific CD8+ T cells but not CD4+ T cells promoted chlamydial pathogenicity in the OT1 mice. Clearly, different infection conditions on different host genetic backgrounds may trigger different subsets of lymphocytes for modulating chlamydial pathogenicity. We recently demonstrated an essential role of gut Chlamydia-induced CD8+ T cells in chlamydial pathogenicity in a CBA/1J mouse model (25, 28). This finding does not necessarily mean that pathogenic CD8+ T cells can only be induced by Chlamydia in the gut. Under different host conditions, the phenotype of a given lymphocyte subset may change. Thus, although C. muridarum infection in the mouse genital tract can induce tubal fibrosis/hydrosalpinx like that observed in C. trachomatis-infected women (2–4), caution should be taken when the information learned from the mouse model is used to understand human C. trachomatis pathogenesis. This is because C. muridarum may be naturally transmitted among mice via the oral-fecal route instead of genital tract transmission while C. trachomatis may have been forced to transmit among humans via sexual contacts. Thus, C. trachomatis may have adapted to the human genital tract. Nevertheless, the information learned from the current mouse model study should still be useful in designing human studies.
MATERIALS AND METHODS
Chlamydial organism growth.
The C. muridarum clone G13.31.1, which was derived from C. muridarum Nigg3 (GenBank accession number CP009760.1) as described previously (14), was used. The G13.32.1 clone, referred to as the wild type C. muridarum, was propagated in HeLa cells (human cervical carcinoma epithelial cells; ATCC catalog number CCL2.1) and purified as elementary bodies (EBs) as described previously (46). Aliquots of purified EBs were stored at −80°C until use. The storage buffer SPG (sucrose/phosphate/glutamate) consists of 220 mM sucrose, 12.5 mM phosphate, and 4 mM L-glutamic acid at pH 7.5.
Mouse infection.
Mouse experiments were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the committee on the ethics of laboratory animal experiments of the University of Texas Health Science Center at San Antonio.
C. muridarum EBs were used to intravaginally inoculate 6 to 7 week old female OT1 transgenic mice [C57BL/6-Tg(TcraTcrb)1100Mjb/J; stock number 003831, Jackson Laboratories, Inc., Bar Harbor, ME.) as described previously (21, 22). Briefly, stock EBs diluted in 10 μL SPG that contains 2 × 105 inclusion forming units (IFUs) were delivered to ectocervix using a 20 μL micropipette tip. Five days prior to inoculation, each mouse was injected subcutaneously with 2.5 mg Depo-Provera (Pharmacia Upjohn, Kalamazoo, MI) suspended in sterile phosphate-buffered saline (PBS). For intragastric inoculation of donor C57BL/6J (0006640) and its congenic strain that expresses the differential Ptprca pan leukocyte marker, CD45.1 (002014), EBs diluted in 100 μL SPG that contained 2 × 105 IFUs were delivered to the stomach using a straight-balled end needle designed for mouse oral gavage (N-PK 020, Braintree Scientific Inc, Braintree, MA). In some experiments, OT1 mice with intravaginal inoculation with the wild type C. muridarum G13.32.1 clone had donor cells adoptively transferred as described below. On day 56 after intravaginal inoculation, all mice were sacrificed for observing genital tract pathology as described below.
Adoptive transfer.
For the adoptive transfer experiments, CD8+ T cells were prepared from donor C57BL/6J mice without or with intragastrical inoculation with C. muridarum. Both spleen and mesenteric lymph nodes harvested from donor mice after 7 to 14 d after chlamydial infection were used for preparing CD8+ T cells using a combination of negative selection. For negative selection, single-cell suspensions that were prepared from the spleen and lymph nodes were treated with a cocktail of biotin-conjugated antibodies to remove CD4+ T cells (clone number GK1.5, Rat IgG2b, κ; catalog number 100404, Biolegend, Inc., San Diego, CA), CD19+ B cells (clone number 6D5, Rat IgG2a, κ; catalog number 115504, Biolegend, Inc.), CD45R/B220+ cells (clone number RA3-6B2, Rat IgG2a, κ; catalog number 103204, Biolegend, Inc.) and MHC class II (I-Ak)+ cells (clone number M5/114.15.2, Rat IgG2b, κ; catalog number 107604, Biolegend, Inc.) by using magnetic beads. The remaining CD8+ T cells were counted and defined as negative selection-enriched donor cells for adoptive transfer experiments. When the donor cells were prepared from the CD45.1+ mice, the donor cells were labeled with anti-CD3-phycoerythrin conjugate (clone#17A2, ratIgG2b/κ; catalog number 100206, Biolegend, Inc.), anti-CD4-allophycocyanin (GK1.5, ratIgG2b/κ, catalog number 100412, Biolegend, Inc.), and anti-CD8-brilliant violet 650 (53 to 6.7, ratIgG2a/κ, catalog number 100741, Biolegend, Inc.). CD4+ and CD8+ T cells were sorted from live CD3+ cells using the BD FACSAriaTM II (catalog number 642886, BD Biosciences San Jose, CA). Both the negative selection-enriched CD8+ T cells and the sorter-purified CD8+ or CD4+ T cells were delivered to the recipient mice retro-orbitally with 2 × 106 cells (for enriched CD8+ T cells) or 1 × 105 cells (for sorter-purified T cells) per injection three times on days 0, 7, and 14 (simultaneous transfer) or days 14, 21, and 35 (for 2-week delay or 2W mice) or days 21, 35, and 42 (for 3-week delay or 3W mice) after the recipient mice were intravaginally infected with wild type C. muridarum.
Titrating live chlamydial organisms recovered from swabs.
To quantitate live chlamydial organisms in vaginal or rectal swabs, each swab was soaked in 0.5 mL of SPG, vortexed with glass beads, and the chlamydial organisms released into the supernatants were titrated on HeLa cell monolayers in duplicate. The infected cultures were processed for immunofluorescence assay as described previously (47) and below. Inclusions were counted in five random fields per coverslip under a fluorescence microscope. For coverslips with less than one IFU per field, entire coverslips were counted. Coverslips showing obvious cytotoxicity of HeLa cells were excluded. The total number of IFUs per swab was calculated based on the mean IFUs per view, the ratio of the view area to that of the well, dilution factor, and inoculation volumes. Where possible, a mean IFU/swab was derived from the serially diluted and duplicate samples for any given swab. The total number of IFUs/swab was converted into log10, which was used to calculate the mean and standard deviation across mice of the same group at each time point.
Immunofluorescence assay.
The immunofluorescence assay used for titration of live organisms was carried out as described previously (48). A rabbit antibody (designated R1604, raised with purified C. muridarum EBs) was used as a primary antibody to label all C. muridarum in HeLa cells, which was visualized with a goat anti-rabbit IgG conjugated with Cy2 (green; catalog number 111-225-144, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). The DNA dye Hoechst 3328 (blue; Sigma-Aldrich, St. Louis, MO) was used to visualize nuclei. The doubly labeled samples were used for counting for C. muridarum under a fluorescence microscope (AX70, Olympus) equipped with a CCD camera (Hamamatsu). For samples containing two different types of C. muridarum, such as the plasmid-free C. muridarum and wild type C. muridarum that express mCherry, we used a rat anti-mCherry monoclonal antibody (M11217, ThermoFisher Scientific) together with R1604 as the primary antibodies to differentiate them. The mCherry-expressing wild type C. muridarum was visualized with a goat anti-rat IgG conjugated with Cy3 (red; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). The wild type C. muridarum was labeled both green and red while plasmid-free C. muridarum was labeled green only. The distinct inclusions were counted separately using AX70 for calculating IFU recoveries.
Evaluating genital tract gross pathology hydrosalpinx macroscopically.
On day 56 after intravaginal infection, mice were euthanized for evaluating genital tract pathology. The focus was on the upper genital tract hydrosalpinx. Before the tissues were removed, an in situ gross examination was performed for evidence of oviduct hydrosalpinx or any other related abnormalities of oviducts. The severity of oviduct hydrosalpinx was scored based on the following criteria: no hydrosalpinx (0), hydrosalpinx detectable only under microscopic examination (1), hydrosalpinx visible with naked eyes but the size was smaller than the ovary on the same side (2), equal to the ovary on the same side (3) or larger than the ovary on the same side (4). Bilateral hydrosalpinx severity was calculated for each mouse as the summed scores of the left and right oviducts. Hydrosalpinx incidence was calculated as the number of mice with a bilateral score of 1 or higher divided by the total number of mice in the group.
Evaluating oviduct dilation and inflammatory infiltration microscopically.
After macroscopic evaluation of hydrosalpinx and photographing for documentation of hydrosalpinx, the same mouse genital tissues were fixed in 10% neutral formalin, embedded in paraffin, and serially sectioned longitudinally (with 5 μm/section). Efforts were made to include the cervix, both uterine horns, the oviducts, and the lumenal structures of each tissue in the same section. The sections were stained with hematoxylin and eosin (H&E). Three representative sections separated by 15 μM or more from each other were used for evaluating both oviduct dilation and inflammatory infiltration. The oviduct dilation was assessed under a 4× subjective lens. The severity of oviduct dilation was semi-quantitatively scored using the following criteria: 0, no significant oviduct lumenal dilatation; 1, mild dilation of a single cross-section; 2, one to three dilated cross sections; 3, more than three dilated cross sections; and 4, confluent pronounced dilation. The median of the three scores served as a single value for each oviduct unilateral dilation score. Both unilateral scores for each mouse were combined to form a bilateral dilation score. Oviduct dilation incidence was calculated as the number of mice with a bilateral score of 1 or higher divided by the total number of mice in the group. The inflammatory infiltrates were semi-quantitatively scored under a 40× objective lens using the following criteria: 0 represents no cellular foci (of mononuclear cells), 1 indicates a single focus, 2 indicates two to four loci, 3 indicates more than four foci, and 4 indicates confluent infiltration in the tissue or one or more clusters of >3 mononuclear cells in the oviduct lumen. The median of the three scores served as a single value for each oviduct unilateral inflammation score, and both unilateral scores for each mouse were combined to form a bilateral score.
Statistics analyses.
All categorical data, including hydrosalpinx and oviduct dilation incidences, were analyzed using Fisher's Exact test (http://vassarstats.net/tab2x2.html). Both quantitative data, such as the time courses of live organism shedding (in terms of area under the curve (AUC) of log10 IFUs per mouse) and semiquantitative data, such as hydrosalpinx, oviduct dilation or inflammatory infiltration scores, were analyzed between two groups using Wilcoxon rank sum test (using an in-house Excel sheet). Prior to any comparisons of two designated groups, an ANOVA was used to determine whether there was an overall significant difference among all groups in the same experiment (https://goodcalculators.com/one-way-anova-calculator/).
ACKNOWLEDGMENTS
This study is supported in part by US NIH grants R01AI047997, R01AI121989, and R21AI151724 to GZ.
We declare no conflict of interest.
Contributor Information
Dabao Xu, Email: dabaoxu@yahoo.com.
Guangming Zhong, Email: Zhongg@UTHSCSA.EDU.
Craig R. Roy, Yale University School of Medicine
REFERENCES
- 1.CDC. 2017. Sexually Transmitted Disease Surveillance, 2016. Services US Department of Health and Human Services, https://www.cdc.gov/std/stats16/default.htm, Atlanta, GA. [Google Scholar]
- 2.Budrys NM, Gong S, Rodgers AK, Wang J, Louden C, Shain R, Schenken RS, Zhong G. 2012. Chlamydia trachomatis antigens recognized in women with tubal factor infertility, normal fertility, and acute infection. Obstet Gynecol 119:1009–1016. 10.1097/AOG.0b013e3182519326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodgers AK, Budrys NM, Gong S, Wang J, Holden A, Schenken RS, Zhong G. 2011. Genome-wide identification of Chlamydia trachomatis antigens associated with tubal factor infertility. Fertil Steril 96:715–721. 10.1016/j.fertnstert.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodgers AK, Wang J, Zhang Y, Holden A, Berryhill B, Budrys NM, Schenken RS, Zhong G. 2010. Association of tubal factor infertility with elevated antibodies to Chlamydia trachomatis caseinolytic protease P. Am J Obstet Gynecol 203:494.e7–494.e14. 10.1016/j.ajog.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de la Maza LM, Pal S, Khamesipour A, Peterson EM. 1994. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun 62:2094–2097. 10.1128/iai.62.5.2094-2097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison RP, Feilzer K, Tumas DB. 1995. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun 63:4661–4668. 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah AA, Schripsema JH, Imtiaz MT, Sigar IM, Kasimos J, Matos PG, Inouye S, Ramsey KH. 2005. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex Transm Dis 32:49–56. 10.1097/01.olq.0000148299.14513.11. [DOI] [PubMed] [Google Scholar]
- 8.Murthy AK, Li W, Guentzel MN, Zhong G, Arulanandam BP. 2011. Vaccination with the defined chlamydial secreted protein CPAF induces robust protection against female infertility following repeated genital chlamydial challenge. Vaccine 29:2519–2522. 10.1016/j.vaccine.2011.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Zhang H, Zhou Z, Yang Z, Ding Y, Zhou Z, Zhong E, Arulanandam B, Baseman J, Zhong G. 2014. Chlamydial induction of hydrosalpinx in 11 strains of mice reveals multiple host mechanisms for preventing upper genital tract pathology. PLoS One 9:e95076. 10.1371/journal.pone.0095076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lei L, Chen J, Hou S, Ding Y, Yang Z, Zeng H, Baseman J, Zhong G. 2014. Reduced live organism recovery and lack of hydrosalpinx in mice infected with plasmid-free Chlamydia muridarum. Infect Immun 82:983–992. 10.1128/IAI.01543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Connell CM, Ingalls RR, Andrews CW, Jr., Scurlock AM, Darville T. 2007. Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease. J Immunol 179:4027–4034. 10.4049/jimmunol.179.6.4027. [DOI] [PubMed] [Google Scholar]
- 12.Zhong G. 2017. Chlamydial plasmid-dependent pathogenicity. Trends Microbiol 25:141–152. 10.1016/j.tim.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Huang Y, Yang Z, Sun Y, Gong S, Hou S, Chen C, Li Z, Liu Q, Wu Y, Baseman J, Zhong G. 2014. Plasmid-encoded Pgp3 is a major virulence factor for Chlamydia muridarum to induce hydrosalpinx in mice. Infect Immun 82:5327–5335. 10.1128/IAI.02576-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conrad TA, Gong S, Yang Z, Matulich P, Keck J, Beltrami N, Chen C, Zhou Z, Dai J, Zhong G. 2016. The chromosome-encoded hypothetical protein tc0668 is an upper genital tract pathogenicity factor of Chlamydia muridarum. Infect Immun 84:467–479. 10.1128/IAI.01171-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison SG, Giebel AM, Toh EC, Spencer HJ, 3rd, Nelson DE, Morrison RP. 2018. Chlamydia muridarum genital and gastrointestinal infection tropism is mediated by distinct chromosomal factors. Infect Immun 86:e00141-18. 10.1128/IAI.00141-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murthy AK, Li W, Chaganty BK, Kamalakaran S, Guentzel MN, Seshu J, Forsthuber TG, Zhong G, Arulanandam BP. 2011. Tumor necrosis factor alpha production from CD8+ T cells mediates oviduct pathological sequelae following primary genital Chlamydia muridarum infection. Infect Immun 79:2928–2935. 10.1128/IAI.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vlcek KR, Li W, Manam S, Zanotti B, Nicholson BJ, Ramsey KH, Murthy AK. 2016. The contribution of Chlamydia-specific CD8(+) T cells to upper genital tract pathology. Immunol Cell Biol 94:208–212. 10.1038/icb.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Z, Conrad T, Zhou Z, Chen J, Dutow P, Klos A, Zhong G. 2014. Complement factor C5 but not C3 contributes significantly to hydrosalpinx development in mice infected with Chlamydia muridarum. Infect Immun 82:3154–3163. 10.1128/IAI.01833-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang J, Karimi O, Ouburg S, Champion CI, Khurana A, Liu G, Freed A, Pleijster J, Rozengurt N, Land JA, Surcel HM, Tiitinen A, Paavonen J, Kronenberg M, Morre SA, Kelly KA. 2012. Interruption of CXCL13-CXCR5 axis increases upper genital tract pathology and activation of NKT cells following chlamydial genital infection. PLoS One 7:e47487. 10.1371/journal.pone.0047487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell J, Huang Y, Liu Y, Schenken R, Arulanandam B, Zhong G. 2014. Bioluminescence Imaging of Chlamydia muridarum Ascending Infection in Mice. PLoS One 9:e101634. 10.1371/journal.pone.0101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Q, Huang Y, Gong S, Yang Z, Sun X, Schenken R, Zhong G. 2015. In vivo and ex vivo imaging reveals a long-lasting chlamydial infection in the mouse gastrointestinal tract following genital tract inoculation. Infect Immun 83:3568–3577. 10.1128/IAI.00673-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao L, Melero J, Zhang N, Arulanandam B, Baseman J, Liu Q, Zhong G. 2017. The cryptic plasmid is more important for Chlamydia muridarum to colonize the mouse gastrointestinal tract than to infect the genital tract. PLoS One 12:e0177691. 10.1371/journal.pone.0177691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shao L, Zhang T, Melero J, Huang Y, Liu Y, Liu Q, He C, Nelson DE, Zhong G. 2018. The genital tract virulence factor pGP3 is essential for Chlamydia muridarum colonization in the gastrointestinal Tract. Infect Immun 86:e00429-17. 10.1128/IAI.00429-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong G. 2018. Chlamydia spreading from the genital tract to the gastrointestinal tract - A two-hit hypothesis. Trends Microbiol 26:611–623. 10.1016/j.tim.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian Q, Zhou Z, Wang L, Abu-Khdeir AH, Huo Z, Sun X, Zhang N, Schenken R, Wang Y, Xue M, Zhong G. 2020. Gastrointestinal coinfection promotes chlamydial pathogenicity in the genital tract. Infect Immun 88:e00905-19. 10.1128/IAI.00905-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao L, Zhang T, Liu Q, Wang J, Zhong G. 2017. Chlamydia muridarum with mutations in chromosomal genes tc0237 and/or tc0668 is deficient in colonizing the mouse gastrointestinal tract. Infect Immun 85:e00321-17. 10.1128/IAI.00321-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Zhang Q, Zhang T, Zhang Y, Zhu C, Sun X, Zhang N, Xue M, Zhong G. 2016. The Chlamydia muridarum organisms fail to auto-inoculate the mouse genital tract after colonization in the gastrointestinal tract for 70 days. PLoS One 11:e0155880. 10.1371/journal.pone.0155880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian Q, Zhou Z, Wang L, Sun X, Arulanandam B, Xu D, Xue M, Zhong G, In press. 2021. Gastrointestinal Chlamydia-induced CD8+ T cells promote chlamydial pathogenicity in the female upper genital tract. Infect Immun 89:e0020521. 10.1128/IAI.00205-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. 1994. T cell receptor antagonist peptides induce positive selection. Cell 76:17–27. 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 30.Manam S, Nicholson BJ, Murthy AK. 2013. OT-1 mice display minimal upper genital tract pathology following primary intravaginal Chlamydia muridarum infection. Pathog Dis 67:221–224. 10.1111/2049-632X.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie L, He C, Chen J, Tang L, Zhou Z, Zhong G. 2020. Suppression of chlamydial pathogenicity by nonspecific CD8(+) T lymphocytes. Infect Immun 88:e00315-20. 10.1128/IAI.00315-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng W, Shivshankar P, Li Z, Chen L, Yeh IT, Zhong G. 2008. Caspase-1 contributes to Chlamydia trachomatis-induced upper urogenital tract inflammatory pathologies without affecting the course of infection. Infect Immun 76:515–522. 10.1128/IAI.01064-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunham RC, Maclean IW, Binns B, Peeling RW. 1985. Chlamydia trachomatis: its role in tubal infertility. J Infect Dis 152:1275–1282. 10.1093/infdis/152.6.1275. [DOI] [PubMed] [Google Scholar]
- 34.Zhang H, Zhou Z, Chen J, Wu G, Yang Z, Zhou Z, Baseman J, Zhang J, Reddick RL, Zhong G. 2014. Lack of long lasting hydrosalpinx in A/J mice correlates with rapid but transient chlamydial ascension and neutrophil recruitment in the oviduct following intravaginal inoculation with Chlamydia muridarum. Infect Immun 82:2688–2696. 10.1128/IAI.00055-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang J, Champion CI, Wei B, Liu G, Kelly KA. 2013. CD8(+)CXCR5(+) T cells regulate pathology in the genital tract. Infect Dis Obstet Gynecol 2013:813238. 10.1155/2013/813238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metwali A, Setiawan T, Blum AM, Urban J, Elliott DE, Hang L, Weinstock JV. 2006. Induction of CD8+ regulatory T cells in the intestine by Heligmosomoides polygyrus infection. Am J Physiol Gastrointest Liver Physiol 291:G253–9. 10.1152/ajpgi.00409.2005. [DOI] [PubMed] [Google Scholar]
- 37.Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, Cua DJ, Goldschmidt M, Hunter CA, Kastelein RA, Artis D. 2006. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med 203:843–849. 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koch KN, Muller A. 2015. Helicobacter pylori activates the TLR2/NLRP3/caspase-1/IL-18 axis to induce regulatory T-cells, establish persistent infection and promote tolerance to allergens. Gut Microbes 6:382–387. 10.1080/19490976.2015.1105427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong X, Liu Y, Chang X, Lei L, Zhong G. 2014. Signaling via tumor necrosis factor receptor 1 but not Toll-like receptor 2 contributes significantly to hydrosalpinx development following Chlamydia muridarum infection. Infect Immun 82:1833–1839. 10.1128/IAI.01668-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asquith KL, Horvat JC, Kaiko GE, Carey AJ, Beagley KW, Hansbro PM, Foster PS. 2011. Interleukin-13 promotes susceptibility to chlamydial infection of the respiratory and genital tracts. PLoS Pathog 7:e1001339. 10.1371/journal.ppat.1001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brodeur TY, Robidoux TE, Weinstein JS, Craft J, Swain SL, Marshak-Rothstein A. 2015. IL-21 Promotes Pulmonary Fibrosis through the Induction of Profibrotic CD8+ T Cells. J Immunol 195:5251–5260. 10.4049/jimmunol.1500777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Long X, Chen Q, Zhao J, Rafaels N, Mathias P, Liang H, Potee J, Campbell M, Zhang B, Gao L, Georas SN, Vercelli D, Beaty TH, Ruczinski I, Mathias R, Barnes KC, Chen X. 2015. An IL-13 promoter polymorphism associated with liver fibrosis in patients with Schistosoma japonicum. PLoS One 10:e0135360. 10.1371/journal.pone.0135360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuschiotti P, Larregina AT, Ho J, Feghali-Bostwick C, Medsger TA. Jr., 2013. Interleukin-13-producing CD8+ T cells mediate dermal fibrosis in patients with systemic sclerosis. Arthritis Rheum 65:236–246. 10.1002/art.37706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson RM, Kerr MS, Slaven JE. 2014. An atypical CD8 T-cell response to Chlamydia muridarum genital tract infections includes T cells that produce interleukin-13. Immunology 142:248–257. 10.1111/imm.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu H, Lin H, Xie L, Tang L, Chen J, Zhou Z, Ni J, Zhong G. 2019. Chlamydia induces pathology in the female upper genital tract via distinct mechanisms. Infect Immun 87doi:10.1128/IAI.00145–19. 10.1128/IAI.00145-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mukhopadhyay S, Clark AP, Sullivan ED, Miller RD, Summersgill JT. 2004. Detailed protocol for purification of Chlamydia pneumoniae elementary bodies. J Clin Microbiol 42:3288–3290. 10.1128/JCM.42.7.3288-3290.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang L, Zhang H, Lei L, Gong S, Zhou Z, Baseman J, Zhong G. 2013. Oviduct Infection and Hydrosalpinx in DBA1/j Mice Is Induced by Intracervical but Not Intravaginal Inoculation with Chlamydia muridarum. PLoS One 8:e71649. 10.1371/journal.pone.0071649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan T, Lu H, Hu H, Shi L, McClarty GA, Nance DM, Greenberg AH, Zhong G. 1998. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med 187:487–496. 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]