Abstract
This study examined Shiga toxin-producing Escherichia coli (STEC) O157, using phage typing, pulsed-field gel electrophoresis, and typing of Shiga toxin variant genes by PCR with restriction fragment length polymorphism in an epidemiological survey of STEC O157 isolated from humans in Finland between 1990 and 1999.
Human infections caused by Shiga toxin-producing Escherichia coli (STEC) O157 have been reported from over 30 countries on six continents (12). In Finland, with a population of 5.1 million, the incidence rate of STEC O157 infections rose from 0.06 in 1990 (9) to 1.0 in 1997 and then decreased to 0.3 in 1999 (13). The only recorded STEC O157 outbreak in Finland occurred in 1997 (14). Pulsed-field gel electrophoresis (PFGE) has been widely used for subtyping of STEC O157 (6). However, an internationally standardized library of PFGE fingerprints has not yet been created, in contrast to phage typing for STEC O157 (1, 10). In this study, 105 STEC O157:H7 and O157:H− strains isolated from cultures sent from Finnish hospital laboratories to the Laboratory of Enteric Pathogens, National Public Health Institute (9), during 1990 to 1999 were phage typed, genotyped by PFGE, and investigated for Shiga toxin variant genes by PCR with restriction fragment length polymorphism. In addition, the relevant patient data, including recent foreign travel, were collected on a special form accompanying the isolate.
For phage typing, the bacteria were grown in double-strength nutrient broth (Difco Laboratories, Detroit, Mich.) shaken for 1.5 h at 37°C, flooded onto double-strength nutrient agar plates (Difco), and phage typed (1, 10). The results were interpreted according to a phage type list of 66 confirmed and 14 provisional phage types. A result of RDNC (for reacts but does not conform) was confirmed by phage typing five additional colonies of the strain.
In PFGE, bacterial growth on nutrient agar was suspended in TEN buffer (0.1 M Tris-HCl, 0.15 M NaCl, 0.1 M EDTA, pH 7.5) to an A600 of 0.280 to 0.310. The suspension was embedded equally in 1.8% InCert agarose (FMC BioProducts, Rockland, Maine) and digested overnight with 0.15 mg of proteinase K (Boehringer Mannheim, Indianapolis, Ind.) per ml at 50°C in 3 ml of ES buffer (0.5 M EDTA, 1% N-lauroylsarcosine). The plugs were washed as described previously (9). The PFGE method and the library of PFGE types were standardized nationally (L. Rantala, M. Saari, S. Hallanvuo, A. Siitonen, and T. Honkanen-Buzalski, Abstr. Nordic PFGE meeting, 25–26, 2000). The PFGE types were coded from 1.1 to 1.59.
PCR-RFLP was executed as described previously (4, 11) with minor modifications. A loopful of bacteria on sorbitol-MacConkey plates was suspended in 500 μl of sterile water, boiled for 5 min, and centrifuged briefly, and 1 μl was used as a template. In the PCR, the final elongation was for 10 min at 72°C. The amplified DNA was restricted with 20 U of HincII and AccI enzymes (New England Biolabs) (4).
Of the 105 strains studied, 98 (93%) belonged to nine confirmed phage types (PTs): PT2, −4, −8, −14, −21/28, −34, −49, −50 and −88 (Table 1). The most common PT was PT2, representing 56% of all isolates, followed by PT4 (11%), PT49 (10%), and PT8 (6%). Seven strains (7%) did not conform to any published phage type and were designated RDNC, which formed three distinct reaction patterns. Of all strains, 93 isolates (89%) were of domestic origin. Among these, the predominant PT was PT2 (62%; 58 isolates), followed by PT49 (11%; 10 isolates) and PT4 (10%; 9 isolates). Three strains (3%) were designated RDNC. In PFGE, the isolates of domestic origin were distributed in 24 types. The main types were 1.1 (53%), 1.3 (5%), and 1.12 (4%). Twelve isolates were associated with recent travel abroad. Among these isolates, the PT distribution was PT2 (8%), PT4 (25%), PT8 (33%), and RDNC (33%). By PFGE, 10 types were detected, of which two types (1.5 and 1.47) were also found indigenously (Table 1, Fig. 1). Among all isolates, the most common PT-PFGE combinations were PT2/1.1 (46%), PT49/1.12 (4%), and PT4/1.47 (4%). In PCR-RFLP, the majority of the strains (70%; 74 strains) were positive for stx2 and either stx2c, stx2vha, or stx2vOX393. These genes associated mainly with PT2/1.1 (48 strains), PT4 (5 strains), and RDNC (3 strains). The strains carrying stx2c, stx2vha, or stx2vOX393 only were all of foreign origin; they were from Turkey (PT8/1.9), Spain (RDNC3/1.11), and Greece (RDNC3/1.59). The five strains carrying stx1 and stx2c, stx2vha, or stx2vOX393 all belonged to PT8; three of them were associated with recent travel to Spain (PT8/1.52), Turkey (PT8/1.29), and the Czech Republic (PT8/1.30).
TABLE 1.
Characteristics of STEC O157:H7 and O157:H− isolates in Finland from 1990 to 2000
| Strain | Origin | Isolation yr/mo | Serotype | Sorbitol fermentation | PT | PFGE type | stx variant gene(s) |
|---|---|---|---|---|---|---|---|
| IH 40962 | Turkey | 1990/5 | O157:H7 | − | 8 | 1.9 | stx2c, stx2vha, or stx2vOX393 |
| IH 40986 | Finland | 1990/7 | O157:H7 | − | 34 | 1.10 | stx1 or stx1v |
| IH 41039 | Spain | 1990/10 | O157:H7 | − | 8 | 1.52 | stx1 or stx1v + stx2c, stx2vha, or stx2vOX393 |
| IH 41285 | Finland | 1992/10 | O157:H7 | − | 49 | 1.8 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53393 | Spain | 1996/7 | O157:H7 | − | RDNC3 | 1.11 | stx2c, stx2vha, or stx2vOX393 |
| IH 53401 | Finland | 1996/12 | O157:H7 | − | 49 | 1.38 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53404 | Finland | 1997/1 | O157:H7 | − | RDNC2 | 1.3 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53407 | Finland | 1997/3 | O157:H7 | − | 4 | 1.15 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53409 | Finland | 1997/5 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53410 | Finland | 1997/5 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53414 | Finland | 1997/7 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53415 | Finland | 1997/7 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53427 | Finland | 1997/7 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53429 | Finland | 1997/7 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53416 | Finland | 1997/7 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53430 | Finland | 1997/7 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53432 | Finland | 1997/7 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53417 | Finland | 1997/7 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53433 | Finland | 1997/7 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53435 | Finland | 1997/7 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53423 | Finland | 1997/7 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53419 | Finland | 1997/7 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53421 | Finland | 1997/7 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53440 | Finland | 1997/7 | O157:H− | + | RDNC1 | 1.4 | stx2 |
| IH 53425 | Finland | 1997/7 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53467 | Finland | 1997/7 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53438 | Finland | 1997/7 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53441 | Finland | 1997/7 | O157:H7 | − | 2 | 1.5 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53442 | Russia | 1997/7 | O157:H7 | − | 2 | 1.5 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53436 | Finland | 1997/7 | O157:H7 | − | 49 | 1.8 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53464 | Finland | 1997/7 | O157:H7 | − | 49 | 1.21 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53443 | Finland | 1997/7 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53461 | Finland | 1997/8 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53459 | Finland | 1997/8 | O157:H7 | − | 49 | 1.21 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53474 | Finland | 1997/8 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53476 | Finland | 1997/9 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53475 | Cruise, Finland and Sweden | 1997/9 | O157:H7 | − | 4 | 1.47 | stx2 |
| IH 53478 | Cruise, Finland and Sweden | 1997/9 | O157:H7 | − | 4 | 1.47 | stx2 |
| IH 53480 | Cruise, Finland and Sweden | 1997/9 | O157:H7 | − | 4 | 1.47 | stx2 |
| IH 53489 | Finland | 1997/9 | O157:H7 | − | 2 | 1.55 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53490 | Finland | 1997/9 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53491 | Finland | 1997/9 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53492 | Finland | 1997/9 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53493 | Finland | 1997/9 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53494 | Finland | 1997/9 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53495 | Finland | 1997/9 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53496 | Finland | 1997/9 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53500 | Finland | 1997/9 | O157:H7 | − | 2 | 1.55 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53497 | Finland | 1997/9 | O157:H7 | − | 2 | 1.55 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 53498 | Finland | 1997/9 | O157:H7 | − | 2 | 1.3 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56726 | Finland | 1997/10 | O157:H7 | − | 2 | 1.3 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56728 | Finland | 1997/10 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56730 | Finland | 1997/11 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56738 | Finland | 1997/12 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56740 | Finland | 1997/12 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56741 | Finland | 1997/12 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56742 | Finland | 1997/12 | O157:H7 | − | 49 | 1.21 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56743 | Finland | 1997/12 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56744 | Finland | 1998/1 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56745 | Finland | 1998/1 | O157:H7 | − | 49 | 1.12 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56746 | Finland | 1998/1 | O157:H7 | − | 21/28 | 1.56 | stx1 or stx1v + stx2 |
| IH 56747 | Finland | 1998/1 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56748 | Finland | 1998/1 | O157:H7 | − | 49 | 1.12 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56767 | Finland | 1998/1 | O157:H7 | − | 49 | 1.12 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56768 | Finland | 1998/1 | O157:H7 | − | 49 | 1.12 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56769 | Finland | 1998/1 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56770 | Finland | 1998/1 | O157:H7 | − | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 | |
| IH 56771 | Finland | 1998/1 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56775 | Finland | 1998/1 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56776 | Finland | 1998/1 | O157:H7 | + | 88 | 1.13 | Not determined |
| IH 56777 | Finland | 1998/1 | O157:H7 | − | 4 | 1.15 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56778 | Finland | 1998/1 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56779 | Finland | 1998/2 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56827 | Finland | 1998/4 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56833 | Greece | 1998/6 | O157:H7 | − | RDNC3 | 1.59 | stx2c, stx2vha, or stx2vOX393 |
| IH 56834 | Finland | 1998/6 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56835 | Finland | 1998/6 | O157:H7 | − | 4 | 1.15 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56836 | Finland | 1998/6 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56839 | Finland | 1998/7 | O157:H7 | − | 2 | 1.1 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56842 | Turkey | 1998/8 | O157:H7 | − | 8 | 1.29 | stx1 or stx1v + stx2c, stx2vha, or stx2vOX393 |
| IH 56843 | Finland | 1998/8 | O157:H7 | − | 2 | 1.1 | stx2 + stx 2c, stx2vha, or stx2vOX393 |
| IH 56844 | Czech Republic | 1998/8 | O157:H− | − | 8 | 1.30 | stx1 or stx1v + stx2c, stx2vha, or stx2vOX393 |
| IH 56845 | Finland | 1998/8 | O157:H7 | − | 14 | 1.1 | stx2 |
| IH 56858 | Finland | 1998/10 | O157:H7 | − | 4 | 1.57 | stx2 |
| IH 56863 | Finland | 1998/10 | O157:H7 | − | 2 | 1.31 | stx2 |
| IH 56868 | Finland | 1998/10 | O157:H7 | − | 4 | 1.58 | stx2 |
| IH 56869 | Finland | 1998/10 | O157:H7 | − | 4 | 1.57 | stx2 |
| IH 56870 | Finland | 1998/10 | O157:H7 | − | 4 | 1.57 | stx2 |
| IH 56893 | Finland | 1999/2 | O157:H7 | − | 50 | 1.3 | stx2 |
| IH 56894 | Finland | 1999/2 | O157:H7 | − | 50 | 1.3 | stx2 |
| IH 56909 | Finland | 1999/3 | O157:H− | + | 88 | 1.53 | stx2 |
| IH 56926 | Finland | 1999/3 | O157:H− | + | 88 | 1.53 | stx2 |
| IH 56927 | Finland | 1999/3 | O157:H− | + | 88 | 1.53 | stx2 |
| IH 56929 | Finland | 1999/3 | O157:H− | + | 88 | 1.54 | stx2 |
| IH 56958 | Finland | 1999/4 | O157:H7 | − | 4 | 1.47 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56966 | Finland | 1999/4 | O157:H7 | − | 4 | 1.37 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 56969 | Finland | 1999/4 | O157:H− | − | 88 | 1.50 | stx2 |
| IH 56970 | Finland | 1999/5 | O157:H7 | − | 2 | 1.28 | stx2 |
| IH 56975 | Finland | 1999/5 | O157:H7 | − | 2 | 1.28 | stx2 |
| IH 57034 | Finland | 1999/6 | O157:H7 | − | 8 | 1.32 | stx1 or stx1v + stx2c, stx2vha, or stx2vOX393 |
| IH 57044 | Finland | 1999/7 | O157:H7 | − | 2 | 1.28 | stx2 |
| IH 57046 | Finland | 1999/7 | O157:H7 | − | 8 | 1.32 | stx1 or stx1v + stx2c, stx2vha, or stx2vOX393 |
| IH 57065 | Greece | 1999/10 | O157:H7 | − | RDNC3 | 1.48 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 57068 | Tunisia | 1999/11 | O157:H7 | − | RDNC3 | 1.49 | stx2 + stx2c, stx2vha, or stx2vOX393 |
| IH 57086 | Finland | 1999/12 | O157:H− | + | RDNC1 | 1.51 | stx2 |
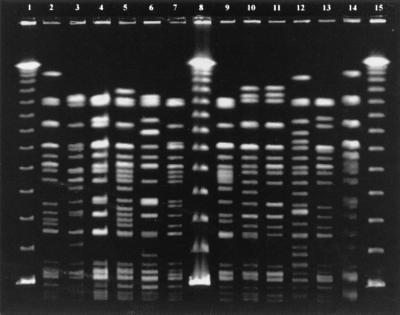
FIG. 1.
PFGE fingerprint patterns of the STEC O157 isolates of foreign and of domestic origin in Finland. Lanes 1, 8, and 15, bacteriophage lambda ladder PFG marker 340 (New England Biolabs Inc., Beverly, Mass.); lane 2, type PT8/1.30 (Czech Republic); lane 3, type RDNC 3/1.49 (Tunisia); lane 4, type RDNC 3/1.11 (Spain); lane 5, type PT8/1.52 (Spain); lane 6, type PT8/1.9 (Turkey); lane 7, type PT8/1.29 (Turkey); lane 9, type RDNC 3/1.59 (Greece); lane 10, type PT2/1.5 (Finland and Russia); lane 11, type PT4/1.47 (Finland and Sweden); lane 12, type RDNC 3/1.48 (Greece); lane 13, type PT8/1.32 (Finland); lane 14, O157:H7 control strain (no. G5244) from the Centers for Disease Control and Prevention.
The distribution of PTs in Finland was similar to that found in several other countries, especially in Europe (2, 3, 7, 8, 15). While the range of PT and PFGE types isolated from human infections in Finland indicates several domestic sources for STEC O157 infections, only one sporadic infection could be linked to a known food item, unpasteurized milk (16). However, various food items or environmental sources have caused STEC O157 infections worldwide (12). Based on these data, further studies of the prevalence of STEC O157 bacteria in Finland will be needed. In this study, 70% of the strains of the most common PT (PT2) possessed stx2 and stx2c, stx2vha, or stx2vOX393. These strains were isolated mainly during the STEC outbreak in Finland in July 1997 (14), although this subtype was seen for the first time in May 1997 (9). The reason for the emergence of these strains can only be speculated on; it took place about 2 years after Finland joined the European Union (EU). In fact, the swift emergence and similarity of the strains in Finland and other European countries even speak for the effect of EU on this issue. For example, in Great Britain, PT2 strains possessing stx2 and stx2c have caused several outbreaks (18). The other common domestic types, PT4 and PT49, have also been common in Great Britain (17). Also, sorbitol-positive O157:H− strains of PT88 carrying the stx2 gene were found in our study. Similar strains have caused a large outbreak in Germany (2) and have been isolated in the Czech Republic (5). In infections of foreign origin, strains of PT8 carrying stx1 and stx2c, stx2vha, or stx2vOX393 have predominated in Finland. Interestingly, PT8 strains possessing the stx1 and stx2 genes have also been found in The Netherlands and Great Britain (7, 17).
This study used STEC O157 phage typing for the first time as an extensive epidemiological surveillance method in Finland. The information gained on the PT distribution in Finland may help to trace O157 infections at the EU level and help to assess the need to standardize new PTs indicated by RDNC.
Acknowledgments
We thank Bernard Rowe and Henry Smith, Central Public Health Laboratory, Colindale, London, England, for cooperation in setting up the phage typing of STEC O157 in Finland. We also thank Sirkku Waarala and Tarja Heiskanen for excellent laboratory assistance.
REFERENCES
- 1.Ahmed R, Bopp C, Borczyk A, Kasatiya S. Phage-typing scheme for Escherichia coli O157:H7. J Infect Dis. 1987;155:806–809. doi: 10.1093/infdis/155.4.806. [DOI] [PubMed] [Google Scholar]
- 2.Ammon A, Petersen L R, Karch H. A large outbreak of hemolytic uremic syndrome caused by an unusual sorbitol-fermenting strain of Escherichia coli O157:H−. J Infect Dis. 1999;179:1274–1277. doi: 10.1086/314715. [DOI] [PubMed] [Google Scholar]
- 3.Barrett T J, Lior H, Green J H, Khakhria R, Wells J G, Bell B P, Greene K D, Lewis J, Griffin P M. Laboratory investigation of a multistate food-borne outbreak of Escherichia coli O157:H7 by using pulsed-field gel electrophoresis and phage typing. J Clin Microbiol. 1994;32:3013–3017. doi: 10.1128/jcm.32.12.3013-3017.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastian S N, Carle I, Grimont F. Comparison of 14 PCR systems for the detection and subtyping of stx genes in Shiga-toxin-producing Escherichia coli. Res Microbiol. 1998;149:457–472. doi: 10.1016/s0923-2508(98)80001-6. [DOI] [PubMed] [Google Scholar]
- 5.Bielaszewska M, Schmidt H, Karmali M A, Khakhria R, Janda J, Blahova K, Karch H. Isolation and characterization of sorbitol-fermenting Shiga toxin (verocytotoxin)-producing Escherichia coli O157:H- strains in the Czech Republic. J Clin Microbiol. 1998;36:2135–2137. doi: 10.1128/jcm.36.7.2135-2137.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grif K, Karch H, Schneider C, Daschner F D, Beutin L, Cheasty T, Smith H, Rowe B, Dierich M P, Allerberger F. Comparative study of five different techniques for epidemiological typing of Escherichia coli O157. Diagn Microbiol Infect Dis. 1998;32:165–176. doi: 10.1016/s0732-8893(98)00103-5. [DOI] [PubMed] [Google Scholar]
- 7.Heuvelink A E, van de Kar N C A J, Meis J F G M, Monnens L A H, Melchers W J G. Characterization of Verocytotoxin-producing Escherichia coli O157 isolates from patients with haemolytic uraemic syndrome in Western Europe. Epidemiol Infect. 1995;115:1–14. doi: 10.1017/s0950268800058064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Izumiya H, Masuda T, Ahmed R, Khakria R, Wada A, Terajima J, Itoh K, Johnson W M, Konuma H, Shinagawa K, Tamura K, Watanabe H. Combined use of bacteriophage typing and pulsed-field gel electrophoresis in the epidemiological analysis of Japanese isolates of enterohemorrhagic Escherichia coli O157:H7. Microbiol Immunol. 1998;42:515–519. doi: 10.1111/j.1348-0421.1998.tb02318.x. [DOI] [PubMed] [Google Scholar]
- 9.Keskimäki M, Saari M, Heiskanen T, Siitonen A. Shiga toxin-producing Escherichia coli in Finland from 1990 through 1997: prevalence and characteristics of isolates. J Clin Microbiol. 1998;36:3641–3646. doi: 10.1128/jcm.36.12.3641-3646.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khakhria R, Duck D, Lior H. Extended phage-typing scheme for Escherichia coli O157:H7. Epidemiol Infect. 1990;105:511–520. doi: 10.1017/s0950268800048135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin Z, Kurazono H, Yamasaki S, Takeda Y. Detection of various variant verotoxin genes in Escherichia coli by polymerase chain reaction. Microbiol Immunol. 1993;37:543–548. doi: 10.1111/j.1348-0421.1993.tb01675.x. [DOI] [PubMed] [Google Scholar]
- 12.Mead P S, Griffin P M. Escherichia coli O157:H7. Lancet. 1998;352:1207–1212. doi: 10.1016/S0140-6736(98)01267-7. [DOI] [PubMed] [Google Scholar]
- 13.National Public Health Institute. Infectious diseases in Finland 1995–1999. Publication series B4/2000. Helsinki, Finland: National Public Health Institute; 2000. [Google Scholar]
- 14.Paunio M, Pebody R, Keskimäki M, Kokki M, Ruutu P, Oinonen S, Vuotari V, Siitonen A, Lahti E, Leinikki P. Swimming associated outbreak of enterohemorrhagic Escherichia coli (EHEC) O157:H7. Epidemiol Infect. 1999;122:1–5. doi: 10.1017/s0950268898001927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith H R, Rowe B, Adak G K, Reilly W J. Shiga toxin (Verocytotoxin)-producing Escherichia coli in the United Kingdom. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other shiga toxin-producing E. coli strains. Washington, D.C.: ASM Press; 1998. pp. 49–58. [Google Scholar]
- 16.Tast E, Keskimäki M, Myllys V, Suomala P, Saari M, Siitonen A, Honkanen-Buzalski T. Proceedings of World Congress on Food Hygiene. Wageningen, The Netherlands: Wageningen Pers; 1997. Vero cytotoxin producing Escherichia coli O157:H7 infection in a 6-year-old child associated with consumption of unpasteurised milk; p. 202. [Google Scholar]
- 17.Thomas A, Cheasty T, Frost J A, Chart H, Smith H R, Rowe B. Vero cytotoxin-producing Escherichia coli, particularly serogroup O157, associated with human infections in England and Wales: 1992–4. Epidemiol Infect. 1996;117:1–10. doi: 10.1017/s0950268800001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willshaw G A, Smith H R, Cheasty T, Wall P G, Rowe B. Vero cytotoxin-producing Escherichia coli O157 outbreaks in England and Wales, 1995: phenotypic methods and genotypic subtyping. Emerg Infect Dis. 1997;3:561–565. doi: 10.3201/eid0304.970422. [DOI] [PMC free article] [PubMed] [Google Scholar]