Abstract
For over 40 years, attempts to develop treatments that protect neurons and other brain cells against the cellular and biochemical consequences of cerebral ischaemia in acute ischaemic stroke (AIS) have been unsuccessful. However, the advent of intravenous thrombolysis and endovascular thrombectomy has taken us into a new era of treatment for AIS in which highly effective reperfusion therapy is widely available. In this context, cytoprotective treatments should be revisited as adjunctive treatment to reperfusion therapy. Renewed efforts should focus on developing new drugs that target multiple aspects of the ischaemic cascade, and previously developed drugs should be reconsidered if they produced robust cytoprotective effects in preclinical models and their safety profiles were reasonable in previous clinical trials. Several development pathways for cytoprotection as an adjunct to reperfusion can be envisioned. In this Review, we outline the targets for cytoprotective therapy and discuss considerations for future drug development, highlighting the recent ESCAPE-NA1 trial of nerinetide, which produced the most promising results to date. We review new types of clinical trial to evaluate whether cytoprotective drugs can slow infarct growth prior to reperfusion and/or ameliorate the consequences of reperfusion, such as haemorrhagic transformation. We also highlight how advanced brain imaging can help to identify patients with salvageable ischaemic tissue who are likely to benefit from cytoprotective therapy.
Subject terms: Neurology, Cerebrovascular disorders
In this Review, Fisher and Savitz consider how the era of reperfusion therapy in ischaemic stroke provides new hope for the development of cytoprotective therapies to further improve outcomes, highlighting how promising recent findings can be built on to benefit patients.
Key points
Highly successful reperfusion therapy with intravenous thrombolysis and endovascular thrombectomy is now widely available for the treatment of acute ischaemic stroke, making cytoprotective therapy a viable additional treatment approach.
Previous attempts to develop cytoprotective therapy have been unsuccessful, but this approach should now be reconsidered as an adjunctive therapy to thrombolysis and thrombectomy.
New cytoprotective drugs should be developed to target multiple aspects of the ischaemic cascade, and previously developed drugs should be reconsidered.
Trials should be conducted to evaluate the effects of cytoprotective drugs when administered before or after reperfusion therapy or both.
Advanced brain imaging should be used to select patients who are most likely to benefit from cytoprotective treatment for enrolment in new trials.
Introduction
The treatment of acute ischaemic stroke (AIS) has been revolutionized by the development of therapies — such as thrombolysis and endovascular thrombectomy — that open occluded cerebral vessels and restore perfusion to the ischaemic brain. Reperfusion salvages ischaemic brain tissue by restoring the supply of oxygen and glucose and arresting the ischaemic cascade initiated by a lack of these vital nutrients. An additional approach in the treatment of AIS is to directly target the ischaemic cascade in the ischaemic region1. Traditionally, this approach has been referred to as neuroprotection, but the term cytoprotection is more appropriate because all cells of the neurovascular unit — including neurons, astrocytes, microglia, pericytes and the endothelia — in the ischaemic region are at risk of damage2,3. Nevertheless, among these cells, neurons are likely to be the most vulnerable and their death is probably the most important contributor to clinical deficits in AIS2.
Cytoprotective therapies for AIS have remained elusive — a long history of failures and disappointments spans more than 40 years. Many pharmaceuticals that target different components of the ischaemic cascade have produced promising results in animal models of stroke with varying degrees of methodological rigour, but none has had clear, reproducible efficacy on primary clinical end points in clinical trials. Reasons for the lack of translational success have been thoroughly discussed for more than 20 years, and include broad deficiencies in preclinical modelling and clinical trial design4 (Box 1). Most clinical trials of neuroprotective approaches have been designed to evaluate one agent in comparison with placebo, and most enrolled patients have not received concomitant thrombolysis and were unlikely to have had substantial reperfusion before or after the neuroprotective drug was administered5. Given that eventual reperfusion is probably a necessity for neuroprotection to be clinically beneficial, the use of neuroprotective agents in isolation is likely to be a major reason for their failure to date.
In this context, the development of cytoprotective drugs should be reconsidered in the current era of highly successful reperfusion, as use of such drugs as an adjunct to thrombolysis, thrombectomy or both considerably increases the likelihood of success. Indeed, use of such an approach with the cytoprotective drug nerinetide in combination with thrombectomy was evaluated in a recent clinical trial6 and seems to have produced therapeutic benefits in some patients, providing new hope. In this Review, we consider how cytoprotection for AIS can now be developed in the era of acute stroke therapy, taking into account recent developments in imaging and advances in health-care delivery services. We discuss the trial of nerinetide in detail and consider the lessons learned and the next steps towards finally achieving approval of a cytoprotective drug for the clinical management of AIS.
Box 1 Potential preclinical and clinical explanations for failure of neuroprotective drugs.
Preclinical
Lack of randomized and blinded outcome assessment.
Lack of allocation concealment so that investigators were aware of the treatment assignment.
Lack of monitoring of physiological measurements, such as blood pressure and temperature.
Inappropriate estimation of required sample size.
Treatment effects not assessed in animals with comorbidities and old age.
Results from all included animals not reported in the study.
Lack of dose–response and therapeutic time window studies.
Lack of reproducibility between models and laboratories.
Incomplete understanding of the pharmacological profile of the drug being tested.
Clinical
Drugs were tested in patients who were unlikely to benefit.
Studies were performed in an insufficient number of patients to detect a treatment effect.
Studies included too many patients with mild or severe stroke, in whom a clinical benefit is difficult to detect.
Drug induced serious adverse events that did not allow adequate serum concentrations to be achieved.
Trials included a substantial number of patients with lacunar stroke with white matter infarction and no evidence suggests that the study drug affects white matter injury.
Patients were assigned to treatment too late after stroke onset to allow substantial ischaemic tissue salvage
Lack of advanced imaging in trials of treatment in later time windows to confirm whether the infarct size precluded beneficial treatment.
Therapeutic targets
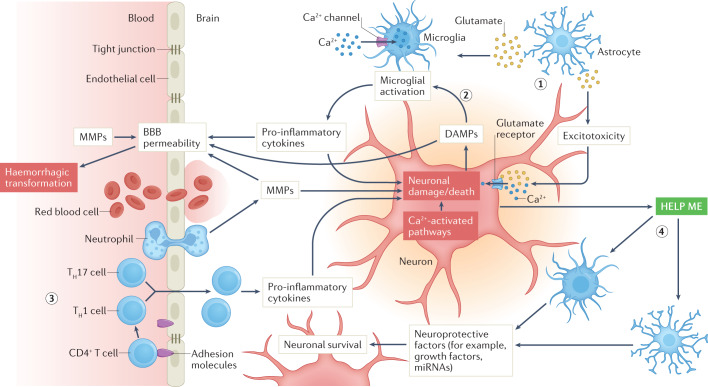
The ischaemic cascade is a multifaceted and complex pathophysiological process with a multitude of interconnected pathways that evolve over time and involve different cell types that are activated in concert or sequentially by marked reductions in oxygen and glucose supply7 (Fig. 1). Among the most salient components of this process are glutamate-mediated excitotoxicity, calcium signalling, oxidative stress and inflammation that can originate from peripheral immune cells that migrate to the brain and/or from the neurovascular unit at the level of the microglia and endothelium. Neuronal injury and trafficking of peripheral immune cells into the CNS amplifies microglial activation, which results in further neuronal damage. In parallel, neuroprotective responses are initiated, in which astrocytes and microglia respond to so-called ‘help me’ signals from injured neurons by releasing trophic factors and extracellular vesicles and by transferring mitochondria to neurons. The complexity of the ischaemic cascade cannot be overstated. Cell-to-cell signalling among the components of the neurovascular unit either amplify injury or promote protection of neurons, and some components of the intercellular signalling can change from being detrimental to being beneficial or vice versa over the course of hours or days after stroke onset. In addition, extracellular signalling can disrupt the blood–brain barrier (BBB), which can lead to haemorrhagic transformation as a consequence of reperfusion injury (see below).
Fig. 1. Elements of the ischaemic cascade in the neurovascular unit during acute ischaemic stroke.
Only selected elements are shown, and the signalling evolves over time after the onset of ischaemia. Oxygen and glucose deprivation leads to release of glutamate from astrocytes (1), which activates excitotoxic signalling within neurons, leading to neuronal injury and death, and activates microglia. Neuronal injury leads to the release of damage-associated molecular patterns (DAMPs), which also activate microglia (2), which consequently release pro-inflammatory cytokines that lead to further damage of neurons and promote endothelial activation and blood–brain barrier (BBB) opening. DAMPS also directly promote BBB breakdown. Leukocyte trafficking and migration to the ischaemic region (3) lead to further release of cytokines, amplification of neuroinflammation and continued neuronal damage. In parallel, neurons release ‘help me’ signals (4) that activate astrocytes and microglia to release factors that protect neurons. MMPs, matrix metalloproteinases; TH1 cell, T helper 1 cell; TH17 cell, T helper 17 cell.
Over the past 40 years, trials of cytoprotective monotherapy have focused on pharmaceutical agents that target one component of the ischaemic cascade. However, such single-target therapeutics are likely to have a limited impact on the complex array of pathophysiological mechanisms unleashed by focal cerebral ischaemia. A more attractive prospect is the use of cytoprotective approaches that affect multiple aspects of the ischaemic cascade; for example, 3K3A-activated protein C acts on protease-activated receptor 1 to alter multiple downstream pathways involved in the ischaemic cascade, and nerinetide is an upstream inhibitor of excitotoxic glutamate signalling8,9. Hypothermia, although not a pharmacological therapy, is an example of an attractive multimodal approach that targets various components of the ischaemic cascade and that cytoprotective therapies could emulate. Hypothermia has not been successful in clinical trials owing to its adverse effects, such as pneumonia, and the need for anti-shivering medication10, but new approaches that achieve rapid reductions in temperature are focusing on focal brain cooling.
A second target for cytoprotective approaches is reperfusion injury, a mechanism that has long been of theoretical interest but is now clinically relevant in the era of reperfusion for AIS11. The concept of reperfusion injury is that the restoration of oxygenated blood flow into previously ischaemic brain tissue can have deleterious consequences in addition to the obvious benefits. For example, oxygenated blood can lead to the generation of reactive oxygen species, such as superoxide and peroxynitrite, that can overwhelm endogenous protective mechanisms, leading to tissue injury12. In addition, oxygenated blood can activate the immune system through the recruitment of leukocytes — activation of microglia can lead to tissue injury through the release of cytokines, complement and chemokines13, and the release of matrix metalloproteinases from neutrophils can lead to degradation of the extracellular matrix and tight junctions, contributing to BBB disruption, leakage and rupture14. The most severe consequence of this BBB disruption is haemorrhagic transformation in the ischaemic territory14,15. The later perfusion is restored in the ischaemic territory, the higher the risk of haemorrhagic transformation16. Finally, the reperfusion treatments can contribute to reperfusion injury themselves: tissue plasminogen activator (tPA) upregulates matrix metalloproteinases with the risk of BBB disruption, and thrombectomy can cause injury to the endothelial lining. These processes can initiate haemorrhagic transformation and lead to intracerebral haemorrhage.
A third cytoprotective strategy in AIS is the activation of endogenous collaterals in the brain to increase blood flow into the ischaemic region, thereby extending survival of the penumbra17. The extent and adequacy of collateral blood flow is one of the most important factors that determines the speed of progression towards irreversible injury and the extent of that injury in AIS. Therefore, enhancing collateral blood flow should be viewed as a valuable way to protect brain cells during ischaemia, especially soon after onset. Several approaches to increasing collateral blood flow are under development, including stimulation of the sphenopalatine ganglion that is close to gaining regulatory approval18.
A fourth potential cytoprotective strategy is the development of immunomodulators. Peripheral immune cells travel to the site of ischaemic injury and promote further damage through the release of cytokines and consequent amplification of pro-inflammatory microglia and astrocytes13. To date, no anti-inflammatory drugs have been proven to improve outcomes in stroke clinical trials. However, our understanding of how and when to modify the inflammatory response to produce beneficial effects needs further study. We have learned from clinical trials that broadly suppressing immune cell trafficking to the brain is not beneficial after stroke19 and that broadly suppressing the inflammatory response can even be harmful20. Approaches that target microglial activation are under development, as are applications of cell-based therapies that selectively downregulate pro-inflammatory signalling and upregulate anti-inflammatory signalling21.
Renewed drug development
With the widespread use of reperfusion therapy in clinical practice, the development of cytoprotective drugs to use in combination with these therapies has been renewed. This research includes the identification and development of novel drugs but also the re-evaluation of the dozens of proposed cytoprotective drugs that have previously been tested in phase II and phase III randomized clinical trials (RCTs)22. Most of these drugs had been evaluated in preclinical testing and produced favourable outcomes to varying degrees, but none provided a reproducible benefit in RCTs. However, these drugs were tested in the absence of reperfusion, so their potential should be reassessed in the reperfusion era.
Selection of drugs for redevelopment
To determine which old cytoprotective drugs could be candidates for redevelopment, several factors should be considered. The previous preclinical studies of the drugs should have demonstrated robust evidence of cytoprotection in temporary occlusion models in several species across multiple laboratories. Candidates should also have been tested in at least phase II RCTs and preferably in a phase III RCT so that a favourable safety profile in humans is well established. Evidence that the drug is compatible and safe with tPA for thrombolysis would also be beneficial but not necessarily required. Furthermore, for the selection of drugs that target the ischaemic cascade, those with pleiotropic effects would be preferable.
Preclinical testing
Preclinical testing of new and repurposed cytoprotective drugs remains important in the development of AIS therapies23. Prior experience shows that efficacy in animal stroke models does not necessarily predict a benefit in patients with AIS, but a lack of efficacy in animal models is highly likely to predict a lack of efficacy in humans. As set out by the Stroke Therapy Academic Industry Roundtable recommendations, which were updated in 2021, initial studies of cytoprotective drug candidates should focus on demonstrating efficacy and safety in rodent models of transient ischaemia in young animals; experiments should be conducted within a few days of stroke onset and at more delayed time points to assess effects on infarcts of different sizes. Long-term behavioural outcomes should also be assessed24. These studies should also provide information about the pharmacokinetics and dynamics of the study drug and should demonstrate either that the drug crosses the BBB to reach the ischaemic brain tissue or that it reaches the cerebral endothelium if vasculoprotection is the goal.
The use of reperfusion stroke models is based on the intention for the drug to be used in conjunction with reperfusion in clinical development. Demonstration of efficacy in permanent occlusion stroke models would also be desirable if the cytoprotective drug is likely to be studied for its ability to prolong survival of the ischaemic penumbra and lengthen the window of opportunity for thrombectomy during, for example, transfer to a tertiary centre2. Drugs that demonstrate efficacy in these domains should be taken forward to later stages of development2 (Box 2).
Later studies in animal models should involve older animals of both sexes with comorbidities, such as hypertension or diabetes mellitus, because patients with stroke are typically elderly and many have comorbid diseases24. Preclinical studies in species with gyrencephalic brains, such as non-human primates, should also be considered. Drugs should enter the last phase of preclinical testing if they have produced favourable outcomes in most, if not all, of these preliminary studies. In this phase, drugs should be tested in multiple laboratories in parallel with standardized protocols25. This last preclinical step is akin to multicentre RCTs to obtain evidence that proceeding to formal clinical studies is warranted3.
In the USA, a structure for such preclinical animal stroke modelling has been implemented through the creation of the Stroke Preclinical Assessment Network (SPAN), funded by the National Institutes of Health (NIH). Six laboratories with well-established histories are testing cytoprotective treatments in preclinical models that were selected by peer review by the NIH. Currently, SPAN is evaluating five cytoprotective drugs (Table 1), each of which was selected by a peer review process based on prior preclinical studies of efficacy. The objective of SPAN is to identify and select promising cytoprotective agents that should be taken into clinical trials in StrokeNet, a preclinical trials network for promising stroke therapies in the USA. Platform trials to test adjunctive treatments with thrombectomy are being established in StrokeNet.
Table 1.
Cytoprotective drugs currently being evaluated by the SPAN network
| Cytoprotective drug57 | Mechanism of action |
|---|---|
| Fingolimod | Immunomodulation |
| Fasudil | Selective RhoA/Rho kinase inhibitor |
| Tocilizumab | IL-6 receptor antagonist |
| Uric acid | Free radical scavenger, antioxidant |
| Veliparib | Poly(ADP-ribose) polymerase (PARP) inhibitor |
Drugs for study were selected on the basis of a peer review process that took into account previous studies of drug properties.
Box 2 Steps for preclinical evaluation of purported cytoprotective drugs.
Early stage
Initial randomized and blinded studies should be performed with appropriate physiological monitoring (including blood pressure, temperature and blood gases) in young or middle-aged rodents of both sexes.
Histological, imaging and behavioural outcomes should be assessed in a blinded manner at early and delayed time points after stroke onset.
An adequate sample size should be studied to provide sufficient statistical power.
A reasonable safety profile should be demonstrated.
Only drugs with favourable outcomes in early-stage studies should be taken forward to late-stage studies.
Late stage
Efficacy studies in rodent models of stroke should be done in elderly animals and those with comorbidities, such as diabetes mellitus and hypertension.
Studies in stroke models should be performed according to the circadian rhythm of the species being studied; given that most patients with acute ischaemic stroke are awake at stroke onset, animals should be studied during the wakeful period of their sleep–wake cycle.
The same modelling procedures and treatment protocols should be used in multiple laboratories to enable data pooling and meta-analysis of the results.
A variety of animal models of stroke should be studied in multiple laboratories.
Interactions of cytoprotective agents with thrombolytics should be studied to rule out negative interactions.
Studies in gyrencephalic species, such as non-human primates, should be considered.
Nerinetide and a promising pathway forward
After years of disappointment, the 2020 publication of the phase III ESCAPE-NA1 trial of nerinetide in patients undergoing thrombectomy provided hope that a cytoprotective drug might finally have a beneficial effect6. Nerinetide is a peptide that disrupts the function of the synaptic folding protein PSD-95 (ref.9). It affects protein–protein interactions and, as a consequence, inhibits neuronal excitotoxicity and downstream neurotoxic signalling. Preclinical studies in rodent and primate stroke models demonstrated robust treatment effects5. A subsequent phase II RCT in patients who were undergoing endovascular treatment of brain aneurysms demonstrated a reasonable safety profile and suggested that nerinetide reduces the development of procedure-related ischaemic brain lesions26.
In the phase III trial, patients who were undergoing thrombectomy were randomly assigned to receive a single intravenous dose of nerinetide or placebo before the procedure6. Eligibility criteria were moderate-to-good filling of collateral vessels on CT angiography and no evidence of an extensive region of early infarction on a CT scan of the head. In the placebo group, 59.2% of patients received tPA. In the nerinetide group, 60.1% received tPA. No significant difference in safety outcomes was observed. At 90 days, a favourable outcome — defined as a score of 0–2 on the modified Rankin scale — was achieved in 59.2% of patients who received placebo and 61.4% of patients who received nerinetide. This difference did not reach statistical significance. However, in a pre-specified subgroup analysis of patients who did not receive tPA, 49.8% of patients who received placebo had a favourable outcome compared with 59.3% of patients who received nerinetide, and this difference was statistically significant. Mortality was also significantly lower in the nerinetide group. Among patients who received tPA, no difference in these outcomes was seen and the peak serum levels of nerinetide were substantially lower among patients who did receive tPA than among those who did not, suggesting an interaction between nerinetide and tPA that altered the therapeutic effect.
To further explore the relationship between nerinetide and tPA, a series of elegant animal experiments was performed. These experiments showed in plasma samples and in vivo that nerinetide is cleaved by plasmin, which is produced when tPA cleaves plasminogen27. In a rat model of embolic stroke, administration of tPA and nerinetide at the same time negated the beneficial effect of nerinetide alone on infarct volume. When nerinetide was administered 30 min before tPA, a significant benefit on infarct volume was observed. On the basis of these findings, a d-enantiomer of nerinetide was developed, which is not degraded in rat or human plasma upon exposure to tPA. Use of the d-enantiomer of nerinetide in the same rat model of embolic stroke led to robust reductions in infarct volume even when it was administered at the same time as tPA.
The results of these preclinical experiments seem to provide an explanation for the outcomes of the ESCAPE-NA1 trial. The findings suggest that nerinetide could have a beneficial effect in AIS if administered earlier than tPA and that the d-enantiomer should be considered for future clinical development. A new phase III clinical trial has begun with a similar design to the ESCAPE-NA1 trial but including only patients who did not receive tPA28. However, other possible explanations for the overall negative results of the ESCAPE-NA1 trial should not be discounted. For example, the infusion of nerinetide was initiated only shortly before thrombectomy, so the time interval might not have been sufficient for it to have a cytoprotective effect. In addition, no advanced imaging was performed to visualize the penumbra so the extent of penumbral tissue available for protection was unknown.
Nevertheless, the findings of ESCAPE-NA1 and the subsequent animal studies provide important lessons on the need to test interactions between thrombolytics and cytoprotective agents. Indeed, this trial is not the first to reveal such interactions. Erythropoietin was considered to be a promising cytoprotective agent (although not as well supported by preclinical studies as nerinetide) but did not improve outcomes in clinical trials and actually increased mortality29. Subsequent studies suggested that erythropoietin was beneficial in patients who did not receive tPA30 and that the combination of tPA and erythropoietin increases haemorrhagic transformation in animal models31.
Moving forward with clinical trials
Given the effectiveness of thrombectomy in some patients and the results of the ESCAPE-NA1 trial, cytoprotective agents could be applied in various clinical scenarios to extend the survival of penumbral tissue before definitive reperfusion therapy (Fig. 2). In the following sections, we discuss clinical trial settings and designs to evaluate cytoprotective therapies in various scenarios. Proof-of-concept phase II trials should involve more homogeneous patient populations than phase III pragmatic trials.
Fig. 2. Outcomes of acute ischaemic stroke with and without cytoprotective therapy and reperfusion.
The aim of cytoprotective therapy administered as early as possible after stroke onset, is to ‘freeze’ the penumbra (left) so that the infarct core does not grow further before definitive reperfusion therapy can be completed. This protection minimizes the size of the final infarct (bottom left). Without cytoprotective therapy, the final infarct size could be larger despite successful reperfusion (bottom right). Cytoprotective therapy can only be beneficial if reperfusion is achieved before the protective effects diminish. If reperfusion therapy is not administered within this time, the final infarct could be as large as it would have been without cytoprotection (bottom centre). Reperfusion injury can lead to haemorrhagic transformation regardless of whether cytoprotective therapy is used.
Bridge therapy before thrombectomy
The ESCAPE-NA1 trial6 provides a model for future clinical trials to evaluate cytoprotective agents in the context of transient ischaemia–reperfusion. The objective in ESCAPE-NA1 was to determine whether a purported anti-excitotoxic agent conferred additional benefits in combination with thrombectomy, a clinical scenario akin to the transient ischaemia–reperfusion that we commonly model in animals. The patients enrolled had moderate-to-good collateral blood flow, indicating high potential for salvageable brain tissue to persist for an extended time period and facilitating drug access to the ischaemic bed. The outcome was chosen on the basis of the trajectories of comparable patients who have undergone thrombectomy in recent RCTs. In considering the design of similar trials in future, some important aspects need to be considered.
One key question is the therapeutic window for administration of cytoprotective agents. In the ESCAPE-NA1 trial, nerinetide was administered up to 12 h after stroke onset as long as the Alberta Stroke Program Early CT Score (ASPECTS) was ≥5, which indicates that infarcts are not large. This wide treatment window enabled enrolment of substantial numbers of patients, including those with wake-up stroke. Another important factor is effect size — the number of patients needed to demonstrate a significant difference in the primary outcome measure. When determining the number of patients needed, several factors need to be taken into account: the predicted percentage of patients who will receive thrombolytics (which will depend on the treatment window chosen); the predicted reperfusion status after thrombectomy; and when the cytoprotective agent is administered relative to the thrombolytic agent. One potential problem with this type of trial is that door-to-groin puncture times have decreased at top stroke centres, leading to implementation of ‘direct to angiosuite’ protocols32. These ultrafast transfer protocols to the angiosuite reduce the feasibility of trials of cytoprotection at this stage, as enrolment into such trials could interfere with time-based performance metrics.
Cytoprotection during thrombectomy
Another possible clinical scenario is the administration of cytoprotective agents intravenously during the thrombectomy procedure. The infusion could even be continued afterwards if the protective agent targets a pathophysiological process that is ongoing after reperfusion or if it targets reperfusion injury, as discussed above. The goal of the cytoprotective therapy would be to add to the clinical benefit at 90 days derived from thrombectomy at 90 days. Trials of this approach are likely to require a substantial sample size because of the treatment effect of thrombectomy in the control group. In addition, depending on the anatomy and collateral circulation, thrombectomy also provides the opportunity for selective intra-arterial delivery of cytoprotective agents into the previously ischaemic arterial territory33. Intra-arterial delivery should first be studied in animal models of stroke to determine whether this route of administration is superior to intravenous delivery.
Pre-hospital cytoprotection
Treatment of patients as early as possible remains the most effective strategy to achieve an excellent outcome after stroke, so the pre-hospital setting seems to be the ideal location for administration of cytoprotective agents. For this reason, the Field Administration of Stroke Therapy–Magnesium (FAST-MAG) trial34 generated an enormous amount of enthusiasm. In this study, patients with suspected stroke were identified by emergency medical personnel in the field and randomly assigned to receive magnesium or placebo. However, unsurprisingly, extensive heterogeneity in ischaemic stroke type was seen among the enrolled patients, and the study population also included people with haemorrhagic stroke and stroke mimics. Other pre-hospital studies have produced similar findings, highlighting that reliable identification of patients who would benefit from cytoprotective treatment is a major challenge in the pre-hospital setting.
Clinical assessment scales can be used to predict large-vessel occlusion (LVO) and could be applied in the pre-hospital setting to selectively enrich the population with patients with LVO. However, each of these scales also has limitations and none has been validated as being far superior to others35,36. Stroke mimic scales have also been developed but have not yet been adopted widely in health-care systems and, to the best of our knowledge, none is used in the pre-hospital setting37. Beyond clinical scales, devices for mobile electroencephalography and measurement of somatosensory-evoked potentials are being developed to facilitate identification of LVOs, but we await studies on the use of these devices in the ambulance38.
Mobile stroke units
Mobile stroke units (MSUs) are transforming pre-hospital care for patients with suspected acute stroke and will provide new opportunities to design better pre-hospital clinical trials of cytoprotection. Access to a CT scanner in the mobile unit enables haemorrhagic stroke to be ruled out, a CT angiogram can confirm an LVO, and CT perfusion is also possible, although brain coverage is limited. These investigations could, therefore, enable selection of patients for cytoprotection trials on the basis of a favourable ASPECTS and a substantial clinical deficit.
MSU trials performed in Houston, Texas, in the USA39, and in Germany40 have demonstrated that initiating tPA in the MSU led to significantly better outcomes at 90 days than treatment initiated at the hospital. The results of these trials are likely to lead to proliferation of MSUs across the world, thereby substantially increasing the opportunities to conduct cytoprotection trials in future. However, given that thrombolysis can be administered in MSUs and reperfusion is the most effective treatment, possible interactions of cytoprotective agents with thrombolytics must be tested and taken into account so as not to delay the administration of thrombolytic agents or the transfer of patients with LVOs to centres for thrombectomy.
Interfacility transfer
On the surface, patients with an LVO who are at a community hospital and are waiting to be transferred to a centre for thrombectomy are excellent candidates for trials to test the hypothesis that cytoprotective agents can extend penumbral survival to improve outcomes after definitive reperfusion therapy41. In prospective studies, the median between arrival at a community hospital and arrival at a hospital with facilities for thrombectomy is 2–3 h42. Given that CT scanners are available in most community hospitals, this time could be used to identify patients with LVOs with CT angiography or on the basis of a high NIH Stroke Scale score and a favourable ASPECTS. Eligible patients could then be randomly assigned to receive a cytoprotective agent or placebo before and during the transfer. Where available, telemedicine connections with advanced stroke centres could be exploited to conduct hub–spoke clinical trials in which an investigator at the hub could enrol patients at the spoke community hospital. Previous work has shown the feasibility of using telemedicine to obtain consent and enrol patients with AIS into clinical trials of acute therapies43, and as a result of the COVID-19 pandemic, electronic consent is now used in many studies.
Despite the apparently ideal scenario described above, several challenges must be considered in the community setting. The rate of infarct progression during transfer is variable and highly dependent on clot location and the status of collateral blood flow44–46. Without at least a CT angiogram to confirm the location of the vascular occlusion and provide quantitative data on the status of collateral blood flow, the rate of infarct progression is difficult to predict. Availability and administration of thrombolytic therapy at the community hospital will also generate substantial variability in the progression of the ischaemic lesion. One retrospective study showed that in some patients with large-artery clots, thrombolysis leads to recanalization and damage from ischaemia is minimized, but in other patients thrombolysis does not lead to complete recanalization, so that infarcts are not minimized and grow substantially to the point that thrombectomy is precluded47. In a third population of patients, thrombolysis can lead to migration of the clot from a proximal to a distal location from which it cannot be retrieved with catheter-based approaches, meaning the patient is no longer eligible for thrombectomy47. Furthermore, some patients might experience substantial delays in transport, which adds to the variability, as does the duration of the cytoprotective effect when a cytoprotective agent is combined with thrombolytic treatment. All of these scenarios must be considered in the design of a clinical trial of a cytoprotective agent combined with thrombolytics.
Further studies are needed to better define which patients are likely to progress quickly or slowly and to understand which patients are most likely to benefit from cytoprotective drugs while in the community hospital awaiting transfer. Poor collateral blood flow could limit drug access to the ischaemic territory, thereby limiting the therapeutic effect48. By contrast, good collateral blood flow alone could effectively preserve ischaemic territory over several hours so that cytoprotective approaches offer only marginal benefits or benefits that are difficult to detect with conventional clinical measures.
Imaging for penumbral protection
In order to better design proof-of-concept and phase III clinical trials to test cytoprotective agents, inclusion of brain imaging in the selection criteria is important because advanced brain imaging can identify patients who are most likely to benefit from the drug being evaluated. Imaging enables identification of patients with severe deficits caused by a large-artery occlusion but who have good collateral blood flow and small infarcts, implying a large penumbra. Such patients were the target population in the ESCAPE-NA1 trial.
In the early time window (0–3 h after AIS onset), the use of CT and CT angiography for patient selection is logical. However, advanced CT and MRI perfusion sequences enable direct identification of potentially salvageable ischaemic brain tissue that is distinct from the infarcted core, especially beyond 3 h. These imaging approaches are moving the stroke field into an era in which decisions on reperfusion therapy can be based on brain tissue status rather than time. Use of standardized perfusion imaging sequences and algorithms to define the penumbra and infarct core in RCTs has proven that, in highly selected patients, thrombectomy is beneficial even up to 24 h after the patient was last known to be well49,50 and beyond50. Consequently, patients with identifiable salvageable tissue could benefit from cytoprotective agents regardless of the time from onset if the intention is to extend penumbral survival and prevent infarct progression before thrombectomy. On this basis, advanced imaging modalities should be used in initial proof-of-concept clinical trials of cytoprotective agents to ensure that the patients who are most likely to benefit from treatment are identified and those who are not are excluded.
Use of imaging to enrich patient populations with those who are most likely to benefit from cytoprotection therapy is most likely to succeed in tertiary centres. Application of imaging in trials to test the hypothesis that cytoprotectants can promote penumbral survival in patients with LVOs at community hospitals before transfer for thrombectomy requires several logistical and practical challenges to be overcome. CT perfusion is not available at most community hospitals51 but CT angiography is now possible at many, at least in the USA, so could be used to screen patients for endovascular therapies and to facilitate implementation of cytoprotection studies. Furthermore, approaches based on machine learning are being studied to determine whether the images acquired with CT angiography can be analysed to distinguish the penumbra from the infarct core52. If successful, this advance will expand the opportunities to identify patients who are good candidates for cytoprotection studies at community hospitals and substantially broaden the population who could benefit from bridging therapies that temporally stabilize the penumbra.
Lastly, portable MRI scanners are emerging and becoming available in clinical settings53. A low-field (0.064 T) portable MRI device can be brought to the bedside, for example in a neurocritical care unit or in the emergency department54. These portable MRI scanners have the potential to be used in MSUs. Though the available imaging sequences are currently limited in their brain coverage and the number of brain slices that can be acquired, the technology is likely to improve over time, just as stationary MRI scanners have been refined since the 1990s. Portable MRI devices in the emergency department and MSUs will enable direct measurement of infarcted tissue and have the potential to enable more direct assessment of the treatment effects of cytoprotective agents.
Reperfusion injury trials
Reperfusion therapy can promote BBB disruption and lead to haemorrhagic transformation, so cytoprotective agents could be beneficial to protect against this risk. First, in the pre-hospital setting, patients could be selected for prophylactic treatment on the basis of clinical rating scales and/or imaging of perfusion with CT or MRI that can predict the risk of haemorrhagic transformation after reperfusion therapy55. Predictive scales are based on variables such as clinical severity, infarct size, age, blood pressure and glucose levels, all of which are associated with the risk of haemorrhagic transformation. Second, clinical trials could be designed to test whether prophylactic treatment preserves BBB integrity during thrombectomy. Studies have shown that patients who undergo multiple catheter passes into the affected artery have a higher risk of BBB disruption56, so trials could test whether prophylactic treatment can protect against this effect. In both scenarios, cytoprotective agents that are intended to protect the BBB could also extend the therapeutic window for reperfusion therapy.
A third clinical scenario would be to use brain imaging immediately after thrombectomy to look for markers of BBB injury as predictors of haemorrhagic transformation (for example, extravasation of contrast material on CT or the hyperintense acute reperfusion marker on MRI) and design clinical trials to test whether vasculoprotective agents that repair the BBB reduce subsequent haemorrhagic injury. The optimal timing of administration for all of these approaches would need to be determined, and this optimization should follow preclinical studies that ensure optimal engagement of the target and rule out detrimental effects.
Immunoprotective therapies
Clinical trials of cytoprotective approaches that target immune responses to stroke typically involve patients who are beyond the hyperacute stage and need careful planning with respect to several important issues. For example, questions that need to be addressed are whether the treatment approach is compatible with reperfusion therapy, when treatment should begin in relation to reperfusion, and whether patients will benefit if they still have substantial penumbra or whether infarcted tissue drives the inflammatory response. Furthermore, the inflammatory response to stroke occurs in a highly orchestrated series of events that involve different types of cells from the peripheral circulation and the brain. Optimal timing for immunoprotective therapies therefore depends on engagement of the desired cellular targets according to their temporal emergence, functionalities and clearance from the brain. Optimal timing is needed to avoid potential detrimental effects. Another consideration for trials of immunoprotective therapy is that, while no specific imaging modalities enable monitoring of immunomodulator targets, MRI could be helpful for the selection of patients on the basis of infarct size and location. For example, in the MASTERS-2 phase III trial of bone marrow cell therapy 18–36 h after ischaemic stroke onset, patient selection is based on the presence of infarctions that involve the cortex on MRI21.
Conclusion
Despite the past failure of attempts to develop cytoprotective drugs to improve outcomes in AIS, developments in the past 5–10 years provide considerable hope that success is close. The development and widespread clinical application of thrombolysis and thrombectomy mean that reperfusion is routinely achievable. Consequently, we have entered an era in which cytoprotective therapies as an adjunct treatment could provide tangible clinical benefits by protecting salvageable tissue in advance of reperfusion therapy. In addition, technological and methodological advances have created conditions that are increasingly favourable for clinical application of cytoprotective therapy. We now have advanced imaging that enables identification of patients in whom the therapeutic target for cytoprotection — the ischaemic penumbra — is present. Trial designs have improved so that the likelihood of detecting treatment effects is higher. We also have better processes for preclinical modelling in animals that emphasize rigour, reproducibility and the use of multicentre studies, making for more efficient identification of promising drugs that should be taken forward to clinical trials. These factors are converging to create an environment that is more likely than ever to produce successful cytoprotective therapies for AIS.
Author contributions
The authors contributed equally to all aspects of the article.
Competing interests
M.F. serves on the data, safety monitoring board for the ESCAPE-Next trial. He also serves as a consultant to Simcere USA and Lumosa of Taiwan. As an employee of the institution (UTHealth), S.I.S. has served in the following roles: as a site investigator in clinical trials sponsored by industry companies (Athersys, ReNeuron, San Bio, KM Pharma, Abbvie) for which UTHealth receives payments on the basis of clinical trial contracts; as an investigator on clinical trials supported by NIH grants, the Department of Defense, Let’s Cure CP, the TIRR Foundation, and the Cord Blood Registry Systems; as a principal investigator or co-investigator on NIH-funded grants in basic science and clinical research; and as principal investigator for an imaging analysis center for clinical trials sponsored by SanBio and ReNeuron. In his capacity as a UTHealth employee, Dr. Savitz has provided consulting services on behalf of UTHealth to ReNeuron, Lumosa, Deck Therapeutics, KM Pharma, Neurexcell, Abbvie, TMC Biodesign and ArunA. All compensation from such consulting arrangements have been paid to UTHealth.
Footnotes
Peer review information
Nature Reviews Neurology thanks M. Yenari and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savitz SI, Baron JC, Fisher M. Stroke Treatment Academic Industry Roundtable X: brain cytoprotection therapies in the reperfusion era. Stroke. 2019;50:1026–1031. doi: 10.1161/STROKEAHA.118.023927. [DOI] [PubMed] [Google Scholar]
- 3.Lyden P, Buchan A, Boltze J, Fisher M. Top priorities for cerebroprotective studies–a paradigm shift: report from STAIR XI. Stroke. 2021;52:3063–3071. doi: 10.1161/STROKEAHA.121.034947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savitz SI, Fisher M. Future of neuroprotection for acute stroke: in the aftermath of the SAINT trials. Ann. Neurol. 2007;61:396–402. doi: 10.1002/ana.21127. [DOI] [PubMed] [Google Scholar]
- 5.Tymianski M. Combining neuroprotection with endovascular treatment of acute stroke: is there hope? Stroke. 2017;48:1700–1705. doi: 10.1161/STROKEAHA.117.017040. [DOI] [PubMed] [Google Scholar]
- 6.Hill MD, et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet. 2020;395:878–887. doi: 10.1016/S0140-6736(20)30258-0. [DOI] [PubMed] [Google Scholar]
- 7.Xing C, Arai K, Lo EH, Hommel M. Pathophysiologic cascades in ischemic stroke. Int. J. Stroke. 2012;7:378–385. doi: 10.1111/j.1747-4949.2012.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffin JH, Fernandez JA, Lyden PD, Zlokovic BV. Activated protein C promotes neuroprotection: mechanisms and translation to the clinic. Thromb. Res. 2016;141:S62–S64. doi: 10.1016/S0049-3848(16)30368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui H, et al. PDZ protein interactions underlying NMDA receptor-mediated excitotoxicity and neuroprotection by PSD-95 inhibitors. J. Neurosci. 2007;2:9901–9915. doi: 10.1523/JNEUROSCI.1464-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyden P, et al. Results of the ICTuS 2 trial (Intravascular Cooling in the Treatment of Stroke 2) Stroke. 2016;47:2888–2895. doi: 10.1161/STROKEAHA.116.014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai J, Lyden PD. Revisiting cerebral postischemic reperfusion injury: new insights in understanding reperfusion failure, hemorrhage, and edema. Int. J. Stroke. 2015;10:143–152. doi: 10.1111/ijs.12434. [DOI] [PubMed] [Google Scholar]
- 12.Jung JE, et al. Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Mol. Neurobiol. 2010;41:172–179. doi: 10.1007/s12035-010-8102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J. Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernardo-Castro S, et al. Pathophysiology of blood–brain barrier permeability throughout the different stages of ischemic stroke and its implication on hemorrhagic transformation and recovery. Front. Neurol. 2020;11:594672. doi: 10.3389/fneur.2020.594672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y, Rosenberg GA. Matrix metalloproteinases as therapeutic targets for stroke. Brain Res. 2015;1623:30–38. doi: 10.1016/j.brainres.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molina CA, et al. Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke. 2001;32:1079–1084. doi: 10.1161/01.str.32.5.1079. [DOI] [PubMed] [Google Scholar]
- 17.Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol. 2011;10:909–921. doi: 10.1016/S1474-4422(11)70195-8. [DOI] [PubMed] [Google Scholar]
- 18.Bornstein NM, et al. An injectable implant to stimulate the sphenopalatine ganglion for treatment of acute ischaemic stroke up to 24h from onset (ImpACT-24B): an international, randomised, double-blind, sham-controlled, pivotal trial. Lancet. 2019;394:219–229. doi: 10.1016/S0140-6736(19)31192-4. [DOI] [PubMed] [Google Scholar]
- 19.Elkind MSV, et al. Natalizumab in acute ischemic stroke (ACTION II): a randomized, placebo-controlled trial. Neurology. 2020;95:e1091–e1104. doi: 10.1212/WNL.0000000000010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poungvarin N, et al. Effects of dexamethasone in primary supratentorial intracerebral hemorrhage. N. Engl. J. Med. 1987;316:1229–1233. doi: 10.1056/NEJM198705143162001. [DOI] [PubMed] [Google Scholar]
- 21.Mays RW, Savitz SI. Intravenous cellular therapies for acute ischemic stroke. Stroke. 2018;49:1058–1065. doi: 10.1161/STROKEAHA.118.018287. [DOI] [PubMed] [Google Scholar]
- 22.Henninger N, Kumar R, Fisher M. Acute ischemic stroke therapy. Expert. Rev. Cardiovasc. Ther. 2010;8:1389–1398. doi: 10.1586/erc.10.128. [DOI] [PubMed] [Google Scholar]
- 23.Bosetti F, et al. Translational stroke research: vision and opportunities. Stroke. 2017;48:2632–2637. doi: 10.1161/STROKEAHA.117.017112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher M, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dirnagl U, et al. A concerted appeal for international cooperation in preclinical stroke research. Stroke. 2013;44:1754–1760. doi: 10.1161/STROKEAHA.113.000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill MD, et al. Safety and efficacy of NA-1 in patients with iatrogenic stroke after endovascular aneurysm repair (ENACT): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2012;11:942–950. doi: 10.1016/S1474-4422(12)70225-9. [DOI] [PubMed] [Google Scholar]
- 27.Mayor-Nunez D, et al. Plasmin-resistant PSD-95 inhibitors resolve effect-modifying drug–drug interactions between alteplase and nerinetide in acute stroke. Sci. Transl. Med. 2021;13(588):eabb1498. doi: 10.1126/scitranslmed.abb1498. [DOI] [PubMed] [Google Scholar]
- 28.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04462536 (2020).
- 29.Ehrenreich H, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40:e647–e656. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- 30.Worthmann H, et al. Asymmetric dimethylarginine in response to recombinant tissue-type plasminogen activator and erythropoietin in acute stroke. Stroke. 2013;44:2128–2133. doi: 10.1161/STROKEAHA.113.001145. [DOI] [PubMed] [Google Scholar]
- 31.Jia L, Chopp M, Zhang L, Lu M, Zhang Z. Erythropoietin in combination of tissue plasminogen activator exacerbates brain hemorrhage when treatment is initiated 6h after stroke. Stroke. 2010;41:2071–2076. doi: 10.1161/STROKEAHA.110.586198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riou-Comte N, et al. Direct transfer to angiosuite for patients with severe acute stroke treated with thrombectomy: the multicentre randomised controlled DIRECT ANGIO trial protocol. BMJ Open. 2021;11:e040522. doi: 10.1136/bmjopen-2020-040522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Link TW, Santillan A, Patsalides A. Intra-arterial neuroprotective therapy as an adjunct to endovascular intervention in acute ischemic stroke: a review of the literature and future directions. Interv. Neuroradiol. 2020;26:405–415. doi: 10.1177/1591019920925677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saver JL, et al. Prehospital use of magnesium sulfate as neuroprotection in acute stroke. N. Engl. J. Med. 2015;372:528–536. doi: 10.1056/NEJMoa1408827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vidale S, Agostoni E. Prehospital stroke scales and large vessel occlusion: a systematic review. Acta Neurol. Scand. 2018;138:24–31. doi: 10.1111/ane.12908. [DOI] [PubMed] [Google Scholar]
- 36.Smith EE, et al. Accuracy of prediction instruments for diagnosing large vessel occlusion in individuals with suspected stroke: a systematic review for the 2018 guidelines for the early management of patients with acute ischemic stroke. Stroke. 2018;49:e111–e122. doi: 10.1161/STR.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 37.Goyal N, et al. FABS: an intuitive tool for screening of stroke mimics in the emergency department. Stroke. 2016;47:2216–2220. doi: 10.1161/STROKEAHA.116.013842. [DOI] [PubMed] [Google Scholar]
- 38.Sergot PB, et al. Portable neuromonitoring device detects large vessel occlusion in suspected acute ischemic stroke. Stroke. 2021;52:1437–1440. doi: 10.1161/STROKEAHA.120.031225. [DOI] [PubMed] [Google Scholar]
- 39.Grotta JC, et al. Prospective, multicenter, controlled trial of mobile stroke units. N. Engl. J. Med. 2021;385:971–981. doi: 10.1056/NEJMoa2103879. [DOI] [PubMed] [Google Scholar]
- 40.Ebinger M, et al. Association between dispatch of mobile stroke units and functional outcomes among patients with acute ischemic stroke in Berlin. JAMA. 2021;325:454–466. doi: 10.1001/jama.2020.26345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savitz SI, Baron JC, Yenari MA, Sanossian N, Fisher M. Reconsidering neuroprotection in the reperfusion era. Stroke. 2017;48:3413–3419. doi: 10.1161/STROKEAHA.117.017283. [DOI] [PubMed] [Google Scholar]
- 42.Wu TC, et al. IAT-TiMeS: intra-arterial thrombectomy transfer metric study in Texas. J. Stroke Cerebrovasc. Dis. 2021;30:105602. doi: 10.1016/j.jstrokecerebrovasdis.2021.105602. [DOI] [PubMed] [Google Scholar]
- 43.Wu TC, et al. Telemedicine-guided remote enrollment of patients into an acute stroke trial. Ann. Clin. Transl. Neurol. 2015;2:38–42. doi: 10.1002/acn3.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reddy ST, et al. Rapid infarct progression in anterior circulation large vessel occlusion ischemic stroke patients during inter-facility transfer. J. Stroke Cerebrovasc. Dis. 2020;29:105308. doi: 10.1016/j.jstrokecerebrovasdis.2020.105308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boulouis G, et al. Clinical imaging factors associated with infarct progression in patients with ischemic stroke during transfer for mechanical thrombectomy. JAMA Neurol. 2017;74:1361–1367. doi: 10.1001/jamaneurol.2017.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ospel JM, et al. Which acute ischemic stroke patients are fast progressors? Results from the ESCAPE trial control arm. Stroke. 2021;52:1847–1850. doi: 10.1161/STROKEAHA.120.032950. [DOI] [PubMed] [Google Scholar]
- 47.Reddy ST, et al. Patients transferred within a telestroke network for large-vessel occlusion. J. Telemed. Telecare. 2020 doi: 10.1177/1357633X20957894. [DOI] [PubMed] [Google Scholar]
- 48.Liebeskind DS. Neuroprotection from a collateral perspective. IDrugs. 2005;8:222–228. [PubMed] [Google Scholar]
- 49.Albers GW, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N. Engl. J. Med. 2018;378:708–718. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nogueira RG, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N. Engl. J. Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 51.Kim Y, et al. Utilization and availability of advanced imaging in patients with acute ischemic stroke. Circ. Cardiovasc. Qual. Outcomes. 2021;14:e006989. doi: 10.1161/CIRCOUTCOMES.120.006989. [DOI] [PubMed] [Google Scholar]
- 52.Sheth SA, et al. Machine learning-enabled automated determination of acute ischemic core from computed tomography angiography. Stroke. 2019;50:3093–3100. doi: 10.1161/STROKEAHA.119.026189. [DOI] [PubMed] [Google Scholar]
- 53.Cahn L, et al. Deployment of portable, bedside, low-field magnetic resonance imaging for evaluation of stroke patients [abstract] Neurology. 2020;94(15 Suppl.):272. [Google Scholar]
- 54.Sheth KN, et al. Assessment of brain injury using portable, low-field magnetic resonance imaging at the bedside of critically ill patients. JAMA Neurol. 2021;78:41–47. doi: 10.1001/jamaneurol.2020.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu G, He Q, Shen Y, Cao F. Potential biomarkers for predicting hemorrhagic transformation of ischemic stroke. Int. J. Neurosci. 2018;128:79–89. doi: 10.1080/00207454.2017.1349766. [DOI] [PubMed] [Google Scholar]
- 56.Luby M, et al. Frequency of blood–brain barrier disruption post-endovascular therapy and multiple thrombectomy passes in acute ischemic stroke patients. Stroke. 2019;50:2241–2244. doi: 10.1161/STROKEAHA.119.025914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stroke Preclinical Assessment Network. Interventionshttps://spannetwork.org/Interventions (2021).