Abstract
Background
Antimicrobial resistance (AMR) has become an emerging threat worldwide, and developing countries such as Bangladesh are considered to be at greater risk of disseminating the resistant bacteria between human–animal interfaces.
Objectives
The present study was carried out to determine the prevalence and AMR profile of Escherichia coli isolated from broiler chickens, the environment, and farmworkers. This study also aimed to identify the risk factors associated with multidrug‐resistant (MDR) E. coli infection in broiler chickens. In addition, the presence of carbapenem resistance gene (NDM‐1) was assessed.
Methods
A total of 114 E. coli isolates, recovered from 150 samples (cloacal swabs = 50, farm sewage = 50, and hand washed water of farmworkers = 50) collected from 50 broiler farms, were identified by biochemical examination and polymerase chain reaction (PCR) assay. Antimicrobial susceptibility test was performed for 10 antibiotics by disk diffusion test. Carbapenem resistance gene (NDM‐1) was detected by PCR. Risk factors were identified through multivariable logistic regression.
Results
The highest prevalence of E. coli was recorded in broiler chickens (86%) and the lowest in farmworkers (66%). For MDR E. coli infection, ‘winter season’, ‘absence of specific shoes for staff’, and ‘use of antibiotics without veterinarian's prescription’ were the significant risk factors. High resistance of the E. coli isolates was observed against levofloxacin (81.6%), doxycycline (78.1%), cefotaxime (78.1%), and ciprofloxacin (70.2%). About 76% of the isolates demonstrated MDR. None of the isolates were positive for the NDM‐1 gene.
Conclusions
The high level and similar pattern of antibiotic resistance in E. coli isolates from broiler chickens, farmworkers, and sewage in poultry farms indicates a good possibility of spreading the antibiotic‐resistant E. coli in such settings.
Keywords: antimicrobial resistance, Bangladesh, broiler chickens, E. coli, multidrug resistance
Winter season, absence of specific shoes for staff, and use of antibiotics without veterinarian's prescription were the significant risk factors of MDR‐E. coli infection in broiler chickens.
A very high resistance of the E. coli isolates against levofloxacin (81.6%), doxycycline (78.1%), cefotaxime (78.1%), and ciprofloxacin (70.2%) was observed.
Similar pattern of resistance phenotype of E. coli was observed among broiler chickens, farm workers, and farm sewage, and about 76% of the isolates demonstrated multidrug resistance.

1. INTRODUCTION
Unquestionably, antibiotics have become the most important solution to many infectious diseases; however, the recent emergence of antimicrobial resistance (AMR) both in the field of human and veterinary medicine has become a significant public health concern worldwide (Ferri et al., 2017; Palma et al., 2020). Exaggerated use of antibiotics in the production facilities of food animals not only for therapeutic purposes but also for growth promotion or prophylaxis purposes is thought to be the crucial factor behind this (Agyare et al., 2019). When bacteria in the guts of animals are exposed to various antimicrobial agents with sub‐therapeutic concentrations and frequencies, they acquire resistance to the antimicrobial agents that have been used through selective pressure (Scott et al., 2002). In intensively reared food animals such as poultry, where antibiotics are often administered as growth promoters to whole flocks, the antibiotic selection pressure in bacteria is high resulting in a high concentration of resistant bacteria in their faecal flora (Marshall & Levy, 2011). Being an essential part of the endogenous microflora, Escherichia coli can easily gain resistance against antimicrobials that are consumed by poultry birds (Hussain et al., 2017). Moreover, the potentiality of E. coli to transfer antibiotic resistance determinants to its other strains as well as different bacteria is well known (Rasheed et al., 2014).
In Bangladesh, poultry farming is expanding day by day and the sector contributes 14% of the total value of livestock output (Hamid et al., 2017). About 37% of the total meat production comes from this industry (Hamid et al., 2017). With the considerable expansion of poultry farming, farmers are more inclined to use antibiotics at sub‐therapeutic doses for growth promotion and infection prevention (K. S. Islam et al., 2016). This tendency is worsening the scene of AMR by increasing the selection of resistant bacteria (Ayukekbong et al., 2017).
Food animals, especially poultry as well as poultry houses, serve as an important reservoir of E. coli and thus a potential source of human infection by its pathogenic strain (Stromberg et al., 2017). Antimicrobial‐resistant E. coli can be transmitted from poultry to humans directly or via food. These resistant bacteria may cause colonization in the human gastrointestinal tract and may also contribute resistance genes to human endogenous microflora. Several studies reported the spread of antimicrobial‐resistant E. coli from chickens to humans in various countries (Amir et al., 2019; Norizuki et al., 2017). The likelihood of transmission of antimicrobial‐resistant E. coli among humans, animals, and the environment is a crucial threat to public health. Therefore, more focus should be given to the people who are occupied in poultry farming to reduce the risk of transmission of AMR.
In Bangladesh, variable prevalence (61.67%–82%) and high rate of AMR of E. coli in cloacal swab of broiler chickens have been reported (Akond et al., 2009; Hossain et al., 2008; Jakaria et al., 2012; Sarker et al., 2019). But there is a scarcity of studies identifying the risk factors for multidrug‐resistant (MDR) E. coli infection in broiler chickens. Furthermore, reports on the AMR pattern of E. coli in one health setting (poultry farm–workers–farm sewage) in Bangladesh are lacking. Notably, at present, the emergence and global spread of carbapenem‐resistant Enterobacteriaceae (CRE) is considered a major public health threat (Hansen, 2021; Jean et al., 2015). Among different carbapenem resistance genes, blaNDM‐1, blaOXA‐1, and blaOXA‐47 have been detected in different bacteria isolated from environmental samples, household water supply, and hospital samples in Bangladesh (Begum & Shamsuzzaman, 2016; M. Islam et al., 2012; Talukdar et al., 2013; Toleman et al., 2015). However, the presence of these genes particularly blaNDM‐1 in commercial poultry farm settings is yet to be investigated. Therefore, a cross‐sectional study was conducted in broiler farms in two selected districts (Mymensingh and Gazipur) of Bangladesh with the aim to determine the prevalence of E. coli and assess their antimicrobial resistance profile in broiler chickens, farm sewage, and farmworkers. Risk factors associated with MDR E. coli infections in broiler chickens were also identified. Additionally, the presence of carbapenem resistance New Delhi metallo β‐lactamase‐1 (NDM‐1) gene was assessed.
2. MATERIALS AND METHODS
2.1. Study area and design
A cross‐sectional study was conducted in commercial broiler farms in Mymensingh and Gazipur districts from December 2018 to April 2019. To get representative data, five upazilas from each district and five broiler farms from each upazila were randomly selected. The selected Upazilas were Fulbaria, Trishal, Ishwarganj, Muktagacha, and Sadar of Mymensingh, and Kapasia, Kaliganj, Kaliakoir, Sreepur, and Sadar of Gazipur district.
2.2. Sample collection
Cloacal swabs were collected from 10 birds in each farm and pooled to make one sample. Additionally, farm sewage (n = 50) and hand washed water of farmworkers (n = 50) were collected from the respective farms. While collecting the cloacal swab, cloacae of broiler chickens were moistened with alcohol dipped cotton and then swabs were collected using sterile swab sticks with 1 ml Buffered Peptone Water (BPW). Farm sewage samples were collected into a sterile falcon tube following aseptic measures. To obtain the hand washed water samples, hands of the workers were washed with 100 ml of sterile distilled water and collected into sterile falcon tube and sealed. Then, the samples were transported in a cool box to the laboratory within no more than 2 h of collection.
2.3. Definition of human traffic control system
Based on the presence of foot‐bath, a fence around the farm, gate, and restriction in the entry, the human traffic control system was graded as good, moderate, and poor. The presence of each item was scored as 1, and the total score was 4. A good human traffic control system was considered when the score was 4, moderate when 3, and poor when <3 score.
2.4. Data collection
A structured questionnaire was developed using KoboCollect (mobile data collection app) and pre‐tested prior to collecting data from the farms. Demographic data of the farmers, flock data along with data on antibiotic usage were collected through face‐to‐face interviews of the farmers, and geo‐location of the farm was also recorded. Farmers who have participated voluntarily were included in this study and written consent was obtained from them before collecting the data.
2.5. E. coli isolation and identification
E. coli was isolated and identified based on standard bacteriological procedure (ISO, 2001). First, samples were pre‐enriched with 1 ml sterile BPW, then transferred into nutrient broth (NB), and incubated at 37°C for 24 h. The culture was then streaked onto MacConkey agar and incubated at 37°C for 24 h. Three presumptive E. coli colonies were then sub‐cultured to obtain pure culture, and identification was performed using Gram staining and biochemical tests including catalase, oxidase, indole, methyl red, Voges‐Proskauer test, and sugar fermentation test using triple sugar iron agar. Biochemically confirmed isolates were then subjected to PCR assay by using malB promoter gene‐specific primers as described earlier (Wang et al., 1996). Bacterial DNA was extracted by the boiling method as described earlier (Dashti et al., 2009). Extracted DNA was measured using nanodrop spectrometer (NanoDrop One; Thermo Fisher Scientific, USA) and more than 100 ng/μl concentration of DNA was maintained to use for PCR assay. The nucleotide primer sequences were ECO‐1 forward: 5′‐GAC CTC GGT TTA GTT CAC AGA‐3′ and ECO‐2 reverse: 5′‐ CAC ACG CTG ACG CTG ACC A‐3′. Amplification was done in a 25 μl reaction volume containing 12.5 μl of master mix (Biolabs, USA), 0.5 μl (50 pmol) forward, 0.5 μl (pmol) reverse primer, 11 μl nuclease‐free water (Life Technologies, USA), and 0.5 μl DNA template in Veriti 96‐Well Thermal Cycler (Thermo Fisher Scientific Inc., USA). The PCR conditions were initial denaturation at 95°C for 5 min, followed by 35 cycles of denaturation at 94°C for 1 min, annealing at 58°C for 1 min, extension at 72°C for 1 min and final extension at 72°C for 7 min. PCR amplified products were subjected to gel electrophoresis (1.5% agarose) with ethidium bromide fluorescence, and visualized by using ultraviolet (UV) transilluminator.
2.6. Antimicrobial susceptibility testing
Antimicrobial susceptibility of E. coli was performed by the disk diffusion test on 10 antimicrobials belonging to six different classes. In brief, after adjusting the turbidity of bacterial suspension equivalent to 0.5 McFarland standard, 150 μl test suspension was inoculated on to Mueller–Hinton agar plates, and then antibiotic disks were placed and incubated at 37°C for 18–24 h. Antibiotic disks, procured from Biomaxima (Poland) and Oxoid (UK), were tetracyclines: doxycycline (DO, 30 μg), polymyxins: colistin (CT, 10 μg), penicillin β‐lactamase inhibitors: amoxyclav (AMC, 30 μg), fluoroquinolones: ciprofloxacin (CIP, 5 μg), levofloxacin (LEV, 5 μg), cephalosporins: ceftazidime (CAZ, 30 μg), ceftriaxone (CRO, 30 μg), cefotaxime (CTX, 30 μg), carbapenems: imipenem (IPM, 10 μg) and meropenem (MEM, 10 μg). The results of the antimicrobial susceptibility test were interpreted according to the guidelines of Clinical and Laboratory Standards Institute (Clinical and Laboratory Standards Institute, 2018). MDR isolates were defined as per the guidelines proposed by Magiorakos et al. (2012) with some modifications. Magiorakos et al. (2012) used the term ‘non‐susceptible’ to denote ‘resistant’ combining both resistant and intermediate isolates. Here, we have considered only the resistant isolates excluding the ‘intermediate’ ones. Thus, isolates resistant to at least 1 antimicrobial agent in ≥3 antimicrobial classes were classified as MDR.
2.7. Detection of NDM‐1 gene
The carbapenem resistance NDM‐1 gene was detected by PCR using specific primers as described earlier (M. Islam et al., 2012). The primer sequences were NDM‐1 forward: 5′‐CTT CCA ACG GTT TGA TCG TC‐3′ and NDM‐1 reverse: 5′‐TAG TGC TCA GTG TCG GCA TC‐3′ with fragment size of 465 bp. The PCR was run with reaction volume of 25 μl containing 12.5 μl of master mix, 0.5 μl (10 pmol) forward, 0.5 μl (10 pmol) reverse primer and 11 μl nuclease‐free water and 0.5 μl DNA templates. The thermal conditions were initial denaturation at 95°C for 7 min, followed by 30 cycles of denaturation at 95°C for 1 min, annealing at 55°C for 1 min, extension at 68°C for 1 min and final extension at 68°C for 7 min. The PCR products were then run on 1.5% agarose gel as described elsewhere.
2.8. Statistical analysis
The SPSS version 22.0 software was used for the statistical analysis. Descriptive analysis was performed to calculate the prevalence of E. coli and resistance percentages. Any significant differences in the prevalence of E. coli and their resistance percentages among different types of samples were analyzed by chi‐square test (Z‐test for proportions) and Fisher's exact test (wherever appropriate). Risk factors associated with MDR E. coli infection in broiler chickens were identified through univariable and multivariable logistic regression. Variables bearing p‐values less than 0.3 in the univariable analysis were included in the multivariable logistic regression (backward selection) analysis, and the Hosmer–Lemeshow test was performed to assess the fit of the final model.
3. RESULTS
3.1. Biosecurity and management practices
The findings of the questionnaire survey revealed that none of the farms were registered with the Department of Livestock Services, Bangladesh. The frequency of farmers with more than 5 years of experience in poultry farming was higher in Gazipur district compared with Mymensingh district. Most of the farms had a flock size of ≤2000 in both the districts (Table 1).
TABLE 1.
Demographic and flock health management information of 50 broiler chicken farms in Mymensingh and Gazipur districts
| Districts | ||||
|---|---|---|---|---|
| Variables | Mymensingh, number of farms (%) (N = 25) | Gazipur, number of farms (%) (N = 25) | Total (N = 50) | |
| Registered | 0 | 0 | 0 | |
| Farming experience (years) | ≤5 | 19 (76) | 13 (52) | 32 (64) |
| >5 | 6 (24) | 12 (48) | 18 (36) | |
| Flock size (number of birds) | ≤2000 | 23 (92) | 21 (84) | 44 (88) |
| >2000 | 2 (8) | 4 (16) | 6 (12) | |
| Other poultry house within 500 m | Yes | 15 (60) | 19 (76) | 34 (68) |
| No | 10 (40) | 6 (24) | 16 (32) | |
| Using of farm premises | Only for poultry farming | 20 (80) | 19 (76) | 39 (78) |
| Integrated with other farming | 5 (20) | 6 (24) | 11 (22) | |
| Human traffic control system † | Poor (<3) | 18 (72) | 12 (48) | 30 (60) |
| Moderate (≥3) | 7 (28) | 13 (52) | 20 (40) | |
| Use of water sanitizer in drinking water | 0 | 9 (36) | 9 (18) | |
| Disposal of litter | Compost | 3 (12) | 2 (8) | 5 (10) |
| Sold as manure | 15 (60) | 20 (80) | 35 (70) | |
| Throw into nearby pit | 9 (36) | 9 (36) | 18 (36) | |
| To biogas plant | 0 | 3 (12) | 3 (6) | |
| Separation of sick birds | 24 (96) | 24 (96) | 48 (96) | |
| Management of dead bird | Burial | 24 (96) | 22 (88) | 46 (92) |
| Thrown away | 3 (12) | 7 (28) | 10 (20) | |
| To garbage bin | 0 | 3 (12) | 3 (6) | |
| Cleaning of feeder | Daily | 8 (32) | 6 (24) | 14 (28) |
| Alternate day | 16 (64) | 13 (52) | 29 (58) | |
| Once a week | 1 (4) | 0 | 1 (2) | |
| Twice a week | 0 | 6 (24) | 6 (12) | |
| Cleaning of waterer | Daily | 19 (76) | 21 (84) | 40 (80) |
| Alternate day | 5 (20) | 2 (8) | 7 (14) | |
| Once a week | 1 (4) | 0 | 1 (2) | |
| Twice a week | 0 | 2 (8) | 2 (4) | |
| Waste management | Compost pit | 0 | 1 (4) | 1 (2) |
| Pond | 16 (64) | 7 (28) | 23 (46) | |
| Drainage system | 9 (36) | 17 (68) | 26 (52) | |
| Use of antibiotics | 23 (92) | 23 (92) | 46 (92) | |
| Purpose of antibiotics use | Therapeutic | 15 (65.2) | 8 (34.8) | 23 (46) |
| Preventive and therapeutic | 6 (26) | 6 (26) | 12 (24) | |
| Therapeutic and growth promoter | 1 (4.34) | 2 (8.7) | 3 (6) | |
| Preventive, therapeutic, and growth promoter | 1 (4.34) | 7 (30.4) | 8 (16) | |
| By whom suggestion antibiotics were used | Company representatives | 7 (28) | 12 (34.3) | 19 (38) |
| Experienced farmer | 0 | 2 (5.7) | 2 (4) | |
| Feed dealer | 9 (36) | 19 (54.3) | 28 (56) | |
| Self | 4 (16) | 0 | 4 (8) | |
| Veterinarian | 5 (20) | 2 (5.7) | 7 (14) | |
Note: N = number of farms.
Total score = 4 (foot‐bath: 1, fence around the farm: 1, Gate: 1, and entry restricted: 1).
Most of the farmers sold the litter materials as manure. The majority of the farmers (96%) responded that they separate the sick birds from the healthy birds and bury the dead birds. Almost all the farmers used to clean waterer and feeder daily or every alternate day. The wastes produced in the farms were found to be evacuated into the drain (52%) or directly into the adjacent ponds (46%). Regarding the use of antibiotics, four farmers claimed that they did not use any antibiotics on their farms. Around half of the farmers used antibiotics for therapeutic purposes. The highest percentage of farmers used antibiotics according to the suggestion of feed dealers (56%), followed by company representatives (38%), while only 14% of farmers followed the prescription of veterinarians. However, the frequency of farmers who follow the prescription of veterinarians was comparatively higher in the Mymensingh district (20%) compared with the Gazipur district (5.7%) (Table 1). Ciprofloxacin (34%) was the highest used antibiotic, followed by amoxycillin (30%) and colistin (26%) in the farms (Figure 1).
FIGURE 1.
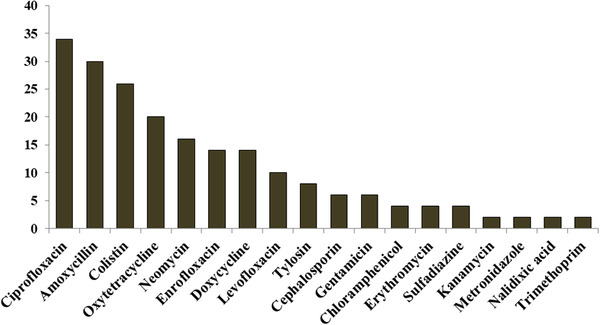
Most commonly used antibiotics in the broiler farms
3.2. Prevalence and distribution of E. coli
Of the 150 samples examined, 114 isolates of E. coli were recovered, and all the isolates were confirmed positive in PCR assay as the target amplicon size of 585 bp was observed. Thus, the overall prevalence of E. coli was 76% (Table 2). Roughly similar prevalence was observed in both the study districts (78.7% and 73.3% in Gazipur and Mymensingh, respectively). Among the three types of samples, the highest prevalence was observed in cloacal swab samples (86%) and the lowest in hand washed water samples (66%). Significant (p < 0.05) difference was observed in the prevalence of E. coli between these two types of samples (Table 2).
TABLE 2.
District and sample type wise prevalence of E. coli
| Variables | Total number of samples | Number of samples positive | Prevalence (%) |
|---|---|---|---|
| Districts | |||
| Mymensingh | 75 | 55 | 73.3* |
| Gazipur | 75 | 59 | 78.7* |
| Total | 150 | 114 | 76 |
| Sample type | |||
| Cloacal swab | 50 | 43 | 86* |
| Farm sewage | 50 | 38 | 76*,** |
| Hand washed water | 50 | 33 | 66** |
| Total | 150 | 114 | 76 |
*,**Values with different superscripts within the same column differ significantly (p < 0.05).
3.3. Antibiotic resistance profile of E. coli
The antimicrobial susceptibility test revealed that the highest resistance was observed in case of levofloxacin (81.6%), cefotaxime (78.1%), and doxycycline (78.1%), followed by ciprofloxacin (70.2%) (Table 3). The lowest percentage of resistance was found in ceftazidime (1.8%) along with ceftriaxone (7.9%) and colistin (14.9%). Notably, imipenem (65.8%) and meropenem (50.9%) belonging to carbapenem have shown significant resistance considering the fact that they are not used in poultry practices in Bangladesh. Resistance percentage based on sample types is illustrated in Figure 2. Almost a similar percentage of resistance was observed among the three types of samples in case of levofloxacin, doxycycline, cefotaxime, colistin and ceftriaxone. However, farm sewage isolates showed a considerably high resistance percentage to amoxyclav and imipenem than the other two types of isolates.
TABLE 3.
Antibiotic resistance profile of E. coli isolates to 10 antibiotics
| Cloacal swab (N = 43) | Farm sewage (N = 38) | Hand washed water (N = 33) | Total (N = 114) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotic name | R (%) | I (%) | S (%) | R (%) | I (%) | S (%) | R (%) | I (%) | S (%) | R (%) | I (%) | S (%) |
| Levofloxacin | 83.7 | 14 | 2.3 | 78.9 | 2.6 | 18.4 | 81.8 | 6.1 | 12.1 | 81.6 | 7.9 | 10.5 |
| Ciprofloxacin | 65.1 | 25.6 | 9.3 | 73.7 | 5.3 | 21.1 | 72.7 | 12.1 | 15.2 | 70.2 | 14.9 | 14.9 |
| Ceftazidime | 4.7 | 0 | 95.3 | 0 | 7.9 | 92.1 | 0 | 3 | 97 | 1.8 | 3.5 | 94.7 |
| Ceftriaxone | 7 | 20.9 | 72.1 | 10.5 | 13.2 | 76.3 | 6.1 | 15.2 | 78.8 | 7.9 | 16.7 | 75.4 |
| Cefotaxime | 74.4 | 9.3 | 16.3 | 81.6 | 10.5 | 7.9 | 78.8 | 9.1 | 12.1 | 78.1 | 9.6 | 12.3 |
| Amoxyclav | 11.6 | 32.6 | 55.8 | 39.5 | 39.5 | 21.1 | 9.1 | 57.6 | 33.3 | 20.2 | 42.1 | 37.7 |
| Colistin | 16.3 | 0 | 83.7 | 13.2 | 0 | 86.8 | 15.2 | 0 | 84.8 | 14.9 | 0 | 85.1 |
| Doxycycline | 79.1 | 18.6 | 2.3 | 76.3 | 13.2 | 10.5 | 78.8 | 21.2 | 0 | 78.1 | 17.5 | 4.4 |
| Imipenem | 62.8 | 25.6 | 11.6 | 73.7 | 10.5 | 15.8 | 60.6 | 21.2 | 18.2 | 65.8 | 19.3 | 14.9 |
| Meropenem | 46.5 | 14 | 39.5 | 52.6 | 13.2 | 34.2 | 54.5 | 6.1 | 39.4 | 50.9 | 11.4 | 37.7 |
Abbreviations: I, intermediate; N, number of isolates; R, resistant; S, susceptible.
FIGURE 2.
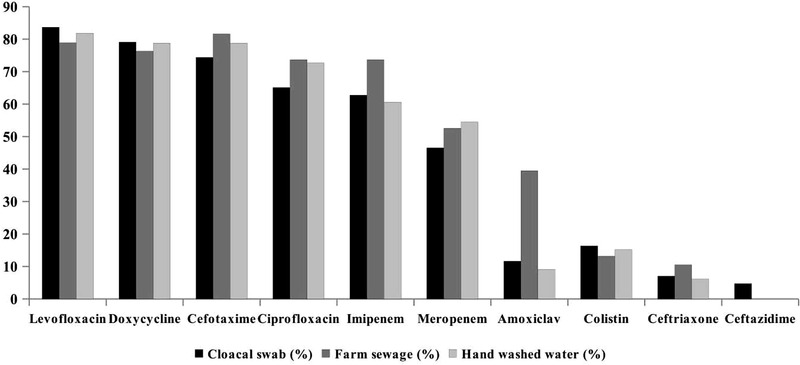
Resistance profile of E. coli isolated from three types of samples
3.4. Multidrug resistance of E. coli
In total, about 76% of E. coli isolates exhibited multidrug resistance which is 78.8%, 76.3%, and 74.4% in case of hand washed water, farm sewage, and cloacal swab, respectively (Table 4). There was no significant difference in MDR percentages among these three types of samples. About 70% isolates were resistant to 4–7 antibiotics, while 27% and 3.5% were resistant to 1–3 and 8–9 antibiotics, respectively (Table 5). High resistance percentages were observed against more than 6 antibiotics in case of farm sewage isolates (52.6%), followed by hand washed water isolates (45.4%). Although, cloacal swab isolates exhibited higher resistance of 51.2% to 4–6 antibiotics. Alarmingly, one farm sewage isolate showed resistance to nine among 10 tested antibiotics.
TABLE 4.
Multidrug resistance (MDR) † was observed among different types of E. coli isolates
| Sample type | Number of isolates | Percentage | p‐Value |
|---|---|---|---|
| Cloacal swab (N = 43) | 32 | 74.4 | 0.906 |
| Farm sewage (N = 38) | 29 | 76.3 | |
| Hand washed water (N = 33) | 26 | 78.8 | |
| Total (N = 114) | 87 | 76.3 |
Note: N = number of isolates.
MDR when isolates are non‐susceptible to at least one antimicrobial agent in ≥3 antimicrobial categories.
TABLE 5.
The distribution of phenotypic resistance of E. coli isolates to number of antibiotics and antibiotic classes
| Number (%) of resistant isolates | ||||
|---|---|---|---|---|
| Number of antibiotics | Cloacal swab (n = 43) | Farm sewage (n = 38) | Hand washed water (n = 33) | Total (n = 114) |
| 1 | 1 (2.3) | 4 (10.5) | 4 (12.1) | 9 (7.9) |
| 2 | 8 (18.6) | 3 (7.9) | 2 (6.1) | 13 (11.4) |
| 3 | 4 (9.3) | 3 (7.9) | 2 (6.1) | 9 (7.9) |
| 4 | 10 (23.3) | 4 (10.5) | 7 (21.2) | 21 (18.4) |
| 5 | 5 (11.6) | 4 (10.5) | 3 (9.1) | 12 (10.5) |
| 6 | 7 (16.3) | 8 (21.1) | 11 (33.3) | 26 (22.8) |
| 7 | 6 (14) | 10 (26.3) | 4 (12.1) | 20 (17.5) |
| 8 | 2 (4.7) | 1 (2.6) | 0 | 3 (2.6) |
| 9 | 0 | 1 (2.6) | 0 | 1 (0.9) |
| Number of antibiotic classes | ||||
| 1 | 3 (7) | 5 (13.2) | 4 (12.1) | 12 (10.5) |
| 2 | 8 (18.6) | 4 (10.5) | 3 (9.1) | 15 (13.2) |
| 3 | 11 (25.6) | 6 (15.8) | 7 (21.2) | 24 (21.1) |
| 4 | 14 (32.6) | 10 (26.3) | 16 (48.5) | 40 (35.1) |
| 5 | 6 (14) | 10 (26.3) | 3 (9.1) | 19 (16.67) |
| 6 | 1 (2.3) | 3 (7.9) | 0 | 4 (3.5) |
Note: n = number of isolates.
In terms of antibiotic class, 72.8% of the total isolates were resistant to 3–5 classes of antibiotics (Table 5). In cloacal swab isolates, resistance to 3–4 antibiotic classes was 58.2%, and more than half of the isolates showed resistance to 4–5 antibiotic classes in case of farm sewage (52.6%) and hand washed water (57.6%). Of note, three farm sewage isolates and one cloacal swab isolate were found resistant to all six antibiotic classes.
All three types of isolates showed a similar trend of resistance to the classes of antibiotics used (Table 6). The highest resistance was observed against quinolones (84.2%) and cephalosporins (80%), followed by tetracyclines (78.1%) and carbapenems (68.4%), while lower resistance was found in polymyxins (14.9%) and penicillin β‐lactamase inhibitors (20.2%) (Table 6).
TABLE 6.
Antibiotic class‐wise resistance percentage of three types of E. coli isolates (tetracyclines: doxycycline; polymyxins: colistin; penicillin β‐lactames inhibitors: amoxyclav; fluoroquinolones: levofloxacin and ciprofloxacin; cephalosporins: ceftazidime, ceftriaxone, and cefotaxime; carbapenems: imipenem, meropenem)
| Number (%) of resistant isolates | ||||
|---|---|---|---|---|
| Antibiotic classes | Cloacal swab (n = 43) | Farm sewage (n = 38) | Hand washed water (n = 33) | Total (n = 114) |
| Fluoroquinolones | 37 (86) | 31 (81.6) | 28 (84.8) | 96 (84.2) |
| Cephalosporins | 33 (76.7) | 31 (81.6) | 26 (78.8) | 90 (80) |
| Penicillin β‐lactames inhibitors | 5 (11.9) | 15 (39.5) | 3 (9.1) | 23 (20.2) |
| Polymyxins | 7 (16.3) | 5 (13.2) | 5 (15.2) | 17 (14.9) |
| Tetracyclines | 34 (79.1) | 29 (76.3) | 26 (78.8) | 89 (78.1) |
| Carbapenems | 28 (65.1) | 28 (73.7) | 22 (66.7) | 78 (68.4) |
Note: n = number of isolates.
3.5. Risk factors for MDR E. coli infection in broilers
The present study revealed the significant risk of infection with MDR E. coli in broiler chickens in the winter season (Tables 7 and 8). Significant risk of MDR E. coli infection was also observed in the broiler chickens of those farms where antibiotics were used without prescription of veterinarians and whose farm personnel did not use specific shoes in the farm.
TABLE 7.
Risk factors for multidrug‐resistant E. coli infection in broiler chickens: results of univariable logistic regression
| Variables | OR | 95% CI | p‐Value | |
|---|---|---|---|---|
| Experience of farming (years) ‡ | ≤5 (n = 28) | 3.07 | 0.74–12.62 | 0.121 |
| >5 (n = 15) | Ref. | – | – | |
| Season ‡ | Winter (December–February) (n = 24) | 5.09 | 1.12–23.14 | 0.035 |
| Pre‐monsoon (March) (n = 19) | Ref. | – | – | |
| Use of farm premises | Only for poultry farming (n = 33) | Ref. | – | – |
| Integrated with other farming (n = 10) | 1.5 | 0.27–8.45 | 0.646 | |
| Distance of natural water body (m) ‡ | <20 (n = 29) | 2.13 | 0.52–8.77 | 0.295 |
| ≥20 (n = 14) | Ref. | – | – | |
| Age of birds (days) | 1–14 (n = 13) | Ref. | – | – |
| 15–35 (n = 30) | 1.46 | 0.34–6.23 | 0.609 | |
| Flock size | ≤1500 (n = 35) | Ref. | – | – |
| >1500 (n = 8) | 0.49 | 0.09–2.53 | 0.397 | |
| Human traffic control system † | Poor (n = 25) | 0.73 | 0.18–3.02 | 0.669 |
| Moderate (n = 18) | Ref. | – | – | |
| Specific shoes for staff ‡ | Yes (n = 18) | Ref. | – | – |
| No (n = 25) | 3.34 | 0.8–13.94 | 0.098 | |
| Litter condition ‡ | Wet (n = 10) | 3.91 | 0.44–35.15 | 0.223 |
| Dry (n = 33) | Ref. | – | – | |
| Litter turning ‡ | Alternate day (n = 16) | Ref. | – | – |
| Once a week (n = 18) | 6.22 | 1.06–36.57 | 0.043 | |
| Twice a week (n = 9) | 2.72 | 0.43–17.42 | 0.29 | |
| Involvement of farmer with other livestock farms ‡ | Yes (n = 18) | 2.35 | 0.53–10.52 | 0.263 |
| No (n = 25) | Ref. | – | – | |
| Other poultry farms within 500 m | Yes (n = 30) | 0.42 | 0.08–2.32 | 0.322 |
| No (n = 13) | Ref. | – | – | |
| Management of dead birds | Burial (n = 33) | Ref. | – | – |
| Put into garbage bin and thrown away (n = 10) | 0.75 | 0.16–3.59 | 0.715 | |
| Use of baits to control rodents ‡ | Yes (n = 14) | Ref. | – | – |
| No (n = 29) | 0.37 | 0.07–2 | 0.25 | |
| Drinkers maintenance | Daily (n = 34) | Ref. | – | – |
| Not daily (n = 9) | 1.26 | 0.22–7.23 | 0.795 | |
| Use of antibiotics | Yes (n = 40) | 1.50 | 0.12–18.36 | 0.749 |
| No (n = 3) | Ref. | |||
| Purpose of antibiotics use | Treatment (n = 19) | Ref. | ||
| Others # (n = 21) | 1.48 | 0.37–5.96 | 0.583 | |
| Follow veterinarian's prescription ‡ | Yes (n = 7) | Ref. | ||
| No (n = 33) | 11.25 | 1.75–72.5 | 0.004 | |
Note: n = number of farms.
Abbreviations: CI, confidence interval; OR, odds ratio.
Total score = 4 (foot‐bath: 1, fence around the farm: 1, gate: 1, and entry restricted: 1).
Variables included in the multivariable logistic model.
Other purposes of antibiotics use include preventive and growth promotion.
TABLE 8.
Risk factors for multidrug‐resistant E. coli infection in broiler chickens: results of multivariable logistic regression
| Variables | OR | 95% CI | p‐Value | |
|---|---|---|---|---|
| Season | Winter (December–February) (n = 24) | 8.39 | 1.10–63.92 | 0.040 |
| Pre‐monsoon (March) (n = 19) | Ref. | – | – | |
| Specific shoes for staff | Yes (n = 18) | Ref. | – | – |
| No (n = 25) | 8.62 | 1.19–62.61 | 0.033 | |
| Follow veterinarian's prescription | Yes | Ref. | – | – |
| No | 18.53 | 1.97–173.9 | 0.011 | |
Note: n = number of farms.
Abbreviations: CI, confidence interval; OR, odds ratio.
3.6. Phenotypic resistance pattern of E. coli isolated from chickens
The distribution of phenotypic resistance patterns among the broiler chicken isolates is illustrated in Figure 3. The most common resistance pattern was levofloxacin–ciprofloxacin‐cefotaxime–doxycycline–imipenem–meropenem found in five isolates. The pattern of levofloxacin‐ciprofloxacin‐cefotaxime‐doxycycline and levofloxacin–ciprofloxacin–cefotaxime–colistin–doxycycline–imipenem–meropenem were found in four isolates followed by levofloxacin‐doxycycline in three isolates of broiler chickens.
FIGURE 3.
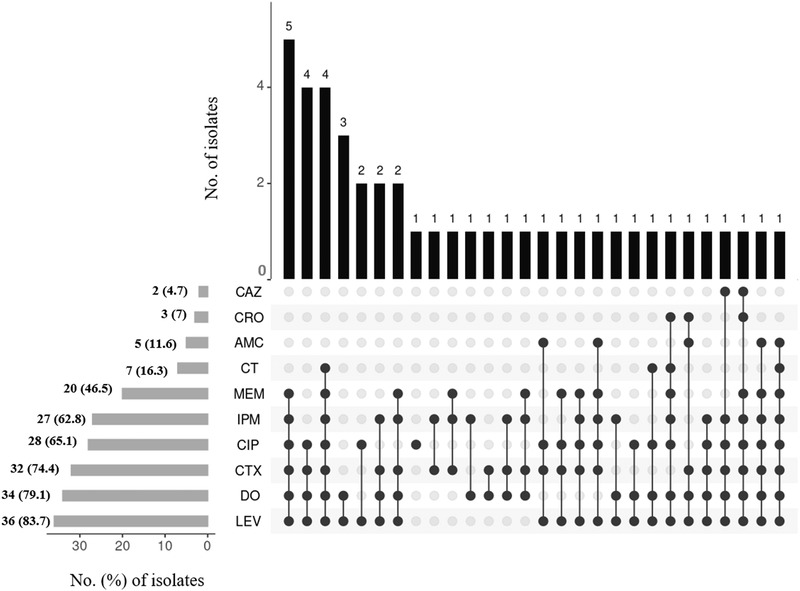
An UpSet plot summarizing the phenotypic resistance pattern of E. coli isolates in broiler chickens. The horizontal bars at the bottom left of the figure show the total number of isolates with percentages resistant to each antimicrobial agent. Joined black circles to the right of these bars indicate that the same phenotypic resistance pattern was common to the number of isolates shown at the top of each vertical bar. Abbreviations: AMC, amoxyclav; CT, colistin; CAZ, ceftazidime; CIP, ciprofloxacin; CRO, ceftriaxone; CTX, cefotaxime; DO, doxycycline; IPM, imipenem; LEV, levofloxacin; MEM, meropenem
3.7. Carbapenem resistance NDM‐1 gene
A total of 114 isolates were screened for the presence of carbapenem resistance NDM‐1 gene. But none of the isolates were found to be positive for the NDM‐1 gene.
4. DISCUSSION
This study documents the updated data on the prevalence along with the AMR profile of E. coli in poultry farms at one health perspective that can be used to establish an integrated AMR surveillance system and can also facilitate the evaluation of interventions used to prevent and control AMR. The overall prevalence of E. coli was 76% and the highest prevalence (86%) was observed in cloacal swab samples which is higher than the previous reports of Hossain et al. (2008), Akond et al. (2009), and Sarker et al. (2019) in Bangladesh. However, Jakaria et al. (2012) and Bashar et al. (2011) reported 82% and 100% prevalence of E. coli in broiler chickens, respectively. Although the reason behind such differences in the prevalence of E. coli is unclear, several factors can contribute to such variations such as regional differences, sample collection techniques, season, and bacterial identification methods. The lower prevalence (66%) in hand washed water samples found in the present study was corroborated by Akond et al. (2009) who reported a lower prevalence of E. coli in hand washed water samples compared to cloacal swab samples. Although there was no report of the prevalence of E. coli in farm sewage samples in the study area, the current study revealed 76% prevalence, which was lower than the prevalence of E. coli isolated from river and sewage water (Nahar et al., 2019; Uddin et al., 2019).
While exploring the risk factors for MDR E. coli infection in broiler chickens, three potential risk factors namely ‘winter season’, ‘absence of specific shoes for staff’, and ‘use of antibiotics without prescription of veterinarians’ were identified. In winter, the birds generally gather together to share the heat allowing to increase the stocking density thus the spread of bacterial infection. Moreover, the stocking density of birds was previously reported as a risk factor for the occurrence of antibiotic‐resistant E. coli in broilers (Persoons et al., 2011). Another variable that was identified as a risk factor for MDR E. coli infection was the absence of specific shoes for the farm staff. The use of no separate shoes allows E. coli to transmit among diverse sources including the human living places. Shared materials between humans and animals may contribute to the transmission of antibiotic‐resistant bacteria from animals to humans and vice versa (Roess et al., 2013). One of the major concerns is the over‐the‐counter sale of antibiotics without prescription that promote irrational use, overuse, and misuse of antibiotics in the animal health as well as human health sectors in most of the developing countries including Bangladesh (Hassan et al., 2021; Kalam et al., 2021; Kumar et al., 2013; Masud et al., 2020). Subsequently, indiscriminate use of antibiotics without prescription contributes to the development and spread of antimicrobial resistance (McEwen & Collignon, 2018; Singer et al., 2003).
In the antimicrobial susceptibility study, we used 10 antibiotics of six different classes. The challenge was not negligible as four (meropenem, ceftriaxone, colistin, and ciprofloxacin) of the antibiotics tested in this study are classified by the World Health Organization as extremely important antibiotics in human medicine, and the other six (levofloxacin, ceftazidime, cefotaxime, amoxyclav, doxycycline, and imipenem) are classified as highly important antibiotics (World Health Organization, 2017). The E. coli isolates in this study exhibited resistance to all classes of the tested antibiotics. High resistance was observed against fluoroquinolones (84.2%), followed by cephalosporin (80%) and tetracycline (78.1%). Among fluoroquinolones, 81.6% and 70.2% of isolates showed resistance to levofloxacin and ciprofloxacin, respectively. A recent study reported 83% resistance to levofloxacin in E. coli isolated from cloacal swab of broiler chickens, which is almost similar to our present finding (Al Azad et al., 2019). A similar high resistance percentage to ciprofloxacin was reported in the previous studies conducted by Akond et al. (2009) (100%) and Bashar et al. (2011) (82%) in poultry birds. Fluoroquinolones particularly ciprofloxacin are widely available and often considered as the most frequently used antibiotic in poultry production in Bangladesh, which was also observed in the present study (K. S. Islam et al., 2016). The increasing tendency to use antibiotics as a preventive measure might be the reason behind this high resistance to fluoroquinolones.
Among cephalosporins, the resistance to cefotaxime was remarkably higher (78.1%) compared to the other two antibiotics of that group. High resistance to cefotaxime was previously reported in broiler farms abroad (Vinueza‐Burgos et al., 2019) and the household water supply in Bangladesh (Talukdar et al., 2013). In addition, the reports on prevalence of cefotaxime resistance genes in poultry, wild birds, and the environment of Bangladesh corroborate the high resistance of cefotaxime found in the present study (Haque et al., 2014; Hasan et al., 2012). The emergence of such resistance genes in poultry production facilities may influence the spread of resistant bacteria among animals and humans. Resistance to tetracycline was found 78.1%, which is lower than the report of Sarker et al. (2019) and Azad et al. (2017) who reported that all of the E. coli isolates from broiler chickens were resistant to tetracycline. Bacterial resistance to tetracycline is of plasmid nature, and the existence of a wide variety of genetic determinants leads to the persistent acquisition of resistance genes by conjugation or transformation (Miles et al., 2006).
It is very alarming to note that in the carbapenem group, 65.8% and 50.9% of the isolates were resistant to imipenem and meropenem, respectively, although there was no record of using those critically used antibiotics in the poultry farms. In the previous studies, 100% susceptibility of E. coli to carbapenem was reported in isolates from poultry (Bashar et al., 2011), household water (Talukdar et al., 2013), and humans (Lina et al., 2014). However, in a recent study, 31.66% and 10% resistance to meropenem and imipenem, respectively, were found in E. coli isolated from cloacal swab of poultry (Sobur et al., 2019). Carbapenem antibiotics are considered as ‘last‐line agents’ as they are used to treat infections due to MDR bacteria that are non‐responsive to other classes of antibiotics (Kamata et al., 2015). Generally, the emergence of carbapenem‐resistant strain could occur through the acquisition of carbapenem‐resistance genes via plasmid‐mediated horizontal gene transfer. However, in this study, none of the E. coli isolates (both carbapenem‐resistant and non‐resistant) harboured NDM‐1 gene, a carbapenem resistance gene. The presence of a carbapenemase is not the only determinant for the resistance to carbapenems. Combinations of different other mechanisms can be responsible for the resistance such as activity of extended‐spectrum beta‐lactamases or ampicillinase C enzymes and decreased permeability of outer membrane (Guerra et al., 2014). Some other important carbapenemases such as Verona imipenemase (VIM), Imipenemase (IMP), K. pneumoniae carbapenemase (KPC), and oxacillinase‐48 (OXA‐48) are responsible for the resistance in gram negative bacteria (Hansen, 2021; Jean et al., 2015), which were not investigated in this study. In addition, not all carbapenemases mediate resistance to all carbapenems (EFSA Panel on Biological Hazards, 2013; Miriagou et al., 2010). Besides, irrational use of antibiotics which allows the long‐term contact between bacteria and antibiotics may contribute to the development and spread of carbapenem resistance (Ye et al., 2018).
In the present study, about 76% of E. coli isolates exhibited multidrug resistance, of which 78.8% isolates from hand washed water, 76.3% isolates from farm sewage, and 74.4% form cloacal swab. Variable percentages (64%–100%) of MDR were reported in E. coli from poultry and its products in Bangladesh (Akhtar et al., 2016; Parvin et al., 2020; Sarker et al., 2019). Concerningly, four isolates from our study showed resistance to all six classes of antibiotics, which poses a risk for the transmission of MDR E. coli from poultry production facilities to human surroundings. It was speculated that the frequent use of antibiotics for preventive purposes or growth‐promoting agents in Bangladesh could reflect this high percentage of MDR E. coli in poultry farms (K. S. Islam et al., 2016). The present study also reported the irrational use of antibiotics as more than half of the respondents use antibiotics based on the suggestion of feed dealers.
The similar phenotypic resistance pattern of E. coli isolates from three different sources observed in the current study implies that there was a possibility of transmission of resistant E. coli between broiler chickens, farmworkers, and the environment. Therefore, the data presented in the current study could act as baseline epidemiological evidence. Further research on genomic and phylogenic relatedness combining with the presented data could help us to accurately identify the transmission networks of E. coli in broiler farms.
5. CONCLUSION
A very high level of antibiotic resistance of E. coli particularly to fluoroquinolones and cephalosporin, was observed, and the isolates from broiler chickens, farmworkers, and sewage showed similar pattern of resistance phenotype. Moreover, the existence of high rates of MDR E. coli was documented, which pose a potential threat to public health. Finally, the data generated in the present study could be useful in formulating and implementing concerted interventions to reduce AMR in human‐animal‐environmental interface in Bangladesh.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
No animal experimentation was done in this study. However, cloacal swabs were collected very softly to avoid any discomfort to the broiler chickens. Farmers who have participated voluntarily were included in this study and the written consent was obtained from them before sampling and data collection.
AUTHOR CONTRIBUTIONS
Conceptualization, investigation, methodology, and writing‐original draft: Amit Kumar Mandal. Data curation, formal analysis, investigation, methodology, and writing‐review & editing: Sudipta Talukder. Investigation and Methodology: Md. Mehedi Hasan and Syeda Tanjina Tasmim. Data curation, project administration, validation, and writing‐review & editing: Mst. Sonia Parvin. Investigation, project administration, and validation: Md. Yamin Ali: Conceptualization, data curation, funding acquisition, investigation, project administration, resources, supervision, validation, and writing‐review & editing: Md. Taohidul Islam:
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.664
ACKNOWLEDGEMENTS
This research was conducted with the financial support from the Bangladesh Agricultural University Research System (Project No. 2018/590/BAU). The authors especially thank the poultry farmers from Mymensingh and Gazipur districts who participated in this study.
Mandal, A. K. , Talukder, S. , Hasan, M. M , Tasmim, S. T. , Parvin, M. S. , Ali, M. Y , & Islam, M. T. (2022). Epidemiology and antimicrobial resistance of Escherichia coli in broiler chickens, farmworkers, and farm sewage in Bangladesh. Veterinary Medicine and Science, 8, 187–199. 10.1002/vms3.664
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Agyare, C. , Boamah, V. E. , Zumbi, C. N. , & Osei, F. B. (2019). Antibiotic use in poultry production and its effects on bacterial resistance. In Y. Kumar (Ed.), Antimicrobial resistance—A global threat. (33–51). IntechOpen. [Google Scholar]
- Akhtar, F. , Rabbani, M. , Muhammad, K. , Younus, M. , Ghafoor, A. , Sheikh, A. , Ahmad, A. , Muhammad, J. , Rasool, A. , & Shaheen, A. (2016). Comparative antibiotic resistance profile of the multidrug resistant E. coli isolated from commercial and backyard poultry. Journal of Animal & Plant Sciences, 26, 1628–1632. [Google Scholar]
- Akond, M. A. , Alam, S. , Hassan, S. , & Shirin, M. (2009). Antibiotic resistance of Escherichia coli isolated from poultry and poultry environment of Bangladesh. Internet Journal of Food Safety, 11, 19–23. [Google Scholar]
- Al Azad, M. , Rahman, A. , Rahman, M. , Amin, R. , Begum, M. , Ara, I. , Fries, R. , Husna, A. , Khairalla, A. S. , & Badruzzaman, A. (2019). Susceptibility and multidrug resistance patterns of Escherichia coli isolated from cloacal swabs of live broiler chickens in Bangladesh. Pathogens, 8, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir, M. , Riaz, M. , Chang, Y. F. , Akhtar, S. , Nadeem, H. , Ahmad, Z. , & Nadeem, M. (2019). Spread of antibiotic resistant Escherichia coli from broiler to human populations. Pakistan Journal of Agricultural Sciences, 56, 977–983. [Google Scholar]
- Ayukekbong, J. A. , Ntemgwa, M. , & Atabe, A. N. (2017). The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrobial Resistance & Infection Control, 6, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad, M. , Amin, R. , Begum, M. , Fries, R. , Lampang, K. , & Hafez, H. (2017). Prevalence of antimicrobial resistance of Escherichia coli isolated from broiler at Rajshahi region, Bangladesh. British Journal of Biomedical and Multidisciplinary Research, 1, 6–12. [Google Scholar]
- Bashar, T. , Rahman, M. , Rabbi, F. A. , Noor, R. , & Rahman, M. M. (2011). Enterotoxin profiling and antibiogram of Escherichia coli isolated from poultry feces in Dhaka District of Bangladesh. Stamford Journal of Microbiology, 1, 51–57. [Google Scholar]
- Begum, N. , & Shamsuzzaman, S. (2016). Emergence of carbapenemase‐producing urinary isolates at a tertiary care hospital in Dhaka, Bangladesh. Tzu Chi Medical Journal, 28, 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute . (2018). Performance standards for antimicrobial susceptibility testing. Twenty eight international supplement. Clinical and Laboratory Standards Institute. [Google Scholar]
- Dashti, A. A. , Jadaon, M. M. , Abdulsamad, A. M. , & Dashti, H. M. , 2009. Heat treatment of bacteria: A simple method of DNA extraction for molecular techniques. Kuwait Medical Journal, 41, 117–122. [Google Scholar]
- Ferri, M. , Ranucci, E. , Romagnoli, P. , & Giaccone, V. (2017). Antimicrobial resistance: A global emerging threat to public health systems. Critical Reviews in Food Science and Nutrition, 57, 2857–2876. [DOI] [PubMed] [Google Scholar]
- Guerra, B. , Fischer, J. , & Helmuth, R. (2014). An emerging public health problem: Acquired carbapenemase‐producing microorganisms are present in food‐producing animals, their environment, companion animals and wild birds. Veterinary Microbiology, 171, 290–297. [DOI] [PubMed] [Google Scholar]
- Hamid, M. , Rahman, M. , Ahmed, S. , & Hossain, K. (2017). Status of poultry industry in Bangladesh and the role of private sector for its development. Asian Journal of Poultry Science, 11, 1–13. [Google Scholar]
- Hansen, G. T. (2021). Continuous evolution: Perspective on the epidemiology of carbapenemase resistance among enterobacterales and other gram‐negative bacteria. Infectious Diseases and Therapy, 10, 75–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque, A. , Yoshizumi, A. , Saga, T. , Ishii, Y. , & Tateda, K. (2014). ESBL‐producing Enterobacteriaceae in environmental water in Dhaka, Bangladesh. Journal of Infection and Chemotherapy, 20, 735–737. [DOI] [PubMed] [Google Scholar]
- Hasan, B. , Sandegren, L. , Melhus, Å. , Drobni, M. , Hernandez, J. , Waldenström, J. , Alam, M. , & Olsen, B. (2012). Antimicrobial drug–resistant Escherichia coli in wild birds and free‐range poultry, Bangladesh. Emerging Infectious Diseases, 18, 2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan, M. M. , Kalam, M. , Alim, M. , Shano, S. , Nayem, M. , Khan, R. , Badsha, M. , Al Mamun, M. , Hoque, A. , & Tanzin, A. Z. (2021). Knowledge, attitude, and practices on antimicrobial use and antimicrobial resistance among commercial poultry farmers in Bangladesh. Antibiotics, 10, 784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Panel on Biological Hazards (2013). Scientific opinion on Carbapenem resistance in food animal ecosystems. EFSA Journal, 11, 3501. [Google Scholar]
- Hossain, M. , Siddique, M. , Hossain, F. , Zinnah, M. , Hossain, M. , Alam, M. , Rahman, M. , & Choudury, K. (2008). Isolation, identification, toxin profile and antibiogram of Escherichia coli isolated from broilers and layers in Mymensingh district of Bangladesh. Bangladesh Journal of Veterinary Medicine, 6, 1–5. [Google Scholar]
- Hussain, A. , Shaik, S. , Ranjan, A. , Nandanwar, N. , Tiwari, S. K. , Majid, M. , Baddam, R. , Qureshi, I. A. , Semmler, T. , & Wieler, L. H. (2017). Risk of transmission of antimicrobial resistant Escherichia coli from commercial broiler and free‐range retail chicken in India. Frontiers in Microbiology, 8, 2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, K. S. , Shiraj‐Um‐Mahmuda, S. , & Hazzaz‐Bin‐Kabir, M. (2016). Antibiotic usage patterns in selected broiler farms of Bangladesh and their public health implications. Journal of Public Health in Developing Countries, 2, 276–284. [Google Scholar]
- Islam, M. , Talukdar, P. K. , Hoque, A. , Huq, M. , Nabi, A. , Ahmed, D. , Talukder, K. , Pietroni, M. , Hays, J. , & Cravioto, A. (2012). Emergence of multidrug‐resistant NDM‐1‐producing Gram‐negative bacteria in Bangladesh. European Journal of Clinical Microbiology & Infectious Diseases, 31, 2593–2600. [DOI] [PubMed] [Google Scholar]
- ISO . (2001). Microbiology of food and animal feeding stuffs—Horizontal method for the detection of Escherichia coli O157. International Organization for Standardization, Geneva, Switzerland. [DOI] [PubMed]
- Jakaria, A. , Islam, M. A. , & Khatun, M. M. , 2012. Prevalence, characteristics and antibiogram profiles of Escherichia coli isolated from apparently healthy chickens in Mymensingh, Bangladesh. Microbes and Health, 1, 27–29. [Google Scholar]
- Jean, S.‐S. , Lee, W.‐S. , Lam, C. , Hsu, C.‐W. , Chen, R.‐J. , & Hsueh, P.‐R. (2015). Carbapenemase‐producing Gram‐negative bacteria: Current epidemics, antimicrobial susceptibility and treatment options. Future Microbiology, 10, 407–425. [DOI] [PubMed] [Google Scholar]
- Kalam, M. , Alim, M. , Shano, S. , Nayem, M. , Khan, R. , Badsha, M. , Mamun, M. , Al, A. , Hoque, A. , & Tanzin, A. Z. (2021). Knowledge, attitude, and practices on antimicrobial use and antimicrobial resistance among poultry drug and feed sellers in Bangladesh. Veterinary Sciences, 8, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata, K. , Suzuki, H. , Kanemoto, K. , Tokuda, Y. , Shiotani, S. , Hirose, Y. , Suzuki, M. , & Ishikawa, H. (2015). Clinical evaluation of the need for carbapenems to treat community‐acquired and healthcare‐associated pneumonia. Journal of Infection and Chemotherapy, 21, 596–603. [DOI] [PubMed] [Google Scholar]
- Kumar, S. G. , Adithan, C. , Harish, B. , Sujatha, S. , Roy, G. , & Malini, A. (2013). Antimicrobial resistance in India: A review. Journal of Natural Science, Biology and Medicine, 4, 286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lina, T. T. , Khajanchi, B. K. , Azmi, I. J. , Islam, M. A. , Mahmood, B. , Akter, M. , Banik, A. , Alim, R. , Navarro, A. , & Perez, G. (2014). Phenotypic and molecular characterization of extended‐spectrum beta‐lactamase‐producing Escherichia coli in Bangladesh. PloS One, 9, e108735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magiorakos, A. P. , Srinivasan, A. , Carey, R. , Carmeli, Y. , Falagas, M. , Giske, C. , Harbarth, S. , Hindler, J. , Kahlmeter, G. , & Olsson‐Liljequist, B. (2012). Multidrug‐resistant, extensively drug‐resistant and pandrug‐resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clinical Microbiology and Infection, 18, 268–281. [DOI] [PubMed] [Google Scholar]
- Marshall, B. M. , & Levy, S. B. (2011). Food animals and antimicrobials: Impacts on human health. Clinical Microbiology Reviews, 24, 718–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masud, A. A. , Rousham, E. K. , Islam, M. A. , Alam, M. ‐U. , Rahman, M. , Mamun, A. A. , Sarker, S. , Asaduzzaman, M. , & Unicomb, L. (2020). Drivers of antibiotic use in poultry production in Bangladesh: Dependencies and dynamics of a patron‐client relationship. Frontiers in Veterinary Science, 7, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen, S. A. , & Collignon, P. J. (2018). Antimicrobial resistance: A one health perspective. Microbiology Spectrum, 6, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles, T. D. , McLaughlin, W. , & Brown, P. D. (2006). Antimicrobial resistance of Escherichia coli isolates from broiler chickens and humans. BMC Veterinary Research, 2, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miriagou, V. , Cornaglia, G. , Edelstein, M. , Galani, I. , Giske, C. , Gniadkowski, M. , Malamou‐Lada, E. , Martinez‐Martinez, L. , Navarro, F. , & Nordmann, P. (2010). Acquired carbapenemases in Gram‐negative bacterial pathogens: Detection and surveillance issues. Clinical Microbiology and Infection, 16, 112–122. [DOI] [PubMed] [Google Scholar]
- Nahar, A. , Islam, M. A. , Sobur, M. A. , Hossain, M. J. , Zaman, S. B. , Rahman, M. B. , Kabir, S. L. , & Rahman, M. T. (2019). Detection of tetracycline resistant E. coli and Salmonella spp. in sewage, river, pond and swimming pool in Mymensingh, Bangladesh. African Journal of Microbiology Research, 13, 382–387. [Google Scholar]
- Norizuki, C. , Wachino, J.‐I. , Suzuki, M. , Kawamura, K. , Nagano, N. , Kimura, K. , & Arakawa, Y. (2017). Specific blaCTX‐M‐8/IncI1 plasmid transfer among genetically diverse Escherichia coli isolates between humans and chickens. Antimicrobial Agents and Chemotherapy, 61, e00663‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma, E. , Tilocca, B. , & Roncada, P. (2020). Antimicrobial resistance in veterinary medicine: An overview. International Journal of Molecular Sciences, 21, 1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvin, M. , Talukder, S. , Ali, M. , Chowdhury, E. H. , Rahman, M. , & Islam, M. (2020). Antimicrobial resistance pattern of Escherichia coli isolated from frozen chicken meat in Bangladesh. Pathogens, 9, 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persoons, D. , Haesebrouck, F. , Smet, A. , Herman, L. , Heyndrickx, M. , Martel, A. , Catry, B. , Berge, A. C. , Butaye, P. , & Dewulf, J. (2011). Risk factors for ceftiofur resistance in Escherichia coli from Belgian broilers. Epidemiology & Infection, 139, 765–771. [DOI] [PubMed] [Google Scholar]
- Rasheed, M. U. , Thajuddin, N. , Ahamed, P. , Teklemariam, Z. , & Jamil, K. (2014). Antimicrobial drug resistance in strains of Escherichia coli isolated from food sources. Revista do Instituto de Medicina Tropical de São Paulo, 56, 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roess, A. A. , Winch, P. J. , Ali, N. A. , Akhter, A. , Afroz, D. , El Arifeen, S. , Darmstadt, G. L. , Baqui, A. H. , & Group, B. P. S. (2013). Animal husbandry practices in rural Bangladesh: Potential risk factors for antimicrobial drug resistance and emerging diseases. American Journal of Tropical Medicine and Hygiene, 89, 965–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker, M. S. , Mannan, M. S. , Ali, M. Y. , Bayzid, M. , Ahad, A. , & Bupasha, Z. B. (2019). Antibiotic resistance of Escherichia coli isolated from broilers sold at live bird markets in Chattogram, Bangladesh. Journal of Advanced Veterinary and Animal Research, 6, 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, T. M. , Rose, J. B. , Jenkins, T. M. , Farrah, S. R. , Lukasik, J. (2002). Microbial source tracking: Current methodology and future directions. Applied and Environmental Microbiology, 68, 5796–5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer, R. S. , Finch, R. , Wegener, H. C. , Bywater, R. , Walters, J. , & Lipsitch, M. (2003). Antibiotic resistance—The interplay between antibiotic use in animals and human beings. The Lancet Infectious Diseases, 3, 47–51. [DOI] [PubMed] [Google Scholar]
- Sobur, M. A. , Ievy, S. , Haque, Z. F. , Nahar, A. , Zaman, S. B. , & Rahman, M. T. (2019). Emergence of colistin‐resistant Escherichia coli in poultry, house flies, and pond water in Mymensingh, Bangladesh. Journal of Advanced Veterinary and Animal Research, 6, 50–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg, Z. R. , Johnson, J. R. , Fairbrother, J. M. , Kilbourne, J. , Van Goor, A. , Curtiss, R. 3rd , & Mellata, M. (2017). Evaluation of Escherichia coli isolates from healthy chickens to determine their potential risk to poultry and human health. PloS One, 12, e0180599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar, P. K. , Rahman, M. , Rahman, M. , Nabi, A. , Islam, Z. , Hoque, M. M. , Endtz, H. P. , & Islam, M. A. (2013). Antimicrobial resistance, virulence factors and genetic diversity of Escherichia coli isolates from household water supply in Dhaka, Bangladesh. Plos One, 8, e61090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toleman, M. A. , Bugert, J. J. , & Nizam, S. A. (2015). Extensively drug‐resistant New Delhi metallo‐β‐lactamase–encoding bacteria in the environment, Dhaka, Bangladesh, 2012. Emerging Infectious Diseases, 21, 1027–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin, M. , Hossain, M. T. , Proshad, R. , Kormoker, T. , Chandra, K. , & Rimi, T. A. (2019). Identification of pathogenic Escherichia coli strain from river and sewage water in Bangladesh. Archives of Agriculture and Environmental Science, 4, 39–44. [Google Scholar]
- Vinueza‐Burgos, C. , Ortega‐Paredes, D. , Narváez, C. , De Zutter, L. , & Zurita, J. (2019). Characterization of cefotaxime resistant Escherichia coli isolated from broiler farms in Ecuador. PLoS One, 14, e0207567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R. F. , Cao, W. W. , & Cerniglia, C. E. (1996). PCR detection and quantitation of predominant anaerobic bacteria in human and animal fecal samples. Applied and Environmental Microbiology, 62, 1242–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2017). Critically important antimicrobials for human medicine: Ranking of antimicrobial agents for risk management of antimicrobial resistance due to non‐human use. Second WHO Expert Meeting. World Health Organization. [Google Scholar]
- Ye, Y. , Xu, L. , Han, Y. , Chen, Z. , Liu, C. , & Ming, L. (2018). Mechanism for carbapenem resistance of clinical Enterobacteriaceae isolates. Experimental and Therapeutic Medicine, 15, 1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


